Abstract
The incidence of acute kidney injury (AKI) rises with age and is associated with multiple risk factors. Here, we compared the risk factors for AKI between younger and older incident diabetic patients to examine the trends in risk alteration for individual factors across different age groups. Between 2007 and 2013, we selected all incident type 2 diabetic adults from the Taiwan National Health Insurance registry, stratified based on age: young (< 65 years), old (≥ 65 but < 75 years), and older-old (≥ 75 years). All factors with potential renal influence (e.g., comorbidities, medications, and diagnostics/procedures) were recorded during the study period, with a nested case-controlled approach utilized to identify independent risk factors for AKI in each age group. Totally, 930,709 type 2 diabetic patients were categorized as young (68.7%), old (17.7%), or older-old (13.6%). Older-old patients showed a significantly higher incidence of AKI than the old and the young groups. Cardiovascular morbidities (hypertension, atrial fibrillation, acute coronary syndrome, and cerebrovascular disease) were shown to increase the risk of AKI, although the risk declined with increasing age. Chronic obstructive pulmonary disease and receiving cardiac catheterization elevated the risk of AKI preferentially in the older-old/old and older-old group, respectively, while the administration of angiotensin-converting enzyme/α-blocker and angiotensin receptor blocker/calcium channel blocker reduced the risk of AKI preferentially in the older-old and older-old/old group, respectively. In conclusion, our findings highlight the importance of devising age-specific risk factor panels for AKI in patients with recently diagnosed type 2 diabetes.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0013-3) contains supplementary material, which is available to authorized users.
Keywords: Acute kidney injury, Diabetes mellitus, Geriatrics, Risk factors
Introduction
Age-related changes confer susceptibility to environmental pressures for almost all physiological systems of our body. Renal aging encompasses a wide range of physiological and structural changes, including a reduction in nephron number and size, glomerulosclerosis, tubulointerstitial fibrosis, and microvascular insufficiency (O’Sullivan et al. 2017). A chronological increase in age is strongly correlated with a decline in measured glomerular filtration rate (GFR). The association can be quite significant, although there are still examples of healthy elderly maintaining measured GFRs equivalent to those of young persons. Despite the finding that age-related histological changes have not been shown to consistently correlate with either a reduction in GFR or albuminuria (Rule et al. 2010; Rule et al. 2011), patients of advanced age are still at a higher risk of developing incident chronic kidney disease (CKD), acute kidney injury (AKI), and adverse renal outcomes (Kaze et al. 2016; Andò et al., 2013; Chao et al. 2012a).
The incidence of AKI rises steadily with aging. Data from the United States Renal Data System survey showed that the incidence of AKI increased progressively from 19.1 episodes per 1000 patient-years among participants aged 66–69 years, to 26.7, 40.2, 57.8, and 89.7 episodes per 1000 patient-years among those aged 70–74 years, 75–79 years, 80–84 years, and ≥ 85 years, respectively (USRDS Annual Report, 2017). Apart from age, there are a number of other risk factors associated with AKI, including medical settings (i.e., hospitalization and intensive care), acute illnesses and interventions (i.e., major surgeries, sepsis, and cardiac procedures), comorbidities or their aggregates, proteinuria, and exposure to nephrotoxins (Rewa and Bagshaw 2014). Existing studies suggest that risk factors for AKI often interact with each other, and that certain factors can modify the relationship between others and AKI, particularly those with diabetes mellitus (DM). In a meta-analysis, James et al. discovered that DM accentuated the risk for AKI for patients irrespective of their baseline estimated GFR levels, while hypertension raised the risk, but only for those with higher estimated GFR (James et al. 2015). Based upon the influence of age on the incidence of AKI, it is highly likely that age might also interact with other risk factors for AKI. Indeed, Grams et al. found that the association between older age and increased risk for AKI was attenuated in those with more advanced CKD (Grams et al. 2015). We also identified that criteria for stratification of prognosis behaved differently between patients of very advanced age (≥ 76 years) and those of 65–76 years (Chao et al. 2012a). In addition, geriatric syndromes, which are rarely detected in younger patients, predispose the elderly to developing AKI. For example, we previously discovered that functional impairment was significantly associated with higher AKI risk, while polypharmacy also raised the risk of AKI by 2.5 fold among affected elderly (Chao et al. 2015a; Chao et al. 2015b). Urinary incontinence may also predispose older adults to urinary tract infection and urosepsis, which is also a recognized risk factor for AKI (Hermandez Hermandez et al., 2013). The above findings lend strong support to our proposition that age represents an under-recognized modifier for the spectrum of AKI risk factors.
We hypothesized that the spectrum of risk factors for AKI found in the general diabetic population would differ from those in the diabetic elderly, an under-recognized reason for the rising incidences of AKI among older diabetic adults. We evaluated risk factor profiles in diabetic patients, a population exhibiting high risk for AKI, of different age groups and compared the results between young and old patients. Furthermore, we attempted to examine the way in which individual risk factors changed across different age groups to identify whether age modifies the relationship between each risk factor and AKI. Judging from the multitude of known risk factors for AKI, and the necessity for including a large number of cases, we aimed to test this hypothesis using a large, well-validated, healthcare registry, the Taiwan National Health Insurance (NHI) database.
Methods
Ethical approval
The protocol for this study was approved by the ethics committee of National Taiwan University (NO.201503028W). The committee waivered the need for informed consent from the participants due to the anonymous nature of the dataset.
Data sources and study population
Study participants were identified from the NHI research database, a comprehensive reimbursement-based registry maintained by the Bureau of NHI in Taiwan, which covers nearly all Taiwanese citizens. All of the health services (medications and surgeries/procedures) received by NHI-covered patients, including outpatient, inpatient, and emergency care, are documented meticulously in an electronic registry (Chao et al. 2012b; Chao et al. 2014; Chao et al. 2017).
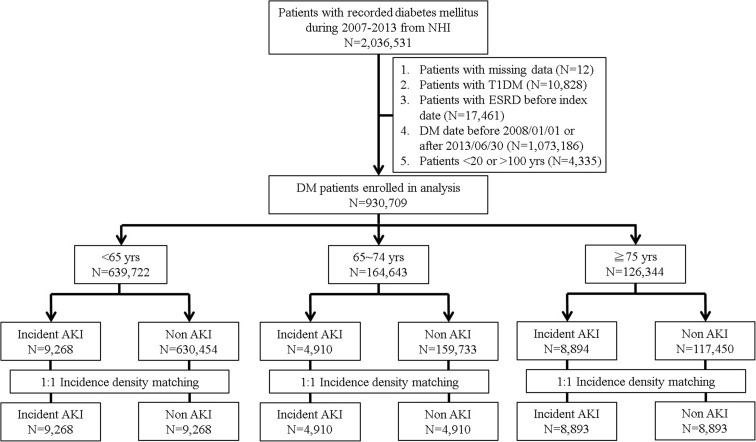
We selected all adults (aged ≥ 20 years) with incident DM in Taiwan between 2007 and 2013 from the registry who had at being hospitalized at least once or had attended two outpatient clinic visits for DM, based on International Classification of Diseases 9th revision—Clinical Modification [ICD-9-CM] codes 250.x. Patients were excluded from the study if they had incomplete demographic data, type 1 DM (codes 250.01, 250.03, 250.11, 250.13, 250.21, 250.23, 250.31, 250.33, 250.41, 250.43, 250.51, 250.53, 250.61, 250.63, 250.71, 250.73, 250.81, 250.83, 250.91, 250.93), DM that pre-existed before 2008 (1 year for verifying the diagnosis and reassuring that participants had recently diagnosed DM) or was recognized after 2013/06/30 (to allow an adequate length of follow-up for AKI event), according to our previous study (Chao et al. 2017), and if they had been diagnosed with pre-enrolment end-stage renal disease (ICD-9-CM code 585 concurrently with the presence of dialysis procedure codes for more than 3 months) (Fig. 1). Patients with prevalent type 2 DM during the study period were excluded due to the reason that the their severity of DM and its complications can vary widely based on their durations of DM, potentially introducing confounding effect on the risk estimation of AKI.
Fig. 1.
Study algorithm for identifying diabetic patients with and without acute kidney injury (AKI) in different age groups. Incidence density matching of these groups were based on age, sex, and the date of DM diagnosis. DM, diabetes mellitus; NHI, National Health Insurance; T1DM, type 1 diabetes mellitus
Definitions for events of interest
The event of interest in this study was hospitalization for AKI, which was further divided into AKI and dialysis-requiring AKI (Chao et al. 2012b; Chao et al. 2017). In this study, we identified incident diabetic participants with any incidence of hospitalization containing the diagnosis codes of ICD-9-CM 584.x. Dialysis-requiring AKI was defined as any hospitalization with a diagnosis of AKI upon admission accompanied by the presence of in-hospital procedure codes for dialysis. In Taiwan, the diagnosis of AKI and dialysis-requiring AKI is frequently based on laboratory criteria (defined as an increase of serum creatinine higher than 0.5 mg/dL from one’s baseline levels), and the accuracy of AKI codes has been validated in multiple prospectively enrolled cohorts (Wu et al. 2014; KDIGO group. 2012). During most clinical practice, the baseline serum creatinine value is usually designated as that obtained before the index hospitalization, which has been adopted widely as a standard for defining baseline renal function among studies evaluating AKI (Siew et al. 2010). We identified AKI episodes systemically from the registry if they occurred between the date of an individual’s DM diagnosis (any time between 2008/01/01 and 2013/06/30) and the end of follow-up (2013/12/31). A criterion that the date of AKI occurrence should be after that of DM diagnosis was set to ensure that our outcome of interest occurred after incident DM.
Definitions of comorbidities, use of medication, and interventions that could potentially influence the risk of developing AKI
We based our selection of risk factors upon previous studies (Rewa and Bagshaw 2014; Chao et al. 2017; Malhotra et al. 2017; Leblanc et al. 2015; Toprak, 2007), and our final analysis included comorbidities, medications, and diagnostics/procedures. Comorbidity profiles for the study population, including hypertension, hyperlipidemia, chronic liver disease (CLD), atrial fibrillation, chronic obstructive pulmonary disease (COPD), acute coronary syndrome (ACS), cerebrovascular disease, malignancy, parkinsonism, CKD, advanced CKD (if CKD patients were receiving erythropoietin concurrently, indicating stage 5 CKD status (Wu et al. 2017)), past experiences of AKI, peripheral vascular disease (PVD), and benign prostatic hyperplasia, were ascertained before the date of DM diagnosis. Recognition of each comorbidity was confirmed if the participant received their diagnosis during at least two outpatient clinics, or during at least one hospital admission prior to the date of DM diagnosis (the index date) (Chao et al. 2017). A complete list of ICD-9-CM codes used for extracting comorbidities could be retrieved from our work published previously (Chao et al. 2017).
We also collected the histories of any participants receiving specific diagnostics/procedures, including computed tomography of any sites (with or without contrast), cardiac catheterization, angiography of any sites, cystoscopy, and transurethral resection of the prostate, between the index date (DM diagnosis) and the occurrence of AKI (Chao et al. 2017). Similarly, we reviewed the prescription records of all medications, including the duration of use for each prescription, between the date of DM diagnosis and that of AKI occurrence (for cases) or matching date (for controls). Those who received each medication for at least 30 consecutive days within the designated study period were categorized as medications users.
Statistical analysis
We first classified the participants by their age on the index date into three groups: young (< 65 years), old (≥ 65 but <75 years) and older-old (≥ 75 years); this age classification was based upon our previous study (Chao et al. 2012a). We then conducted a nested case-controlled study to investigate independent risk factors for developing AKI, using an incidence density sampling approach, which matched each case of AKI to a control who was at risk for AKI at the time of case occurrence. Propensity-score matching was not used, since we aimed to examine the influence of all potential risk factors within this study cohort but not neutralize some of them. We first examined baseline characteristics, including age, sex, residential locations (rural vs. urban), risk-modifying interventions, comorbidities, and medications among participants from different age groups at the time of AKI occurrence (for cases) or matching date (for controls). The clinical characteristics listed above were compared using the chi-square test or the Student’s t test, as appropriate. We mitigated potential selection bias between the case (AKI) and control groups using one-to-one matching strategy by age (± 1 year), sex, and the date of DM diagnosis (± 90 days) across the three age groups (Richardson 2004). Within each age group, univariate analyses were performed to identify those with significant differences (p < 0.05) between the cases and controls, followed by a conditional multivariate logistic regression to estimate the odds ratios (ORs), and the 95% confidence intervals (CIs), of each significant variable identified by the univariate analyses (Chao et al. 2012c). C-statistics were used to estimate model validity.
All statistical tests were two-sided and P values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 2,036,531 patients with type 2 DM were identified between 2007 and 2013. After applying our exclusion criteria, we enrolled 930,709 diabetic patients for the final study, categorized as young (n = 639,722; 68.7%), old (n = 164,643; 17.7%), and older-old (n = 126,344; 13.6%) (Fig. 1). The clinical features of our participants are shown in Table 1. Older-old patients were significantly more likely to be female (p < 0.01), more likely to have the most comorbidities recorded (all p < 0.01) (except hyperlipidemia and CLD), and were less likely to live in urban areas (p < 0.01), than either the old or young participants. Older-old patients were also more likely to receive diagnostics/procedures with nephrotoxic potential (all p < 0.01).
Table 1.
Clinical features of all identified diabetic participants of different age groups
| Variables | < 65 years (n = 639,722) | 65–74 years (n = 164,643) | ≥ 75 years (n = 126,344) | p value |
|---|---|---|---|---|
| Demographic and socioeconomic profiles | ||||
| Age (years) | 51.2 ± 9.1 | 69.7 ± 2.9 | 81.4 ± 4.8 | < 0.01 |
| Gender (female %) | 277,492 (43.4) | 87,364 (53.1) | 65.451 (51.8) | < 0.01 |
| Residential location (urban %) | 444,656 (69.5) | 104,279 (63.3) | 77.992 (61.7) | < 0.01 |
| Comorbidities | ||||
| Hypertension (%) | 296,299 (46.3) | 115,549 (70.2) | 99,891 (79.1) | < 0.01 |
| Hyperlipidemia (%) | 264,071 (41.3) | 70,962 (43.1) | 39,633 (31.4) | < 0.01 |
| Chronic liver disease (%) | 125,981 (19.7) | 28,608 (17.4) | 16,426 (13.0) | < 0.01 |
| Atrial fibrillation (%) | 26,783 (4.2) | 16,569 (10.1) | 22,952 (18.2) | < 0.01 |
| Chronic obstructive pulmonary disease (%) | 19,154 (3.0) | 14,684 (8.9) | 23,931 (18.9) | < 0.01 |
| Acute coronary syndrome (%) | 53,741 (8.4) | 32,860 (20.0) | 36,698 (29.0) | < 0.01 |
| Cerebrovascular disease (%) | 36,683 (5.7) | 27,355 (16.6) | 39,837 (31.5) | < 0.01 |
| Malignancy (%) | 24,989 (3.9) | 12,881 (7.8) | 13,275 (10.5) | < 0.01 |
| Parkinsonism (%) | 2300 (0.4) | 3008 (1.8) | 7503 (5.9) | < 0.01 |
| Chronic kidney disease (%) | 56,875 (8.9) | 22,581 (13.7) | 28,689 (22.7) | < 0.01 |
| Advanced chronic kidney disease (%) | 342 (0.1) | 274 (0.2) | 425 (0.3) | < 0.01 |
| Past experience of AKI (%) | 6218 (1.0) | 2850 (1.7) | 5748 (4.5) | < 0.01 |
| Peripheral vascular disease (%) | 5371 (0.8) | 3320 (2.0) | 4356 (3.4) | < 0.01 |
| Benign prostatic hyperplasia (%) | 20,773 (3.2) | 18,496 (11.2) | 23,912 (18.9) | < 0.01 |
| Intervention before the index date | ||||
| Computed tomography of any site (%) | 48,993 (7.7) | 19,554 (11.9) | 22,278 (17.6) | < 0.01 |
| Cardiac catheterization (%) | 7933 (1.2) | 4475 (2.7) | 4397 (3.5) | < 0.01 |
| Angiography of any site (%) | 7379 (1.2) | 4205 (2.6) | 4160 (3.3) | < 0.01 |
| Cystoscopy with or without biopsy (%) | 10,444 (1.6) | 4379 (2.7) | 4525 (3.6) | < 0.01 |
| Transurethral resection of prostate (%) | 438 (0.1) | 1181 (0.7) | 1622 (1.3) | < 0.01 |
| Chronic medication use | ||||
| Aspirin (%) | 140,428 (22.0) | 61,523 (37.4) | 47,224 (37.4) | < 0.01 |
| β-blocker (%) | 198,102 (31.0) | 65,605 (39.8) | 42,452 (33.6) | < 0.01 |
| ACEI (%) | 100,158 (15.7) | 31,863 (19.4) | 22,292 (17.6) | < 0.01 |
| ARB (%) | 239,566 (37.4) | 82,353 (50.0) | 57,976 (45.9) | < 0.01 |
| Clopidogrel (%) | 19,270 (3.0) | 10,713 (6.5) | 11,144 (8.8) | < 0.01 |
| Statin (%) | 307,840 (48.1) | 80,933 (49.2) | 41,023 (32.5) | < 0.01 |
| Fibrate (%) | 117,017 (18.3) | 19,796 (12.0) | 8618 (6.8) | < 0.01 |
| Ezetimibe (%) | 27,531 (4.3) | 6582 (4.0) | 2897 (2.3) | < 0.01 |
| Calcium channel blocker (%) | 261,502 (40.9) | 99,499 (60.4) | 74,815 (59.2) | < 0.01 |
| α-blocker (%) | 33,735 (5.3) | 20,341 (12.4) | 18,070 (14.3) | < 0.01 |
| Wafarin (%) | 6265 (1.0) | 4493 (2.7) | 4773 (3.8) | < 0.01 |
| Platinum-based anti-neoplastic agents (%) | 5361 (0.8) | 1662 (1.0) | 604 (0.5) | < 0.01 |
| Atypical anti-psychotics (%) | 13,837 (2.2) | 5803 (3.5) | 11,002 (8.7) | < 0.01 |
| Nephrotoxic anti-bacterials (%)* | 1096 (0.2) | 454 (0.3) | 376 (0.3) | < 0.01 |
| Nephrotoxic anti-virals (%)& | 1128 (0.2) | 193 (0.1) | 66 (0.1) | < 0.01 |
| Cyclosporin/tacrolimus (%) | 678 (0.1) | 73 (< 0.1) | 2 (< 0.1) | < 0.01 |
| Lithium (%) | 1760 (0.3) | 144 (0.1) | 31 (< 0.1) | < 0.01 |
| Anti-diabetic medications | ||||
| Insulin (%) | 31,840 (5.0) | 4836 (2.9) | 4451 (3.5) | < 0.01 |
| Biguanides (%) | 426,930 (66.7) | 96,100 (58.4) | 5,0051 (39.6) | < 0.01 |
| Sulfonylurea (%) | 316,085 (49.4) | 68,697 (41.7) | 36,986 (29.3) | < 0.01 |
| Meglitinide (%) | 40,731 (6.4) | 11,847 (7.2) | 10,388 (8.2) | < 0.01 |
| α-glucosidase (%) | 71,674 (11.2) | 16,516 (10.0) | 10,895 (8.6) | < 0.01 |
| Thiazolidinedione (%) | 52,333 (8.2) | 8819 (5.4) | 3832 (3.0) | < 0.01 |
| DPP4 inhibitor (%) | 111,001 (17.4) | 21,283 (12.9) | 12,010 (9.5) | < 0.01 |
Data are expressed as mean ± standard deviation for continuous variable and number (percentage) for categorical variables
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; DPP4i, dipeptidyl peptidase 4 inhibitor
*Including vancomycin, aminoglycosides, and colistin
&Including acyclovir and ganciclovir
Following the diagnosis of DM, older-old patients showed a significantly higher incidence of AKI (25.5 episodes/1000 person-years) compared to the old patients (9.1 episodes/1000 person-years) and the young patients (4.4 episodes/1000 person-years). Similarly, older-old patients had a significantly higher incidence of dialysis-requiring AKI (4.4 episodes/1000 person-years) compared to the old patients (1.9 episodes/1000 person-years) and young patients (1.0 episodes/1000 person-years), lending support to the notion that aging increases the susceptibility of developing AKI.
Next, we determined the potential risk factors for AKI among patients from the three age groups, using a case-control approach. In total, 9268, 4910, and 8894 patients with AKI were selected from the young, old, and older-old groups, respectively; these were matched 1:1 to a non-AKI control from their age group (Fig. 1). Univariate analyses in young, old, and older-old patients with and without AKI are shown in supplementary Tables 1, 2, and 3. Conditional regression analyses disclosed that young patients with hypertension, CLD, atrial fibrillation, ACS, cerebrovascular disease, malignancy, Parkinsonism, CKD, advanced CKD, past AKI, and PVD, receiving computed tomography, using atypical anti-psychotics, insulin, sulfonylurea, and meglitinide, had a significantly higher risk of developing AKI than those without, while those with hyperlipidemia, receiving cystoscopy, using aspirin, β-blockers, statin, fibrate, biguanide, and dipeptidyl peptidase 4-inhibitor (DPP4i) had a significantly lower risk of developing AKI than those without (Table 2). Among old patients, those with hypertension, atrial fibrillation, COPD, ACS, cerebrovascular disease, malignancy, CKD, past AKI, PVD, using insulin, sulfonylurea, and meglitinide had a significantly higher risk of developing AKI than those without, while those with hyperlipidemia, receiving cystoscopy, using aspirin, β-blocker, angiotensin receptor blocker (ARB), statins, fibrate, calcium channel blockers (CCBs), biguanide, and DPP4i had a significantly lower risk of developing AKI than those without (Table 3). Finally, older-old patients with hypertension, atrial fibrillation, COPD, ACS, cerebrovascular disease, malignancy, CKD, advanced CKD, past AKI, receiving cardiac catheterization, using insulin, and sulfonylurea had a significantly higher risk of developing AKI than those without, while those with hyperlipidemia, using aspirin, β-blockers, angiotensin-converting enzyme inhibitor (ACEI), ARB, statins, fibrate, CCBs, α-blocker, biguanide, and DPP4i had a significantly lower risk of developing AKI than those without (Table 4). The areas under receiver-operating-curve (AUC) for analyses of young, old, and older-old group were 0.805, 0.775, and 0.723, respectively.
Table 2.
Crude and adjusted odds ratio of risk factors for AKI among participants lower than 65 years
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Demographic and socioeconomic profiles | ||||||
| Residential location (urban %) | 0.87 | 0.82–0.93 | < 0.01 | 0.94 | 0.86–1.02 | 0.14 |
| Comorbidities | ||||||
| Hypertension (%) | 1.91 | 1.79–2.04 | < 0.01 | 1.86 | 1.69–2.04 | < 0.01 |
| Hyperlipidemia (%) | 0.61 | 0.57–0.65 | < 0.01 | 0.78 | 0.71–0.85 | < 0.01 |
| Chronic liver disease (%) | 1.46 | 1.36–1.55 | < 0.01 | 1.33 | 1.22–1.46 | < 0.01 |
| Atrial fibrillation (%) | 1.73 | 1.54–1.94 | < 0.01 | 1.37 | 1.17–1.61 | < 0.01 |
| Chronic obstructive pulmonary disease (%) | 1.77 | 1.55–2.01 | < 0.01 | 1.17 | 0.98–1.39 | 0.09 |
| Acute coronary syndrome (%) | 1.57 | 1.45–1.71 | < 0.01 | 1.52 | 1.34–1.73 | < 0.01 |
| Cerebrovascular disease (%) | 2.41 | 2.19–2.66 | < 0.01 | 2.34 | 2.05–2.67 | < 0.01 |
| Malignancy (%) | 3.68 | 3.29–4.12 | < 0.01 | 3.5 | 3.02–4.05 | < 0.01 |
| Parkinsonism (%) | 2.42 | 1.73–3.38 | < 0.01 | 1.74 | 1.12–2.68 | < 0.01 |
| Chronic kidney disease (%) | 5.78 | 5.33–6.27 | < 0.01 | 4.62 | 4.18–5.1 | < 0.01 |
| Advanced chronic kidney disease (%) | 8.71 | 6.14–12.36 | < 0.01 | 2.94 | 1.94–4.44 | < 0.01 |
| Past experience of AKI (%) | 11.61 | 9.25–14.56 | < 0.01 | 3.26 | 2.51–4.24 | < 0.01 |
| Peripheral vascular disease (%) | 2.72 | 2.21–3.35 | < 0.01 | 2.42 | 1.84–3.19 | < 0.01 |
| Benign prostatic hyperplasia (%) | 1.22 | 1.08–1.37 | < 0.01 | 0.84 | 0.71–0.99 | 0.04 |
| Intervention before the index date | ||||||
| Computed tomography of any site (%) | 2.33 | 2.1–2.58 | < 0.01 | 1.31 | 1.13–1.52 | < 0.01 |
| Cardiac catheterization (%) | 1.62 | 1.27–2.05 | < 0.01 | 1.13 | 0.72–1.78 | 0.6 |
| Angiography of any site (%) | 1.84 | 1.44–2.33 | < 0.01 | 0.69 | 0.44–1.08 | < 0.01 |
| Cystoscopy with or without biopsy (%) | 1.66 | 1.32–2.1 | < 0.01 | 0.51 | 0.37–0.7 | < 0.01 |
| Transurethral resection of prostate (%) | 0.8 | 0.22–2.98 | 0.74 | |||
| Chronic medication use | ||||||
| Aspirin (%) | 0.84 | 0.79–0.9 | < 0.01 | 0.7 | 0.63–0.78 | < 0.01 |
| β-blocker (%) | 1.1 | 1.04–1.17 | < 0.01 | 0.84 | 0.77–0.92 | < 0.01 |
| ACEI (%) | 1.08 | 1–1.16 | < 0.01 | 0.97 | 0.88–1.08 | < 0.01 |
| ARB (%) | 0.98 | 0.93–1.04 | 0.57 | |||
| Clopidogrel (%) | 1.28 | 1.11–1.47 | < 0.01 | 1.01 | 0.82–1.25 | 0.94 |
| Statin (%) | 0.52 | 0.49–0.55 | < 0.01 | 0.54 | 0.49–0.6 | < 0.01 |
| Fibrate (%) | 0.58 | 0.54–0.63 | < 0.01 | 0.67 | 0.6–0.74 | < 0.01 |
| Ezetimibe (%) | 0.84 | 0.73–0.97 | 0.02 | 1.04 | 0.85–1.27 | 0.7 |
| Calcium channel blocker (%) | 1.04 | 0.98–1.1 | 0.23 | |||
| α-blocker (%) | 1.39 | 1.25–1.55 | < 0.01 | 1.06 | 0.91–1.24 | < 0.01 |
| Wafarin (%) | 1.89 | 1.5–2.38 | < 0.01 | 1.25 | 0.92–1.71 | 0.16 |
| Platinum-based anti-neoplastic agents (%) | 2.54 | 1.91–3.37 | < 0.01 | 1.44 | 0.99–2.1 | 0.06 |
| Atypical anti-psychotics (%) | 1.58 | 1.33–1.87 | < 0.01 | 1.55 | 1.23–1.94 | 0.01 |
| Nephrotoxic anti-bacterials (%)* | 1.83 | 1.03–3.26 | 0.04 | 1.28 | 0.57–2.9 | 0.55 |
| Nephrotoxic anti-virals (%)& | 1.42 | 0.68–2.97 | 0.36 | |||
| Cyclosporin/tacrolimus (%) | 2.74 | 1.62–4.63 | < 0.01 | 0.94 | 0.49–1.83 | 0.86 |
| Lithium (%) | 1.61 | 0.9–2.9 | 0.11 | |||
| Anti-diabetic medications | ||||||
| Insulin (%) | 2.68 | 2.4–2.99 | < 0.01 | 2.43 | 2.1–2.81 | < 0.01 |
| Biguanides (%) | 0.5 | 0.47–0.53 | < 0.01 | 0.59 | 0.53–0.65 | < 0.01 |
| Sulfonylurea (%) | 0.87 | 0.82–0.92 | < 0.01 | 1.36 | 1.24–1.5 | < 0.01 |
| Meglitinide (%) | 1.82 | 1.64–2.01 | < 0.01 | 1.36 | 1.18–1.56 | < 0.01 |
| α-glucosidase (%) | 1.02 | 0.94–1.11 | 0.65 | |||
| Thiazolidinedione (%) | 0.86 | 0.78–0.96 | < 0.01 | 1.1 | 0.95–1.28 | 0.19 |
| DPP4 inhibitor (%) | 0.67 | 0.62–0.73 | < 0.01 | 0.66 | 0.58–0.74 | < 0.01 |
*Including vancomycin, aminoglycosides, and colistin
&Including acyclovir and ganciclovir
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; DPP4, dipeptidyl peptidase 4; OR, odds ratio
Table 3.
Crude and adjusted odds ratio of risk factors for AKI among participants between 65 and 75 years
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Demographic and socioeconomic profiles | ||||||
| Residential location (urban %) | 0.88 | 0.81–0.96 | < 0.01 | 0.98 | 0.88–1.08 | 0.66 |
| Comorbidities | ||||||
| Hypertension (%) | 1.79 | 1.61–2 | < 0.01 | 2.11 | 1.8–2.47 | < 0.01 |
| Hyperlipidemia (%) | 0.61 | 0.57–0.67 | < 0.01 | 0.82 | 0.73–0.93 | < 0.01 |
| Chronic liver disease (%) | 1.24 | 1.13–1.36 | < 0.01 | 1.01 | 0.9–1.14 | 0.86 |
| Atrial fibrillation (%) | 1.58 | 1.42–1.77 | < 0.01 | 1.27 | 1.1–1.47 | < 0.01 |
| Chronic obstructive pulmonary disease (%) | 1.83 | 1.63–2.04 | < 0.01 | 1.45 | 1.26–1.67 | < 0.01 |
| Acute coronary syndrome (%) | 1.51 | 1.39–1.65 | < 0.01 | 1.38 | 1.22–1.57 | < 0.01 |
| Cerebrovascular disease (%) | 1.98 | 1.81–2.17 | < 0.01 | 1.74 | 1.55–1.96 | < 0.01 |
| Malignancy (%) | 2.66 | 2.36–3.01 | < 0.01 | 2.25 | 1.93–2.64 | < 0.01 |
| Parkinsonism (%) | 1.69 | 1.38–2.07 | < 0.01 | 1.2 | 0.93–1.55 | 0.17 |
| Chronic kidney disease (%) | 4.26 | 3.85–4.71 | < 0.01 | 3.53 | 3.13–3.99 | < 0.01 |
| Advanced chronic kidney disease (%) | 4.19 | 2.73–6.43 | < 0.01 | 1.4 | 0.83–2.36 | 0.2 |
| Past experience of AKI (%) | 6.72 | 5.18–8.72 | < 0.01 | 2.17 | 1.6–2.94 | < 0.01 |
| Peripheral vascular disease (%) | 1.87 | 1.52–2.31 | < 0.01 | 1.6 | 1.23–2.09 | < 0.01 |
| Benign prostatic hyperplasia (%) | 1.12 | 1–1.25 | 0.05 | |||
| Intervention before the index date | ||||||
| Computed tomography of any site (%) | 1.87 | 1.66–2.12 | < 0.01 | 1.06 | 0.9–1.25 | 0.46 |
| Cardiac catheterization (%) | 1.98 | 1.57–2.51 | < 0.01 | 1.27 | 0.82–1.97 | 0.28 |
| Angiography of any site (%) | 2.18 | 1.7–2.79 | < 0.01 | 1.23 | 0.79–1.91 | 0.35 |
| Cystoscopy with or without biopsy (%) | 1.55 | 1.22–1.96 | < 0.01 | 0.68 | 0.5–0.92 | < 0.01 |
| Transurethral resection of prostate (%) | 0.87 | 0.55–1.38 | 0.56 | |||
| Chronic medication use | ||||||
| Aspirin (%) | 0.78 | 0.72–0.85 | < 0.01 | 0.77 | 0.68–0.86 | < 0.01 |
| β-blocker (%) | 0.87 | 0.81–0.95 | < 0.01 | 0.89 | 0.79–0.99 | 0.04 |
| ACEI (%) | 0.91 | 0.83–1 | 0.05 | |||
| ARB (%) | 0.81 | 0.75–0.88 | < 0.01 | 0.87 | 0.78–0.98 | 0.02 |
| Clopidogrel (%) | 1.27 | 1.1–1.46 | < 0.01 | 1.11 | 0.92–1.35 | 0.26 |
| Statin (%) | 0.48 | 0.44–0.52 | < 0.01 | 0.59 | 0.52–0.67 | < 0.01 |
| Fibrate (%) | 0.62 | 0.55–0.71 | < 0.01 | 0.78 | 0.66–0.91 | < 0.01 |
| Ezetimibe (%) | 0.71 | 0.57–0.87 | < 0.01 | 0.83 | 0.63–1.09 | 0.17 |
| Calcium channel blocker (%) | 0.72 | 0.67–0.79 | < 0.01 | 0.6 | 0.53–0.68 | < 0.01 |
| α-blocker (%) | 0.98 | 0.88–1.1 | 0.75 | |||
| Wafarin (%) | 1.29 | 1.05–1.59 | 0.02 | 1.04 | 0.79–1.37 | 0.79 |
| Platinum-based anti-neoplastic agents (%) | 1.61 | 1.11–2.35 | < 0.01 | 1.39 | 0.88–2.19 | 0.16 |
| Atypical anti-psychotics (%) | 1.58 | 1.31–1.89 | < 0.01 | 1.19 | 0.95–1.5 | 0.14 |
| Nephrotoxic anti-bacterials (%)* | 0.62 | 0.31–1.24 | 0.17 | |||
| Nephrotoxic anti-virals (%)& | 0.25 | 0.05–1.18 | 0.08 | |||
| Cyclosporin/tacrolimus (%) | 1.00 | 0.25–4 | 1 | |||
| Lithium (%) | 3.00 | 0.97–9.3 | 0.06 | |||
| Anti-diabetic medications | ||||||
| Insulin (%) | 2.14 | 1.76–2.61 | < 0.01 | 1.79 | 1.4–2.3 | < 0.01 |
| Biguanides (%) | 0.51 | 0.47–0.56 | < 0.01 | 0.73 | 0.65–0.82 | < 0.01 |
| Sulfonylurea (%) | 0.78 | 0.71–0.84 | < 0.01 | 1.25 | 1.11–1.41 | < 0.01 |
| Meglitinide (%) | 1.5 | 1.31–1.71 | < 0.01 | 1.4 | 1.18–1.66 | < 0.01 |
| α-glucosidase (%) | 0.88 | 0.77–1 | 0.04 | 0.91 | 0.77–1.07 | 0.25 |
| Thiazolidinedione (%) | 0.82 | 0.69–0.97 | 0.02 | 1.04 | 0.84–1.29 | 0.75 |
| DPP4 inhibitor (%) | 0.69 | 0.61–0.78 | < 0.01 | 0.82 | 0.7–0.96 | 0.02 |
*Including vancomycin, aminoglycosides, and colistin
&Including acyclovir and ganciclovir
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; DPP4, dipeptidyl peptidase 4; OR, odds ratio
Table 4.
Crude and adjusted odds ratio of risk factors for AKI among participants ≥ 75 years
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Demographic and socioeconomic profiles | ||||||
| Residential location (urban %) | 0.90 | 0.85–0.96 | < 0.01 | 0.93 | 0.87–1 | 0.06 |
| Comorbidities | ||||||
| Hypertension (%) | 1.41 | 1.3–1.54 | < 0.01 | 1.63 | 1.46–1.82 | < 0.01 |
| Hyperlipidemia (%) | 0.71 | 0.67–0.76 | < 0.01 | 0.88 | 0.81–0.96 | < 0.01 |
| Chronic liver disease (%) | 1.20 | 1.1–1.3 | < 0.01 | 1.03 | 0.94–1.14 | 0.51 |
| Atrial fibrillation (%) | 1.55 | 1.45–1.66 | < 0.01 | 1.35 | 1.25–1.47 | < 0.01 |
| Chronic obstructive pulmonary disease (%) | 1.50 | 1.4–1.61 | < 0.01 | 1.17 | 1.08–1.27 | < 0.01 |
| Acute coronary syndrome (%) | 1.40 | 1.32–1.49 | < 0.01 | 1.31 | 1.21–1.41 | < 0.01 |
| Cerebrovascular disease (%) | 1.54 | 1.45–1.64 | < 0.01 | 1.4 | 1.3–1.5 | < 0.01 |
| Malignancy (%) | 1.60 | 1.47–1.75 | < 0.01 | 1.52 | 1.37–1.69 | < 0.01 |
| Parkinsonism (%) | 1.39 | 1.25–1.55 | < 0.01 | 1.11 | 0.98–1.25 | 0.12 |
| Chronic kidney disease (%) | 3.13 | 2.92–3.34 | < 0.01 | 2.65 | 2.46–2.87 | < 0.01 |
| Advanced chronic kidney disease (%) | 3.05 | 2.29–4.05 | < 0.01 | 1.56 | 1.13–2.17 | < 0.01 |
| Past experience of AKI (%) | 3.81 | 3.33–4.37 | < 0.01 | 1.61 | 1.38–1.89 | < 0.01 |
| Peripheral vascular disease (%) | 1.34 | 1.18–1.53 | < 0.01 | 1.12 | 0.96–1.31 | 0.14 |
| Benign prostatic hyperplasia (%) | 1.21 | 1.12–1.31 | < 0.01 | 1.03 | 0.94–1.14 | 0.5 |
| Intervention before the index date | ||||||
| Computed tomography of any site (%) | 1.37 | 1.27–1.49 | < 0.01 | 1.00 | 0.9–1.1 | 0.95 |
| Cardiac catheterization (%) | 1.58 | 1.35–1.85 | < 0.01 | 1.35 | 1.01–1.79 | 0.04 |
| Angiography of any site (%) | 1.55 | 1.31–1.83 | < 0.01 | 0.97 | 0.72–1.29 | 0.82 |
| Cystoscopy with or without biopsy (%) | 1.16 | 0.99–1.36 | 0.06 | |||
| Transurethral resection of prostate (%) | 1.03 | 0.79–1.35 | 0.84 | |||
| Chronic medication use | ||||||
| Aspirin (%) | 0.69 | 0.65–0.74 | < 0.01 | 0.76 | 0.7–0.82 | < 0.01 |
| β-blocker (%) | 0.75 | 0.71–0.8 | < 0.01 | 0.83 | 0.77–0.9 | < 0.01 |
| ACEI (%) | 0.79 | 0.74–0.86 | < 0.01 | 0.87 | 0.79–0.95 | < 0.01 |
| ARB (%) | 0.69 | 0.65–0.73 | < 0.01 | 0.86 | 0.8–0.93 | < 0.01 |
| Clopidogrel (%) | 1.05 | 0.95–1.15 | 0.36 | |||
| Statin (%) | 0.52 | 0.48–0.55 | < 0.01 | 0.68 | 0.62–0.75 | < 0.01 |
| Fibrate (%) | 0.55 | 0.49–0.63 | < 0.01 | 0.71 | 0.62–0.83 | < 0.01 |
| Ezetimibe (%) | 0.58 | 0.47–0.72 | < 0.01 | 0.95 | 0.74–1.23 | 0.72 |
| Calcium channel blocker (%) | 0.6 | 0.56–0.64 | < 0.01 | 0.64 | 0.59–0.69 | < 0.01 |
| α-blocker (%) | 0.74 | 0.68–0.81 | < 0.01 | 0.77 | 0.7–0.85 | < 0.01 |
| Wafarin (%) | 1.08 | 0.94–1.25 | 0.27 | |||
| Platinum-based anti-neoplastic agents (%) | 0.90 | 0.58–1.41 | 0.65 | |||
| Atypical anti-psychotics (%) | 0.93 | 0.85–1.03 | 0.16 | |||
| Nephrotoxic anti-bacterials (%)* | 0.93 | 0.56–1.56 | 0.79 | |||
| Nephrotoxic anti-virals (%)& | 0.50 | 0.09–2.73 | 0.42 | |||
| Cyclosporin/tacrolimus (%) | – | |||||
| Lithium (%) | 1.33 | 0.3–5.96 | 0.71 | |||
| Anti-diabetic medications | ||||||
| Insulin (%) | 1.36 | 1.18–1.56 | < 0.01 | 1.32 | 1.12–1.55 | < 0.01 |
| Biguanides (%) | 0.51 | 0.48–0.54 | < 0.01 | 0.76 | 0.69–0.82 | < 0.01 |
| Sulfonylurea (%) | 0.72 | 0.68–0.77 | < 0.01 | 1.10 | 1.01–1.2 | 0.03 |
| Meglitinide (%) | 1.02 | 0.92–1.12 | 0.85 | |||
| α-glucosidase (%) | 0.77 | 0.7–0.86 | < 0.01 | 0.98 | 0.86–1.11 | 0.71 |
| Thiazolidinedione (%) | 0.62 | 0.52–0.74 | < 0.01 | 0.96 | 0.77–1.18 | 0.67 |
| DPP4 inhibitor (%) | 0.60 | 0.54–0.67 | < 0.01 | 0.79 | 0.69–0.9 | < 0.01 |
*Including vancomycin, aminoglycosides, and colistin
&Including acyclovir and ganciclovir
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; DPP4, dipeptidyl peptidase 4; OR, odds ratio
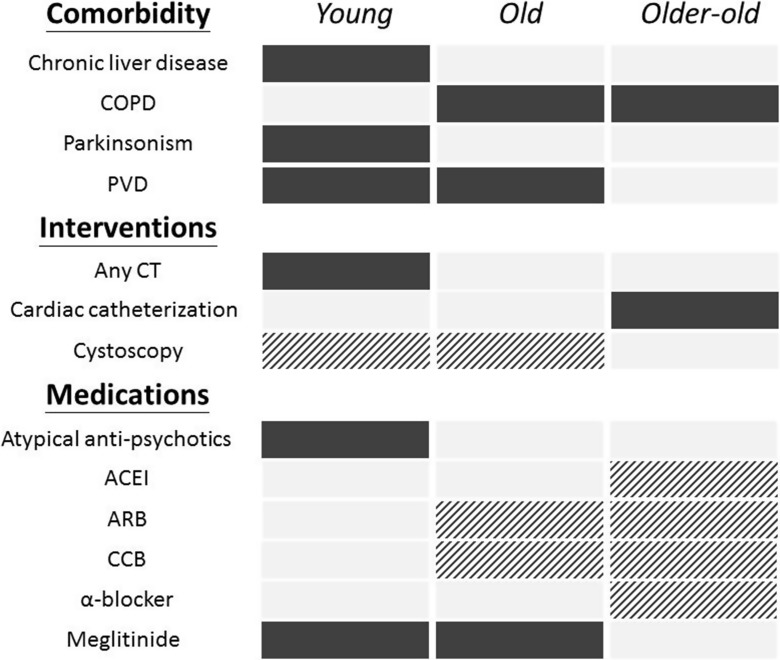
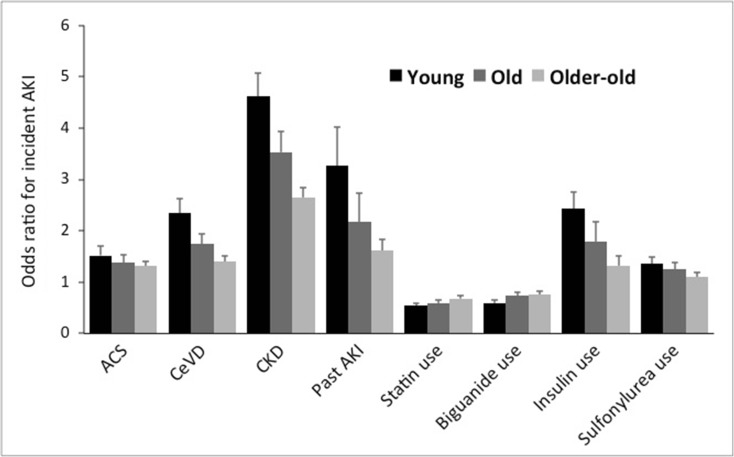
It is interesting to find that a common panel of risk factors (hypertension, atrial fibrillation, ACS, cerebrovascular disease, malignancy, CKD, past AKI, insulin use) were consistently associated with a higher risk of AKI among type 2 diabetic patients, while aspirin, β-blocker, statin, fibrate, biguanide, sulfonylurea, and DPP4 inhibitor use correlated with a lower risk. A summary of differential risk factors across patients of increasing age is shown in Fig. 2. Furthermore, we identified that the risk of AKI declined successively with increasing age, regarding multiple variables (Fig. 3).
Fig. 2.
A diagram illustrating discrepancies in risk factors for acute kidney injury (AKI) between incident diabetic patients of different age groups. The solid black box represents a significant increase in the risk for AKI, while the dashed black box represents a significant reduction in risk. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; CT, computed tomography; PVD, peripheral vascular disease
Fig. 3.
The trend of risk estimates for acute kidney injury from individual factors listed in Tables 2, 3, and 4, among incident diabetic patients with different age groups. Convergence of risk toward neutrality was noted for all the risk factors among older participants. ACS, acute coronary syndrome; AKI, acute kidney injury; CeVD, cerebrovascular disease; CKD, chronic kidney disease
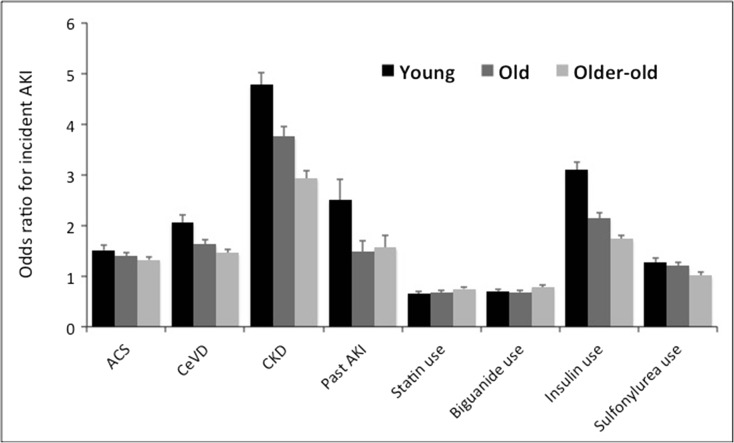
Sensitivity analyses were done to affirm the validity of our findings. First, we combined the three age groups into one and analyzed the relationship between age and the risk of AKI among all participants with incident diabetes, using a similar case-control design. After matching for gender, we found that increasing age was still significantly associated with a higher risk of developing AKI among incident type 2 diabetic patients (n = 23,071), independent of other covariates (OR 1.19 per 1 year increase, 95% CI 1.13–1.25; p < 0.001). In addition, we intended to address whether similar findings could occur among those with prevalent type 2 DM, by analyzing those excluded during the first step in our study flowchart (Fig. 1); that is, those who already had a diagnosis of diabetes between 2007 and 2008, or the prevalent diabetic cases. A total of 25,784, 22,884, and 22,306 prevalent diabetic patients were identified from this database and enrolled into the young, old, and the older-old groups, respectively. After matching for age and gender, the results of the conditional logistic regression analyses were provided in supplementary Tables 4, 5, and 6. We found that among the three groups of participants with prevalent diabetes, the age-dependent attenuations in the magnitude of the risk of AKI were still present (Fig. 4). Similarly, multiple risk factors were still present among certain age groups of prevalent diabetic patients but not the others; for example, the use of calcium channel blockers were associated with a lower risk of AKI among the old and the older-old groups but not the young one. α-blocker or ACEI use was associated with a lower risk of AKI only in the old group but not the young, while meglitinide use was associated with a higher risk of AKI among the young and the old but the older-old group.
Fig. 4.
The trend of risk estimates for acute kidney injury from individual factors listed in Tables 2, 3, and 4, among prevalent diabetic patients with different age groups. Convergence of risk toward neutrality was noted for all the risk factors among older participants. ACS, acute coronary syndrome; AKI, acute kidney injury; CeVD, cerebrovascular disease; CKD, chronic kidney disease
However, there are also differences between the risk factor profiles for AKI between prevalent and incident diabetic cases. ß-blocker and clopidogrel use was associated with an increased risk for AKI among young patients with prevalent diabetes, while their use was associated with a decreased risk among young patients with incident diabetes (Table 2 and supplementary Table 4).
Discussion
In the current study, we assembled a nationwide cohort of diabetic patients from different age groups and evaluated their risk factor profiles for administrative code-identified AKI. We discovered that comorbidities, interventions, and the use of certain medications (listed in Fig. 2) influenced the risk of AKI in diabetic patients of certain age groups but not others. These findings highlight the importance of incorporating age into AKI risk estimation and elucidate the interactions between age and a range of risk factors for AKI.
Several cardiovascular morbidities consistently increased the risk of developing AKI (Tables 2, 3, and 4), and this association has been reported among different populations with a similar degree of risk elevation (Yi et al. 2016; Mesropian et al. 2016; Madhavan et al. 2014), lending support to the validity of our findings. In addition, the phenomenon that the risk of adverse outcome declined successively with increasing age has been reported anecdotally, but not for the prediction of risk for AKI; Iso et al. reported that the risk of mortality among smokers conferred by cerebrovascular disease and ACS was more evident among younger patients than among older ones (Iso et al. 2005). There are several plausible reasons for these observations. Firstly, elderly patients tend to have a significantly higher risk of mortality compared to younger ones, and a competing risk between AKI and mortality in the old and older-old groups might weaken the ORs of individual risk factor for AKI. In addition, patients of advanced age frequently exhibit functional decline and are at an increased risk of developing geriatric syndromes including frailty, polypharmacy, and malnutrition (Chao et al. 2015a; Chao et al. 2015b), all of which potentially dilute the risk posed by traditional risk factors. Finally, a distinct pattern of risk factor interplay might exist in the old and older-old groups, resulting in an alteration in the risk of AKI. We believe that subsequent studies aiming to estimate the risk of AKI should obtain age-specific criteria to improve result accuracy.
Existing studies have identified a higher incidence of AKI among COPD patients with or without acute exacerbation (Barakat et al. 2015), through inducing chronic hypoxemia, hypercapnia, and low grade inflammation, leading to the reduction in renal blood flow and GFR, predisposing them to AKI (Husain-Syed et al. 2016; Domenech et al. 2017). This pathophysiological association is expected to worsen in older-old patients since they have a higher prevalence of CKD, other concurrent pulmonary pathologies, and far greater deterioration in respiratory mechanisms than their younger counterparts (Yoshikawa et al. 2017). Older-old patients are less likely to be offered mechanical cardiopulmonary support during the peri-catheterization period than younger patients due to the concern of medical futility; these differences in medical decision-making can potentially lead to a higher risk of AKI among older patients (Prondzinsky et al. 2010; Flaherty et al. 2017; Swetz et al. 2013).
Multiple anti-hypertensive agents showed favorable effects upon the risk of AKI only in the old or older-old patients. Existing literature appears to be controversial in terms of the influences of ACEI/ARB upon AKI (Shah et al. 2014; Arora et al. 2008; Friedl et al. 2012; Whiting et al. 2017). Elderly who stay on ACEI/ARB have more stable serum creatinine and may derive more benefit from blood pressure control. Despite the abundance of reports addressing ACEI/ARB and AKI risk, very few evaluate the relationship between AKI risk and either CCB or α-blockers. Clinical and experimental studies suggested that the use of CCB and α-blockers might be associated with a lower AKI risk (Passaroni et al. 2010; Fujii et al. 2007). In light of the multitude of anti-hypertensive agents exhibiting AKI-reduction effect in patients of advanced age (Fig. 2), we propose that these medications work through a common mechanism involving blood pressure (BP) reduction and the amelioration of CKD progression, instead of a class-specific effect against AKI. Indeed, an earlier meta-analysis discovered that among diabetic patients, the use of anti-hypertensive agents could reduce the risk of renal decline if their baseline BP was greater than 150 mmHg; furthermore, older adults were more commonly affected due to arterial stiffness (Brunström et al. 2016). In the present study, the prevalence of hypertension was higher in older-old and old patients than young ones (Table 1), providing support for this hypothesis. Alternatively, the target BP can differ between the three age groups, and clinicians often opt for a less stringent goal for those of advanced age, leading to a lower likelihood of hypotension-related AKI. Since we did not have data regarding BP goals or hypertensive severity, it might be prudent to select anti-hypertensive medications that we identified with favorable renal effects among the older-old or old patients, with the target BP set appropriately based on the best available evidence. Finally, certain drugs are frequently avoided in patients with past AKI or CKD, and physically fit patients are more likely to receive these medications (ex. ACEI/ARB). This is to be remembered when interpreting our results.
CLD, including hepatitis and cirrhosis, has been reported by many researchers as a significant predictor of AKI (Wu et al. 2014; Lacave et al. 2017). Age itself is an important risk factor for mortality among cirrhotic patients (Das et al. 2010), and the cross-sectional sampling approach in our study would retrieve fewer elderly patients with liver disease (Table 1). The absence of an association between CLD and AKI risk in the old and older-old groups may have arisen from the lower prevalence of CLD in these two groups than in young patients. On the contrary, Parkinsonism, receiving computed tomography, and atypical anti-psychotics use did not increase AKI in the older-old group. Patients with Parkinsonism receiving deep brain stimulation were at higher risk of AKI, and those with young-onset Parkinsonism and few comorbidities are more likely surgical candidates (Guimarães et al. 2010; Pollak 2013). This may partially explain the preferential existence of Parkinsonism-AKI relationship in young patients. Although computed tomography was less commonly arranged in young patients, we found that they were more likely to receive contrast-enhanced examinations than the old and older-old patients, since most physicians were aware that increasing age predisposes patients to contrast-induced nephropathy, leading to a higher risk of AKI in the young. Atypical anti-psychotics were recently found to elevate the risk of AKI in Western countries (Hwang et al. 2014; Jiang et al. 2017). Ethnic differences in study population, a relatively low prevalence of benign prostatic hyperplasia in the old and the older-old group (Table 1), or a difference in the severity of treatment-related hypotension (Hwang et al. 2014) may all be potential reasons to explain our findings.
Several reasons might be responsible for the discrepancy of risk factors between incident and prevalent cases. We propose that prevalent cases may have a longer period of diabetes, and likely more severe vascular morbidities. The use of ß-blocker and clopidogrel in these patients might be a surrogate for the severity of their vascular morbidity; thus their use significantly correlated with a higher risk of AKI in prevalent diabetic cases but not incident ones.
Several oral anti-diabetic agents exhibit protective effect against AKI among diabetic patients, especially metformin. Bell et al., through analyzing electronic health records from Scotland, found that current metformin use was not associated with an increased risk of AKI among diabetic patients, but was associated with a better survival among those with AKI (Bell et al. 2017). Similar findings can be found in studies addressing the renal influence of metformin in patients after percutaneous coronary intervention (Zeller et al. 2016). Experiments on renal tubular epithelia and animals showed that metformin mitigated tubular apoptosis and the severity of AKI through the induction of AMPK phosphorylation (Li et al. 2016), and a call for trials to affirm this renoprotective effect has been proposed (De Broe et al. 2017). However, a retrospective population-based study reported that metformin use was associated with a 53% higher risk of acute dialysis than those receiving sulphonylurea (Carlson et al. 2016), rendering the relationship between metformin and AKI controversial. In this study, we found that metformin use was consistently associated with 20–40% lower risk of developing AKI among incident type 2 diabetic patients (Tables 2, 3, and 4) than non-users, favoring a positive effect of metformin on renal outcomes. Further evidence is needed before we can make a conclusion on this issue.
Limitations
To our knowledge, this is the first population-based study to investigate the differential risk factor profiles for AKI among incident diabetic patients of different age groups. Population-based cohort studies, performed in a real-world setting such as ours, are well-suited for addressing the interactions between a wide spectrum of potential risk factors due to their larger sample size and the possibility of evaluating the influence of uncommon factors. The definitions used herein for the event of interest (AKI), and the panels of risk factors investigated, have all been validated previously (Chao et al. 2017; Wu et al. 2014) with a reasonable consistency. Nonetheless, multiple limitations do apply. Observational studies tend to be confounded by unrecognized features that vary between age groups, although we believe that some of these features can be balanced toward neutrality if the number of cases can be augmented. The lack of laboratory data (e.g., serum creatinine levels) in the current study also made it difficult to ascertain the severity and courses of AKI. We tightened the definition of “medication users; to those who took the same medications for 30 days continuously, an approach that increased specificity. Finally, due to the limitation of our data source, we could only focus on diabetic participants during the given study period, although we did recruit all diabetic participants in this country without random sampling.
In conclusion, risk factors for AKI in diabetic patients differ between age groups, with some factors only exhibiting influence on young patients and others exerting their effects predominantly in old and older-old patients. Subsequent studies on AKI should focus on developing age-specific categorization to better select the appropriate patients for instituting preventive measures.
Electronic supplementary materials
(DOC 8 kb)
Funding disclosure
The study is financially sponsored by National Taiwan University Hospital BeiHu branch.
Author contributions
Study design: CTC, KLC; Data analysis: CTC, JW, HYW, KLC; Article drafting: CTC, JW, JWH, KLC; Article approval: all authors.
Compliance with ethical standards
Sponsor’s role
The sponsors have no role in the study design, data collection, analysis, and result interpretation of this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0013-3) contains supplementary material, which is available to authorized users.
References
- Andò G, Morabito G, de Gregorio C, Trio O, Saporito F, Oreto G. Age, glomerular filtration rate, ejection fraction, and the AGEF score predict contrast-induced nephropathy in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82(6):878–885. doi: 10.1002/ccd.25023. [DOI] [PubMed] [Google Scholar]
- Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, Lohr J. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266–1273. doi: 10.2215/CJN.05271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat MF, McDonald HI, Collier TJ, Smeeth L, Nitsch D, Quint JK. Acute kidney injury in stable COPD and at exacerbation. Int J Chron Obstruct Pulmon Dis. 2015;10:2067–2077. doi: 10.2147/COPD.S88759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S, Farran B, McGurnaghan S, McCrimmon RJ, Leese GP, Petrie JR, McKeigue P, Sattar N, Wild S, McKnight J, Lindsay R, Colhoun HM, Looker H. Risk of acute kidney injury and survival in patients treated with metformin: an observational cohort study. BMC Nephrol. 2017;18:163. doi: 10.1186/s12882-017-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson N, Hommel K, Olesen JB, Gerds TA, Soja AM, Vilsboll T, Kamper AL, Torp-Pedersen C, Gislason G. Metformin-associated risk of acute dialysis in patients with type 2 diabetes: a nationwide cohort study. Diabetes Obes Metab. 2016;18(12):1283–1287. doi: 10.1111/dom.12764. [DOI] [PubMed] [Google Scholar]
- Chao CT, Hou CC, Wu VC, Lu HM, Wang CY, Chen L, Kao TW. The impact of dialysis-requiring acute kidney injury on long-term prognosis of patients requiring prolonged mechanical ventilation: nationwide population-based study. PLoS One. 2012;7(12):e50675. doi: 10.1371/journal.pone.0050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CT, Jui W, Wu HY, Chien KL, Hung KY. Dipeptidyl peptidase inhibitor use is associated with a lower risk of incident acute kidney injury in patients ith diabetes. Oncotarget. 2017;8(32):53028–53040. doi: 10.18632/oncotarget.18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CT, Lai CF, Huang JW. Risk factors for herpes zoster reactivation in maintenance hemodialysis patients. Eur J Intern Med. 2012;23(8):711–715. doi: 10.1016/j.ejim.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Chao CT, Tsai HB, Wu CY, Hsu NC, Lin YF, Chen JS, Hung KY. Cross-sectional study of the association between functional status and acute kidney injury in geriatric patients. BMC Nephrol. 2015;16(1):186. doi: 10.1186/s12882-015-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, Hung KY. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine. 2015;94(31):e1251. doi: 10.1097/MD.0000000000001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CT, Wang CY, Lai CF, Huang TM, Chen YY, Kao TW, Chu TS, Chang CH, Wu VC, Ko WJ, Chen L, Wu KD. Dialysis-requiring acute kidney injury increases risk of long-term malignancy: a population-based study. J Cancer Res Clin Oncol. 2014;140(4):613–621. doi: 10.1007/s00432-014-1600-z. [DOI] [PubMed] [Google Scholar]
- Chao CT, Wu VC, Lai CF, Shiao CC, Huang TM, Wu PC, Tsai IJ, Hou CC, Wang WJ, Tsai HB, Lin YF, Chiang WC, Lin SL, Tsai PR, Ko WJ, Wu MS, Wu KD, NSARF group Advanced age affects the outcome-predictive power of RIFLE classification in geriatric patients with acute kidney injury. Kidney Int. 2012;82(8):920–927. doi: 10.1038/ki.2012.237. [DOI] [PubMed] [Google Scholar]
- Das V, Boelle P-Y, Galbois A, Guidet B, Maury E, Carbonell N, Moreau R, Offenstadt G. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38(11):2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- De Broe ME, Kajbaf F, Lalau JD (2017) Renoprotective effects of metformin. Nephron. 10.1159/000481951 [DOI] [PubMed]
- Domenech P, Perez T, Saldarini A, Uad P, Musso CG. Kidney–lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 2017;49(7):1211–1215. doi: 10.1007/s11255-017-1585-z. [DOI] [PubMed] [Google Scholar]
- Flaherty MP, Pant S, Patel SV, Kilgore T, Dassanayaka S, Loughran JH, Rawasia W, Dawn B, Cheng A, Bartoli CR. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res. 2017;120(4):692–700. doi: 10.1161/CIRCRESAHA.116.309738. [DOI] [PubMed] [Google Scholar]
- Friedl A, Peric S, Masghati S, Wolzt M, Hori WH, Soleiman A, Fuhrmann V, Haider DG. No association of angiotensin-converting enzyme inhibitor or angiotensin 2 receptor blocker intake with acute kidney injury in patients undergoing kidney biopsy. Kidney Blood Press Res. 2012;35(6):558–560. doi: 10.1159/000339707. [DOI] [PubMed] [Google Scholar]
- Fujii T, Sugiura T, Ohkita M, Kobuchi S, Takaoka M, Matsumura Y. Selective antagonism of the postsynaptic α1-adrenoceptor is protective against ischemic acute renal failure in rats. Eur J Pharmacol. 2007;574(2):185–191. doi: 10.1016/j.ejphar.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, Oien C, Smith DH, Coresh J, Sarnak MJ, Stengel B, Tonelli M, CKD Prognosis Consortium A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66(4):591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães J, Vieira-Coelho A, Rosas MJ, Moura E, Vaz R, Garrett C. Acute renal failure in patients with bilateral deep brain stimulation. Mov Disord. 2010;25(14):2462–2464. doi: 10.1002/mds.23228. [DOI] [PubMed] [Google Scholar]
- Hermandez Hermandez D, Tesouro RB, Castro-Diaz D. Urinary retention. Urologia. 2013;80(4):257–264. doi: 10.5301/RU.2013.11688. [DOI] [PubMed] [Google Scholar]
- Husain-Syed F, Slutsky AS, Ronco C. Lung–kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402–414. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- Hwang Y, Dixon SN, Reiss JP, Wald R, Parikh CR, Gandhi S, Shariff SZ, Pannu N, Nash DM, Rehman F. Garg AX (2014) atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161(4):242–248. doi: 10.7326/M13-2796. [DOI] [PubMed] [Google Scholar]
- Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A, JACC Study Group Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC study. Am J Epidemiol. 2005;161(2):170–179. doi: 10.1093/aje/kwi027. [DOI] [PubMed] [Google Scholar]
- James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ, CKD prognosis Consortium A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, McCombs JS, Park SH. A retrospective cohort study of acute kidney injury risk associated with antipsychotics. CNS Drugs. 2017;31(4):319–326. doi: 10.1007/s40263-017-0421-4. [DOI] [PubMed] [Google Scholar]
- Kaze FF, Kengne A-P, Magatsing CT, Halle MP, Yiagnigni E, Ngu KB. Prevalence and determinants of chronic kidney disease among hypertensive Cameroonians according to three common estimators of the glomerular filtration rate. J Clin Hypertens. 2016;18(5):408–414. doi: 10.1111/jch.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease Improving Global Outcomes (KDIGO) group Section 2: AKI definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacave G, Caille V, Bruneel F, Palette C, Legriel S, Grimaldi D, Eurin M, Bedos JP. Incidence and risk factors of acute kidney injury associated with continuous intravenous high-dose vancomycin in critically ill patients: a retrospective cohort study. Medicine. 2017;96(7):e6023. doi: 10.1097/MD.0000000000006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc M, Kellum JA, Gibney RTN, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2015;11(6):533–536. doi: 10.1097/01.ccx.0000183666.54717.3d. [DOI] [PubMed] [Google Scholar]
- Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, He W, Yang J, Dai C. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKa-regulated autophagy induction. Sci Rep. 2016;6:23975. doi: 10.1038/srep23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan MV, Généreux P, Rubin J, Palmerini T, Caixeta A, Xu K, Weisz G, Mehran R, Stone GW. Usefulness of the SYNTAX score to predict acute kidney injury after percutaneous coronary intervention (from the acute catheterization and urgent intervention triage strategy trial) Am J Cardiol. 2014;113(8):1331–1337. doi: 10.1016/j.amjcard.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Kashani KB, Macedo E, Kim J, Bouchard J, Wynn S, Li G, Ohno-Machado L, Mehta R. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2017;32(5):814–822. doi: 10.1093/ndt/gfx026. [DOI] [PubMed] [Google Scholar]
- Mesropian PD, Othersen J, Mason D, Wang J, Asif A, Mathew RO. Community-acquired acute kidney injury: a challenge and opportunity for primary care in kidney health. Nephrology. 2016;21(9):729–735. doi: 10.1111/nep.12751. [DOI] [PubMed] [Google Scholar]
- O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–420. doi: 10.1681/ASN.2015121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaroni AC, Silva MAM, Martins AS, Kochi AC. Uso de nifedipina e incidência de lesão renal aguda em pós-operatório de cirurgia de revascularização do miocárdio com CEC. Braz J Cardiovasc Surg. 2010;25:32–37. doi: 10.1590/S0102-76382010000100010. [DOI] [Google Scholar]
- Pollak P. Deep brain stimulation for Parkinson’s disease— patient selection. Handb Clin Neurol. 2013;116:97–105. doi: 10.1016/B978-0-444-53497-2.00009-7. [DOI] [PubMed] [Google Scholar]
- Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, Schmidt H, Winkler M, Thiery J, Werdan K, Buerke M. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. doi: 10.1097/CCM.0b013e3181b78671. [DOI] [PubMed] [Google Scholar]
- Rewa O, Bagshaw SM. Acute kidney injury: epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12):e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD. THe association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152(9):561–567. doi: 10.7326/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule AD, Cornell LD, Poggio ED. Senile Nephrosclerosis—does it explain the decline in glomerular filtration rate with aging. Nephron Physiol. 2011;119(Suppl 1):6–11. doi: 10.1159/000328012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Jain AK, Brunelli SM, Coca SG, Devereaux PJ, James MT, Luo J, Molnar AO, Mrkobrada M, Pannu N, Parikh CR, Paterson M, Shariff S, Wald R, Walsh M, Whitlock R, Wijeysundera DN, Garg AX. Association between angiotensin converting enzyme inhibitor or angiotensin receptor blocker use prior to major elective surgery and the risk of acute dialysis. BMC Nephrol. 2014;15(1):53. doi: 10.1186/1471-2369-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77(6):536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetz KM, Cook KE, Ottenberg AL, Chang N, Mueller PS. Clinicians’ attitudes regarding withdrawal of left ventricular assist devices in patients approaching the end of life. Eur J Heart Fail. 2013;15(11):1262–1266. doi: 10.1093/eurjhf/hft094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak O. Conflicting and new risk factors for contrast induced nephropathy. J Urol. 2007;178(6):2277–2283. doi: 10.1016/j.juro.2007.08.054. [DOI] [PubMed] [Google Scholar]
- Annual Report USRDS. Chapter 5: acute kidney injury. Am J Kidney Dis. 2017;69(3 Suppl 1):S107–S132. [Google Scholar]
- Whiting P, Morden A, Tomlinson LA, Caskey F, Blakeman T, Tomson C, Stone T, Richards A, Savovic J, Horwood J. What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open. 2017;7(4):e012674. doi: 10.1136/bmjopen-2016-012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Yang YW, Hung SC, Kuo KL, Wu KD, Wu VC, Hsieh TC. Ketoanalogues supplementation decreases dialysis and mortality risk in patients with anemic advanced chronic kidney disease. PLoS One. 2017;12(5):e0176847. doi: 10.1371/journal.pone.0176847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ, NSARF group Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q, Li K, Jian Z, Xiao YB, Chen L, Zhang Y, Ma RY. Risk factors for acute kidney injury after cardiovascular surgery: evidence from 2,157 cases and 49,777 controls—a meta-analysis. Cardiorenal Med. 2016;6(3):237–250. doi: 10.1159/000444094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Yamamoto Y, Tomoda K, Fujita Y, Yamauchi M, Osa T, Uyama H, Okamoto N, Kurumatani N, Kimura H. Prevalence of chronic obstructive pulmonary disease in independent community-dwelling older adults: the Fujiwara-kyo study. Geriatr Gerontol Int. 2017;17:2421–2426. doi: 10.1111/ggi.13091. [DOI] [PubMed] [Google Scholar]
- Zeller M, Labalette-Bart M, Juliard JM, Potier L, Feldman LJ, Steg PG, Cottin Y, Roussel R. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: a multicenter study. Int J Cardiol. 2016;220:137–142. doi: 10.1016/j.ijcard.2016.06.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 8 kb)