Abstract
Background
Presence of dementia is a contraindication for DBS treatment of Parkinson’s disease. Recent evidence suggests that borderline cognitive function, as measured with a common screening measure, the Mattis Dementia Rating Scale, has a negative impact on quality of life (QoL) after DBS of the STN.
Methods
We attempted to replicate and extend this finding in a larger group of patients with a wider range of preoperative global cognitive performance.
Results
Our data indicate that performance on the screening measure is not associated with QoL or medical outcomes, even with scores well below the cutoff for identifying dementia.
Conclusions
This cognitive screening measure lacks sufficient sensitivity to warrant its use in predicting which patients will show QoL benefit from DBS.
Keywords: Parkinson’s disease, deep brain stimulation, outcome research, quality of life, cognition
Substantial effort has gone into studying the factors that affect motor outcome in patients with Parkinson’s disease (PD) who undergo DBS of the STN (STN DBS). There is also growing interest in determining the factors that predict or accompany quality of life (QoL) changes after this procedure. However, there are little data regarding the role of cognitive impairments in QoL outcome. A recent study1 looked at QoL outcomes as a function of preoperative performance on a cognitive screening measure, the Mattis Dementia Rating Scale (DRS).2 The study showed that patients with the poorest performance (bottom quartile) in their sample—still above the recommended cut-off for dementia in PD—failed to show significant improvements in QoL post–STN DBS. Our clinical experience suggests that the DRS lacks sufficient sensitivity and specificity to the cognitive impairments most relevant to DBS QoL outcome.3 As such, we attempted to replicate this finding in a larger DBS sample with similar or poorer preoperative DRS scores. Unlike the previous study,1 we hypothesized that baseline DRS score would not be related to QoL outcome post-DBS.
Patients and Methods
The study was approved by the Cleveland Clinic Institutional Review Board (IRB; Cleveland, OH).
Patients
We retrospectively identified patients from an IRB-approved data registry of all patients diagnosed with idiopathic PD observed for DBS evaluations at Cleveland Clinic. Patients who had undergone previous neurosurgery were excluded. One hundred six patients (79 males) who had undergone STN DBS placement between 2006 and 2013 had completed the DRS-2nd Edition (DRS-2) and QoL measures (Parkinson Disease Questionnaire-39; PDQ-39)4 both before and 6 months after surgery. Our decision-making procedure for DBS candidacy and surgical plan has been described elsewhere.3– 5 Forty-four patients underwent bilateral STN-DBS implantations and 62 patients had unilateral procedures (20 right [7 left-handed]; 42 left [38 right-handed]).
DRS-2 and PDQ-39
The DRS-2 is a standardized cognitive screening tool that yields a maximum total score of 144. The interpretative manual recommends a cut-off score of less than 123 for diagnosis of dementia; however, lower cutoffs (i.e., 116–123 points) have been recommended for use in individuals with PD to correct for the negative impact of motor impairment on test performance.6–8 Patients completed the DRS-2 during both pre- and 6-month postoperative neuropsychological evaluations. Patients were grouped into one of five categories based on preoperative DRS-2 performance. The first four groups were formed using the same criteria as Witt et al.1 in order to directly compare our results with theirs. We created a fifth group of patients who scored lower than the range included in that study and with greater likelihood of clinically significant cognitive impairment. Raw scores permitted inclusion of patients below the normative age range. DRS score ranges for each group are provided in Table 1.
TABLE 1.
Baseline characteristics and change scores post–STN DBS
| Overall (n = 106)
|
Group 1 (n =4) 144–143 |
Group 2 (n = 21) 142–141 |
Group 3 (n =17) 140–138 |
Group 4 (n = 40) 137–130 |
Group 5 (n = 24) 129–104 |
Group
|
Tukey’s HSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F value | P value | ||
| Preoperative sample characteristics | |||||||||||||||
| Age, years | 62.4 | 7.9 | 66.0 | 8.1 | 57.8 | 7.2 | 61.8 | 6.4 | 62.4 | 8.0 | 66.0 | 7.8 | 3.59 | 0.01 | 2<5 |
| Education, years | 14.1 | 2.9 | 16.0 | 3.7 | 15.8 | 2.3 | 14.3 | 2.8 | 13.9 | 2.6 | 12.6 | 3.2 | 4.19 | 0.01 | 2>5 |
| LEDD | 958.5 | 517.7 | 989.6 | 490.6 | 971.5 | 430.7 | 1,143.8 | 713.1 | 889.8 | 490.8 | 925.3 | 483.1 | 0.75 | 0.56 | |
| PD duration | 10.6 | 5.1 | 8.8 | 5.7 | 11.3 | 4.7 | 8.9 | 4.2 | 10.9 | 5.2 | 10.6 | 6.1 | 0.70 | 0.60 | |
| UPDRS-III off medsa | 39.3 | 10.3 | 31.0 | 4.1 | 36.9 | 10.5 | 37.0 | 10.6 | 42.4 | 11.0 | 38.3 | 7.6 | 2.10 | 0.09 | |
| UPDRS-III on medsa | 20.8 | 8.9 | 12.3 | 6.5 | 17.5 | 7.1 | 19.1 | 9.3 | 22.4 | 8.4 | 23.0 | 8.6 | 2.80 | 0.03 | ns |
| PDQ-39 Summary Index | 28.8 | 14.2 | 17.3 | 17.9 | 27.0 | 11.2 | 31.1 | 15.9 | 31.0 | 13.5 | 26.8 | 15.6 | 1.20 | 0.31 | |
| Normal/MCI-SD/MCI-MD | 18/33/55 | 0/1/3 | 7/8/6 | 3/3/11 | 5/12/23 | 3/9/12 | |||||||||
| WASI, FSIQa | 101.8 | 14.4 | 123.3 | 9.2 | 112.8 | 11.0 | 105.8 | 11.0 | 98.8 | 11.8 | 91.2 | 12.8 | 13.78 | 0.00 | 1,2>4,5;3>5 |
| BNT | 11.0 | 9.2 | 11.8 | 1.9 | 11.4 | 2.7 | 11.1 | 3.2 | 12.6 | 14.4 | 7.8 | 3.9 | 1.01 | 0.41 | |
| COWAT | 9.7 | 2.9 | 12.5 | 1.3 | 11.2 | 2.1 | 10.6 | 2.4 | 9.7 | 2.5 | 7.1 | 3.0 | 9.61 | 0.00 | 1,2,3,4>5 |
| Category | 9.2 | 3.0 | 10.8 | 1.0 | 10.5 | 3.1 | 10.2 | 2.3 | 9.2 | 2.8 | 7.1 | 2.9 | 5.10 | 0.00 | 2,3,4>5 |
| JOLO | 10.1 | 3.5 | 13.8 | 1.5 | 12.2 | 2.5 | 10.3 | 3.3 | 9.7 | 3.2 | 8.0 | 3.6 | 6.38 | 0.00 | 1>5; 2>4,5 |
| Oral SDMTa | 87.3 | 13.7 | 90.4 | 11.2 | 96.1 | 11.2 | 94.9 | 11.5 | 84.6 | 12.0 | 77.7 | 13.5 | 8.25 | 0.00 | 2,3>4,5 |
| Digit Span | 9.8 | 3.2 | 13.5 | 2.4 | 11.1 | 2.7 | 10.6 | 3.0 | 9.0 | 2.1 | 8.8 | 4.4 | 4.02 | 0.01 | 1>4,5 |
| WCST Persev Errorsa | 89.9 | 14.0 | 96.0 | 20.8 | 94.4 | 10.4 | 90.4 | 16.1 | 88.2 | 12.3 | 87.2 | 16.3 | 1.11 | 0.36 | |
| RAVLT Trial 1 | 8.5 | 2.9 | 9.0 | 1.4 | 8.1 | 3.5 | 8.8 | 2.7 | 8.7 | 2.5 | 7.9 | 3.4 | 0.40 | 0.81 | |
| WMS-3, LM II | 9.7 | 2.7 | 11.8 | 1.5 | 10.5 | 2.5 | 10.0 | 3.3 | 9.5 | 2.6 | 8.7 | 2.6 | 1.88 | 0.12 | |
| BDI-IIb | 10.4 | 6.7 | 6.0 | 6.7 | 9.6 | 6.6 | 10.5 | 6.1 | 11.0 | 6.5 | 11.0 | 7.7 | 0.62 | 0.65 | |
| Change scores | |||||||||||||||
| LEDD | 305.1b | 544.6 | 491.5 | 364.6 | 332.8 | 543.9 | 305.6 | 725.9 | 229.6 | 507.9 | 321.2 | 487.5 | 0.44 | 0.78 | |
| UPDRS-III off medsa | 18.5b | 11.4 | 10.5 | 7.8 | 16.9 | 12.5 | 17.0 | 12.9 | 19.0 | 12.8 | 18.9 | 8.3 | 0.53 | 0.72 | |
| UPDRS-III on medsa | 4.1b | 8.3 | −4.3 | 9.1 | 3.9 | 7.1 | 3.7 | 7.6 | 5.3 | 10.1 | 5.4 | 7.7 | 1.04 | 0.39 | |
| DRSa | 0.3 | 6 | 4.8 | 2.9 | 2.9 | 4.4 | 1.0 | 3.5 | −0.1 | 6.0 | −2.7 | 7.3 | 3.3 | 0.01 | 1,2,3<4<5 |
| BDI-II | 1.3 | 6.4 | 2.5 | 5.8 | 0.6 | 7.2 | 1.3 | 6.5 | 1.6 | 5.9 | 0.2 | 7.4 | 0.25 | 0.92 | |
| PDQ-39 | 9.1b | 11.5 | 12.6 | 15.6 | 5.9 | 12.3 | 12.3 | 15.1 | 9.9 | 10.4 | 7.9 | 8.9 | 0.94 | 0.44 | |
| Unilateral/bilateral DBS | 62/44 | 3/1 | 11/10 | 8/9 | 26/14 | 14/10 | |||||||||
| Improve/stable/decline | 48/54/4 | 2/2/0 | 8/11/2 | 7/10/0 | 21/18/1 | 10/13/1 | |||||||||
All neuropsychological data presented as scaled scores, except where designated by (a) =standard score or (b) =raw score.
Group 4: n =37; group 5: n =23.
Significant change pre-post: P <0.05.
LEDD, levodopa equivalent daily dose; BNT, Boston Naming Test9; COWAT, Controlled Oral Word Association Test10; Category, Category fluency; JOLO, Judgment of Line Orientation11; Oral SDMT, Oral Symbol Digit Modalities Test12; Digit Span, Wechsler Memory Scale-III13; WCSTPE, Wisconsin Card Sorting Test14 Perseverative Errors; RAVLT trial 1, Rey Auditory Verbal Learning Test15 Trial 1; WMS LM2, Wechsler Memory Scale-III Logical Memory II13; MCI-SD, Mild Cognitive Impairment-Single Domain; MCI-MD, Mild Cognitive Impairment-Multiple Domain; SD, standard deviation; ns, not significant.
QoL was assessed using the PDQ-394 during preand postoperative neuropsychological evaluations. This measure assesses the degree to which patients experience negative effects of PD in eight domains.
Neuropsychological Evaluation
Neuropsychological variables for the sample are summarized in Table 1. We selected measures of language, visuospatial function, processing speed, attention span, executive function, single-trial learning, and delayed memory based on sensitivity to PD, frequency of reporting in the STN-DBS literature, and to minimize colinearity and the number of statistical comparisons. Depression symptoms were assessed using the Beck Depression Inventory-2nd Edition (BDI-II).9
Motor Function
All patients underwent evaluation by a movement disorders neurologist to confirm diagnosis, gauge surgical appropriateness, establish levodopa daily dosage, and complete the UPDRS-III in the off and on medication states.
Statistical Analysis
Preoperative characteristics of the five DRS-based groups were examined using one-way analysis of variance (ANOVA). Post-hoc comparisons (Tukey’s honestly significant difference [HSD]) were employed where omnibus testing indicated significant group differences. Repeated-measures ANOVAs examined preto postoperative changes in PDQ-39 and other clinical measurements across the five DRS groups. Paired-samples t tests with Bonferroni’s correction for multiple comparisons were used to identify significant changes in PDQ-39 subdomains. The five groups were combined and Pearson’s correlations evaluated the relationship between preoperative DRS-2 scores, preoperative QoL, and clinical change scores post–STN DBS. To examine individual differences in QoL outcome, each patient was also classified as reporting improved, worsened, or stable Qol using published PD-specific Reliable Change Indices (RCIs),10 and Phi statistic examined group difference in individual outcomes.
Results
Preoperative Characteristics
Table 1 shows the overall characteristics of the sample and baseline clinical and cognitive characteristics for patients in the five DRS-2 groups. Using International Parkinson and Movement Disorder Society Task Force Diagnostic Criteria,11 55 patients (51.9%) had Mild Cognitive Impairment (MCI)-Multiple Domain, 33 (31.1%) had MCI-Single Domain, and 18 (17%) did not show clear evidence of change from their presumed cognitive baseline. Note that the latter group is labeled “normal” despite the fact that long-standing cognition may actually fall in the impaired range. There was no significant difference between DRS-based groups in the number of patients who underwent unilateral or bilateral procedures (χ2=2.3; P=0.7) or in the proportion of patients meeting different MCI criteria (Φ=0.31; P=0.28). One-way ANOVAs indicated significant group differences in age, UPDRS-III on medications score, and all age-corrected cognitive variables, except naming, perseverative errors, single-trial learning, and delayed memory. With the exception of the Oral Symbol Digit Modalities processing speed test, the average scaled/standard scores on all neuropsychological measures for group 5 were within broad normal limits. Likewise, most patients endorsed minimal-to-mild depression. Two patients with severe BDI-II scores were included in group 4, whereas 11 with moderate BDI-II scores were included in groups 2 to 5.
Group Differences in STN-DBS Outcomes
Table 1 displays mean pre- to postoperative change scores on clinical measures for each DRS-2 group. Overall, QoL ratings improved postsurgery (F(1,101)=41.8; P<0.001), but the magnitude of change did not significantly differ across the DRS-based groups. A similar main effect of surgery, but no effect of DRS group, was also observed for medication reductions (F(1,101)=22.3; P<0.001) and improvements in UPDRS-III scores both on medication (F(1,82)=5.7; P=0.02) and off medication (ON stimulation; F(1,73)>102.4; P<0.001). Independent t test indicated that there was no difference in QoL change between patients who underwent uni- versus bilateral procedures (t(104)=−0.36; P>0.7).
There was no overall effect of surgery on DRS-2 scores, although there was an interaction between time (pre-post) and group (F(1,101)=3.3; P=0.01). Tukey’s HSD comparisons indicated that groups 1, 2, and 3 showed similar declines in DRS-2 scores postsurgery (likely reflecting a ceiling effect, such that higher scores are more able to decrease), whereas improvements in groups 4 and 5 were significantly different from all other groups, including one another (likely reflecting some floor effect/regression to the mean, such that lower scores are more likely to increase). There was no effect of surgery or group on depression scores.
Correlations Between Preoperative DRS-2 and STN-DBS Outcomes
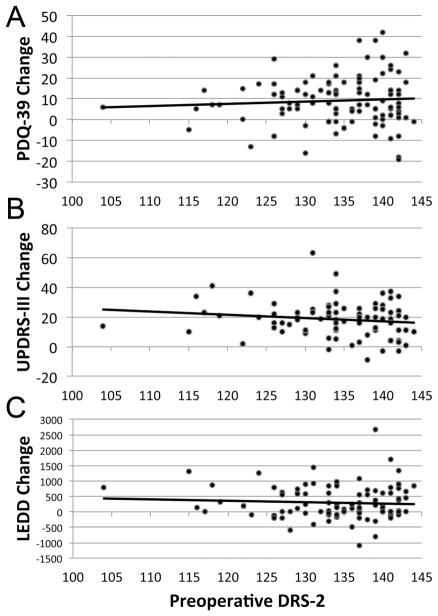
Figure 1 depicts the relationships between baseline DRS-2 score and change scores on outcome measures. There were no significant relationships between preoperative DRS-2 score and QoL change postsurgery (Fig. 1A), improvements in off medication motor scores (off med vs. off-med/ON-stim; Fig. 1B), improvements in on medication motor scores (on med vs. on-med/ON-stim), or reduction in dopaminergic dosage (Fig. 1C). Moreover, preoperative DRS-2 score was not associated with preoperative QoL ratings.
FIG. 1.
Nonsignificant correlations between preoperative DRS-2 score and outcome measures. (A) PDQ-39 change. Positive scores represent improved QoL. (B) UPDRS-III change (off medication minus off medication/ON stimulation). Positive scores represent improved motor function. (C) LEDD change. Positive scores represent postsurgical reductions in medication. LEDD, levodopa equivalent daily dose.
Individual Difference Analysis
Similar to previous work,10 we found that 45% of our sample reported improvements in QoL on the PDQ-39 postsurgery based on RCIs. Approximately 51% of patients reported stable QoL, whereas 4% reported worsening of QoL. Phi statistic indicated no group differences in the proportion of patients reporting improved, stable, or worsened QoL across DRS-2 categories (Φ=0.20; P=0.84; see Table 1). Of the 13 patients who reported moderate-to-severe preoperative depression, 7 reported improved QoL and 6 reported stable QoL.
Discussion
In our sample, QoL and UPDRS-III motor scores improved and medications were reduced postSTN DBS. Preoperative DRS-2 scores, however, were not related to postoperative QoL, motor, or medication outcome. Our efforts to replicate and expand on an earlier finding 1 demonstrate that there were no differences in QoL changes in patients grouped according to DRS-2 performance. Moreover, similar proportions of patients within each DRS-based group showed improved or stable PDQ-39 scores. These data argue against the use of the DRS-2 as an indicator of which patients are likely to benefit from surgery. Rather, our earlier work suggests that a combination of particularly sensitive variables are helpful for predicting QoL post-DBS, including Rey Auditory Verbal Learning Test (RAVLT) T1, BDI-II, and QoL presurgery.3 Note that these were found to be the most useful variables among nondemented patients who were judged to be good surgical candidates on the basis of full cognitive and motor evaluations and are not considered to be stand-alone indicators of QoL outcome or surgical candidacy.
There are several important factors that may explain the discrepancy between these two studies. First, the sample sizes of patients with poor dementia screening scores were vastly different. The conclusion regarding worse outcomes with lower DRS scores was originally based on a small sample where the poorest performing group contained only 12 patients. Here, we more than tripled the number of patients scoring in that range, but failed to replicate the original finding. Of note, the characteristics of the samples (e.g., age, self-reported depression, UPDRS-ON scores) were fairly comparable.
A potentially important difference between the studies is that the DRS-based groups in the previous study differed in their preoperative performance on the immediate recall trials of the RAVLT, whereas our patient groups did not differ on the first recall trial of the same test. We have previously published data to show that single-trial learning on the RAVLT is the best cognitive predictor of QoL outcome.3 The German and English versions of this language-based memory measure may be psychometrically disparate. As such, the potential immediate-recall differences in the two populations under study may account for the incongruent findings regarding the association of the DRS and QoL outcomes.
In summary, our study of a large sample of nondemented patients with PD, whose baseline DRS scores range widely, argues against using the DRS as a predictive tool in determining QoL post-DBS. These data serve as a cautionary note for placing undue emphasis on cognitive screening measures in ascertaining surgical candidacy.
Acknowledgments
Funding agencies: Portions of this research were funded by the Cleveland Clinic Center for Neurological Restoration and the National Institutes of Health (R01 NS058706-01 [to D.F.], KL2TR000440 [to R.M.B.], and R01 HD061363-01 [to A.G.M.]).
The authors thank the anonymous reviewers for helpful suggestions and Dr. Hyun-Joo Park for his help in constructing the figure.
Footnotes
Relevant conflicts of interest/financial disclosures: Dr. Machado reports the following: Potential future distribution from Intellectual Property licensed to Enspire, Cardionomics and ATI.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Witt K, Daniels C, Krack P, et al. Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Sci. 2011;310:261–266. doi: 10.1016/j.jns.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 3.Floden DP, Cooper SE, Griffith SD, et al. Predicting quality of life outcomes after subthalamic nucleus deep brain stimulation. Neurology. 2014;83:1627–1633. doi: 10.1212/WNL.0000000000000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peto V, Jenkinson C, Fitzpatrick R, et al. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4:241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 5.Abboud H, Machado A, Deogaonkar M, et al. Comprehensive, multi-disciplinary DBS screening for Parkinson patients: no room for “short cuts”. Mov Disord Clin Pract. 2014;1:336–341. doi: 10.1002/mdc3.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llebaria G, Pagonabarraga J, Kulisevsky J, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson’s disease. Mov Disord. 2008;23:1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- 7.Brown GG, Rahill AA, Gorell JM, et al. Validity of the demential rating scale in assessing cognitive function in Parkinson’s disease. J Geriatr Psychiatry and Neurol. 1999;12:180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- 8.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 10.Daniels C, Krack P, Volkmann J, et al. Is improvement in the quality of life after subthalamic nucleus stimulation in Parkinson’s disease predictable? Mov Disord. 2011;26:2516–2521. doi: 10.1002/mds.23907. [DOI] [PubMed] [Google Scholar]
- 11.Litvan I, Goldman J, Tröster A, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]