Abstract
The effects of two different external carbon sources (acetate and ethanol) and electron acceptors (dissolved oxygen, nitrate, and nitrite) were investigated under aerobic and anoxic conditions with non-acclimated process biomass from a full-scale biological nutrient removal-activated sludge system. When acetate was added as an external carbon source, phosphate release was observed even in the presence of electron acceptors. The release rates were 1.7, 7.8, and 3.5 mg P/(g MLVSS·h) (MLVSS: mixed liquor volatile suspended solids), respectively, for dissolved oxygen, nitrate, and nitrite. In the case of ethanol, no phosphate release was observed in the presence of electron acceptors. Results of the experiments with nitrite showed that approximately 25 mg NO2-N/L of nitrite inhibited anoxic phosphorus uptake regardless of the concentration of the tested external carbon sources. Furthermore, higher denitrification rates were obtained with acetate (1.4 and 0.8 mg N/(g MLVSS·h)) compared to ethanol (1.1 and 0.7 mg N/(g MLVSS·h)) for both anoxic electron acceptors (nitrate and nitrite).
Keywords: Biological nutrient removal (BNR), Denitrification, Enhanced biological phosphorus removal (EBPR), External carbon source, Electron acceptor
1. Introduction
In the field of wastewater treatment, biological nutrient removal (BNR) processes have been accepted as the most economical and sustainable technology for simultaneous nitrogen and phosphorus removal. One of the key aspects of BNR processes is the presence of available biodegradable organic carbon, which is essential for providing an electron donor for denitrification and energy for enhanced biological phosphorus removal (EBPR). However, the supply of biodegradable organic substrates in the influent wastewater, required for both processes, is normally insufficient. In many cases, external carbon sources are usually added to achieve satisfactory overall BNR performance and low effluent levels for nitrogen and phosphorus. Acetate is an efficient external carbon source for denitrification as well as a preferred carbon source for EBPR. Ethanol is another kind of readily biodegradable compound which has been proven as a carbon source for denitrification (Peng et al., 2007; Li et al., 2008). Moreover, ethanol has successfully been tested as a substrate for EBPR; however, a long acclimation period (50–140 d) was required (Puig et al., 2008; Wang et al., 2013).
In the BNR-activated sludge systems, the external carbon sources also interact with the EBPR process. The EBPR process is based on the enrichment of activated sludge with polyphosphate accumulating organisms (PAOs), usually under sequential anaerobic and aerobic (or anoxic) conditions so that the electron donor (organic matter) and the electron acceptor (dissolved oxygen (DO) or nitrate) are physically separated (Metcalf & Eddy Inc. et al., 2003). However, the mechanisms of PAOs are not yet fully established under conditions that differ from the classical anaerobic/aerobic (or anoxic) conditions, e.g. in the presence of both electron donor and electron acceptor. Several studies have focused on the effect of the simultaneous presence of both acetate (electron donor) and DO (electron acceptor). Guisasola et al. (2004) observed that PAOs behave in a similar way under aerobic conditions in the presence of acetate to the classical anaerobic/aerobic cycle of the EBPR process. Specifically, PAOs are capable of taking up acetate under aerobic conditions, linking this uptake to phosphate release, poly-β-hydroxyalkanoates (PHA) formation and glycogen degradation. Ultimately, when the substrate is depleted, PAOs take up phosphate from the medium linked with PHA degradation, glycogen synthesis, and PAOs growth, achieving a net phosphorus removal (Ahn et al., 2002; Guisasola et al., 2004; Pijuan et al., 2005). A similar behavior was observed by Swinarski et al. (2009) and Yuan and Oleszkiewicz (2010) in anoxic batch experiments with acetate. Several studies have reported that the effect of the presence of nitrate on P release is related to both the nature of the carbon source (Guerrero et al., 2011) and operational conditions of the reactor (Guerrero et al., 2012). Guerrero et al. (2011) reported that when the influent COD composition was a mixture of different carbon sources (acetic acid, propionic acid, and sucrose), the anaerobic-anoxic-aerobic (A2/O) system, fed with excess of nitrate in the anaerobic reactor, was able to maintain EBPR, even with the internal recycle ratios up to ten times of the influent flow rate and COD limiting conditions. However, EBPR failed when a more complex compound (sucrose) was used as a sole carbon source. This indicates that the nitrate presence has an inhibitory effect on EBPR, not to inhibit the P release process itself but to prevent the fermentation process for volatile fatty acid (VFA) production. Guerrero et al. (2012) found that the operational conditions of the A2/O pilot plant selected a PAO population capable of (1) coexisting with nitrate without an inhibitory effect and (2) outcompeting denitrifying bacteria for the carbon source, in contrast to the SBR pilot plant, where nitrate had an inhibitory effect on EBPR.
It should be noted that nitrite is a key intermediate in nitrogen transformations and may play an essential role in the novel treatment process, such as mainstream deammonification. Therefore, nitrite should also be carefully examined as a potential electron acceptor for PAOs in BNR-activated sludge systems. However, a few studies with PAOs have revealed that elevated concentrations of nitrite would negatively affect the activity of PAOs under either anoxic or aerobic conditions, but different threshold values of inhibition by nitrite have been reported in the literature. Meinhold et al. (1999) found that a nitrite level of 6–8 mg NO2-N/L completely halted the anoxic P uptake for sludge from a BNR pilot plant. Saito et al. (2004) observed that nitrite concentrations higher than 12 mg NO2-N/L reduced the anoxic P uptake rate to 65%. In contrast, Sin et al. (2008) found no inhibitory effect on the anoxic P uptake activity of both sequencing batch reactor (SBR) and membrane biological reactor (MBR) sludge within the studied range of nitrite concentrations (up to 25 mg NO2-N/L). Also, Ahn et al. (2001) and Hu et al. (2003) did not find any severe effect on the anoxic P uptake at elevated nitrite concentrations, even up to 40 and 35 mg NO2-N/L, respectively.
The factors such as the available carbon source and DO (elector acceptor) have significant impacts on the effective conduct of BNR processes, as confirmed by Smolders et al. (1994), Guisasola et al. (2004), or Pijuan et al. (2005). The aim of this study was to investigate the effect of not only the parameters mentioned above but also other electron acceptors such as nitrite and nitrate on denitrification and phosphorus removal. Tests take into account the individual effect of the analyzed parameters, as well as unique, simultaneous effects of several factors on the processes carried out under both aerobic and anoxic conditions.
2. Materials and methods
2.1. Origin of the process biomass
The process biomass, used in batch experiments, originated from the “Wschód” wastewater treatment plant (WWTP; 600 000 population equivalents (PE), 1 PE=0.5 m3/d) in the city of Gdansk (northern Poland). The biological step of the plant, retrofitted in 2012, consists of six parallel A2/O-activated sludge bioreactors and twelve circular secondary clarifiers. In the settled wastewater, concentrations of total nitrogen (TN) and phosphorus (TP) are relatively high, exceeding 80 mg N/L and 15 mg P/L, respectively. The plant meets the most stringent effluent criteria of the European Union Urban Wastewater Directive (Council of the European Union, 1991) for large WWTPs, i.e. TN=10 mg N/L and TP=1 mg P/L.
2.2. Laboratory apparatus
The main parts of the experimental apparatus (Fig. 1) were two parallel batch reactors with a maximum working volume of 4 L. The reactors were equipped with electrodes and probes (WTW, Germany) for a continuous monitoring of pH (SenTix 21), oxidation-reduction potential (SenTix ORP), temperature and DO (CellOx 325). The automated control systems for aeration and heating/cooling allowed maintenance of the DO concentration (under aerobic conditions) and temperature around the set points of 6 mg O2/L and 20 °C, respectively. The content of the reactors was mixed by mechanical stirrers at the rate of 180 r/min. Under aerobic conditions, oxygen uptake rates (OURs) were measured in small chambers connected to the main reactors. The measurements were conducted in a controlled, cyclic mode of filling/emptying with an adjustable length of the measurement phase (3 min assumed in this study).
Fig. 1.
Schematic of the experimental set-up for the laboratory batch experiments
M: motor; DO: dissolved oxygen; T: temperature
2.3. Batch experiments
Fresh mixed liquor withdrawn from the aerobic zone of the “Wschód” WWTP in Gdansk (northern Poland) was prepared in the batch reactors with the mixed liquor suspended solids (MLSS) concentrations of approximately 4000 mg/L, mixed liquor volatile suspended solids (MLVSS) concentrations around 3000 mg/L, and the resulting MLVSS/MLSS ratio averaged approximately 0.75. The actual MLSS and MLVSS concentrations in each reactor were measured at the beginning of the tests. Several types of batch experiments were carried out, including one-phase experiments under anaerobic, aerobic, and anoxic conditions as well as two-phase experiments under anaerobic/anoxic and anaerobic/aerobic conditions. Two different kinds of external carbon sources, acetate vs. ethanol, were tested under different electron acceptor conditions (DO, nitrate, and nitrite). Under aerobic conditions, the nitrification inhibitor (allylthiourea) was added in the amount of 10 mg/L to inhibit the activity of nitrifiers.
2.3.1 One-phase experiments
In the anaerobic P release tests, acetate or ethanol was added in excess as a sole carbon source for phosphate release. The initial concentration of the readily biodegradable chemical oxygen demand (COD) was approximately 500 mg COD/L and the test was run for 4 h. In the aerobic P uptake experiments, the external carbon source and phosphorus (NaH2PO4/Na2HPO4) were added in the ratio of 10 g COD/g PO4-P at the beginning of the test and the test was run for 4 h. In the anoxic P uptake experiments, a source of nitrate (KNO3) or nitrite (KNO2) and the external carbon source were added in the ratio of 6 g COD/g NO3-N or 6 g COD/g NO2-N at the beginning of the test and the test was run for 4 h. For comparison, a reference anoxic test without the external carbon source was also performed.
2.3.2 Two-phase experiments
In the two-phase (anaerobic/aerobic or anaerobic/anoxic) experiments, process biomass and acetate were mixed and kept first under anaerobic conditions for 2.5 h. At the beginning of the second phase, the external carbon source (acetate or ethanol) was added to increase the COD concentration by 150 mg COD/L. When the second phase was run under aerobic conditions, the aeration system was turned on to maintain the DO concentration around 6 mg O2/L during the entire aerobic phase. When the second phase was run under anoxic conditions, nitrate (KNO3) or nitrite (KNO2) was injected to raise the NO3-N or NO2-N concentration by 25 mg N/L. The second phase of the experiments lasted for 4.5 h (aerobic) or 3.5 h (anoxic). For comparison, a reference test without the external carbon source in the anoxic phase was also performed.
2.4. Sampling frequency and analytical methods
Samples of the mixed liquor were frequently (every 5–60 min) withdrawn from the batch reactors and filtered under vacuum pressure through a 1.2-μm pore size Millipore nitrocellulose filter (Billerica, MA, USA). Concentrations of PO4-P, NO3-N, NO2-N, and COD were determined using a Xion 500 spectrophotometer (Dr Lange GmbH, Berlin, Germany). The analytical procedures, which were adopted by Dr. Lange, followed the standard methods (APHA, 1998). The MLSS and MLVSS concentrations were determined at the beginning of the tests by the gravimetric method according to the standard methods (APHA, 1998). Free nitrous acid (FNA) concentrations under anoxic conditions were calculated through the nitrite concentration (), pH, and temperature, from the following formula (Anthonisen et al., 1976):
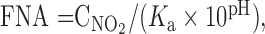 , ,
|
(1) |
where K a=e−2300/ T and T is temperature (K).
3. Results and discussion
3.1. Effects of different carbon sources on the anaerobic behavior of PAOs
Results of the anaerobic P release test with acetate and ethanol are shown in Fig. 2. With acetate, a nearly complete P release was obtained after 4 h implicitly because the internal level of polyphosphate in the PAOs biomass was very low. The ultimate PO4-P concentration reached 84.0 mg P/L. This corresponded to the value of 34.70 mg P/g MLVSS for the specific amount of P released. The observed P release/acetate uptake ratio, , was 0.41 g P/g COD (r 2=0.9887) and 0.43 g P/g COD (r 2=0.9924) for the initial 90 min and the whole experimental period, respectively. Moreover, it was found in a previous study that the activity of glycogen accumulating organisms (GAOs) is negligible in the studied plant (Swinarski et al., 2012). The results of a similar experiment with acetate in excess in this work confirm this finding, revealed by the fact that the acetate utilization hardly continued after polyphosphate depletion in the biomass. In contrast, in a similar experiment with ethanol, the specific rate and ultimate amount of P released were very small and reached only 2.44 mg P/g MLVSS and 6.92 mg P/L, respectively. Using process biomass from the same WWTP, Swinarski et al. (2009) also found that ethanol insignificantly induced the anaerobic P release. For comparison, in the study of Puig et al. (2008), ethanol was dosed to the sludge non-acclimated to ethanol and the PO4-P profile followed a similar trend to that in this study. The observed specific amount of P released was a little higher and reached 3.8 mg P/g MLVSS.
Fig. 2.

Observed phosphate release and COD consumption during the anaerobic experiments with different carbon sources (added in excess)
(a) Acetate; (b) Ethanol. Data are expressed as mean±standard deviation (n=3)
Since the non-acclimated biomass was not able to perform suitable EBPR with ethanol, only acetate was used to induce anaerobic P release in the subsequent two-phase batch experiments. In all these experiments, the maximum P release rate was maintained for approximately the initial 90 min because of the sufficient amounts of acetate and internally stored polyphosphate and glycogen in the PAO cells. The average P release rate and P release/acetate uptake ratio from nine experiments were (8.4±0.7) mg P/(g MLVSS·h) and (0.42±0.02) g P/g COD, respectively, which confirmed the results of the one-phase anaerobic experiment (8.7 mg P/(g MLVSS·h) and 0.41 g P/g COD, respectively). Similar ratios with acetate (0.50 g P/g COD) have also been reported in the literature (Smolders et al., 1994; Puig et al., 2007).
3.2. Effects of different external carbon sources on the aerobic behavior of PAOs and “ordinary” heterotrophs
In the aerobic phase of the two-phase experiments, either acetate or ethanol was added as a sole external carbon source. In the experiments with ethanol, the PO4-P profile followed a similar trend to that without any external carbon addition (Figs. 3a and 3c). In both cases, low PO4-P concentrations were achieved at the end of the 4.5-h aerobic phase, i.e. 2.3 vs. 0.9 mg P/L, respectively, for no external carbon and ethanol addition. In the case of acetate, a short lag phase (lasting approximately 30 min) was observed with respect to P uptake. Such PO4-P behavior is implicitly related to the effect of simultaneous P uptake due to aerobic conditions and P release because of acetate uptake. In this study, the PO4-P uptake rate was faster than the PO4-P release rate, achieving a net PO4-P uptake and a short lag occurred. This resulted in a higher final PO4-P concentration (6.9 mg P/L) than those in the other two experiments. The highest and lowest maximum P uptake rates were obtained for ethanol (12.40 mg P/(g MLVSS·h)) and acetate (9.50 mg P/(g MLVSS·h)), respectively (Table 1). It seemed that the addition of ethanol as external carbon enhanced the aerobic P uptake by PAOs, whereas the addition of acetate adversely affected the P uptake to a certain extent. The enhancement of aerobic P uptake with ethanol could be related to the P requirements for growth of ordinary heterotrophic organisms (OHOs) based on ethanol as a carbon source under aerobic conditions.
Fig. 3.

Observed phosphate and COD behavior during the two-phase (anaerobic/aerobic) experiments with different carbon sources
(a) No external carbon source added during the aerobic phase; (b) Acetate; (c) Ethanol. Data are expressed as mean±standard deviation (n=3)
Table 1.
Phosphorus release and uptake rates under different carbon sources and electron acceptors in one-(aerobic) and two-phase (anaerobic/aerobic) experiments (T=293 K)
| Carbon source | Electron acceptor | One-phase |
Two-phase |
||
| Prelease (mg/(g MLVSS·h)) | Puptake (mg/(g MLVSS·h)) | Prelease (mg/(g MLVSS·h)) | Puptake (mg/(g MLVSS·h)) | ||
| Acetate | O2 | 1.70±0.20 | 2.12±0.30 | 9.50±0.50 | |
| NO3 | 7.80±0.30 | 1.10±0.20 | 3.70±0.52 | 0.34±0.03 | |
| NO2 | 3.50±0.46 | 3.70±0.31a; 2.90±0.18b | ≈0 | ||
| Ethanol | O2 | 2.14±0.20 | 12.40±0.40 | ||
| NO3 | 0.04±0.01 | 1.50±0.10 | |||
| NO2 | 0.09±0.01 | 0.65±0.21 | ≈0 | ||
| Not added | O2 | 10.20±0.70 | |||
| NO3 | 1.80±0.20 | ||||
| NO2 | 0.31±0.11 | ≈0 | |||
This rate is related to the initial hour;
This rate is related to the whole anoxic experimental period. Prelease: P release rate; Puptake: P uptake rate. Data are expressed as mean±standard deviation (n=3)
In order to further investigate the behavior of PAOs under aerobic conditions in the presence of the electron donor (acetate or ethanol), the one-phase (aerobic) experiments were carried out (Fig. 4). In the case of ethanol (Fig. 4b), however, no PO4-P release was observed and P was continuously taken up at the average rate of 2.14 mg P/(g MLVSS·h), with the final PO4-P concentration reaching 0.29 mg P/L. The measured OUR kept almost constant during the entire experiment (Fig. 4d). This can be attributed to the abundance of the soluble substrate which was not fully utilized by the process biomass. The results of Adouani et al. (2010) also revealed a similar, almost constant OUR profile when feeding ethanol as the carbon source.
Fig. 4.
Observed phosphate, COD, and OUR behaviors during the aerobic experiments with different external carbon sources
(a, c) Acetate; (b, d) Ethanol. Data are expressed as mean±standard deviation (n=3)
In the experiment with acetate (Fig. 4a), P release was observed in the initial 45 min until acetate was depleted. The P release rate was 1.70 mg P/(g MLVSS·h) and the peak PO4-P concentration reached 12.7 mg P/L. Afterwards, when the substrate was depleted, P uptake was observed with a P uptake rate of 2.12 mg P/(g MLVSS·h), a little higher than the previous P release rate (1.70 mg P/(g MLVSS·h)). The final PO4-P concentration was 2.5 mg P/L at the end of the experiment. The P uptake rate (2.12 mg P/(g MLVSS·h)) was almost the same as in the case of ethanol.
Such PO4-P behavior confirmed that storage of PHA could also occur under aerobic conditions, provided that suitable readily biodegradable substrates (volatile fatty acids) are available and PO4-P is not completely released from the PAO cells. Hence, in the presence of acetate, PAOs tend to behave under aerobic conditions in a similar way to the classical EBPR process occurring under the alternating anaerobic/aerobic conditions. Similar results have been reported in several studies with acetate-fed under aerobic conditions (Ahn et al., 2002; Guisasola et al., 2004; Pijuan et al., 2005). Pijuan et al. (2005) tested a response to acetate under aerobic conditions of the same PAOs-enriched biomass with high and low levels of intracellular storage compounds, observing in both cases aerobic P release linked to acetate uptake. However, higher P release/C uptake ratio was observed in the experiment with biomass withdrawn at the end of the aerobic phase where higher level of polyphosphate was present in the biomass. Afterwards, when the substrate was depleted, PAOs took up the phosphate from the medium, achieving a net phosphorus removal. The measured OUR clearly exhibited a biphasic pattern during the experiment (Fig. 4c), indicating the aerobic storage of acetate in the first phase and consumption of the stored compounds in the second phase. In order to describe this aerobic acetate consumption and PHA storage, two different hypotheses were proposed by Guisasola et al. (2004) including the PAO hypothesis vs. OHO hypothesis. Both hypotheses assume that PAOs, under aerobic conditions, take up acetate coupled to PHA storage, glycogen degradation, and P release as under anaerobic conditions. However, the PAO hypothesis assumes that PAOs are capable of storing acetate as PHA linked to oxygen consumption whereas the OHO hypothesis assumes that this storage is due to the activity of OHOs. Further research is still needed to clarify this issue.
3.3. Effects of different carbon sources and nitrate/nitrite on the anoxic behavior of PAOs and denitrifying heterotrophs
The behaviors of PO4-P, COD, and NO3-N/NO2-N in the one-phase (anoxic) and two-phase (anaerobic/anoxic) experiments are presented in Figs. 5 and 6, respectively. The measured anoxic P uptake rates and nitrate/nitrite utilization rates (NURs) are listed in Table 2.
Fig. 5.
Denitrification rate experiments with different external carbon sources and electron acceptors
(a, b) Acetate, NO3-N and NO2-N, respectively; (c, d) Ethanol, NO3-N and NO2-N, respectively. Data are expressed as mean±standard deviation (n=3)
Fig. 6.
Anaerobic/anoxic experiments with different carbon sources and electron acceptors
(a, b) No external carbon source added during the anoxic phase, NO3-N and NO2-N, respectively; (c, d) Acetate, NO3-N and NO2-N, respectively; (e, f) Ethanol, NO3-N and NO2-N, respectively. Data are expressed as mean±standard deviation (n=3)
Table 2.
Denitrification rates under different carbon sources and electron acceptors in one-(anoxic) and two-phase (anaerobic/anoxic) experiments (T=293 K)
| Carbon source | Electron acceptor | One-phase |
Two-phase |
||
| NUR (mg N/(g MLVSS∙h)) | ΔCOD/ΔN (g COD/g N) | NUR (mg N/(g MLVSS∙h)) | ΔCOD/ΔN (g COD/g N) | ||
| Acetate | NO3 | 1.4±0.0 | 5.7±0.6 | 2.3±0.1 | 5.6±0.5 |
| NO2 | 0.8±0.1 | 8.4±1.3 | 1.2±0.2 | 3.3±0.4 | |
| Ethanol | NO3 | 1.1±0.1 | 4.2±0.2 | 1.5±0.0 | 2.6±0.1 |
| NO2 | 0.7±0.0 | 7.2±0.0 | 1.0±0.2 | 2.4±0.7 | |
| Not added | NO3 | 1.1±0.1 | 0 | ||
| NO2 | 0.7±0.1 | 0 | |||
NUR: nitrite utilization rates. Data are expressed as mean±standard deviation (n=3)
It should be noted that the behavior of PAOs under anoxic conditions was significantly different when adding acetate or ethanol as the external carbon source. As shown in Fig. 5a, P release was observed during the initial hour when the biodegradable substrate was present under anoxic conditions with nitrate (NO3) as an electron acceptor. Afterwards, when the substrate was depleted, PAOs took up the phosphate from the medium. When nitrite (NO2) served as an electron acceptor (Fig. 5b), P release was observed during the entire anoxic experiment because the biodegradable substrate was present in the batch reactor until the end of the experiment. In the two-phase experiments, when nitrate served as the electron acceptor, the release of PO4-P was initially observed at the rate of 3.70 mg P/(g MLVSS·h), despite high concentrations of NO3-N (9–17 mg N/L) (Fig. 6c). The anoxic P uptake (0.34 mg P/(g MLVSS·h)) was observed once acetate was depleted after approximately 1 h of the anoxic phase (Fig. 6c). In contrast, insignificant P release occurred with ethanol as the carbon source during both one-phase (Figs. 5c and 5d) and two-phase (Figs. 6e and 6f) experiments. These observations are in accordance with the conclusion of Guerrero et al. (2011) that the availability of VFA is the key factor in triggering the EBPR activity and the complex compound (e.g. ethanol) must be fermented to VFAs to maintain the EBPR activity. When nitrite was used as the electron acceptor, a significant P release was also observed despite high concentrations of NO2-N (12–20 mg N/L) during the entire anoxic phase with the addition of acetate (Fig. 6d). The P release rate in the initial hour under anoxic conditions was 3.70 mg P/(g MLVSS·h), the same as the case when nitrate was used as the electron acceptor. Afterwards, the P release rate in the remaining anoxic cycle with nitrite was decreased to 0.53 mg P/(g MLVSS·h). The overall amount of P released reached 5.02 mg P/g MLVSS with nitrite, 35.7% higher than the amount of P released with nitrate (3.70 mg P/g MLVSS). When nitrite served as the electron acceptor with ethanol, no P uptake was observed and much lower P was released in the absence of any external carbon (0.31 mg P/(g MLVSS·h)) (Fig. 6b) or in the presence of ethanol (0.65 mg P/(g MLVSS·h)) (Fig. 6f). These results suggest that the non-acclimated PAOs are not able to use nitrite under anoxic conditions with the tested initial NO2-N concentration of approximately 25 mg NO2-N/L. In the literature, high concentration of nitrite has indeed been reported to be a severe inhibitor of the anoxic P uptake by PAOs (Meinhold et al., 1999; Saito et al., 2004). However, some recent studies reported that the protonated species of nitrite, FNA, rather than nitrite itself, is likely the actual inhibitor of the microbial activity in BNR systems, including the PAOs responsible for the anoxic phosphorus uptake, depending on the concentration of nitrite, operating pH, and temperature (Zhou et al., 2007, 2008). At a temperature of 20 °C and initial pH of 7.2–7.4, the calculated FNA concentrations for the initial 1.5 h of the anoxic phase in the two-phase anaerobic-anoxic experiments with acetate and ethanol were in the range of 0.0014–0.0032 and 0.0015–0.0027 mg FNA/L, respectively (Figs. 6d and 6f). The literature data (Zhou et al., 2007, 2011) suggest that the FNA inhibition could occur during the experiments. Zhou et al. (2007) reported that the anoxic P uptake activity of PAOs was inhibited by FNA at concentrations in the range of 0.001–0.002 mg FNA/L, which is substantially lower than those in this study. Furthermore, FNA completely halted the anoxic P uptake at 0.02 mg FNA/L. Zhou et al. (2007, 2011) proposed several mechanisms for the FNA inhibition, including the effect of the enzymes involved, energy production, and nitric oxide (NO) inhibition triggered by FNA inhibition. The extent to which the inhibition mechanism plays a role during FNA inhibition on the anoxic P uptake process is still unclear and requires further research. It is, however, clear that the inhibitory effect of FNA may not be due to a single mechanism or pathway and varies for different microorganisms.
In the one-phase anoxic experiment with acetate and nitrate, the measured NURs averaged 1.4 mg N/(g MLVSS·h). For comparison, this value is higher than that (1.1 mg N/(g MLVSS·h)) obtained with a full-scale nitrifying sludge at 20 °C (Kristensen et al., 1992) and in the range of 1–3 mg N/(g MLVSS·h) at 15–20 °C reported by Kujawa and Klapwijk (1999) for a bench-scale N removing system. However, this value is much lower than the rates reported in several studies for denitrifying systems, in which the NURs remain in the range of 2.8–13.9 mg N/(g MLVSS·h) at 17–21.5 °C (Isaacs and Henze, 1995; Rodríguez et al., 2007; Morgan-Sagastume et al., 2008; Kampas et al., 2009). The NUR (1.1 mg N/(g MLVSS·h)) measured with ethanol and nitrate was also slightly lower than the values in the range of 1.6–2.5 mg N/(g MLVSS·h) reported in other studies (Hagman et al., 2008; Morgan-Sagastume et al., 2008). The possible explanation for the differences of the NURs between this study and other studies is linked to the origin of the non-acclimated biomass and its activity (Kampas et al., 2009). It should be noted that throughout the one-phase experiments, P release was observed to indicate anoxic consumption of acetate by PAOs until the readily biodegradable substrate was depleted in the batch reactor (Figs. 5a and 5b). Conversely, significant P release did not occur with ethanol during the one-phase (anoxic) experiments with either nitrate or nitrite (Figs. 5c and 5d).
In order to evaluate the effect of an anaerobic phase on denitrification, the measurements of NURs under anoxic P uptake were also conducted similarly to other studies (Swinarski et al., 2009) (Fig. 6). It was found that higher NURs were obtained in the two-phase (anaerobic/anoxic) experiments than those in the one-phase (anoxic) experiments (Table 2). This is because PHA synthesized by PAOs under anaerobic conditions was utilized by a fraction of PAOs for denitrification in the anoxic phase. In the two-phase experiments with nitrite, the measured NURs with acetate and ethanol averaged 1.2 and 1.0 mg N/(g MLVSS·h), respectively. Both rates were lower in comparison with those obtained with nitrate as the electron donor (2.3 and 1.5 mg N/(g MLVSS·h) for acetate and ethanol, respectively), but higher than the endogenous denitrification rate (0.7 mg N/(g MLVSS·h)) obtained without any carbon addition (Table 2).
Notably, in both one-phase and two-phase experiments with acetate, higher NURs were always obtained compared to ethanol regardless of the electron acceptors. The highest NURs, (2.3±0.1) mg N/(g MLVSS·h), were observed with acetate and nitrate in the two-phase experiments. This could be explained by the fact that acetate can be directly assimilated by microorganisms, whereas ethanol must be first converted to acetate (Constantin and Fick, 1997).
4. Conclusions
The combined effects of different external carbon sources (acetate and ethanol) and electron acceptors (DO, nitrate, and nitrite) revealed significantly different behavior of PAOs and denitrifying “ordinary” heterotrophs in BNR processes. Phosphate release with acetate by PAOs occurred in the presence of electron acceptors (DO, nitrate, and nitrite), whereas no phosphate release was observed with ethanol in the presence of electron acceptors. Regardless of the carbon sources, nitrite inhibited anoxic P uptake in the studied initial concentrations of approximately 25 mg N/L. Furthermore, higher NURs were obtained with acetate than those with ethanol with non-acclimated biomass in the cases of both anoxic electron acceptors (nitrate/nitrite).
Acknowledgments
During the time of the study at Gdansk University of Technology, Ms. Xiang HU was a researcher supported by the project “CARbon BALAncing for Nutrient Control in Wastewater Treatment (CARBALA)” (PIRSES-GA-2011-295176) under the International Research Staff Exchange Scheme FP7-PEOPLE-2011-IRSES.
Footnotes
Project supported by the European Regional Development Fund within the Framework of the Innovative Economy Operational Program 2007-2013 (No. UDA-POIG.01.03.01-22-140/09-04), the CARbon BALAncing for Nutrient Control in Wastewater Treatment (CARBALA) (No. PIRSES-GA-2011-295176), and the National Water Pollution Control and Management of Science and Technology in China (No. 2015ZX07218001)
Compliance with ethics guidelines: Xiang HU, Dominika SOBOTKA, Krzysztof CZERWIONKA, Qi ZHOU, Li XIE, and Jacek MAKINIA declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Adouani N, Lendormi T, Limousy L, et al. Effect of the carbon source on N2O emissions during biological denitrification. Resour Conserv Recy. 2010;54(5):299–302. doi: 10.1016/j.resconrec.2009.07.011. [DOI] [Google Scholar]
- 2.Ahn J, Daidou T, Tsuneda S, et al. Metabolic behavior of denitrifying phosphate-accumulating organisms under nitrate and nitrite electron acceptor conditions. J Biosci Bioeng. 2001;92(5):442–446. doi: 10.1016/S1389-1723(01)80293-0. [DOI] [PubMed] [Google Scholar]
- 3.Ahn J, Daidou T, Tsuneda S, et al. Transformation of phosphorus and relevant intracellular compounds by a phosphorus accumulating enrichment culture in the presence of both the electron acceptor and electron donor. Biotechnol Bioeng. 2002;79(1):83–93. doi: 10.1002/bit.10292. [DOI] [PubMed] [Google Scholar]
- 4.Anthonisen AC, Loehr RC, Prakasam TBS, et al. Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Contr Fed. 1976;48(5):835–852. [PubMed] [Google Scholar]
- 5.APHA (American Public Health Association) Standard Methods for the Examination of Water and Wastewater, 20th Ed. American Public Health Association, Washington, DC, USA; 1998. [Google Scholar]
- 6.Constantin H, Fick M. Influence of C-sources on the denitrification rate of a high-nitrate concentrated industrial wastewater. Water Res. 1997;31(3):583–589. doi: 10.1016/S0043-1354(96)00268-0. [DOI] [Google Scholar]
- 7.Council of the European Union. Council Directive 91/271/EEC of 21 May 1991 concerning urban wastewater treatment. Off J Eur Union. 1991;135:40–52. [Google Scholar]
- 8.Guerrero J, Guisasola A, Baeza JA. The nature of the carbon source rules the competition between PAO and denitrifiers in systems for simultaneous biological nitrogen and phosphorus removal. Water Res. 2011;45(16):4793–4802. doi: 10.1016/j.watres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero J, Taya C, Guisasola A, et al. Understanding the detrimental effect of nitrate presence on EBPR systems: effect of the plant configuration. J Chem Technol Biotechnol. 2012;87(10):1508–1511. doi: 10.1002/jctb.3812. [DOI] [Google Scholar]
- 10.Guisasola A, Pijuan M, Baeza JA, et al. Aerobic phosphorus release linked to acetate uptake in bio-P sludge: process modelling using oxygen uptake rate. Biotechnol Bioeng. 2004;85(7):722–733. doi: 10.1002/bit.10868. [DOI] [PubMed] [Google Scholar]
- 11.Hagman M, Nielsen JL, Nielsen PH, et al. Mixed carbon sources for nitrate reduction in activated sludge-identification of bacteria and process activity studies. Water Res. 2008;42(6-7):1539–1546. doi: 10.1016/j.watres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Hu JY, Ong SL, Ng WJ, et al. A new method for characterizing denitrifying phosphorus removal bacteria by using three different types of electron acceptor. Water Res. 2003;37(14):3463–3471. doi: 10.1016/S0043-1354(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs SH, Henze M. Controlled carbon source addition to an alternating nitrification-denitrification wastewater treatment process including biological P removal. Water Res. 1995;29(1):77–89. doi: 10.1016/0043-1354(94)E0119-Q. [DOI] [Google Scholar]
- 14.Kampas P, Parsons SA, Pearce P, et al. An internal carbon source for improving biological nutrient removal. Bioresour Technol. 2009;100(1):149–154. doi: 10.1016/j.biortech.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen GH, Jørgensen PE, Henze M. Characterization of functional microorganism groups and substrate in activated sludge and wastewater by AUR, NUR and OUR. Water Sci Technol. 1992;25(6):43–57. [Google Scholar]
- 16.Kujawa K, Klapwijk B. A method to estimate denitrification potential for predenitrification systems using NUR batch tests. Water Res. 1999;33(10):2291–2300. doi: 10.1016/S0043-1354(98)00459-X. [DOI] [Google Scholar]
- 17.Li QH, Li P, Zhu PP, et al. Effects of exogenous organic carbon substrates on nitrous oxide emissions during the denitrification process of sequence batch reactors. Environ Eng Sci. 2008;25(8):1221–1228. doi: 10.1089/ees.2007.0172. [DOI] [Google Scholar]
- 18.Meinhold J, Arnold E, Isaacs S. Effect of nitrite on anoxic phosphorus uptake in biological phosphorus removal activated sludge. Water Res. 1999;33(8):1871–1883. doi: 10.1016/S0043-1354(98)00411-4. [DOI] [Google Scholar]
- 19.Metcalf & Eddy Inc, Tchobanoglous G, Burton FL. Wastewater Engineering: Treatment and Reuse, 4th Ed. McGraw-Hill Higher Education, New York; 2003. [Google Scholar]
- 20.Morgan-Sagastume F, Nielsen JL, Nielsen PH. Substrate-dependent denitrification of abundant probe-defined denitrifying bacteria in activated sludge. FEMS Microbiol Ecol. 2008;66(2):447–461. doi: 10.1111/j.1574-6941.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Peng YZ, Ma Y, Wang SY. Denitrification potential enhancement by addition of external carbon sources in a pre-denitrification process. J Environ Sci. 2007;19(3):284–289. doi: 10.1016/S1001-0742(07)60046-1. [DOI] [PubMed] [Google Scholar]
- 22.Pijuan M, Guisasola A, Baeza JA, et al. Aerobic phosphorus release linked to acetate uptake: influence of PAO intracellular storage compounds. Biochem Eng J. 2005;26(2-3):184–190. doi: 10.1016/j.bej.2005.04.014. [DOI] [Google Scholar]
- 23.Puig S, Coma M, van Loosdrecht MCM, et al. Biological nutrient removal in a sequencing batch reactor using ethanol as carbon source. J Chem Technol Biotechnol. 2007;82(10):898–904. doi: 10.1002/jctb.1754. [DOI] [Google Scholar]
- 24.Puig S, Coma M, Monclusa H, et al. Selection between alcohols and volatile fatty acids as external carbon sources for EBPR. Water Res. 2008;42(3):557–566. doi: 10.1016/j.watres.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez L, Villasenor J, Fernandez FJ. Use of agro-food wastewaters for the optimisation of the denitrification process. Water Sci Technol. 2007;55(10):63–70. doi: 10.2166/wst.2007.307. [DOI] [PubMed] [Google Scholar]
- 26.Saito T, Brdjanovic D, van Loosdrecht MCM. Effect of nitrite on phosphate uptake by phosphate accumulating organisms. Water Res. 2004;38(17):3760–3768. doi: 10.1016/j.watres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Sin G, Niville K, Bachis G, et al. Nitrite effect on the phosphorus uptake activity of phosphate accumulating organisms (PAOs) in pilot-scale SBR and MBR reactors. Water SA. 2008;34:249–260. [Google Scholar]
- 28.Smolders GJF, van der Meij J, van Loosdrecht MCM, et al. Model of the anaerobic metabolism of the biological phosphorus removal process; stoichiometry and pH influence. Biotechnol Bioeng. 1994;43(6):461–470. doi: 10.1002/bit.260430605. [DOI] [PubMed] [Google Scholar]
- 29.Swinarski M, Makinia J, Czerwionka K, et al. Comparison of the effects of conventional and alternative external carbon sources for enhancing the denitrification process. Water Environ Res. 2009;81(9):896–906. doi: 10.2175/106143009X407438. [DOI] [PubMed] [Google Scholar]
- 30.Swinarski M, Makinia J, Stensel HD, et al. Modeling external carbon addition in biological nutrient removal processes with an extension of the International Water Association Activated Sludge Model. Water Environ Res. 2012;84(8):646–655. doi: 10.2175/106143012X13373550426670. [DOI] [PubMed] [Google Scholar]
- 31.Wang DB, Zheng W, Li XM, et al. Evaluation of the feasibility of alcohols serving as external carbon sources for biological phosphorus removal induced by the oxic/extended-idle regime. Biotechnol Bioeng. 2013;110(3):827–837. doi: 10.1002/bit.24753. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Q, Oleszkiewicz J. Interaction between denitrification and phosphorus removal in a sequencing batch reactor phosphorus removal system. Water Environ Res. 2010;82(6):536–540. doi: 10.2175/106143009X12529484815476. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Pijuan M, Yuan Z. Free nitrous acid inhibition on anoxic phosphorus uptake and denitrification by poly-phosphate accumulating organisms. Biotechnol Bioeng. 2007;98(4):903–912. doi: 10.1002/bit.21458. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Pijuan M, Yuan Z. Development of a 2-sludge, 3-stage system for nitrogen and phosphorous removal from nutrient-rich wastewater using granular sludge and biofilms. Water Res. 2008;42(12):3207–3217. doi: 10.1016/j.watres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Oehme A, Lim M, et al. The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res. 2011;45(15):4672–4682. doi: 10.1016/j.watres.2011.06.025. [DOI] [PubMed] [Google Scholar]






