Abstract
Microorganisms provide both beneficial and harmful effects to human beings. Beneficial effects come from the symbiotic relationship that exists between humans and microbiota, but then several human illnesses have turned some friendly microbes into opportunistic pathogens, causing several microbial-related diseases. Various efforts have been made to create and utilize antimicrobial agents in the treatment and prevention of these infections, but such efforts have been hampered by the emergence of antimicrobial resistance. Despite extensive studies on drug discovery to alleviate this problem, issues with the toxicity and tolerance of certain compounds and continuous microbial evolution have forced researchers to focus on screening various phytochemical dietary compounds for antimicrobial activity. Linolenic acid and its derivatives (eicosapentaenoic acid and docosahexaenoic acid) are omega-3 fatty acids that have been studied due to their role in human health, being important for the brain, the eye, the cardiovascular system, and general human growth. However, their utilization as antimicrobial agents has not been widely appreciated, perhaps due to a lack of understanding of antimicrobial mechanisms, toxicity, and route of administration. Therefore, this review focuses on the efficacy, mechanism, and toxicity of omega-3 fatty acids as alternative therapeutic agents for treating and preventing diseases associated with pathogenic microorganisms.
Keywords: Linolenic acid, Omega-3 fatty acid, Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Antimicrobial agent, Fatty acid
1. Introduction
A substance capable of killing or inhibiting the growth of microorganisms is known as an antimicrobial agent. Antimicrobial agents are classified as -cidal or -static based on their ability to kill or suppress the growth of microorganisms, respectively. These agents involve a wide range of medicinal compounds that include antibacterial, antifungal, antiviral, and anti-parasitic drugs (Leekha et al., 2011; CDC, 2015). Antimicrobial agents came to light with the discovery of penicillin in 1928 by Alexander Fleming (CDC, 2015) and its use in the 1940s (Wainwright, 1989; CDC, 2015). Since then, conventional antimicrobial agents have been produced and are preferred over natural compounds like fatty acids (FAs) because of their greater effectiveness and selectivity (Thormar, 2011).
However, their application in preventing and treating infections in humans, animals, and plants has led to the emergence and spread of antimicrobial resistance (CDC, 2015). As well as the development of drug resistance, conventional drugs also possess undesired adverse effects (Sousa et al., 2016), prompting researchers to look for alternative antimicrobial agents (with little or no adverse effects) to evade the drawbacks that accompany drug resistance.
There is a plethora of data that elucidates the antimicrobial value of various phytochemicals and dietary compounds (Morais-Braga et al., 2012; Kalia et al., 2015). Phytochemical compounds are naturally occurring compounds produced by plants and their biological activity has an impact on human health (Babajide et al., 2013; Mukherjee et al., 2013; Moss and Ramji, 2016). Sources include vegetables, legumes, nuts, fruits, and seeds (Nascimento et al., 2000; Wall et al., 2010). Studies have shown that such dietary products can provide humans with nourishment, and are the reservoirs of several therapeutic compounds for various diseases such as cardiovascular diseases, diabetes, hypertension, and cancer (Burlingame et al., 2009; Lopez-Romero et al., 2015; Pagliaro et al., 2015; Mattos et al., 2016; Mileski et al., 2016; Moss and Ramji, 2016). The FAs, among several phytochemical compounds, are equally important for human development and growth. Several types of FAs including omega-3 polyunsaturated FAs (PUFAs) have been found to play significant roles in human eye-and brain-related conditions (Carballeira, 2008; Das, 2008; Richard et al., 2008; Desbois and Smith, 2010; Desbois and Lawlor, 2013). However, the use of FAs as antimicrobial agents has yet to receive much attention in clinical medicine. This paper summarizes the recent and existing literature on the effectiveness, mechanism, and toxicity of omega-3 FAs, demonstrating that these FAs possess antimicrobial properties and are therefore potential therapeutic targets for clinical use in future.
2. Classification and utilization of fatty acids
FAs are essential for living organisms and are widely found in dairy products, vegetation, and seeds. In humans, fats can provide an absorption medium for fat-soluble vitamins and improve the palatability of food, and play a pivotal role in human growth and development, from embryonic development through infancy and childhood (Burlingame et al., 2009). FAs can be classified as either saturated or unsaturated, based on their chemical and physical structures. Saturated FAs with a single carbon to carbon (C–C) bond in their chemical structure are mostly solid at room temperature, while unsaturated FAs with one or more carbon to carbon (C=C) double bonds are usually in liquid state at room temperature. The unsaturated FAs can be subdivided into monounsaturated FAs with one C=C double bond and PUFAs with two or more C=C double bonds. Depending on the arrangement of hydrogen atoms in relation to the double bonds, unsaturated FAs can also be classified into a cis configuration (hydrogen atoms on the same side) or trans configuration (hydrogen atoms on the opposite side).
The omega-6 and omega-3 FA families are widely discussed due to their nutritional and human health benefits. The inability of the human body to derive linoleic acid (LA) and arachidonic acid (AA) from compounds such as carbohydrates or proteins means that they, and all omega-3 FAs, are commonly considered to be essential FAs (Lawrence, 2010). LA is the parent FA of the omega-6 family and AA is the most important omega-6 PUFA as the primary precursor of omega-6-derived eicosanoids (WHO, 1995). On the other hand, linolenic acid (LNA) is the parent FA of the omega-3 family with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) as the most essential omega-3 FAs in human diet (WHO, 1995). Despite the essentiality of these FAs, they are not produced by the human body but are acquired through nutritional diet and supplements. Moreover, the presence of a double bond specifically at omega-3 position in LNA and at omega-6 position in LA allows these FAs to play important functions in human bodies, but human enzymes cannot modify the FA chain beyond carbon 10 of the carboxylic acid end (Neitzel, 2010). This makes LA and LNA essential FAs for human health.
The human body can metabolize LNA from a nutritious diet using a series of enzymes such as desaturases and elongases. Since FAs are firstly synthesized in saturated forms, specific enzymes desaturate them through insertion of a double bond in the carbon chain on positions 5, 6, and 9, and the chain may be further elongated by adding two carbon atoms to the elongase enzymes (Lawrence, 2010). It is through these metabolic enzymatic reactions that EPA and DHA are produced (Fig. 1).
Fig. 1.
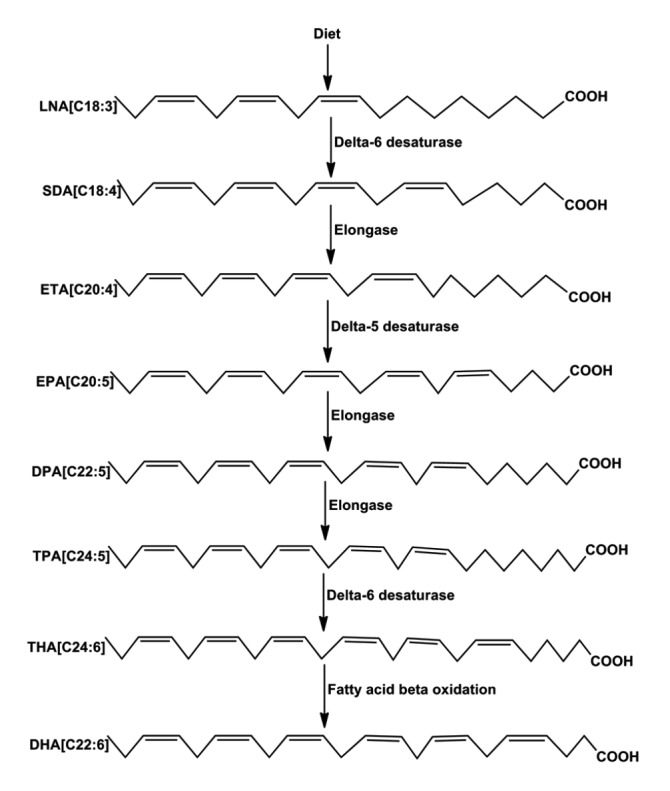
Metabolism of linolenic acid to docosahexaenoic acid
Linolenic acid is firstly desaturated at carbon 6, then elongated and desaturated at carbon 5, followed by a couple of elongation processes, a final desaturation at carbon 6 and then removal of the two added carbons through fatty acid β-oxidation to produce DHA. LNA: linolenic acid; SDA: stearidonic acid; ETA: eicosatetraenoic acid; EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; TPA: tetracosapentaenoic acid; THA: tetracosahexaenoic acid; DHA: docosahexaenoic acid. The schematic drawing was prepared with ChemBioDraw Ultra 14.0 software
LNA and its derivatives (EPA and DHA) play a vital role in human health and development (Hoffman, 2013; Igennus, 2016). The body requires a certain amount of EPA and DHA to function properly. Lower levels of EPA especially in adolescents and adults, for instance, has been associated with neurodegenerative diseases, depression, joint and bone conditions, heart problems, dyslexia, and dyspraxia (Igennus, 2016). The effects of increasing omega-3 FAs levels reduce the synthesis of inflammatory eicosanoids, reactive oxygen species, and cytokines, and give rise to anti-inflammatory mediators such as resolvins (Calder, 2006). These effects have been linked to EPA and the ratio of EPA/AA on prostanoid metabolism and function (Smith, 2005). In addition, EPA displaces AA, the pro-inflammatory precursor, by regulating the action of phospholipase A2 and cyclooxygenase enzymes to produce anti-inflammatory products, which inhibit the production of AA from dihomo-γ-LNA (Igennus, 2016).
3. Biological activity and mechanism of omega-3 fatty acids
3.1. Antimicrobial activity
Pathogenic microorganisms cause infections in various ways, such as the production of virulence factors, formation of biofilms to survive hostile environments, the modification of access points for antimicrobial agents, and acquisition of resistance genes as a way to evade host defense systems to ensure their survival (Prince, 2002; Cavalieri et al., 2005; Bordi and de Bentzmann, 2011; Sharma et al., 2014). However, in vitro and in vivo studies indicate that LNA and its derivatives, used alone or in combination with conventional drugs, possess antimicrobial properties (Table 1). For instance, with the aim of enhancing the biosynthesis of omega-3 PUFAs in transgenic zebrafish, Cheng et al. (2015) studied the effects of expressing delta-6 desaturase and elongase genes in the liver of Vibrio vulnificus-infected transgenic fish. Interestingly, the survival rate of transgenic strains was 70%, while that of wild type was 20% 24 h post-infection. Since delta-6 desaturase and elongase enzymes are involved in metabolizing LNA (Fig. 1), increased production of EPA and DHA inhibited bacterial growth and enhanced production of anti-inflammatory cytokines, thereby protecting zebrafish from V. vulnificus infection (Cheng et al., 2015). From this study, it can also be deduced that virulence production of cytotoxins could have been suppressed by the presence of biosynthesized omega-3 PUFAs, indicating the antibacterial effect of these FAs. Moreover, EPA and DHA are reported to induce the death of the Plasmodium species, inhibit viral replication, exert anti-hepatitis C virus activity, and exhibit bactericidal and fungicidal effects, suggesting that omega-3 PUFAs function as endogenous antimicrobial molecules (Das, 2008) and a detailed spectrum of antimicrobial activity exerted by EPA is reviewed elsewhere (Desbois, 2013). Furthermore, Mil-Homens et al. (2012) studied the antimicrobial effects of DHA on Burkholderia cenocepacia using a Galleria mellonella caterpillar in vivo model, which showed that omega-3 FAs have antimicrobial properties.
Table 1.
Antimicrobial activity of omega-3 PUFAs
| Treatment | Dose | Spectrum | Pathogen(s) | Mode of action | Model system | Reference |
| EPA | 100 μmol/L | Antibacterial, anti-biofilm | Porphyromonas gingivalis/Fusobacterium nucleatum | ↓ virulence factor gene expression | In vitro | Sun et al., 2016 |
| DHA | 100 μmol/L | Antibacterial, anti-biofilm | P. gingivalis/F. nucleatum | ↓ virulence factor gene expression | In vitro | Sun et al., 2016 |
| DHA | 100 μmol/L | Antibacterial | Helicobacter pylori | Cell morphology alteration | In vitro | Correia et al., 2012 |
| DHA | 50 μmol/L | Antibacterial | H. pylori | Inhibit gastric colonisation | In vitro | Correia et al., 2012 |
| DHAstandard therapy | 50 μmol/L | Antibacterial | H. pylori | Prevent infection recurrence | In vitro | Correia et al., 2012 |
| EPA and DHA | Antibacterial | Vibrio vulnificus | Inhibit bacterial growth | In vitro | Cheng et al., 2015 | |
| EPA | Antibacterial | Methicillin resistant Staphylococcus aureus (MRSA) | In vitro | Desbois and Smith, 2009 | ||
| EPAneomycin | Antibacterial | S. aureus | In vitro | Desbois and Lawbor, 2013 | ||
| ALA, DHA and EPA | 2.5, 25, 250 μg/ml | Antibacterial | Streptococcus mutans | Inhibit bacterial growth | In vitro | Huang et al., 2010 |
| ALA, DHA and EPA | 2.5, 25, 250 μg/ml | Anti-fungal | Candida albicans | Inhibit bacterial growth | In vitro | Huang et al., 2010 |
| ALA, DHA and EPA | 2.5, 25, 250 μg/ml | Antibacterial | Aggregatibacter actinomycetemcomitans | Inhibit bacterial growth | In vitro | Huang et al., 2010 |
| ALA, DHA and EPA | 2.5, 25, 250 μg/ml | Antibacterial | P. gingivalis | Inhibit bacterial growth | In vitro | Huang et al., 2010 |
| ALA, DHA and EPA | 2.5, 25, 250 μg/ml | Antibacterial | F. nucleatum | Inhibit bacterial growth | In vitro | Huang et al., 2010 |
| DHA | 50 mmol/L | Antibacterial | Burkholderia cenocepacia | Inhibit bacterial growth | In vivo/in vitro | Mil-Homens et al., 2012 |
| LNA | 0.05–0.40 mmol/L | Antibacterial | S. aureus Staphylococcus pyogenes | Inhibit FabI enzyme activity | In vitro | Zheng et al., 2005 |
| DHA | 20–40 μg/ml | Antiparasitic | Plasmodium falciparum | Inhibit FabI enzyme activity | Carballeira, 2008 |
EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; ALA: α linolenic acid; LNA: linolenic acid; ↓: reduced
3.2. Mechanisms behind the function of omega-3 fatty acids
The survival of bacteria in any environment depends on how they alter their membrane viscosity by regulating the formation and structure of their FAs in order to meet environmental requirements (Zhang and Rock, 2008). The phospholipid acyl chains regulate the viscosity of the cell membrane, and it is through this viscosity adjustment that impacts are made on the vital functions of the cell membrane, such as the transportation of active solutes, passive permeability of hydrophobic molecules, and protein-to-protein interactions (Zhang and Rock, 2008). However, FAs are reported to alter cell membrane hydrophobicity, cell surface charge, and membrane integrity, which lead to electron leakage resulting in cell death (Desbois and Smith, 2010; Lawrence, 2010; Calo et al., 2015; Lopez-Romero et al., 2015). Similarly, omega-3 FAs exert some of their activity on the cell membrane. For instance, scanning electron microscope analysis revealed Porphyromonas gingivalis and Fusobacterium nucleatum cellular distortion in the presence of 100 μmol/L DHA or EPA, which led to growth inhibition and the expression of the virulence factor gene was downregulated resulting in suppression of the pathogenesis of periodontal disease (Sun et al., 2016).
The precise adjustment of membrane lipid composition is performed by bacteria through altering the types of FAs formed by the biosynthetic pathway (Zhang and Rock, 2008). Phospholipids are produced via the FA biosynthetic pathway to serve the particular purpose of electron transport, solute transport, initiation of DNA replication, and cell division (Zhang and Rock, 2008). Unlike mammalian FA synthesis, which is regulated by type I enzyme (a single multifunctional enzyme-acyl carrier protein complex), bacterial FA is facilitated by a set of individual enzymes, the type II enzymes (Zheng et al., 2005). One of these enzymes is an enoyl-acyl carrier protein reductase (FabI), which plays an important role in the final elongation process for type II FA synthesis (Zheng et al., 2005; Zhang and Rock, 2008). Blocking the action of FabI can reduce the rate of FA elongation (Zhang and Rock, 2008), which may negatively affect the FA biosynthetic pathway such as the malfunctioning of phospholipids. It is believed that some exogenous FAs, such as DHA, oleic acid, LNA, LA, AA, and palmitoleic acid (Zheng et al., 2005; Carballeira, 2008), can alter the activity of FabI enzyme in the biosynthetic pathway. For instance, the anti-parasitic activity of marine FAs has been reviewed and found to be effective inhibitors of the Plasmodium falciparum FabI enzyme (Carballeira, 2008). DHA is reported to kill more than 90% of P. falciparum with the concentration of 20–40 µg/ml, and also as effective inhibitors of N-myristoyltransferase for antifungal activity (Carballeira, 2008). Similarly, various unsaturated FAs with a concentration ranging from 0.05 to 0.40 mmol/L inhibit the growth of Staphylococcus aureus and Staphylococcus pyogenes by blocking the FabI enzyme (Zheng et al., 2005).
The question on whether omega-3 PUFAs are pro-or anti-oxidant agents is divisive. This is due to their high susceptibility to free radical oxidation (Grundt et al., 2003), which can result in the induction of oxidative stress, negatively impacting human health by eliciting atherogenesis. Some studies have shown the antioxidant effects of PUFAs (Richard, et al., 2008; Casos et al., 2010), but were not clear on their modulatory role in the cellular antioxidant defense system. Emerging evidence has shown that the anti-inflammatory mechanism of omega-3 PUFAs is due to the G protein-coupled receptor 120 (GPR120) as an omega-3 PUFA receptor/sensor (Oh et al., 2010), suggesting that omega-3 PUFAs can be anti-inflammatory and antioxidant compounds. The supplementation of omega-3 PUFAs on human aortic endothelial cells reduced the formation of reactive oxygen species much better than cells supplemented with saturated, monounsaturated, or omega-6 PUFAs (Richard et al., 2008). A study by di Nunzio et al. (2011) revealed that omega-3 PUFAs supplementation positively modulated the antioxidant defenses as antioxidants, and DHA supplementation showed no adverse effects and induced antioxidant system activity in comparison with other tested PUFAs on HepG2 cells. This study also showed that some PUFAs had antioxidant activity coupled with adverse effects while others like DHA did not exhibit any adverse effects. This suggested that some of the PUFA family may support the generation of pro-oxidant effects while others may support antioxidant effects. Therefore, it may be illogical to label all FAs as pro-oxidant agents, and instead consider them as individual agents, which may help with separating pro-oxidant from antioxidant agents.
Various compounds with antioxidant activity have been shown to possess antimicrobial activity (Mattos et al., 2016; Mileski et al., 2016; Albonetti et al., 2017). The effectiveness of a compound as an antioxidant is directly proportional to its antimicrobial effect (Fung et al., 1985), a phenomenon that is still unclear. Studies seem to suggest that antioxidant compounds attenuate microbial growth through (1) inhibition or competition with the electron donor within the cell, (2) leakage of intracellular proteins and alteration of vital FAs in the organisms, and (3) suppression of yeast growth and hyphae transition in fungal species (Fung et al., 1985). Therefore, it is plausible that the antioxidant nature of omega-3 PUFAs (Richard et al., 2008; di Nunzio et al., 2011) could be contributing to their antimicrobial actions (Hilmarsson et al., 2007; Carballeira, 2008; Desbois and Smith, 2010; Huang and Ebersole, 2010), which needs further exploration.
The antimicrobial mechanisms of PUFAs including omega-3 FAs may therefore include: (1) disruption of cell-to-cell communication, (2) disruption of adenosine triphosphate (ATP) production, (3) alteration in membrane hydrophobicity, (4) blocking FabI enzymes to disrupt FA synthesis, (5) causing cellular leakages via increasing membrane poles, and (6) disruption of the electron transport system (Zheng et al., 2005; Carballeira, 2008; Desbois and Smith, 2010). This should stimulate the interest of researchers and clinicians, in considering these compounds as potential adjunctive or alternative antimicrobial therapeutic agents.
4. Possible application of omega-3 fatty acids
Because of the vast antagonistic effects of FAs against pathogenic growth, biofilm formation, and virulence factor production (Huang and Ebersole, 2010; Desbois, 2012; Desbois and Lawlor, 2013; Sun et al., 2016), omega-3 FAs can be applied in consumable products to maintain and ensure the supply of desired antimicrobial concentrations. The incorporation of omega-3 FAs into products such as chewing gums, drinks, tea, milk, sugar, tooth paste, or dentifrice may provide the antimicrobial requirements for treating and preventing oral diseases (Huang, 2011). In an attempt to understand the antimicrobial action of bioconversion extracts of EPA and DHA on foodborne pathogenic bacteria such as Bacillus subtilis, Listeria monocytogenes, S. aureus, Enterobacter aerogenes, Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritidis, and Salmonella typhimurium, Shin et al. (2007) revealed their potency in controlling foodborne pathogens, suggesting that the integration of these FAs into food preservatives may suppress bacterial growth, making them potential agents for improving food safety.
Products like polypropylene meshes that are utilized in hernia repair are coated with a mixture of esterified omega-3 FAs to prevent microbial colonization and inflammatory reaction, reviewed by Desbois (2012). In this review, the author made mention of various developments for the application of FAs in the food industry, agriculture, and aquaculture to avoid or suppress microbial growth and proliferation. Therefore, it seems plausible to hypothesize that the inclusion of omega-3 FAs on some prosthetics might help to lessen inflammatory reactions and inhibit microbial growth.
5. Drawbacks associated with the use of omega-3 fatty acids
5.1. Route of administration
Various routes of administration including oral, intravenous, inhalation, topical, or other administrations are employed to take substances into the body. The route of delivery for an active substance to the targeted site is usually considered. Clinical trials investigating the effects of LNA on several human conditions such as cardiovascular diseases, lipid metabolism disorders, and many others employed oral administration as the route of entry (https://clinicaltrials.gov/ct2/results?term=linolenic+acid&pg=2; accessed on Nov. 6, 2016). However, poor solubility and delivery have an impact on the bioavailability of FAs for antimicrobial actions (Jung and Lee, 2016), resulting in conflicting in vivo findings between human and mouse studies (Frieri et al., 2000). In order to improve the bioavailability of these compounds, several technological approaches are studied to ascertain the effective targeted delivery. Approaches involving controlled release of hydrogels, solid lipid nanoparticles, and liposomes are potentials in generating bioavailable antimicrobial solutions (Jackman et al., 2016). Therefore, more studies in both human and animal subjects are especially needed to determine the route of administration with better delivery proficiency.
5.2. Toxic effect
The toxic effect of any therapeutic agent under consideration is cardinal, as this information plays a vital role in public health and regulatory decision-making. Omega-3 PUFAs, being components of natural products or extracts such as fish oil or algal oil, are risk-free in toxicity (El-Sharkawy et al., 2010; Schmitt et al., 2012). For instance, in assessing the impact of DHA on Helicobacter pylori growth in a mouse model, Correia et al. (2012) did not observe any toxic effects resulting from DHA treatment, as there were no signs of weight variations or liver toxicity. Further studies on clinical safety revealed DHA oils to be safe for human consumption (Kroes et al., 2003; Arterburn et al., 2007). Schmitt et al. (2012) evaluated the toxic effects of DHA-rich algal oil in rats and found no neurotoxic effects even after using a high concentration of 2000 mg/(kg·d). The authors also concluded that DHA-rich algal oil was safe for using in foods. Similarly, an evaluation of EPA-rich algal oil was performed to assess the mutagenic activity and acute oral toxicity in Sprague-Dawley rats with the single gavaged dose of 2000 mg/kg body weight (Collins et al., 2014). A fortnight observation in this study found no evidence of mutagenicity or any toxic-related signs of morbidity. Furthermore, there was no cytotoxicity or other undesirable effects that were reported even after administration of higher doses of oleic acid, EPA, or DHA (360 mg/kg body weight per day) in Wistar rats (Calviello et al., 1997). Therefore, it is reasonable to deduce that omega-3 PUFAs may be safe for human use. Toxic effects are a subset of adverse side effects that the drug may present and may be considered serious or detrimental to health (https://medlineplus.gov/drugreactions.html; accessed on Apr. 2, 2017).
The existing conventional therapeutic agents possess mild to severe side effects that in patients could be bearable or unbearable. However, natural products, especially those from edible substances, have been perceived to possess little to no side effects (Kabara, 1979; US FDA, 2004) and attention is now drawn to them in the search for alternative therapeutic agents that will counteract emerging antimicrobial resistance and unbearable side effect problems. It is worth mentioning that side effects such as belching, epigastralgias, and gastroesophageal reflux were reported in adults with cystic fibrosis after taking FA supplements (Olveira et al., 2010).
5.3. Insufficient clinical trials
Lipid imbalance of essential FAs is believed to be a major contributing factor in the vicious infection-inflammation cycle of cystic fibrosis (Matouk, 2014). The innate variations such as reduced DHA levels, elevated AA levels, and raised AA/DHA ratio exacerbate cystic fibrosis infection (Matouk, 2014), which call for proper monitoring and supplementation. For instance, FA supplementation in a clinical study in adults with cystic fibrosis revealed a linear increase in DHA levels and a linear decrease in the AA/DHA ratio (Olveira et al., 2010), suggesting that dietary FA supplementation can modulate the disease. However, clinical studies on the effectiveness of omega-3 PUFAs against pathogenic microbes are lacking. Previous studies have shown that pathogenesis associated with microbial diseases, such as periodontal diseases, involves tissue damage resulting from the activation of host immune response (Page, 1991; van Dyke and Serhan, 2003). Thus, omega-3-supplemented studies typically focus on ameliorating tissue damage caused by activated host response rather than getting rid of the etiological agents. With the prevailing evidence of the broad spectrum nature of antimicrobial FAs (Desbois and Smith, 2010) that has been exhibited in various in vitro and in vivo studies, together with their significance in non-microbial conditions (Igennus, 2016), clinical studies are of utmost importance to substantiate the antimicrobial potential of omega-3 PUFAs.
6. Conclusions
LNA and its derivatives (EPA and DHA) are omega-3 FAs that have been widely studied for their beneficial effects on human health, mainly the brain, eye, cardiovascular system, and general human growth. However, their utilization as antimicrobial agents has not been widely appreciated perhaps because of little understanding on antimicrobial mechanism. Nonetheless, the efficacy of these agents on microbial cell membranes and their antioxidant properties have been shown to inhibit the growth of microorganisms and thereby promote human health and animal health. Hence, omega-3 FAs can be considered as potential alternative or adjunctive therapeutic agents because of their antimicrobial and immunomodulatory properties. Moreover, the escalating levels of resistance may be minimized because they have been reported to have little effect on evolving antimicrobial resistance (Desbois and Smith, 2010), and are also safe for human use. Since the development of antimicrobial resistance outruns antimicrobial drug development, it is worthwhile to consider omega-3 FAs in the list of potential antimicrobial agents. However, more clinical studies are required to support this hypothesis.
Acknowledgments
We would like to convey our sincere appreciations to Mr. Richardson JOSEPH (Center for Infrastructure Engineering, Western Sydney University, Australia), Dr. Brian AYUKA (MD) (Department of Orthopedic, the Second Affiliated Hospital of Dalian Medical University, China), Mr. Emeka OKOYE (Department of Human Anatomy, Histology and Embryology, Dalian Medical University, China), and Mr. Tesfaldet Osman MOHAMMED (Department of Epidemiology and Biostatistics, Dalian Medical University, China) for taking time to review the manuscript.
Footnotes
Compliance with ethics guidelines: Warren CHANDA, Thomson P. JOSEPH, Xue-fang GUO, Wen-dong WANG, Min LIU, Miza S. VUAI, Arshad A. PADHIAR, and Min-tao ZHONG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Albonetti S, Minardi P, Trombetti F, et al. In vivo and in vitro effects of selected antioxidants on rabbit meat microbiota. Meat Sci. 2017;123:88–96. doi: 10.1016/j.meatsci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Arterburn LM, Oken HA, Hoffman JP, et al. Bioequivalence of docosahexaenoic acid from different algal oils in capsules and in a DHA-fortified food. Lipids. 2007;42(11):1011–1024. doi: 10.1007/s11745-007-3098-5. [DOI] [PubMed] [Google Scholar]
- 3.Babajide JM, Olaluwoye AA, Taofik Shittu TA, et al. Physicochemical properties and phytochemical components of spiced cucumber-pineapple fruit drink. Niger Food J. 2013;31(1):40–52. doi: 10.1016/S0189-7241(15)30055-2. [DOI] [Google Scholar]
- 4.Bordi C, de Bentzmann S. Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care, 1:19. 2011 doi: 10.1186/2110-5820-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlingame B, Nishida C, Uauy R, et al. Fats and fatty acids in human nutrition: introduction. Ann Nutr Metab. 2009;55(1-3):5–7. doi: 10.1159/000228993. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. N3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6):S1505–S1519. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 7.Calo JR, Crandall PG, O'Bryan CA, et al. Essential oils as antimicrobials in food systems–a review. Food Control. 2015;54:111–119. doi: 10.1016/j.foodcont.2014.12.040. [DOI] [Google Scholar]
- 8.Calviello G, Palozza P, Franceschelli P, et al. Low-dose eicosapentaenoic or docosahexaenoic acid administration modifies fatty acid composition and does not affect susceptibility to oxidative stress in rat erythrocytes and tissues. Lipids. 1997;32(10):1075–1083. doi: 10.1007/s11745-997-0139-4. [DOI] [PubMed] [Google Scholar]
- 9.Carballeira NM. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog Lipid Res. 2008;47(1):50–61. doi: 10.1016/j.plipres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casos K, Zaragoza MC, Zarkovic N, et al. A fish-oil-rich diet reduces vascular oxidative stress in apoE−/− mice. Free Radic Res. 2010;44(7):821–829. doi: 10.3109/10715762.2010.485992. [DOI] [PubMed] [Google Scholar]
- 11.Cavalieri SJ, Rankin ID, Harbeck RJ, et al. Antimicrobial modes of action. In: Coyle MB (Ed.), Manual of Antimicrobial Susceptibility Testing. American Society for Microbiology, Washington DC; 2005. pp. 3–14. [Google Scholar]
- 12.CDC (Centers for Disease Control and Prevention) Antimicrobial Resistance. http://www.cdc.gov/drugresistance/about.html [Accessed on Oct. 17, 2016].2015. [Google Scholar]
- 13.Cheng CL, Huang SJ, Wu CL, et al. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. [j journal] J Biomed Sci. 2015;22(1):103. doi: 10.1186/s12929-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins ML, Lynch B, Barfield W, et al. Genetic and acute toxicological evaluation of an algal oil containing eicosapentaenoic acid (EPA) and palmitoleic acid. Food Chem Toxicol. 2014;72:162–168. doi: 10.1016/j.fct.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Correia M, Michel V, Matos AA, et al. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS ONE. 2012;7(4):e35072. doi: 10.1371/journal.pone.0035072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das UN. Can essential fatty acids reduce the burden of disease(s)? Lipids Health Dis. 2008;7(1):9. doi: 10.1186/1476-511X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desbois AP. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat Antiinfect Drug Discov. 2012;7(2):111–122. doi: 10.2174/157489112801619728. [DOI] [PubMed] [Google Scholar]
- 18.Desbois AP. Antimicrobial properties of eicosapentaenoic acid (C20:5n−3) In: Kim SK (Ed.), Marine Microbiology. Wiley Online Library; 2013. pp. 351–367. [DOI] [Google Scholar]
- 19.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85(6):1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 20.Desbois AP, Lawlor KC. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus . Marine Drugs. 2013;11(11):4544–4557. doi: 10.3390/md11114544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Nunzio M, Valli V, Bordoni A. Pro-and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostag Leukotr Ess. 2011;85(3-4):121–127. doi: 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 22.El-Sharkawy H, Aboelsaad N, Eliwa M, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low-dose aspirin. J Periodontol. 2010;81(11):1635–1643. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- 23.Frieri G, Pimpo MT, Palombieri A, et al. Polyunsaturated fatty acid dietary supplementation: an adjuvant approach to treatment of Helicobacter pylori infection. Nutr Res. 2000;20(7):907–916. doi: 10.1016/S0271-5317(00)00182-2. [DOI] [Google Scholar]
- 24.Fung DYC, Sheree Lin CC, Gailani MB. Effect of phenolic antioxidants on microbial growth. CRC Crit Rev Microbiol. 1985;12(2):153–183. doi: 10.3109/10408418509104428. [DOI] [PubMed] [Google Scholar]
- 25.Grundt H, Nilsen DWT, Mansoor MA, et al. Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (PUFAs) following an acute myocardial infarction. Eur J Clin Nutr. 2003;57(6):793–800. doi: 10.1038/sj.ejcn.1601730. [DOI] [PubMed] [Google Scholar]
- 26.Hilmarsson H, Traustason BS, Kristmundsdottir T, et al. Virucidal activities of medium-and long-chain fatty alcohols and lipids against respiratory syncytial virus and parainfluenza virus type 2: comparison at different pH levels. Arch Virol. 2007;152(12):2225–2236. doi: 10.1007/s00705-007-1063-5. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman DR. What are EPA/DHA?. http://drhoffman.com/wp-content/plugins/post2pdf-converter/post2pdf-converter-pdf-maker.php?id=2513 [Accessed on Aug. 30, 2016].2013. [Google Scholar]
- 28.Huang C. Omega polyunsaturated fatty acids for the treatment of oral diseases. http://www.google.com/patents/WO2011056327A1?cl=en [Accessed on Jan. 3, 2017].2011. [Google Scholar]
- 29.Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol. 2010;25(1):75–80. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 30.Igennus EPA vs DHA–understand the difference. https://igennus.com/nutrition/omega-3-science/epa-vs-dha/# [Accessed on Jan. 3, 2017].2016. [Google Scholar]
- 31.Jackman JA, Yoon BK, Li D, et al. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules. 2016;21(3):305. doi: 10.3390/molecules21030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung SW, Lee SW. The antibacterial effect of fatty acids on Helicobacter pylori infection. Korean J Intern Med. 2016;31(1):30–35. doi: 10.3904/kjim.2016.31.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabara JJ. Toxicological, bacteriocidal and fungicidal properties of fatty acids and some derivatives. J Am Oil Chem Soc. 1979;56(11):760A–767A. doi: 10.1007/BF02667439. [DOI] [Google Scholar]
- 34.Kalia M, Yadav VK, Singh PK, et al. Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa . PLoS ONE. 2015;10(8):e0135495. doi: 10.1371/journal.pone.0135495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroes R, Schaefer EJ, Squire RA, et al. A review of the safety of DHA45-oil. Food Chem Toxicol. 2003;41(11):1433–1446. doi: 10.1016/S0278-6915(03)00163-7. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence GD. The Fats of Life: Essential Fatty Acids in Health and Disease. Rutgers University Press; 2010. pp. 11–43. [Google Scholar]
- 37.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–167. doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Romero JC, Gonzalez-Rios H, Borges A, et al. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus . Evid-Based Compl Altern Med, 2015:795435. 2015 doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matouk E. Safety study of fenretinide in adult patients with cystic fibrosis. https://clinicaltrials.gov/ct2/show/ NCT02141958?term=Docosahexaenoic&phase=0467%20&rank=20 [Accessed on Jan. 3, 2017].2014. [Google Scholar]
- 40.Mattos GN, Tonon RV, Furtado AA, et al. Grape by-products extracts against microbial proliferation and lipid oxidation: a review. J Sci Food Agric. 2016;97(4):1055–1064. doi: 10.1002/jsfa.8062. [DOI] [PubMed] [Google Scholar]
- 41.Mil-Homens D, Bernardes N, Fialho AM, et al. The antibacterial properties of docosahexaenoic omega-3 fatty acid against the cystic fibrosis multiresistant pathogen Burkholderia cenocepacia . FEMS Microbiol Lett. 2012;328(1):61–69. doi: 10.1111/j.1574-6968.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 42.Mileski KS, Ciric AD, Trifunovic SS, et al. Heracleum orphanidis: chemical characterisation, and comparative evaluation of antioxidant and antimicrobial activities with specific interest in the influence on Pseudomonas aeruginosa PAO1. Food Funct. 2016;7(9):4061–4074. doi: 10.1039/C6FO01018K. [DOI] [PubMed] [Google Scholar]
- 43.Morais-Braga MF, Souza TM, Santos KK, et al. Phenolic compounds and interaction between aminoglycosides and natural products of Lygodium venustum SW against multiresistant bacteria. Chemotherapy. 2012;58(5):337–340. doi: 10.1159/000343044. [DOI] [PubMed] [Google Scholar]
- 44.Moss JWE, Ramji DP. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol. 2016;13(9):513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee PK, Nema NK, Maity N, et al. Phytochemical and therapeutic potential of cucumber. Fitoterapia. 2013;84:227–236. doi: 10.1016/j.fitote.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Nascimento GGF, Locatelli J, Freitas PC, et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31:247–256. doi: 10.1590/S1517-83822000000400003. [DOI] [Google Scholar]
- 47.Neitzel JJ. Fatty acid molecules: fundamentals and role in signaling. Nat Educ. 2010;3(9):57. [Google Scholar]
- 48.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olveira G, Olveira C, Acosta E, et al. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch Bronconeumol. 2010;46(2):70–77. doi: 10.1016/j.arbres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26(3):230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 51.Pagliaro B, Santolamazza C, Simonelli F, et al. Phytochemical compounds and protection from cardiovascular diseases: a state of the art. Biomed Res Int, 2015:918069. 2015 doi: 10.1155/2015/918069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prince AS. Biofilms, antimicrobial resistance, and airway infection. New Engl J Med. 2002;347(14):1110–1111. doi: 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 53.Richard D, Kefi K, Barbe U, et al. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57(6):451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt D, Tran N, Peach J, et al. Toxicologic evaluations of DHA-rich algal oil in rats: developmental toxicity study and 3-month dietary toxicity study with an in utero exposure phase. Food Chem Toxicol. 2012;50(11):4149–4157. doi: 10.1016/j.fct.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 55.Sharma G, Rao S, Bansal A, et al. Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals. 2014;42(1):1–7. doi: 10.1016/j.biologicals.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Shin SY, Bajpai VK, Kim HR, et al. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int J Food Microbiol. 2007;113(2):233–236. doi: 10.1016/j.ijfoodmicro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 57.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17(2):174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Sousa AS, Prates RA, de Santi MESO, et al. Photodynamic inactivation of Candida albicans biofilm: influence of the radiant energy and photosensitizer charge. Photodiagn Photodyn. 2016;14:111–114. doi: 10.1016/j.pdpdt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Sun M, Zhou Z, Dong J, et al. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb Pathogenesis. 2016;99:196–203. doi: 10.1016/j.micpath.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Thormar H. Antibacterial effects of lipids: historical review (1881 to 1960) Lipids and Essent Oils as Antimicro Agents. John Wiley & Sons, Ltd; 2011. pp. 25–45. [Google Scholar]
- 61.US FDA (Food and Drug Administration) Substances affirmed as generally recognized as safe: menhaden oil [Docket No. 1999P-5332]. US Federal Register, p.2313-2317. https://www.federalregister.gov/documents/2004/01/15/04-811/substances-affirmed-as-generally-recognized-as-safe-menhaden-oil [Accessed on Apr. 3, 2017].2004. [Google Scholar]
- 62.van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82(2):82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 63.Wainwright M. Moulds in ancient and more recent medicine. Mycologist. 1989;3(1):21–23. doi: 10.1016/S0269-915X(89)80010-2. [DOI] [Google Scholar]
- 64.Wall R, Ross RP, Fitzgerald GF, et al. Fatty acids from fish: the anti-inflammatory potential of long-chain ω-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 65.WHO. WHO and FAO joint consultation: fats and oils in human nutrition. Nutr Rev. 1995;53(7):202–205. doi: 10.1111/j.1753-4887.1995.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microl. 2008;6(3):222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 67.Zheng CJ, Yoo JS, Lee TG, et al. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579(23):5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]


