Abstract
With the increasing occurrence of haze during the summer, the physicochemical characteristics and toxicity differences in PM2.5 in different seasons are of great concern. Hangzhou is located in an area that has a subtropical monsoon climate where the humidity is very high during both the summer and winter. However, there are limited studies on the seasonal differences in PM2.5 in these weather conditions. In this test, PM2.5 samples were collected in the winter and summer, the morphology and chemical composition of PM2.5 were analyzed, the toxicity of PM2.5 to human bronchial cells BEAS-2B was compared, and the correlation between PM2.5 toxicity and the chemical composition was discussed. The results showed that during both the winter and summer, the main compounds in the PM2.5 samples were water-soluble ions, particularly SO4 2−, NO3 −, and NH4 +, followed by organic components, while heavy metals were present at lower levels. The higher the mass concentration of PM2.5, the greater its impact on cell viability and ROS levels. However, when the mass concentration of PM2.5 was similar, the water extraction from the summer samples showed a greater impact on BEAS-2B than that from the winter samples. The cytotoxicity of PM2.5 was closely associated with heavy metals and organic pollutants but less related to water-soluble ions.
Keywords: PM2.5, Seasonal difference, Physical and chemical characteristics, Cytotoxicity
1. Introduction
Air pollution is a major problem in many modern cities and influences billions of people worldwide (Shi et al., 2015). PM2.5 (aerodynamic diameter ≤2.5 μm) is defined by fine particles that contain carbon and absorb various chemical compounds, such as metals, organic compounds, and salts, and biological groups, such as toxins and pollen (Spurny, 1996). These PM2.5 compounds have a great effect on human health and can cause respiratory and cardiovascular diseases.
Most of the national heavy haze events occur during the spring and winter, but in recent years the frequency of summer haze has increased in North China (Chen and Wang, 2015). Scholars found that the source and composition of PM2.5 in an area varied in different seasons. Some studies have shown that the inorganic ions, heavy metals, and organic compounds in PM2.5 were generally lower in the summer than in the winter (Manzanoleón et al., 2015; Huang et al., 2016; Meng et al., 2016; Wang et al., 2016). However, the results have been inconsistent. For example, Perrone et al. (2010) found that PM2.5 in Milan during the summer was richer in sulfates, Al, As, Cr, Cu, and Zn than PM2.5 during the winter. The sources of PM2.5 also showed seasonal differences. Zhang et al. (2013) found that the concentration of secondary inorganic aerosol was higher during the warm and rainy summers than during the winter, while secondary organic aerosols tended to be formed during the dry and cold winters. Manzanoleón et al. (2015) found that the chemical compounds of PM2.5 during the warm and rainy season were intimately associated with the soil source, while they were closely related to combustion sources during the cold and dry season.
PM2.5 is small enough to invade even the smallest airways and penetrate to the lungs (Shi et al., 2015), and will cause oxidative stress and inflammation in the respiratory system, and then may cause epithelial damage and abnormal cardiovascular function. The particle in vitro toxicity test with human bronchial cells is a typical method for simulating the toxicity of PM2.5 to the respiratory system (Borgie et al., 2015a). The toxicity of PM2.5 is not only related to its physical characteristics but is also related to various toxic chemicals adsorbed onto PM2.5 (Ma et al., 2013). Organic compounds in PM2.5, such as aliphatic/chlorinated hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), and nitro PAHs/ketones/quinones, may be an important part of PM2.5 toxicity (Borgie et al., 2015b). The water-soluble components of PM2.5 are also very harmful to human health (Gualtieri et al., 2009). For example, the transition metal elements Zn and Pb have a strong correlation with the A549 cells cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (Liu et al., 2014).
There are some studies regarding the differences in PM2.5 toxicity between the winter and summer (Becker et al., 2005; Perrone et al., 2010; Happo et al., 2013; Longhin et al., 2013). Some researchers have found that reactive oxygen species (ROS) levels in alveolar macrophages and the activity of A549 cells were affected more significantly by PM2.5 during the summer than during the winter (Becker et al., 2005; Perrone et al., 2010). However, Longhin et al. (2013) found that winter PM2.5 would cause a significant ROS level increase in cells after only one hour of exposure. Happo et al. (2013) also found that the cytotoxicity of outdoor PM2.5 samples of the spring and summer was more cytotoxic than those collected during the winter.
Obviously, neither the differences in the chemical composition nor the cytotoxicities of PM2.5 during different seasons have consistent conclusions. It is important to understand this issue because it is related to the mechanism and governance of haze and the protection of public health. The current comparison studies between the winter and summer PM2.5 have focused on the dry and cold winters and warm and rainy summers. Hangzhou (Zhejiang, China) is located in a wet area with subtropical monsoons, and the humidity is very high during both the summer and winter; however, there are limited studies regarding the seasonal differences in PM2.5 in these weather conditions. In this paper, PM2.5 samples from different seasons in Hangzhou were collected, the main chemical components were analyzed, the influences of PM2.5 water extraction on the cell viability and ROS levels of BEAS-2B cells were compared, and the relationship between the cell toxicity of PM2.5 over different seasons and its chemical factors was investigated.
2. Materials and methods
2.1. Collection and selection of PM2.5 samples
The sampling point was located at the third floor of the Agricultural Biological and Environment Building in the Zijingang Campus of Zhejiang University, Hangzhou, Zhejiang Province, China. There are a few major roads within 1 km of the sampling point. A residential area is approximately 200 m east of the sampling point (Fig. 1). PM2.5 samples were collected on 90-mm quartz microfiber filters (prebaked, QMA, Whatman-GE Healthcare Biosciences Corp., UK) using an air impactor monitor (Qingdao Laoying Corp., Qingdao, China) that was operated at a flow rate of 100 L/min, as recommended by China’s standard method for PM2.5 collection (Environmental Protection Department of the People’s Republic of China, 2013). Samples were collected from Jan. 5 to 14 and May 20 to June 25 in 2015. The filters were changed every morning at 10:00 a.m. during the measurement period. The quartz fiber filters were baked for 5 h at 500 °C to remove impurities and then placed into desiccators for 24 h and weighted prior to deployment for PM2.5 measurement. After weighing, the filters were stored in a refrigerator at −18 °C before the chemical analysis.
Fig. 1.
Position of sampling point
Based on the filter quality differences between the before and after samplings and the sampling volume under standard conditions, the PM2.5 mass concentration could be obtained. Then, the samples from Jan. 5 (WH), 6 (WM), and 14 (WL) were selected, representing severe, light, and good winter weather conditions, respectively, and May 20 (SH), June 25 (SM), and June 24 (SL) were selected, representing summer weather (Table 1).
Table 1.
Concentrations of PM2.5 and the chemical compounds in the samples
| Samplemark | Samplingtime | Mass concentration (μg/m3) | Chemical compound concentration (ng/m3) |
||||||||
| V | Mn | Ni | Cu | Cd | Ba | Pb | |||||
| WH | 2015.1.5 | 290 | 27.93 | 250.16 | 6.57 | 60.86 | 7.92 | 70.85 | 196.85 | ||
| WM | 2015.1.6 | 96 | 71.41 | 61.71 | 6.57 | 10.96 | 1.87 | 36.1 | 84.53 | ||
| WL | 2015.1.14 | 35 | 77.69 | 20.2 | 1.62 | 8.53 | 1.21 | 17.23 | 47.48 | ||
| SH | 2015.5.20 | 171 | 112.23 | 115.1 | 16.22 | 32 | 1.4 | 84.43 | 50.38 | ||
| SM | 2015.6.25 | 92 | 79.75 | 45.84 | 5.2 | 10.01 | 1.39 | 22.49 | 66.31 | ||
| SL | 2015.6.24 | 47 | 117.62 | 40.33 | 4.11 | 9.91 | 2.2 | 32.47 | 33.86 | ||
|
| |||||||||||
|
| |||||||||||
| Samplemark | Chemical compound concentration (μg/m3) |
||||||||||
| Cl- | NO3 - | SO4 2- | Na+ | NH4 + | K+ | Mg2+ | Ca2+ | OC | EC | SOC | |
|
| |||||||||||
| WH | 6.72 | 32.24 | 22.54 | 0.56 | 8.12 | 2.49 | 0.09 | 1.9 | 39.2 | 10.31 | 23.74 |
| WM | 5.49 | 16.89 | 21.42 | 1.09 | 9.31 | 1.33 | 0.06 | 0.96 | 12.15 | 2.65 | 8.17 |
| WL | 1.31 | 4.58 | 9.91 | 0.21 | 3.73 | 0.6 | 0.02 | 0.42 | 5.25 | 1.42 | 3.12 |
| SH | 1.13 | 10.76 | 25.74 | 0.86 | 2.78 | 0.69 | 0.32 | 7.16 | 20.39 | 4.79 | 13.2 |
| SM | 0.16 | 11.86 | 24.19 | 0.23 | 6.07 | 0.27 | 0.07 | 0.74 | 12.24 | 2.47 | 8.53 |
| SL | 0.77 | 4.39 | 10.88 | 0.11 | 0.93 | 0.1 | 0.06 | 0.51 | 10.2 | 2.31 | 6.73 |
OC: organic carbon; EC: elemental carbon; SOC: secondary organic carbon. SOC=OC−OC×(OC/EC)min, where (OC/EC)min is the mini-mum value of the OC/EC during the sampling period, and 1.5 is used in this test
2.2. PM2.5 morphology and chemical composition analysis
All the selected filters were divided into four parts, which were used for the morphology, chemical composition analysis, and cell toxicity tests. The main chemical analysis methods were discussed by Liuet al. (2014).
2.2.1PM2.5 morphology analysis
A small piece of filter (approximately 0.5 cm×0.5 cm) was cut from SM, WM, and blank sample filters, and placed in a 3-cm cylindrical stage by conductive adhesive. After gold platting, the PM2.5 morphology was analyzed under SU-8010 type field emission scanning electron microscope (SEM) (SU-8010 Hitachi, Japan).
2.2.2Trace heavy metal analysis
Inductively coupled plasma-mass spectrometer (ICP-MS) was used to analyze the trace heavy metals in PM2.5. The sample filter was cut into pieces, placed in a Teflon crucible with 10.0 ml of mixed acids (5.55% (v/v) HNO3/16.75% (v/v) HCl), and then the crucibles were heated at 100 ¡for 2 h. After cooling, ultra-pure water was added to the extraction for 0.5 h.
Finally, the filter pieces were removed, and the extracting solution was reduced to a constant volume of 50.0 ml. The extracting solution was filtered through a 0.45-¦˭ Millipore filter and analyzed by NexION(tm) 300X ICP-MS (NexION 300, PerkinElmer, USA) to test for seven trace heavy metals (V, Mn, Ni, Cu, Cd, Ba, and Pb).
2.2.3Water soluble ion analysis
The water solution ions were analyzed by ion chromatography. A quarter of each sample filter was cut into pieces and placed in a cleaning centrifuge tube with 20.0 ml of ultra-pure water; then, the tubes were placed in an ultrasonic water bath for 1 h. The extracting solution was filtered through a 0.22-¦˭ Millipore filter and analyzed by ICS-3000 Multifunctional Ion Chromatography (Dionex ICS-3000, Thermo Scientific, USA) to test for eight water-soluble ions (Cl?, SO42?, NO3?, Na+, NH4+, K+, Mg2+, and Ca2+).
2.2.4Organic carbon and elemental carbon analyses
The concentrations of organic carbon (OC) and elemental carbon (EC) in the samples were analyzed with a thermal/optical carbon analyzer (DRI 2001A, Atmoslytic, Inc., USA) using the IMPROVE temperature program.
2.3. Cell cytotoxicity
2.3.1Preparation of PM2.5 extraction solution
Based on the result of pre-test, a quarter of each sample filter was cut into pieces and placed in a clean sterile centrifuge tube with 10.0 ml of serum-free Dulbecco's modified Eagle's medium (DMEM) (Gibco, USA); then the tube was placed in an ultrasonic water bath for 1 h and the temperature was kept at 30 ¡ The step was repeated three times. After filtering through a 0.22-¦˭ sterile filter, the PM2.5 extractions were kept in ?20 ¡storage before the cytotoxicity tests.
2.3.2Cytotoxicity test
Human bronchial cells BEAS-2B (ATCC No. ATCC(r) CRL-9609(tm)) were cultured at 37 ¡in a 5% CO2 box (Eppendorf China Ltd., Shanghai, China) before the cell viability and ROS tests. When the culture dish was covered in mono-layer cells, the cells were digested and matched into a single cell suspension by a DMEM medium containing 10% (v/v) fetal bovine serum and 1% (v/v) Penicillin-Streptomycin solution, and then vaccinated to Corning 96-well plates in 10 000 densities and cultured for 24 h before the PM2.5 cytotoxicity experiments.
A CCK-8 cell activity detection kit (Beyotime Biotechnology Co., Ltd., Shanghai, China) was used to test the cell activity, and an ROS detection kit (Beyotime Biotechnology Co., Ltd., Shanghai, China) was used to test intracellular ROS. In the experiment, the non-cell group was used as a blank control, and none of the PM2.5 exposure groups was used as a control group. Each sample was provided with three sets of parallel samples. The experimental operation was carried out strictly in accordance with the specification. After 24 h of exposure, the morphology of the cells was observed under a Nikon Eclipse TS100 microscope (Eclipse TS100 Nikon, Japan).
2.4. Data analyses
Data were analyzed using Origin 8.0 and Microsoft Excel 2010. The correlation analysis was finished by Pearson's correlation analysis program in IBM Statistics SPSS 19.0.
3. Results and discussion
3.1. Chemical analysis of PM2.5 samples
The PM2.5 mass concentration and chemical components of the samples are shown in Table 1, and the percentages of the main compounds are shown in Table 2. The PM2.5 mass concentrations of the six samples were much higher than the daily average concentration limit of the World Health Organization (WHO) (25 ¦˧/m3). The most abundant components in the PM2.5 of each sample were water-soluble ions (especially SO42?, NO3?, and NH4+), which accounted for 25.74%-59.37%. The concentrations of SO42? and NO3? are directly related to the contents of SO2 and NOx, which are mainly from fixed sources, such as coal combustion, and mobile sources, such as vehicle emissions, indicating that the use of fossil fuels, such as coal and oil, has a significant impact on the ionic composition of PM2.5. The mass ratio of NO3? and SO42? ([NO3?]/[SO42?]) is typically used to compare the contribution of fixed sources (such as coal combustion) and mobile sources (such as vehicle emission) to sulfur and nitrogen in the atmosphere. The results showed that except for WH, the [NO3?]/[SO42?] mass ratios for the remainder of the samples were less than 1, while the [NO3?]/[SO42?] mass ratios of WM and WL were closer to 1, which indicated that although the fixed sources have a great contribution in the winter, the impact of mobile sources should not be ignored. It should be noted that Cl? and K+were higher in the winter than in the summer, since Cl? and K+come from biomass burning, indicating that PM2.5 is affected more by biomass burning in the winter than in the summer.
Table 2.
Percentages of water-soluble ions, total carbon, total metals, and SOC in the samples
| Sample mark | Water-soluble ions (%) | Total carbon (%) | Total metals (%) | SOC (%) |
| WH | 25.74 | 17.07 | 0.21 | 8.18 |
| WM | 58.91 | 15.42 | 0.28 | 8.51 |
| WL | 59.37 | 19.06 | 0.50 | 8.90 |
| SH | 28.91 | 14.73 | 0.24 | 7.71 |
| SM | 47.38 | 15.99 | 0.25 | 9.22 |
| SL | 37.77 | 26.62 | 0.51 | 14.26 |
SOC: secondary organic carbon
The contents of OC and EC were 14.73%–26.63%, which were slightly inferior to the water-soluble ions. The percentage of EC in the summer samples was similar to that in the winter, while the percentages of OC and SOC in the summer samples were higher than those in the winter samples. Because EC is mainly from primary sources, such as coal emission, and OC is derived from primary sources and secondary reactions in the atmosphere, which indicated that the contribution of primary sources to PM2.5 was relatively stable in the winter and summer, while PM2.5 in the summer is influenced more by secondary sources than that in the winter. This differed from the prior results (Zhang et al., 2013), maybe because the temperature has a greater effect on the formation of SOC when the humidity was high both in the summer and winter, and thus the summer climate in Hangzhou was more conducive to the formation of SOC.
The heavy metals only accounted for less than 1% of all selected samples. V, Mn, Pb, and Cu were relatively rich among the seven metals. Mn, Pb, and Cu increased with increases in the PM2.5 mass concentration and were higher in the winter than in the summer, while V decreased with increases in the PM2.5 mass concentration and was higher in the summer. Coal, fossil fuels, and industrial emissions were the main sources of V, Mn, and Pb. There are no industrial parks around the sampling point, which indicated a contribution of long distance transmission to PM2.5. In addition, the resuspension of soil could also increase the Mn content in the PM2.5. Notably, although the concentration of Pb did not exceed the WHO limit value (500 ng/m3), it was higher than that in other cities in China, such as Guangdong, Nanjing, and Macao, and was slightly higher in the winter (Zheng et al., 2014).
3.2. PM2.5 morphology
The morphological characteristics of PM2.5 affect its adsorption of toxic and harmful substances and PM2.5 toxicity. SEM images are widely used in the study of atmospheric particle morphology, and can directly show the particle size, morphology, aggregation characteristics, composition, and even sources of fine particles (McMurry, 2000). In this report, SEM was used to analyze the morphological characteristics of PM2.5 on the filter. There was no significant difference between the winter and summer particle samples (Fig. 2). In contrast to the blank quartz filter, the sample filter was not as smooth and the adhered fine particles made the fiber coarser. PM2.5 was not only comprised mainly of irregularly shaped agglomerate particles but also contained spherical, elongated, and flocculent particles.
Fig. 2.
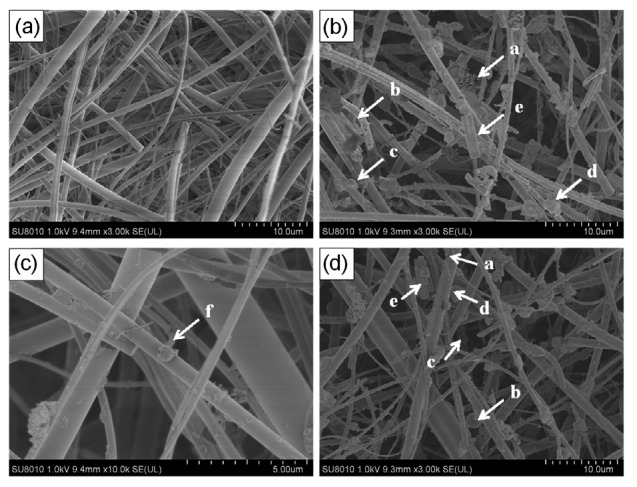
SEM images of PM2.5
(a) SEM results of blank filter (magnified by 3000 times); (b) SEM results of SM sample (magnified by 3000 times), (c) SEM results of SM sample (magnified by 10 000 times); (d) SEM results of WM sample (magnified by 3000 times). a: flocculent particles; b: spherical particles; c: deformation of spherical particles; d: irregularly shaped agglomerate particles; e: elongated particles; f: spongy spherical particles
Irregularly shaped agglomerate particles might be large particles that absorbed various substances and mainly came from soil or construction dust; spherical particles and disportionate spherical particles could be coal fly ash, which was mainly from coal burning or nitrate and sulfate particles formed through atmospheric reactions (Du et al., 2015). Spongy spherical particles were probably spongy carbon particles (Masiol et al., 2013). Elongated particles had a regular shape and a smooth surface, which were supposedly mineral particles, such as sulfate and nitrate particles, and mainly came from secondary particles formed in atmospheric chemical reactions. Flocculent particles were fluffy and might be soot and soot aggregates, which were mainly from coal burning and motor vehicle exhaust (Du et al., 2015).
It could be speculated that the spherical particles and soot aggregates in PM2.5 can enable the fine particles to easily adsorb toxic and harmful substances, such as heavy metals, volatile organic contaminants, and semivolatile organic pollutants. The study of nanoscale characteristics of PM2.5 confirmed that soot aggregates (flocculent particles) had high adhesion to adsorb other types of particles, resulting in an enhanced complexity and toxicity, and spherical carbon particles will be associated with soot aggregates when transferred, which increased the toxicity of PM2.5 (Shi et al., 2015).
3.3. Impacts of PM2.5 on cell vitality and ROS level
Cell viability refers to the percentage of living cells in total cells among a group of cells. It is a simple and intuitive index to test whether the cell is damaged in the experiment. The balance of oxidation and antioxidant system in cells is a key factor in determining the survival state of cells. Intracellular ROS, which are oxygen free radical and its derivatives, will rapidly increase when the body suffers from harmful stimuli. ROS will attack DNA, RNA, protein, fat, and other macromolecules in cells and ultimately lead to cell damage and even cell death.
The states of cells under the microscope (magnified by 200 times) after being exposed to PM2.5 extractions of different seasons for 24 h were showed in Fig. 3. The changes in the cell states were similar when exposed to PM2.5 extractions from different seasons. The cells of the control group grew well, and cell transparency was good, but after being exposed to PM2.5 extractions for 24 h, the cells became irregular and detached from the bottom of the culture dish. Moreover, the gap between the cells increased. The number of living cells in the field of vision decreased sharply with increases in the PM2.5 sample mass concentration.
Fig. 3.
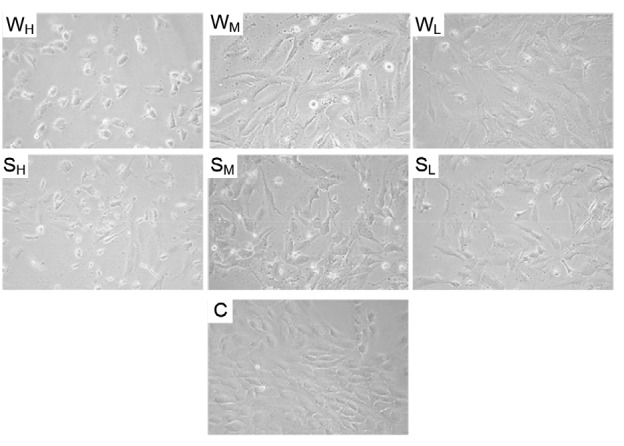
States of cells under the microscope after exposure to the water extraction solution of PM2.5 (magnified by 200 times)
Fig. 4 shows the results of changes of the cell viability after exposure to PM2.5 extractions from different seasons for 24 and 48 h. The results showed that all PM2.5 extractions had an effect on the cell viability, and the higher the mass concentration of PM2.5, the lower the cell viability. In addition, the longer the exposure time, the greater the effect on the cell viability. The results also showed that the PM2.5 extraction of WH had a significant effect on the cell viability. The cell viability decreased to only 9% after 24 h exposure, and the cells almost died after 48 h, while the viability decreased to approximately 10% after 48 h in the other experimental groups. For the exposure groups of PM2.5 samples of the winter, the cell viability showed a dose-response relationship with the PM2.5 concentration in the early exposure period, which implied that the higher the PM2.5 sample mass concentration, the lower the cell viability. However, the cell viability of the group exposed to PM2.5 extraction of SL was lower than that of SM. This may due to the equal content of heavy metals in SL and SM, where some elements were even higher in SL than in SM. For example, the concentration of the toxic element V was 117.62 ng/m3 in SL, while it was only 79.75 ng/m3 in SM. Meanwhile, OC (10.20 μg/m3) was higher in SL, which may lead to an increase in the release of organic compounds in the water and have a notable effect on cell viability.
Fig. 4.
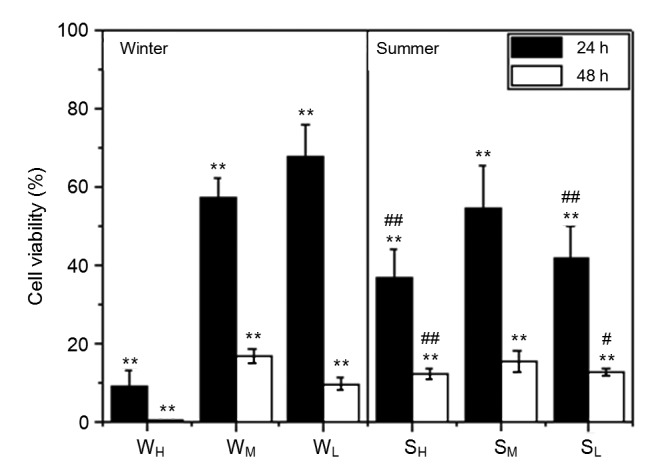
Cell viability after exposure to the water extraction solution of PM2.5
Sample groups are compared to control groups, where ** indicates a significant difference. Summer severe, mild, and good weather groups are compared to the corresponding winter samples, where # indicates a significant difference at 0.05, and ## indicates a significant difference at 0.01
In addition to the ROS components of the cells, PM2.5 can also stimulate cells to produce a large number of ROS. As shown in Fig. 5, the ROS content increased and was approximately two times greater compared to the control group after 3 h of PM2.5 stimulation. As time elapsed, ROS secretion in the 6 h experimental groups decreased. This may be the result of the balance between intracellular oxidation and antioxidant system, e.g. the intracellular superoxide dismutase (SOD) might play a role in scavenging free radicals so that the level of ROS decreased. This was similar to the results reported previously which used A549 cells (Deng et al., 2013).
Fig. 5.
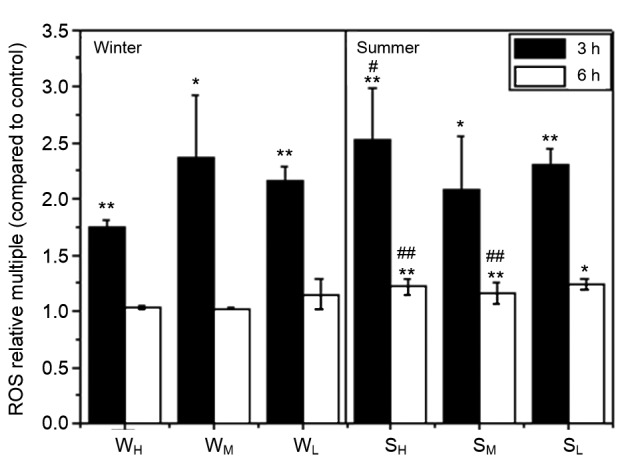
Relative multiple of ROS in cells compared to the control group after exposure to the water extraction solution of PM2.5
Sample groups are compared to control groups, where * indicates a significant difference at 0.05, and ** indicates a significant difference at 0.01. Summer severe, mild, and good weather groups are compared to the corresponding winter samples, where # indicates a significant difference at 0.05, and ## indicates a significant difference at 0.01
In addition, the PM2.5 extraction samples of the summer induced higher increments of intracellular ROS than the winter samples after 6 h of exposure (Fig. 5). In contrast to the cell viability data, the effect of PM2.5 extractions of the summer on cell viability was also greater in the early 24 h exposure time, which is similar to the results reported before (Becker et al., 2005; Perrone et al., 2010). It could be conjectured that a considerable mass concentration of PM2.5 in the warm months may have a greater effect on the cell viability of BEAS-2B cells and the increase in intracellular ROS than that in the cold months.
3.4. Correlation between the chemical composition and PM2.5 cytotoxicity
Previous studies have shown that the cytotoxicity of PM2.5 may be related to the metal element and organic pollutant content (de Kok et al., 2006; Borgie et al., 2015b). According to the study of Xiang et al. (2016), the ingredients in water extraction of PM2.5 should be water-soluble ions and heavy metals, which are consistent with the chemical compounds of PM2.5 in Section 3.1. From the perspective of human health, when the PM2.5 invades the lungs, the encounter is mainly inorganic salt water extraction and lung fluid. Thus, the correlation between the chemical components and the cytotoxicity of PM2.5 was analyzed, and the results showed that the metal element and OC, EC, and SOC content had different correlations with the cytotoxicity results.
3.4.1 Correlation between heavy metals and PM2.5 cytotoxicity
Many studies have confirmed that heavy metals in PM2.5 were harmful to human health (de Kok et al., 2006). For example, transition metal elements can induce cells to produce ROS, which leads to excessive free radicals that can cause lipid peroxidation and the breakdown of DNA strands, possibly causing cell death.
Based on Table 3, it was notable that V had relevance to cell viability and intracellular ROS formation, while the correlations between other elements and the cell viability and the intracellular ROS content were not significant. V had a high correlation with the change in the intracellular ROS content, indicating that it had a substantial influence on the oxidative stress of cells. This agreed with Okeson’s conclusion that the cytotoxicity of PM2.5 was significantly correlated with the content of V (Okeson et al., 2003). Pb had a strong correlation with the cell viability and intracellular ROS content, which meant that although the content of Pb in PM2.5 did not exceed the concentration limit of the WHO, its toxicity could not be ignored. Meanwhile, Cu, Cd, and Mn also showed high negative correlations with the cell viability. The results agreed with previous studies (de Kok et al., 2006; Gualtieri et al., 2009), which demonstrated that transition metals, such as Cu and Mn, were toxic to cells and could damage the membrane lipid, protein, and DNA, possibly causing cell death. Because the concentrations of V, Mn, and Pb differed between the summer and winter, this may be one of the reasons for the differences in the toxicity of PM2.5 between the summer and winter.
Table 3.
Correlation coefficients of different chemical compounds in PM2.5 and the cell viability and intracellular ROS level
| Chemical component | Cell viability |
Intracellular ROS level |
||
| 24 h | 48 h | 3 h | 6 h | |
| V | 0.5440* | 0.8456* | 0.8456* | 0.8714* |
| Mn | −0.9370 | −0.2874 | −0.2874 | −0.4838 |
| Ni | −0.4064 | 0.0296 | 0.4770 | 0.1994* |
| Cu | −0.9237 | −0.8479 | −0.5440 | −0.3936 |
| Cd | −0.8284 | −0.8432 | −0.7824 | −0.5694 |
| Ba | −0.7257 | −0.4213 | 0.0586 | −0.0560 |
| Pb | −0.8169 | −0.8177 | −0.7887 | −0.7605 |
| OC | −0.9639 | −0.7943 | −0.5750 | −0.4507 |
| EC | −0.9436 | −0.8360 | −0.6092 | −0.4644 |
| SOC | −0.9729 | −0.7621 | −0.5490 | −0.4392 |
| Na+ | −0.1803 | 0.1112 | 0.3471 | −0.5110 |
| NH4 + | −0.3339 | −0.2694 | −0.4487 | −0.9418 |
| K+ | −0.6900 | −0.7343 | −0.6019 | −0.8352 |
| Mg2+ | −0.3634 | −0.0062 | 0.4817 | 0.3741 |
| Ca2+ | −0.3632 | −0.0739 | 0.4537 | 0.2974 |
| Cl− | −0.4896 | −0.4885 | −0.4589 | −0.9101 |
| NO3 − | −0.8384 | −0.7056 | −0.6617 | −0.7764 |
| SO4 2− | −0.6335 | −0.1494 | −0.0206 | −0.2741 |
Significant correlation at the 0.05 probability level
3.4.2 Correlations between OC, EC and PM2.5 cytotoxicity
Organic compounds in PM2.5 have been linked to its toxicity (Borgie et al., 2015b; Liu et al., 2016). The correlation coefficients (Table 3) showed that OC, EC, and SOC were negatively correlated with the cell viability, and the correlations were especially high during the early exposure (24 h). This indicated that there were some organic matters in the extraction of PM2.5, which affected the growth of the cells. Therefore, cell viability may be affected by the synergism or overlapping of water-soluble heavy metals and organic compounds. Moreover, this phenomenon was most obvious during the early exposure period. This result was similar to that reported by Huang et al. (2015), who used a water extraction of dust and PM2.5 to interact with human cells and obtained a similar conclusion. However, OC, EC, and SOC had a smaller effect on ROS, which may be because the cells had no phagocytic function. Because of the phagocytic function of macrophages, when exposed to PM2.5 extractions, the non-water-soluble components, which are presented as a foreign matter, can be easily identified by macrophages. In the process of its phagocytosis, the oxygen resulted in a large number of ROS, which led to the death of the macrophages (Imrich et al., 2000). However, cells do not have phagocytosis, such as A549 cells, non-water-soluble composition has little influence on cells, and water-soluble transition metal ions and other substances can more easily induce the production of free radicals and aggravate oxidative stress damage (Cao et al., 2008). Therefore, it could be conjectured that because of the BEAS-2B cells not having phagocytosis, OC, EC, and SOC have a low impact on ROS production. The different contents of OC and EC in the summer and winter would influence PM2.5 cytotoxicity during the two seasons.
3.4.3 Correlation between water-soluble ions and PM2.5 cytotoxicity
Previous studies have found that the high levels of SO4 2− and NO3 − in PM2.5 could change the pH of the exposed liquid, thereby affecting the growth of cells (Huang et al., 2015). From the correlation results (Table 3), water-soluble ions showed negative correlations with cell viability and intracellular ROS level. In addition to the higher correlations between K+, NO3 − and other indices, other components showed a low correlation with the cytotoxicity results.
The toxic effect of K+ on cells could be related to the change in the membrane potential of cells, while NO3 − may change the pH of the exposure solution and indirectly affect the growth of cells.
4. Conclusions
During both the summer and winter, the main chemical components of PM2.5 in Hangzhou were water-soluble ions, particularly SO4 2−, NO3 −, and NH4 +, followed by organic compounds and then heavy metals. The toxicity tests of water extractions of PM2.5 showed that PM2.5 had greater influences on cell viability and ROS levels when the mass concentration increased. However, when the mass concentration of PM2.5 was considerable in the summer and winter, the extraction of PM2.5 collected in the summer showed greater effects on cell viability and ROS levels. The difference in the contents of trace heavy metals, such as V and Pb, and organic compounds, such as OC and EC, during the winter and summer was one of the reasons for the seasonal toxicity difference of PM2.5. Organic compounds in the extracts of PM2.5 together with trace heavy metals would produce synergistic or overlapping toxic effects on cells, causing the cell viability decreasing. However, because of the low content of organic compounds released into the aqueous extraction and BEAS-2B cells did not have the function of phagocytosis, water-soluble organic compounds had less effect on ROS level. Although water-soluble ions were the main components in PM2.5, most of them were not related to the cytotoxicity of PM2.5.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 41371447) and the National Key Technologies R&D Program of China (No. 2013BAC16B04)
Compliance with ethics guidelines: Hui-hui ZHANG, Zheng LI, Yu LIU, Ping XINAG, Xin-yi CUI, Hui YE, Bao-lan HU, and Li-ping LOU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Becker S, Dailey LA, Soukup JM, et al. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Persp. 2005;113(8):1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgie M, Dagher Z, Ledoux F, et al. Comparison between ultrafine and fine particulate matter collected in Lebanon: chemical characterization, in vitro cytotoxic effects and metabolizing enzymes gene expression in human bronchial epithelial cells. Environ Pollut. 2015;205:250–260. doi: 10.1016/j.envpol.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Borgie M, Ledoux F, Verdin A, et al. Genotoxic and epigenotoxic effects of fine particulate matter from rural and urban sites in Lebanon on human bronchial epithelial cells. Environ Res. 2015;136:352–362. doi: 10.1016/j.envres.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q, Qian X, Zhang S, et al. Cytotoxicity of soluble and insoluble components of atmospheric fine particles. Acta Sci Circum. 2008;28(6):1167–1172. [Google Scholar]
- 5.Chen H, Wang H. Haze days in North China and the associated atmospheric circulations based on daily visibility data from 1960 to 2012. J Geophys Res Atmos. 2015;120(12):5895–5909. doi: 10.1002/2015JD023225. [DOI] [Google Scholar]
- 6.Deng X, Zhang F, Rui W, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol in Vitro. 2013;27(6):1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Du D, Li J, Tao Y, et al. Seasonal variations of microscopic characteristics of PM2.5 in Guiyang City. Acta Sci Circum. 2015;35(6):1645–1650. [Google Scholar]
- 8.Environmental Protection Department of the People’s Republic of China. Specifications and Test Procedures for PM10 and PM25 Sampler. China Environmental Science Press, Beijing; 2013. (in Chinese) [Google Scholar]
- 9.Gualtieri M, Mantecca P, Corvaja V, et al. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549) Toxicol Lett. 2009;188(1):52–62. doi: 10.1016/j.toxlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Happo M, Markkanen A, Markkanen P, et al. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicol in Vitro. 2013;27(5):1550–1561. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Kang Y, Wang W, et al. Potential cytotoxicity of water-soluble fraction of dust and particulate matters and relation to metal (loid)s based on three human cell lines. Chemosphere. 2015;135:61–66. doi: 10.1016/j.chemosphere.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Huang T, Chen J, Zhao W, et al. Seasonal variations and correlation analysis of water-soluble inorganic ions in PM2.5 in Wuhan, 2013. Atmosphere. 2016;7(4):49. doi: 10.3390/atmos7040049. [DOI] [Google Scholar]
- 13.Imrich A, Ning Y, Kobzik L. Insoluble components of concentrated air particles mediate alveolar macrophage responses in vitro. Toxicol Appl Pharm. 2000;167(2):140–150. doi: 10.1006/taap.2000.9002. [DOI] [PubMed] [Google Scholar]
- 14.de Kok TMCM, Driece HAL, Hogervorst JGF, et al. Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat Res Rev. 2006;613(2-3):103–122. doi: 10.1016/j.mrrev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Baumgartner J, Zhang Y, et al. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ Sci Technol. 2014;48(21):12920–12929. doi: 10.1021/es5029876. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Baumgartner J, Zhang Y, et al. Source apportionment of Beijing air pollution during a severe winter haze event and associated pro-inflammatory responses in lung epithelial cells. Atmos Environ. 2016;126:28–35. doi: 10.1016/j.atmosenv.2015.11.031. [DOI] [Google Scholar]
- 17.Longhin E, Pezzolato E, Mantecca P, et al. Season linked responses to fine and quasi-ultrafine Milan PM in cultured cells. Toxicol in Vitro. 2013;27(2):551–559. doi: 10.1016/j.tiv.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Chen Z, Wu M, et al. Airborne PM2.5/PM10-associated chlorinated polycyclic aromatic hydrocarbons and their parent compounds in a suburban area in Shanghai, China. Environ Sci Technol. 2013;47(14):7615–7623. doi: 10.1021/es400338h. [DOI] [PubMed] [Google Scholar]
- 19.Manzanoleón N, Serranolomelin J, Sanchez BN, et al. TNF-α and IL-6 responses to particulate matter in vitro: variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environ Health Persp. 2015;57(2):133–135. doi: 10.1289/ehp.1409287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masiol M, Ceccato D, Squizzato S, et al. An integrated analytical approach using ion chromatography, PIXE and electron microscopy to point out the differences in composition of PM10 individual particles. AIP Confer Proc. 2013;1530:111–118. doi: 10.1063/1.4812912. [DOI] [Google Scholar]
- 21.McMurry PH. A review of atmospheric aerosol measurements. Atmos Environ. 2000;34(12-14):1959–1999. doi: 10.1016/S1352-2310(99)00455-0. [DOI] [Google Scholar]
- 22.Meng CC, Wang LT, Zhang FF, et al. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos Res. 2016;171:133–146. doi: 10.1016/j.atmosres.2015.12.013. [DOI] [Google Scholar]
- 23.Okeson CD, Riley MR, Fernandez A, et al. Impact of the composition of combustion generated fine particles on epithelial cell toxicity: influences of metals on metabolism. Chemosphere. 2003;51(10):1121–1128. doi: 10.1016/S0045-6535(02)00721-X. [DOI] [PubMed] [Google Scholar]
- 24.Perrone MG, Gualtieri M, Ferrero L, et al. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere. 2010;78(11):1368–1377. doi: 10.1016/j.chemosphere.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Ji Y, Sun H, et al. Nanoscale characterization of PM2.5 airborne pollutants reveals high adhesiveness and aggregation capability of soot particles. Sci Rep. 2015;5(3):181–183. doi: 10.1038/srep11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spurny KR. Chemical mixtures in atmospheric aerosols and their correlation to lung diseases and lung cancer occurence in the general population. Toxicol Lett. 1996;88(1-3):271–277. doi: 10.1016/0378-4274(96)03749-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Guo Z, Lin T, et al. Seasonal variation of carbonaceous pollutants in PM2.5 at an urban supersite in Shanghai, China. Chemosphere. 2016;146:238–244. doi: 10.1016/j.chemosphere.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Xiang P, Liu RY, Sun HJ, et al. Molecular mechanisms of dust-induced toxicity in human corneal epithelial cells: water and organic extract of office and house dust. Environ Int. 2016;92-93:348–356. doi: 10.1016/j.envint.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Jing J, Tao J, et al. Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmos Chem Phys. 2013;13(14):7053–7074. doi: 10.5194/acp-13-7053-2013. [DOI] [Google Scholar]
- 30.Zheng N, Tan J, Duan J, et al. Research progress on water-soluble heavy metal in atmospheric particulate mattters. Environ Chem. 2014;33(12):2109–2116. [Google Scholar]