Abstract
Cellular senescence is a central component of the aging process. This cellular response has been found to be induced by multiple forms of molecular damage and senescent cells increase in number with age in all tissues examined to date. We have examined the correlation with age of two key proteins involved in the senescence program, p16INK4a and HMGB2. These proteins are involved in cell cycle arrest and chromatin remodeling during senescence. Circulating levels of these markers increases with age and correlates with functional status. The levels of HMGB2 appear to be significantly correlated with functional status, whereas p16INK4a levels are more weakly associated. Interestingly, there is a strong correlation between the two proteins independent of age. In particular, a single high-functioning individual over 90 years of age displays a disproportionately low level of HGMB2. The results suggest that with improved testing methodology, it may be possible to monitor circulating protein markers of senescence in human populations.
Keywords: Aging, Senescence, p16, HMGB2, Cognition, Frailty, Biomarker, Chromatin
Introduction
Cellular senescence is a state of permanent growth arrest that can be triggered by damage to the genome in the form of telomere erosion, hypereplication due to oncogene activation, γ-irradiation, or metabolic imbalances caused by mitochondrial dysfunction (Lu and Finkel 2008; Nacarelli and Sell 2017; von Zglinicki et al. 2005). Evidence for the role of senescent cells in the aging process includes the identification of senescent cells in aged tissues such as the skin, cardiovascular system, bone, and brain (Zhu et al. 2014). Direct evidence for a role in age-related dysfunction for senescent cells has been provided by mouse model in which the elimination of senescent cells can alleviate age-related dysfunction (Baker et al. 2011) and improve recovery following exposure to high doses of radiation, which induces senescence in multiple cell types (Yosef et al. 2016).
A barrier to the evaluation of senescence in human populations is the lack of reliable markers for senescence (Niedernhofer et al. 2017). Such markers are important to evaluate individual variation in the rate of aging in human populations and the impact of interventions that target the aging process. Efforts to estimate the burden of senescence have focused on RNA levels of the cell cycle regulator, p16INK4a, and mediators of the senescence-associated secretory phenotype (SASP) (Sanoff et al. 2014; Maas et al. 2016). These mediators include inflammatory cytokines such as IL-6 and IL-8. One limitation to the usefulness of these markers, however, is their dual roles in both inflammation and senescence. Inflammatory processes are known to increase with age and the level of IL-6 increases in multiple pathologies associated with aging, such as Alzheimer’s disease (He and Sharpless 2017; Swardfager et al. 2010).
One class of proteins that has not been explored as potential markers of senescence are the high-mobility group (HMG) proteins). The HMG proteins are structural components of chromatin and are ubiquitously distributed in the nuclei of higher eukaryotes (Bianchi and Agresti 2005; Thomas 2001). One member of this protein family, HMGB1, has been found to function as a chemokine and is secreted during necrosis to activate macrophages, monocytes, and dendritic cells (Scaffidi et al. 2002). A second member of this family, HMBG2 shows a more limited pattern of expression and has recently been found to be excluded from the nucleus of senescent cells (Davalos et al. 2013). The loss of HMGB2 is a part of the chromatin remodeling that is central to the establishment of the senescent state and secretion of the inflammatory cytokines that are a part of the SASP (Aird et al. 2016).
We have examined the circulating levels of HMGB2 in a population of healthy, free living adults to determine whether a relationship exists between age and HMGB2 levels in the circulation.
Methods
Patient recruitment and health assessment
Patients over the age of 70 were recruited at the Drexel University Internal Medicine Clinical Practices under an approved IRB protocol. Patient samples were processed by the Drexel University Department of Pathology Tissue Procurement Facility and both DNA and plasma samples were stored at − 80 °C. For Cognitive Assessment, the Montreal Cognitive Assessment (MOCA) was used as a guide but patients were assigned to one of three categories of functional status with scores ranging from 1 to 3 based on clinical observations to allow segregation into defined functional groups for correlation with HMGB2 levels. Similarly, for Functional Assessment, the Clinical Frailty Index (CFI) was used as a guide. Patients were classified using the CFI based on Instrumental Activities of Daily Living (ADls), ability to exercise and ambulate. Using the Katz index of Activities of Daily Living which consists of independence in the following: Bathing, Dressing, Toileting, Continence, Feeding, Transferring. Patients received a score of 1 is a CFI of 1–3, a score of 2 is a CFI of 4–6, and a score of 3 relates to a CFI of 7–9. No patients were in the extreme ranges of the CFI of extreme frailty as all patients were recruited in the outpatient clinics.
HMGB2 measurements
Levels of HMGB2 and p16INK4A were measured in plasma samples and cell culture supernatants using an ELISA kit produced by Lifespan Biosciences (LS-F11643-1, LS-F11076). Cell culture supernatants were prepared from human umbilical vein endothelial cells (HUVEC) purchased from the American Type Culture Collection (ATCC). Senescence was induced through exposure to 10 μg/ml nucleoside analogs tenofovirdisoproxilfumarate (TDF) and emtricitabine (FTC) which were provided by the NIH AIDS Reagent Program. These concentrations of nucleoside analogs have been demonstrated to induce senescence through mitochondrial stress (Nacarelli and Sell 2017). Cells were exposed to nucleoside analogs for 7 days conditioned media was collected by removing culture media, washing cells 3 times with phosphate buffered saline and placing cultures into serum free medium for 24 h. Cell number was determined for normalization. Plasma samples (100 μl) or cell culture supernatant were analyzed by ELISA and samples from cell cultures were adjusted for cell number from direct cell counts.
Results
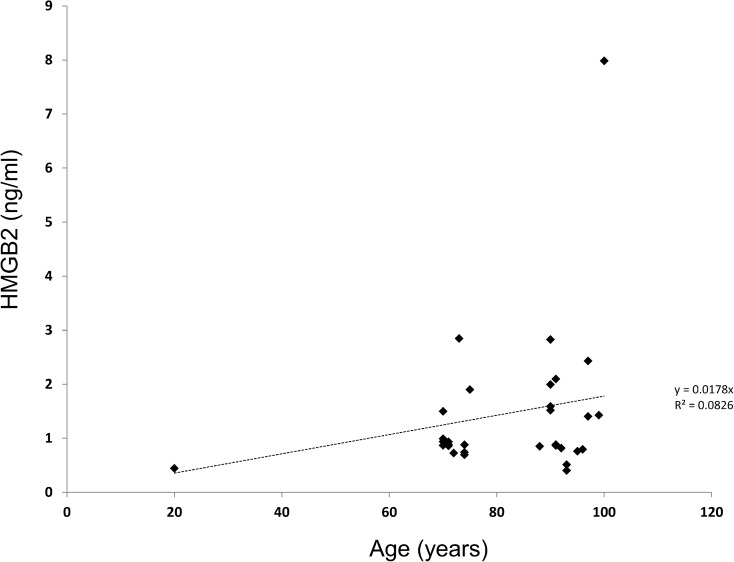
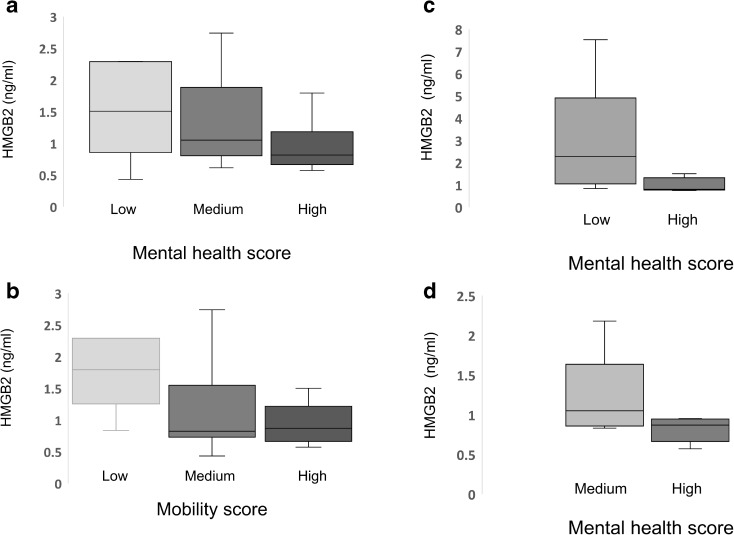
We examined plasma levels of HMGB2 in a cohort of 108 patients ranging from 70 to 100 years who had been recruited into the Drexel Healthy Aging Study at the Drexel University Internal Medicine Clinical Practices. Plasma from four healthy young controls aged 20–24 years was included to provide an early life baseline. HMGB2 levels increased with age (R2 value of 0.0826 and a Pearson’s correlation of 0.546) (Fig. 1). Absolute HMGB2 levels ranged from 0.4 to 7.9 ng/ml (Fig. 1). We also examined mean HMGB2 levels in the patients > 90 years old following stratification by functional status. HMGB2 levels in the blood correlated with both mental health and functional status when all patients in the cohort were evaluated (Fig. 2). The difference in HMGB2 stratified by mental health status was significant by ANOVA at P = 0.0299. The difference in HMGB2 relative to mobility was less robust at P = 0.058. The results were also stratified by age in patients > 90 years, and in patients aged 70–80 years (Fig. 2). This evaluation revealed the same trend, but differences did not reach significance when analyzed by ANOVA. When the cohort of patients > 90 years were evaluated by activities of daily living, HMGB2 levels were also significantly higher in the group of low-functioning patients relative to the group of high-functioning patients but the difference was not statistically significant.
Fig. 1.
HMGB2 levels correlate with age and functional status in humans. HMGB2 levels were measured in a cohort of patients ranging in age from 60 to 100 as well as four healthy donors aged 20–24. HMGB2 levels are plotted against age
Fig. 2.
HMGB2 levels correlate with functional status. Patients were grouped by functional status, either mental health (Panel A) or mobility (Panel B) as assessed by clinical observation. Mean HMGB2 levels in each group (high, medium, or low) were then graphed and analyzed by ANOVA and student’s T test. The difference between high and low groups was significant by ANOVA analysis at P < 0.05. In panel C and D, patients over 90 years of age (C), or between 70 and 80 years of age (D) were subjected to the same analysis. Values for the medium group and the low-functioning groups are not included respectively due to low numbers, less than two per group. Differences between groups were not significant by ANOVA
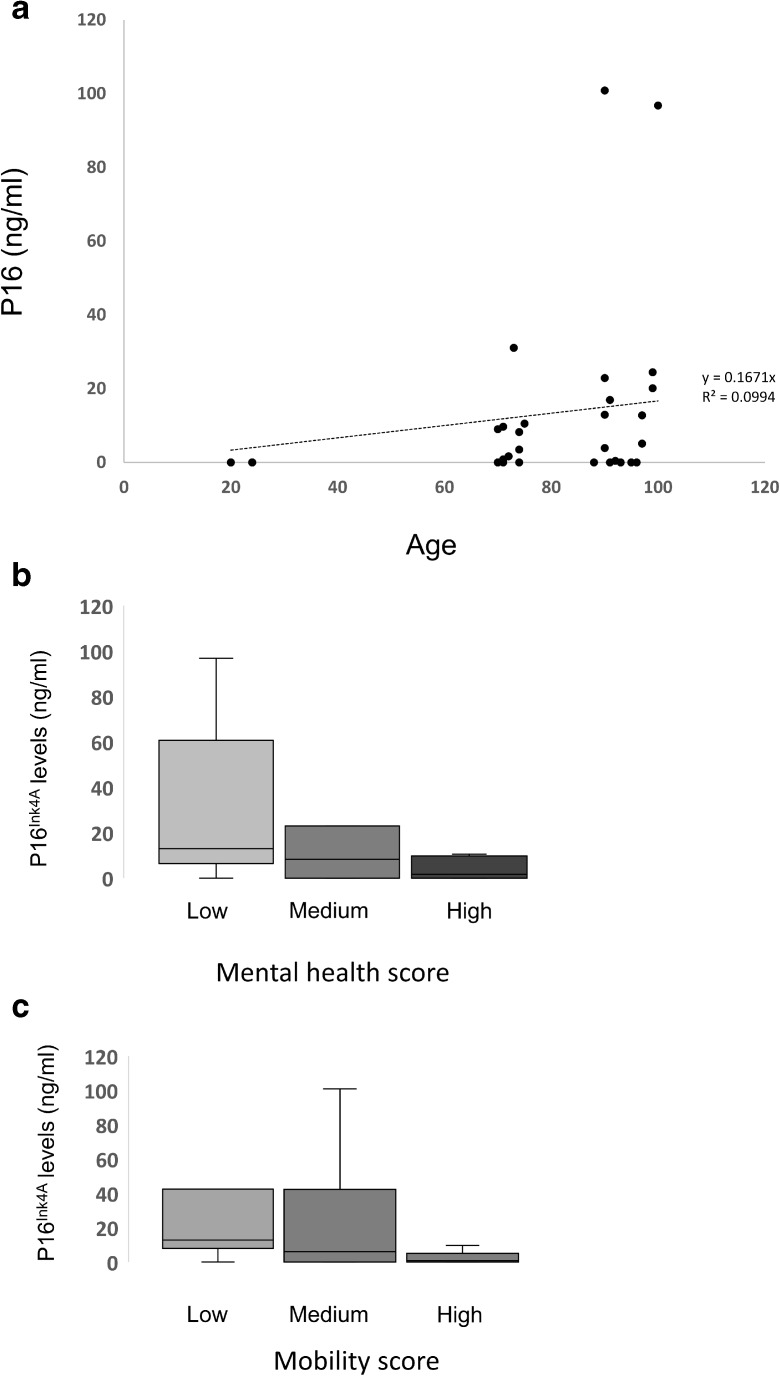
In order to determine if levels of HMGB2 correlated with additional markers of senescence, levels of the p16 INK4a protein were evaluated by ELISA in the plasma of a subset of patients in the cohort. The levels of p16 INK4a increased with age with an R2 value of 0.099 and Pearson’s correlation coefficient of 0.035 (Fig. 3). Levels of p16 followed similar trends as HMGB2 when patients were stratified by either mental health or mobility. However, the correlation between p16 INK4a levels in the plasma and functionality was lower than the association observed for HMGB2 (Fig. 3).
Fig. 3.
Levels of ranging in age from 60 to 100 as well as four healthy donors aged 20–24. HMGB2 levels are plotted against age. Correlate with age and functional status. In panel A, p16Ink4a levels are plotted against age in patients ranging in age from 60 to 100 as well as 4 healthy donors aged 20–24. HMGB2 levels are plotted against age. In panel B and C, patients were grouped according to functional status and the mean levels of p16Ink4a are presented for each group according to mental health score (C) or mobility score (D). Differences between groups were not significant by ANOVA
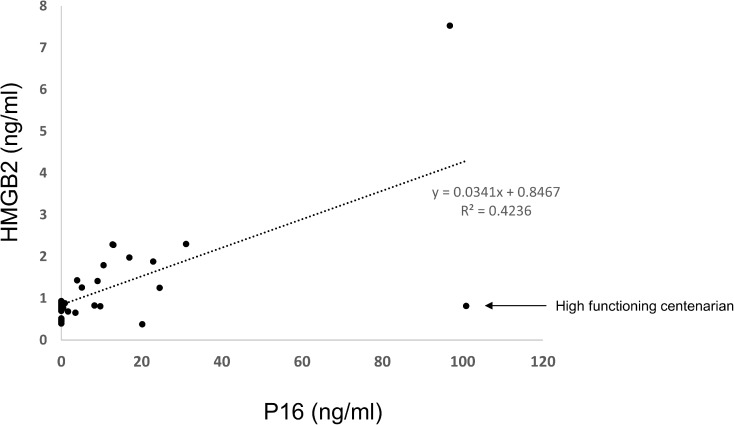
In order to determine whether the two markers of senescence, HMGB2 and p16 INK4a, bore any relationship to each other, we looked for a correlation between HMGB2 and p16 levels in the blood of patients in the cohort (Fig. 4). The levels of HMGB2 and p16 INK4a showed a robust correlation (R2 = 0.4236 and Pearson’s correlation coefficient 0.65). Interestingly, two of the oldest patients in the cohort showed very different HMGB2 levels. One 91 year old had quite high levels relative to the rest of the cohort and one centenarian had quite low levels relative to the rest of the cohort. The patient with elevated HMGB2 showed low mental health and mobility whereas, the patient with low HMGB2 showed high mental health status and high mobility. Removal of the high-functioning patient increased the R value for the correlation between HMGB2 and p16 to 0.8644 and the Pearson’s coefficient to 0.929.
Fig. 4.
Levels of HMGB2 and p16Ink4a correlate regardless of patient age. Levels of HMGB2 and p16Ink4a are plotted independently of other variables. Removal of the single high-functioning individual at 100 years results in a correlation of 0.864 and a Pearson’s correlation coefficient of 0.929
In order to evaluate the potential source of HMGB2, we examined endothelial cells for changes in HMGB2 reasoning that these cells may release the protein directly into the circulation upon entering senescence. Endothelial cells were driven into senescence by exposure to nucleoside analogs, which impair mitochondrial function, leading to increased levels of ROS, metabolic stress, and senescence (Nacarelli et al. 2016). Senescent endothelial cells showed a reduced HMGB2 levels and loss of nuclear stain relative to non-senescent cells, and an evaluation of HMGB2 levels in the supernatant revealed that senescent endothelial cells released HMGB2 into the culture medium (Fig. 5).
Fig. 5.
HMGB2 is released from senescent endothelial cells. Panel A contains the results of an ELISA for HMGB2 protein in the supernatant of pre senescent human endothelial cells and human endothelial cells induced to enter senescence through mitochondrial stress. Differences in HMGB2 levels in the ELISA are significant at P < 0.05 by Student’s T test
Discussion
The identification of accurate biomarkers for the intrinsic aging process is essential for the successful development of therapies targeting age-related disorders (Niedernhofer et al. 2017). One potentially significant biomarker is the cell cycle regulator p16Ink4a, which has emerged as a key regulator of cellular senescence (He and Sharpless 2017).To determine whether p16Ink4a may serve as a marker of biological age, we assayed free p16Ink4a levels in the serum of patients and found that free p16Ink4a protein can be detected in the serum of patients and correlate with donor age. One caveat to p16Ink4a as a marker of biological age is the very low levels detected in many patients, which likely decreases the power of the marked and limits the usefulness of the test in its current form. Interestingly, we find that an additional marker of senescence, HMGB2 is also correlated with donor age and with functional status. There is a striking correlation between levels of HMGB2 and p16Ink4a in our cohort which is independent of donor age or functional status. This correlation suggests that the markers detect the intrinsic aging rate but the relatively lower correlation with age for each of the markers indicates that additional influences impact each marker. Perhaps most surprising is the outlying value in a 91-year old patient with very high-functional status. This patient (the outlier with a low HMGB2 level in Fig. 5) had a score of 6 on the Katz activities of daily living and a score of 6 on the Lawtons Instrumental Activities of Daily living. Based on her Katz, Lawtons score and her ability to exercise, she was classified as Class 2 in the CFI. The other oulier in Fig. 5, with a high HMGB2 score scored 0 on Activities of Daily living and a 0 in Instrumental Activities of daily living. Also, based on the fact that she was completely dependent and approaching end of life, her CFI score was 8. Greater numbers of patients in these age ranges with high functionality are required to confirm these results but it is worthwhile investigating for other markers of senescence in the geriatric population especially those who are classified as being Clinical Frailty Index class 1,2, or 3, and advanced age. It is also important to evaluate whether race and cognition play a role in the levels of these markers. It will be of interest to utilize the markers of senescence identified in our study in a longitudinal study to follow patients with divergent HMGB2 levels in their 70s as these patients reach advanced age. One would predict that patients with low HMGB2 levels would maintain functional and mental status better than patients with high HMGB2 levels.
Obvious limitations to the present analysis include the number of patients within the cohort and the relative insensitivity of the p16Ink4a assay. It is likely that more sensitive assays will provide greater insight into the relationships between the senescence process and late-life functionality. However, the data strongly suggests that utilizing markers of senescence will be a useful approach to evaluate functionality both in normal aging and in the evaluation of interventions that target the intrinsic aging process of senescence.
References
- Aird KM, Iwasaki O, Kossenkov AV, Tanizawa H, Fatkhutdinov N, Bitler BG, le L, Alicea G, Yang TL, Johnson FB, Noma KI, Zhang R. HMGB2 orchestrates the chromatin landscape of senescence-associated secretory phenotype gene loci. J Cell Biol. 2016;215(3):325–334. doi: 10.1083/jcb.201608026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15(5):496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201(4):613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314(9):1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas BM, Francis O, Mollan KR, Lee C, Cottrell ML, Prince HMA, Sykes C, Trezza C, Torrice C, White N, Malone S, Hudgens MG, Sharpless NE, Dumond JB. Concentrations of pro-inflammatory cytokines are not associated with senescence marker p16INK4a or predictive of intracellular emtricitabine/tenofovir metabolite and endogenous nucleotide exposures in adults with HIV infection. PLoS One. 2016;11(12):e0168709. doi: 10.1371/journal.pone.0168709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Sell C. Targeting metabolism in cellular senescence, a role for intervention. Mol Cell Endocrinol. 2017;455:83–92. doi: 10.1016/j.mce.2016.08.049. [DOI] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Sell C. Mitochondrial stress induces cellular senescence in an mTORC1-dependent manner. Free Radic Biol Med. 2016;95:133–154. doi: 10.1016/j.freeradbiomed.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Kirkland JL, Ladiges W. Molecular pathology endpoints useful for aging studies. Ageing Res Rev. 2017;35:241–249. doi: 10.1016/j.arr.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001;29(Pt 4):395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126(1):111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]