Introduction
Pain is the primary reason that individuals seek medical care. While the cause of some forms of pain can be readily determined, as many as 1 in 5 adults in America still suffer from some form of persistent pain [1]. There is currently great interest in the co-occurrence of persistent pain conditions within the same individual that is associated with widespread pain across the body. Such widespread pain may be independent of pain severity and is thought to involve a restructuring of pain processing at the level of the brain, as suggested in the widespread pain condition fibromyalgia.
Fibromyalgia may be considered the prototypical centralized pain disorder, wherein pain is primarily originating from the central nervous system [6; 12; 15]. This is supported by the observations of generalized hyperalgesia that these patients experience throughout the body [53], as well as enhanced brain responses to experimental pain [25; 37], altered brain connectivity patterns [22; 44], regional increases and decreases in brain gray matter [14; 33; 39; 51], and changes in brain neurotransmitter levels [9; 23; 29; 47] and their associated receptors [27; 55]. Some of these same brain outcomes dynamically change following successful pharmacologic [28; 49] as well as non-pharmacologic [30; 31; 42] therapy, and these changes concomitantly track with chronic pain improvement. If, as suggested, the brain is the primary locus for pathology in widespread pain patients, these individuals may be more likely to benefit from strategies that go beyond targeting an individual’s focal peripheral pain symptom [2; 15; 43].
A highly prevalent but poorly-understood chronic pain condition is urologic chronic pelvic pain syndrome (UCPPS), encompassing interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) [17]. Despite the clinical presentation of UCPPS, primarily characterized by chronic and often debilitating pain in the pelvic region, no generally effective treatments have been identified [17]. The lack of generally effective treatments may be related to unidentified heterogeneities within the UCPPS population.
To identify underling pathological pain factors that may be related to widespread pain in some UCPPS patients, we designed a study addressing three hypotheses: (1) UCPPS patients would display a reliable distribution of widespread pain, with some patients reporting highly localized pain in the pelvic region and others additionally reporting pain in other body locations as in fibromyalgia, (2) UCPPS individuals reporting pain at more body locations would have lower measures of physical and mental function even after controlling for overall pain severity, and (3) UCPPS patients reporting widespread pain will have common neurologic brain alterations independent of clinical diagnoses (i.e. UCPPS, fibromyalgia).
Here we demonstrate that UCPPS patients are heterogeneous in their degree of widespread pain, and widespread pain is accompanied by poor daily function. Moreover, we show for the first time, that widespread pain has valid markers in brain structure and function within pelvic pain patients that are indistinguishable from fibromyalgia. These neurobiologic changes, which translate across diagnoses, may be critical to the initial development of chronic overlapping pain conditions, and ultimately inform the design of personalized analgesic treatments, a concept unexplored in chronic pain.
Methods
Participants and Study Design
Data were selected for analysis from 1079 participants in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Study (Fig. 1; ClinicalTrials.gov number NCT01098279) [17]. Data were available for patients with a clinical diagnosis of UCPPS, healthy controls without a history or clinical diagnosis of chronic pain, and case-controls with the centralized pain diagnosis of fibromyalgia (but not UCPPS). MAPP study participants were recruited at seven sites: Northwestern University, University of California Los Angeles, University of Iowa, University of Michigan, University of Washington, Washington University St. Louis, and Stanford University. At each site, the Institutional Review Board approved the study, and all participants provided informed consent according to the Declaration of Helsinki. All of the authors vouch for the accuracy and completeness of the data, analyses reported, and the fidelity of the study protocol [41].
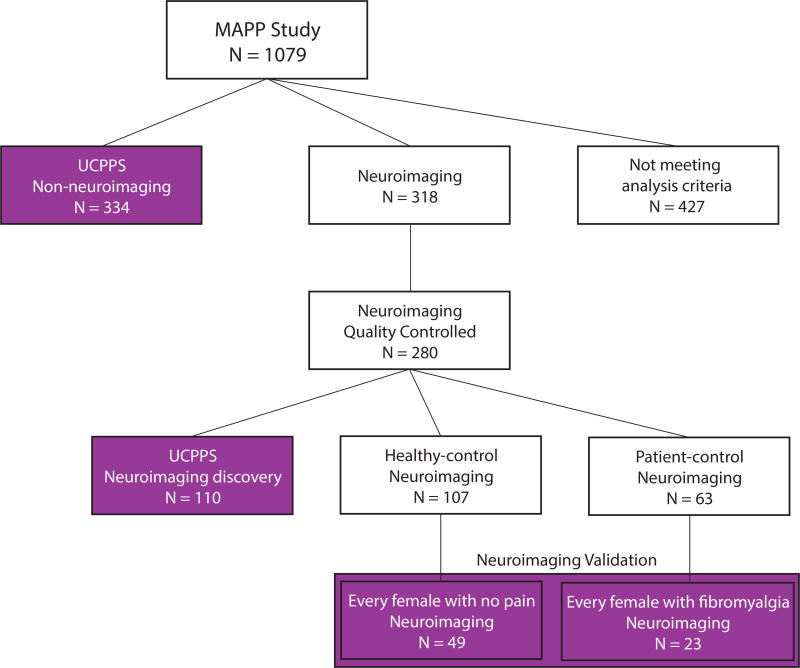
Figure 1.
Selection criteria for participants used in analysis. Participants were selected from a study sample of 1079 participants. 427 participants did not meet the criteria for analysis in this study (e.g. did not have neuroimaging data or were not UCPPS patients). 334 UCPPS patients were identified with self-reported symptoms but no neuroimaging data and were called the non-neuroimaging UCPPS cohort. These UCPPS patients completed the Brief Pain Inventory questionnaire (including body map of pain and pain severity questions) as well as the SF-12 questionnaire about daily function. These 334 were used for the analysis shown in Figures 2 and 5. A separate set of 318 participants were identified with both self-reported symptoms and neuroimaging data. 280 of these participants had high-quality structural and resting-state functional neuroimaging determined independently of the investigators [3]. 110 of these participants had a diagnosis of UCPPS and constituted the UCPPS neuroimaging discovery cohort (localized n=33, intermediate n=37, and widespread n=40) analyzed in Figures 3 and 4. In order to validate a neural signature related to widespread pain in fibromyalgia patients, we constructed a set independent of the UCPPS neuroimaging discovery cohort that contained individuals with very widespread pain (fibromyalgia) and individuals without widespread pain (healthy controls). This neuroimaging validation cohort was constructed by creating a sex-matched (all female) set of every healthy female with no self-reported pain anywhere on the body map (healthy controls n=49) and every female participant with a clinical diagnosis of fibromyalgia (but no diagnosis of UCPPS; n=23).
The inclusion/exclusion criteria for the MAPP study have been described previously [41]. In brief, inclusion criteria for UCPPS participants were: 1) a diagnosis of Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) or Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), with urologic symptoms present a majority of the time during any 3 of the past 6 months (CP/CPPS) or the most recent 3 months (IC/BPS); 2) at least 18 years old; 3) reporting a non-zero score for bladder/prostate and/or pelvic region pain, pressure or discomfort during the past 2 weeks; and 4) consented to provide a blood or cheek swab sample to test DNA (not analyzed here). Exclusion criteria for UCPPS consisted of the following: symptomatic urethral stricture, on-going neurological conditions affecting the bladder or bowel, active auto- immune or infectious disorders, history of cystitis caused by tuberculosis or radiation or chemotherapies, history of non-dermatologic cancer, current major psychiatric disorders, or severe cardiac, pulmonary, renal, or hepatic disease. In addition, males diagnosed with unilateral orchalgia without pelvic symptoms, and males with a history of microwave thermotherapy, trans-urethral or needle ablation or other specified prostate procedures were also excluded. To ensure a clearly defined healthy control subgroup, potential control participants were excluded if they reported any pain in the pelvic or bladder region or chronic pain in more than one non-urologic body region. Pain free controls were also excluded if they had any ongoing chronic illness or acute pain symptoms. Like healthy controls, fibromyalgia participants needed to be free of pain in the pelvic region, but also needed to qualify on the Complex Multi-Symptom Inventory as having fibromyalgia [41].
The study used a cross-sectional design with validation in independent cohorts (Figure 1). We selected participants for analysis according to the following criteria. All UCPPS participants with clinical data but lacking neuroimaging data were selected to map the distribution of pain across the body and assess the impact of widespread pain on physical and mental function. This group is referred to as the UCPPS non-neuroimaging cohort (N = 334). MAPP study participants with neuroimaging data (N = 318) were quality-controlled (independently of the study investigators) according to standardized procedures [3] to yield a set of participants with high-quality structural and resting state functional brain magnetic resonance imaging scans (N = 280). From this a UCPPS neuroimaging discovery cohort (N = 110) provided a sample to address the distribution of pain, impact on physical and mental function, and discover a neurological correlates of pain distributed across the body. A neuroimaging validation cohort (N = 72) was constructed as a sex-matched group of females in the healthy control neuroimaging cohort without pain anywhere in the body (N = 49) and females with fibromyalgia (N = 23; pain reported at many body locations except the pelvis). The validation cohorts were limited to women due to gender differences in the prevalence of fibromyalgia [58].
Identifying patients with widespread pain
To address our first hypothesis, we analyzed the spatial distribution of pain across the body in UCPPS patients. All patients in the MAPP study completed a questionnaire called the Brief Pain Inventory (BPI) [16]. The BPI captured a body map of pain, self-reported by the participant, indicating in 45 regions across the body whether or not the participant experienced pain in the last week that they considered more than an “everyday” kind of pain (given the examples of minor headaches, sprains, and toothaches). A statistical distribution of the number of body regions reported as painful for each participant was developed for the non-neuroimaging UCPPS cohort and the neuroimaging UCPPS cohort. These distributions were separately divided into approximate thirds (tertiles) by an algorithm that optimized the equality of the number of participants in each tertile by examining all possible integer values of the number of painful body regions that separated the tertiles. The tertile with the smallest number of painful body regions was referred to as “localized”, the tertile with the middle number of painful body regions was referred to as “intermediate”, and the tertile with the greatest number of painful body regions was referred to as “widespread”.
Quantifying the functional impact of widespread pain
To address our second hypothesis, we used the tertiles derived from the BPI body map of pain along with two additional pieces of self-reported data. From the BPI questionnaire, an overall pain severity score was derived according to standard calculations [16], and the Short Form 12 (SF-12) questionnaire was used to assess mental and physical function [24]. Using the same algorithm for generating widespread pain tertiles described above, patients were categorized by pain severity as mild, moderate, or severe according to overall pain severity from the BPI. The relationship between pain spread and overall pain severity, on function (physical and mental, separately) was assessed using a 2-way ANOVA with a post-hoc multiple comparison tests (Bonferroni corrected for multiple comparisons; significance p < 0.05). These methods allowed us to assess whether reporting pain in more body regions made an independent contribution from overall pain severity to functional decline. We also explored the amount of shared variance between pain severity and spread using a Pearson’s correlation between these two outcomes. This analysis was performed on 444 participants (combined 334 participants in the UCPPS non-neuroimaging cohort and 110 participants in the UCPPS neuroimaging discovery cohort); however missing pain severity data or SF-12 physical function data were identified in 32 participants out of the 444 so analyses were conducted only on the 412 participants with complete data.
Discovering and validating neural correlates of widespread pain: MRI neuroimaging acquisition and analyses
Overview
To address our third hypothesis, we investigated differences in brain structure and functional connectivity between UCPPS patients in the widespread category compared to patients in the localized category using methods previously described [7; 26; 33; 34; 38]. We studied brain structure by examining regional gray matter volume using Voxel Based Morphometry and we studied brain functional connectivity by examining activity differences in known neural networks using independent components analyses (ICA). To ensure that our connectivity approach was comprehensive, we also used an atlas-based approach to examine the interaction between all possible pairs of functional signals extracted from 165 cortical and subcortical regions of a previously-described anatomical brain atlas [20; 35; 40]. In all cases, we used the UCPPS neuroimaging discovery cohort to identify potential changes in brain structure and functional connectivity associated with widespread pain. These neurobiological markers were then validated in the neuroimaging validation cohort, by determining if the same changes occurred in fibromyalgia patients and not pain-free controls using General Linear Models controlling for study site, total intracranial volume (structural analyses only), and age with significance at p < 0.05 Bonferroni corrected.
Image acquisition for voxel based morphometry and resting functional connectivity
3D T1-Weighted Structural MRI data were acquired as follows. A magnetization prepared rapid gradient echo (MPRAGE) pulse sequence was used for high-resolution, 3D T1-weighted structural MRI scanners at Northwestern University, University of California Los Angeles, University of Michigan, and University of Alabama Birmingham (scanning site associated with Washington University St. Louis), while an inversion-recovery fast spoiled gradient echo (IR-FSPGR) sequence was used for 3D T1-weighted structural MRI scanners at Stanford University. This particular pulse sequence has been standardized across vendors and software platforms as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI), which used the MPRAGE and IR-FSPGR sequences as the primary structural imaging method. MPRAGE/IR-FSPGR sequences provide excellent tissue contrast at an isotropic spatial resolution around 1mm3. Details of the MAPP multi-site acquisition structural protocol have been published previously [3].
Resting-state fMRI (rs-fMRI) acquisition parameters followed recommendations from the functional bioinformatics research network (fBIRN). Briefly, the rs-fMRI acquisition protocol used a target run length of 10 minutes to allow for adequate filtering of low frequency fluctuations from raw temporal data. rs-fMRI volumes were acquired with a TR of 2 seconds. Details of the MAPP multi-site acquisition resting state protocol have also been published previously [3].
Preprocessing for voxel-based morphometry analysis
Voxel based morphometry preprocessing was described previously [38]. In brief raw T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid maps using the New Segment tool in SPM8 (Statistical parametric mapping; Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), run with MATLAB 7.10 (The Mathworks, Inc., Natick, Massachusetts, United States). Resultant gray matter images were then preprocessed using the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) toolbox [8]. In doing so, the accuracy of the inter-subject alignment is increased by modeling the shape of each participant’s brain using millions of parameters (3 parameters per voxel). DARTEL works by simultaneously aligning gray matter and white matter images. In doing so, an increasingly high resolution average template was created to which the data were aligned. Data were then normalized to a standard brain in Montreal Neurological Institute (MNI) space. Because spatial normalization expands and contracts some brain regions, the gray matter images were modulated so that the total amount of gray matter remained the same as in the original images. Normalized, modulated images were then smoothed with a Gaussian kernel of 8 full-width, half-maximum. To avoid possible edge effects between the border of gray matter and white matter, an absolute threshold mask of 0.1 (to remove voxels with gray matter values less than 0.1 from the analysis) was implemented to only include relatively homogenous voxels.
Preprocessing for voxel-based functional connectivity analysis
Functional MRI data was preprocessed using SPM8 (Statistical parametric mapping; Wellcome Department of Cognitive Neurology, London, UK) software package running under Matlab 7.10. Canonical preprocessing steps involved slicetime correction, reangulation of images to center at the anterior commissure, realignment of all images to the first volume to correct for intra-scan movements, coregistration to T1 anatomical image, spatial normalization to standard MNI space and smoothing with a Gaussian kernel of 8mm full width half maximum to compensate for small residual anatomic variations across participants. For atlas-based functional connectivity, raw rs-fMRI data were preprocessed using the FMRIB Expert Analysis Tool (FEAT, http://www.fmrib.ox.ac.uk) [36], which included skull extraction using the brain extraction tool (BET), slice timing correction, motion correction, spatial smoothing using a Gaussian kernel with full-width half-maximum of 5 mm and nonlinear high-pass temporal filtering (150 s). The first 4 acquired volumes were discarded to allow for image stabilization.
Voxel based morphometry and functional connectivity analyses: discovering and validating neural correlates of centralized pain
To address our third hypothesis, we adopted two complementary approaches to discover and validate the neural correlates of pain in multiple body regions. The first was a voxel-based approach, and the second was an atlas-based network approach. These approaches view the brain at different levels of spatial resolution – the voxel-based approach examines the brain with higher spatial resolution but involves more statistical comparisons while the atlas-based approach examines the brain with lower spatial resolution but fewer statistical comparisons.
In the voxel-based approach, we performed two analyses: one investigating volumetric gray matter structural differences, and another exploring differences in functional connectivity to known neural networks using data driven independent component analysis (ICA). For the structural analysis we performed a whole brain voxel based morphometry analysis of gray matter tissue. Within each individual subject, T1 structural images were segmented into gray matter, white matter, and cerebrospinal fluid maps. Gray matter and white matter maps for all subjects were simultaneously aligned to create a high resolution average template to which the images were ultimately aligned. These images were then registered and normalized to a standard template [8] as previously described [38]. Because spatial normalization expands and contracts some brain regions, the gray matter images were modulated so that the total amount of gray matter remained the same as in the original images.
For our voxel-based functional connectivity approach, we performed independent component analysis using GIFT toolbar [13] and component estimates were validated using ICASSO software [32] for 10 iterations. The number of independent components (ICs) was limited to 20 to minimize splitting into subcomponents. Subject specific spatial maps and time courses were back-reconstructed using spatio-temporal regression (STR) or dual regression option available in GIFT. Using STR the original subject data is regressed onto the combined spatial ICA maps to estimate subject specific time courses for each component, then the estimated time course matrices are regressed back to estimate subject specific spatial maps. Thus the original aggregate spatial map and the later estimated spatial maps represent the best approximation for the individual subject specific network component maps. From the estimated aggregate components six resting state networks (RSN) were identified by spatially correlating to standard RSN templates [10]. These individual resting state network maps (salience network (SLN), default mode network (DMN), dorsal attention network (DAN), right and left frontal control network (FCN) and sensorimotor network (SMN)) were then passed onto group second level analyses in SPM.
For the voxel-based approach in the UCPPS neuroimaging discovery cohort, all participant preprocessed gray matter images and independent network maps were entered into separate ANCOVA analyses each using a General Linear Model within SPM8 with age, total intracranial volume (structural analyses only), and neuroimaging site as regressors of no interest. We then compared differences in gray matter volume or brain connectivity between the three pain tertiles using a contrast involving increasing or decreasing connectivity across all three groups. As this first step focused on discovery, we used a more liberal threshold of significance to identify potentially validatable regions. Results were deemed significant at a whole brain cluster-level corrected (either FWE or FDR) threshold of p < 0.05 derived from an uncorrected voxel-level threshold of p < 0.005. Significant results were then extracted from the peak cluster voxel using the MarsBaR region of interest toolbox and plotted to confirm significance and determine any outliers in SPSS (version 21). Validation analyses were then performed in the neuroimaging validation cohort by simply extracting peak cluster voxel values, originating from the significant discovery result regions, from the identical regions in the healthy control and fibromyalgia patients (i.e. the validation cohort). These values were then entered into a univariate General Linear Model in SPSS with the gray matter or connectivity values as dependent variables, cohort as a fixed factor, as well as total brain volume (for structural analyses only), age, and neuroimaging site as confound variables of no interest. Unidirectional results were deemed significant at a one-sided test with p < 0.05 (Bonferroni corrected for multiple comparisons across the number of significant regional differences found from the discovery analyses).
In the atlas-based network approach, we began with division of each participant’s brain into 165 cortical and subcortical regions as described previously [20; 35; 40]. An average time series during a 10 minute resting state scan was extracted from each region, as well as 9 additional time series: the whole brain time series (global signal), the ventricle time series, the white matter, and the 6 time series rotations/translations of the brain across the duration of the scan. For each pair of regions (i,j), a general linear model was fit to the time series in region i using the time series in region j and the 9 time series of no interest described above. The connectivity strength between atlas regions i and j in each participant was quantified by the coefficient βi,j weighting the time series from brain region j in the model of time series from brain region i. With 165 regions, there were 27,060 region pairs (βi,i were not examined). βi,j and βj,i were averaged to create a single value representing the connectivity strength of 13,530 unique pairs of regions.
An identical cross-participant model was used for the atlas-based approach as in the voxel-based approach. Within the UCPPS neuroimaging discovery cohort, all 13,530 unique region pairs were sequentially entered into a cross-participant General Linear Model in MATLAB with the functional connectivity as a dependent variable, pain spread category (localized, intermediate, widespread) as a fixed factor, as well as age and neuroimaging site as confound variables of no interest. Within the neuroimaging validation cohort, all 13,530 unique region pairs were sequentially entered into a cross-participant General Linear Model in MATLAB with the functional connectivity as a dependent variable, participant type (fibromyalgia or pain-free control) as a fixed factor, as well as age and neuroimaging site as confound variables of no interest. In the UCPPS neuroimaging discovery cohort, for each unique pair of brain regions, coefficients in the cross-participant General Linear Model were contrasted between the UCPPS patients with widespread pain and the UCPPS patients with localized pain. In the neuroimaging validation cohort, for each unique pair of brain regions, coefficients in the cross-participant General Linear Model were contrasted between the fibromyalgia patients and the pain-free healthy controls.
We used the following approach to assign significance within the atlas-based approach: Denote the number of unique region pairs with significant (p < 0.05 two sided) differences (widespread greater than localized) in the discovery cohort as ND and the number of unique region pairs with significant differences in the validation cohort (fibromyalgia greater than pain-free controls) as NV. The number of common connections significant in both cohorts was tested to ensure that it exceeded what would be expected by chance: in each of 50,000 iterations, ND connections were chosen at random from 13,530 possible connections, and the number in common with the actual NV connections identified as significant in the validation cohort were counted. We computed probability that number of connections ND&V that had significant differences in both the UCPPS neuroimaging discovery cohort and the neuroimaging validation cohort could occur due to chance as the fraction of the iterations in which the ND randomly chosen connections had greater than or equal to ND&V connections in common with the NV connections identified as significant in the validation cohort.
Results
Robust distribution of widespread pain
As expected, UCPPS patients reported pain in the pelvic region on the pain body map, but surprisingly many patients in both the non-neuroimaging (Fig. 2A) and neuroimaging cohorts (Fig. 2B), also displayed pain in a wide range of other body locations. The localized tertile of UCPPS patients reported fewer than (or equal to) two painful body locations, and the widespread tertile of UCPPS patients reporting over five painful body locations. The distribution of the number of painful body locations was robust as it was identical in the two independent samples of UCPPS participants.
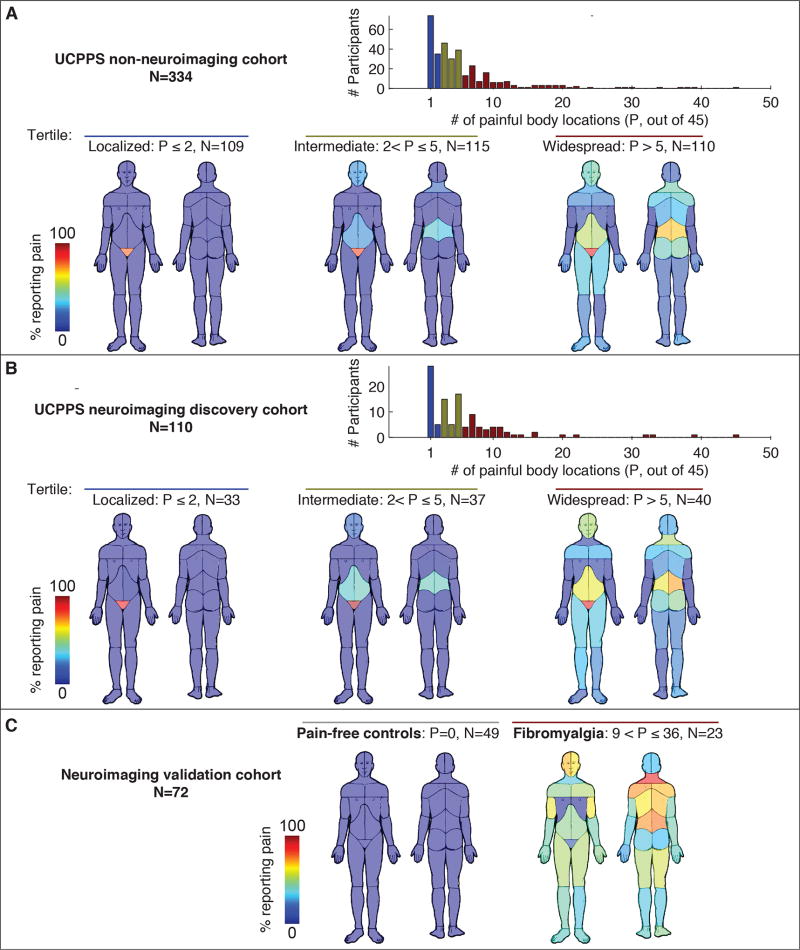
Figure 2. Distribution of painful body locations in three independent cohorts.
Panel A shows data from 334 participants in the UCPPS non-neuroimaging cohort. Histogram of number of painful body regions (out of 45) was divided into 3 tertiles (localized n=109, intermediate n=115, widespread n=110). The body maps show the percentage of patients in each tertile reporting pain in each body region with P being the number of body locations with pain. Panel B shows the same data analysis arising from a separate smaller group of 110 participants in the UCPPS neuroimaging discovery cohort (localized n=33, intermediate n=37, widespread n=40). Panel C shows similar data in the neuroimaging validation cohort of pain-free controls (n=49) and patients diagnosed with fibromyalgia (n=23).
For validation of neuroimaging findings, we used an independent sample of participants that were not UCPPS patients and were at least as extreme in the number of painful body sites as the localized and widespread tertiles of UCPPS patients. This validation cohort was provided by fibromyalgia patients and comparison healthy controls and their body maps are located in Figure 2C.
Cohort demographics and clinical data
Participant demographics and clinical data within our cohorts are displayed in Tables 1 and 2. Of greatest interest was that the UCPPS neuroimaging discovery cohort had patients in the localized and widespread pain categories well matched for variables of no-interest such as age and sex, but differed in variables of interest expected to accompany widespread pain such as overall pain severity, anxiety, and depression.
Table 1.
Demographics and clinical data of UCPPS patients in the neuroimaging and non-neuroimaging cohorts for localized and widespread pain groups.
| UCPPS Neuroimaging (110) | UCPPS Non-neuroimaging (334) | |||||
|---|---|---|---|---|---|---|
| Local Pain (33) |
Widespread Pain (40) |
p-value | Local Pain (109) |
Widespread Pain (110) |
p-value | |
| Age (years)* | 42.3 ± 13.8 | 39.5 ± 10.2 | 0.33 | 47.4 ± 15.2 | 41.7 ± 15.4 | 0.006 |
| Sex | 22 F, 14 M | 26 F, 10 M | 0.11 | 46 F, 63 M | 71 F, 39 M | <0.001 |
| Body mass index* | 25.2 ± 4.5 | 27.1 ± 6.0 | 0.13 | 26.3 ± 5.1 | 26.5 ± 5.9 | 0.78 |
| Race | ||||||
| North American | 0 % | 2.8 % | 0.9% | 0.9% | ||
| Asian | 2.8 % | 0 % | 3.7% | 3.6% | ||
| African American | 5.6 % | 5.6 % | 4.6% | 4.5% | ||
| Native Hawaiian | 0 % | 0 % | 0 % | 0 % | ||
| Caucasian | 94 % | 94 % | 92.6% | 90.0% | ||
| Other | 0 % | 2.8 % | 0.9% | 4.6% | ||
| Ethnicity | ||||||
| Hispanic or Latino | 0 % | 14 % | 5.5% | 7.3% | ||
| Not Hispanic or Latino | 100% | 86 % | 94.5% | 92.7% | ||
| Urologic Symptom duration (yrs)* | 10.3 ± 13.3 | 11.8 ± 10.6 | 0.61 | 8.2 ± 10.2 | 10.0 ± 11.3 | 0.21 |
| Hospital Anxiety and Depression Scale (HADS)* | ||||||
| Anxiety | 6.5 ± 3.5 | 8.8 ± 4.4 | 0.02 | 6.1 ± 3.9 | 9.3 ± 5.2 | <0.001 |
| Depression | 3.9 ± 3.0 | 7.2 ± 4.0 | 0.0002 | 4.3 ± 3.4 | 7.2 ± 4.8 | <0.001 |
| Brief Pain Inventory (BPI)* | ||||||
| Severity | 3.6 ± 1.8 | 4.7 ± 2.0 | 0.01 | 3.6 ± 2.0 | 5.0 ± 2.1 | <0.001 |
| Interference | 3.4 ± 2.3 | 4.8 ± 2.7 | 0.02 | 2.9 ± 2.3 | 5.1 ± 2.8 | <0.001 |
| Short Form-12 Function* | ||||||
| Physical Function | 51.3 ± 8.7 | 42.7 ± 11.6 | 0.0009 | 51.8 ± 7.6 | 42.1 ± 11.0 | <0.001 |
| Mental Function | 45.0 ± 9.0 | 39.8 ± 11.9 | 0.05 | 47.0 ± 9.9 | 39.7 ± 10.8 | <0.001 |
mean±SD
Table 2.
Demographics and clinical data within the neuroimaging validation sample.
| Healthy (n = 49) | Fibromyalgia (n = 23) | p-value | |
|---|---|---|---|
| Age (yrs)* | 33.6 ± 9.2 | 38.4 ± 14.5 | 0.09 |
| Sex | 49 F, 0 M | 23 F, 0 M | - |
| Body mass index* | 25.4 ± 5.5 | 27.4 ± 4.9 | 0.15 |
| Race | |||
| North American | 8.2 % | 8.7 % | |
| Asian | 16 % | 0 % | |
| African American | 16 % | 8.7 % | |
| Native Hawaiian | 4.1 % | 4.3 % | |
| Caucasian | 76 % | 91 % | |
| Other | 6.1 % | 8.7 % | |
| Ethnicity | |||
| Hispanic or Latino | 4.1 % | 17 % | |
| Not Hispanic or Latino | 96 % | 83 % | |
| Urologic symptom duration (yrs) | - | - | - |
| Hospital Anxiety and Depression Scale (HADS)* | |||
| Anxiety | 3.4 ± 2.6 | 7.1 ± 4.9 | <0.001 |
| Depression | 1.9 ± 1.9 | 6.7 ± 3.7 | <0.001 |
| Brief Pain Inventory (BPI)* | |||
| Severity | 0.0 ± 0.1 | 4.5 ± 2.0 | <0.001 |
| Interference | 0.0 ± 0.0 | 4.7 ± 1.7 | <0.001 |
| Short Form-12 Function* | |||
| Physical Function | 57.7 ± 2.5 | 38.3 ± 12.2 | <0.001 |
| Mental Function | 55.3 ± 5.5 | 41.9 ± 10.6 | <0.001 |
mean±SD
To ensure that medication usage would not adversely affect our neuroimaging analyses, we compared medication use in the UCPPS neuroimaging discovery cohort (Supplementary Fig. 1). There were no significant differences (all p > 0.05) in medication usage between the localized and widespread category of patients in the UCPPS neuroimaging discovery cohort. To examine the prevalence of multiple comorbid diagnoses, we also compared the relationship between the distribution of self-reported pain on the BPI body map and the presence of comorbid conditions (Supplementary Fig. 2). As expected, we found significant increases in the symptoms of fibromyalgia, irritable bowel syndrome, and temporomandibular joint disorder in UCPPS patients with widespread pain. Only 20% of UCPPS widespread pain patients had a diagnosis of fibromyalgia, whereas over 80% displayed fibromyalgia symptoms.
Validated brain correlates of widespread pain
Voxel based morphometry and ICA functional connectivity: Voxel-based approach
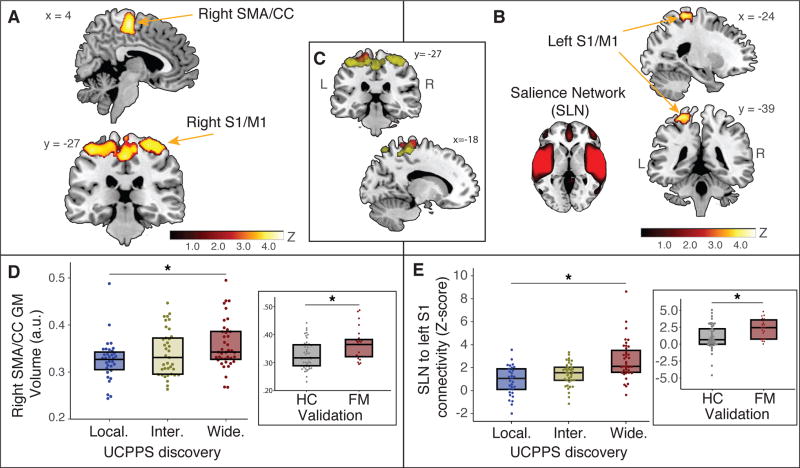
Regional gray matter volume analysis within the UCPPS neuroimaging discovery cohort revealed increasing gray matter volume from the localized, to intermediate, to widespread pain groups within several sensorimotor areas (Figs. 3A and D, p < 0.05 corrected). These brain regions included the: right supplementary motor area/mid cingulate cortex (SMA/CC) (peak voxel coordinates: x = −45, y = −53, z = 57; z-score = 4.17; PFDR = 0.001), right inferior parietal lobule (peak voxel coordinates: x = 45, y = −65, z = 38; z-score = 4.66; PFDR = 0.027), left inferior parietal lobule (peak voxel coordinates: x = −45, y = −53, z = 57; z-score = 4.17; PFDR = 0.001), right medial primary somatosensory cortex/primary motor cortex (S1/M1) (peak voxel coordinates: x = 38, y = −35, z = 64; z-score = 3.93; PFDR = 0.027), right lateral SI/MI (peak voxel coordinates: x = 48, y = −15, z = 54; z-score = 3.93; PFDR = 0.027). The UCPPS patients with localized pain did not have any brain regions with significantly greater gray matter volume compared to UCPPS patients with widespread pain (all p > 0.05). All regions showing significant increases in gray matter volume in UCPPS individuals, were then examined in the validation analysis comparing female healthy control and female fibromyalgia participants. Interestingly, one of the five structural results from the discovery analyses was found to validate in the fibromyalgia patients versus healthy controls analyses. Fibromyalgia patients displayed greater gray matter volume when compared to healthy controls within the right SMA/CC (Figure 3D inset; p < 0.05 Bonferroni corrected for multiple comparisons). The other remaining brain regions (right inferior parietal lobule, left inferior parietal lobule, right medial SI/MI, right lateral S1/M) did not validate between these two groups (all p > 0.05).
Figure 3. Structural and functional brain signature of widespread pain.
Panel A shows increases in gray matter volume (GMV) in right supplementary motor area/cingulate cortex (SMA/CC) and bilateral primary sensory/motor cortex (S1/M1) regions among UCPPS patients with widespread pain as compared to localized pain. Panel B shows an increase in brain connectivity between salience network (SLN) and a left S1/M1 region among UCPPS with widespread pain as compared to localized pain. Panel C shows overlap of the S1/M1 cluster from structural (Panel A) and functional connectivity (Panel B) analyses. Panel D shows box plots of median and inter-quartile range (25–75%), highlighting significant SMA/CC GMV increases from localized (local. - blue), to intermediate (inter. – yellow), to widespread (wide. - red) tertiles. This same region is validated by displaying increased GMV in patients diagnosed with fibromyalgia (FM - red) as compared to pain-free healthy controls (HC - gray; Panel D inset). Panel E shows box plots of median and inter-quartile range (25 – 75%), highlighting a significant increase in functional connectivity between SLN to left S1/M1 region from localized (local. – blue), to intermediate (inter. – yellow), to widespread (wide. - red) pain groups. This result is validated by FM patients (red) having greater connectivity in this same region as compared to HCs (gray; Panel E inset).
Results from the whole brain network ICA analysis within the UCPPS discovery cohort revealed increasing connectivity between the salience network (SLN) and left S1/M1 region from the localized, to intermediate, to widespread pain groups (Fig. 3B and 3E; peak voxel coordinates x = −19, y = −27, z = 71; z-score = 4.31; PFWE = 0.04). This result was validated comparing female healthy controls and fibromyalgia participants (Figure 3E inset; p = 0.003; no Bonferroni correction was required as this was the only region showing significant connectivity differences to the SLN). Interestingly this region overlapped with the S1/M1 cluster identified as showing greater gray matter volume in widespread patients with UCPPS (Figure 3C). We also found significantly increased connectivity between the sensorimotor network (SMN) and bilateral anterior cingulate region (peak voxel coordinates x = −9, y = 19, z = 29; z-score = 4.74; PFWE = 0.009) from the localized, to intermediate, to widespread pain groups, but this RSN connectivity did not validate when comparing female healthy controls and fibromyalgia patients (p > 0.05). No other significant results were found with any other RSNs with either increases or decreases in connectivity.
To investigate the specificity of our voxel-based neuroimaging results to widespread pain, we examined correlations between our voxel-based brain signatures and other variables such as pain severity, anxiety, and depression (see Supplementary Figure 3A). We did not find any significant associations between the structural SMA/CC result and pain severity (rho = −0.241, p = 0.135), anxiety (rho = 0.036, p = 0.828), or depression (rho = −0.212, p = 0.194). Results from our voxel-based ICA network signatures also did not show any significant associations between SLN to left S1/M1 and pain severity (see Supplementary Figure 3B; rho = 0.286, p = 0.073), anxiety (rho = 0.005, p = 0.977) or depression (rho = 0.101, p = 0.539). This suggests that our neuroimaging findings are largely related to the degree of widespread pain and surprisingly not pain severity (or depression and anxiety).
Functional Connectivity: Atlas-based approach
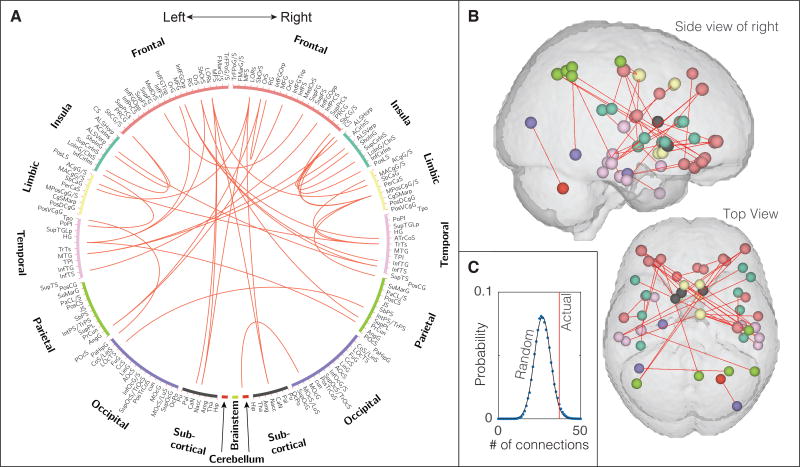
Probing for additional brain changes in connectivity using our Atlas-based approach (Supplementary Table 1), we found that the functional interaction between 37 pairs of brain regions increased significantly (p < 0.05, corrected) in UCPPS patients with widespread as compared to localized pain as well as increased significantly in patients with fibromyalgia compared to pain-free controls (Fig. 4A and B). These altered connections focused on frontal, insula, limbic, and temporal cortices, as well as subcortical regions including bi-lateral nucleus accumbens. The number of connections significantly greater in UCPPS patients with widespread pain compared to UCPPS patients with localized pain was found to be ND = 525. The number of connections significantly greater in patients with fibromyalgia compared to pain-free controls was found to be NV = 697. The number of these connections significantly increased in both the UCPPS neuroimaging discovery cohort and the neuroimaging validation cohort was found to be ND&V = 37. In a randomization test using 50,000 iterations, the probability of 525 randomly chosen connections from a possible set of 13,530 possible connections having 37 connections in common with the 697 identified in the validation cohort is only 3.3% (p = 0.033), indicating that the number of connections observed to increase in both of these cohorts related to widespread pain was not likely to occur simply by chance (Fig. 4C). Since the voxel-based approach only identified increases in functional connectivity and increases in gray matter volume, we did not explore decreases in functional connectivity with the atlas-based approach.
Figure 4. Functional connectivity associated with widespread pain.
Panel A shows 165 anatomically-defined brain regions, arranged around a circle for visualization, with red lines indicating the 37 pairs of regions for which functional connectivity increased in the UCPPS neuroimaging discovery cohort (UCPPS patients with widespread pain compared to UCPPS patients with localized pain) as well as in the neuroimaging validation cohort (patients with fibromyalgia compared to pain-free controls). Panel B shows these same connections spatially in the brain. Panel C shows that the number of connections identified to increase in both cohorts exceeds what would be expected by chance (p = 0.033, 50000 iterations, see Materials and Methods).
Functional impact of widespread pain
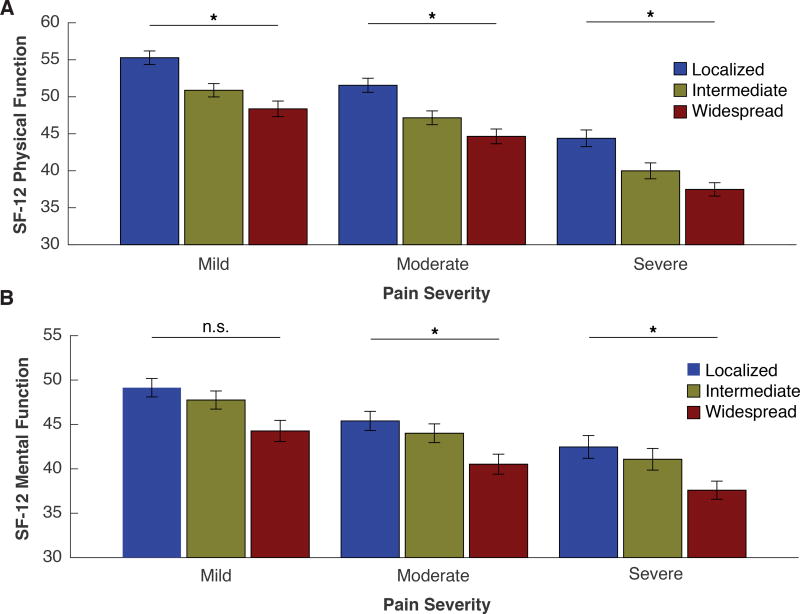
Widespread pain contributed to diminished physical and mental function in UCPPS patients independent of overall pain severity (Fig. 5A and B). Patients were found across all combinations of widespread pain categories (localized, intermediate, widespread) and pain severity categories (mild, moderate, severe), which would not be the case if widespread pain and pain severity were highly correlated. Physical function was reduced in UCPPS patients with more widespread as compared to localized pain in all categories of pain severity (Fig. 5A). The main effect of widespread pain category yielded an F ratio of F(2,403) = 17.2, p < 0.001, indicating that the physical function was reduced in UCPPS patients with widespread pain (mean = 41.9, SD = 11.0) compared to UCPPS patients with localized pain (mean = 52.0, SD = 7.6). The main effect of overall pain severity category yielded an F ratio of F(2,403) = 41.5, p < 0.001, indicating that the physical function was also reduced in UCPPS patients with more severe pain (mean = 39.4, SD = 11.1) compared to more mild pain (mean = 52.1, SD = 8.2). The interaction effect of widespread pain category and pain severity category on physical function was non-significant F(4,403) = 1.16, p = 0.33.
Figure 5. Functional impact of widespread pain on physical and mental function is independent of overall pain severity.
UCPPS patients divided into tertiles according to pain spread were independently divided into tertiles according to overall pain severity to provide a classification as mild, moderate (mod.) or severe (sev.). Each grouping shows the average physical (Panel A) and mental (Panel B) function scores across pain widespread and severity tertiles. A decline in physical (p < 0.001) and mental function (p < 0.001) scores as a function of pain spread is observed even after controlling for pain severity. * Denotes post hoc p < 0.05 significant relationships between decline in function (physical or mental) and pain spread, within individual pain severity tertiles.
Mental function was reduced in UCPPS patients with more widespread as compared to localized pain irrespective of pain severity (Fig. 5B). The main effect of widespread pain category yielded an F ratio of F(2,403) = 8.6, p < 0.001, indicating that mental function was reduced in UCPPS patients with widespread pain (mean = 39.9, SD = 11.0) compared to UCPPS patients with localized pain (mean = 46.6, SD = 9.7). The main effect of overall pain severity category yielded an F ratio of F(2,403) = 12.03, p < 0.0001, indicating that mental function was also reduced in UCPPS patients with more severe pain (mean = 39.3, SD = 11.3) compared to more mild pain (mean = 47.6, SD = 8.9). The interaction effect of widespread pain category and pain severity category on mental function was non-significant F(4,403) = 0.98, p = 0.42. The data above suggest that widespread pain is associated with worse physical as well as mental function, irrespective of the severity dimension of pain. The widespread and severity pain outcomes were largely independent as pain sensitivity and spread only had a 9% overlap in variation with a Pearson correlation coefficient of 0.30. This was significant with a p-value less than 0.001.
Discussion
We identified patient subtypes within a clinical diagnosis (UCPPS) that expressed differing degrees of widespread body pain. Importantly, more widespread body pain was associated with reduced daily function independent of pain severity, and it had a pattern of brain structure and function that was also observed in fibromyalgia, the prototypical centralized pain state [6]. To our knowledge this is the first report of increased widespread pain being associated with altered brain structure and function co-occurring in different, but potentially overlapping, clinical chronic pain diagnoses.
The widespread brain outcomes identified are consistent with research in neural correlates of chronic pain in general, but have unique differences as well. Sensorimotor and insular cortices are known to play an important role in pain processing and response to pain threat, and have structural and functional abnormalities in a number of different chronic pain states [5; 18; 54]. Our results here show that these specific regions are further important in distinguishing subtypes of patients within a particular chronic pelvic pain diagnosis. It is not yet known if the structural and functional abnormalities in sensorimotor and insular cortices associated with widespread pain are present within other regional pain conditions, particular genetic subtypes [48], or become accentuated in certain patients after the onset of persistent focal pain.
Our findings have implications for neuroimaging studies of pain in general. Since we find changes in gray matter volume and connectivity in specific regions within patients with more widespread pain, neuroimaging studies may be confounded if participants displaying differing degrees of widespread pain are enrolled. This may very well be a contributing factor in the variability of brain imaging findings observed in the pain field. For example while the predominant finding is decreased brain gray matter volume, increases have also been reported [33; 45; 50–52]. A previous investigation suggested that this discrepancy across studies may be attributable to differing ages, with younger fibromyalgia patients showing increases in brain gray matter and older individuals showing decreases [14]. However, since there were no age differences between our localized and widespread UCPPS groups, age is likely not a major contributing factor in our analyses. Although it is unknown what increased gray matter volume may reflect on the neuronal level, a current study suggests that these increases may actually involve neuronal plasticity [46]. We speculate that the increased gray matter volume seen in widespread patients may reflect a dynamic strengthening in synaptic strength (increased size/number of synapses/neurons) expressed as an increase in neuronal volume. If true, this may occur more so in younger fibromyalgia patients during the beginning stages of pain centralization, and therefore might actually be related to the increased volume seen in younger fibromyalgia patients. However, this remains to be tested. We are aware of no other neuroimaging reports that have specifically addressed the spread of pain dimension.
In the future, the brain-based outcomes associated with widespread pain that we identified could be useful clinically as an objective marker of pain that can identify and quantify the amount of this pain dimension within a given individual: the degree to which an individual has centralized pain (pain arising primarily from the central nervous system). Indeed, chronic overlapping pain conditions have been shown to have commonalities among a number of separate diagnoses and this has clinical impact on how an individual patient should and should not be treated. For example, there is evidence that knee and hip arthritis patients that display more widespread pain respond poorly to peripherally targeted treatments (arthroplasty) [11]. We suspect that this may also be true for UCPPS. The need for more “personalized” or “mechanism-based” treatments for chronic pain has been emphasized in the literature [56], but has yet to be realized. The clinical need for tests that enable diagnoses and treatment decisions based on objective criteria, in addition to patient self-report, has also been highlighted [4; 49]. While the current state of the field precludes the use of brain imaging in the diagnosis and treatment of chronic pain patients, current trends in other neurobiological disorders suggests that objective MRI outcomes may provide useful future information to assist in clinical decisions [19; 21; 57]. For example, brain MRI outcomes could be used to predict, at the individual patient level, who would benefit from a specific treatment. This is important because it could ultimately result in less time and money spent on the current “trial-and-error” approach to chronic pain treatment. While the purpose of our work was to identify neural correlates and functional impact of widespread pain at the population level, this investigation also opens up unique research avenues for diagnostic criteria and potentially novel individualized treatment approaches for chronic pain.
Supplementary Material
Acknowledgments
This work was supported by a cooperative agreement from the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant numbers DK082370, DK082342, DK082315, DK082344, DK082325, DK082345, DK082333, DK082316, DK103260, DK103277, and DK103271). We thank all of the volunteers who participated in the study. We also would like to thank Dr. Thomas Chenevert at the University of Michigan for assistance with neuroimaging. Dr. Daniel J. Clauw has consulted for Forest Laboratories, Pfizer, Inc., Cerephex Corporation, Eli Lilly and Company, Merck & Co., Nuvo Research Inc., Tonix Pharmaceuticals, Johnson & Johnson, Pierre Fabre, Cypress Biosciences, Wyeth Pharmaceuticals, UCB, AstraZeneca, Jazz Pharmaceuticals, Abbott Laboratories, and Iroko Pharmaceuticals. Dr. Richard E. Harris has received grant funding and has consulted for Pfizer, Inc.
References
- 1.Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): 2011. [PubMed] [Google Scholar]
- 2.Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med. 2001;134(9 Pt 2):868–881. doi: 10.7326/0003-4819-134-9_part_2-200105011-00011. [DOI] [PubMed] [Google Scholar]
- 3.Alger JR, Ellingson BM, Ashe-McNalley C, Woodworth DC, Labus JS, Farmer M, Huang L, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Deutsch G, Harris RE, Clauw DJ, Glover GH, Parrish TB, Hollander J, Kusek JW, Mullins C, Mayer EA Investigators MRN. Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP Research Network. Neuroimage Clin. 2016;12:65–77. doi: 10.1016/j.nicl.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allsop SA, Erstad DJ, Brook K, Bhai SF, Cohen JM, Dimitrakoff JD. The DABBEC phenotyping system: towards a mechanistic understanding of CP/CPPS. Nat Rev Urol. 2011;8(2):107–113. doi: 10.1038/nrurol.2010.227. [DOI] [PubMed] [Google Scholar]
- 5.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Arnold LM, Choy E, Clauw DJ, Goldenberg DL, Harris RE, Helfenstein M, Jr, Jensen TS, Noguchi K, Silverman SL, Ushida T, Wang G. Fibromyalgia and Chronic Pain Syndromes: A White Paper Detailing Current Challenges in the Field. Clin J Pain. 2016;32(9):737–746. doi: 10.1097/AJP.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–1394. doi: 10.1002/art.39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014;44(1):68–75. doi: 10.1016/j.semarthrit.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn Reson Imaging. 2004;22(9):1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin. 2013;3:249–260. doi: 10.1016/j.nicl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 17.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ Group MRNS. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis KD. Neuroimaging of pain: what does it tell us? Curr Opin Support Palliat Care. 2011;5(2):116–121. doi: 10.1097/SPC.0b013e3283458f96. [DOI] [PubMed] [Google Scholar]
- 19.Dazzan P. Neuroimaging biomarkers to predict treatment response in schizophrenia: the end of 30 years of solitude? Dialogues Clin Neurosci. 2014;16(4):491–503. doi: 10.31887/DCNS.2014.16.4/pdazzan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon N, Chiu Y, Nurmikko T, Stancak A. Functional Connectivity with the Default Mode Network Is Altered in Fibromyalgia Patients. PLoS One. 2016;11(7):e0159198. doi: 10.1371/journal.pone.0159198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64(2):579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 26.Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin. 2015;8:305–313. doi: 10.1016/j.nicl.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 29.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58(3):903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 31.Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009;47(3):1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143(3):262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014;15(8):815–826. e811. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irimia A, Chambers MC, Torgerson CM, Van Horn JD. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage. 2012;60(2):1340–1351. doi: 10.1016/j.neuroimage.2012.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1–2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2015;193(1):131–137. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ Group MRNS. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazaridou A, Kim J, Cahalan CM, Loggia ML, Franceschelli O, Berna C, Schur P, Napadow V, Edwards RR. Effects of Cognitive-Behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin J Pain. 2017;33(3):215–221. doi: 10.1097/AJP.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napadow V, Harris RE. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of 'centralized' pain? Arthritis Res Ther. 2014;16(5):425. doi: 10.1186/s13075-014-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Pomares FB, Funck T, Feier NA, Roy S, Daigle-Martel A, Ceko M, Narayanan S, Araujo D, Thiel A, Stikov N, Fitzcharles MA, Schweinhardt P. Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging. J Neurosci. 2017;37(5):1090–1101. doi: 10.1523/JNEUROSCI.2619-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyke T, Osmotherly PG, Baines S. Measuring Glutamate Levels in the Brains of Fibromyalgia Patients and a Potential Role for Glutamate in the Pathophysiology of Fibromyalgia Symptoms: A Systematic Review. Clin J Pain. 2016 doi: 10.1097/AJP.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 48.Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Lemaitre H, Mann KF, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Schumann G, Hawrylycz M, Poline JB, Greicius MD consortium I. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348(6240):1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, Clauw DJ, Harris RE. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014;6:252–261. doi: 10.1016/j.nicl.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1–2):89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Sorensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25(1):152–155. [PubMed] [Google Scholar]
- 54.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7(3):173–181. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- 55.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8(1):51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology. 2001;95(1):241–249. doi: 10.1097/00000542-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 57.Ye Z, Rae CL, Nombela C, Ham T, Rittman T, Jones PS, Rodriguez PV, Coyle-Gilchrist I, Regenthal R, Altena E, Housden CR, Maxwell H, Sahakian BJ, Barker RA, Robbins TW, Rowe JB. Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson's disease with clinical and neuroimaging measures. Hum Brain Mapp. 2016;37(3):1026–1037. doi: 10.1002/hbm.23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med. 2002;5(2):42–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.