Abstract
Japanese encephalitis virus (JEV) is the most commonly identified cause of acute encephalitis syndrome (AES) in Asia. The WHO recommended test is anti-JEV IgM-antibody-capture-enzyme-linked-immunosorbent-assay (JEV MAC-ELISA). However, data suggest this has low positive predictive value, with false positives related to other Flavivirus infections and vaccination. JEV RT-PCR in cerebrospinal fluid (CSF) and/or serum is highly specific, but is rarely positive; 0–25% of patients that fulfil the WHO definition of JE (clinical Acute Encephalitis Syndrome (AES) and JEV MAC-ELISA positive). Testing other body fluids by JEV RT-qPCR may improve the diagnosis. As a pilot study thirty patients admitted to Mahosot Hospital 2014–2017, recruited to the South-East-Asia-Encephalitis study, were tested by JEV MAC-ELISA and two JEV real-time RT-PCR (RT-qPCR) assays (NS2A and NS3). Eleven (36.7%) were JEV MAC-ELISA positive. Available CSF and serum samples of these patients were JEV RT-qPCR negative but 2 (7%) had JEV RNA detected in their throat swabs. JEV RNA was confirmed by re-testing, and sequencing of RT-qPCR products. As the first apparent report of JEV RNA detection in human throat samples, the provides new perspectives on human JEV infection, potentially informing improving JEV detection. We suggest that testing patients’ throat swabs for JEV RNA is performed, in combination with molecular and serological CSF and serum investigations, on a larger scale to investigate the epidemiology of the presence of JEV in human throats. Throat swabs are an easy and non-invasive tool that could be rolled out to a wider population to improve knowledge of JEV molecular epidemiology.
Introduction
Evidence continues to implicate Japanese encephalitis virus (JEV) as a major cause of encephalitis in Asia, with recent evidence of possible autochthonous transmission in Africa1–3. Among the key factors in its persistent role as a public health problem are the limitations of existing diagnostic tests, our understanding of its epidemiology and inadequate implementation of vaccination programmes.
The conventional mainstay of JEV encephalitis diagnosis is serology, with serum and cerebrospinal fluid (CSF) anti-JEV IgM antibody capture enzyme-linked immunosorbent assays (JEV MAC-ELISA) recommended by the World Health Organization (WHO)4. However, there are concerns about the accuracy of JEV MAC-ELISA for diagnosing JEV. Low positive predictive value of JEV MAC-ELISA has been reported5, and there are recognised difficulties with false positives related to vaccination and in areas where other Flavivirus infections are endemic6,7.
Diagnosis of JEV by Reverse-Transcription (RT)-PCR is highly specific, and enables improved understanding of the molecular epidemiology of JEV8. However, JEV RT-PCR testing of CSF and serum samples has low sensitivity, rarely positive in patients at presentation (0–25% that fulfil the WHO definition of Acute Encephalitis Syndrome (AES) and JEV MAC-ELISA positivity)5,9. Other body fluids may provide additional source of JEV RNA but there has been little investigation of this possibility. Other Flaviviruses, such as West Nile virus, Zika virus and Dengue virus, have been detected in urine, and JEV RNA has recently been detected in the urine of two patients10–13.
Human throat swab samples have apparently not been used to test for JEV RNA, although there is recent evidence of high JEV viral loads in the oronasal secretions in the pigs, the amplifying host, and that the tonsils may be a site of viral replication and contribute to transmission14.
We therefore hypothesised that JEV may be present in the throats of human patients. If this is the case, the use of non-invasive throat swab clinical samples could potentially improve the diagnosis of JEV and our understanding of JEV molecular epidemiology. We performed a pilot study to evaluate if JEV RNA could be detected by RT-qPCR in throat swabs of patients admitted to Mahosot Hospital, Vientiane, Laos, with AES clinical presentation.
Results
From November 2014 to June 2017, 129 patients admitted to Mahosot Hospital who had lumbar puncture presented with clinical AES presentation. Of these, 61 patients presented within 7 days of disease onset and 40 of these were anti-JEV IgM positive on CSF and/or serum or undiagnosed following routine testing. Throat swab samples from 30 patients were available and were included in this study. Eleven (36.7%) patients were anti-JEV IgM MAC-ELISA positive in CSF and admission serum (Table 1). CSF samples were available for 9 patients and sera from 6 patients. All CSF and serum samples were negative by JEV RT-qPCR, but 2 (7%) patients had JEV RNA detected in their throat swabs, patient 5 and patient 10 (Table 1). Anti-JEV IgM was detected in CSF and admission sera from both patients.
Table 1.
Patients with positive results for JE MAC-ELISA or JEV RT-qPCR.
| Patient number | Age (yr) | Sex | JE MAC-ELISA (ISR)° | JEV RT-qPCR (Ct value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission Serum | Convalescent Serum | CSF | Throat swab | CSF | Serum | ||||||
| NS2A | NS3 | NS2A | NS3 | NS2A | NS3 | ||||||
| 1 | 19 | M | Pos (18.4) | Pos (45.1) | Pos (29.8) | Neg | Neg | Neg | Neg | Neg | Neg |
| 2 | 50 | M | Pos (26.0) | Pos (40.8) | Pos (34.2) | Neg | Neg | Neg | Neg | Neg | Neg |
| 3 | 33 | M | Pos (40.6) | Neg (3.2) | Pos (35.1) | Neg | Neg | Neg | Neg | — | — |
| 4 | 7 | M | Pos (17.7) | — | Pos (44.4) | Neg | Neg | Neg | Neg | — | — |
| 5 | 16 | M | Pos (7.9) | Pos (18.0) | Pos (21.6) | Pos (32) | Pos (37) | Neg | Neg | — | — |
| 6 | 13 | M | Pos (11.3) | Pos (27.9) | Pos (60.3) | Neg | Neg | Neg | Neg | — | — |
| 7 | 14 | M | Pos (21.7) | — | Pos (38.2) | Neg | Neg | Neg | Neg | Neg | Neg |
| 8 | 3 | F | Pos (13.6) | — | Pos (33.9) | Neg | Neg | — | — | Neg | Neg |
| 9 | 3 | M | Pos (18.0) | — | Pos (41.8) | Neg | — | Neg | — | Neg | — |
| 10 | 13 | M | Pos (22.3) | — | Pos (42.8) | Pos (36) | — | Neg | — | Neg | — |
| 11 | 23 | M | Pos (6.3) | Neg (4.8) | Pos (6.4) | Neg | Neg | — | — | — | — |
M: male, F: female. CSF: cerebrospinal fluid. °Anti-JEV IgM detection using JE Detect™ IgM Antibody Capture ELISA Kit (InBios, Washington, USA). Positive (Pos) = Anti-JEV IgM positive, negative (Neg) = Anti-JEV IgM negative or equivocal, with ISR (Immune status Ratio) cut-offs calculated according to the manufacturer’s instructions. RT-qPCR negative (Neg) = no amplification curve or curve with a Cq > 40. RT-qPCR positive (Pos) = amplification curve with Cq < 40. “−“ = no sample available for testing due to small sample volume collected and/or the use in its entirety for previous assays.
For patient 5, both NS2A and NS3 RT-qPCRs from the throat swab were positive (Ct 32 and 37, respectively). This throat swab was re-extracted and retested, and results demonstrated a NS2A RT-qPCR Ct of 34 and a negative NS3 RT-qPCR. For patient 10, only NS2A RT-qPCR was performed from the throat swab and was found positive with a Ct of 36. Unfortunately, sample volume was insufficiency to permit repetition or NS3 RT-qPCR.
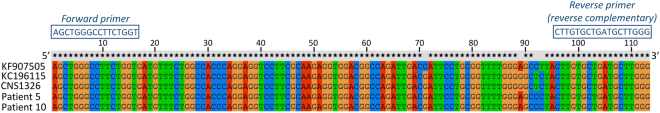
Sequences of NS2A RT-qPCR products for patients 5 and 10 are displayed in Fig. 1. BLAST search on the NCBI website (blastn) showed 100% identities with described JEV isolates. Sequence alignment (Fig. 1) shows two nucleotide differences (C/G at position 71 and T/C at position 93) with the sequence of the JEV RNA used as positive control. We also compared the NS2A sequences from patients 5 and 10 with the two JEV strains that were isolated for patients CSF in 2009 and 2013 and observed two nucleotide differences (G/A at position 89 and T/C at position 92).
Figure 1.
Sequences of the amplicons obtained by NS2A JEV RT-qPCR from throat swabs of patients 5 and 10. Sequences obtained for the 2 patients were aligned (using ClustalX 2.1) with the corresponding sequences of all JEV strains ever handled in the laboratory. KF907505: JEV RNA used as positive control for all JEV RT-qPCR runs. KC196115 and CNS1326: the two JEV strains isolated on cell culture from CSF patients in 2009 and 2013 respectively. Sequence identities are indicated by stars. Sequences of the forward and reverse primers used for the JEV RT-qPCR are indicated above the sequence alignment.
Discussion
We provide strong evidence of JEV RNA detection in throat swab from two patients with clinical presentation of AES. Sequencing of JEV RT-qPCR products confirmed the detection of JEV sequences with nucleotide differences as compared to all JEV strains handled in the laboratory, reducing the likelihood of contamination having occurred. JEV RNA was detected from patient 5 using two different RT-qPCR systems targeting two different genes (NS2A and NS3) and in two sample aliquots from same patient extracted at different time.
Consistent with the literature that JEV RNA is rarely detected in CSF or serum samples, neither of the throat swab JEV positive patients had JEV RNA detected in CSF or serum. Therefore, the use of throat swabs in addition to serum and CSF increased JEV molecular detection. In contrast to JEV MAC-ELISA, the detection of JEV RNA is highly specific for confirming JEV infection. Throat swabs are non-invasive samples, which may be useful to improve diagnosis, especially in patients in whom a lumbar puncture (LP) is not possible or where LP facilitates are not available. Throat swab collection does not require specific facilities, and therefore could facilitate sampling from a wider population to improve our understanding of JEV molecular epidemiology.
There are minimal data on the detection of JEV RNA in other body fluids of patients. One retrospective study did not detect JEV RNA in urine of 52 patients in China15. More recently, JEV RNA has been detected in the urine of two patients10,13. It is likely that JEV RNA may be excreted in urine intermittently and at low levels. We are not aware of any published studies testing for JEV RNA in human throat swabs. However, our findings are consistent with the study by Ricklin et al., in pigs (14). However, the current study was performed on only a small number patients’ samples, and larger studies are needed to better understand the role of throat samples in the detection of JEV RNA by RT-PCR, especially on the frequency and duration of throat swab positivity in relation to clinical presentation, anti-JEV IgM and CSF positivity and patient age.
Our findings also raise the question of possible throat excretion of live JEV virus in infected patients. Throat swab samples from patients 5 and 10 were inoculated on Vero and C6/36 cells but unfortunately the virus could not be isolated, probably due to the low virus titre, but we cannot exclude that JEV RNA fragments alone were present in the throat. More extensive studies are required to investigate the epidemiology of live JEV in human throats.
Methods
Patients
We performed a retrospective study of patients recruited in the South-East Asia Encephalitis study16 conducted at Mahosot Hospital, Vientiane, Laos, between November 2014 to June 2017. CSF, serum and throat swab (using Sigma Virocult®, Medicale Wire, Wiltshire, England) samples at patient inclusion, and follow-up serum at hospital discharge, were collected and stored at −80 °C for subsequent testing. Patients included in our study met all the following criteria: (i) were admitted to hospital with sings/symptoms fulfilling the WHO clinical case definition for AES4, (ii) had no contraindication for lumbar puncture, (iii) presented within 7 days of onset of symptoms, (iv) gave written informed consent, (v) were found anti-JEV IgM positive or undiagnosed following routine diagnosis (vi) had throat swabs available for testing.
Ethical clearance was granted by the Ethical Review Committee of the Faculty of Medical Sciences, National University of Lao, and the Oxford University Tropical Ethics Research Committee, Oxford, UK. The study was performed in accordance with relevant guidelines and regulations.
Anti-JEV IgM ELISA (JE MAC-ELISA)
A WHO recommended commercial JE MAC-ELISA assay is the InBios JE Detect kit (Washington, USA)17. As hospital routine diagnosis procedure, CSF and serum samples from patients with suspicion of CNS infections were tested using this kit following manufacturer’s instruction. A 1/10 dilution was used for CSF samples as recommended by WHO. For each tested sample, ISR (Immune Status Ratio) was calculated and interpreted for the qualitative detection of anti-JEV IgM following manufacturer’s instructions: positive if >6.0, equivocal if 4.0–6.0, or negative if <4.0.
RNA extraction
After defrosting, 200 µL of each sample was extracted and eluted in 60 µL using EZ1 Virus Mini Kit v2.0 (Qiagen, Hilden, Germany) following manufacturer’s instruction.
Real-time RT-PCR (RT-qPCR)
Two in-house JEV RT-qPCR assays were performed, targeting different parts of the JEV genome, NS2A and NS3. Primers and probes were designed to identify the best in-silico matching from a multiple genome alignment of all JEV sequences (303) above 9,000 base pairs available on GenBank. Optimisation and validation assays (Bharucha et al., in prep., Supplementary Data Fig. 1) show good analytical sensitivity (approximately 4 copies/reaction) and 100% specificity when tested on a set of patients and on other viruses from JEV serocomplex. NS2A RT- qPCR was performed with TaqMan® Fast Virus 1-Step kit (Thermo Fisher, Waltham, USA): 50 μL reaction volume; 600 nM forward (5′-AGCTGGGCCTTCTGGT-3′) and reverse (5′-CCCAAGCATCAGCACAAG-3′) primers; 300 nM probe (5′ FAM-CTTCGCAAGAGGTGGACGGCCA-TAMRA 3′) and 30 μL RNA. Thermocycling conditions: 50 °C for 5 minutes, 95 °C for 20 seconds, 45 × [95 °C for 15 seconds +62 °C for 60 seconds]. NS3 RT-qPCR was performed with SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase kit (Thermo Fisher, Waltham, USA): 50 μL reaction volume; 600 nM forward (5′-GCAATGTGYCTCCAAAGAGC-3′) and reverse (5′-GTCGATGACCCTGCTCGC-3′) primers; 300 nM probe (5′FAM-TCCTATGAYACAGAATAYCCAAA-MGB NFQ 3′); 15 μL RNA. Thermocycling conditions: 50 °C for 15 minutes; 95 °C for 2 minutes; 45 × [95 °C for 15 seconds +56 °C for 45 seconds]. RNA positive control (UVE/JEV/UNK/TW/RP9-190 strain, GenBank KF907505, EVA 001V-02344) and no template control were also included to each RT-qPCR run. JEV RT-qPCR result with Ct < 40 was classified as positive.
Due to limitation in sample volume available, NS2A RT- qPCR was performed first, then NS3 when sample still available. In case of any positive the corresponding RT-qPCR was repeated on new extraction, when sample available.
An internal control (MS2 phage) was added to each patient sample prior to extraction. MS2 RT-qPCR was performed to control the extraction process and to exclude inhibition as previously described18.
Sequencing
When available, positive RT-qPCR results were further investigated by sending the RT-qPCR product for next-generation sequencing using Ion S5 system (Thermo Fisher, Waltham, USA), to Unité des Virus Emergents, Faculty of Medicine, Marseille, France.
Sequences were aligned, using ClustalX 2.119, with the sequence of the positive control used for the JEV RT-qPCR and the sequences of any other JEV RNA handled in the laboratory.
Data availability
Every effort has been made to present the data generated and analysed during the study in entirety. The corresponding author will be able to provide further details on reasonable request.
Electronic supplementary material
Acknowledgements
We are very grateful to the patients and to Ass. Prof. Bounthaphany Bounxouei, the Director of Mahosot Hospital, the late Dr Rattanaphone Phetsouvanh, Director of the Microbiology Laboratory, and the staff of the wards and Microbiology Laboratory of Mahosot Hospital particularly Ooyanong Phonemixay for technical help and support, Ass. Prof. Chanphomma Vongsamphan, the Director of Department of Health Care, Ministry of Health, and Prof. Bounkong Syhavong, Minister of Health, Lao PDR for their very kind help and support. We thank all the stakeholders of the SEAe project (www.seaeproject.org). The SEAe project was supported by Total Foundation, Horizon 2020 research and innovation programme EVAg under grant agreement N° 653316, the Institute of Research for Development (IRD), Aix-Marseille University and the Wellcome Trust of Great Britain. The research was implemented in collaboration with ComAcross project (www.onehealthsea.org/comacross) thanks to the financial support of the European Union (EuropeAid, INNOVATE contract 315-047).
Author Contributions
T.B. conceived the concept and design of the study, performed the laboratory testing, data analysis and manuscript preparation. O.S. performed the laboratory testing, and reviewed the submitted manuscript. M.S. performed the laboratory testing, and reviewed the submitted manuscript. Mal.V. performed the laboratory testing, and reviewed the submitted manuscript. Man.V. supervised the laboratory testing and reviewed the submitted manuscript. S.R. recruited and managed patients, and reviewed the submitted manuscript. G.P. performed the sequencing, and reviewed the submitted manuscript. M.L. has jointly had overall responsibility and direction of the SEAe study, and reviewed the submitted manuscript. C.G. has jointly had overall responsibility and direction of the SEAe study, and reviewed the submitted manuscript. J.D.P. provided the clinical datasets, has jointly had overall responsibility and direction of the SEAe study, and reviewed the submitted manuscript. P.N.N. conceived the concept and design of the study, has jointly had overall responsibility and direction of the SEAe study, supervised drafting and submission of the manuscript. X.D.L. conceived the concept and design of the study, has jointly had overall responsibility and direction of the SEAe study, and reviewed the submitted manuscript. A.D.P. conceived the concept and design of the study, supervised the laboratory testing, has jointly had overall responsibility and direction of the SEAe study, supervised drafting and submission of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26333-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Granerod J, Crowcroft NJ. The epidemiology of acute encephalitis. Neuropsychol Rehabilitation. 2007;17:406–428. doi: 10.1080/09602010600989620. [DOI] [PubMed] [Google Scholar]
- 2.Simon-Loriere E, et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. New England Journal of Medicine. 2017;376:1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- 3.Tarantola A, et al. Estimating the burden of Japanese encephalitis virus and other encephalitides in countries of the mekong region. PLoS neglected tropical diseases. 2014;8:e2533. doi: 10.1371/journal.pntd.0002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization, South-East Asia and the Western Pacific. Asia Pacific Strategy for Strengthening Health Laboratory Services (2010–15). WHO Report (2010).
- 5.Dubot-Peres A, Sengvilaipaseuth O, Chanthongthip A, Newton PN, de Lamballerie X. How many patients with anti-JEV IgM in cerebrospinal fluid really have Japanese encephalitis? The Lancet. Infectious diseases. 2015;15:1376–1377. doi: 10.1016/S1473-3099(15)00405-3. [DOI] [PubMed] [Google Scholar]
- 6.Khalakdina, A. et al. Field evaluation of commercial Immunoglobulin M antibody capture ELISA diagnostic tests for the detection of Japanese encephalitis virus infection among encephalitis patients in Nepal. International Journal of Infectious Diseases14, 10.1016/j.ijid.2009.11.020 (2010). [DOI] [PubMed]
- 7.Robinson JS, et al. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. The American journal of tropical medicine and hygiene. 2010;83:1146–1155. doi: 10.4269/ajtmh.2010.10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swami R, Ratho RK, Mishra B, Singh M. Usefulness of RT-PCR for the diagnosis of Japanese encephalitis in clinical samples. Scandinavian Journal of Infectious Diseases. 2008;40:815–820. doi: 10.1080/00365540802227102. [DOI] [PubMed] [Google Scholar]
- 9.Touch S, et al. Epidemiology and burden of disease from Japanese encephalitis in Cambodia: results from two years of sentinel surveillance. Tropical medicine & international health: TM & IH. 2009;14:1365–1373. doi: 10.1111/j.1365-3156.2009.02380.x. [DOI] [PubMed] [Google Scholar]
- 10.Mai NTH, et al. Central Nervous System Infection Diagnosis by Next-Generation Sequencing: A Glimpse Into the Future? Open Forum Infect Dis. 2017;4:ofx046. doi: 10.1093/ofid/ofx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andries AC, et al. Value of Routine Dengue Diagnostic Tests in Urine and Saliva Specimens. PLoS neglected tropical diseases. 2015;9:e0004100. doi: 10.1371/journal.pntd.0004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourinat A-C, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika Virus in Urine. Emerging infectious diseases. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang GKL, et al. Prolonged Detection of Japanese Encephalitis Virus in Urine and Whole Blood in a Returned Short-term Traveler. Open Forum Infectious Diseases. 2017;4:ofx203. doi: 10.1093/ofid/ofx203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricklin ME, et al. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, et al. Japanese encephalitis virus RNA not detected in urine. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:157–158. doi: 10.1093/cid/cit169. [DOI] [PubMed] [Google Scholar]
- 16.South East Asia Encephalitis Project (SEAe), https://research.pasteur.fr/en/program_project/the-southeast-asia-encephalitis-project/ (2013).
- 17.World Health Organisation Regional Office for the Western. Training Report Fourth Hands-on Training Workshop on the Laboratory Diagnosis of Japanese Encephalitis. WHO Report (2014).
- 18.Ninove L, et al. RNA and DNA Bacteriophages as Molecular Diagnosis Controls in Clinical Virology: A Comprehensive Study of More than 45,000 Routine PCR Tests. PloS one. 2011;6:e16142. doi: 10.1371/journal.pone.0016142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Every effort has been made to present the data generated and analysed during the study in entirety. The corresponding author will be able to provide further details on reasonable request.