Abstract
Developmental dyslexia is a neurodevelopmental disorder with a strong genetic basis. Previous studies observed white matter alterations in the left posterior brain regions in adults and school-age children with dyslexia. However, no study yet has examined the development of tract-specific white matter pathways from the pre-reading to the fluent reading stage in children at familial risk for dyslexia (FHD+) versus controls (FHD−). This study examined white matter integrity at pre-reading, beginning, and fluent reading stages cross-sectionally ( n = 78) and longitudinally (n = 45) using an automated fiber-tract quantification method. Our findings depict white matter alterations and atypical lateralization of the arcuate fasciculus at the pre-reading stage in FHD+ versus FHD− children. Moreover, we demonstrate faster white matter development in subsequent good versus poor readers and a positive association between white matter maturation and reading development using a longitudinal design. Additionally, the combination of white matter maturation, familial risk, and psychometric measures best predicted later reading abilities. Furthermore, within FHD+ children, subsequent good readers exhibited faster white matter development in the right superior longitudinal fasciculus compared with subsequent poor readers, suggesting a compensatory mechanism. Overall, our findings highlight the importance of white matter pathway maturation in the development of typical and atypical reading skills.
Keywords: developmental dyslexia, familial risk, longitudinal, tractography, white matter development
Introduction
Learning to read is a developmental process that requires the seamless integration of neural circuits involved in vision, audition, and language, and is accompanied by prominent experience-dependent functional and gray matter plasticity in various brain regions, including temporo-parietal, temporo-occipital, superior temporal, and inferior frontal regions ( Turkeltaub et al. 2003 ). Studies show that 5–17% of all children persistently struggle with learning to read and suffer from developmental dyslexia (DD), the most common learning disability ( Lyon et al. 2003 ; McCandliss and Noble 2003 ). DD has an estimated heritability of 58% ( Pennington and Olson 2005 ) and has been genetically linked to at least 4 candidate susceptibility genes ( DYX1C1 , KIAA0319 , DCDC2 , and ROBO1 ) ( Galaburda et al. 2006 ). Notably, experimental interference with these genes leads to abnormal cortical neuronal migration during cortical development in rodents ( Galaburda 1993 ; Taipale et al. 2003 ; Cope et al. 2005 ; Meng et al. 2005 ; Fisher and Francks 2006 ; Galaburda et al. 2006 ). Children with a family history of DD have a 34–56% chance of developing DD ( Pennington and Lefly 2001 ). However, while there is increasing evidence of the neurobiological substrates of DD, the causes of DD are still debated.
An abundance of neuroimaging studies have shown atypical neurobiological substrates of DD in all components of the reading circuitry. For example, functional magnetic resonance imaging (fMRI) studies have reported reduced activation of left temporo-parietal and temporo-occipital regions during reading and reading-related tasks in school-age children and adults with reading impairment or a diagnosis of DD ( Shaywitz and Shaywitz 2008 ; Gabrieli 2009 ; Richlan et al. 2009 ; Martin et al. 2015 ), as well as in pre-readers with or without familial risk for DD ( Specht et al. 2009 ; Yamada et al. 2011 ; Raschle, Zuk, Ortiz-Mantilla, et al. 2012 ; Raschle, Zuk, Gaab 2012 ; Bach et al. 2013 ; Raschle et al. 2013 ). Furthermore, structural MRI using voxel-based morphometry (VBM) has revealed decreased gray matter volume indices in left posterior brain regions in individuals with DD ( Eckert et al. 2005 ; Hoeft et al. 2007 ; Krafnick et al. 2011 , 2014 ; Linkersdorfer et al. 2015 ) and pre-readers at familial risk for DD ( Raschle et al. 2011 ; Simon et al. 2013 ). Additionally, structural MRI data have demonstrated atypical sulcal patterns in school-age children with DD and kindergartners at familial risk for DD, which might originate from altered organization of white matter and cortical function ( Im et al. 2014 ).
The structural connections between these functional cortical regions are formed through axonal bundles that can be quantified in vivo using diffusion tensor imaging (DTI) ( Mori and van Zijl 2002 ; Mori et al. 2009 ). This technique provides quantitative measures including fractional anisotropy (FA) as a normalized scalar measure of the degree of diffusion anisotropy, axial diffusivity (AD) as a measure of diffusion parallel to white matter tracts, and radial diffusivity (RD) as a measure of diffusion perpendicular to white matter tracts ( Le Bihan et al. 2001 ). Differences in FA values can either be attributed to myelination, which has also been associated with RD, or axonal properties (including the number of axons, axon density, and axon caliber), which have been linked to AD ( Vandermosten, Boets, Wouters, et al. 2012 ). Importantly, learning to read requires transformation from print to speech and print to meaning, which involves multiple functional cortical regions connected through white matter pathways. Three white matter pathways that have been most strongly linked to language and reading include the left arcuate fasciculus (AF), the left superior longitudinal fasciculus (SLF), and the left inferior longitudinal fasciculus (ILF) ( Hoeft et al. 2011 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Saygin et al. 2013 ; Myers et al. 2014 ). The left AF consists of a long medial segment connecting the superior temporal gyrus (STG) and inferior frontal gyrus (IFG); a lateral anterior segment connecting the IFG and the inferior parietal lobule (IPL); and a lateral posterior segment linking the STG and IPL. These regions have been related to various reading and reading-related skills ( Anderson et al. 1999 ; Duffau 2008 ; Vandermosten, Boets, Poelmans, et al. 2012 ). The left SLF connects inferior parietal and inferior frontal/premotor regions. It has been shown to map phonemic representations to motor representations and to sustain the phonological aspects of speech perception ( Hickok and Poeppel 2004 , 2007 ; Wandell and Yeatman 2013 ; Qi et al. 2015 ). The left ILF carries signals from the posterior inferior temporal gyrus to the anterior and medial temporal lobe, which have been proposed to play an important role in mapping visual information about words to their lexical meaning ( Anwander et al. 2007 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Yeatman, Rauschecker, et al. 2012 ; Wandell and Yeatman 2013 ; Cummine et al. 2015 ; Qi et al. 2015 ). The reading-related functions of these tracts stem from lesion studies ( Epelbaum et al. 2008 ; Szwed et al. 2011 ) and studies that examined illiterate adults before and after reading instruction ( Dehaene and Cohen 2011 ), as well as cross-sectional studies examining correlations between white matter microstructure and (pre-) reading skills in beginning, emergent, and fluent readers ( Hoeft et al. 2011 ; Saygin et al. 2013 ; Cummine et al. 2015 ). Furthermore, 1 study also revealed anomalies in the left inferior fronto-occipital fasciculus (IFOF) in Dutch-speaking pre-reading children at risk for dyslexia, but the study failed to observe alterations in the left AF and right homologs ( Vandermosten et al. 2015 ). Notably, evidence for white matter microstructure prior to formal reading instruction is limited and inconclusive since most neuroimaging studies focus on school-age children or adults with reading impairment or DD. Moreover, to date, no study has examined white matter microstructure across early reading development from the pre-reading to the fluent reading stages, cross-sectionally or longitudinally.
Previous studies have demonstrated lower FA in the left AF, SLF, and ILF in children and adults with reading impairment or DD ( Richards et al. 2008 ; Hoeft et al. 2011 ; Yeatman et al. 2011 ; Vandermosten, Boets, Poelmans, et al. 2012 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Boets et al. 2013 ; Saygin et al. 2013 ; Myers et al. 2014 ). In addition, polymorphism in the dyslexia susceptibility genes has been related to alterations in white matter structure ( Darki et al. 2012 ; Scerri et al. 2012 ). Decreased FA in white matter tracts relevant to language or reading was also evident in beginning readers ( Hosseini et al. 2013 ) and pre-readers ( Vandermosten et al. 2015 ), as well as infants ( Langer et al. 2015 ) who are at familial risk for DD, suggesting that white matter alterations precede DD. However, the lack of longitudinal data in the current literature limits our understanding of how white matter developmental changes differ due to the genetic predisposition to dyslexia.
Recent advancement in diffusion tractography allows researchers to compute FA at multiple nodes along a white matter pathway (tract) instead of merely a global mean value ( Mori and van Zijl 2002 ; Yeatman, Dougherty, Myall, et al. 2012 ). Using this technique, named Automatic Fiber Quantification (AFQ) ( Yeatman, Dougherty, Myall, et al. 2012 ), Johnson et al. (2013) have reported not only that diffusion properties vary significantly along a tract, but also the relationship between age and diffusion metrics changes along a given tract. These findings suggest focal/regional variability in age-related white matter development across a tract ( Johnson et al. 2013 ). White matter consists mostly of glial cells and myelinated axons that transmit signals between brain regions. Thousands of axons enter and excite at different special locations along a white matter fiber. Thus, summarizing the entire tract with a single diffusion parameter may lose valuable information, whereas examining regional diffusion properties may provide a detailed characterization of development of white matter tract structure during early childhood ( Basser et al. 1994 ). Especially, during brain development in early and middle childhood (birth to 12 years), rapid changes are happening in the white matter ( Dubois et al. 2006 ; Hermoye et al. 2006 ). White matter development includes 2 major processes: myelination that leads to increased FA, and pruning that leads to decreased FA. For healthy children, FA increases with age until early adolescence, which is primarily driven by brain maturation consisting of myelination and pruning ( Giedd et al. 1999 ; Schmithorst et al. 2002 ). Meanwhile, intervention studies have reported FA increases in response to reading intervention ( Keller and Just 2009 ; Hoeft et al. 2011 ), suggesting the possibility of additional experience/environment-driven increases of FA. Using AFQ, Yeatman, Dougherty, Ben-Shachar, et al. ( 2012 ) demonstrated that children with above-average reading skills initially show low fractional anisotropy (FA) at age 7 that increased over the examined 3-year period in the left AF and ILF when compared with children with below-average reading skills. They suggested that the dual process which consists of myelination increasing FA and pruning decreasing FA during brain maturation differs depending on reading skill. However, their study does not capture the process of learning to read, which a typically developing child usually accomplishes between age 5 and 6 (kindergarten). The present study aims to examine this relationship between white matter development and (pre-) reading skills in younger children, from the pre-reading to the fluent reading stage (age 5–12).
Furthermore, several studies have discussed reduced lateralization as 1 contributing factor of DD. In 1985, Galaburda and colleagues reported reduced left-hemispheric asymmetry of the planum temporale, which is part of the superior temporal lobe, in post-mortem analyses of adults with dyslexia ( Galaburda et al. 1985 ). Based on this result, the authors hypothesized that specific influences in utero can reduce the developmental rate of left-hemispheric structures within the language/reading network, thus diminishing the magnitude of the well-established left-hemispheric lateralization in individuals with dyslexia ( Geschwind and Galaburda 1985 ; Galaburda et al. 2006 ). KIAA0319, one of the candidate dyslexia susceptibility genes that is involved in neuronal migration, has been associated with the left–right asymmetry of brain activations for reading in temporal cortex using fMRI ( Pinel et al. 2012 ). Additionally, recent studies have proposed a tentative model that hypothesizes genetic anomalies of the cortical micro-architecture in the left-hemispheric temporal lobe, where phonological deficits could arise ( Lehongre et al. 2011 ; Giraud and Ramus 2013 ). Using modern neuroimaging techniques like fMRI and DTI, Vernooij et al. (2007 ) reported significant leftward asymmetry in the relative fiber density of the AF in 20 healthy adults, whereas a recent study demonstrated reduced left lateralization of the AF in adults with dyslexia ( Vandermosten et al. 2013 ). This evidence suggests a relationship between atypical hemispheric lateralization of AF and dyslexia. It will be of great importance to examine how lateralization evolves over time in children with or without a family history of DD/with and without subsequent diagnosis of DD.
Several previous studies suggested a potential compensatory mechanism that enables individuals with dyslexia to overcome dysfunctions in left posterior cortical areas by utilizing the homologous regions of the right hemisphere. For instance, Hoeft et al. have reported that greater right IFG activation during a reading task and higher FA values in the right SLF (including AF) significantly predicted future reading gains over a 2.5-year period in children with dyslexia ( Hoeft et al. 2011 ). Their findings suggested that the right IFG and SLF may be critical for longitudinal reading improvement in children with dyslexia. Moreover, it has been shown that individuals with dyslexia demonstrated hyperactivation in the right IFG ( Shaywitz et al. 1998 ; Milne et al. 2002 ; Shaywitz et al. 2003 ) which suggests that compensatory mechanism can be observed structurally and functionally. However, the characteristics of a compensatory mechanism have not yet been explored in children with and without family history of DD across the time course of early reading development.
Furthermore, several neuroimaging studies have revealed that brain structure and function in early readers are effective predictors of later reading abilities ( Hoeft et al. 2007 , 2011 ; Giedd and Rapoport 2010 ; McNorgan et al. 2011 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Bach et al. 2013 ). Hoeft et al. ( 2007 ) reported that a combined regression model including fMRI data, VBM data, and standardized behavioral measures explained about 81% of the variance of later reading ability, which is more predictive than unimodal models. Recently, 2 DTI studies demonstrated that FA-development rates in elementary school children also yield relatively strong prediction values. In one, about 43% of reading variance was predicted by the FA-development rates of the left AF and ILF ( Yeatman, Dougherty, Ben-Shachar, et al. 2012 ), while in the other study, volume changes in temporo-parietal white matter tracts combined with preliteracy measures predicted 56% of the variance in reading outcomes ( Myers et al. 2014 ). These results emphasized that the developmental trajectory of brain structure might provide more information about the underlying brain mechanisms related to reading ( Wandell and Yeatman 2013 ). Nevertheless, no study to date has examined how well the combination of FA-development rates and behavioral measures in preliterate children, along with familial risk, predict later reading skills in elementary school.
To investigate these open questions, we utilized an AFQ method ( Yeatman, Dougherty, Myall, et al. 2012 ) in children with (FHD+) and without (FHD−) a family history of DD to 1) examine the regional tract-specific white matter microstructure in the left AF, ILF, and SLF from the pre-reading to the fluent reading stage in a cross-sectional cohort of 78 FHD− and FHD+ children; 2) examine the differences of FA-developmental trajectories between FHD− and FHD+ children and the differences of FA-developmental trajectories between subsequent good and poor readers in a longitudinal cohort of 45 children; 3) examine whether the longitudinal rate of FA-development correlates with the development of reading skills; and 4) discern the optimal combination of development of white matter tracts, preliteracy behavioral measures, and familial risk status to best predict later reading outcomes in elementary school.
Materials and Methods
Participants
A sample of 78 healthy, native English-speaking children was recruited between May and November of their kindergarten entry year and followed longitudinally thereafter as participants in the Boston Longitudinal Dyslexia study (BOLD) ( Raschle, Zuk, Ortiz-Mantilla, et al. 2012 ; Raschle, Zuk, Gaab 2012 ) (see Supplementary Table 1 ). For the cross-sectional cohort, 78 children were divided into 3 developmental groups. Children who recognized fewer than 9 single words were regarded as pre-readers. Children who had either entered kindergarten or 1st/2nd grade were regarded as beginning readers. Children who had entered 3rd/4th/5th grade were regarded as fluent readers. Forty-five children (age range: 59–150 months, 24 boys, 21 girls) who had at least one first-degree relative with a clinical diagnosis of DD were classified as FHD+ (with a family history of DD). Thirty-three children (age range: 60–134 months 18 boys/15 girls) who had no first-degree relatives with DD or reading difficulties were classified as FHD− (without a family history of DD).
Of the 78 children, 45 had >1 scan point and formed the longitudinal cohort (FHD−: n = 22, mean age at the first data point: 80 months 10 boys/12 girls; FHD+: n = 23, mean age at the first data point: 81 months 13 boys/10 girls), with a total of 103 scans (see Supplementary Table 2 ). None of the participants had any history of neurological or psychological symptoms, head injuries, visual problems, or hearing loss. The study was approved by the Institutional Review Board at Boston Children's Hospital. Written informed consent was obtained from each participant's parents, and verbal assent was obtained from each participant.
Behavioral Assessments
At the time of initial recruitment, all participants were screened for pre-reading status using the Word Identification (ID) subtest of the Woodcock Reading Mastery Test-Revised (WRMT-R) ( Woodcock 1987 ). In addition, a set of standardized assessments examining language functions and intelligence was administered, including the Clinical Evaluation of Language Fundamentals-Fourth Edition (CELF-4) ( Semel et al. 1999 ), the Rapid Automatized Naming/Rapid Alternating Stimulus Tests (RAN/RAS) ( Wolf and Denckla 2005 ), the Comprehensive Test Of Phonological Processing (CTOPP) ( Torgesen et al. 1999a , b ), the Test of Silent Word Reading Fluency (TOSWRF) ( Hammill et al. 2004 ), the Test Of Memory And Learning Second Edition (TOMAL2) ( Reynolds and Voress 2007 ), and the Kaufman Brief Intelligence Test Second Edition (KBIT-2) ( Kaufman and Kaufman 1990 ). After the first-year imaging scan, reading assessments were added into the behavioral testing battery, including the Test Of Word Reading Efficiency (TOWRE) ( Torgesen et al. 1999a , b ), the Gray Oral Reading Test Fifth Edition (GORT-5) ( Wiederholt and Bryant 2001 ), the Passage Comprehension subtest of WRMT-R, and the Reading Fluency subtest of the Woodcock-Johnson Test of Achievement Third Edition (WJ-III) ( Woodcock et al. 2001 ). Supplementary Table 1 presents descriptive statistics for the 2 groups (FHD− and FHD+).
Image Acquisition
All participants underwent MRI scans on a 3.0 T Siemens Tim Trio whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany) using multi-echo magnetization-prepared rapid gradient-echo sequences with prospective motion correction (mocoMEMPRAGE) for structural T1 -weighted images and echo planar image (EPI) sequence of 30 gradient directions for diffusion-weighted images with a 12-channel head coil. The imaging parameters for structural MRI were as follows: flip angle/TE/TR/TA = 7 degrees/1450 ms/2270 ms/4:51 min; field of view (FOV) = 220 × 220 mm; in-plane acceleration (GRAPPA) factor of 2; spatial resolution = 1.1 × 1.1 × 1.0 mm (176 slices). The imaging parameters for diffusion-weighted imaging (DWI) were as follows: flip angle/TE/TR/TA/FOV = 90 degrees/88 ms/8320 ms/5:59 min/256 × 256 mm; b = 1000 s/mm 2 . Each child underwent extensive preparation and training in the mock MR scanner area before the actual MRI scan session, which has been shown to improve the child's compliance and imaging data quality ( Raschle et al. 2009 ; Raschle, Zuk, Ortiz-Mantilla, et al. 2012 ; Raschle, Zuk, Gaab 2012 ).
Image Processing
The T1 -weighted structural image was used to generate a brain mask by removal of nonbrain tissue using the Brain Extraction Tool (BET) ( Smith 2002 ) from Functional MRI of the Brain (FMRIB) software Library (Oxford, UK). DWI DICOM data were converted into NRRD ( teem.sourceforge.net/nrrd/ ) format using DicomToNrrdConverter software from Slicer4 ( www.slicer.org ). DWI quality control (QC) procedures were conducted using DTIprep software ( Liu et al. 2010 ) and visual inspection. Motion artifacts were defined by translation threshold of 2 mm and rotation threshold of 0.5° through rigid registration-based volume-by-volume measures. Volumes with motion artifacts were excluded from diffusion tensor estimation. After QC, DWI data were processed using mrDiffusion, a toolbox from the VISTALab (Stanford Vision and Imaging Science and Technology) diffusion MRI software suite ( www.vistalab.com ) including Eddy current correction and tensor-fitting estimations ( Rohde et al. 2004 ). Diffusion tensors were fitted using a linear least-squares (LS) fit, and eigenvalues from the diffusion tensor estimation were used to compute FA, AD, and RD ( Basser et al. 1994 ).
Automatic Fiber Quantification
The AFQ ( github.com/jyeatman/AFQ ) software package ( Yeatman, Dougherty, Myall, et al. 2012 ) was used to identify white matter tracts. The AFQ analysis pipeline is described in greater detail in Yeatman, Dougherty, Myall, et al. ( 2012 ). A brief description of the steps used in this study is provided here: 1) whole-brain tractography using a deterministic streamlines tracking algorithm (STT) ( Mori et al. 1999 ; Basser et al. 2000 ) with an FA threshold of 0.2 and angle threshold of 40°, 2) region of interest (ROI)-based fiber tract segmentation, 3) fiber-tract cleaning using a statistical outlier rejection algorithm, and 4) diffusion characteristics quantification at each node along the trajectory of the fiber. Each fiber is sampled to 100 equidistant nodes that can be used to compute FA value at each node (certain spatial location) along the fiber. AFQ segments the whole-brain fiber group into 20 white matter tracts that are defined in the white matter atlas ( Wakana et al. 2007 ). The analyses here focused on 3 left-hemispheric tracts that have been linked to reading ability including the AF, SLF, and ILF ( Yeatman et al. 2011 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Saygin et al. 2013 ) (see Supplementary Fig. 1 ). Moreover, instead of computing mean diffusion parameters (FA, AD, RD), AFQ computes diffusion parameters using a weighted sum of each fiber's value at a given node where a fiber is weighted based on its Mahalanobis distance from the core or mean location of the tract ( Johnson et al. 2013 ). This improves detection power for group differences. For each tract, 100 nodes along the tract were resampled to 50 nodes by discarding the portion of fiber tract where individual fibers separate from the core fascicle toward their destination in the cortex. This approach normalizes the fiber end points across participants and improves the co-registration of each fiber tract among all participants ( Yeatman et al. 2011 ).
Statistical Analyses
All statistical analyses were executed using the R system (version 3.1.0 64 bit) ( Ihaka and Gentleman 1996 ). The Shapiro–Wilk Normality test was used to determine whether a 2-sample t -test or Mann–Whitney U test should be used for group comparison. Significance was set at P = 0.05 for all analyses, and false discovery rate (FDR) correction was applied to adjust for multiple comparisons ( Benjamini and Hochberg 1995 ).
Cross-Sectional Analyses
Tract-specific white matter microstructure at 3 developmental stages
To examine group difference at each developmental stage, FA, AD, and RD values of each node along the tract of interest were compared between FHD− and FHD+ children using 2-sample Wilcoxon rank-sum tests controlling for age and sex.
Lateralization of white matter microstructure at 3 developmental stages
The lateralization index (LI) for each tract of interest was computed using the following equation: LI (FA) = 100 x [right (FA) − left (FA)]/[right (FA) + left (FA)] ( Vandermosten et al. 2013 ). A positive LI indicated right lateralization, whereas a negative LI indicated left lateralization. To examine the group effect, the LI of each node of the tract of interest was compared between FHD− and FHD+ children using 2-sample Wilcoxon rank-sum tests controlling for age and sex.
Longitudinal Analyses
Tract-specific white matter maturation
Among the cross-sectional cohort of 78 children, 45 children had at least 2 but no more than 4 DWI scans. The total number of imaging scans was 103. Based on the cross-sectional results that indicated lower FA in FHD+ compared with FHD− children at all reading stages in 1 temporo-parietal segment of the left AF (node 24, see Fig. 1 ), this segment (node 24) was chosen to examine the relationship between FA and age. FA values for FHD− and FHD+ children were modeled as FA = (Age) × β1 + β0 using a linear mixed-effect model in the lme4 R package ( Bates 2005 ). This allowed for a general variance–covariance for random effects for each child. The 45 children within the longitudinal cohort were divided into good readers and poor readers based on their standard scores for TOWRE PDE and SWE, WRMT-R Passage Comprehension, and WJ-III Reading Fluency at 2nd grade or later. Children were classified as poor readers if they earned standard scores below 85 on any of the 4 reading measures and as good readers if they earned standard scores above 90 on all of the 4 reading measures. Then, FA values for good and poor readers were also modeled as FA = (Age) × β1 + β0 using a linear mixed-effect model. Furthermore, the rate of FA-development from each child demonstrates the average change year to year. Due to missing data, the initial starting point is different for some children. To eliminate differences in FA-development rate due to initial age, the rate of FA-development was age-adjusted using general linear regression. At last, 2 sample t -tests were used to examine the group differences of FA-development rate between FHD− and FHD+ children, as well as between subsequent good and poor readers.
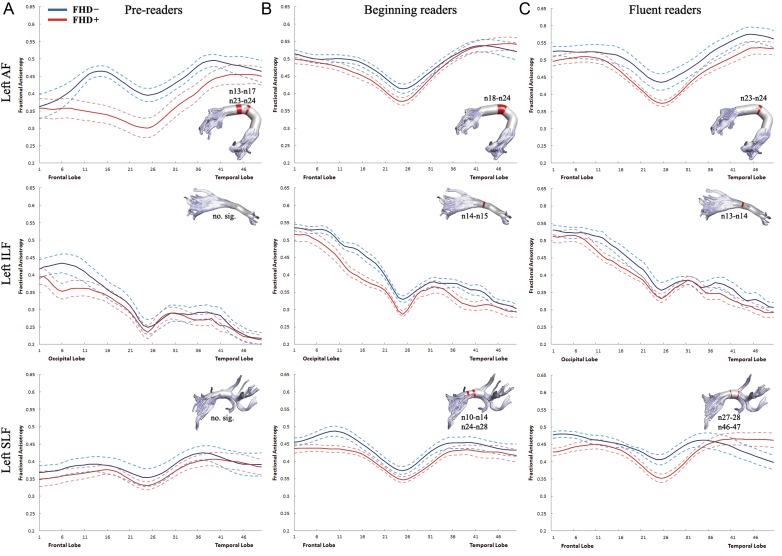
Figure 1.
Tract FA profiles between the 1st and 50th nodes for the left AF, ILF, SLF are shown for 3 developmental stages of reading. Solid lines represent the mean FA and dotted lines denote standard error of the mean. Blue lines: FHD− children. Red lines: FHD+ children. Nodes that show significantly higher FA in FHD− than in FHD+ children are marked in red on the 3-dimensional rendered tract ( P < 0.05, FDR-corrected). AF: arcuate fasciculus; ILF: inferior longitudinal fasciculus; SLF: superior longitudinal fasciculus.
Brain development–behavioral relationship
Previous studies have shown that the change in FA values over time for each child was approximately linear ( Giedd 2004 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ). Thus, a linear model was employed to determine each child’s rate of FA development. In addition, each child's reading development was calculated by computing the annual changes of WRMT-R Word ID and TOSWRF raw scores. These 2 reading assessments were chosen because the majority of our longitudinal cohort completed both measures, thus maximal children could be included in this correlation analysis. The rate of FA development was adjusted for the initial starting age and was then correlated with the annual changes of WRMT-R Word ID and TOSWRF raw scores using Pearson's correlation tests.
Prediction of Later Reading Comprehension and Fluency (Longitudinal)
To identify the optimal set of predictors for reading comprehension and fluency, backward stepwise regression was used with an initial linear regression model, including WMRT-R Passage Comprehension or WJ-III Reading Fluency standard score (SS) as the dependent variable and 10 independent variables, including age at the first scan, familial risk status, gender, the mean FA-development rates of left AF, ILF, and SLF, WRMT-R Letter ID SS, RAN Object SS, CTOPP Nonword Repetition SS, and KBIT-2 Nonverbal IQ SS at the pre-reading stage before entering kindergarten (initial model: reading ability = β0 + β1 × age + β2 × familial risk + β3 × gender + β4 × left AF + β5 × left ILF + β6 × left SLF + β7 × WRMT-R letter ID + β8 × RAN Object + β9 × CTOPP Nonword Repetition SS + β10 × KBIT-2 Nonverbal IQ + ε ). Each tract had to be summarized with mean FA due to the limitation of sample size. Including all nodes in each tract within the initial model would penalize the model with no degree of freedom. Reading abilities were quantified using 2 aspects of reading including reading comprehension and reading fluency: 1) WRMT-R Passage Comprehension SS as the measure of reading comprehension at the beginning reading and reading stages; 2) WJ-III Reading Fluency SS as the measure of reading fluency at the beginning reading and fluent reading stages. Stepwise regression analyses were utilized to pick the best final model using the Akaike information criterion (AIC). In general, a smaller AIC indicates a better model fit. The contribution of each independent variable included in the final model was computed using the relaimpo package in R ( Gromping 2006 ). Ninety-five percent bootstrap confidence intervals were computed with 1000 iterations.
Potential Protective or Compensatory Factors in the Right SLF (Longitudinal)
Previously, Hoeft et al. (2011 ) observed a positive correlation between white matter anisotropy in the right SLF and a reading gain over 2.5 years in children with DD, but not in typical readers ( Hoeft et al. 2011 ), suggesting that the right SLF might play an important compensatory/protective role during reading development in at-risk children or children with a DD diagnosis. In the present study, only 1 out of 21 FHD− children developed into a poor reader (5%), whereas 10 out of 21 FHD+ children developed into poor readers (48%). Thus, there is not enough data to compare good readers with poor readers within FHD− children. Within FHD+ children, we further examine the differences of FA-developmental rates in the right SLF between a group of 11 FHD+ children who subsequently developed into good readers and a group of 10 FHD+ children who subsequently developed into poor readers using 2-sample Wilcoxon rank-sum tests.
Results
Demographic Information and Behavioral Assessments
Descriptive statistics of demographic characteristics and behavioral assessments are summarized for the cross-sectional data in Supplementary Table 1 and for the longitudinal data in Supplementary Table 2 . At the pre-reading stage, FHD− compared with FHD+ children showed significantly higher scores only in rapid automatized naming. At the beginning reading stage, FHD− compared with FHD+ children showed significantly better performance on receptive and expressive language and single word reading (timed and untimed). At the fluent reading stage, FHD− compared with FHD+ children showed significantly better performance in phonological processing, verbal memory, and reading fluency.
Cross-Sectional Analyses
Tract-Specific White Matter Microstructure at 3 Developmental Stages
Differences in FA values at each node along the left AF, ILF, and SLF were examined between FHD− and FHD+ children at 3 developmental stages (see Fig. 1 ). For the left AF, the significantly higher FA in FHD− compared with FHD+ children was present on nodes 13–17 and 23–24 at the pre-reading stage (Fig. 1A ), on nodes 18–24 at the beginning reading stage (Fig. 1B ), and on nodes 23–24 at the fluent reading stage (Fig. 1C ). For the left ILF, no group difference was present at the pre-reading stage. FHD− children had significantly higher FA on nodes 14–15 at the beginning reading stage and on nodes 13–14 at the fluent reading stage. For the left SLF, FHD− children displayed significantly higher FA on nodes 10–14 and 24–28 at the pre-reading stage and on nodes 27–28 and 46–47 at the beginning reading stage. No group difference was observed for AD values. Differences in RD values between FHD− and FHD+ children at 3 developmental stages are shown in Supplementary Figure 2 .
Lateralization of White Matter Microstructure at 3 Developmental Stages
Differences in the LI values at each node along AF between FHD− and FHD+ were examined at 3 developmental stages (see Fig. 2 ). At the pre-reading stage, there were significant differences between FHD− and FHD+ children on nodes 8–19 and nodes 23–26. FHD+ children showed right lateralization in AF, whereas FHD− children showed left lateralization in AF. At the beginning reading stage, nodes 14–15 showed group differences between FHD− and FHD+ children. FHD+ children showed right lateralization in AF, whereas FHD− children showed left lateralization in AF. At the fluent reading stage, there was no significant group difference. There was no difference in the LI of ILF and SLF between FHD− and FHD+ children.
Figure 2.
Tract lateralization index (LI) profiles between the 1st and 50th nodes for AF are shown for 3 reading developmental stages. Solid lines represent the mean LI and dotted lines denote standard error of the mean. Negative LI indicates left lateralization. Nodes that show significant group differences between FHD− and FHD+ children are marked in red on the 3-dimensional rendered tract ( P < 0.05, FDR-corrected).
Longitudinal Analyses
Tract-Specific White Matter Maturation
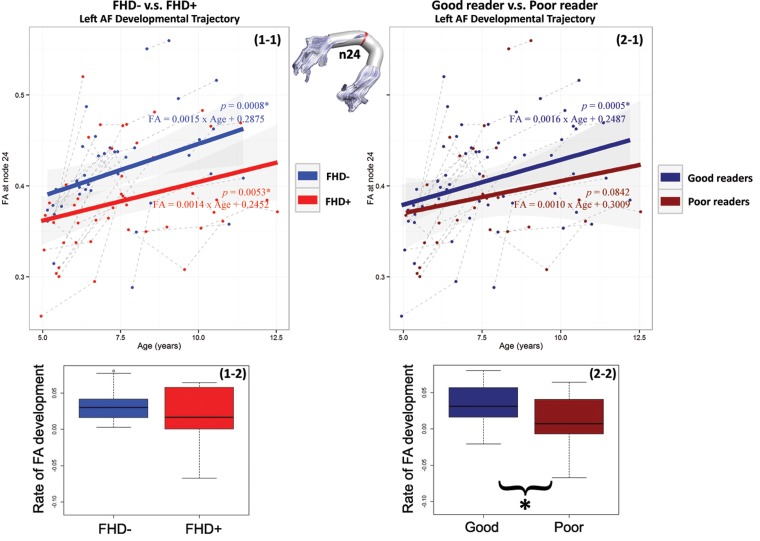
Our cross-sectional results showed significantly lower FA in the left AF for FHD+ compared with FHD− children on node 24 for all 3 reading development stages, and therefore, tract-specific white matter maturation was examined on this node. The FA-developmental trajectories for node 24 of the left AF were shown in Figure 3 . FHD− children's FA-developmental trajectory is FA = (Age) × 0.0015 + 0.2875 ( P = 0.0008), while FHD+ children's FA-developmental trajectory is FA = (Age) × 0.0014 + 0.2452 ( P = 0.0053). Subsequent good readers' FA-developmental trajectory is FA = (Age) × 0. 0016 + 0.2487 ( P = 0.0005), while subsequent poor readers' FA-developmental trajectory is FA = (Age) × 0.0010 + 0.3009 ( P = 0.0842). Furthermore, the age-adjusted rate of FA development from each child was summarized in boxplots for FHD− and FHD+ children (see Fig. 3 , 1–2) and for subsequent good and poor readers (see Fig. 3 , 2-2). There was no significant difference between FHD- and FHD+ children ( P = 0.9313), whereas subsequent good readers showed a significantly higher rate of FA development compared with subsequent poor readers ( P = 0.0062).
Figure 3.
Scatter plots of FA at node 24 for the left AF are grouped by familial risk status (1-1) and reading ability (2-1). Solid lines represent the linear relationship between FA and age (years) for each group. Gray-dotted lines represent the linear relationship between FA and age (years) for each child. Node 24 is marked in red on the 3-dimensional rendered tract. (1-2) shows the boxplot for FA-development rates by familial risk status. (2-2) shows the boxplot for FA-development rates by reading ability.
Brain Development–Behavioral Relationship
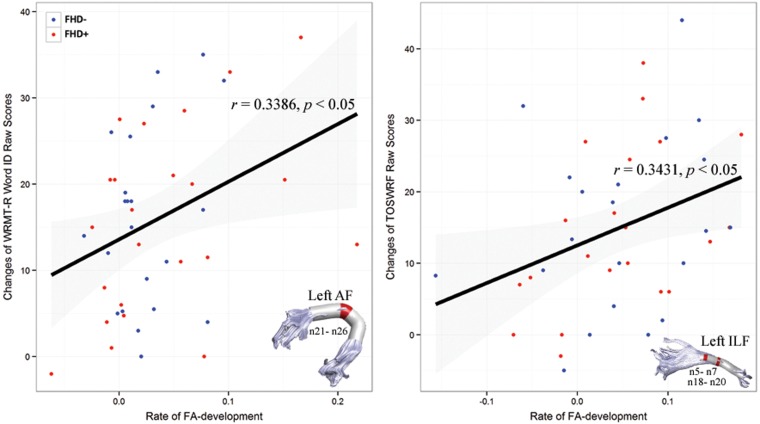
Both FHD- and FHD+ children showed a positive association between the rate of FA-development and reading development. Thus, the 2 groups were pooled together. For the left AF, rates of FA development and word identification skill development correlated positively on nodes 21–26 (see Fig. 4 ). A higher rate of FA development in the temporo-parietal segments was associated with greater increase in word identification skill ( r = 0.3386, P < 0.05). On nodes 5–7 and 18–20 of the left ILF, rates of FA development correlated positively with greater increase in silent reading fluency skill measured by the Test of Silent Word Reading Fluency (TOSWRF) ( r = 0.3431, P < 0.05) (see Fig. 4 ).
Figure 4.
Scatter plots of rates of FA development versus changes of reading development for the left AF and ILF. The representative node 23 is plotted for the left AF, and the representative node 19 is plotted for the left ILF. Solid black lines represent the linear relationship between rates of FA development and changes of behavioral assessments. Nodes that show significant correlation between rates of FA development and changes of behavioral assessments are marked in red on the 3-dimensional rendered tract ( P < 0.05, FDR-corrected).
Prediction of Later Reading Comprehension and Fluency (Longitudinal)
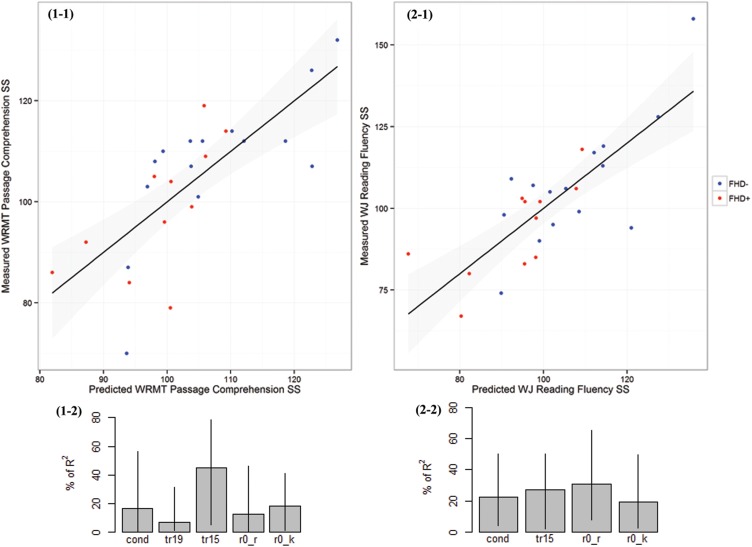
The mean FA-development rate of all tracts, familial risk, and psychometric measures at the pre-reading stage were combined in backward stepwise regression analyses to identify the optimal set of predictors for later reading abilities (see Fig. 5 ). When using the WRMT-R Passage Comprehension standard scores as a quantitative measure of reading comprehension skill, R2 was 0.56 ( P = 0.004) and the best set of predictors included the following: 1) the mean FA-development rate of the left AF (contributing 7% of R2 ); 2) left SLF (contributing 45% of R2 ); 3) RAN Objects (contributing 13% of R2 ); 4) KBIT-2 Nonverbal IQ standard scores (contributing 18% of R2 ) at the pre-reading stage; 5) familial risk (contributing 17% of R2 ). When using the WJ-III Reading Fluency subtest standard scores as a quantitative measure of reading fluency, R2 was 0.62 ( P = 0.0003) and the best set of predictors included the mean FA-development rate of left SLF (contributing 27% of R2 ), RAN Objects (contributing 31% of R2 ), KBIT-2 Nonverbal IQ standard scores (contributing 19% of R2 ) at the pre-reading stage, as well as familial risk (contributing 23% of R2 ).
Figure 5.
Prediction of reading comprehension (1-1) and fluency (2-1) using backward stepwise regression analysis to identify the best set of predictors which includes the combination of behavioral and FA-development rate (slope) measures along with familial risk: (1-1) Woodcock Reading Mastery Tests-Revised Passage Comprehension subtest, (2-1) Woodcock-Johnson Tests of Achievement-III Reading Fluency subtest. For each scatter plot, predicted scores are shown on the x -axis, and measured scores are shown on the y -axis. Blue dots: FHD− children, Red dots: FHD+ children. (1-2) and (2-2) are the relative importance bar plot with 95% bootstrap confidence intervals for reading comprehension and fluency, respectively. Tract names and behavioral measures are abbreviated as follows: tr19 = left arcuate fasciculus, tr15 = left superior longitudinal fasciculus, r0_r = RAN Objects standard scores at the pre-reading stage and r0_k = KBIT-2 Nonverbal IQ standard scores at the pre-reading stage.
Potential Compensatory or Protective Factor in the Right SLF (Longitudinal)
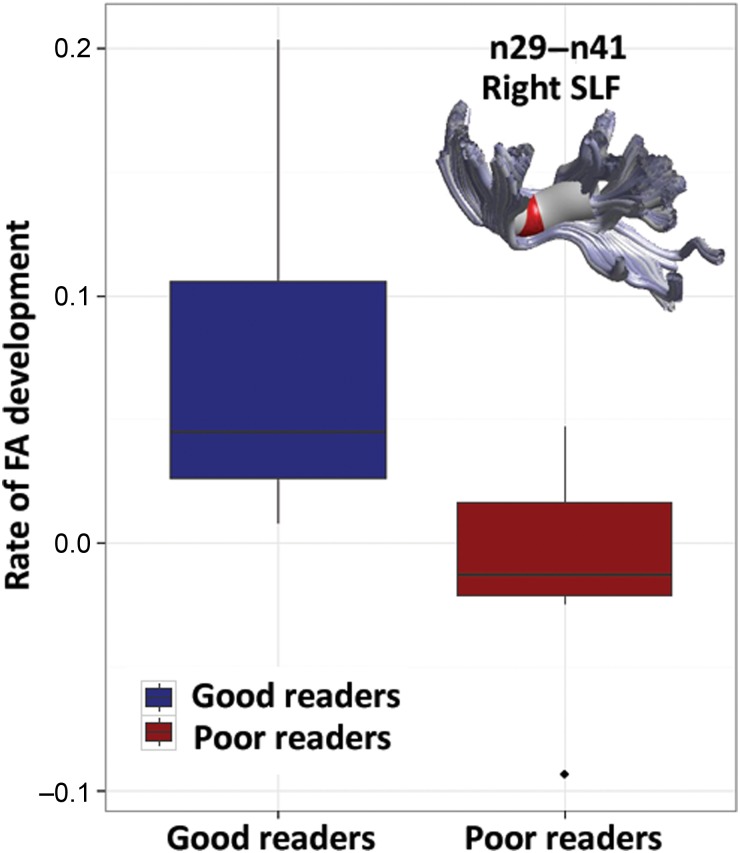
Of 21 FHD+ children, 11 children subsequently developed into good readers, while 10 children subsequently developed into poor readers. The FHD+ children who subsequently developed into good readers showed a significantly higher rate of FA development in the temporo-parietal segments of the right SLF compared with those FHD+ children who subsequently developed into poor readers ( P = 0.015) (see Fig. 6 ).
Figure 6.
In FHD+ children, boxplots of rate of FA development on nodes 29–41 of the right SLF are shown by reading ability (subsequent good versus poor readers). Nodes that show significant differences in FA-development rate between subsequent good and poor readers within FHD+ children are marked in red on the 3-dimensional rendered tract ( P < 0.05, FDR-corrected). Dark blue: FHD+ children who subsequently developed into good readers, Dark red: FHD+ children who subsequently developed into poor readers.
Discussion
The present study is the first to examine tract-specific white matter microstructure in a cross-sectional cohort and characterize white matter development in a longitudinal cohort including both FHD− and FHD+ children across early reading development from the pre-reading to fluent reading stage. Significant lower FA was observed for FHD+ compared with FHD− children in 3 major left hemispheric white matter tracts including AF, SLF, and ILF after the onset of reading instruction. In addition, reduced FA and reduced lateralization in the temporo-parietal segments of the AF in FHD+ compared with FHD− children were observed at the pre-reading stage, indicating that tract-specific white matter alterations predate reading onset. Furthermore, we observed a significantly positive correlation between the rate of white matter development and reading development across both groups, suggesting that the maturation of white matter pathway plays an important role in atypical and typical reading development. Interestingly, both FHD− and FHD+ children exhibit a positive slope of white matter development, but there were significant differences in the rate of FA development between subsequent good and poor readers. Moreover, a subset of FHD+ children who developed into good readers show faster white matter development in the right SLF compared with those FHD+ children who developed into poor readers, suggesting a potential right-hemispheric compensatory mechanism for DD. Finally, white matter maturation from the pre-reading to the fluent reading stage in the longitudinal cohort combined with familial risk and psychometric measures at the pre-reading stage best predict later reading abilities, emphasizing the importance of considering white matter development as a dynamic variable when examining typical and atypical reading development and their brain correlates.
Tract-Specific White Matter Microstructure (Cross-Sectional)
For the left AF, the cross-sectional results demonstrate reduced FA in the temporo-parietal segments in FHD+ children compared with FHD− children at the pre-reading/beginning reading/fluent reading stages. This aligns with previous studies showing reduced FA in left fronto-temporal and temporo-parietal regions in school-age children and adults with DD or reading impairment ( Klingberg et al. 2000 ; Niogi and McCandliss 2006 ; Steinbrink et al. 2008 ; Carter et al. 2009 ; Rimrodt et al. 2010 ; Vandermosten, Boets, Poelmans, et al. 2012 ), kindergartner at familial risk for DD ( Vandermosten et al. 2015 ) and infants at familial risk for DD ( Langer et al. 2015 ). In addition, previous neuroimaging studies have shown atypical event-related potentials (ERP) to auditory speech stimuli in toddlers or infants at familial risk for DD ( Guttorm et al. 2001 , 2010 ; Molfese et al. 2001 , 2002 ; Espy et al. 2004 ; van Herten et al. 2008 ; Leppanen et al. 2012 ), reduced gray matter volume and functional activation within a left hemispheric network in FHD+ children at the pre-reading stage ( Raschle et al. 2011 ; Raschle, Zuk, Ortiz-Mantilla, et al. 2012 ; Raschle, Zuk, Gaab 2012 ; Raschle et al. 2013 ), and atypical sulcal pattern in early beginning readers with DD and kindergartner at familial risk for DD ( Im et al. 2014 ). Thus, together with previous findings of functional and neuroanatomical alterations related to DD prior to formal reading instruction, our results provide further evidence that the reduced FA of the left AF observed in school-age children and adults with DD is not a consequence of reading impairment, but predate the onset of formal reading instruction. On the contrary, a recent study failed to reveal altered FA in the left AF in Dutch-speaking kindergartner at familial risk for DD ( Vandermosten et al. 2015 ). The lack of significant differences between FHD− and FHD+ children in Vandermosten et al. may be explained by the fact that they examined children who speak Dutch, a more transparent language, or by reduced sensitivity, since only the mean FA of 2 long segments of the left AF was computed, and no fine grained analysis of AF segments was conducted. Similarly, no significant differences of FA could be observed in the left AF between FHD− and FHD+ children at the pre-reading stage when we merely computed the mean FA of the left AF. The AFQ method employed in the present study computes FA at multiple equidistant nodes along the tract with more precise spatial localizations, thereby providing greater sensitivity for detecting group differences between FHD− and FHD+ children. However, Vandermonsten et al. also observed a positive correlation between FA in the left AF and phonological awareness, similar to Saygin et al. in kindergartner, which is in line with previous studies suggesting that the left AF plays an important role in phonological awareness ( Saygin et al. 2013 ; Thiebaut de Schotten et al. 2014 ; Dehaene et al. 2015 ; Vandermosten et al. 2015 ).
For the left ILF, our results showed no significant group difference between FHD− and FHD+ children at the pre-reading stage, but significantly reduced FA in FHD+ children compared with FHD− children at later reading stages. Our finding indicates that diffusion anisotropy is tract-specific and differs between FHD− and FHD+ children. This is in agreement with the dual route theory of reading ( Jobard et al. 2003 ). Reading requires a network of brain regions communicating with each other through white matter pathways. While the left AF pathway has been related to speech production, auditory comprehension, and reading ( Rauschecker et al. 2009 ; Yeatman et al. 2011 ; Vandermosten, Boets, Poelmans, et al. 2012 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Vandermosten et al. 2015 ), the left ILF pathway has been associated with visual processing of words ( Cohen 2003 ; Jobard et al. 2003 ; Yeatman et al. 2011 ; Vandermosten, Boets, Wouters, et al. 2012 ). The left ILF is also suggested to constitute the neuroanatomical pathway that facilitates the orthographic reading route, which transfers visual information from occipital areas to the VWFA, where information is orthographically processed ( Anwander et al. 2007 ; Vandermosten, Boets, Poelmans, et al. 2012 ; Qi et al. 2015 ). In line with a study by Saygin et al. ( 2013 ) that demonstrated no association between the volume of the left ILF and phonological awareness scores in 40 kindergartners who had received little or no reading instruction, the absence of group differences between FHD− and FHD+ children in the present study at the pre-reading stage reflects their lack of reading experience. Moreover, the left ILF might be insufficiently developed in pre-readers and might be shaped by learning experience. Furthermore, observed increases in activation of the VWFA in previously illiterate adults who learned to read during adulthood ( Dehaene and Cohen 2011 ), and increases of FA in the white matter pathway induced by literacy acquisition ( Thiebaut de Schotten et al. 2014 ; Dehaene et al. 2015 ), as well as in children with DD after reading intervention ( Heim et al. 2015 ), have demonstrated that the VWFA is highly plastic and strongly influenced by environmental experiences, such as learning to read.
For the left SLF, the frontal and temporo-parietal segments presented significantly lower FA in FHD+ children compared with FHD− children at later reading stages, but not at the pre-reading stage. In agreement with earlier DTI studies of DD ( Klingberg et al. 2000 ; Beaulieu et al. 2005 ; Deutsch et al. 2005 ; Niogi and McCandliss 2006 ; Carter et al. 2009 ), which have shown reduced white matter anisotropy in individuals with DD, the present results revealed similar reduction of FA in children at familial risk for DD.
Animal studies have shown that increased RD is associated with reduced myelination, whereas decreased AD is related to axonal degeneration ( Song et al. 2005 ; Ashtari et al. 2007 ; Harsan et al. 2007 ; Vandermosten, Boets, Wouters, et al. 2012 ). However, their underlying biophysical properties are still under debate ( Wheeler-Kingshott and Cercignani 2009 ). Thus, in the present study, in addition to FA values, we also examined AD and RD indices to acquire more information about white matter microstructure differences between FHD− and FHD+ children. The segments of tracts of interest that showed significantly lower FA also showed higher RD in FHD+ children compared with FHD− children. This aligns with the majority of research studies that previously suggested that white matter abnormalities due to reading differences are rooted in atypical RD rather than AD ( Dougherty et al. 2007 ; Keller and Just 2009 ; Yeatman et al. 2011 ; Vandermosten, Boets, Wouters, et al. 2012 ). Despite limitations of current DTI techniques, which are unable to disentangle microscopic and macroscopic factors, a possible explanation for the present findings could be that genetic influences during the development of white matter tracts may cause less diffusivity perpendicular to the principle axis of diffusion in white matter tracts. Therefore, the reduced FA along with increased RD indicates reduced myelination of axons in FHD+ children, leading to slower transmission of action potentials between neurons ( Klingberg et al. 2000 ; Glasser and Rilling 2008 ).
Lateralization of White Matter Microstructure (Cross-Sectional)
Previous studies have suggested a leftward asymmetry of the AF in typically developing toddlers ( Dubois et al. 2009 ; Lebel et al. 2012 ) and young adults ( Lebel and Beaulieu 2009 ; Johnson et al. 2013 ). Here, at the pre-reading stage, FHD+ children demonstrated right lateralization in the anterior segment of the AF, whereas FHD− children showed left lateralization. This is the first study to report a rightward asymmetry of the frontal and temporo-parietal segments of the AF for pre-readers at familial risk for DD. Notably, reduced leftward asymmetry in the posterior region of the superior temporal gyrus and the AF has previously been shown in adults with dyslexia ( Vandermosten et al. 2013 ), as well as children with reading impairment ( Niogi and McCandliss 2006 ). A clinical case report also described 4 patients with dyslexia who showed symmetry of the planum temporale instead of left-lateralized asymmetry ( Galaburda et al. 1985 ). Interestingly, the section along the AF showing right lateralization in FHD+ children reduced in size over the course of brain development, which suggests that white matter alterations in the AF in FHD+ children remain capable of plastic changes through brain development, most likely as a result of postnatal factors such as home literacy environments and quality of reading instruction.
Tract-Specific White Matter Maturation (Longitudinal)
A unique aspect of the present study was the longitudinal cohort, which allowed for differentiation of tract-specific white matter development between FHD− and FHD+ children and between subsequent good and poor readers. During brain development, synaptic pruning and ongoing axonal myelination depend on both intrinsic genetic and extrinsic environmental factors ( Emery 2010 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Wandell and Yeatman 2013 ). These white matter maturation processes are quantified by changes of FA values and have been shown to be integral to the development of cognition ( Paus et al. 1999 ; Schmithorst et al. 2005 , 2011 ; Wang et al. 2012 ; Yeatman, Dougherty, Ben-Shachar, et al. 2012 ; Saygin et al. 2013 ; Myers et al. 2014 ). For the first time, this study provides insights on how FA-development rate differs between FHD− and FHD+ children along a tract instead of a merely global mean FA of the tract, and how it relates to the development of reading abilities. Diffusion properties have been shown to vary substantially along a tract using the novel AFQ technique ( Johnson et al. 2013 ). Thus, it is essential to examine regional diffusion properties along the tract to achieve better spatial specificity and understanding of underlying biological processes. Based on the cross-sectional results that indicated lower FA in FHD+ compared with FHD− children at all reading stages in one temporo-parietal segment of the left AF, this segment was examined using the longitudinal study design. At this specific spatial section, both FHD− and FHD+ children displayed positive FA-developmental slopes. In addition, FHD+ children show reduced FA compared with FHD− children, which aligns with our cross-sectional results. Furthermore, the significantly lower rate of white matter development in subsequent poor readers suggests an atypical white matter developmental pattern in children who subsequently developed into poor readers. This finding aligns with Klingberg et al. ( 2000 ) and partially aligns with Yeatman, Dougherty, Ben-Shachar, et al. ( 2012 ). Klingberg et al. observed lower FA in the temporo-parietal regions of white matter in poor adult readers compared with typical adult readers. Interestingly, Yeatman et al. observed that 7-year-old children with low initial FA and a positive FA-developmental slope subsequently developed into good readers, whereas children with high initial FA and a negative FA-developmental slope subsequently developed into poor readers. The authors proposed a synchronous dual process for subsequent good readers and an asynchronous dual process for subsequent poor readers. Subsequent good readers experience that FA increases monotonically and then approaches its mature level, whereas subsequent poor readers experience that FA overshoots at about age 7.5 and then decreases afterwards. Thus, they suggested that both components (myelination and pruning) of the dual process of white matter maturation are driven by the same experiential factors for subsequent good readers, but are driven by different experiential factors for subsequent poor readers. The present study also identified different rates of FA development in subsequent good readers compared with subsequent poor readers, suggesting that children with different subsequent reading ability present different profiles of white matter maturation. However, some substantial differences between our study and the study by Yeatman et al. (2012) could be observed. First of all, the children in our study who subsequently develop into poor readers show higher initial (at age 5 years) FA values than the subsequent poor readers. This is in line with previous work that has shown higher FA values in the left AF in infants with a familial risk of DD ( Langer et al. 2015 ) using the AFQ. Furthermore, the subsequent poor readers in the present study still present an overall positive FA-developmental slope rather than a negative slope as shown by Yeatman et al. (2012) . Additionally, there is no overshoot of FA in subsequent poor readers at age 7.5 in the present study. These discrepancies may be due to the difference of the initial age (youngest subject) between their study sample and ours. The initial age of our study sample is approximately 5 years old, rather than 7 years old as reported in Yeatman et al. (2012) . Previous literature on white matter maturation in humans has shown that FA increases with age until early adolescence, which is primarily driven by brain maturation consisting of 2 main processes, including myelination and pruning ( Giedd et al. 1999 ; Hermoye et al. 2006 ). Animal studies have further shown that both myelin extension and myelin pruning co-existed and changed during early development ( Liu et al. 2014 ). Thus, children at age 5 may experience a different dual process of myelination and pruning compared with children at age 7. However, the oldest children in our sample size were 12 years old, the same age that reported in Yeatman et al., and we still did not observe a negative slope. This difference may be explained by the fact that we examined only one segment of the AF in our study, which was selected based on our cross-sectional results (located in the parieto-temporal portion), and Yeatman et al. examined the mean of the entire tract. Since Johnson et al. (2013) have reported that not only diffusion properties, but also the relationship between age and diffusion metrics, vary significantly along a tract, the dual process as described above and its relationship to reading development may vary substantially along the tract. Based on our result, we hypothesize that myelin extension in the parieto-temporal portion of the tract is dominant for children at younger age and accompanied with less myelin pruning compared with children at older age. Moreover, our results suggest that subsequent poor readers may experience slower myelination and a similar pruning process compared with subsequent good readers. Nonetheless further quantitative studies are needed to explain the underlying driving force for different FA-developmental rates during the development of reading. Taken together, the present findings suggest that the positive FA-developmental slope of white matter tracts is essential for successful reading development, which is supported by studies that showed that the acquisition of literacy or word learning leads to an increase in the FA of the left AF ( Lopez-Barroso et al. 2013 ; Thiebaut de Schotten et al. 2014 ) and that reading remediation can lead to an increase in the FA of the left anterior AF in poor readers ( Keller and Just 2009 ). Furthermore, alterations in white matter tracts connecting temporo-parietal and frontal cortices in individual, with DD can lead to a disruption in communication between functional regions ( Klingberg et al. 2000 ; Ben-Shachar et al. 2007 ). In accordance with previous findings, the present results demonstrate that slower FA development in the temporo-parietal segment of the left AF can lead to insufficient transmission between reading-related functional regions, which might indirectly lead to subsequent poor reading development.
Brain Development–Behavioral Relationship (Longitudinal)
The longitudinal design of the present study allowed us to investigate how developmental changes of white matter integrity relate to changes of reading skills. A recent longitudinal DTI study observed that white matter development was associated with gains in cognitive performance, including response inhibition and working memory ( Simmonds et al. 2014 ). Additionally, white matter development supports the development of cognitive abilities such as cognitive control and reasoning ( Chaddock-Heyman et al. 2013 ; Ferrer et al. 2013 ; Deoni et al. 2014 ). Previous cross-sectional studies have associated development of reading ability with changes of white matter maturation in the left temporo-parietal region ( Klingberg et al. 2000 ; Nagy et al. 2004 ). White matter maturation has been suggested to relate to cognitive development during childhood and adolescence ( Barnea-Goraly et al. 2005 ; Tamnes et al. 2010 ; O'Muircheartaigh et al. 2014 ). The present study extends these cross-sectional findings by using a longitudinal design, allowing the application of linear mixed-effects regression to quantify FA-developmental rate in each tract. The rates of FA development in the left AF and ILF positively correlated with improved reading abilities. This finding agrees with previous studies ( Klingberg et al. 2000 ; Beaulieu et al. 2005 ) identifying regional brain structural correlations with untimed single word reading. The present findings provide evidence that faster increases of white matter integrity in the left temporal segments of fiber tracts relate to faster development of reading abilities, indicating that the underlying biological process of white matter maturation may play an important role in reading development. On the contrary, a recent study failed to detect an association between the rate of reading development and the rate of white matter development ( Yeatman, Dougherty, Ben-Shachar, et al. 2012 ). This could have resulted from the similar rates of reading improvement across good and poor readers in this study with few changes in rank order, which may have led to low statistical power. However, the present study has a more heterogeneous sample that included both FHD− and FHD+ children, resulting in various rates of reading improvement among subsequently poor and good readers, and thus, the statistical power for detecting association between the 2 rates of development was higher.
Prediction of Later Reading Comprehension and Fluency (Longitudinal)
Previously, Hoeft et al. ( 2007 ) demonstrated that a model that combined functional and structural characteristics with behavioral test scores accounted for 81% of the variance on the WRMT-R Word Attack subtest, which is a standardized test of decoding and phonemic awareness. The same group also reported that functional activation combined with white matter integrity predicted reading gain over 2.5 years with 72% accuracy in children with DD ( Hoeft et al. 2011 ). Moreover, a recent study revealed that volume changes in temporo-parietal white matter, together with preliteracy measures, predicted 56% of the variance in reading outcomes ( Myers et al. 2014 ). Consistent with previous findings, the present study demonstrates that combined behavioral and neuroanatomical data, along with familial risk, accounted for 56% of variance in reading comprehension and 62% of variance in reading fluency. Our findings further suggest that the addition of white matter development rates improves the prediction of later reading comprehension and fluency over behavioral scores. Using both WRMT-R Passage Comprehension and WJ-III Reading Fluency standard scores, the FA-developmental rate of the left SLF plays an important role in predicting later reading abilities. This finding aligns with a previous study suggesting that damage to the left SLF pathway led to impaired language fluency ( Catani et al. 2005 ). In addition, the left SLF pathway has been associated with language perception and articulation ( Baddeley 2003 ; Duffau 2008 ). Thus, the development of the left SLF is crucial for facilitating structural pathways to support reading fluency. Moreover, the FA-development rate of the left AF only improves the prediction of reading comprehension assessed by the WRMT-R Passage Comprehension subtest. This finding seems somewhat surprising. However, previous studies have suggested that the left AF consists of various subcomponents which have been related to different reading and reading-related abilities ( Anderson et al. 1999 ; Duffau 2008 ; Vandermosten, Boets, Poelmans, et al. 2012 ; Thiebaut de Schotten et al. 2014 ; Gullick and Booth 2015 ). Thus, using the mean FA along the entire tract may have confounded regional differences within the tract and therefore may have resulted in reduced sensitivity for detecting microstructural differences. Further, the contribution of RAN alone to reading fluency was 18%, supporting previous studies that reported RAN as one of the best predictors of reading fluency ( Krasowicz-Kupis et al. 2009 ; Norton and Wolf 2012 ). Additionally, familial risk for DD explained 20% of the variance for both regression models, which aligns with previous studies suggesting a strong genetic basis for DD ( Galaburda et al. 2006 ; Snowling et al. 2007 ; Raschle et al. 2011 , 2013 ; Black et al. 2012 ; Raschle, Zuk, Ortiz-Mantilla, et al. 2012 ; Raschle, Zuk, Gaab 2012 ; Costa et al. 2013 ; Hosseini et al. 2013 ; Myers et al. 2014 ). Finally, KBIT-2 Nonverbal IQ explained 20% of the variance for both regression models. The present finding supports evidence that nonverbal IQ plays an important role in predicting later reading outcomes, maybe because of its least dependency on education opportunities ( Fathi-Ashtiani and Ahmadi 2006 ), which align with previous findings suggesting nonverbal IQ to be a protective factor for children at familial risk for DD ( Stanovich 1996 ; van der Leij et al. 2013 ). Overall, the present study suggests that white matter development is an important specific structural risk factor for DD.
Potential Compensatory or Protective Factor in the Right SLF (Longitudinal)
Previous auditory ERP studies have shown significant differences between 6.5-year-old kindergartner at familial risk for DD who were later diagnosed with DD at school age and those who were not ( Maurer et al. 2003 , 2009 ; Hämäläinen et al. 2013 ), but it remains relatively unclear which one or set of protective factors and/or compensatory mechanisms play a significant role and how they develop over time. In addition, Hoeft et al. ( 2011 ) observed a significantly positive correlation between longitudinal reading gains over 2.5 years and FA of the right SLF in 17 adolescents with DD but not in typical readers, suggesting the existence of a potential compensatory mechanism in the right SLF in adolescents with DD. Moreover, hyperactivation in the right superior frontal and mid-temporal regions has been reported in compensated readers ( Shaywitz et al. 2003 ). In the present study, FHD+ children who later developed into good readers demonstrated significantly higher rates of FA development in the right SLF than those FHD+ children who later emerged as poor readers. This suggests that the higher rate of FA development in the right SLF may be a protective factor for FHD+ children. A possible explanation is that FHD+ children who subsequently develop into good readers despite a familial risk for DD experience faster maturation in the right SLF, which may facilitate rapid neural transmission in the right hemisphere and compensate for white matter alterations in the left hemisphere. However, the present study cannot answer the question of whether the hypothesized faster maturation in the right hemisphere is a consequence of genetic predisposition or caused by environmental influences, or a combination of the 2.
Limitations and Future Directions
There are several limitations in the present study. First, due to missing data points, the longitudinal sample size is relatively small, thus limiting the number of independent variables that could be included in the regression analyses. As a consequence, the mean FA of each tract of interest was used to characterize the whole tract, which confounded regional differences within the tract and reduced sensitivity for the detection of microstructural differences. Second, the developmental trajectories of each tract are over the age span from 5 to 12 years. Previous studies have shown that white matter development begins in utero and continues through late adolescence or early adulthood ( Paus et al. 1999 ; Giedd 2008 ). Thus, to understand the entire process of white matter development, it is necessary to collect longitudinal data staring from as early as infancy and continuing through late adolescence.
Summary
This cross-sectional and longitudinal study, for the first time, identified altered development of regional tract-specific white matter microstructure from the pre-reading to the fluent reading stage in children at familial risk for DD compared with controls. A positive association between the rates of white matter development and reading development, along with faster white matter development in subsequent good readers compared with poor readers suggest that white matter development plays a prominent role in typical and atypical reading development. Finally, the rate of white matter development combined with familial risk and psychometric measures at the pre-reading stage best predicted later reading abilities, emphasizing the importance of white matter maturation, as a dynamic variable, in predicting typical and atypical reading outcome.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development #R01HD65762-01/04 (awarded to N.G.), Charles H. Hood Foundation (awarded to N.G.), and Boston Children's Hospital Pilot Grant (awarded to N.G.).
Supplementary Material
Notes
The authors especially appreciate the families for their participation in this study and acknowledge the assistance of Mr Michael Figuccio, Ms Jennifer Zuk, Ms Michelle Lee, Drs Nicolas Langer, Nora Raschle, and Sara Smith for helping with initial recruitment and data collection. We also thank Ms Grace Coviello and Ms Sonal Sharda for their help with proofreading and anonymous reviewers for their helpful comments on the manuscript. Conflict of Interest : None declared.
References
- Anderson JM , Gilmore R , Roper S , Crosson B , Bauer RM , Nadeau S , Beversdorf DQ , Cibula J , Rogish M III , Kortencamp S et al. . 1999. . Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke-Geschwind model . Brain Lang . 70 : 1 – 12 . [DOI] [PubMed] [Google Scholar]
- Anwander A , Tittgemeyer M , von Cramon DY , Friederici AD , Knösche TR . 2007. . Connectivity-based parcellation of Broca's area . Cereb Cortex . 17 : 816 – 825 . [DOI] [PubMed] [Google Scholar]
- Ashtari M , Cervellione KL , Hasan KM , Wu J , McIlree C , Kester H , Ardekani BA , Roofeh D , Szeszko PR , Kumra S . 2007. . White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study . Neuroimage . 35 : 501 – 510 . [DOI] [PubMed] [Google Scholar]
- Bach S , Richardson U , Brandeis D , Martin E , Brem S . 2013. . Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade . Neuroimage . 82 : 605 – 615 . [DOI] [PubMed] [Google Scholar]
- Baddeley A . 2003. . Working memory: looking back and looking forward . Nat Rev Neurosci . 4 : 829 – 839 . [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N , Menon V , Eckert M , Tamm L , Bammer R , Karchemskiy A , Dant CC , Reiss AL . 2005. . White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study . Cereb Cortex . 15 : 1848 – 1854 . [DOI] [PubMed] [Google Scholar]
- Basser PJ , Mattiello J , LeBihan D . 1994. . MR diffusion tensor spectroscopy and imaging . Biophys J . 66 : 259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ , Pajevic S , Pierpaoli C , Duda J , Aldroubi A . 2000. . In vivo fiber tractography using DT-MRI data . Magn Reson Med . 44 : 625 – 632 . [DOI] [PubMed] [Google Scholar]
- Bates D . 2005. . Fitting linear mixed models in R . R News . 5 : 27 – 30 . [Google Scholar]
- Beaulieu C , Plewes C , Paulson LA , Roy D , Snook L , Concha L , Phillips L . 2005. . Imaging brain connectivity in children with diverse reading ability . NeuroImage . 25 : 1266 – 1271 . [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M , Dougherty RF , Wandell BA . 2007. . White matter pathways in reading . Curr Opin Neurobiol . 17 : 258 – 270 . [DOI] [PubMed] [Google Scholar]
- Benjamini Y , Hochberg Y . 1995. . Controlling the false discovery rate: a practical and powerful approach to multiple testing . J R Stat Soc Ser B . 289 – 300 . [Google Scholar]
- Black JM , Tanaka H , Stanley L , Nagamine M , Zakerani N , Thurston A , Kesler S , Hulme C , Lyytinen H , Glover GH et al. . 2012. . Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers . Neuroimage . 59 : 3021 – 3032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B , Op de Beeck HP , Vandermosten M , Scott SK , Gillebert CR , Mantini D , Bulthe J , Sunaert S , Wouters J , Ghesquiere P . 2013. . Intact but less accessible phonetic representations in adults with dyslexia . Science . 342 : 1251 – 1254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC , Lanham DC , Cutting LE , Clements-Stephens AM , Chen X , Hadzipasic M , Kim J , Denckla MB , Kaufmann WE . 2009. . A dual DTI approach to analyzing white matter in children with dyslexia . Psychiatry Res . 172 : 215 – 219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M , Jones DK , ffytche DH . 2005. . Perisylvian language networks of the human brain . Ann Neurol . 57 : 8 – 16 . [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L , Erickson KI , Voss MW , Powers JP , Knecht AM , Pontifex MB , Drollette ES , Moore RD , Raine LB , Scudder MR et al. . 2013. . White matter microstructure is associated with cognitive control in children . Biol Psychol . 94 : 109 – 115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L . 2003. . Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias . Cereb Cortex . 13 : 1313 – 1333 . [DOI] [PubMed] [Google Scholar]
- Cope N , Harold D , Hill G , Moskvina V , Stevenson J , Holmans P , Owen MJ , O'Donovan MC , Williams J . 2005. . Strong evidence that KIAA0319 on chromosome 6p is a susceptibility gene for developmental dyslexia . Am J Hum Genet . 76 : 581 – 591 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa HC , Perdry H , Soria C , Pulgar S , Cusin F , Dellatolas G . 2013. . Emergent literacy skills, behavior problems and familial antecedents of reading difficulties: a follow-up study of reading achievement from kindergarten to fifth grade . Res Dev Disabil . 34 : 1018 – 1035 . [DOI] [PubMed] [Google Scholar]
- Cummine J , Dai W , Borowsky R , Gould L , Rollans C , Boliek C . 2015. . Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging . Brain Struct Funct . 220 : 445 – 455 . [DOI] [PubMed] [Google Scholar]
- Darki F , Peyrard-Janvid M , Matsson H , Kere J , Klingberg T . 2012. . Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure . Biol Psychiatry . 72 : 671 – 676 . [DOI] [PubMed] [Google Scholar]
- Dehaene S , Cohen L . 2011. . The unique role of the visual word form area in reading . Trends Cogn Sci . 15 : 254 – 262 . [DOI] [PubMed] [Google Scholar]
- Dehaene S , Cohen L , Morais J , Kolinsky R . 2015. . Illiterate to literate: behavioural and cerebral changes induced by reading acquisition . Nat Rev Neurosci . 16 : 234 – 244 . [DOI] [PubMed] [Google Scholar]
- Deoni SC , O'Muircheartaigh J , Elison JT , Walker L , Doernberg E , Waskiewicz N , Dirks H , Piryatinsky I , Dean DC III , Jumbe NL . 2014. . White matter maturation profiles through early childhood predict general cognitive ability . Brain Struct Funct . 2212 : 1189 – 1203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK , Dougherty RF , Bammer R , Siok WT , Gabrieli JD , Wandell B . 2005. . Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging . Cortex . 41 : 354 – 363 . [DOI] [PubMed] [Google Scholar]
- Dougherty RF , Ben-Shachar M , Deutsch GK , Hernandez A , Fox GR , Wandell BA . 2007. . Temporal-callosal pathway diffusivity predicts phonological skills in children . Proc Natl Acad Sci USA . 104 : 8556 – 8561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J , Hertz-Pannier L , Cachia A , Mangin JF , Le Bihan D , Dehaene-Lambertz G . 2009. . Structural asymmetries in the infant language and sensori-motor networks . Cereb Cortex . 19 : 414 – 423 . [DOI] [PubMed] [Google Scholar]
- Dubois J , Hertz-Pannier L , Dehaene-Lambertz G , Cointepas Y , Le Bihan D . 2006. . Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography . Neuroimage . 30 : 1121 – 1132 . [DOI] [PubMed] [Google Scholar]
- Duffau H . 2008. . The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography . Neuropsychologia . 46 : 927 – 934 . [DOI] [PubMed] [Google Scholar]
- Eckert MA , Leonard CM , Wilke M , Eckert M , Richards T , Richards A , Berninger V . 2005. . Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures . Cortex . 41 : 304 – 315 . [DOI] [PubMed] [Google Scholar]
- Emery B . 2010. . Regulation of oligodendrocyte differentiation and myelination . Science . 330 : 779 – 782 . [DOI] [PubMed] [Google Scholar]
- Epelbaum S , Pinel P , Gaillard R , Delmaire C , Perrin M , Dupont S , Dehaene S , Cohen L . 2008. . Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept . Cortex . 44 : 962 – 974 . [DOI] [PubMed] [Google Scholar]
- Espy KA , Molfese DL , Molfese VJ , Modglin A . 2004. . Development of auditory event-related potentials in young children and relations to word-level reading abilities at age 8 years . Ann Dyslexia . 54 : 9 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi-Ashtiani A , Ahmadi K . 2006. . Verbal and non-verbal intelligence: dyslexic-dysgraphic students and normal students . Arch Med Sci . 2 : 42 . [Google Scholar]
- Ferrer E , Whitaker KJ , Steele JS , Green CT , Wendelken C , Bunge SA . 2013. . White matter maturation supports the development of reasoning ability through its influence on processing speed . Dev Sci . 166 : 941 – 951 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SE , Francks C . 2006. . Genes, cognition and dyslexia: learning to read the genome . Trends Cogn Sci . 10 : 250 – 257 . [DOI] [PubMed] [Google Scholar]
- Gabrieli JD . 2009. . Dyslexia: a new synergy between education and cognitive neuroscience . Science . 325 : 280 – 283 . [DOI] [PubMed] [Google Scholar]
- Galaburda AM . 1993. . Neurology of developmental dyslexia . Curr Opin Neurobiol . 3 : 237 – 242 . [DOI] [PubMed] [Google Scholar]
- Galaburda AM , LoTurco J , Ramus F , Fitch RH , Rosen GD . 2006. . From genes to behavior in developmental dyslexia . Nat Neurosci . 9 : 1213 – 1217 . [DOI] [PubMed] [Google Scholar]
- Galaburda AM , Sherman GF , Rosen GD , Aboitiz F , Geschwind N . 1985. . Developmental dyslexia: four consecutive patients with cortical anomalies . Ann Neurol . 18 : 222 – 233 . [DOI] [PubMed] [Google Scholar]
- Geschwind N , Galaburda AM . 1985. . Cerebral lateralization: Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research . Arch Neurol . 42 : 428 . [DOI] [PubMed] [Google Scholar]
- Giedd JN . 2004. . Structural magnetic resonance imaging of the adolescent brain . Ann NY Acad Sci . 1021 : 77 – 85 . [DOI] [PubMed] [Google Scholar]
- Giedd JN . 2008. . The teen brain: insights from neuroimaging . J Adolesc Health . 42 : 335 – 343 . [DOI] [PubMed] [Google Scholar]
- Giedd JN , Blumenthal J , Jeffries NO , Castellanos FX , Liu H , Zijdenbos A , Paus T , Evans AC , Rapoport JL . 1999. . Brain development during childhood and adolescence: a longitudinal MRI study . Nat Neurosci . 2 : 861 – 863 . [DOI] [PubMed] [Google Scholar]
- Giedd JN , Rapoport JL . 2010. . Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron . 67 : 728 – 734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L , Ramus F . 2013. . Neurogenetics and auditory processing in developmental dyslexia . Curr Opin Neurobiol . 23 : 37 – 42 . [DOI] [PubMed] [Google Scholar]
- Glasser MF , Rilling JK . 2008. . DTI tractography of the human brain's language pathways . Cereb Cortex . 18 : 2471 – 2482 . [DOI] [PubMed] [Google Scholar]
- Gromping U . 2006. . Relative importance for linear regression in R: The Package relaimpo . J Stat Softw . 17 : 27 . [Google Scholar]
- Gullick MM , Booth JR . 2015. . The direct segment of the arcuate fasciculus is predictive of longitudinal reading change . Dev Cogn Neurosci . 13 : 68 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK , Leppanen PH , Hamalainen JA , Eklund KM , Lyytinen HJ . 2010. . Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia . J Learn Disabil . 43 : 391 – 401 . [DOI] [PubMed] [Google Scholar]
- Guttorm TK , Leppanen PH , Richardson U , Lyytinen H . 2001. . Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia . J Learn Disabil . 34 : 534 – 544 . [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA , Guttorm TK , Richardson U , Alku P , Lyytinen H , Leppanen PH . 2013. . Auditory event-related potentials measured in kindergarten predict later reading problems at school age . Dev Neuropsychol . 38 : 550 – 566 . [DOI] [PubMed] [Google Scholar]
- Hammill DD , Wiederholt JL , Allen EA . 2004. . Test of silent word reading fluency . TX: : PRO-ED Austin; . [Google Scholar]
- Harsan LA , Poulet P , Guignard B , Parizel N , Skoff RP , Ghandour MS . 2007. . Astrocytic hypertrophy in dysmyelination influences the diffusion anisotropy of white matter . J Neurosci Res . 85 : 935 – 944 . [DOI] [PubMed] [Google Scholar]
- Heim S , Pape-Neumann J , van Ermingen-Marbach M , Brinkhaus M , Grande M . 2015. . Shared vs. specific brain activation changes in dyslexia after training of phonology, attention, or reading . Brain Struct Funct . 2204 : 2191 – 2207 . [DOI] [PubMed] [Google Scholar]
- Hermoye L , Saint-Martin C , Cosnard G , Lee SK , Kim J , Nassogne MC , Menten R , Clapuyt P , Donohue PK , Hua K et al. . 2006. . Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood . Neuroimage . 29 : 493 – 504 . [DOI] [PubMed] [Google Scholar]
- Hickok G , Poeppel D . 2004. . Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language . Cognition . 92 : 67 – 99 . [DOI] [PubMed] [Google Scholar]
- Hickok G , Poeppel D . 2007. . The cortical organization of speech processing . Nat Rev Neurosci . 8 : 393 – 402 . [DOI] [PubMed] [Google Scholar]
- Hoeft F , McCandliss BD , Black JM , Gantman A , Zakerani N , Hulme C , Lyytinen H , Whitfield-Gabrieli S , Glover GH , Reiss AL et al. . 2011. . Neural systems predicting long-term outcome in dyslexia . Proc Natl Acad Sci USA . 108 : 361 – 366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F , Ueno T , Reiss AL , Meyler A , Whitfield-Gabrieli S , Glover GH , Keller TA , Kobayashi N , Mazaika P , Jo B et al. . 2007. . Prediction of children's reading skills using behavioral, functional, and structural neuroimaging measures . Behav Neurosci . 121 : 602 – 613 . [DOI] [PubMed] [Google Scholar]
- Hosseini SMH , Black JM , Soriano T , Bugescu N , Martinez R , Raman MM , Kesler S , Hoeft F . 2013. . Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties . Neuroimage . 71 : 260 – 274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R , Gentleman R . 1996. . R: a language for data analysis and graphics . J Comput Graph Statist . 5 : 299 – 314 . [Google Scholar]
- Im K , Raschle NM , Smith SA , Grant PE , Gaab N . 2014. . Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners . Cereb Cortex. 10.1093/cercor/bhu305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G , Crivello F , Tzourio-Mazoyer N . 2003. . Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies . Neuroimage . 20 : 693 – 712 . [DOI] [PubMed] [Google Scholar]
- Johnson RT , Yeatman JD , Wandell BA , Buonocore MH , Amaral DG , Nordahl CW . 2013. . Diffusion properties of major white matter tracts in young, typically developing children . Neuroimage . 88C : 143 – 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 1990. K-BIT: Kaufman brief intelligence test. American Guidance Service. Circle Pines MN 55014 . [Google Scholar]
- Keller TA , Just MA . 2009. . Altering cortical connectivity: remediation-induced changes in the white matter of poor readers . Neuron . 64 : 624 – 631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T , Hedehus M , Temple E , Salz T , Gabrieli JD , Moseley ME , Poldrack RA . 2000. . Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging . Neuron . 25 : 493 – 500 . [DOI] [PubMed] [Google Scholar]
- Krafnick AJ , Flowers DL , Luetje MM , Napoliello EM , Eden GF . 2014. . An investigation into the origin of anatomical differences in dyslexia . J Neurosci . 34 : 901 – 908 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ , Flowers DL , Napoliello EM , Eden GF . 2011. . Gray matter volume changes following reading intervention in dyslexic children . Neuroimage . 57 : 733 – 741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowicz-Kupis G , Borkowska AR , Pietras I . 2009. . Rapid automatized naming, phonology and dyslexia in Polish children . Med Sci Monit . 15 : CR460 – CR469 . [PubMed] [Google Scholar]
- Langer N , Peysakhovich B , Zuk J , Drottar M , Sliva DD , Smith S , Becker BL , Grant PE , Gaab N . 2015. . White matter alterations in infants at risk for developmental dyslexia . Cereb Cortex . bhv281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D , Mangin JF , Poupon C , Clark CA , Pappata S , Molko N , Chabriat H . 2001. . Diffusion tensor imaging: concepts and applications . J Magn Reson Imaging . 13 : 534 – 546 . [DOI] [PubMed] [Google Scholar]
- Lebel C , Beaulieu C . 2009. . Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children . Hum Brain Mapp . 30 : 3563 – 3573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C , Gee M , Camicioli R , Wieler M , Martin W , Beaulieu C . 2012. . Diffusion tensor imaging of white matter tract evolution over the lifespan . Neuroimage . 60 : 340 – 352 . [DOI] [PubMed] [Google Scholar]
- Lehongre K , Ramus F , Villiermet N , Schwartz D , Giraud A-L . 2011. . Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia . Neuron . 72 : 1080 – 1090 . [DOI] [PubMed] [Google Scholar]
- Leppanen PH , Hamalainen JA , Guttorm TK , Eklund KM , Salminen H , Tanskanen A , Torppa M , Puolakanaho A , Richardson U , Pennala R et al. . 2012. . Infant brain responses associated with reading-related skills before school and at school age . Neurophysiol Clin . 42 : 35 – 41 . [DOI] [PubMed] [Google Scholar]
- Linkersdorfer J , Jurcoane A , Lindberg S , Kaiser J , Hasselhorn M , Fiebach CJ , Lonnemann J . 2015. . The association between gray matter volume and reading proficiency: a longitudinal study of beginning readers . J Cogn Neurosci . 272 : 308 – 318 . [DOI] [PubMed] [Google Scholar]
- Liu P , Du J-L , He C . 2014. . Developmental pruning of early-stage myelin segments during CNS myelination in vivo . Cell Res . 237 : 2013 – 2023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z , Wang Y , Gerig G , Gouttard S , Tao R , Fletcher T , Styner M . 2010. . Quality control of diffusion weighted images. In: SPIE medical imaging. International Society for Optics and Photonics. p. 76280J-76280J . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barroso D , Catani M , Ripolles P , Dell'Acqua F , Rodriguez-Fornells A , de Diego-Balaguer R . 2013. . Word learning is mediated by the left arcuate fasciculus . Proc Natl Acad Sci USA . 110 : 13168 – 13173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR , Shaywitz SE , Shaywitz BA . 2003. . A definition of dyslexia . Ann Dyslexia . 53 : 1 – 14 . [Google Scholar]
- Martin A , Schurz M , Kronbichler M , Richlan F . 2015. . Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies . Hum Brain Mapp . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U , Bucher K , Brem S , Benz R , Kranz F , Schulz E , van der Mark S , Steinhausen HC , Brandeis D . 2009. . Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school . Biol Psychiatry . 66 : 341 – 348 . [DOI] [PubMed] [Google Scholar]
- Maurer U , Bucher K , Brem S , Brandeis D . 2003. . Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk . Neuroreport . 14 : 2245 – 2250 . [DOI] [PubMed] [Google Scholar]
- McCandliss BD , Noble KG . 2003. . The development of reading impairment: a cognitive neuroscience model . Ment Retard Dev Disabil Res Rev . 9 : 196 – 205 . [DOI] [PubMed] [Google Scholar]
- McNorgan C , Alvarez A , Bhullar A , Gayda J , Booth JR . 2011. . Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading . J Neurosci Nurs . 31 : 9641 – 9648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H , Smith SD , Hager K , Held M , Liu J , Olson RK , Pennington BF , DeFries JC , Gelernter J , O'Reilly-Pol T . 2005. . DCDC2 is associated with reading disability and modulates neuronal development in the brain . Proc Natl Acad Sci USA . 102 : 17053 – 17058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RD , Syngeniotis A , Jackson G , Corballis MC . 2002. . Mixed lateralization of phonological assembly in developmental dyslexia . Neurocase . 83 : 205 – 209 . [DOI] [PubMed] [Google Scholar]
- Molfese D , Molfese V , Key S , Modglin A , Kelley S , Terrell S . 2002. . Reading and cognitive abilities: Longitudinal studies of brain and behavior changes in young children . Ann Dyslexia . 52 : 99 – 119 . [Google Scholar]
- Molfese VJ , Molfese DL , Modgline AA . 2001. . Newborn and preschool predictors of second-grade reading scores: an evaluation of categorical and continuous scores . J Learn Disabil . 34 : 545 – 554 . [DOI] [PubMed] [Google Scholar]
- Mori S , Crain BJ , Chacko V , Van Zijl P . 1999. . Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging . Ann Neurol . 45 : 265 – 269 . [DOI] [PubMed] [Google Scholar]
- Mori S , Oishi K , Faria AV . 2009. . White matter atlases based on diffusion tensor imaging . Curr Opin Neurol . 22 : 362 – 369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S , van Zijl PC . 2002. . Fiber tracking: principles and strategies - a technical review . NMR Biomed . 15 : 468 – 480 . [DOI] [PubMed] [Google Scholar]
- Myers CA , Vandermosten M , Farris EA , Hancock R , Gimenez P , Black JM , Casto B , Drahos M , Tumber M , Hendren RL et al. . 2014. . White matter morphometric changes uniquely predict children's reading acquisition . Psychol Sci . 25 : 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z , Westerberg H , Klingberg T . 2004. . Maturation of white matter is associated with the development of cognitive functions during childhood . J Cogn Neurosci . 16 : 1227 – 1233 . [DOI] [PubMed] [Google Scholar]
- Niogi SN , McCandliss BD . 2006. . Left lateralized white matter microstructure accounts for individual differences in reading ability and disability . Neuropsychologia . 44 : 2178 – 2188 . [DOI] [PubMed] [Google Scholar]
- Norton ES , Wolf M . 2012. . Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities . Annu Rev Psychol . 63 : 427 – 452 . [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J , Dean DC , Ginestet CE , Walker L , Waskiewicz N , Lehman K , Dirks H , Piryatinsky I , Deoni SCL . 2014. . White matter development and early cognition in babies and toddlers . Hum Brain Mapp . 35 : 4475 – 4487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T , Zijdenbos A , Worsley K , Collins DL , Blumenthal J , Giedd JN , Rapoport JL , Evans AC . 1999. . Structural maturation of neural pathways in children and adolescents: in vivo study . Science . 283 : 1908 – 1911 . [DOI] [PubMed] [Google Scholar]
- Pennington BF , Lefly DL . 2001. . Early reading development in children at family risk for dyslexia . Child Dev . 72 : 816 – 833 . [DOI] [PubMed] [Google Scholar]
- Pennington BF , Olson RK . 2005. . Genetics of dyslexia . In: Snowling MJ, Hulme C, editors. The science of reading: A handbook. Blackwell handbooks of developmental psychology. Malden, Hoboken, NJ: Blackwell Publishing. p. 453–472. [Google Scholar]
- Pinel P , Fauchereau F , Moreno A , Barbot A , Lathrop M , Zelenika D , Le Bihan D , Poline J-B , Bourgeron T , Dehaene S . 2012. . Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions . J Neurosci Nurs . 32 : 817 – 825 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z , Han M , Garel K , San Chen E , Gabrieli JDE . 2015. . White-matter structure in the right hemisphere predicts Mandarin Chinese learning success . J Neurolinguistics . 33 : 14 – 28 . [Google Scholar]
- Raschle N , Zuk J , Ortiz-Mantilla S , Sliva DD , Franceschi A , Grant PE , Benasich AA , Gaab N . 2012. . Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines . Ann N Y Acad Sci . 1252 : 43 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM , Chang M , Gaab N . 2011. . Structural brain alterations associated with dyslexia predate reading onset . Neuroimage . 57 : 742 – 749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM , Lee M , Buechler R , Christodoulou JA , Chang M , Vakil M , Stering PL , Gaab N . 2009. . Making MR imaging child's play - pediatric neuroimaging protocol, guidelines and procedure . J Vis Exp . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM , Stering PL , Meissner SN , Gaab N . 2013. . Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia . Cereb Cortex . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM , Zuk J , Gaab N . 2012. . Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset . Proc Natl Acad Sci USA . 109 : 2156 – 2161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker AM , Deutsch GK , Ben-Shachar M , Schwartzman A , Perry LM , Dougherty RF . 2009. . Reading impairment in a patient with missing arcuate fasciculus . Neuropsychologia . 47 : 180 – 194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR , Voress J . 2007. . Test of memory and learning (TOMAL-2) . Austin, TX: : Pro-Ed; . [Google Scholar]
- Richards T , Stevenson J , Crouch J , Johnson LC , Maravilla K , Stock P , Abbott R , Berninger V . 2008. . Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia . Am J Neuroradiol . 29 : 1134 – 1139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F , Kronbichler M , Wimmer H . 2009. . Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies . Hum Brain Mapp . 30 : 3299 – 3308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL , Peterson DJ , Denckla MB , Kaufmann WE , Cutting LE . 2010. . White matter microstructural differences linked to left perisylvian language network in children with dyslexia . Cortex . 46 : 739 – 749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK , Barnett AS , Basser PJ , Marenco S , Pierpaoli C . 2004. . Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI . Magn Reson Med . 51 : 103 – 114 . [DOI] [PubMed] [Google Scholar]
- Saygin ZM , Norton ES , Osher DE , Beach SD , Cyr AB , Ozernov-Palchik O , Yendiki A , Fischl B , Gaab N , Gabrieli JD . 2013. . Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children . J Neurosci . 33 : 13251 – 13258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri TS , Darki F , Newbury DF , Whitehouse AJ , Peyrard-Janvid M , Matsson H , Ang QW , Pennell CE , Ring S , Stein J . 2012. . The dyslexia candidate locus on 2p12 is associated with general cognitive ability and white matter structure . PLoS ONE . 7 : e50321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ , Holland SK , Plante E . 2011. . Diffusion tensor imaging reveals white matter microstructure correlations with auditory processing ability . Ear Hear . 32 : 156 – 167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ , Wilke M , Dardzinski BJ , Holland SK . 2002. . Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study . Radiology . 222 : 212 – 218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ , Wilke M , Dardzinski BJ , Holland SK . 2005. . Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study . Hum Brain Mapp . 26 : 139 – 147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel EM , Wiig EH , Sabers D . 1999. . CELF: clinical evaluation of language functions . Columbus, OH: CE Merrill; . [Google Scholar]
- Shaywitz SE , Shaywitz BA . 2008. . Paying attention to reading: the neurobiology of reading and dyslexia . Dev Psychopathol . 20 : 1329 . [DOI] [PubMed] [Google Scholar]
- Shaywitz SE , Shaywitz BA , Fulbright RK , Skudlarski P , Mencl WE , Constable RT , Pugh KR , Holahan JM , Marchione KE , Fletcher JM et al. . 2003. . Neural systems for compensation and persistence: young adult outcome of childhood reading disability . Biol Psychiatry . 54 : 25 – 33 . [DOI] [PubMed] [Google Scholar]
- Shaywitz SE , Shaywitz BA , Pugh KR , Fulbright RK , Constable RT , Mencl WE , Katz L . 1998. . Functional disruption in the organization of the brain for reading in dyslexia . Ann NY Acad Sci . 955 : 2636 – 2641 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ , Hallquist MN , Asato M , Luna B . 2014. . Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study . Neuroimage . 92 : 356 – 368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G , Lanoe C , Poirel N , Rossi S , Lubin A , Pineau A , Houde O . 2013. . Dynamics of the anatomical changes that occur in the brains of schoolchildren as they learn to read . PLoS ONE . 8 : e81789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM . 2002. . Fast robust automated brain extraction . Hum Brain Mapp . 17 : 143 – 155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MJ , Muter V , Carroll J . 2007. . Children at family risk of dyslexia: a follow-up in early adolescence . J Child Psychol Psychiatry . 48 : 609 – 618 . [DOI] [PubMed] [Google Scholar]
- Song SK , Yoshino J , Le TQ , Lin SJ , Sun SW , Cross AH , Armstrong RC . 2005. . Demyelination increases radial diffusivity in corpus callosum of mouse brain . Neuroimage . 26 : 132 – 140 . [DOI] [PubMed] [Google Scholar]
- Specht K , Hugdahl K , Ofte S , Nygard M , Bjornerud A , Plante E , Helland T . 2009. . Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children . Scand J Psychol . 50 : 79 – 91 . [DOI] [PubMed] [Google Scholar]
- Stanovich KE . 1996. . Toward a more inclusive definition of dyslexia . Dyslexia . 2 : 154 – 166 . [Google Scholar]
- Steinbrink C , Vogt K , Kastrup A , Muller HP , Juengling FD , Kassubek J , Riecker A . 2008. . The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T . Neuropsychologia . 46 : 3170 – 3178 . [DOI] [PubMed] [Google Scholar]
- Szwed M , Dehaene S , Kleinschmidt A , Eger E , Valabrègue R , Amadon A , Cohen L . 2011. . Specialization for written words over objects in the visual cortex . Neuroimage . 56 : 330 – 344 . [DOI] [PubMed] [Google Scholar]
- Taipale M , Kaminen N , Nopola-Hemmi J , Haltia T , Myllyluoma B , Lyytinen H , Muller K , Kaaranen M , Lindsberg PJ , Hannula-Jouppi K . 2003. . A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain . Proc Natl Acad Sci . 100 : 11553 – 11558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK , Ostby Y , Fjell AM , Westlye LT , Due-Tonnessen P , Walhovd KB . 2010. . Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure . Cereb Cortex . 20 : 534 – 548 . [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M , Cohen L , Amemiya E , Braga LW , Dehaene S . 2014. . Learning to read improves the structure of the arcuate fasciculus . Cereb Cortex . 24 : 989 – 995 . [DOI] [PubMed] [Google Scholar]
- Torgesen J , Wagner R , Rashotte C . 1999. a . Comprehensive test of phonological processing . Austin, TX: : Pro-Ed; . [Google Scholar]
- Torgesen J , Wagner R , Rashotte C . 1999. b . Test of Word Reading Efficiency (TOWRE) . London: : Psychological Corporation; . [Google Scholar]
- Turkeltaub PE , Gareau L , Flowers DL , Zeffiro TA , Eden GF . 2003. . Development of neural mechanisms for reading . Nat Neurosci . 6 : 767 – 773 . [DOI] [PubMed] [Google Scholar]
- van der Leij A , van Bergen E , van Zuijen T , de Jong P , Maurits N , Maassen B . 2013. . Precursors of developmental dyslexia: an overview of the longitudinal Dutch Dyslexia Programme study . Dyslexia . 19 : 191 – 213 . [DOI] [PubMed] [Google Scholar]
- van Herten M , Pasman J , van Leeuwen TH , Been PH , van der Leij A , Zwarts F , Maassen B . 2008. . Differences in AERP responses and atypical hemispheric specialization in 17-month-old children at risk of dyslexia . Brain Res . 1201 : 100 – 105 . [DOI] [PubMed] [Google Scholar]
- Vandermosten M , Boets B , Poelmans H , Sunaert S , Wouters J , Ghesquière P . 2012. . A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing . Brain . 135 : 935 – 948 . [DOI] [PubMed] [Google Scholar]
- Vandermosten M , Boets B , Wouters J , Ghesquière P . 2012. . A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia . Neurosci Biobehav Rev . 36 : 1532 – 1552 . [DOI] [PubMed] [Google Scholar]
- Vandermosten M , Poelmans H , Sunaert S , Ghesquiere P , Wouters J . 2013. . White matter lateralization and interhemispheric coherence to auditory modulations in normal reading and dyslexic adults . Neuropsychologia . 51 : 2087 – 2099 . [DOI] [PubMed] [Google Scholar]
- Vandermosten M , Vanderauwera J , Theys C , De Vos A , Vanvooren S , Sunaert S , Wouters J , Ghesquiere P . 2015. . A DTI tractography study in pre-readers at risk for dyslexia . Dev Cogn Neurosci . 14 : 8 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij M , Smits M , Wielopolski P , Houston G , Krestin G , van der Lugt A . 2007. . Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right-and left-handed healthy subjects: a combined fMRI and DTI study . Neuroimage . 35 : 1064 – 1076 . [DOI] [PubMed] [Google Scholar]
- Wakana S , Caprihan A , Panzenboeck MM , Fallon JH , Perry M , Gollub RL , Hua K , Zhang J , Jiang H , Dubey P . 2007. . Reproducibility of quantitative tractography methods applied to cerebral white matter . Neuroimage . 36 : 630 – 644 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA , Yeatman JD . 2013. . Biological development of reading circuits . Curr Opin Neurobiol . 23 : 261 – 268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y , Adamson C , Yuan W , Altaye M , Rajagopal A , Byars AW , Holland SK . 2012. . Sex differences in white matter development during adolescence: a DTI study . Brain Res . 1478 : 1 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA , Cercignani M . 2009. . About “axial” and “radial” diffusivities . Magn Reson Med . 61 : 1255 – 1260 . [DOI] [PubMed] [Google Scholar]
- Wiederholt J , Bryant B . 2001. . GORT 4: Gray Oral Reading Tests examiner's manual . Austin, TX: : Pro-Ed; . [Google Scholar]
- Wolf M , Denckla MB . 2005. . RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests . Austin, TX: Pro-ed Inc; . [Google Scholar]
- Woodcock RW . 1987. . Woodcock reading mastery tests, revised . MN: : American Guidance Service Circle Pines; . [Google Scholar]
- Woodcock RW , McGrew K , Mather N . 2001. . Woodcock-Johnson tests of achievement . Itasca, IL: : Riverside Publishing; . [Google Scholar]
- Yamada Y , Stevens C , Dow M , Harn BA , Chard DJ , Neville HJ . 2011. . Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study . Neuroimage . 57 : 704 – 713 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD , Dougherty RF , Ben-Shachar M , Wandell BA . 2012. . Development of white matter and reading skills . Proc Natl Acad Sci USA . 109 : E3045 – E3053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD , Dougherty RF , Myall NJ , Wandell BA , Feldman HM . 2012. . Tract profiles of white matter properties: automating fiber-tract quantification . PLoS ONE . 7 : e49790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD , Dougherty RF , Rykhlevskaia E , Sherbondy AJ , Deutsch GK , Wandell BA , Ben-Shachar M . 2011. . Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children . J Cogn Neurosci . 23 : 3304 – 3317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD , Rauschecker AM , Wandell BA . 2012. . Anatomy of the visual word form area: Adjacent cortical circuits and long-range white matter connections . Brain Lang . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.