Abstract
Streptococcus suis is an emerging zoonotic pathogen causing streptococcal toxic shock like syndrome (STSLS), meningitis, septicemia, and even sudden death in human and pigs. Serious septicemia indicates this bacterium can evade the host complement surveillance. In our previous study, a functionally unknown protein SntA of S. suis has been identified as a heme-binding protein, and contributes to virulence in pigs. SntA can interact with the host antioxidant protein AOP2 and consequently inhibit its antioxidant activity. In the present study, SntA is identified as a cell wall anchored protein that functions as an important player in S. suis complement evasion. The C3 deposition and membrane attack complex (MAC) formation on the surface of sntA-deleted mutant strain ΔsntA are demonstrated to be significantly higher than the parental strain SC-19 and the complementary strain CΔsntA. The abilities of anti-phagocytosis, survival in blood, and in vivo colonization of ΔsntA are obviously reduced. SntA can interact with C1q and inhibit hemolytic activity via the classical pathway. Complement activation assays reveal that SntA can also directly activate classical and lectin pathways, resulting in complement consumption. These two complement evasion strategies may be crucial for the pathogenesis of this zoonotic pathogen. Concerning that SntA is a bifunctional 2′,3′-cyclic nucleotide 2′-phosphodiesterase/3′-nucleotidase in many species of Gram-positive bacteria, these complement evasion strategies may have common biological significance.
Keywords: Streptococcus suis, surface protein SntA, C1q, complement evasion, pathogenesis
Introduction
Streptococcus suis are recognized as an important swine and human pathogen (1). Among the 33 serotypes, S. suis serotype 2 (SS2) is the most virulent and prevalent one, which is also an emerging zoonotic pathogen (2). Two large-scale outbreaks of severe human SS2 infection occur in 1998 and 2005 in China causing 229 infections and 52 deaths (3, 4). In 2005, the streptococcal toxic shock like syndrome (STSLS) is first reported to occur in the human. An early burst of inflammatory cytokines could result in the STSLS with death as quickly as 13 h after SS2 infection, and subsequently SS2 breaks through blood–brain barrier (BBB) to cause disease, particularly meningitis (1, 5). Bacterial pathogens evade host innate immune defenses and maintain a high dose in blood causing bacteremia and septicemia. During these processes, the host complement system is an important factor facilitating clearance of bacterial pathogens (6, 7).
The complement system consists of more than 50 plasma and cell surface proteins. As a first line of defense against pathogenic intruders and a mediator between the innate and adaptive immune response, it plays an essential and efficient role in rapid recognition and elimination for invading pathogens. The complement has three independent but interactive activation pathways: the classical pathway, alternative pathway, and lectin pathway (8). These three different complement pathways are stimulated by different foreign substance through specific recognition molecules (4). All the complement cascades result in the deposition of C3b to amplify the cascades, and mediate phagocytosis and adaptive immune responses by binding to complement receptors; the release of pro-inflammatory anaphylatoxins and chemoattractant C5a and C3a; and formation of membrane attack complex (MAC; C5b-9) then lead to direct lysis of Gram-negative bacteria (9).
Although host complement can rapidly recognized and eliminated foreign microorganisms, it also offers many interference sites that can disrupt this balanced network of protein interactions by complement-binding proteins leading to failure of elimination by host. Complement-binding proteins can be identified from both host and pathogens. These complement evasion mechanisms include (I) recruiting or mimicking of complement regulators; (II) modulating or inhibiting complement by direct interactions; and (III) enzymatic degradation by complement components (4).
C1q is the recognition subunit of C1 complex to trigger the classical complement pathway, following the recognition of IgG or IgM-bearing immune complexes (10). Proteins that interact with C1q have been identified widely in Gram-negative bacteria, such as Salmonella Minnesota (11), Escherichia coli (12–14), Klebsiellapneumoniae (15), Legionella pneumophila (16), Bacillus anthracis (17), Moraxella catarrhalis (12), nontypeable Haemophilus influenzae (12), but in Gram-positive bacteria the research is not much, except for Group B Streptococci (18, 19), Streptococcus pyogenes (20–22) and Staphylococcus aureus (23).
In our previous study, surface protein SntA of S. suis without any unknown function has been characterized to be a heme-binding protein which involved in the pathogenesis of S. suis in pigs. SntA can interact with the host antioxidant protein AOP2 and consequently inhibit its antioxidant activity (24). Complement C1q is identified as another interacting partner of SntA when we screen the SntA binding proteins in the host. In the present study, we demonstrate that SntA is an important player in complement evasion of this important zoonotic pathogen.
Materials and Methods
Strains and Culture Conditions
Streptococcus suis serotype 2 strain SC-19 (GenBank accession number: NZ_CP020863.1) used in this study was isolated from a sick pig during the epidemic outbreak in Sichuan province of China in 2005. The S. suis strains were grown in tryptic soy broth (TSB; Difco, France) or on tryptic soy agar (TSA; Difco) plates supplemented with 5% newborn bovine serum (Sijiqing, Hangzhou, China) at 37°C. The E. coli DH5α and BL21 (DE3) strains were grown in LB broth or on LB agar plates at 37°C. The bacterial strains, plasmids, and primers used in this study are listed in Table 1.
Table 1.
Bacterial strains, plasmids, and primers used in this study.
| Strains, plasmids, and primers | Description and sequence (5′ → 3′) | Source, reference, and products |
|---|---|---|
| Escherichia coli strains | ||
| DH5α | Host for cloning vector | Kangwei |
| BL21 (DE3) | Host for expression vector | Kangwei |
| S. suis strains | ||
| SC-19 | S. suis serotype 2, the wild type | (25) |
| ΔsntA | The sntA-deleted strain; Emr | (24) |
| CΔsntA | Complemented strain of ΔsntA; Emr, Spcr | This study |
| Plasmids | ||
| pET28a | Expression vector to produce His-fused proteins; lacZ, Kanar | Novagen |
| pET28a-SUMO | Expression vector to produce His-and SUMO-fused proteins; lacZ, Kanar | (26) |
| pQE32 | Expression vector to produce His-fused proteins; lacZ, Ampr | Qiagen |
| pSET2 | E. coli-S. suis shuttle vector; Spcr | (27) |
| pSET2:sntA | pSET2 containing the intact sntA gene and its upstream promoter; Spcr Emr | This study |
| Primers | ||
| C1qA_F | GCGTCGACGCTGGCCGTATCCCTG | ORF of c1qA gene |
| C1qA_R | CCAAGCTTTCAGGTGGAGGGGAAG | |
| C1qB_F | AGAGAACAGATTGGTGGATCCCAGGTCTTCTGCATGCCTGGC | ORF of c1qB gene |
| C1qB_R | CTCGAGTGCGGCCGCAAGCTTTCATGCCTCTGCATCTGGGAAG | |
| C1qC_F | AGAGAACAGATTGGTGGATCCCAGGACAACTTAAGATGCCATGGG | ORF of c1qC gene |
| C1qC_R | CTCGAGTGCGGCCGCAAGCTTCTAGTTAGGGAAGAGCAAGAAGCCG | |
| CΔsntA_F | AAAACTGCAGCTCGTTTTTCAAACGAACGTTCAG | sntA gene and its upstream promoter |
| CΔsntA_R | GCGGGATCCTTAATGTTCTTTTTTCTTAGTCCATG | |
| 10065_F | ATGAAAGAAATTAACTACACTGGGGA | ORF of B9H01_10065 gene |
| 10065_R | TTATCCGTTGGTCCTTTTCACTGA | |
| SntA_F | ATGAATTTTCGTTTCAGTAAGTGTGC | ORF of sntA gene |
| SntA_R | TTAATGTTCTTTTTTCTTAGTCCATGC | |
| 10075_F | ATGAAAAGAATAGGATATGTTTTCATAT | ORF of B9H01_10075gene |
| 10075_R | CTACCAAATATAGGTTCTAATAAAACGG | |
Construction of sntA Gene Deletion and Complementary Strains
The sntA-deleted strain ΔsntA was constructed in our previous study (24). To construct sntA complementary strain, a DNA fragment containing the entire sntA coding sequence and its promoter and terminator was amplified by using primers CΔsntA_F/CΔsntA_R. The amplicon was subsequently cloned into E. coli-S. suis shuttle vector pSET2, resulting in the recombinant plasmid pSET2:sntA. This plasmid was transformed into the ΔsntA strain, and the resulting complementary strain CΔsntA was screened on TSA agar plates supplemented with 100 µg/ml spectinomycin (Spc). The Spc-resistant colonies were verified by PCR and reverse transcription (RT)-PCR analyses by using three pairs of primers SntA_F/SntA_R, 10065_F/10065_R, and 10075_F/10075_R (Table 1).
Experimental Infection of Mice
To detect the virulence of sntA in S. suis, a total of 24 female 5-week-old specific-pathogen-free (SPF) Kunming mice (8 mice per group) were intraperitoneally infected with 2 × 109 colony forming unit (CFU)/mouse of SC-19 and ΔsntA. Physiological saline was served as a negative control. Then the morbidity, mortality, and clinical symptoms such as limping, swollen joints, shivering, and central nervous system failure of all the mice were observed for 5 days. To evaluate the effect of sntA on survival in blood and organs colonization, competitive colonization experiment of SC-19 and ΔsntA was performed. 5-week-old SPF Kunming mice (7 mice per group) were co-infected with SC-19 and ΔsntA at the ratio 1:1 (5 × 108 CFU/mouse). Bacterial counts in blood, brain, and lung were collected at 6, 12, 24, and 48 h post infection (hpi). Colonization of bacteria in various tissues were analyzed by serial diluted and plated brain and lung samples after homogenizing, and blood samples on TSA agar plates with (Em+) and without (Emc) 100 µg/ml erythromycin (Em) as described previously (28). The number of live bacteria on TSA agar plates with or without Em was calculated as ΔsntA, sum of SC-19 and ΔsntA, respectively. The number of SC-19 was calculated as .
Bactericidal Assays
In human blood and serum killing assays, experiments to evaluate the survival rate of S. suis were carried out as described previously (29). Overnight cultures of the S. suis strains in TSB were diluted in 1:100 and grown to mid-log phase without agitation at 37°C. The cultures were diluted to 5 × 107 CFU/ml with physiological saline. 50 µl diluted cultures was mixed with 450 µl fresh human blood for human blood killing assay and 20 µl diluted cultures were mixed with 180 µl of normal human serum (NHS) and complement heat inactivated serum by incubation at 56°C for 30 min for human serum killing assay. The resulting mixtures were incubated at 37°C for 30 min. Live bacteria were counted by plating the serial diluted samples on TSA agar plates. The percentage of live bacteria was subsequently calculated as (CFUafter incubation/CFUin original inoculum) × 100%.
In PMNs killing assay, PMNs were isolated from heparinized venous blood by human peripheral blood PMN isolation kit (Haoyang, Tianjin, China). Experiment to investigate the survival rate of S. suis in PMNs was carried out as described previously (6). PMNs were mixed with bacteria at MOI = 1:10 in RPMI-1640 medium (Hyclone, USA) supplemented with 20% freshly non-immune human serum and incubated at 37°C under 5% CO2 for 30 min. The percentage of live bacteria was subsequently calculated as .
In phagocytosis assay, the experiment was carried out by our previous study (30). Briefly, the 1.0 × 106 RAW264.7 cells for each well were pooled into 12-well plates. Then S. suis strains in mid-log phase were added to the plates (MOI = 10:1) and incubated for 30 min at 37°C to allow cells to be phagocytized. After incubation, ampicillin was applied to plates for 1 h to kill the extracellular bacteria. Then, the cells were lysed with sterile distilled water on ice and the bacterial CFU were counted on TSA agar plates. These data are presented as means ± SDs from three separate experiments.
Complement C3 Deposition and MAC Formation Analysis
C3 deposition and MAC formation assays were performed as previous study (31). Overnight cultures of S. suis were diluted in 1:100 in TSB and grown to mid-log phase without agitation at 37°C. Bacteria were collected by centrifugation at 6,000 rpm for 5 min. After two washes, cultures were suspended to 5 × 107 CFU/ml with physiological saline. 300 µl S. suis suspensions were mixed with 240 µl freshly undiluted and 1/500 diluted non-immune human serum and incubated at 37°C for 30 min. After two washes, S. suis was resuspended in 300 µl anti-C3b monoclonal antibody (20 µg/ml; Abcam, England) to detect C3 deposition and monoclonal anti-C5b-9 antibody (20 µg/ml; Abcam) to detect MAC formation. After incubation for 10 min at room temperature, FITC-conjugate goat-anti-mouse IgG (BD, USA) was used as the second antibody and incubated at room temperature for 10 min. Finally, bacteria were washed and resuspended in 800 µl physiological saline for flow cytometry analysis performed by FACSCalibur (BD). Bacteria were detected by log-forward and log-side scatter dot-plot at middle flow rate. A gating region was set to include the majority of bacteria except debris. 10,000 bacteria/events were acquired and analyzed for fluorescence using log-scale amplifications. C3 deposition and MAC formation capacities were measured by the geometric mean fluorescence intensity (GMFI). GMFI value was measured from three independent experiments performed in duplicate.
SntA Immunodetection
Western-blot analysis was used to determine the location of SntA expressed in the SC-19, ΔsntA, and CΔsntA strains. For cell lysate, cell cultures of S. suis strains were collected, resuspended with 1 ml bacterial lysis buffer (0.05 M Tris–HCl, 2.5 mM EDTA, 0.1 M NaCl, 0.25% Triton X-100, pH 8.5~9.0) and boiled 20 min to lysis, the resulting cell lysate were used to prepare SDS-PAGE samples. For cell wall proteins, samples were prepared as described previously (32). Briefly, bacterial cultures were collected; washed once in 50 mM Tris–HCl, pH 7.3; resuspended in 1 ml of osmoprotective buffer (50 mM Tris–HCl, pH 7.3, 20% sucrose, 2.5 µM PMSF) supplemented with 175 U/ml mutanolysin (Sigma-Aldrich, Shanghai, IL, USA); incubated at 37°C for 90 min under constant gentle agitation. After centrifugation at 12,000 g, 4°C for 15 min, supernatants containing the cell wall proteins were used to prepare SDS-PAGE samples. For secreted proteins, culture supernatants were collected, filtered (Millipore, 0.22 µm, Shanghai, China), and concentrated to 10 mg/ml, and then the proteins were sedimentated with pre-cooled 10% TCA-acetone solution at 4°C overnight. After centrifugation at 12,000 g, 4°C for 15 min, washed sediment with pre-cooled 90% acetone supplemented with 10 mM DTT for three times. The last sediment was resuspended with 8 M urea, 2% CHAPS, 10 mM DTT for next step. For all SDS-PAGE samples immunodetection, total proteins were quantified by BCA protein assay kit (Kangwei, Beijing, China), and then 48 µg cell lysate, secreted, and cell wall proteins were applied to 10% SDS-PAGE. Proteins transferred onto PVDF membrane (Millipore, 0.45 µm). Mouse SntA polyclonal antibody (24) was used for SntA immunodetection, and chemiluminescence detection (Bio-Rad, USA) with HRP-conjugate goat anti-mouse IgG (Antgene, Wuhan, China) by MF-Chemi BIS 3.2 (DNR, Israel).
Preparation of Recombinant Proteins
Recombinant SntA protein (Protein ID: WP_012027972.1) was expressed and purified as our previous report (24). To obtain the recombinant proteins of the complement C1q subunits, the cDNAs of C1qA, C1qB, and C1qC were amplified from the total cDNA of porcine lung tissue with primers C1qA_F/R, C1qB_F/R, C1qC_F/R, respectively (Table 1). Total cDNA was prepared from total RNA of porcine lung tissue by using RT-PCR. Then the C1qA cDNA was cloned into pQE-32 plasmid (Qiagen, Shanghai, China), while the cDNAs of C1qB and C1qC were cloned into pET-28a-SUMO plasmid (26) to promote soluble expression. The resultant recombinant plasmids were transformed into E. coli BL21 (DE3), respectively. The expression of C1qA, C1qB, and C1qC proteins was induced by 1 mM isopropyl-β-d-thiogalactoside for 4 h at 37°C. The recombinant proteins were purified by using Ni-NTA agarose column (Bio-Rad) and quantified by BCA protein assay kit (Kangwei).
Interaction Between C1q and SntA Proteins
ELISA was used to confirm the interaction between SntA and C1q proteins as described previously (33). For SntA bound assay, the proteins C1q (Quidel, USA), three C1q subunits C1qA, C1qB, C1qC, and BSA were coated to 96-well plates (BIOFIL, Guangzhou, China) with increasing concentrations of 0–7 µg/ml at 4°C for 16 h, respectively. After blocking with blocking buffer (PBS supplemented with 0.05% Tween20 and 1% BSA), 5 µg/ml SntA was poured to each well, and then incubated at 37°C for 1 h. Mouse SntA polyclonal antibody was applied at 37°C for 1 h to test the direct binding capacity. For C1q bound assay, SntA, BSA, and heme were coated to 96-well plates with increasing concentrations of 0–7 µg/ml at 4°C for 16 h, respectively, After blocking with blocking buffer, 5 µg/ml C1q was applied to each well at 37°C for 1 h. Rabbit C1qA polyclonal antibody (Abclonal, Wuhan, China) was applied at 37°C for 1 h to test the direct binding capacity. For inhibition assay, 5 µg/ml human aggregated IgG (Sigma-Aldrich) was coated to plates at 4°C overnight. Human aggregated IgG was prepared by incubating at 63°C for 20 min, immediately placing on ice for 1 h, centrifuging at 16,000 g for 5 min and quantification (34). C1q was pre-incubated with increasing concentrations of 0–25 µM SntA, heme, or BSA, and then applied to plates at 37°C for 1 h. Rabbit C1qA polyclonal antibody was applied to plates at 37°C for 1 h to test the indirect binding capacity. All the ELISA assays, the HRP-conjugate goat-anti-rabbit IgG (Antgene), or goat-anti-mouse IgG was used as the second antibody at 37°C for 1 h before chromogenic reaction.
Competitive binding assays were performed as described previously (35). For C1q-IgG binding assay, microtiter plates were pre-coated with 30 µg/ml human C1q overnight at 4°C. After blocking, plates were incubated with the mixtures of 50 µl 2.5 mg/ml human aggregated IgG and 50 µl increasing concentrations of 0–50 µM SntA or BSA in HB++ buffer (10 mM Hepes, pH 7.4, 100 mM NaCl, 5 mM CaCl2, 1 mM MgCl2). After incubation for 1 h at 37°C, plates were washed with washing buffer (HB++ buffer supplemented with 0.05% Tween20) and developed with HRP-conjugate goat anti-human IgG Fc (1:4,000; Sigma-Aldrich) at 37°C for 1 h before chromogenic reaction. For C1q–Ag–Ab binding assay, microtiter plates were coated with 2.5 µg/ml tetanus toxoid (Millipore) as an antigen for immune complex formation. After blocking, human tetanus toxin immunoglobulin (1:1,000; Hualan Bio, Xinxiang, China) was applied to the tetanus toxoid-coated plates to form immune complexes for 1 h at 37°C. 50 µl 2 µg/ml human C1q were mixed with 50 µl increasing concentrations of 0–50 µM SntA or BSA. The resulting mixtures were applied to each well at 37°C for 1 h. After washed, polyclonal goat antiserum to human C1q (1:5,000; Quidel, USA) was incubated to plates at 37°C for 1 h. HRP-conjugate rabbit anti-goat IgG (Antgene) was used as the second antibody at 37°C for 1 h before Chromogenic reaction. These data are presented as mean ± SD from three separate experiments.
Binding of C1q to S. suis
Overnight cultures of the S. suis strains in TSB were diluted in 1:100 and grown to mid-log phase without agitation at 37°C. The cultures were washed and resuspended in DGHB++ buffer to 5 × 108 CFU/ml. S. suis suspensions were incubated with 0, 10, and 20 µg/ml C1q proteins at 37°C for 1 h and the total volume was 100 µl. After incubation and two washes, FITC-rabbit anti-human C1q antibody (Abcam) was applied for 30 min at 37°C. Finally, the bacteria was washed and resuspended in 500 µl PBS for flow cytometry analysis by FACSCalibur. Data from three independent experiments in duplicate were analyzed by Flow Jo 7.6.1 software (33). The assay was repeated three times.
Hemolytic Assay
The hemolytic activity of SntA protein was measured as previous study (33). For the classical pathway, sheep red blood cells (SRBCs) (Baiji, Zhengzhou, China) were washed with pre-cooled DGHB++ buffer for three times and diluted to a concentration of 1 × 109 cells/ml. Then the SRBCs solution was mixed with equal volume of ambocepter (1:1,000; Baiji) and rotated at 37°C for 20 min. After two washes, 0.2% NHS was incubated with 0–100 µg/ml SntA at 37°C for 15 min, then the mixtures were incubated with 3 × 108 cells/ml SRBCs at 37°C for 1 h. BSA was used as a negative and the total volume is 150 µl. After centrifugation at 800 g, the hemolytic activity was calculated by spectrophotometric measurement of absorbance at 405 nm. For the alternative pathway, rabbit red blood cells (RRBCs) (Baiji) were washed with pre-cooled Mg2+-EGTA-DGHB buffer (4.2 mM Hepes, pH 7.4, 59 mM NaCl, 2.08% glucose, 0.08% gelatin, 7 mM MgCl2, 10 mM EGTA) for three times and diluted to a concentration of 1 × 109 cells/ml. 1.25% NHS was incubated with 0–100 µg/ml SntA at 37°C for 15 min, then the mixtures were incubated with 3 × 108 cells/ml RRBCs at 37°C for 1 h. The hemolytic activity of alternative pathway was determined as the classical pathway. These data are presented as mean ± SD from three separate experiments.
Complement Activation Assay
To detect whether SntA could activate complement directly, complement activation ELISA were performed as described previously (33). 2 µg/ml SntA, 2 µg/ml BSA, 10 µg/ml IgM (Berseebio, Beijing, China) for the classical pathway, 100 µg/ml Mannan (Sigma) for the lection pathway, and 20 µg/ml Zymosan (Sigma) for the alternative pathway were coated to 96-well plates in PBS. The plates were washed with PBS supplemented with 0.05% Tween20 for each step. After blocking with the blocking buffer, 0–7% NHS in DGHB++ buffer was applied for classical pathway, 0–7% depleted of protein C1q serum (Quidel) in DGHB++ buffer was applied for lectin pathway, and 0–7% NHS in Mg2+-EGTA-DGHB buffer was applied for alternative pathway. After incubation for 1 h, the plates were washed and C3b rabbit polyclonal antibody (1:1,000; Proteintech, Wuhan, China) was poured to plates to detect C3 deposition at 37°C for 1 h. All the ELISA assays, the HRP-conjugate goat-anti-rabbit IgG was used as the second antibody at 37°C for 1 h before chromogenic reaction. These data are presented as mean ± SD from three separate experiments.
Results
SntA Contributes to the Virulence of S. suis
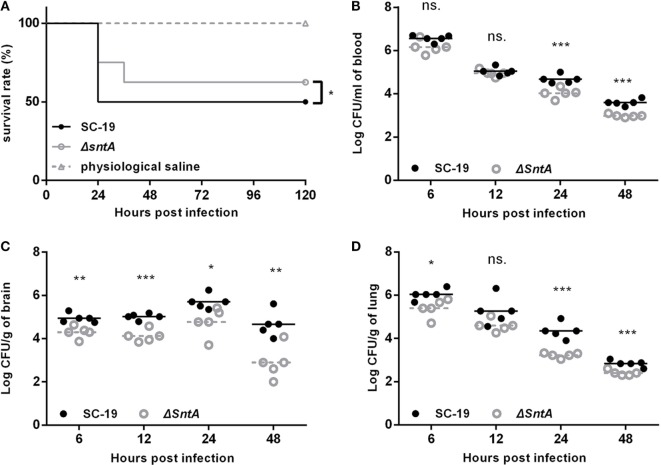
Two mouse infection models were performed to investigate the contribution of sntA on virulence. The growth curves of S. suis presented by OD and CFU were measured before infection in mice. Results showed that no significant differences were observed in SC-19, ΔsntA, and CΔsntA in both OD and CFU (Figure S2 in Supplementary Material). First, groups of eight mice were intraperitoneally infected with 2 × 109 CFU/mouse SC-19 and ΔsntA. Physiological saline was used as a negative control. Survival rate of infected mice was measured within 5 days post infection (dpi). We observed that four of mice infected with SC-19 died within 24 hpi, another four showed obvious limping, shivering, and central nervous system failure within 5 dpi. By contrast, only two mice infected with ΔsntA died within 24 hpi, one mouse died within 36 hpi, and another five mice were survived during the observation within 5 dpi (Figure 1A). This revealed that significant difference was observed in survival rate of SC-19 and ΔsntA infected mice. Second, groups of seven mice were intraperitoneally infected with the mixtures of SC-19 and ΔsntA at the ratio 1:1 (5 × 108 CFU/mouse). Blood and brain, lung colonization were recovered at 6, 12, 24, and 48 hpi, respectively. The efficiency of colonization of SC-19 was much higher at 24 and 48 hpi than that of ΔsntA in blood (Figure 1B) and lung (Figure 1D). In addition, significant differences were observed in brain from 6 to 48 hpi (Figure 1C). The results showed that SntA contributed to the survival in blood and the colonization in specific organs.
Figure 1.
SntA contributes to pathogenesis in mouse infection model. (A) Survival curves for mice challenged with Streptococcus suis. Groups of eight mice were injected separately and intraperitoneally with 2 × 109 colony forming units (CFU)/mouse of SC-19 and ΔsntA. Eight mice inoculated with physiological saline were served as a negative control. Significant difference in survival between different groups was analyzed by Log Rank test for trend. Bacterial loads in blood (B), brain (C), and lung (D) were shown. Seven mice per group were intraperitoneally infected with the mixtures of SC-19 and ΔsntA at ratio 1:1 (dose of 5 × 108 CFU/mouse). Results were shown as log10 of recovered bacteria counts after deletion of the highest and lowest value (CFU/ml in blood and CFU/g in organs). The statistical significance was shown by asterisks (unpaired t test; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).
SntA Possess Obvious Anti-Phagocytic Activity
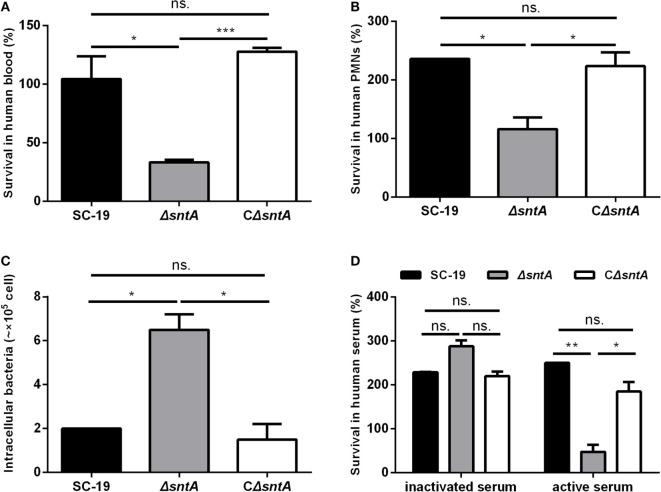
We constructed a complementary strain of sntA, as confirmed by genome PCR (Figure S1A in Supplementary Material) and RT-PCR (Figure S1B in Supplementary Material). To investigate the anti-phagocytosis mediated by SntA, three bacteria killing assays and one phagocytosis assay were performed. In blood killing assay, the survival rate of SC-19 in human blood was 105%, but the absence of sntA decreased the survival rate to only 33% (Figure 2A). Similar results were observed in mouse blood killing assay (Figure S3A in Supplementary Material). In PMNs killing assay, PMNs supplemented with 20% active human serum were used to test the survival abilities of S. suis in human neutrophils. Results revealed that the survival rate of ΔsntA was significantly decreased compared to SC-19 and CΔsntA (Figure 2B). In phagocytosis assay, RAW 264.7 cells were used to test anti-phagocytic activity of S. suis. Results demonstrated that the phagocytic numbers of ΔsntA were much higher comparing with SC-19 and CΔsntA (Figure 2C). In the human serum killing assay, the active and inactivated human serum were used to investigate the role of complement on S. suis clearance. For the sntA-deleted mutant strain, only 48% viable ΔsntA bacteria survived in freshly active human serum. The presence of sntA significantly increased the survival rate of S. suis up to 250% (SC-19) and 185% (CΔsntA), respectively. No obvious differences were observed in the survival rates of S. suis in inactivated serum (Figure 2D). Normal mouse serum was also used in this assay, and similar results were obtained (Figure S3B in Supplementary Material). No significant differences were observed between SC-19 and CΔsntA in all tests. These data demonstrated that SntA was involved in resistance to phagocytosis and the bactericidal activity of blood, PMNs, and NHS dependent on complement. This reveled that SntA could obviously contribute to anti-phagocytosis and this may be involved in complement.
Figure 2.
SntA possess anti-phagocytic activity. (A) Survival rate of Streptococcus suis in human blood. (B) Survival rate of S. suis in PMNs separated from freshly human blood. (C) The numbers of S. suis phagocytized in mouse macrophage RAW264.7 cells. (D) Survival rate of S. suis in human active and inactivated serum. Results were expressed from three independent experiments performed in triplicate. The statistical significance was showed by asterisks (unpaired t test; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).
SntA Inhibits the C3 Deposition and MAC Formation on S. suis
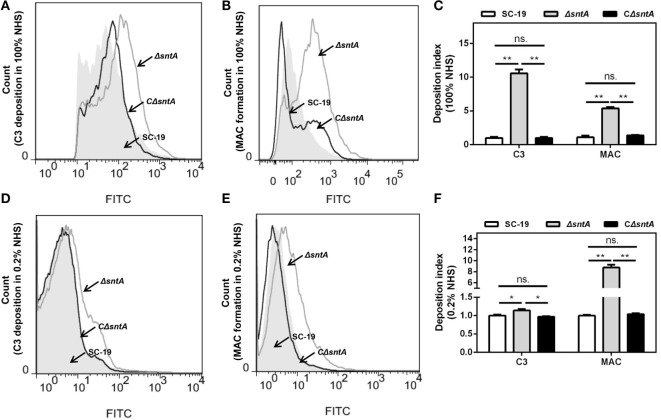
SntA possessed anti-phagocytosis, but the effect of SntA on complement activation on S. suis was still unclear. To investigate that, the C3 deposition and MAC formation assays were performed in both undiluted and 1:500 diluted human serum. Results showed that the viable bacteria number aggregated fluorescence of ΔsntA in C3 deposition was obviously increased compared to SC-19 and CΔsntA in undiluted human serum (Figures 3A,C). The efficiency of MAC formation was significantly higher comparing with SC-19 and CΔsntA (Figures 3B,C) as well. To assess the effect of SntA on complement activation through the classical pathway, 1/500 NHS was used. Results revealed that C3 deposition and MAC formation on surface of ΔsntA was significantly higher compared with SC-19 and CΔsntA in 1/500 diluted human serum (Figures 3D–F). No significant differences were observed between SC-19 and CΔsntA in all tests. These assays demonstrated that SntA could inhibit the complement pathway.
Figure 3.
SntA inhibits C3 deposition and membrane attack complex (MAC) formation. (A–F) The C3 deposition and MAC formation capacities were presented by count of positive cell aggregated with fluorescence in the presence of complement (100 and 0.2% normal human serum). The representative histogram of flow cytometry from three independent experiments was exhibited (A,B,D,E). Geometric mean fluorescence intensity (GMFI) value was measured from three independent experiments performed in duplicate. The GMFI value of C3 deposition and MAC formation of SC-19 in all tests was set as 1.00, the corresponding ΔsntA and CΔsntA values were proportional achieved. The resulting C3 deposition and MAC formation index were presented (C,F). The statistical significance was calculated by two-way ANOVA of Sidak’s correct for multiple comparison and shown by asterisks (**p < 0.01; *p < 0.05; ns, p > 0.05).
SntA Is a Cell Wall Anchored Protein
The cell wall attached protein LPXTG motif region was found at C terminal of amino acid sequence of SntA (Figure 4A). To confirm this prediction, western-blot analysis was performed. Results showed that SntA expressed in cell lysate extracted from SC-19 and CΔsntA, but not detected in ΔsntA. Cell wall proteins extracted by mutanolysin and secreted proteins sedimentated by TCA-acetone were used to further determine the location of SntA in S. suis. SntA was demonstrated to express in cell wall proteins, and not in secreted proteins (Figure 4B). These assays indicated that SntA existed in cell wall.
Figure 4.
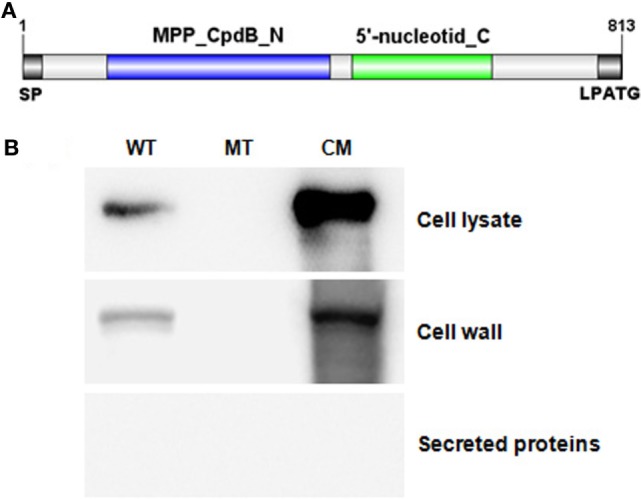
SntA was a conserved cell wall anchored protein. (A) Conserved domain of SntA was predicted in NCBI database (https://www.ncbi.nlm.nih.gov/cdd). SntA had two conserved domain: MPP_CpdB_N and 5′-nucleotid_C, and a cell wall attached LPATG motif. (B) Western-blot was used to determine surface location of SntA. Cell lysate, cell wall proteins, and secreted proteins were ran 10% SDS-PAGE, transferred onto PVDF membrane and detected by mouse anti-SntA poly-antibody following by HRP-conjugate goat-anti-mouse IgG. WT, SC-19; MT, ΔsntA; CM, CΔsntA.
SntA Interacts With Complement C1q
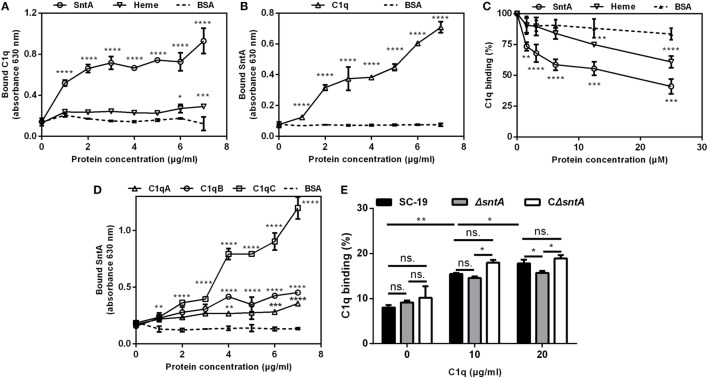
To assess the binding capacity between SntA and C1q, the direct and indirect bound assays were performed. In direct bound assay, C1q could significantly bind with SntA in a dose-dependent manner in both C1q-coated and SntA-coated plates (Figures 5A,B). The known C1q-binding molecule heme was used as a positive control and BSA as a negative control. In inhibition assay, both SntA and heme could significantly inhibit the binding capacity between plasma purified C1q and aggregated human IgG on IgG-coated plates, and the inhibitory effect of SntA was much stronger than the positive control heme (Figure 5C). Furthermore, three subunits of C1q including C1qA, C1qB, and C1qC could obviously interact with SntA in a dose-dependent manner as well (Figure 5D). These results indicated that recombinant SntA could bind to C1q. To investigate whether SntA bound with C1q on bacterial surface, flow cytometry was performed. A dose-dependent increase of C1q-binding capacity was observed on S. suis. Compared with SC-19 and CΔsntA, a significant reduction of C1q-binding capacity was observed in ΔsntA when 20 µg/ml C1q was added (Figure 5E). Taken together, SntA could interact with C1q and act as a C1q ligand on bacterial surface.
Figure 5.
SntA interacts with C1q. (A) C1q was applied to SntA-, heme-, and BSA-coated plates to test the C1q bound capacity. (B) SntA was applied to C1q- and BSA-coated plates to test the SntA bound capacity. (C) SntA, heme, or BSA was premixed with C1q and applied to IgG-coated plates to test the binding capacity between C1q and IgG, which indicating the binding capacity between SntA and C1q. (D) SntA was applied to C1qA-, C1qB-, C1qC-, and BSA-coated plates to test the SntA bound capacity. (E) Increasing concentrations of C1q were incubated with Streptococcus suis to detect the binding capacity by FITC-anti-human C1q antibody. Results were expressed from three independent experiments performed in triplicate. The statistical significance was calculated by two-way ANOVA of Sidak’s correct for multiple comparison and shown by asterisks (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).
SntA Competitively Bind With C1q
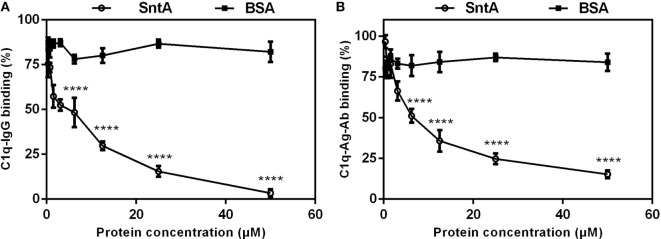
The classical pathway is triggered through the recognition of antigen-antibody complex on micro surface by recognition molecule C1q (4). To assess the effect of C1q-binding protein SntA on the recognition step of the classical complement pathway, competitive binding assays were performed. In C1q–IgG binding assay, result showed that SntA strongly decreased the C1q–IgG binding capacity, and 50 µM SntA inhibited the binding capacity down to 3.34% (Figure 6A). In C1q–Ag–Ab binding assay, the binding capacity was significantly decreased in a dose-dependent manner and 84.7% of it was remarkably inhibited with 50 µM SntA (Figure 6B). Results demonstrated that SntA could inhibit C1q–IgG binding and C1q–Ag–Ab binding by competitive interaction with C1q.
Figure 6.
SntA competitively binds with C1q. (A) Inhibition of IgG–C1q binding. Increasing concentrations of SntA were premixed with C1q and applied to IgG-coated plates to test the inhibitory capacity of SntA to C1q–IgG binding. (B) Inhibition of C1q–Ag–Ab binding. IgG was applied to tetanus toxoid-coated plates for IgG-containing immune complex formation. Increasing concentrations of SntA were premixed with C1q and added to plates to test the inhibitory capacity of SntA to C1q–Ag–Ab binding. BSA was served as a negative control. Results were expressed from three independent experiments performed in triplicate. The statistical significance was calculated by two-way ANOVA of Sidak’s correct for multiple comparison and shown by asterisks (****p < 0.0001; ns, p > 0.05).
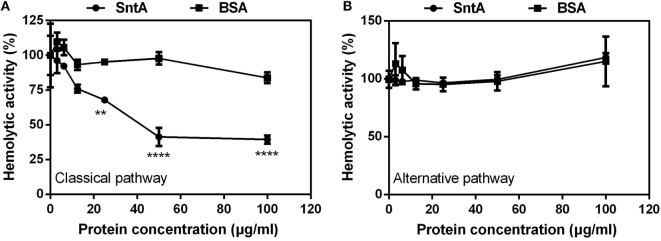
SntA Inhibits Hemolytic Activity
SntA inhibited complement pathway, in which pathway SntA acted remain unclear. Hemolytic activity assay is often used to assess the activity of the classical pathway of complement activation with sheep erythrocytes (SRBCs) and of alternative pathway with rabbit erythrocytes (RRBCs) (33). So, to test in which pathway SntA inhibited complement, the hemolytic activity assays were performed. Results showed that SntA remarkably inhibited the hemolytic activity of SRBCs in a dose-dependent manner mediated by the classical pathway in the presence of NHS (Figure 7A). No inhibitory effect was observed in the negative control of BSA. However, SntA did not inhibit the hemolytic activity of RRBCs mediated by the alternative pathway (Figure 7B). These data revealed that SntA could inhibit the hemolytic activity mediated by the complement classical pathway.
Figure 7.
SntA inhibits hemolytic activity. (A) To measure the inhibitory effect of SntA on the classical pathway, antibody-coated sheep erythrocytes were subjected to complement attack from normal human serum (NHS) in the presence of the increasing concentrations of SntA. BSA was used as a negative control. The degree of lysis was estimated by measurement of the release of hemoglobin. (B) To measure the inhibitory effect of SntA on the alternative pathway, rabbit erythrocytes were subjected to complement attack from NHS in the presence of the increasing concentrations of SntA. BSA was used as a negative control. Cell lysis was measured like (A). The absorbance without inhibitor was set to 100%. Results were expressed from three independent experiments performed in triplicate. The statistical significance was calculated by two-way ANOVA of Sidak’s correct for multiple comparison and shown by asterisks (****p < 0.0001; **p < 0.01; ns, p > 0.05).
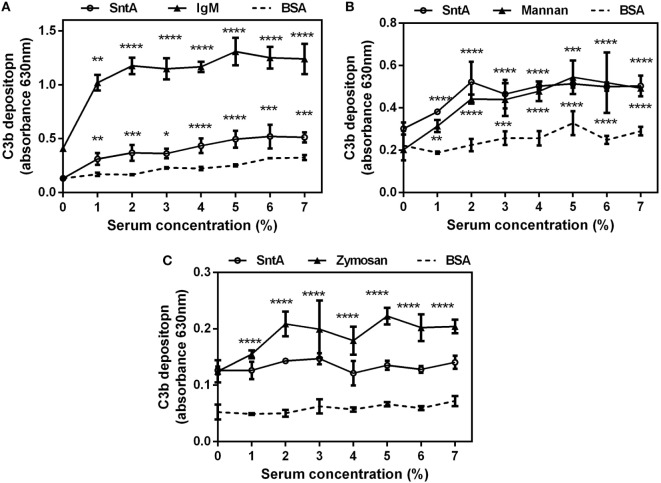
SntA Directly Activates Complement Pathways
To test whether SntA could activate complement directly, the complement activation assays were performed. In the classical pathway, the increasing concentrations of 0–7% NHS were applied to the SntA pre-coated plates to detect C3b deposition with specific C3b polyclonal antibody. IgM acted as a positive control and BSA as a negative control. Significant activation was observed on the SntA pre-coated plates and not on the BSA pre-coated plates (Figure 8A). This showed that SntA could activate complement through the classical pathway, although the activation level was lower than the positive control. In the lectin pathway, microtiter plates pre-coated with SntA and positive control Mannan exhibited strong complement activation mediated by the lectin pathway when human serum depleted of C1q was used (Figure 8B). Result demonstrated that SntA could activate the lectin pathway directly. In the alternative pathway, NHS in Mg2+-EGTA-DGHB buffer was used to block classical activation pathway and detect complement activation mediated by the alternative pathway. Result showed that no significant activation was observed in the plates pre-coated with SntA, while the positive control Zymosan obviously activated the alternative pathway (Figure 8C). Thus, SntA could directly activate the complement by the classical and lectin pathway, not the alternative pathway.
Figure 8.
SntA can directly activate the classical and lectin complement pathways. (A) Normal human serum (NHS) in DGHB++ buffer was applied to the plates pre-coated with SntA, IgM was acted as a positive control and BSA as a negative control to detect C3b deposition. (B) Human serum depleted of C1q in DGHB++ buffer was applied to the plates pre-coated with SntA, Mannan as a positive control to detect C3b deposition. (C) NHS in Mg2+-EGTA-DGHB buffer was applied to the plates pre-coated with SntA, Zymosan as a positive control to detect C3b deposition. Results were expressed from three independent experiments performed in triplicate. The statistical significance was calculated by two-way ANOVA of Sidak’s correct for multiple comparison and shown by asterisks (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).
Discussion
Streptococcal toxic shock like syndrome, meningitis, and septicemia are the most important characteristics of the recently emerging S. suis infection, especially in Southeast Asia. During infection, the pathogen must compete with the normal microflora, resist defense mechanisms of the local mucosal immunity, adhere and invade the mucosal epithelial cell barrier, subsequently reach and survive in the blood stream, and finally invade multiple organs including spleen, liver, kidney, and lung, even entry BBB (36). A plenty of factors have been demonstrated to be associated with these processes [reviewed in Ref. (37)]. However, how S. suis escape the immune surveillance to invade into blood and most organs is largely unknown. In this study, we found that the surface heme-binding protein SntA of S. suis (24) contributes to the resistance of C3 deposition and MAC formation on bacterial surface, the abilities of anti-phagocytosis, survival in blood, and in vivo colonization. SntA can interact with C1q, and inhibit the hemolytic activity via the classical pathway. SntA could also directly activate classical and lectin pathways, resulting in complement consumption. These two complement evasion strategies may be crucial for the pathogenesis of the zoonotic pathogen S. suis.
In our previous study, SntA is identified as a heme-binding protein which can interact with the host antioxidant protein AOP2 and inhibit its antioxidant activity, and thus contribute to the survival and pathogenesis of S. suis in pigs (24). This protein is a bifunctional 2′,3′-cyclic nucleotide 2′-phosphodiesterase/3′-nucleotidase, which is distributed in many species of Gram-positive bacteria (24). In the present study, mouse infection model is used to further study the function of SntA involving in the pathogenesis. Results show that SntA facilitates in S. suis survival in blood (Figure 1B) and subsequent colonization in some specific organs (Figures 1C,D). To entry the bloodstream and spread to distant organs, the Gram-positive pathogens usually utilize opsonophagocytic clearance function of neutrophils and macrophages (38). SntA is demonstrated to contribute to the anti-phagocytosis of S. suis mediated by neutrophils and macrophages and the survival in whole blood and serum in vitro (Figure 2). The efficient phagocytosis is an important innate immune mechanism for controlling infection of extracellular bacteria such as Streptococci, and this process requires serum with intact complement activity (39). Recently, many complement inhibitors target at complements such as FH (29), C1q (23, 40), C3 (41), C4BP (42), mediate complement evasion, and subsequently contribute to pathogenesis in Gram-positive bacterium. In order to investigate whether SntA involves in the complement evasion of S. suis, a series of experimentations are carried out to this end.
First, the complement deposition assays are performed. The C3 deposition and MAC formation on the surface of the mutant strain ΔsntA are significantly higher than those on the parental strain SC-19 and complementary strain CΔsntA (Figure 3). This indicates that SntA involved in complement activation by S. suis. The undiluted and 1:500 diluted human serum (NHS) are used in the complement deposition assays, respectively, C3 deposition and MAC formation on the surface of ΔsntA are always significantly higher than those on SC-19 and CΔsntA (Figure 3). This suggests that SntA could inhibit both classical pathway and alternative pathway because it has been documented that the classical pathway is activated by using diluted NHS (35), whereas alternative pathway is probably activated by using undiluted NHS in the complement activation assays (30).
Hemolytic assay is often used to assess the activation of the classical pathway by using sheep erythrocytes and the activation of alternative pathway by using rabbit erythrocytes (33). To confirm which complement pathway is inhibited by SntA, hemolytic assays have been performed using recombinant SntA protein. The results show that SntA could inhibit the hemolytic activity of SRBCs, but not of rabbit erythrocytes (Figure 7). This indicates that SntA could only inhibit the classical pathway. Furthermore, SntA is confirmed as a cell wall anchored protein that could interact with C1q in a dose-dependent manner (Figures 4 and 5). ELISA experiments also suggest that SntA could interact with the three C1q subunits C1qA, C1qB, and C1qC, respectively (Figure 5). It is very interesting whether this interaction results in the complement evasion. To this end, the competitive binding assays have been done by ELISAs reported previously (35). The results show that SntA could competitively bind C1q with IgG and Ag–Ab complex (Figure 6). Thus, SntA can interact with C1q and inhibit the hemolytic activity mediated by the classical pathway, consequently contribute to complement evasion. Similar inhibitory mechanism has been identified in other Gram-positive strain. Streptococcus pneumococcal (33) and S. aureus (23) infection. These bacterial proteins interact with C1q and specially inhibit the classical complement pathway, consequently facilitate pneumococcal complement escape (23, 33).
Concerning that C1q is a complex of 18 polypeptide chains consisting of six C1qA, C1qB, and C1qC subunits, the binding assays using each subunit could not properly reveal the real interactions between SntA and C1q although it has been reported that the globular heads of the recombinant subunits can assemble to form the high molecular weight oligomers (43). Interestingly, heme has also been demonstrated to inhibit the classical pathway through binding with C1q (35), and SntA is a heme-containing protein (24), it warrants further investigation whether the heme in SntA or SntA as a hemeprotein involves in the complement evasion. To this end, we are trying to obtain the recombinant SntA without heme and holo-form SntA although this is not an easy thing.
To look at whether SntA can directly activate complement pathways, the recombinant SntA protein is subjected to activate the complement C3. The results show that SntA could directly activate both classical and lectin pathways but not alternative pathway (Figure 8). This indicates that SntA could mediate complement evasion through complement consumption as well (33). In S. pneumococcal, PepO can activate complement through the classical and alternative pathway (33), while anther complement inhibitor phosphoglycerate kinase cannot activate complement (44). Research about complement evasion mediated by complement consumption is not much. The mechanism of this process warrants further study.
In conclusion, S. suis cell wall anchored heme-binding protein SntA mediates complement evasion by inhibition of complement activation and complement consumption. The inhibition of complement activation may be via the SntA–C1q interaction. These two complement evasion strategies may be crucial for the pathogenesis of S. suis.
Ethics Statement
Animal experiments were approved by the Laboratory Animal Monitoring Committee of Huazhong Agricultural University and performed strictly according to the recommendations in the Guide for the Care and Use of Laboratory Animals of Hubei Province, China. Venous blood samples were provided by healthy donors and collected in accordance with the approved guidelines. Approval was obtained from the Institutional Medical Ethics Committee of Huazhong Agricultural University and the healthy donors provided written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
The experiments were performed mainly by SD, some experiment material and data were provided by TX and LY, and some experiments were performed with the assistance of TX, QF, JZ, LC, and JL. SD analyzed the data. The study was conceived and designed by SD and RZ. SD and RZ wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Qi Huang from University of Dundee in United Kingdom for revising the grammar in this manuscript and Dr. Takamatsu for providing the S. suis- E. coli shuttle cloning vector pSET2.
Footnotes
Funding. This work was supported by the Natural Science Foundation of China (NSFC; 31472202), the National Key R&D Program of China (2017YFD0500201), and Hubei Province Natural Science Foundation for Innovative Research Groups (2016CFA015).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01063/full#supplementary-material.
Identification of Streptococcus suis strains SC-19, ΔsntA, and CΔsntA. The sntA gene and its flanked genes (B9H01_10065 and B9H01_10075) were amplified by genome PCR (A) and reverse transcription-PCR (B) using primers SntA_F/R, 10065_F/R, and 10075_F/R, respectively. WT, wild-type strain SC-19; MT, sntA gene mutant strain ΔsntA; CM, complementary strain CΔsntA.
The growth abilities of Streptococcus suis strains SC-19, ΔsntA, and CΔsntA. (A) Bacterial cell density was measured by spectrophotometer at 600 nm. (B) Bacterial colony forming unit count. Results were expressed from three independent experiments performed in triplicate.
SntA possess anti-phagocytic activity. (A) Survival rate of Streptococcus suis in mouse blood. (B) Survival rate of S. suis in mouse active and inactivated serum. Results were expressed from three independent experiments performed in triplicate. The statistical significance was showed by asterisks (unpaired t test; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).
References
- 1.Feng Y, Zhang H, Ma Y, Gao GF. Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent. Trends Microbiol (2010) 18(3):124–31. 10.1016/j.tim.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Li W, Liu L, Qiu D, Chen H, Zhou R. Identification of Streptococcus suis serotype 2 genes preferentially expressed in the natural host. Int J Med Microbiol (2010) 300(7):482–8. 10.1016/j.ijmm.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 3.Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med (2006) 3(5):e151. 10.1371/journal.pmed.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol (2008) 6(2):132–42. 10.1038/nrmicro1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol (2010) 5(3):371–91. 10.2217/fmb.10.2 [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Pian Y, Li X, Liu R, Xie W, Zhang C, et al. Streptococcus suis adenosine synthase functions as an effector in evasion of PMN-mediated innate immunit. J Infect Dis (2014) 210(1):35–45. 10.1093/infdis/jiu050 [DOI] [PubMed] [Google Scholar]
- 7.Doran KS, Fulde M, Gratz N, Kim BJ, Nau R, Prasadarao N, et al. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol (2016) 131(2):185–209. 10.1007/s00401-015-1531-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghebrehiwet B. The complement system: an evolution in progress. F1000Res (2016) 5:2840. 10.12688/f1000research.10065.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laarman A, Milder F, van Strijp J, Rooijakkers S. Complement inhibition by Gram-positive pathogens: molecular mechanisms and therapeutic implications. J Mol Med (Berl) (2010) 88(2):115–20. 10.1007/s00109-009-0572-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojouharova M, Reid K, Gadjeva M. New insights into the molecular mechanisms of classical complement activation. Mol Immunol (2010) 47(13):2154–60. 10.1016/j.molimm.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Stemmer F, Loos M. Evidence for direct binding of the first component of complement, C1, to outer membrane proteins from Salmonella minnesota. Curr Top Microbiol Immunol (1985) 121:73–84. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal V, Ahl J, Riesbeck K, Blom AM. An alternative role of C1q in bacterial infections: facilitating Streptococcus pneumoniae adherence and invasion of host cells. J Immunol (2013) 191(8):4235–45. 10.4049/jimmunol.1300279 [DOI] [PubMed] [Google Scholar]
- 13.Aubert B, Chesne S, Arlaud GJ, Colomb MG. Antibody-independent interaction between the first component of human complement, C1, and the outer membrane of Escherichia coli D31 m4. Biochem J (1985) 232(2):513–9. 10.1042/bj2320513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenner AJ, Ziccardi RJ, Cooper NR. Antibody-independent C1 activation by E. coli. J Immunol (1984) 133(2):886–91. [PubMed] [Google Scholar]
- 15.Alberti S, Marques G, Camprubi S, Merino S, Tomas JM, Vivanco F, et al. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun (1993) 61(3):852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mintz CS. Gene transfer in Legionella pneumophila. Microbes Infect (1999) 1(14):1203–9. 10.1016/S1286-4579(99)00241-5 [DOI] [PubMed] [Google Scholar]
- 17.Xue Q, Gu C, Rivera J, Hook M, Chen X, Pozzi A, et al. Entry of Bacillus anthracis spores into epithelial cells is mediated by the spore surface protein BclA, integrin alpha2beta1 and complement component C1q. Cell Microbiol (2011) 13(4):620–34. 10.1111/j.1462-5822.2010.01558.x [DOI] [PubMed] [Google Scholar]
- 18.Butko P, Nicholson-Weller A, Wessels MR. Role of complement component C1q in the IgG-independent opsonophagocytosis of group B Streptococcus. J Immunol (1999) 163(5):2761–8. [PubMed] [Google Scholar]
- 19.Eads ME, Levy NJ, Kasper DL, Baker CJ, Nicholson-Weller A. Antibody-independent activation of C1 by type Ia group B streptococci. J Infect Dis (1982) 146(5):665–72. 10.1093/infdis/146.5.665 [DOI] [PubMed] [Google Scholar]
- 20.Koroleva IV, Sjoholm AG, Schalen C. Binding of complement subcomponent C1q to Streptococcus pyogenes: evidence for interactions with the M5 and FcRA76 proteins. FEMS Immunol Med Microbiol (1998) 20(1):11–20. 10.1111/j.1574-695X.1998.tb01106.x [DOI] [PubMed] [Google Scholar]
- 21.Agarwal V, Kuchipudi A, Fulde M, Riesbeck K, Bergmann S, Blom AM. Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J Biol Chem (2013) 288(10):6849–63. 10.1074/jbc.M112.405530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal V, Blom AM. Roles of complement C1q in pneumococcus-host interactions. Crit Rev Immunol (2015) 35(3):173–84. 10.1615/CritRevImmunol.2015012177 [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Han D, Liu C, Gao Y, Li D, Liu Y, et al. Staphylococcus aureus VraX specifically inhibits the classical pathway of complement by binding to C1q. Mol Immunol (2017) 88:38–44. 10.1016/j.molimm.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 24.Wan Y, Zhang S, Li L, Chen H, Zhou R. Characterization of a novel streptococcal heme-binding protein SntA and its interaction with host antioxidant protein AOP2. Microb Pathog (2017) 111:145–55. 10.1016/j.micpath.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 25.Li W, Liu L, Chen H, Zhou R. Identification of Streptococcus suis genes preferentially expressed under iron starvation by selective capture of transcribed sequences. FEMS Microbiol Lett (2009) 292(1):123–33. 10.1111/j.1574-6968.2008.01476.x [DOI] [PubMed] [Google Scholar]
- 26.Butt TR, Edavettal SC, Hall JP, Mattern MR. SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif (2005) 43(1):1–9. 10.1016/j.pep.2005.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamatsu D, Osaki M, Sekizaki T. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid (2001) 45(2):101–13. 10.1006/plas.2000.1510 [DOI] [PubMed] [Google Scholar]
- 28.de Greeff A. Contribution of fibronectin-binding protein to pathogenesis of Streptococcus suis serotype 2. Infect Immun (2002) 70(3):1319–25. 10.1128/IAI.70.3.1319-1325.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pian Y, Gan S, Wang S, Guo J, Wang P, Zheng Y, et al. Fhb, a novel factor H-binding surface protein, contributes to the antiphagocytic ability and virulence of Streptococcus suis. Infect Immun (2012) 80(7):2402–13. 10.1128/IAI.06294-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Wan Y, Tao Z, Chen H, Zhou R. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet Microbiol (2013) 162(1):186–94. 10.1016/j.vetmic.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Carlsson F, Berggard K, Stalhammar-Carlemalm M, Lindahl G. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J Exp Med (2003) 198(7):1057–68. 10.1084/jem.20030543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firon A, Dinis M, Raynal B, Poyart C, Trieu-Cuot P, Kaminski PA. Extracellular nucleotide catabolism by the Group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood. J Biol Chem (2014) 289(9):5479–89. 10.1074/jbc.M113.545632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal V, Sroka M, Fulde M, Bergmann S, Riesbeck K, Blom AM. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J Biol Chem (2014) 289(22):15833–44. 10.1074/jbc.M113.530212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansari AA, St. Clair JB, Detanico T, Aviszus K, Kirchenbaum GA, Christie M, et al. Immunogenicity of isogenic IgG in aggregates and immune complexes. PLoS One (2017) 12(1):e0170556. 10.1371/journal.pone.0170556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roumenina LT, Radanova M, Atanasov BP, Popov KT, Kaveri SV, Lacroix-Desmazes S, et al. Heme interacts with c1q and inhibits the classical complement pathway. J Biol Chem (2011) 286(18):16459–69. 10.1074/jbc.M110.206136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segura M, Calzas C, Grenier D, Gottschalk M. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: fighting against nonspecific defenses. FEBS Lett (2016) 590(21):3772–99. 10.1002/1873-3468.12364 [DOI] [PubMed] [Google Scholar]
- 37.Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol (2012) 7(2):259–79. 10.2217/fmb.11.149 [DOI] [PubMed] [Google Scholar]
- 38.Okumura CY, Nizet V. Subterfuge and sabotage: evasion of host innate defenses by invasive gram-positive bacterial pathogens. Annu Rev Microbiol (2014) 68:439–58. 10.1146/annurev-micro-092412-155711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuste J, Sen A, Truedsson L, Jonsson G, Tay LS, Hyams C, et al. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect Immun (2008) 76(8):3761–70. 10.1128/IAI.00291-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honda-Ogawa M, Sumitomo T, Mori Y, Hamd DT, Ogawa T, Yamaguchi M, et al. Streptococcus pyogenes endopeptidase O contributes to evasion from complement-mediated bacteriolysis via binding to human complement factor C1q. J Biol Chem (2017) 292(10):4244–54. 10.1074/jbc.M116.749275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynskey NN, Reglinski M, Calay D, Siggins MK, Mason JC, Botto M, et al. Multi-functional mechanisms of immune evasion by the streptococcal complement inhibitor C5a peptidase. PLoS Pathog (2017) 13(8):e1006493. 10.1371/journal.ppat.1006493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ermert D, Weckel A, Agarwal V, Frick IM, Bjorck L, Blom AM. Binding of complement inhibitor C4b-binding protein to a highly virulent Streptococcus pyogenes M1 strain is mediated by protein H and enhances adhesion to and invasion of endothelial cells. J Biol Chem (2013) 288(45):32172–83. 10.1074/jbc.M113.502955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Z, Li M, Ma J, Zhang S. An amphioxus gC1q protein binds human IgG and initiates the classical pathway: implications for a C1q-mediated complement system in the basal chordate. Eur J Immunol (2014) 44(12):3680–95. 10.1002/eji.201444734 [DOI] [PubMed] [Google Scholar]
- 44.Blom AM, Bergmann S, Fulde M, Riesbeck K, Agarwal V. Streptococcus pneumoniae phosphoglycerate kinase is a novel complement inhibitor affecting the membrane attack complex formation. J Biol Chem (2014) 289(47):32499–511. 10.1074/jbc.M114.610212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of Streptococcus suis strains SC-19, ΔsntA, and CΔsntA. The sntA gene and its flanked genes (B9H01_10065 and B9H01_10075) were amplified by genome PCR (A) and reverse transcription-PCR (B) using primers SntA_F/R, 10065_F/R, and 10075_F/R, respectively. WT, wild-type strain SC-19; MT, sntA gene mutant strain ΔsntA; CM, complementary strain CΔsntA.
The growth abilities of Streptococcus suis strains SC-19, ΔsntA, and CΔsntA. (A) Bacterial cell density was measured by spectrophotometer at 600 nm. (B) Bacterial colony forming unit count. Results were expressed from three independent experiments performed in triplicate.
SntA possess anti-phagocytic activity. (A) Survival rate of Streptococcus suis in mouse blood. (B) Survival rate of S. suis in mouse active and inactivated serum. Results were expressed from three independent experiments performed in triplicate. The statistical significance was showed by asterisks (unpaired t test; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05).