Abstract
Processes such as aberrant redox signaling and chronic low-grade systemic inflammation have been reported to modulate age-associated pathologies such as cognitive impairment. Curcumin, the primary therapeutic component of the Indian spice, Turmeric (Curcuma longa), has long been known for its strong anti-inflammatory and antioxidant activity attributable to its unique molecular structure. Recently, an interest in this polyphenol as a cognitive therapeutic for the elderly has emerged. The purpose of this paper is to critically review preclinical and clinical studies that have evaluated the efficacy of curcumin in ameliorating and preventing age-associated cognitive decline and address the translational progress of preclinical to clinical efficacy. PubMed, semantic scholar, and Google scholar searches were used for preclinical studies; and clinicaltrials.gov, the Australian and New Zealand clinical trials registry, and PubMed search were used to select relevant completed clinical studies. Results from preclinical studies consistently demonstrate curcumin and its analogues to be efficacious for various aspects of cognitive impairment and processes that contribute to age-associated cognitive impairment. Results of published clinical studies, while mixed, continue to show promise for curcumin’s use as a therapeutic for cognitive decline but overall remain inconclusive at this time. Both in vitro and in vivo studies have found that curcumin can significantly decrease oxidative stress, systemic inflammation, and obstruct pathways that activate transcription factors that augment these processes. Future clinical studies would benefit from including evaluation of peripheral and cerebrospinal fluid biomarkers of dementia and behavioral markers of cognitive decline, as well as targeting the appropriate population.
Keywords: Curcumin, Cognition, Aging
Introduction
Age-associated cognitive decline lowers the quality of life and increases the cost of living for the affected individual and their families. Known risk factors for cognitive decline include heart disease, stroke, high blood pressure, high cholesterol, and diabetes. Aging, however, remains the greatest risk factor due to processes such as oxidative stress and inflammation that increase with age, both of which exacerbate cognitive decline via homeostatic imbalance. Neuroinflammation has been tied to disease progression and severity in Alzheimer’s disease (AD) where misfolded and aggregated proteins trigger an immune response resulting in neuronal death and progressive cognitive decline (Cai et al. 2014; Di Benedetto et al. 2017; Heneka et al. 2015). Alzheimer’s disease affects 10% of individuals over the age of 65, rising to over 40% in those aged over 85 (Qiu et al. 2009). Oxidative stress, which results in cumulative oxidative damage with aging, is generated by the accumulation of oxygen free radicals. Cumulative oxidative damage has been mechanistically implicated in cell death and over time systemic functional debilitation (Droge and Schipper 2007; Mariani et al. 2005; Niedzielska et al. 2016). Suzuki and colleagues examined a set of Okinawan centenarians and their family members to examine what factors contributed to their long life expectancy and healthy life span. They reported that there is a progressive increase in lipid peroxidation levels with age, which was not the case for the successfully aging centenarians. The drop in oxidative stress in centenarians compared to those younger was attributed to their adherence to a high antioxidant dietary regimen (Suzuki et al. 2010).
Dietary modifications and/or nutraceutical supplementation are attractive avenues for the treatment of age-associated diseases; as monotarget therapies are showing less and less promise (Doody et al. 2014; Salloway et al., 2014). In order to understand how dietary interventions affect aging at the molecular level, much attention is being given to their individual components, most of which either have high polyphenol component or produce polyphenols as secondary metabolites. Polyphenols are the largest group of phytochemicals and have been reported to have strong antioxidant activities in both in vitro and in vivo studies (Molino et al. 2016; Schaffer et al. 2012). Curcumin, a polyphenol with strong antioxidant activity, is the active component responsible for the yellow color of the spice turmeric and makes up ~ 5% of the spice. Turmeric is one of the most widely used spices throughout the world, with a rich history as a herbal supplement in both ancient China and India (Goel et al. 2008; Gupta et al. 2013; Lee et al. 2013). Curcumin is the key component responsible for the major therapeutic properties attributed to turmeric including antioxidant, anti-inflammatory, anti-mutagenic, and anti-microbial activities among others (Anand et al. 2011; Shehzad et al. 2013). Curcumin’s molecular structure and its ability to cross the blood-brain barrier provide a promising avenue for neuroprotection. It is the presence of both oxidative stress and inflammation around neurons and glial cells that is significantly associated with brain aging and injury (Polazzi and Monti 2010). Reports have suggested lower dementia prevalence in South Asia to be directly attributable to the amount of turmeric used in daily cooking (Ng et al. 2006).
Epidemiology studies
The lower dementia prevalence and high consumption of turmeric in South Asia has led to much anecdotal evidence and epidemiological studies attributing lower dementia rates to higher curry consumption. A 2-year prospective study in an Indian village using the Clinical Dementia Rating (CDR) scale to determine the age-specific incidence of AD found a significantly higher incidence of AD in an age-matched rural US community (Chandra et al. 2001).The authors however noted that there were certain confounding factors that could have affected the result such as ε4 allele of the apolipoprotein E gene (APOE4) status and mortality rates. Another study reported on the prevalence of all types of dementia in an urban Indian population. They used a diagnostic determination based on joint decisions by neurologists, psychiatrists, and psychologists using criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), CDR, and analysis daily living activities. While the prevalence of dementia based on age was the same, the incidence was lower than that reported in developed countries (Vas et al. 2001). In 2006, Ng and colleagues reported that non-demented individuals in Singapore between the ages of 60–93 who “often or very often” or “occasionally” consumed curry performed significantly better on the Mini-Mental State Exam (MMSE) compared to those who “never or rarely” consumed curry (Ng et al. 2006). This is one of the few population-based studies that reported on life-long curry consumption and memory rating within the population itself, thereby avoiding some of the cultural biases and genetic confounding factors that were reported by the predecessors.
Results from these studies and public health data from this region when compared to Western countries indicated an association between curry consumption particularly turmeric and better cognitive function in elderly subjects. Further, due to curcumin’s multi-faceted pharmacology, extensive effort has been put toward the possibility of using curcumin to treat or prevent age-related neurodegenerative diseases such as AD, Parkinson’s disease (PD), and cerebrovascular disease (Bigford and Del Rossi 2014; Mourtas et al. 2014; Siddique et al. 2014).
Curcumin molecular structure
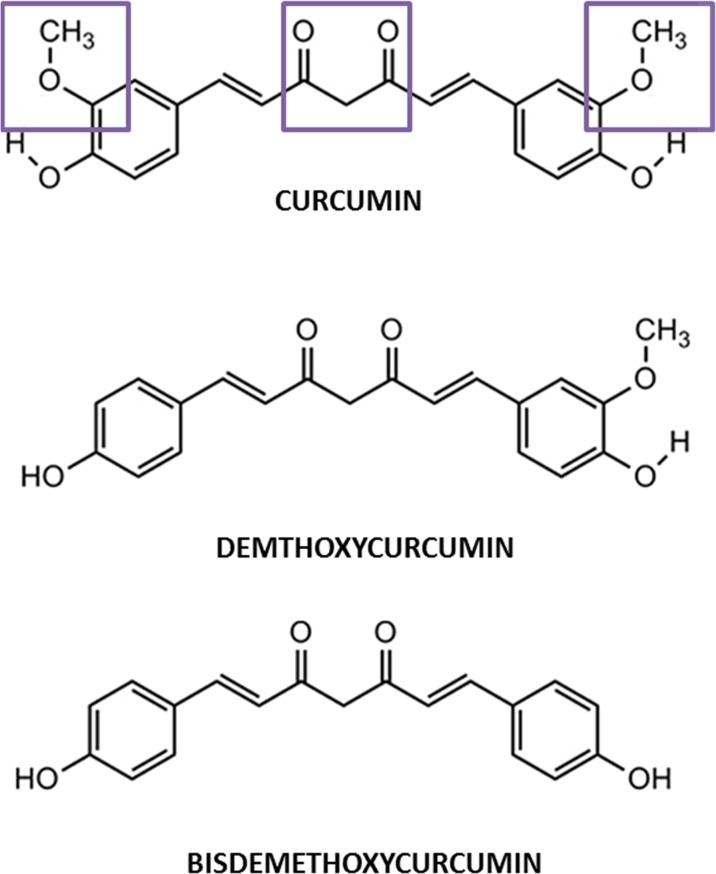
Turmeric consists of three important components also called curcuminoids, curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC) (Fig. 1). Curcumin, also known as diferuloylmethane, is a low molecular mass polyphenol compound and is the most abundant among the three making up about 77% of the curcuminoids (Lee et al. 2013). It also has been reported to have better antioxidant and anti-inflammatory action compared to the other two curcuminoids (Anand et al., 2008). Curcumin consists of two aryl rings containing ortho-methoxy phenolic hydroxyl (OH) groups that are symmetrically linked to a β-diketone moiety (Fig. 1). The occurrence of intramolecular hydrogen atoms transfer at the β-diketone chain of curcumin leads to the existence of keto and enol tautomeric conformations in equilibrium. Curcumin’s unique molecular structure contributes to its strong antioxidant and anti-inflammatory properties.
Fig. 1.
Molecular structures of curcumin, demethoxycurcumin, and bisdemethoxycurcumin
Curcumin and the KEAP1-NRF2-ARE system
The KEAP1-NRF2-ARE is an endogenous antioxidant system that is activated by mild to moderate levels of stress-induced reactive oxygen species (ROS)/free radicals (Motohashi and Yamamoto 2004). In a state of mild to moderate stress, the conformation of Kelch-like ECH-associated protein 1 (KEAP1) changes due to modification of its cysteine residues undergoing a Michael addition reaction (Nguyen et al. 2009). The conformational change in KEAP1 breaks the bond between KEAP1 and nuclear factor E2-related factor 2 (NRF2) releasing a phosphorylated NRF2, which then undergoes nuclear translocation binding and inducing transcription of antioxidant response elements (ARE) (Itoh et al. 2015; Petri et al. 2012; Satoh et al. 2013). The induction of ARE upregulates endogenous antioxidant enzyme synthesis and increases detoxification enzymes production (Itoh et al. 2015; Sykiotis et al. 2011). One of these enzymes includes the glutamate cysteine ligase catalytic subunit (GCLc) (Bea et al. 2003; Mani et al. 2013). GCLc is a rate-limiting enzyme in glutathione synthesis. Glutathione in its native form (GSH) is a strong antioxidant, which is synthesized in the liver and is the body’s principal non-protein thiol tripeptide (DeLeve and Kaplowitz 1990). The redox state of a cell is often defined by the ratio of reduced (GSH) to oxidized form of glutathione, called glutathione disulfide (GSSG) (Zhang et al. 2013). This ratio has been used in preclinical and clinical studies as a sensitive marker for systemic oxidative stress. Depletion of GSH or a decrease in its redox state (GSH/GSSG) has been implicated in several neurodegenerative pathologies like AD and PD as well as in normal aging (Bermejo et al. 2008; Zhu et al. 2006).
The o-methoxy group that flanks the curcumin base molecular structure makes it a strong free radical scavenger. The hydrogen bonding interaction between the phenolic OH and the o-methoxy groups greatly modulates the OH bond energy enabling the hydrogen atom to be acquired by free radicals, stabilizing them (Esatbeyoglu et al. 2012). Along with curcumin’s molecular structure enabling it to be a strong antioxidant, its binding capability to KEAP1 due to its electrophilic properties makes it a strong NRF2 inducer. Curcumin acts as an electrophile and binds to KEAP1 via the sulfhydryl (SH) group, which leads to cysteine modification and stabilizes NRF2 (Magesh et al. 2012) (Fig. 2). The stabilization and separation of NRF2 from KEAP1 leads to its nuclear translocation, and transcription of ARE induces phase II antioxidant genes which activates enzymes that synthesize GSH (Esatbeyoglu et al. 2012; Trujillo et al. 2014).
Fig. 2.
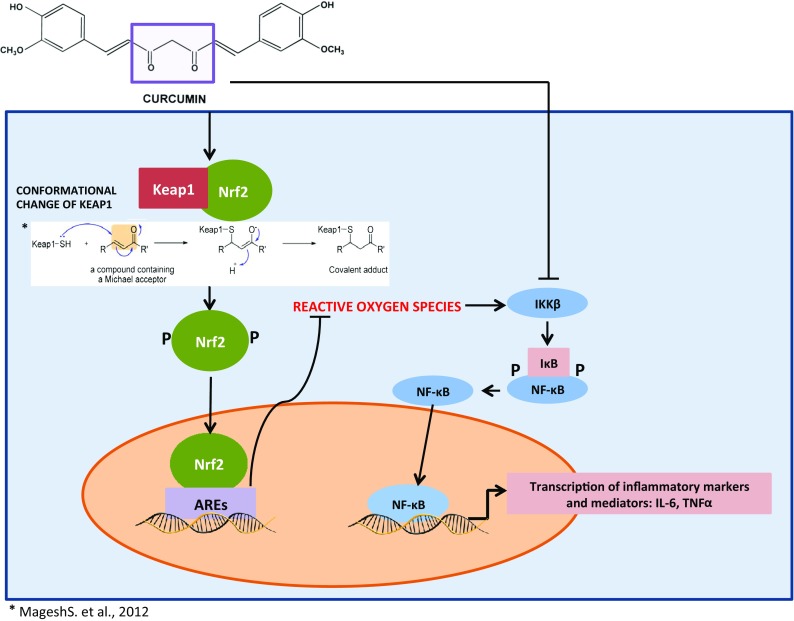
Curcumin modulating antioxidant and anti-inflammatory pathway
Curcumin and the NF-κB system
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor has been reported to be the master regulator of inflammatory processes and is extremely sensitive to oxidative stimuli sharing a reciprocal relationship. NF-κB activity is modulated by upstream signaling pathways, which includes the inhibitor of nuclear factor kappa-B kinase subunit B (IKKβ). The activated IΚΚ complexes phosphorylate the IκΒ subunits of NF-κB leading to the degradation of IκΒ allowing the nuclear translocation of NF-κB and transcription of proinflammatory cytokines. This system is an integral part of the body’s immune system and is strictly regulated. However, as individuals age, this well-controlled system gradually loses its integrity and results in continued transcription of NF-κB, inducing systemic chronic low-grade inflammation. Some of the downstream NF-κB induced proteins, tumor necrosis factor-α (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6) themselves, are potent NF-κB activators and form an auto-activating loop (Hoesel and Schmid 2013; Lawrence 2009).
In human studies, circulating levels of pro-inflammatory cytokines have been reported to increase with aging; increased concentrations of IL-6, TNF-α, and IL-1β have been reported in both the periphery and central nervous system (CNS) leading to age-associated chronic low-grade systemic inflammation, also known as inflammaging (Sankowski et al. 2015; Winklewski et al. 2015). The β-diketone moiety in the curcumin molecular structure is a Michael reaction acceptor, which by binding to IKKβ inhibits nuclear translocation of NF-κB (Anand et al. 2008; Anand et al. 2011; Fujisawa et al. 2004) (Fig. 2). In addition, Devi and colleagues recently reported curcumin strongly inhibiting the expression of glycoprotein 130, a critical molecule in IL-6 signaling, along with inhibiting phosphorylation and nuclear localization of the signal transducer and activator of transcription 3 (STAT3), another well-known downstream mediator of IL-6 signaling. In the same study, expression of IL-1β-induced mRNA-binding protein YB-1 (p50) in uterine decidual cells was repressed by curcumin (Bhattacharya et al. 2015). The synthesis of high sensitivity C-reactive protein (CRP), a biomarker for systemic inflammation, in hepatocytes is induced by proinflammatory cytokines primarily IL-6. Elevated CRP and IL-6 were reported to be indicative of cognitive decline in both obesity and aging (Puzianowska-Kuznicka et al. 2016).
Reviews of the literature have extensively covered various mechanisms by which curcumin can modulate inflammation and oxidative stress, but have focused on its potential as a therapeutic for conditions such as obesity, diabetes, and cancer (Alappat and Awad 2010; Jimenez-Osorio et al. 2016; Shanmugam et al. 2015). Several separate preclinical and clinical reviews have reported on curcumin but most have been in comparison with other nutritional strategies and/or polyphenolic compounds in their effect on cognitive impairment (Mazzanti and Di Giacomo 2016; Vivar 2015).
In the past 10 years, a body of preclinical studies using different models of aging has emerged that investigates curcumin’s potential in preserving cognition in normal aging as well as in neurodegenerative disorders. While previous reviews have highlighted how curcumin and compounds similar to it in structure and function can affect cognition, none have provided a detailed overview on the recent preclinical and clinical studies on curcumin’s efficacy for cognition and what mechanisms of action may contribute to this effect on memory. Therefore, the current report fills this gap by reviewing curcumin’s mechanism of action associated with cognitive improvement and the translational progress to date in curcumin’s potential as a cognitive therapeutic agent. The research question addressed by this report is as follows: what is the current state in the translation of research for curcumin as a therapeutic agent for cognitive aging and aging-associated pathological cognitive decline? To answer this question, this narrative review has been divided into three parts: (1) in-depth comparison and contrast of current preclinical studies to inform the future development of clinical trials, (2) examination of completed clinical studies, and (3) identification of translational successes and key barriers through an integrated interprofessional perspective.
Methods
PubMed and the Semantic Scholar database search engines, and the clinicaltrials.gov and anzctr.org.au sites were used to identify the studies relevant to this narrative review. The paper includes preclinical studies that have reported on the effects of curcumin on cognition in the past 10 years as well as completed clinical trials/studies/cases. Keywords used for the preclinical/animal studies search were “curcumin” AND “aging” AND “cognition.” The search output 24 results in PubMed and 15 in Semantic Scholar. Previous reviews that resulted from the search were omitted from this paper. Only reports that tested for curcumin’s efficacy for cognition in different animal experimental models of aging were included, resulting in a total of 15 preclinical studies. The keywords used for the clinical studies section were “curcumin,” AND “memory,” AND “aging” with the filter “human” applied. Among the papers generated, five published studies were selected for direct relevance to this review. Because this is a narrative review, all clinical studies were included regardless of sample size. To identify completed clinical trials, we searched for studies on clinicaltrials.gov and the Australian New Zealand clinical trials registry. Due to the lack of consistency in methodologies between studies, we have provided methodological details for the purposes of comparative review.
Preclinical studies
Transgenic studies
Several preclinical studies have been conducted on rodent models of AD since it accounts for approximately 70% late-onset cases of dementia. Most of these rodent models are transgenic models, which include mutations at the gene for amyloid precursor protein (APP) and Presenilin 1 (PS1) and Presenilin 2 (PS2) such as APPswe/ind, Tg2576, and 3xTgAD; some of these mutations have been identified in a subset of familial cases of AD (Bilkei-Gorzo 2014). Familial forms of AD, just like sporadic AD, have identical behavioral deficits such as cognitive decline and molecular morbidities such as neuroinflammation and oxidative stress. A classic presentation of AD also includes neuritic plaques and neuron loss, which these transgenic models present.
The senescence accelerated mouse (SAM)-prone 8 (SAMP8) mice display a phenotype of accelerated aging and are frequently used for aging studies (Morley et al. 2012). Sun and colleagues evaluated the efficacy of curcumin in this model of accelerated aging. The mice were randomly assigned to four different groups where SAM-resistant mice were the negative control and the untreated SAMP8 mice were the positive control. In the treated SAMP8 mice, intragastric administration of curcumin (20 or 50 mg/kg) for 25 days had a dose-dependent effect on cognition (decreased latency in the Morris Water Maze (MWM)) where the mice given the highest dose had the same latency as that reported with SAMR1 mice. The decreased expression of oxidative stress biomarkers (hippocampal malondialdehyde (MDA) and superoxide dismutase (SOD) content) were also dose dependent. Both doses also improved the expression of phosphorylated N-methyl-d-aspartic acid receptor 1 (NMDAR1) in the hippocampal membrane. There was also increased intensity in the immunohistochemical staining of the p-calcium/calmodulin-dependent kinase II (CAMKII) in the stratum lucidem area of the CA3 region in the hippocampus of the mice that received the highest dose indicating an improvement in synaptic plasticity (Sun et al. 2013).
In a study using a double knockout model (APP/PS1), cognitive and autophagy markers were measured in mice that were either on a low (160 ppm) or a high (1000 ppm) curcumin supplemented diet for 6 months. Autophagy is a degradative process where damaged organelles, cell constituents, and protein aggregates are recycled in special lysosomes called autophagosomes (Murrow and Debnath 2013; Ravanan et al. 2017). After a 6-month dietary intervention, the mice underwent cognitive tests using MWM. Mice fed a curcumin diet had better working and long-term memory in a dose-dependent manner. These mice also had a reduction in the amyloid beta 42 (Aβ42) aggregates and better clearance of the dissolved aggregates. There was a significantly higher number of autophagosomes in the CA1 region of the curcumin groups along with an increased expression of Beclin 1 and downregulation of the PI3K/Akt/mTOR signaling pathway; these are biomarkers of autophagy (Wang et al. 2014b). Other studies have used the same double knockout model to study a structural analog of curcumin named J147, which was reported to have better outcomes than the parent molecule. Qi Chen and colleagues conducted a study using J147 on both young rats and mice and a transgenic double knockout (APP/PS1) mouse strain. J147 is a derivative of CNB-001, which is pyrazole derivative of curcumin. J147 proved to be more potent than both CNB-001 and curcumin, and had the best neuroprotective effects among the various derivatives as indicated from results from the different assays in the authors’ drug discovery scheme. J147 facilitated long-term potentiation (LTP) induction in rat hippocampal slices and improved recognition index in young male Sprague-Dawley rats tested on a novel object recognition test. The rats were injected with different doses of J147 1 h before habituation. The improvements were dose dependent and comparable to that reported after galantamine injection. They used young male C57BL/6 mice to test for memory in other behavioral assays; Y-maze, novel object location (NOL), and Barnes maze. Mice were fed J147 at 200 ppm in their food for 2 weeks before the administration of the behavioral assays. In the NOL test, J147 supplemented mice experienced a significant improvement in spatial memory as indicated by their increased recognition index. On the Y-maze, J147 mice made a higher percentage of spontaneous alterations indicating better short-term memory. Finally, these mice were tested on the Barnes maze, which is also a spatial learning and memory test (Barnes, 1979) where the J147 supplemented mice made significantly fewer errors than control mice on the retention test portion of the assessment. The authors, after noticing the success of J147 in normal mice and rats, tested this compound on a transgenic model of AD, the huAPP/PS1 mice. These transgenic mice were fed J147 at 200 ppm from 4 months of age. At 9 months of age, the mice were assayed for memory deficits in the MWM. By day 5 of the MWM test, J147 mice learned the task but the untreated AD controls did not. In the probe trial, J147 mice spent more time around and at the target location compared to untreated controls and comparable to the wild-type controls. The cortex and hippocampus of the transgenic mice were tested for proteins involved in synaptic functionality. There was a decrease in neuroinflammation as indicated by the reduction in the microglial marker Iba1 in the hippocampus and a significant reduction in pro-oxidant enzymes such as inducible nitric oxide synthase (iNOS). Further, treatment increased proteins that are needed for proper synaptic function (Drebrin, synapsin 1, and synaptophysin) compared to untreated AD controls. There was also increased expression of brain-derived neurotrophic factor (BDNF) without affecting the expression of BDNF’s receptor, tropomycin receptor kinase B (TrkB) in both the normal rats and transgenic mice (Maher et al. 2010). Yanagisawa and colleagues also utilized the APP/PS1 double knockout model to evaluate another derivative of curcumin FMeC, which bears a substitution at C4 influencing the ratio of keto to enol tautomers of curcumin. Two of the derivatives, FMeC1 and FMeC2, and curcumin alone at a dose of 500 ppm were tested on the 9-month-old mice for a period of 6 months. Mice fed FMeC1 for 6 months showed a reduction in insoluble Aβ deposits and glial cell activity together with reduced cognitive deficits as indicated by shorter escape latencies and longer time period in the target quadrant in the MWM, compared to those under a control diet, curcumin alone, or FMeC2. Curcumin alone and FMeC1 changed the formation of Aβ aggregates; however, only FMeC1 significantly attenuated the cellular toxicity of Aβ. This study indicated that FMeC1 and not curcumin can potentially prevent behavioral and physiological symptoms of AD (Yanagisawa et al. 2015).
Tau aggregates are responsible for the neurofibrillary tangles, which are one of the hallmark features in the AD brain, and tau pathologies have been closely linked to the levels of adversity in cognitive function (Pooler et al. 2013; Tomita 2017). Miyasaka and colleagues developed a transgenic nematode (Caenorhabditis elegans) model to express R406W (human tau) in all the neurons, which displayed the Unc phenotype. The Unc phenotype exhibited uncontrolled movement and neuritic abnormalities with loss of microtubules compared to healthy controls. The authors used tau-induced morphological abnormalities and neuronal dysfunction in this model as an analog for tau-induced cognitive decline in humans. First, they performed a drug screening process comparing the effects of different doses of methylene blue, resveratrol, trehalose, and curcumin on the behavioral activity of this transgenic model. High doses of methylene blue (200 μM) reduced the behavioral abnormalities in these worms; however, curcumin (30 μM) not only significantly reduced behavioral abnormalities, it also reduced the proportion of worms affected by this mutation. None of the other compounds improved neuronal dysfunction. The dose and the range of improvements with curcumin made it the most promising candidate for their study. Treatment of mutated worms with curcumin significantly reduced the uncontrolled movements and the neuritic abnormalities by stabilizing microtubules. Curcumin increased levels of acetylated α-tubulin, which most likely contributed to microtubule stabilization, since it did not reduce tau aggregates (Miyasaka et al. 2016).
None of the transgenic studies indicated any negative effects of curcumin and for most of the studies curcumin had a dose-dependent effect on biomarkers of cognition. Results from these studies suggest curcumin to improve some aspects of cognition as indicated by differences in performance in the cognitive tasks assessing spatial and non-spatial memory. Based on these results, curcumin tested on transgenic models have had positive results in regard to cognition and a general slowing of the aging process. In one study, a curcumin analogue cleared amyloid aggregates better than the parent molecule (Yanagisawa et al. 2015). This animal model of amyloid aggregates induced by genetic mutations does not mimic AD pathology in its entirety, as presence of amyloid in the brain does not always lead to AD. As reported from clinical trials, monocloncal antibody therapy targeting amyloid has had minimal, if any positive outcomes (Salloway et al. 2014); neither do tau mutation models mimic complete AD pathology, even though there are symptomatic similarities. A major clinical trial on the efficacy of a methylene blue analog for tau protein failed to meet its primary end points (Gauthier et al. 2016). Part of the problem lies in the fact that most cases of AD are sporadic and not familial, therefore outcomes from transgenic models may not necessarily translate to humans. Further, the heterogeneity of the disease complicates the targeting process. Nonetheless, these models provide helpful mechanistic insight on disease processes.
Studies of experimentally induced accelerated aging
Often studies incorporate different methods to induce oxidative or inflammatory insult through the use of chemicals or environmental factors to accelerate aging and mimic aging diseased pathologies instead of using aged animals. d-Galactose (DGAL) is a physiological nutrient and a reducing sugar that reacts with free amines of amino acids in proteins to form advanced glycation end (AGEs) products through nonenzymatic glycation (Song et al. 1999). AGEs have been implicated in the production of free radicals, and chronic administration of DGAL for 6–10 weeks induces significant cognitive and motor impairment. Chronic DGAL also increases oxidative stress and systemic inflammation (Cui et al. 2004).
Several investigators have tested the efficacy of curcumin on memory and molecular insults in DGAL-induced aging rodent models. Nam and colleagues subcutaneously injected young adult male C57BL/6 mice with 100 mg/kg of DGAL to induce pathological aging for 10 weeks. Mice were gavaged with curcumin (300 mg/kg) alone or in addition to DGAL (CUR-DGAL) the last 3 weeks of the 10-week DGAL treatment. The CUR-DGAL mice and those administered curcumin alone performed better in the memory test with shorter escape latencies (MWM) than untreated DGAL controls. Curcumin treatment alone and CUR-DGAL increased nuclei involved in cell proliferation (Ki67 immunoreactive nuclei), nuclei involved in neuroblast differentiation (doublecortin immunoreactive nuclei), phosphorylated cyclic AMP (cAMP) response elements, and brain-derived neurotropic factor (BDNF) in the hippocampal dentate gyrus area compared to their respective controls (Nam et al. 2014). Banji and colleagues used a lower dose of DGAL (60 mg/kg) and injected it intraperitoneally (IP) to induce aging in young Wistar rats (3 to 4 months old) 1 week after starting prophylactic treatment with curcumin alone (20 and 40 mg/kg), piperine alone (6 and 12 mg/kg), or a combination treatment of curcumin and piperine. Piperine was used to enhance bioavailability of curcumin as reported by previous papers (Khajuria et al. 2002; Suresh and Srinivasan 2010) and also to evaluate whether there would be a synergistic benefit to combining two antioxidants. The therapeutic treatments were administered orally for 7 weeks. The results from this study indicate the combination therapy to be better than monotherapy. The combination therapy significantly improved spatial memory, reduced oxidative stress via significant reduction of MDA, and increase in GSH, SOD, and catalase (CAT), whereas monotherapy showed only marginal responses. The combination therapy and curcumin alone increased hippocampal volume and neuronal density. Results from this study suggest a combination of curcumin and piperine to be a superior therapy compared to curcumin alone (Banji et al. 2013).
Homocysteine (Hcy), a sulfur-containing amino acid derived from methionine, is another chemical commonly used to induce aging in animals. Chronic administration of Hcy increases oxidative stress and accelerates aging processes in rodents, including cognitive impairment (Xie et al. 2016). Human studies have suggested elevated Hcy levels in the elderly contribute to brain atrophy and cognitive decline (de Jager 2014). Ataie and colleagues tested a Hcy-induced aging model to study the dose-dependent prophylactic therapeutic effect of curcumin in adult male Wistar rats. Two different doses of curcumin were administered IP 5 days prior to the Hcy intrahippocampal administration, after which both Hcy and curcumin was administered for 10 days. Hcy induced lipid peroxidation in the hippocampus and the rats had memory retention problems as indicated by their performance in the passive avoidance test. Curcumin treatment rescued the Hcy-induced memory impairment and decreased MDA and superoxide levels in the hippocampus (Ataie et al. 2010).
Streptozotocin (STZ) is a chemical commonly used to induce diabetes and also a commonly used agent to induce aging in preclinical studies. Glucose metabolism impairment in the brain has been suggested to be an important contributor to late onset AD (Malkki 2015; Willette et al. 2015). Two different laboratories examined the effect of curcumin supplementation in an intracerebroventricular (ICV) STZ injection-induced cognitive impairment model. Ishrat et al. tested one dose of curcumin (80 mg/kg) given orally to the rats for 3 weeks. Behavioral tests indicated curcumin to improve memory and learning impaired by STZ. The curcumin gavaged rats also had lower levels of lipid peroxidation products and an improved redox state in the cerebral cortex and hippocampus (Ishrat et al. 2009). Bassani et al. also induced accelerated aging via ICV-STZ injections testing three different oral doses of curcumin (25, 50, and 100 mg/kg) for 4 weeks. In contrast to Ishrat et al.’s study, the curcumin-supplemented rats did not exhibit better spatial memory, regardless of dose. Also, only the two higher doses improved object recognition memory (Bassani et al., 2017a). There were only slight improvements in neuroinflammation with minimal but significant attenuation in the ionized calcium binding adaptor molecule 1 and glial fibrillary acidic protein expression in the corpus callosum only.
Lipopolysaccharide (LPS) is the major component of the outer membrane of gram-negative bacteria and when injected induces a major immune response which includes a strong pro-inflammatory response (Catorce and Gevorkian 2016). Lipopolysaccharide (LPS) is often used to induce inflammation in rodent studies. Kawamoto and colleagues wanted to test whether the anti-inflammatory benefits of curcumin on neuroinflammation and its associated comorbidities like cognitive impairment was dependent on proper functioning of the inflammatory signaling pathway. In their study, they used a wild-type strain C57BL/6 as well as TNFR2 knockout mice. The experimental mice received 50 mg/kg curcumin IP for 4 days. On the last day of treatment, the mice received one single dose of LPS 2 h after curcumin injection. One dose of LPS is enough to induce cognitive impairment as well as inflammation in the CNS and the periphery. The 4-day pretreatment with curcumin ameliorated the LPS-induced memory and motor impairment. Curcumin-pretreated mice also exhibited less anxiety. The authors examined inflammatory biomarkers in the serum and hippocampus. LPS as expected significantly increased the concentration of pro-inflammatory cytokines and expression of GFAP in the hippocampus. Mice under curcumin supplementation had significantly lower levels of cytokines in the serum and a lower expression of GFAP in the hippocampus. However, in the absence of functional TNF receptors, curcumin supplementation failed to ameliorate the detrimental effects of LPS (Kawamoto et al. 2013).
All of the studies included a wild-type untreated control that exhibited behavioral and pathophysiological aging processes induced by the chemicals. Overall, studies using chemically induced aging models indicate curcumin to significantly reduce both inflammation and oxidative stress. Treatment with curcumin did not have any side effects. The DGAL studies used different doses of DGAL, different doses of curcumin, and different species but their mode of curcumin treatment was similar. The lower doses of curcumin failed to show an improvement in memory when administered alone, indicating curcumin to have a synergistic effect with piperine and that successful treatment can be achieved with lower doses of curcumin when combined with piperine. The STZ studies used different doses of STZ and curcumin but used the same species and same mode of treatment administration. The dosage of STZ in Bassani’s study (3 mg/kg) was double that of what was used in Ishrat’s study (1.5 mg/kg). The two studies also used two different methods of analyzing spatial memory, which could have contributed to the discrepancy in spatial memory results; Ishrat et al. used the MWM, whereas Bassani and colleagues used the Y maze. Lastly, in the LPS-induced inflammation study, functional TNF receptors were necessary for the mice to benefit from curcumin treatment. These studies provide useful information about the effects of curcumin in its ability to reduce inflammation and oxidative stress. However, it is harder to translate these results for clinical outcomes of regular aging and even that of pathological aging because they are not reliable analogues of the time-dependent process of human pathological processes in age-associated diseases such as AD. Generally, the use of experimentally induced aging studies is done for time, money, and aged rodents’ availability constraints. Therefore, one should be cautious while interpreting results from chemically induced models and avoid large-scale extrapolations from these studies to explain how curcumin modulates the aging processes.
Studies of aging induced by environmental factors
Chronic stress has been identified as a risk factor for AD and has been utilized in pre-clinical studies to induce AD (Leonard 2017; Lin et al. 2016; McEwen 2005; Piirainen et al. 2017). Rinwa and Kumar used a 28-day chronic stress paradigm made up of random patterns of mild stressors. Mice were injected with different doses of curcumin with or without piperine (20 mg/kg) 30 min before each stressor administration. Behavioral tests were conducted between days 20 and 28. Interestingly, chronic stress significantly reduced sucrose consumption, which was rectified by curcumin. There was a dose-dependent effect to this behavior, where 100 mg/kg of curcumin by itself had no beneficial effect; however, 200 and 400 mg/kg of curcumin increased sucrose consumption. Further, a combination of 100 mg/kg of curcumin with 20 mg/kg reversed the sucrose consumption to normal levels. Chronic treatment with curcumin (200 and 400 mg/kg) significantly improved spatial learning and memory in a dose-dependent manner. The same improvement was reported for mice on the combination treatments with lower doses of curcumin. Curcumin supplementation also significantly decreased corticosterone levels indicating stress alleviation. Additionally, the authors found that curcumin supplementation improved mitochondrial enzymes (complex I to V) and redox state indicators such as GSH, CAT, SOD, MDA, and nitrite. Addition of piperine to the lower doses of curcumin (100 and 200 mg/kg) potentiated their beneficial effects. Results from this study confirmed the deleterious effects of chronic stress on memory and how curcumin can alleviate these symptoms potentially via reduction of corticosterone. In addition, the results indicated that a smaller dose of curcumin could be used for the same beneficial effects by combining treatment with piperine (Rinwa and Kumar 2012).
The Rinwa and Kumar study was the only study found that reported on the effects of environmental factors such as chronic stress on memory and the efficacy of curcumin for cognitive decline induced by chronic stress. Chronic stress and depression have been reported to share some of the same mechanistic pathways and behavioral symptoms. Curcumin has been clinically studied for depression and anxiety with positive outcomes being reported (Sanmukhani J 2014; Esmaily H 2015; Ng QX 2017). There needs to be more preclinical studies with different stress paradigms to evaluate the effect of curcumin on chronic stress, since the Rinwa and Kumar study showed promising results with regard to behavior and corticosterone levels.
Studies using a normal aging model
Aging is the main risk factor for cognitive decline, therefore it is imperative to know how curcumin supplementation would impact a normal aging model. Belviranli and colleagues gavaged aged (20 months) female Wistar rats with 300 mg/kg of curcumin for 12 days and behavioral tests were conducted at 7 days of curcumin treatment. The authors chose this dose based on previous studies that demonstrated antioxidant and neuroprotective effects on rats. Curcumin supplementation improved spatial memory and decreased MDA in the brain but there were no changes in plasma MDA and no significant changes in the protein carbonyl and GSH concentration in both the plasma and brain (Belviranli et al. 2013). Another study tested three doses of curcumin (100, 200, and 400 mg/kg) in normally aging middle-aged rats (12 months) for 6 months. To analyze the effects of aging, the authors collected serum to measure albumin, globulin, and albumin/globulin ratio; they also measured CRP, lipid peroxidation products, total antioxidant capacity, SOD, and nitric oxide (NO). The rats fed the two higher doses of curcumin had significantly lower levels of CRP compared to sham control and those gavaged with the lowest dose of curcumin. However, rats fed the highest dose also had high levels of MDA, indicating curcumin can induce lipid peroxidation at higher doses. Rats receiving the two highest doses also had high levels of NO in their plasma. Results from this study indicate that curcumin’s beneficial effects are dose dependent and higher doses of curcumin may not necessarily be beneficial, displaying a hormetic effect (Shailaja et al. 2017). Dong and colleagues studied the short-term (6 weeks) and long-term (12 weeks) effects of curcumin (480 mg/kg of feed) in aged male Sprague-Dawley rats (15 months old) evaluating both behavioral and physiological effects (Dong et al. 2012). Curcumin did not have any effect on motor behavior but both treatment periods had significant cognitive effects. Both treatment periods improved social recognition index, which is a measurement for non-spatial learning; however, only those that underwent longer treatment had better spatial learning. Further, the mice with the longer treatment also had enhanced cell proliferation in the dentate gyrus indicating neurogenesis. The authors also studied modulation of gene expression by curcumin, which was evident for both treatment periods. The results indicated major changes in genes involved in neurogenesis, brain development, and cognitive function. The authors concluded that a longer treatment would promote overall cognitive improvement.
The authors of this review paper conducted a study on curcumin on ad-libitum fed aged 15-month-old C57BL/6 male mice (Sarker et al. 2015). We tested 1000 ppm of curcumin for 3 months in middle-aged male C57BL/6 mice. The accumulated weight and adipose tissue of middle-aged, ad-libitum fed laboratory mice was targeted as an analog for human mid-life obesity in this study. The mice fed a curcumin-supplemented diet were compared to that under caloric restriction. Caloric restriction is a dietary regimen that has been reported to successfully increase healthy lifespan which includes delaying cognitive decline, decreasing oxidative stress and inflammation along with reducing body weight (Forster and Lal 1999; Forster et al. 2003; Joseph et al. 2009). Results from our study indicated curcumin improved executive function but not spatial memory. Also, curcumin supplementation decreased systemic inflammation and improved redox state (Sarker et al. 2015).
Results from studies using a normal aging model indicate that even without severe pathological conditions, curcumin can slow down age-associated cognitive decline, and oxidative stress and inflammation, two processes that have been implicated in the progression of cognitive impairment. In Shailaja et al. (2017) study, the different effect of the highest and lower doses of curcumin indicates a hormetic effect where the highest dose in that study actually increased lipid peroxidation. This suggests that the dose of choice for studying efficacy of curcumin is important when translating results. There was also a difference in the spatial memory results between two studies that used the same species but had different modes, dosages, and intervention periods. This discrepancy however can be attributed to the difference in doses with the dose of curcumin being almost 100 times higher in the Belviranli et al. (2013) study compared to Dong et al. (2012) study. However, curcumin may produce the same effects at a lower dose but with a longer treatment period in a normal aging model as indicated by improvement in spatial learning when rats were kept on the treatment for 12 weeks and not 6 weeks (Dong et al. 2012). This maybe favorable since there have been some reports of gastrointestinal issues with higher doses of curcumin in humans. Our study used an intermediate dose as compared to Belviranli and Dong but did not show favorable effects on spatial learning, despite an equivalent intervention period to Dong and colleagues. The difference in spatial memory results could be the difference in species. Further, we found an improvement in executive function, which was not measured by Belviranli or Dong.
Results reported from these studies provide a vast range of data from doses to use and the ideal intervention period for maximal beneficial physiological and functional changes. However, the varying doses and treatment periods also make it harder to translate to the clinic due to the lack of consistency in the preclinical data. It should be noted that the studies using different animal models to test the efficacy of curcumin is pertinent to treatment efficacy. Reporting of the rationale for dosing decisions would benefit clinical research by providing information for the optimal dosing regimen to test for maximal efficacy. Another limitation in preclinical studies studying curcumin efficacy for cognition is the lack of data from female specimens in a majority of these studies. Our follow up study comparing male and female middle-aged and aged C57BL/6 mice indicated sex-dependent metabolic and behavioral differences after a high-dose curcumin treatment (unpublished results). Studies across species have firmly established differences in male and female brains (Huang and Woolley 2012; Jazin and Cahill 2010). Addressing sex differences in relation to curcumin intake will provide more robust preclinical data to inform translation to broader, inclusive clinical studies on curcumin efficacy. Even with these commonly encountered design variabilities in the early stages of pre-clinical investigation, the results of the present review indicate efficacy of curcumin in the amelioration of cognitive decline. Table 1 includes preclinical studies and their main findings discussed in this section.
Table 1.
Preclinical/animal models
| Model | Author(s) | Species/chemical/environmental factor and age/study sample size | Effect size (d), standard deviation (SD), and sample size (n) (80% power) | Dose and time period | Biochemical results | Behavioral results |
|---|---|---|---|---|---|---|
| Genetically modified | Sun et al., 2013 | SAMP8, 6-month-old male mice n = 22 |
d = 0.59 SD = 11.069 n = 13 Escape latency on the MWM |
20 and 50 mg/kg per day intragastrically, once daily for 25 days |
• Dose-dependent decrease in hippocampal MDA • No difference in hippocampal SOD content • Highest dose significantly increased p-NMDAR expression • Increase in p-CAMKII expression in the hippocampus of mice given the highest dose. |
• Dose-dependent effect on escape latencies (MWM) |
| P. Wang et al., 2014a | APP/PS1, 6-month-old male and female mice n = 12 |
d = 0.434 SD = 3.46 n = 22 Target crossing on the MWM |
160 and 1000 ppm for 6 months | • Reduced Aβ42 generation and increased clearance in a dose-dependent manner • Increased autophagy indicated by increased LC3 immunofluroscence and increased number of autophagosomes in the CA1 region of the hippocampus • Decreased expression of PI3K, phosphorylated Akt, and ratio of phosphorylated mTOR to mTOR |
• Dose-dependent significant effect on working and spatial memory | |
| Chen et al. 2011 | APP/PS1 4-month-old male mice n = 12 |
d = 0.578 SD = 5.194 n = 13 Novel object recognition index |
200 ppm for 5 months of J147, a derivative of CNB-001 CNB-001: pyrazole derivative of curcumin |
• Decrease in Iba1 in the hippocampus • Decrease in pro-oxidant enzymes: HO-1 and iNOS • Increase in synaptic protein expression: Drebrin, synapsin 1, and synaptophysin • Increase in BDNF expression without affecting BNDF receptor expression |
• Shorter escape latencies comparable to that of wild type • Increased time spent in target quadrant during probe trial |
|
| Yanagisawa et al. 2015 | APP/PS1, 9 months n = 6–12 |
d = 0.996 SD = 7.785 n = 6 Time in target quadrant in MWM |
500 ppm for 6 months of curcumin, FMec1, or FMec2 | • Reduction in insoluble Aβ deposits in FMec1 mice • GFAP-1 reduced in cerebral cortex and Iba1 reduced both in cortex and hippocampus in FMec1 mice • Significant increase in Aβ40 in curcumin treated mice |
• Significant reduction in escape latencies and increased time in target quadrant comparative to that of wild type only in mice fed FMec1 • No significant difference in the Y-maze test |
|
| Miyasaka et al. 2016 | R406W (human tau) in C. elegans Unc phenotype: Uncoordinated moves n = 56–60 |
d = 0.288 SD = 1.91 n = 49 No. of protrusions |
Low dose: 10 μg/plate High dose: 100 μg/plate |
• High dose significantly reduced the proportion of severely affected worms • Did not affect tau phosphorylation • Improvement in morphological abnormalities: reduced number of kinks in affected neurites • Amount of acetylated α-tubulin significantly increased contributing to microtubule stability |
• High dose reduced population of worms displaying Unc phenotype independent of effect on tau aggregation | |
| Experimentally induced accelerated aging | Nam et al. 2014 |
d-galactose-injected SC (100 mg/kg) C57Bl/6 male mice—10 weeks n = 10 |
d = 0.672 SD = 0.632 n = 10 No. of target crossings on the MWM |
300 mg/kg gavage for 3 weeks w/ (CUR-DGAL) or w/o D-galactose (CUR) | • CUR increased Ki67 immunoreactive nuclei in the subgranular zone of dentate gyrus compared to regular controls • CUR-DGAL increased Ki67 immunoreactive nuclei in the subgranular zone of dentate gyrus compared to DGAL • CUR and CUR-DGAL increased DCX-immunoreactive neuroblasts in dentate gyrus compared to their respective controls • CUR and CUR-DGAL increased pCREB expression in dentate gyrus compared to their respective controls • CUR and CUR-DGAL increased BDNF levels in dentate gyrus compared to their respective controls |
CUR-DGAL mice performed better spatially than untreated DGAL controls CUR did not affect spatial performance |
| Banji et al. 2013 |
d-galactose-injected IP (60 mg/kg) Male Wistar rats—6 weeks n = 6 |
d = 4.61 SD = 0.282 n < 2 No. of target crossings on the MWM |
Curcumin: 20 and 40 mg/kg Piperine: 6 and 12 mg/kg For 7 weeks |
• Combination high dose Tx increased hippocampal volume and neuron number • Combination decreased MDA levels and increased GSH, CAT, and SOD levels compared to untreated DGAL • Combination high dose Tx decreased lipofuscin accumulation than untreated DGAL • Combination had better neuronal structural integrity compared to untreated DGAL |
• Combination Tx significantly reduced escape latency and increased number of target crossings | |
| Ataie et al. 2010 | Homocysteine- 5 days, Intrahippocampal administration (0.2 μmol/μl) Male Wistar rats n = 8 |
d = 1.692 SD = 63.55 n = 3 First retention latency on passive avoidance task |
5 or 50 mg/kg injected IP for 10 days | • Decrease in MDA and SOD concentration was dose dependent compared to untreated Hcy controls | • Both doses reversed the Hcy-mediated decrease in retention latencies in the passive avoidance task | |
| Ishrat et al. 2009 | Streptozotocin (STZ)—1.5 mg/kg ICV injection Male Wistar rats n = 10 |
d = 0.556 SD = 126 n = 14 Retention latency on passive avoidance task |
80 mg/kg gavaged w/ or w/o ICV-STZ for 3 weeks | • Tx reduced 4-HNE + MDA, H2O2, and TBARS concentration in the hippocampus and cortex of ICV-STZ rats • Tx reduced protein carbonyl concentration in the hippocampus and cortex of ICV-STZ rats • Tx reversed the effect of STZ on GSH/GSSG ratio and antioxidant enzymes in the hippocampus and cortex |
• Tx increased retention latency on the passive avoidance task compared to untreated ICV-STZ • Treated group had lower escape latency and spent more time in the target quadrant compared to untreated ICV-STZ |
|
| Bassani et al., 2017b | Streptozotocin (STZ)—3 mg/kg ICV injection Male Wistar rats n = 6–8 |
d = 0.688 SD = 0.349 n = 10 Discrimination index on the Object recognition test |
25, 50, or 100 mg/kg gavaged w/ ICV-STZ for 4 weeks (CUR25, CUR50, CUR100) |
• Tx did not improve neurogenesis (Ki67 immunoreactive nuclei) • Tx did not reduce inflammation in different brain regions caused by STZ. Iba-1 and GFAP expression similar to that in untreated STZ rats except in corpus callosum |
• No significant differences in the open field test, CUR100, and STZ spent more time in closed arms in the elevated plus maze • No improvements in spatial memory as indicated by Y maze results • CUR50 and CUR100 improved short-term recognition memory compared to untreated STZ controls |
|
| Kawamoto et al. 2013 | LPS: 250 μg/kg—one time 2 h after last curcumin administration 2.5-month-old male mice n = 10 |
d = 0.768 SD = 11.064 n = 8 Percent time in the target quadrant |
50 mg/kg IP for 4 days | • PreTx significantly reduced systemic inflammation induced by LPS: lower concentration of TNFα and IL-1β • PreTx significantly reduced CNS inflammation induced by |
• PreTx decreases the memory consolidation deficit induced by LPS: longer time spent in target quadrant • PreTx decreases (longer rotorod latency) the motor deficit induced by LPS • PreTx increases freezing percentage time in the contextual fear paradigm test compared to that of untreated LPS mice |
|
| Environmental factor | Rinwa and Kumar 2012 | Chronic unpredictable stress paradigm for 28 days. Male Laca mice |
9 groups • 100, 200, 400 mg/kg • 100, 200 mg/kg w/ 20 mg/kg piperine • 20 mg/kg piperine • 400 mg/kg naïve • Naïve • CUS (untreated stressed control) Gavaged Tx for 28 days |
• Tx dose dependently decreased MDA and nitrite concentrations compared to CUS • Tx dose dependently prevented the decrease of GSH, catalase and SOD induced by CUS • Combined Tx with piperine enhanced the protected effect for lower dose treatments • Tx dose dependently decreased acetylcholinesterase activity compared to CUS; piperine combination potentiates effect for lower dosages. Also, highest dose on naïve mice have similar dampening effect on acetylcholinesterase activity • Tx and combination Tx improved mitochondrial respiratory enzyme activity • Tx and combination Tx decreased serum corticosterone concentration |
• Improvement in locomotor activity was dose dependent and lower doses had improvements too when potentiated with piperine compared to CUS • Higher doses (200 and 400) decreased return latencies on EPM and lower doses when potentiated with piperine had similar decreased latencies compared to CUS • Higher doses significantly shortened mean escape latencies; lower doses (100) had same results when potentiated with piperine compared to CUS |
|
| Normal aging | Belviranli et al. 2013 | Female Wistar rats 20 months n = 10 |
d = 0.738 SD = 1.4236 n = 9 No. of platform crossings on MWM |
300 mg/kg gavaged for 12 days | • Treated rats had significantly lower levels of MDA in the brain but not plasma • No changes in SOD and protein carbonyl concentration in the brain and plasma |
• Treated rats crossed the platform significantly more than untreated rats (MWM) |
| Dong et al. 2012 | Male Sprague-Dawley rats 15 months n = 15 |
d = 0.841 SD = 0.328 n = 7 Recognition index |
480 ppm for 6 and 12 weeks |
• The long Tx period increased neurogenesis in the dentate gyrus | • Both Tx periods improved social recognition index; however, only longer Tx period increased time spent in target quadrant | |
| Sarker et al. 2015 | Male C57BL/6 mice 15 months n = 19 |
d = 0.692 SD = 1.59 n = 10 Avg. no. of trials to reach criterion on the discriminated avoidance task |
1000 ppm for 3 months | • Significant increase in erythrocyte GSH/GSSG ratio • Significant decrease in plasma CRP but no difference in IL-6 concentration |
• Significant improvement in executive function, less trials to reach correct arm; comparable to that of mice under caloric restriction • No difference in spatial performance |
Human studies and clinical trials
Although preclinical studies have repeatedly demonstrated positive therapeutic effects of curcumin in regard to age-related memory loss and neuronal integrity as well as established biological rationale, similar levels of success have yet to be demonstrated in clinical studies. The number of clinical trials testing the therapeutic potential of curcumin to slow or prevent cognitive aging has increased in the last few years. However, since most of them are ongoing, only some of the results have been published. Table 2 includes published results of human studies/clinical trials.
Table 2.
Clinical studies of curcumin efficacy for cognitive decline
| Author(s) | Study design | Sample size/subject type | Effect size (d), standard deviation (SD) and sample size (n) (80% power) | Time period (months) | Dosage | Other medications | Main findings | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Baum et al. 2008 | Randomized, placebo-controlled, double-blind study | 34 subjects with AD n = 8–11 completed study |
d = 0.359 SD = 2.648 n = 32 Change in MMSE scores |
6 | 1000 to 4000 mg/day | Standard treatment of gingko leaf extract | • No cognitive decline in subjects under placebo in 6 months • No cognitive improvement in patients taking curcumin • Vitamin E levels increased with curcumin • Trend of serum Aβ40 rising with curcumin |
None. 3 subjects on 1 g did withdraw due to minor gastrointestinal issues |
| Hishikawa et al. 2012 | Case studies. | Geriatric patients with AD | 3 | 100 mg/day | 2 subjects were taking Yokukansan | • Improved mood as there was a noticeable decrease in their behavioral and psychological symptoms of dementia • One subject had a 5 point improvement on the MMSE |
None | |
| Ringman et al. 2012 | Randomized, placebo-controlled, double-blind study | 36 subjects randomized with mild or moderate AD n = 9–11 completed study |
d = 0.088 SD = 8.212 n > 500 Change in MMSE scores |
6 | 2000 or 4000 mg/day of curcumin C3 complex | Most subjects on AchE inhibitors and memantine | • No significant clinical or biochemical efficacy of curcumin against AD • No significant improvement on the MMSE and ADAS-Cog and no changes noted after treatment in plasma Aβ, CSF Aβ, CSF t-tau, p-tau181, and CSF isoprostanes • There was noticeable variability on baseline disease severity |
• Adverse events occurred in both placebo and curcumin group • Diarrhea and joint pain complaints for both placebo and the two curcumin groups • Authors noted that no serious adverse events occurred |
| Cox et al. 2015) | Randomized, placebo-controlled, double-blind study | 60 healthy elderly subjects with no diagnosed dementia | Acute and chronic (1/3 h) or 1 month | 400 mg/day of Longvida | None | • Improvement in sustained attention and working memory compared to placebo • Significant reduction in LDL cholesterol under curcumin |
None. | |
| Rainey-Smith et al. 2016 | Randomized, placebo-controlled, double-blind study | 96 subjects non-demented indicated by MoCA (score >26) n = 39 Curcumin group n = 57 Placebo |
d = 0.75 SD = 0.4 n = 9 Change in MoCA scores |
12 | 1500 mg/day of Biocurcumax | None | • No cognitive performance differences between groups • Significant time X treatment group interaction observed for MoCA scores driven by decline in function in placebo group at 6 months • Curcumin did not improve mood as indicated by self- reports measuring depression and anxiety |
None |
Baum and colleagues conducted a 24-week double-blind randomized clinical trial testing curcumin in patients with progressive cognitive decline according to diagnostic criteria laid out by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer Disease and Related Disorders Association for probable or possible AD. Thirty-four subjects between the ages of 59 and 85 were selected to participate in the study and randomized to 3 groups; 4, 1, and 0 g (placebo) of curcumin. Subjects ingested curcumin in either capsule or powder form. Additionally, subjects were given 120 mg standardized gingko leaf extract as previous studies with gingko had displayed a moderate cognitive benefit. Plasma was measured at baseline, 1 and 6 months for isoprostanes and antioxidants, and serum was collected to measure Aβ. At 6 months, there were no significant differences in MMSE scores between any of the groups compared to placebo for subjects taking either form, and the controls also did not have a decline in their MMSE score after 6 months. Serum Aβ concentration did not differ between groups. Changes in plasma isoprostanes concentration between baseline and at 6 months were not significantly different for the different doses. The authors also measured the bioavailability of curcumin at various time points by measuring the concentration of curcumin after 4 g of curcumin intake in the plasma of 1 non-demented individual. The level of curcumin peaked at 250 nM at 1.5 h and at 270 nM in 4 h. At 24 h, the level of curcumin fell to 60 nM. Curcumin supplementation also increased serum Aβ levels, although not significantly. Since curcumin has been reported in other studies to disaggregate Aβ in the brain, the authors conclude this ability may lead to releasing Aβ into circulation. The authors concluded that curcumin use was safe but was not efficacious for cognitive improvement at this intervention period. However, the authors note that the lack of decline in cognition as measured by the MMSE in placebo could have hampered their ability to detect any effect of curcumin. They also recommend the use of a more sensitive cognitive screen like the Alzheimer’s Disease Assessment Scale–cognitive scale (ADAS-Cog). Further, since there were not any metabolite differences between the 1 and 4 g cohorts, the authors suggest using a lower dose and increase the duration of the study (Baum et al. 2008).
Ringman and colleagues performed a 24-week randomized, double-blind, placebo-controlled study of curcumin with an open label extension to 48 weeks. Thirty-six subjects diagnosed with mild to moderate AD were selected to participate with a study partner. Subjects were assigned to either receive placebo, 2 or 4 g of curcumin C3 complex in capsule form. The curcumin C3 complex includes curcumin and the two major curcuminoids in smaller proportions, 15 to 25% of demethoxycurcumin and 2.5 to 6.5% of bisdemethoxycurcumin. The primary outcome measures for this study were tolerability and cognitive scores which included the MMSE scores and the ADAS-Cog scores. Secondary outcome measures included the Neuropsychiatric Inventory (NPI) and the Alzheimer’s Diseases Cooperative Study Activities of Daily Living (ADL), which were administered at baseline, 24, and 48 weeks. Plasma and cerebrospinal fluid (CSF) were collected at baseline and 24 weeks to measure levels of Aβ1–40 and Aβ1–42 in plasma and levels of Aβ1–42, t-tau, p-tau181, and F2-isoprostanes in CSF. The curcumin supplementation was reportedly well tolerated, although there were a few subjects who opted out due to gastrointestinal issues both in the placebo and the curcumin group. However, there was no demonstrable clinical or biochemical efficacy against AD. There was however a trend of memory worsening in subjects taking 2 and 4 g of curcumin according to the MMSE as compared to subjects on the placebo. Biochemical measures of isoprostanes did not show any significant differences from baseline with or without curcumin. Also, there were not any significant differences in the plasma and CSF levels of Aβ40/42 compared to control; in fact, plasma levels of Aβ40/42 were lower than baseline at 6 months after curcumin treatment regardless of dose, unlike Baum et al. study. The authors concluded that the lack of translation could be attributed to the differences in rodent and human models of amyloidosis, behavioral symptomology of AD, and the difference in metabolism of curcumin in rodents versus humans (Ringman et al. 2012).
Hishiwaka and colleagues presented three case studies where elderly subjects experienced a significant improvement in behavioral symptoms associated with AD after 12 weeks of turmeric treatment. These subjects were already diagnosed with AD and were on donepezil. Two subjects were also on Yokukanasan, a traditional Japanese medicine prescribed for cognitive difficulties. All three had an MRI indicating symmetrical bilateral temporal atrophy, although subject 3 also had atrophy in the parietal and posterior cingulate area. Cognitive decline and functional behavior were assessed with NPI and MMSE. Case 1 received 764 mg/day of turmeric (100 mg/day of curcumin) for 12 weeks. Compared to baseline, the NPI indicated a significant decrease in agitation, apathy, anxiety, and irritability. However, there was no improvement in the cognitive score on the MMSE from a baseline score of 1/30. Case 2 was administered 100 mg/day of curcumin for 12 weeks after which there was a significant decrease in the NPI score and a lessening of reported caregiver burden. Case 3 was administered 100 mg/day of curcumin for 12 weeks. The MMSE score went up by 5 points from a baseline of 12/30 and behavioral symptoms stabilized. The authors concluded based on these three cases that curcumin relieves behavioral symptoms associated with AD (Hishikawa et al. 2012).
Cox and colleagues investigated the effect of a lipid curcumin formulation (Longvida optimized curcumin lipidated 400 mg with 80 mg of curcumin) on mood and cognition in a randomized, double-blind, placebo-controlled trial of 60 non-demented adults between the ages of 60 and 85. The purpose was to test for the efficacy of curcumin for improving cognition in an older population. Subjects were tested either after acute (1 or 3 h) or chronic (4 weeks) administration of curcumin or acute-on-chronic administration (1 and 3 h after single dose following chronic administration). The authors reported an improvement in working memory after both acute and chronic administration of a solid lipidated curcumin formulation. Subjects on curcumin did significantly better on the digit vigilance task after acute treatment. Subjects on both the acute and chronic curcumin treatment had significantly better performance on the serial 3 subtraction task than their counterparts on placebo. Chronic treatment also improved mood and general contentedness as well as decreased overall and physical fatigue as measured by the Chalder Fatigue Scale. Other non-behavioral improvements included a significantly reduced total and LDL cholesterol (Cox et al. 2014). The authors of this study are currently conducting a phase 3 study registered with the Australian and New Zealand clinical trial registry (ACTRN12616000484448) where they are testing the same dose as their pilot study but in a 12-week period. They are collecting data on different cognitive and other behavioral measures. They are also measuring blood biomarkers of inflammation such as cytokines, CRP, and erythrocyte sedimentation rate; and oxidative stress, 8-hydroxy-2′-deoxyguanosine, MDA, and total antioxidant capacity. In addition, the authors are also measuring blood biomarkers of beta amyloid and neurogenesis. Another unique aspect of this trial is the proposed fMRI assessments to measure cerebral oxygen metabolism in response to curcumin treatment during cognitive tasks.
Another group from Australia, Rainey-Smith et al. (2016), reported on the results of a 48-week randomized placebo-controlled, double-blind study that investigated the efficacy of a curcumin formulation in preventing cognitive decline in an elderly non-demented population. Normal cognitive decline was based on score on the Montreal Cognitive Assessment (MoCA). A total of 160 subjects participated in the study. The curcumin group ingested 500 mg of Biocurcumax (BCM-95) three times a day for a dose of 1500 mg/day. The authors selected this curcumin formulation due to its reported enhanced oral bioavailability. They implemented the final dosage incrementally to prevent gastrointestinal-related adverse events reported in other studies. Comprehensive cognitive assessments were conducted at baseline, 24 weeks, and 48 weeks of treatment. DNA samples were also genotyped for APOE status. The only significant difference in cognition measures occurred at the 24-week mark where the subjects in the curcumin group did significantly better on the MoCA compared to the placebo group. However, at 48 weeks, the cognitive difference was not present. The subjects did not display any significant differences at either time point in non-cognitive assessments such as stress and anxiety. This study did not measure any inflammatory or oxidative stress physiological markers (Rainey-Smith et al. 2016). Based on the demonstrated cognitive benefits, the authors of this study are conducting a phase II sister study (ACTRN12613000681752) with individuals who have reported memory complaints and score less than or equal to 17 on the MoCA during their baseline assessment. They are using the same dosage, same incremental way of implementing the final dose, and will study the same primary and secondary outcome measures but with individuals in the mild cognitive impairment (MCI) and early AD stage.
Based on published results of clinical studies thus far, the efficacy of curcumin for cognitive improvement or amelioration of age-associated cognitive decline has been inconsistent, but nonetheless informative. The mixed translational results may be due to the use of relatively insensitive or incomplete assessments of cognition. The use of a more sensitive and valid assessment such as the CDR Worksheet (Hughes et al. 1982), which is a semi-structured interview and rates patients on a 5-point scale characterizing six domains of cognitive and functional performance, increasing data reliability through its combined emphasis on both cognitive and behavioral data and staging criteria.
Another issue related to lack of translation is likely due to lower curcumin bioavailability as reported in several pharmacology studies (Adiwidjaja et al. 2017; Kumar et al. 2010). Based on our review, studies that have utilized a lipidated curcumin pill for better bioavailability have yielded positive results. An additional issue in translation is the lack of power inherent in many early smaller clinical studies, which typically result in mixed findings across the literature.
Conclusion
This narrative review of curcumin found that preclinical studies consistently demonstrated its efficacy for ameliorating or preventing cognitive decline. The early clinical studies to date are less consistent and highlight the problems in translating basic research to clinical research. The problems range from a lack of consistency in defining aging in preclinical animal models as well as an over reliance on behavioral symptomology of AD to use as an analog for animal models. Even with the different animal models and the difference in cognitive benefits, curcumin consistently decreases both systemic and neuroinflammation as well as improves redox state. Unfortunately, the clinical studies to date did not include different measures of inflammatory and oxidative biomarkers. Considering that numerous studies (both preclinical and clinical) have reported these two processes to be closely associated with age-associated cognitive decline, including these biomarkers in clinical studies may yield further insight into why curcumin has not shown consistent cognitive effects in humans. Further, since curcumin clears amyloid beta in the brain, this might also be a future target for clinical investigations.
In sum, preclinical studies demonstrate benefit for the use of curcumin regardless of the mode, intervention period, or dosage, although the specific cognitive and physiological benefits varied between studies. There have been too few clinical studies to date to provide conclusive evidence for the efficacy of curcumin for cognitive decline, although some have demonstrated beneficial effects. In order to better demonstrate the efficacy of curcumin for cognitive improvement in clinical trials, future studies should define dementia by using biomarkers such as amyloid and tau status in addition to more sensitive behavioral assays.
This review attempted to present a comprehensive and unbiased review of the preclinical and clinical studies on curcumin and its efficacy for cognition. As with any narrative review, the lack of a meta-analysis is a limitation. Additionally, instead of utilizing a set criterion, this review included all studies that were pertinent to address the question of the current state in the translation of research for curcumin as a therapeutic agent for cognitive aging and aging-associated pathological cognitive decline.
Abbreviations
- AD
Alzheimer’s disease
- ADAS-Cog
Alzheimer’s Disease Assessment Scale-cognitive subscale
- ADL
Activities of daily living
- AGE
Advanced glycation end products
- APP
Amyloid precursor protein
- AREs
Antioxidant response elements
- Aβ
Amyloid beta
- BDMC
Bisdemethoxycurcumin
- BDNF
Brain-derived neurotrophic factor
- CA
Cornu Ammonis (hippocampal subfield)
- CAMKII
Calcium/calmodulin-dependent kinase II
- CNS
Central nervous system
- cAMP
Cyclic AMP
- CREB
Cyclic AMP-responsive element-binding protein
- CRP
C-reactive protein
- DMC
Demethoxycurcumin
- DSM
Diagnostic and statistical manual of mental disorders
- GCLc
Glutamate cysteine ligase catalytic subunit
- GFAP
Glial fibrillary acidic protein
- GSH
Reduced glutathione
- GSSG
Oxidized glutathione
- Hcy
Homocysteine
- Iba1
Ionized calcium binding adaptor molecule 1
- IKKβ
Inhibitor of nuclear factor kappa-B kinase subunit B
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- IP
Intraperitoneal
- KEAP1
Kelch-like ECH-associated protein 1
- LPS
Lipopolysaccharide
- LTP
Long-term potentiation
- MCI
Mild cognitive impairment
- MDA
Malondialdehyde
- MMSE
Mini Mental Status Exam
- MoCA
Montreal Cognition Assessment
- mTOR
Mammalian target of rapamycin
- MWM
Morris water maze
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDAR
p-N-methyl-d-aspartate receptor
- NO
Nitric oxide
- NOL
Novel object location
- NPI
Neuropsychiatric Inventory
- NRF2
Nuclear factor (erythroid-derived 2)-like 2
- OH
Hydroxyl
- PD
Parkinson’s disease
- PI3K
Phosphoinositide 3-kinase
- PS1 & 2
Presenilin 1 and 2
- ROS
Reactive oxygen species
- SAMP8
Senescence accelerated mouse–Prone 8
- SAMR1
Senescence resistant
- SH
Sulfhydryl
- SOD
Superoxide dismutase
- STZ
Streptozotocin
- TNF-α
Tumor necrosis factor alpha
- TrkB
Tropomycin receptor kinase B
- Tx
Treatment
References
- Adiwidjaja J, McLachlan AJ, Boddy AV. Curcumin as a clinically-promising anti-cancer agent: pharmacokinetics and drug interactions. Expert Opin Drug Metab Toxicol. 2017;13(9):953–972. doi: 10.1080/17425255.2017.1360279. [DOI] [PubMed] [Google Scholar]
- Alappat L, Awad AB. Curcumin and obesity: evidence and mechanisms. Nutr Rev. 2010;68(12):729–738. doi: 10.1111/j.1753-4887.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Anand P, Sung B, Kunnumakkara AB, Rajasekharan KN, Aggarwal BB. Suppression of pro-inflammatory and proliferative pathways by diferuloylmethane (curcumin) and its analogues dibenzoylmethane, dibenzoylpropane, and dibenzylideneacetone: role of Michael acceptors and Michael donors. Biochem Pharmacol. 2011;82(12):1901–1909. doi: 10.1016/j.bcp.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B. Neuroprotective effects of the polyphenolic antioxidant agent, curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav. 2010;96(4):378–385. doi: 10.1016/j.pbb.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Banji D, Banji OJ, Dasaroju S, Annamalai AR. Piperine and curcumin exhibit synergism in attenuating D-galactose induced senescence in rats. Eur J Pharmacol. 2013;703(1–3):91–99. doi: 10.1016/j.ejphar.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bassani, T. B., Bonato, J. M., Machado, M. M. F., Coppola-Segovia, V., Moura, E. L. R., Zanata, S. M., …, Vital, M. (2017a). Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer's disease in rats. Mol Neurobiol doi:10.1007/s12035-017-0645-9 [DOI] [PubMed]
- Bassani, T. B., Turnes, J. M., Moura, E. L. R., Bonato, J. M., Coppola-Segovia, V., Zanata, S. M., …, Vital, M. (2017b). Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer's type. Behav Brain Res, 335, 41–54. doi:10.1016/j.bbr.2017.08.014 [DOI] [PubMed]
- Baum, L., Lam, C. W., Cheung, S. K., Kwok, T., Lui, V., Tsoh, J., …, Mok, V. (2008). Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol, 28(1), 110–113. doi:10.1097/jcp.0b013e318160862c [DOI] [PubMed]
- Bea F, Hudson FN, Chait A, Kavanagh TJ, Rosenfeld ME. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circ Res. 2003;92(4):386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- Belviranli M, Okudan N, Atalik KE, Oz M. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology. 2013;14(2):187–196. doi: 10.1007/s10522-013-9422-y. [DOI] [PubMed] [Google Scholar]
- Bermejo, P., Martin-Aragon, S., Benedi, J., Susin, C., Felici, E., Gil, P., …, Villar, A. M. (2008). Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer's disease from mild cognitive impairment. Free Radic Res, 42(2), 162–170. doi:10.1080/10715760701861373 [DOI] [PubMed]
- Bhattacharya, I., Basu, S., Sarda, K., Gautam, M., Nagarajan, P., Pradhan, B. S., …, Majumdar, S. S. (2015). Low levels of Galphas and Ric8b in testicular sertoli cells may underlie restricted FSH action during infancy in primates. Endocrinology, 156(3), 1143–1155. doi:10.1210/en.2014-1746 [DOI] [PubMed]
- Bigford GE, Del Rossi G. Supplemental substances derived from foods as adjunctive therapeutic agents for treatment of neurodegenerative diseases and disorders. Adv Nutr. 2014;5(4):394–403. doi: 10.3945/an.113.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer's disease. Pharmacol Ther. 2014;142(2):244–257. doi: 10.1016/j.pharmthera.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Cai Z, Hussain MD, Yan LJ. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer's disease. Int J Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- Catorce MN, Gevorkian G. LPS-induced murine Neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. 2016;14(2):155–164. doi: 10.2174/1570159X14666151204122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/WNL.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Prior, M., Dargusch, R., Roberts, A., Riek, R., Eichmann, C., … Schubert, D. (2011). A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One, 6(12), e27865. doi:10.1371/journal.pone.0027865 [DOI] [PMC free article] [PubMed]
- Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. 2014;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. 2015;29(5):642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W, Liu J. D-galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology. 2004;5(5):317–325. doi: 10.1007/s10522-004-2570-3. [DOI] [PubMed] [Google Scholar]
- de Jager CA. Critical levels of brain atrophy associated with homocysteine and cognitive decline. Neurobiol Aging. 2014;35(Suppl 2):S35–S39. doi: 10.1016/j.neurobiolaging.2014.03.040. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Kaplowitz N. Importance and regulation of hepatic glutathione. Semin Liver Dis. 1990;10(4):251–266. doi: 10.1055/s-2008-1040481. [DOI] [PubMed] [Google Scholar]
- Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Zhao Z. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7(2):e31211. doi: 10.1371/journal.pone.0031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Farlow M, Aisen PS, Alzheimer's Disease Cooperative Study Data A, Publication C. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med. 2014;370(15):1460–1460. doi: 10.1056/NEJMc1402193. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6(3):361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin—from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20(2):167–176. doi: 10.1016/S0197-4580(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17(6):690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24(2B):563–569. [PubMed] [Google Scholar]
- Gauthier, S., Feldman, H. H., Schneider, L. S., Wilcock, G. K., Frisoni, G. B., Hardlund, J. H., …, Wischik, C. M. (2016). Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet, 388(10062), 2873–2884. doi:10.1016/S0140-6736(16)31275-2 [DOI] [PMC free article] [PubMed]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., …, Kummer, M. P. (2015). Neuroinflammation in Alzheimer's disease. Lancet Neurol, 14(4), 388–405. doi:10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed]
- Hishikawa, N., Takahashi, Y., Amakusa, Y., Tanno, Y., Tuji, Y., Niwa, H., … Krishna, U. K. (2012). Effects of turmeric on Alzheimer's disease with behavioral and psychological symptoms of dementia. Ayu, 33(4), 499–504. doi:10.4103/0974-8520.110524 [DOI] [PMC free article] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Ishrat, T., Hoda, M. N., Khan, M. B., Yousuf, S., Ahmad, M., Khan, M. M., . . . Islam, F. (2009). Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer's type (SDAT). Eur Neuropsychopharmacol, 19(9), 636–647. doi:10.1016/j.euroneuro.2009.02.002 [DOI] [PubMed]
- Itoh K, Ye P, Matsumiya T, Tanji K, Ozaki T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr. 2015;56(2):91–97. doi: 10.3164/jcbn.14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci. 2010;11(1):9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- Jimenez-Osorio AS, Monroy A, Alavez S. Curcumin and insulin resistance-molecular targets and clinical evidences. Biofactors. 2016;42(6):561–580. doi: 10.1002/biof.1302. [DOI] [PubMed] [Google Scholar]
- Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29(41):12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Scavone C, Mattson MP, Camandola S. Curcumin requires tumor necrosis factor alpha signaling to alleviate cognitive impairment elicited by lipopolysaccharide. Neurosignals. 2013;21(1–2):75–88. doi: 10.1159/000336074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9(3):224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ahuja A, Ali J, Baboota S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst. 2010;27(4):279–312. doi: 10.1615/CritRevTherDrugCarrierSyst.v27.i4.10. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11(4):338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. Major depression as a neuroprogressive prelude to dementia: what is the evidence? Mod Trends Pharmacopsychiatry. 2017;31:56–66. doi: 10.1159/000470807. [DOI] [PubMed] [Google Scholar]
- Lin, L. Y., Zhang, J., Dai, X. M., Xiao, N. A., Wu, X. L., Wei, Z., … Chen, X. C. (2016). Early-life stress leads to impaired spatial learning and memory in middle-aged ApoE4-TR mice. Mol Neurodegener, 11(1), 51. doi:10.1186/s13024-016-0107-2 [DOI] [PMC free article] [PubMed]
- Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Schubert D, Abe K. A pyrazole derivative of curcumin enhances memory. Neurobiol Aging. 2010;31(4):706–709. doi: 10.1016/j.neurobiolaging.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Malkki H. Alzheimer disease: insulin resistance could be linked to risk of AD via reduced glucose uptake. Nat Rev Neurol. 2015;11(9):485. doi: 10.1038/nrneurol.2015.147. [DOI] [PubMed] [Google Scholar]
- Mani, M., Khaghani, S., Gol Mohammadi, T., Zamani, Z., Azadmanesh, K., Meshkani, R., …, Mostafavi, E. (2013). Activation of Nrf2-antioxidant response element mediated glutamate cysteine ligase expression in hepatoma cell line by homocysteine. Hepat Mon, 13(5), e8394. doi:10.5812/hepatmon.8394 [DOI] [PMC free article] [PubMed]
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Giacomo S (2016) Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules 21(9). 10.3390/molecules21091243 [DOI] [PMC free article] [PubMed]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Miyasaka T, Xie C, Yoshimura S, Shinzaki Y, Yoshina S, Kage-Nakadai E, et al. Curcumin improves tau-induced neuronal dysfunction of nematodes. Neurobiol Aging. 2016;39:69–81. doi: 10.1016/j.neurobiolaging.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Molino S, Dossena M, Buonocore D, Ferrari F, Venturini L, Ricevuti G, Verri M. Polyphenols in dementia: from molecular basis to clinical trials. Life Sci. 2016;161:69–77. doi: 10.1016/j.lfs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Morley JE, Armbrecht HJ, Farr SA, Kumar VB. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer's disease. Biochim Biophys Acta. 2012;1822(5):650–656. doi: 10.1016/j.bbadis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG. Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem. 2014;80:175–183. doi: 10.1016/j.ejmech.2014.04.050. [DOI] [PubMed] [Google Scholar]
- Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, S. M., Choi, J. H., Yoo, D. Y., Kim, W., Jung, H. Y., Kim, J. W., … Hwang, I. K. (2014). Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food, 17(6), 641–649. doi:10.1089/jmf.2013.2965 [DOI] [PMC free article] [PubMed]
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164(9):898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M. Oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2016;53(6):4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri S, Korner S, Kiaei M. Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurol Res Int. 2012;2012(878030):1–7. doi: 10.1155/2012/878030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirainen S, Youssef A, Song C, Kalueff AV, Landreth GE, Malm T, Tian L. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer's disease: the emerging role for microglia? Neurosci Biobehav Rev. 2017;77:148–164. doi: 10.1016/j.neubiorev.2017.01.046. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92(3):293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Polydoro M, Wegmann S, Nicholls SB, Spires-Jones TL, Hyman BT. Propagation of tau pathology in Alzheimer's disease: identification of novel therapeutic targets. Alzheimers Res Ther. 2013;5(5):49. doi: 10.1186/alzrt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka, M., Owczarz, M., Wieczorowska-Tobis, K., Nadrowski, P., Chudek, J., Slusarczyk, P., …Mossakowska, M. (2016). Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing, 13, 21. doi:10.1186/s12979-016-0076-x [DOI] [PMC free article] [PubMed]
- Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey-Smith SR, Brown BM, Sohrabi HR, Shah T, Goozee KG, Gupta VB, Martins RN. Curcumin and cognition: a randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br J Nutr. 2016;115(12):2106–2113. doi: 10.1017/S0007114516001203. [DOI] [PubMed] [Google Scholar]
- Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Ringman, J. M., Frautschy, S. A., Teng, E., Begum, A. N., Bardens, J., Beigi, M., …, Cole, G. M. (2012). Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther, 4(5), 43. doi:10.1186/alzrt146 [DOI] [PMC free article] [PubMed]
- Rinwa P, Kumar A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2012;1488:38–50. doi: 10.1016/j.brainres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Brashear HR. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med. 2014;370(15):1460. doi: 10.1056/NEJMc1402193. [DOI] [PubMed] [Google Scholar]
- Sankowski R, Mader S, Valdes-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker MR, Franks S, Sumien N, Thangthaeng N, Filipetto F, Forster M. Curcumin mimics the neurocognitive and anti-inflammatory effects of caloric restriction in a mouse model of midlife obesity. PLoS One. 2015;10(10):e0140431. doi: 10.1371/journal.pone.0140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73(13):4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Asseburg H, Kuntz S, Muller WE, Eckert GP. Effects of polyphenols on brain ageing and Alzheimer's disease: focus on mitochondria. Mol Neurobiol. 2012;46(1):161–178. doi: 10.1007/s12035-012-8282-9. [DOI] [PubMed] [Google Scholar]
- Shailaja M, Damodara Gowda KM, Vishakh K, Suchetha Kumari N. Anti-aging role of curcumin by modulating the inflammatory markers in albino Wistar rats. J Natl Med Assoc. 2017;109(1):9–13. doi: 10.1016/j.jnma.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Shanmugam, M. K., Rane, G., Kanchi, M. M., Arfuso, F., Chinnathambi, A., Zayed, M. E., …Sethi, G. (2015). The multifaceted role of curcumin in cancer prevention and treatment. Molecules, 20(2), 2728–2769. doi:10.3390/molecules20022728 [DOI] [PMC free article] [PubMed]
- Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39(1):69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- Siddique YH, Naz F, Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson's disease. Biomed Res Int. 2014;2014(606928):1–6. doi: 10.1155/2014/606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999;108(3):239–251. doi: 10.1016/S0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Sun CY, Qi SS, Zhou P, Cui HR, Chen SX, Dai KY, Tang ML. Neurobiological and pharmacological validity of curcumin in ameliorating memory performance of senescence-accelerated mice. Pharmacol Biochem Behav. 2013;105:76–82. doi: 10.1016/j.pbb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682–691. [PubMed] [Google Scholar]
- Suzuki M, Willcox DC, Rosenbaum MW, Willcox BJ. Oxidative stress and longevity in Okinawa: an investigation of blood lipid peroxidation and tocopherol in okinawan centenarians. Curr Gerontol Geriatr Res. 2010;2010(380460):1–10. doi: 10.1155/2010/380460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14(1):41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Aberrant proteolytic processing and therapeutic strategies in Alzheimer disease. Adv Biol Regul. 2017;64:33–38. doi: 10.1016/j.jbior.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Trujillo J, Granados-Castro LF, Zazueta C, Anderica-Romero AC, Chirino YI, Pedraza-Chaverri J. Mitochondria as a target in the therapeutic properties of curcumin. Arch Pharm (Weinheim) 2014;347(12):873–884. doi: 10.1002/ardp.201400266. [DOI] [PubMed] [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13(4):439–450. doi: 10.1017/S1041610201007852. [DOI] [PubMed] [Google Scholar]
- Vivar C. Adult hippocampal neurogenesis, aging and neurodegenerative diseases: possible strategies to prevent cognitive impairment. Curr Top Med Chem. 2015;15(21):2175–2192. doi: 10.2174/1568026615666150610141524. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi: 10.1016/j.ejphar.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Wang, P., Su, C., Li, R., Wang, H., Ren, Y., Sun, H., …Jiang, S. (2014a). Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J Neurosci Res, 92(2), 218–231. doi:10.1002/jnr.23322 [DOI] [PubMed]
- Willette, A. A., Bendlin, B. B., Starks, E. J., Birdsill, A. C., Johnson, S. C., Christian, B. T., …Asthana, S. (2015). Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol, 72(9), 1013–1020. doi:10.1001/jamaneurol.2015.0613 [DOI] [PMC free article] [PubMed]
- Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation. 2015;12:85. doi: 10.1186/s12974-015-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, F., Zhao, Y., Ma, J., Gong, J. B., Wang, S. D., Zhang, L., …, Qian, L. J. (2016). The involvement of homocysteine in stress-induced Abeta precursor protein misprocessing and related cognitive decline in rats. Cell Stress Chaperones, 21(5), 915–926. doi:10.1007/s12192-016-0718-0 [DOI] [PMC free article] [PubMed]
- Yanagisawa, D., Ibrahim, N. F., Taguchi, H., Morikawa, S., Hirao, K., Shirai, N., …, Tooyama, I. (2015). Curcumin derivative with the substitution at C-4 position, but not curcumin, is effective against amyloid pathology in APP/PS1 mice. Neurobiol Aging, 36(1), 201–210. doi:10.1016/j.neurobiolaging.2014.07.041 [DOI] [PubMed]
- Zhang X, Xiao Z, Yao J, Zhao G, Fa X, Niu J. Participation of protein kinase C in the activation of Nrf2 signaling by ischemic preconditioning in the isolated rabbit heart. Mol Cell Biochem. 2013;372(1–2):169–179. doi: 10.1007/s11010-012-1458-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090(1):35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]