Abstract
(R)-2-hydroxy-3-phenylpropionic acid (PLA) is an ideal antimicrobial compound with broad-spectrum activity against a wide range of Gram-positive bacteria, some Gram-negative bacteria, and fungi. We studied the bioconversion of phenylpyruvate (PPA) to PLA using whole recombinant Escherichia coli cells in a series of buffer/organic solvent systems. Octane was found to be the best organic solvent. The optimum volume ratio of the water phase to the n-octane phase, conversion temperature, substrate concentration, and cell concentration were 6:4, 40 °C, 12.5 g/L, and 30 g/L wet cells, respectively. Under the optimized conditions, the average PLA productivity in the aqueous/n-octane system was 30.69% higher than that in the aqueous system, and 32.31 g/L PLA was obtained with the use of a stirred reactor (2-L scale). Taken together, our findings indicated that PLA biosynthesis was more efficient in an aqueous/n-octane biphasic system than in a monophasic aqueous system. The proposed biphasic system is an effective strategy for enhancing PLA yield and the biosynthesis of its analogues.
Keywords: (R)-2-hydroxy-3-phenylpropionic acid, Phenylpyruvate, Aqueous/n-octane biphasic system, Whole cell bioconversion, Recombinant Escherichia coli
1. Introduction
(R)-2-hydroxy-3-phenylpropionic acid (PLA) is an organic acid that is abundantly found in the products of lactic acid bacterial fermentation (Li et al., 2015) and in honey (Tan et al., 1989; Tuberoso et al., 2011). PLA is an ideal antimicrobial compound with broad-spectrum activity against a wide range of Gram-positive bacteria, some Gram-negative bacteria (Dieuleveux et al., 1998), and fungi (Ohhira et al., 2004). PLA production by biocatalytic asymmetric synthesis has recently received extensive attention in industry and academia owing to its advantages of simple operation, high conversion, and high efficiency. PLA can be synthesized by many lactic acid bacteria (LAB) and some non-LAB such as Bacillus coagulans SDM (Zheng et al., 2011) and Propionibacterium spp. (Yu et al., 2014). The synthesis of PLA using recombinant Escherichia coli has also been investigated in several studies, and the PLA yield obtained using recombinant E. coli (pET-28a-ldh Y52V) is typically about 15.6 g/L (Zhu et al., 2015). Some researchers have been successful in increasing this PLA yield using simultaneous saccharification and fermentation (SSF) (Kawaguchi et al., 2014). Lactobacillus sp. SK007 produces 17.38 g/L PLA by substrate feeding and pH control (Mu et al., 2009). However, the low dissolution of phenylpyruvate (PPA) and the inhibitory effects of PPA and PLA reduce the final yield of PLA. To overcome this problem, a biphasic system was designed to extract PLA from the aqueous phase for relieving the inhibitory effects of PLA and PPA in this study. So far, there has been no report on any suitable biphasic system to be applied to the biosynthesis of PLA using recombinant E. coli. Some studies have shown that the yield of the target product can be improved by increasing enzymatic activity (Kobayashi et al., 2000; Ni et al., 2013; Delbecq et al., 2016), increasing the solubility of the substrate (Zou et al., 2013; Resasco, 2014), and reducing the inhibitory effects of the substrate and products (Li et al., 2007; Wang et al., 2015) in an aqueous/organic solvent biphasic system. Li et al. (2007) reported successful increase in the concentration and e.e. (S) (enantiomeric excess of substrate) value of 2-octanol by reduction of 2-octanone in a water/n-dodecane biphasic system, which served to relieve the inhibitory effect of production (Sakdaronnarong et al., 2016). Wang et al. (2015) also reported an increase in the production of cholest-4-en-3-one with the use of a biphasic system by solving the problem of low substrate solubility. The synthesis of cyclohexyl-α and β-D-glucoside, dehydration of xylose to furfural, and bio-resolution of epichlorohydrin have also been shown to be better in an organic solvent/buffer biphasic system than in aqueous solvents (Yi et al., 1998).
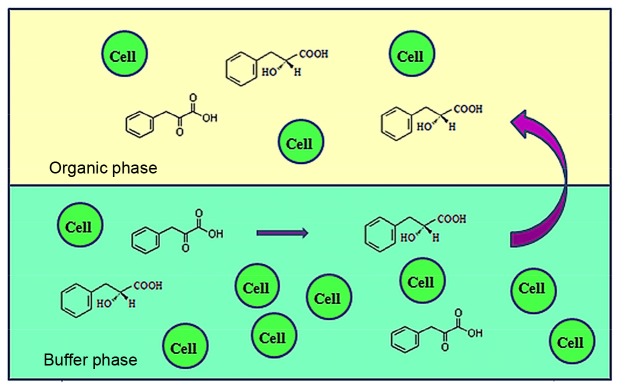
In this study, we used recombinant E. coli pET-28a-ldh Y52V in a biphasic system to synthesize PLA from PPA using lactate dehydrogenase (LDH). The product PLA was partially moved into the organic solvent phase (Fig. 1), which alleviated the inhibitory effect of PLA, thus enhancing the production of PLA.
Fig. 1.
Schematic diagram of the biphasic system
2. Materials and methods
2.1. Strains and chemicals
E. coli pET-28a-ldh Y52V (Zhu et al., 2015) bearing the gene of Tyr52Val-mutated D-LDH was preserved in our lab. D-PLA and PPA were purchased from Sinopharm Chemical Reagent Co., Ltd. (Suzhou, China). The organic solvents were purchased from Qiangshun Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of analytical grade.
2.2. Cell culture
E. coli pET-28a-ldh Y52V was cultured in Luria-Bertani (LB) medium (10.0 g/L tryptone, 5.0 g/L yeast extract, 10.0 g/L NaCl; pH 7.0) supplemented with kanamycin (50 μg/ml) at 37 °C with shaking at 200 r/min. When the optical density at 600 nm (OD600) value reached 0.6–0.8, 1 mmol/L isopropyl-β-D-thiogalactoside was added and induction culture was carried out at 25 °C with shaking at 200 r/min for 8 h. The cells were then harvested by centrifugation at 4 °C and 6000 r/min for 5 min and washed twice with phosphate buffer (0.1 mol/L, pH 7.0).
2.3. Selection of the biphasic systems
The synthesis of PLA in different biphasic systems was conducted in 50-ml shake flasks. We suspended 10 g/L of wet cells in 10 ml of the reaction mixture containing 5 ml phosphate buffer (pH 7.0) with 40 g/L glucose and 5 g/L PPA, and 5 ml organic solvent. The catalysis process was performed at 37 °C and 200 r/min for 60 min.
2.4. Synthesis of PLA in a flask with substrate feeding
The synthesis of PLA in the aqueous/n-octane biphasic system was performed in 500-ml shake flasks. One hundred milliliters of the reaction mixture contained 60 ml phosphate buffer (pH 7.0) with 40 g/L glucose, 30 g/L cell, and 40 ml n-octane. At the beginning of catalysis, 5 ml of 250 g/L PPA was added into the biphasic system at 40 °C with shaking at 200 r/min; 5 ml of 250 g/L PPA and 2 g glucose were supplemented twice at 30 and 70 min during the process.
2.5. Synthesis of PLA in a mechanically stirred reactor
We used a 5-L reactor for the synthesis of PLA in a mechanically stirred reactor (Winpact FS-05). We inoculated 0.02 g/ml E. coli pET-28a-ldh Y52V in 4 L of LB medium supplemented with kanamycin (50 μg/ml) and cultured it at 37 °C with stirring at 200 r/min. When the OD600 reached 0.6–0.8, 1 mmol/L isopropyl-β-D-thiogalactoside was added and the cells were cultured at 25 °C for 8 h. The cells were then collected by centrifugation and suspended in the 2-L biphasic system (30 g/L wet cells) containing 0.8 L n-octane and 1.2 L phosphate buffer with 40 g dissolved glucose and 25 g PPA. The conversion was performed at 40 °C for 150 min. The pH was maintained at 7.0 and the dissolved oxygen (DO) level was kept at 35% by automatically adjusting the stirring speed; the ventilatory capacity was 0.5 vvm (air volume/culture volume per min). The substrate was supplemented by feeding 100 ml of 250 g/L PPA. Glucose (40 g) was added into the reaction system at 30, 60, and 90 min. The aqueous and organic phases were separately subjected to high-performance liquid chromatography (HPLC).
2.6. Analytical methods
The concentrations of PPA and PLA were determined using an LC-20A chromatograph (Shimadzu, Japan) equipped with a Shim-pack VP-ODS C18 chromatographic column (Shimadzu, Japan); mobile phase A consisted of 0.05% trifluoroacetic acid solution and mobile phase B comprised 0.05% trifluoroacetic acid methanol solution. The flow rate was 1 ml/min. The ratio of B ranged from 10% to 100% between 0 and 20 min, was maintained at 100% for 3 min, ranged from 100% to 10% between 23 and 25 min, and was then again maintained at 10% for 5 min. The temperature of the column was 30 °C. The sampling volume was 5 μl, and the detection wavelength was 210 nm. The distribution coefficient was defined as PLA (PPA) in the organic solvent/PLA (PPA) in buffer. The total concentration (C) of PLA was calculated as C (g/L)=(W A+W O)/V, where W A and W O indicate the quality of PLA in the aqueous and organic phases, respectively, and V indicates the total volume of the biphasic system. The molar conversion ratio was defined as mol(PLA)/mol(PPA)×100%, where mol(PLA) and mol(PPA) denote the molar masses of synthetic PLA and consumed PPA, respectively.
3. Results and discussion
3.1. Selection of organic solvents
Generally, organic solvents have toxic effects on biological cells, which results in decreased biological activity. Therefore, in this study, we tested eight biphasic systems consisting of different polar organic solvents (methylbenzene, ethyl acetate, cyclohexane, n-hexane, n-octane, isoamylol, trichloromethane, and oleic acid) and phosphate buffer, and studied the distribution coefficients of PPA and PLA and their effects on the production of PLA by whole cells (Table 1). As is evident from Fig. 2, n-octane was found to be the best organic solvent (logP=4.5). In the aqueous/n-octane biphasic system, the distribution ratios of PPA and PLA were 0.40 and 0.37, respectively, and the conversion ratio (67.23%) was higher than that obtained using an aqueous-phase system. Although the distribution ratios of PLA in aqueous/isoamylol and aqueous/ethyl acetate were better than that in aqueous/n-octane, the production of PLA was not increased due to the polarity and toxicity to cells. Part of the PLA produced migrated into the n-octane phase, and aggrandized cell membrane permeability, which decreased the inhibitory effect of PLA, and thus increased the yield of PLA.
Table 1.
Distribution coefficients of PPA and PLA in different biphasic systems
| Organic solvent | LogP | Distribution coefficient |
|
| PPA | PLA | ||
| Control | |||
| Isoamylol | 1.3 | 0.210±0.050 | 1.440±0.330 |
| Trichloromethane | 1.6 | 0.220±0.036 | 0.230±0.015 |
| Ethyl acetate | 1.7 | 0.280±0.011 | 0.630±0.056 |
| Methylbenzene | 2.5 | 1.280±0.025 | 0.230±0.047 |
| Cyclohexane | 3.2 | 0.330±0.140 | 0.200±0.076 |
| n-Hexane | 3.5 | 0.290±0.042 | 0.290±0.130 |
| n-Octane | 4.5 | 0.400±0.074 | 0.370±0.065 |
| Oleic acid | 7.7 | 0.330±0.006 | 0.280±0.059 |
LogP: polarity of organic solvents. Distribution coefficients are expressed as mean±standard deviation (SD), with n=4
Fig. 2.
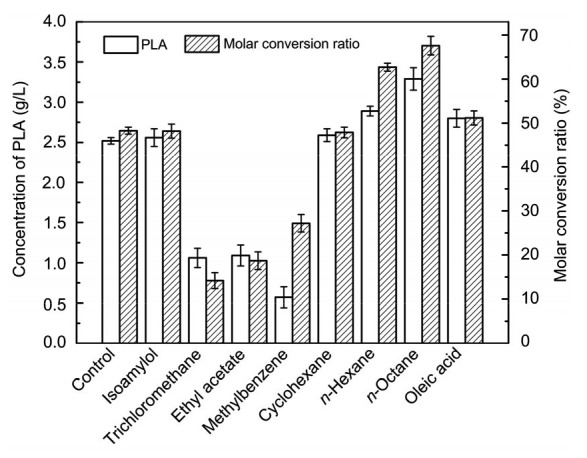
Effects of various organic solvents on the production and molar conversion ratio in biphasic systems
Reaction conditions: 5 ml organic solvent, 5 ml phosphate buffer, 5 g/L PPA, 20 g/L glucose, 15 g/L cells, 37 °C, and 200 r/min. Data are expressed as mean±SD, with n=4
3.2. Effect of the volume ratio of n-octane and the aqueous phase on PLA synthesis
The volume ratio of the organic and aqueous phases was defined as V o/V a, where V o is the volume of the organic solvent and V a is the volume of the aqueous phase. The V o/V a ratio would affect the distribution ratios of the substrate and the product as well as cell viability. In this study, the n-octane content ranged from 20% to 60%. When the volume fraction of n-octane was 40%, the concentration of PLA produced was 6.34 g/L and the conversion ratio was the highest (Fig. 3).
Fig. 3.
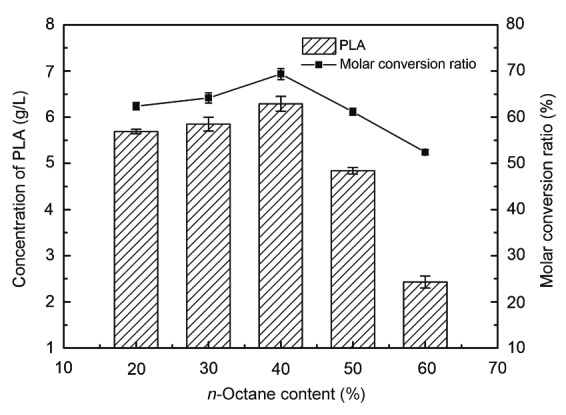
Effects of n-octane volume ratio on the production of PLA and molar conversion ratio
Reaction conditions: 10 ml biphasic system containing different volumes of n-octane and the aqueous phase, 10 g/L PPA, 30 g/L cells, 37 °C, 200 r/min, and 60 min. Data are expressed as mean±SD, with n=4
Organic solvents are virulent to enzymes and microbial cells. The higher the volume of the organic solvent, the greater is the toxicity. An appropriate volume fraction of n-octane balanced the inhibitory effect of PLA and the toxicity of n-octane, thus resulting in an increase in the production of PLA in the biphasic system.
3.3. Effect of reaction temperature on PLA synthesis
Reaction temperature is a key factor in whole-cell conversion as it can influence the activity of critical enzymes such as LDH. In a biphasic system, temperature is also a principal parameter as it influences the stability of the solvent phase as well as the reaction equilibrium (Zou et al., 2013; Resasco, 2014). In this study, we varied the temperature of the conversion system in the range of 30–45 °C and found that the concentration of PLA and the conversion ratio increased with temperature (Fig. 4). The PLA concentration was 6.76 g/L at 40 °C, which was 18.35% higher than that in an aqueous system; the conversion ratio was 72.23%. The change in reaction temperature also influenced enzyme activity and substrate dissolution: enzyme activity and substrate dissolution improved gradually as the temperature increased. High temperature adversely affects the activity of LDH and microbial cells. Therefore, the production of PLA decreased when the reaction temperature was over 40 °C.
Fig. 4.
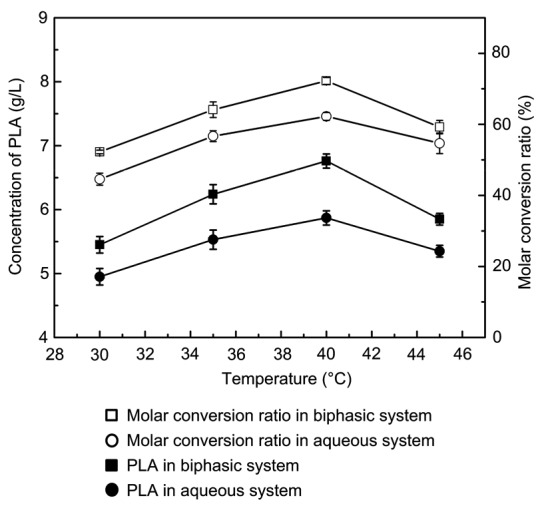
Effects of reaction temperature on the production of PLA and molar conversion ratio
Reaction conditions: 10 g/L PPA, 30 g/L cells, 200 r/min, and 60 min. Aqueous phase system: 10 ml buffer; Biphasic system: 4 ml n-octane and 6 ml buffer. Data are expressed as mean±SD, with n=4
3.4. Effect of cell concentration on PLA synthesis
Cell concentration is directly related to reaction efficiency and enzyme quantity. When the substrate concentration is constant, an increase in cell concentration will result in a higher conversion ratio. However, higher cell concentrations reduce the production per gram cell (Resasco, 2014). When 10 g/L PPA was added to the system, the concentration of PLA increased with increasing cell concentration. The PLA concentration and conversion ratio were the highest at 60 g/L cell concentration (Fig. 5). However, at cell concentrations higher than 60 g/L, the PLA production per gram cell began to decrease, although the production and conversion ratio were not significantly affected. Taking into account the cost and efficiency of the process, a cell concentration of 30 g/L was determined to be optimum for further studies.
Fig. 5.
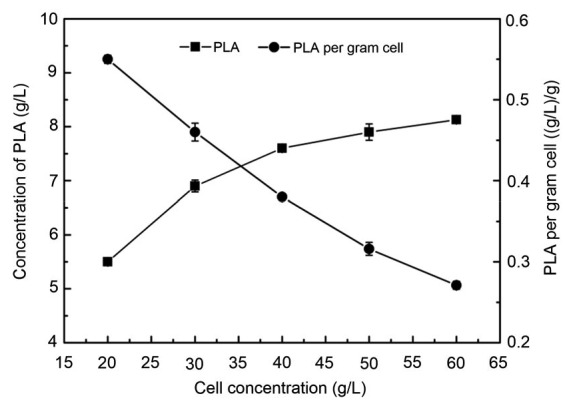
Effect of cell concentration on the production of PLA
Reaction conditions: 4 ml n-octane, 6 ml buffer, 10 g/L PPA, different cell concentrations, 40 °C, 200 r/min, and 60 min. Data are expressed as mean±SD, with n=4
3.5. Effect of substrate concentration on PLA synthesis
PPA concentration is a significant factor that affects the production of PLA. Low PPA concentration will affect PLA production, but high concentration is toxic to cells (Zou et al., 2013). Therefore, we investigated the effect of substrate concentration on the production of PLA in an aqueous phase system and an aqueous/n-octane biphasic system (Fig. 6). When 12.5 g/L PPA was added to the conversion system, the PLA concentration in the biphasic system (9.7 g/L) increased by 14.11% compared with that in the aqueous phase system (8.5 g/L). PPA was toxic to cells at high concentrations and influenced cell activity; PLA concentration and conversion ratio decreased when the PPA concentration exceeded 12.5 g/L.
Fig. 6.
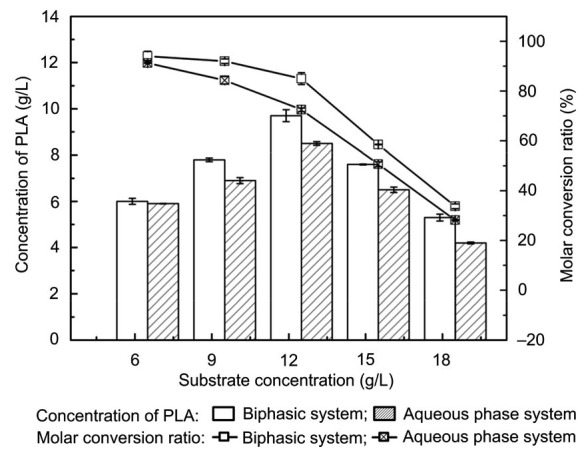
Effects of substrate concentration on the production of PLA and molar conversion ratio
Reaction conditions: 30 g/L cells, 40 °C, 200 r/min, and 60 min. Aqueous phase system: 10 ml buffer; Biphasic system: 4 ml n-octane and 6 ml buffer. Data are expressed as mean±SD, with n=4
3.6. Biosynthesis of PLA by fed-batch in flasks
Under the optimized conditions, we added the substrate at 30 and 70 min and found that the PLA concentration increased to 20.98 g/L at 150 min, which was higher than that reported previously for PLA synthesis in an aqueous phase (Zhu et al., 2015). In our study, the productivity was 8.392 g/(L∙h), and the final conversion ratio was 85.13% (Fig. 7). At the beginning of the reaction, the conversion reaction was rapid, PLA was synthesized rapidly, and the conversion ratio was high. After 100 min, the high PLA concentration had an inhibitory effect on the cells and the rate of PLA generation decreased.
Fig. 7.
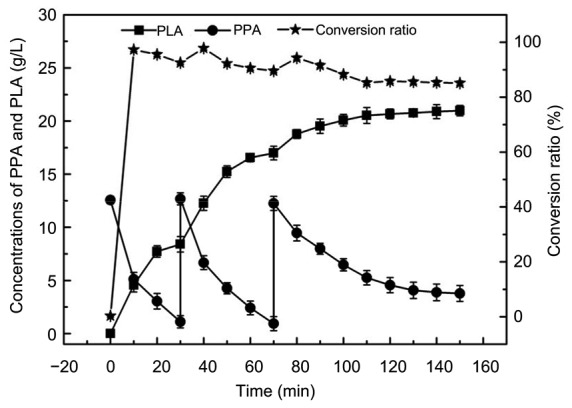
Production of PLA by fed-batch in a flask
Reaction conditions: 40 ml n-octane, 60 ml buffer (0.1 mol/L, pH 7.0), 30 g/L cells, 40 °C, 200 r/min, fed-batch, and 150 min. Data are expressed as mean±SD, with n=4
3.7. Biosynthesis of PLA in a mechanically stirred reactor
Under the optimized conditions, the substrate was added at 30, 60, and 90 min. The PLA concentration peaked at 32.31 g/L at 150 min, which implied that the PLA yield increased by 54% compared to that achieved in the shaking flasks. The productivity was also boosted to 12.92 g/(L∙h), which is higher than that reported in the latest study (Xu et al., 2016) (Fig. 8). In the mechanically stirred reactor, the DO was maintained at 35% and pH was maintained at 7.0 at all times. The DO provided adequate oxygen for whole-cell conversion and the pH level was most suitable for cell activity and could reduce the acid stress caused by PLA accumulation. Taken together, these results indicated that fermentation in a stirred tank was more suitable for PLA synthesis than that in a flask.
Fig. 8.
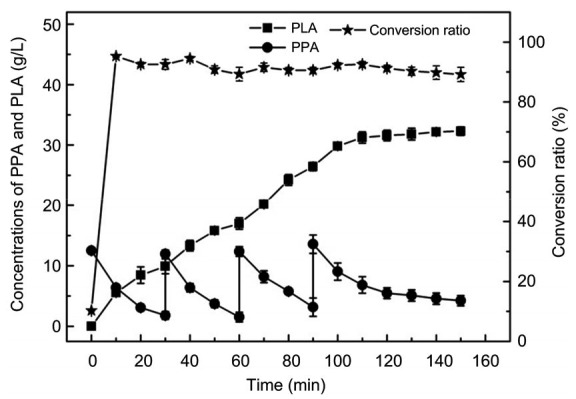
PLA production in a fermentation tank
Reaction conditions: 0.8 L n-octane, 1.2 L buffer (0.1 mol/L, pH 7.0), 30 g/L cells, 40 °C, 200 r/min, fed-batch, and 150 min. Data are expressed as mean±standard error, with n=4
The PLA concentrations obtained using whole-cell conversion in recent studies using aqueous monophonic systems are shown in Table 2. From Table 2, it is evident that the concentration and productivity of PLA obtained by whole-cell conversion in any of these previously reported studies are not high. Wang M et al. (2016) and Wang Y et al. (2016) achieved high PLA production using E. coli pET-28a-DLDH744 and recombinant E. coli BL21 (DE3), respectively, in aqueous monophonic systems, but the productivity of PLA was only 4.82 and 4.94 g/(L∙h), respectively. This implies that a biphasic whole-cell conversion system can help obtain higher yield and productivity of PLA than an aqueous system.
Table 2.
PLA yield by whole-cell conversion reported in previous studies
| Strain | Reaction system | PLA concentration (g/L) | Conversion ratio (%) | Productivity (g/(L∙h)) | Reference |
| Lactobacillus sp. SK007 | Aqueous | 17.38 | 51.10 | 0.241 | Mu et al., 2009 |
| E. coli pET-28a-LDHY52L | Aqueous | 18.47 | 65.96 | 3.078 | Jiang et al., 2016 |
| E. coli pET-28a-ldh Y52V | Aqueous | 15.60 | 77.00 | 3.805 | Zhu et al., 2015 |
| E. coli pET-28a-DLDH744 | Aqueous | 21.43 | 82.38 | 4.820 | Wang M et al., 2016 |
| E. coli pET-28a-ldhL | Aqueous | 19.75 | 65.32 | 4.940 | Wang Y et al., 2016 |
| E. coli BL21 pET28-gdh-T7-ppr | Aqueous | n.i. | 91.30 | 10.120 | Xu et al., 2016 |
| E. coli pET-28a-ldh Y52V | Biphasic | 32.31 | 88.45 | 12.920 | This study |
n.i.: no information
4. Conclusions
We studied the application of aqueous/non-aqueous biphasic system in the synthesis of PLA by recombinant E. coli pET-28a-ldh Y52V cells, and the conversion conditions were optimized. The presence of n-octane resulted in a partial transfer of PLA to the organic phase, which relieved the inhibitory effect of PLA. Under the optimum conditions, the production of PLA by fed-batch in an aqueous/n-octane biphasic system was significantly higher than that in an aqueous system. In the future, this method of synthesis of PLA in the aqueous/n-octane biphasic system can be applicable to scale-up of the PLA production. In addition, the aqueous/non-aqueous biphasic system can be applied to the production of PLA analogues.
Acknowledgments
We thank Editage for providing language services.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31470092 and 31501459) and the Natural Science Youth Foundation of Jiangsu Province (No. BK20130380), China
Compliance with ethics guidelines: Yi-bo ZHU, Yan XU, Li-mei WANG, and Bin QI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Delbecq F, Wang Y, Len C. Conversion of xylose, xylan and rice husk into furfural via betaine and formic acid mixture as novel homogeneous catalyst in biphasic system by microwave-assisted dehydration. J Mol Catal A: Chem. 2016;423:520–525. doi: 10.1016/j.molcata.2016.07.003. [DOI] [Google Scholar]
- 2.Dieuleveux V, Lemarinier S, Guéguen M. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int J Food Microbiol. 1998;40(3):177–183. doi: 10.1016/S0168-1605(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 3.Jiang ZY, Ji W, Qian BB, et al. The recombinant expression of D-lactic dehydrogenase mutant D-LDHY52L in E. coli and its application in D-phenyllactic acid synthesis research. Food Ferment Ind. 2016;42:1–6. doi: 10.13995/j.cnki.11-1802/ts.201601001. (in Chinese) [DOI] [Google Scholar]
- 4.Kawaguchi H, Uematsu K, Ogino C, et al. Simultaneous saccharification and fermentation of kraft pulp by recombinant Escherichia coli for phenyllactic acid production. Biochem Eng J. 2014;88:188–194. doi: 10.1016/j.bej.2014.04.014. [DOI] [Google Scholar]
- 5.Kobayashi T, Adachi S, Nakanishi K, et al. Synthesis of alkyl glycosides through β-glucosidase-catalyzed condensation in an aqueous–organic biphasic system and estimation of the equilibrium constants for their formation. J Mol Catal B: Enzym. 2000;11(1):13–21. doi: 10.1016/S1381-1177(00)00190-9. [DOI] [Google Scholar]
- 6.Li X, Ning Y, Liu D, et al. Metabolic mechanism of phenyllactic acid naturally occurring in Chinese pickles. Food Chem. 2015;186:265–270. doi: 10.1016/j.foodchem.2015.01.145. [DOI] [PubMed] [Google Scholar]
- 7.Li YN, Shi XA, Zong MH, et al. Asymmetric reduction of 2-octanone in water/organic solvent biphasic system with Baker’s yeast FD-12. Enzyme Microb Technol. 2007;40(5):1305–1311. doi: 10.1016/j.enzmictec.2006.10.016. [DOI] [Google Scholar]
- 8.Mu W, Liu F, Jia J, et al. 3-Phenyllactic acid production by substrate feeding and pH-control in fed-batch fermentation of Lactobacillus sp. SK007. Bioresour Technol. 2009;100(21):5226–5229. doi: 10.1016/j.biortech.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Ni Y, Su Y, Li H, et al. Scalable biocatalytic synthesis of optically pure ethyl (R)-2-hydroxy-4-phenylbutyrate using a recombinant E. coli with high catalyst yield. J Biotechnol. 2013;168(4):493–498. doi: 10.1016/j.jbiotec.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Ohhira I, Kuwaki S, Morita H, et al. Identification of 3-phenyllactic acid as a possible antibacterial substance produced by Enterococcus faecalis TH 10. Biocontrol Sci. 2004;9(3):77–81. doi: 10.4265/bio.9.77. [DOI] [Google Scholar]
- 11.Resasco DE. Carbon nanohybrids used as catalysts and emulsifiers for reactions in biphasic aqueous/organic systems. Chin J Catal. 2014;35(6):798–806. doi: 10.1016/S1872-2067(14)60119-4. [DOI] [Google Scholar]
- 12.Sakdaronnarong C, Saengsawang A, Siriyutta A, et al. An integrated system for fractionation and hydrolysis of sugarcane bagasse using heterogeneous catalysts in aqueous biphasic system. Chem Eng J. 2016;285:144–156. doi: 10.1016/j.cej.2015.09.098. [DOI] [Google Scholar]
- 13.Tan ST, Wilkins AL, Molan PC, et al. A chemical approach to the determination of floral sources of New Zealand honeys. J Apicult Res. 1989;28(4):212–222. doi: 10.1080/00218839.1989.11101187. [DOI] [Google Scholar]
- 14.Tuberoso CI, Bifulco E, Caboni P, et al. Lumichrome and phenyllactic acid as chemical markers of thistle (Galactites tomentosa Moench) honey. J Agric Food Chem. 2011;59(1):364–369. doi: 10.1021/jf1039074. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Li W, Song JR, et al. Production, purification, and identification of cholest-4-en-3-one produced by cholesterol oxidase from Rhodococcus sp. in aqueous/organic biphasic system. Biochem Insights. 2015;8(s1):1–8. doi: 10.4137/BCI.S21580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Zhu L, Xu X. Efficient production of enantiomerically pure D-phenyllactate from phenylpyruvate by structure-guided design of an engineered D-lactate dehydrogenase. Appl Microbiol Biotechnol. 2016;100(17):7471–7478. doi: 10.1007/s00253-016-7456-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, He H, Hu FG, et al. Optimization of D-phenyllactic acid production by whole cells of recombinant Escherichia coli using response surface methodology. Food Sci. 2016;37(7):88–92. doi: 10.7506/spkx1002-6630-201607017. (in Chinese) [DOI] [Google Scholar]
- 18.Xu GC, Zhang LL, Ni Y. Enzymatic preparation of D-phenyllactic acid at high space-time yield with a novel phenylpyruvate reductase identified from Lactobacillus sp. CGMCC 9967. J Biotechnol. 2016;222:29–37. doi: 10.1016/j.jbiotec.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Yi Q, Sarney DB, Khan JA, et al. A novel approach to biotransformations in aqueous-organic two-phase systems: enzymatic synthesis of alkyl β-[D]-glucosides using microencapsulated β-glucosidase. Biotechnol Bioeng. 1998;60(3):385–390. doi: 10.1002/(sici)1097-0290(19981105)60:3<385::aid-bit16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Zhu L, Zhou C, et al. Enzymatic production of D-3-phenyllactic acid by Pediococcus pentosaceus D-lactate dehydrogenase with NADH regeneration by Ogataea parapolymorpha formate dehydrogenase. Biotechnol Lett. 2014;36(3):627–631. doi: 10.1007/s10529-013-1404-2. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Z, Ma C, Gao C, et al. Efficient conversion of phenylpyruvic acid to phenyllactic acid by using whole cells of Bacillus coagulans SDM. PLoS ONE. 2011;6(4):e19030. doi: 10.1371/journal.pone.0019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Hu F, Zhu Y, et al. Enhancement of phenyllactic acid biosynthesis by recognition site replacement of D-lactate dehydrogenase from Lactobacillus pentosus . Biotechnol Lett. 2015;37(6):1233–1241. doi: 10.1007/s10529-015-1778-4. [DOI] [PubMed] [Google Scholar]
- 23.Zou S, Yan H, Hu Z, et al. Enzymatic resolution of epichlorohydrin catalyzed by whole cells in an organic solvent/buffer biphasic system. Chin J Catal. 2013;34(7):1339–1347. doi: 10.1016/S1872-2067(12)60576-2. [DOI] [Google Scholar]