Abstract
Objective
To determine the effects of medical cannabinoids on pain, spasticity, and nausea and vomiting, and to identify adverse events.
Data sources
MEDLINE, the Cochrane Database, and the references of included studies were searched.
Study selection
Systematic reviews with 2 or more randomized controlled trials (RCTs) that focused on medical cannabinoids for pain, spasticity, or nausea and vomiting were included. For adverse events, any meta-analysis for the conditions listed or of adverse events of cannabinoids was included.
Synthesis
From 1085 articles, 31 relevant systematic reviews were identified including 23 for pain, 5 for spasticity, 6 for nausea and vomiting, and 12 for adverse events. Meta-analysis of 15 RCTs found more patients taking cannabinoids attained at least a 30% pain reduction: risk ratio (RR) of 1.37 (95% CI 1.14 to 1.64), number needed to treat (NNT) of 11. Sensitivity analysis found study size and duration affected findings (subgroup differences, P ≤ .03), with larger and longer RCTs finding no benefit. Meta-analysis of 4 RCTs found a positive global impression of change in spasticity (RR = 1.45, 95% CI 1.08 to 1.95, NNT = 7). Other results were not consistently statistically significant, but when positive, a 30% or more improvement in spasticity had an NNT of 10. Meta-analysis of 7 RCTs for control of nausea and vomiting after chemotherapy found an RR of 3.60 (95% CI 2.55 to 5.09) with an NNT of 3. Adverse effects caused more patients to stop treatment (number needed to harm [NNH] of 8 to 22). Individual adverse events were very common, including dizziness (NNH = 5), sedation (NNH = 5), confusion (NNH = 15), and dissociation (NNH = 20). “Feeling high” was reported in 35% to 70% of users. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) evaluation reduced evidence ratings of benefit to low or very low.
Conclusion
There is reasonable evidence that cannabinoids improve nausea and vomiting after chemotherapy. They might improve spasticity (primarily in multiple sclerosis). There is some uncertainty about whether cannabinoids improve pain, but if they do, it is neuropathic pain and the benefit is likely small. Adverse effects are very common, meaning benefits would need to be considerable to warrant trials of therapy.
Résumé
Objectif
Déterminer les effets du cannabis médical sur la douleur, la spasticité, et les nausées et les vomissements, et vérifier les effets indésirables du cannabis.
Source des données
MEDLINE, la base de données Cochrane et les références bibliographiques des études consultées.
Choix des études
On a choisi les revues systématiques comprenant au moins 2 essais randomisés contrôlés (ERC) portant principalement sur l’emploi du cannabis médical contre la douleur, la spasticité, ou les nausées et les vomissements, ou sur les effets indésirables des cannabinoïdes.
Synthèse
Sur 1085 articles, on a retenu 31 revues systématiques pertinentes, dont 23 portaient sur la douleur, 5 sur la spasticité, 6 sur les nausées et les vomissements, et 12 sur les effets indésirables observés. Une méta-analyse de 15 ERC a révélé que plus de patients obtenaient une réduction de la douleur d’au moins 30 % avec le cannabis : risque relatif (RR) de 1,37 (IC à 95% 1,14 à 1,64), nombre de patients à traiter (NPT = 11). Une analyse de sensibilité a observé que la taille de l’étude et sa durée affectaient les résultats (différences entre sous-groupes, P ≤ .03), alors qu’une amplitude et une durée plus grandes des observations des ERC n’avaient aucun avantage. Une méta-analyse de 4 ERC a révélé que les patients avaient l’impression d’une amélioration de la spasticité (RR = 1,45, IC à 95 % 1,08 à 1,95, NPT = 7). Les autres résultats n’étaient pas toujours statistiquement significatifs, mais quand ils étaient positifs, une amélioration d’au moins 30 % de la spasticité avait un NPT de 10. Une autre méta-analyse de 7 ERC portant sur le contrôle des nausées et des vomissements causés par la chimiothérapie a révélé un RR de 3,60 (IC à 95 % 2,55 à 5,09) avec un NPT de 3. Les effets indésirables ont amené plus de patients à cesser le traitement (NPT de 8 à 22). Les différents effets indésirables du cannabis étaient très fréquents, dont les étourdissements (NPT pour les observer = 5), la sédation (NPT = 5), la confusion (NPT = 15) et l’état de dissociation (NPT = 20). Entre 35 et 70 % des utilisateurs ont mentionné avoir ressenti une sensation d’euphorie. L’évaluation GRADE (Grading of Recommendation Assessment, Development and Evaluation) a réduit à faibles ou très faibles les scores obtenus pour les données probantes indiquant un avantage.
Conclusion
Des données probantes raisonnables semblent indiquer que les cannabinoïdes ont un effet positif sur les nausées et les vomissements causés par la chimiothérapie. Ils pourraient aussi réduire la spasticité, surtout dans la sclérose en plaques. Il n’est pas absolument certain qu’ils diminuent la douleur, mais si c’est le cas, il s’agirait surtout de la douleur neuropathique, et les avantages seraient plutôt minimes. Les effets indésirables sont très fréquents, ce qui signifie que les avantages doivent être importants pour justifier un essai de traitement.
Medical cannabinoids have been advocated for an extensive variety of conditions, from glaucoma to cancer.1 Unfortunately, bias is pervasive throughout the medical cannabinoid literature, including in randomized controlled trials (RCTs).2 This is compounded by poor reporting in the media, with 79% of medical cannabinoid newspaper stories providing inappropriate information, most of which was sensationalism.3
The interest in medical cannabinoids has varied broadly among prescribers, from enthusiasm4 to reluctance.5 A survey found that about one-quarter of physicians in a region of Quebec prescribed medical cannabinoids, primarily (about 90%) nabilone, but they thought more education on prescribing would be helpful.6 A needs assessment survey found that Canadian physicians wanted more information about the risks and potential therapeutic uses of medical cannabinoids.7 While Canadian organizations have responded by providing guidance documents8 and patient information,9 these documents lack numeric information and GRADE (Grading of Recommendations Assessment, Development and Evaluation) evaluation10 regarding risks and benefits to adequately promote shared, informed decision making.
Two large and comprehensive reviews have examined the use of cannabinoids for various medical conditions.1,2 If cannabinoids are effective, the evidence suggests that they are most likely to work for chronic pain, nausea and vomiting associated with chemotherapy, and spasticity associated with chronic neurologic conditions like multiple sclerosis.1,2 However, a key consideration for any medical intervention is the potential adverse events or harms that could arise from the therapy.
Our purpose was to complete a systematic review to provide evidence for a medical cannabinoid prescribing guideline. We focused on the conditions for which medical cannabinoids have the best evidence base and the highest likelihood of having medical advantages. Therefore, our objective was to complete 4 distinct systematic reviews of systematic reviews on medical cannabinoids for pain, nausea and vomiting, spasticity, and adverse events. On completion, we hoped to have clear guidance for prescribers and their patients, as well as to provide adequate information to promote shared, informed decision making.
METHODS
We followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)11 as a guide for completion of this systematic review, augmented with the guide to systematic reviews of systematic reviews.12
Data sources
A medical librarian (K.C.) searched MEDLINE via Ovid from 1946 to April 2017 using English-language and systematic review limits. To start, a MEDLINE search protocol was created for cannabis and cannabinoids that used MeSH terms cannabis or medical marijuana or key words cannabinoid/s or nabilone or Cesamet or dronabinol or Marinol or levonantradol or tetrahydrocannabinol or delta-9-THC or delta-9-tetrahydrocannabinol. Next, 4 MEDLINE searches used the above protocol with unique terms added. Nausea and vomiting was searched with and MeSH and key word terms nausea or vomit/ing or antiemetic/s or key word emesis. Spasticity was searched with the above protocol and MeSH and key word terms spasm or multiple sclerosis or key words spasticity or MS. The pain search combined the search protocol with and MeSH and key word term pain. Finally, the adverse events search added the MeSH terms patient harm or harm reduction or key words harm/s or adverse events or side effects.
Next, Cochrane Library searches were conducted in May 2017 with all searches limited to Cochrane reviews (excluding protocols). One set of searches was conducted with the terms marijuana or cannabis and nausea, vomiting, pain, spasticity, MS, harm, adverse events, and side effects (with the terms searched separately).
References from all included articles were reviewed to identify missed systematic reviews, particularly within the gray literature. Last, any relevant references from the authors’ personal collections were added.
Study selection
To be included, studies had to be systematic reviews (with or without meta-analysis) of RCTs examining medical cannabinoids for the management of pain, spasticity, or nausea and vomiting. Studies were excluded if they were systematic reviews not focused on medical cannabinoids or if they were focused on conditions other than those listed. Systematic reviews of observational studies or of other systematic reviews, systematic reviews published as abstracts only, systematic reviews in which more than 50% of the RCTs involved pediatric patients, and systematic reviews with less than 2 RCTs were also excluded.
For the adverse events systematic review, systematic reviews of RCTs with meta-analysis focused on the harms of medical cannabinoids or systematic reviews of RCTs with meta-analysis of adverse events identified in the pain, spasticity, or nausea and vomiting systematic reviews were included. Exclusion criteria were the same as previously listed.
Dual independent review (G.M.A. with C.R.F., D.P., J.R., or J.T.) was performed on study titles and abstracts identified in the librarian search, with dual independent full-article review as necessary. Additionally, a single reviewer (G.M.A.) assessed titles and abstracts of all studies identified from reference lists of included systematic reviews, with dual independent review of any studies requiring full-article review. Study inclusion disagreement was resolved by consensus. While our search was originally limited to English-only articles, we included any relevant article located during any stage of the search, regardless of language.
Synthesis
Data extraction
Paired, independent data extraction (G.M.A. with C.R.F., D.P., J.R., or J.T.) was performed, with disagreement resolved by consensus. Data were extracted on number of RCTs, number of patients, specific focus (eg, neuropathic pain), baseline characteristics (average age or sex proportion), cannabinoid intervention (types and doses), control intervention (placebo or specific active control), risk-of-bias tool used to assess RCTs, risk of bias found, other quality issues, and findings (benefits and harms). When considering the number of RCTs in a given systematic review, only those focused on pain, spasticity, nausea and vomiting, or adverse events were considered. The total number of patients reported for each systematic review was, whenever possible, the summed number of patients randomized in (not those completing) all included relevant RCTs. If the number of RCTs or patients was not reported for adverse events, we reported the number of RCTs and patients included in the largest meta-analysis of adverse events for that systematic review.
For findings of benefit, in order, we prioritized data extraction on responder rates, mean change in scales of symptoms and signs, patient-reported improvement (eg, global impression of change), and standard mean difference analyses. Responder rate analyses are the proportion of patients who attained an established improvement on a scale, such as a 30% improvement in a visual analogue pain score or reaching a defined minimal clinically important difference. Pooled meta-analytic results were extracted preferentially. In systematic reviews without meta-analysis, it is possible that multiple results from multiple studies are presented. These have the potential to be selectively reported (either for or against the intervention), so we placed less value on these results. When we extracted data from these descriptive systematic reviews, we minimized the risk of selective reporting by focusing on the largest and highest-quality RCTs reported. We also extracted representative data from different types of cannabinoids or different relevant populations as defined by the systematic review authors. For example, a pain systematic review might have grouped results into cancer pain and neuropathic pain RCTs, so we would report the results from the largest and highest-quality RCT for each pain subtype.
For findings of harm (adverse events), we used only results of meta-analyses. We extracted data on total adverse events, total serious adverse events, adverse events leading to withdrawal, and any specific adverse event symptom, sign, or condition (eg, dizziness).
Risk-of-bias assessment
Risk of bias for the included systematic reviews was assessed using a modified version of the AMSTAR score.13 The AMSTAR score is quite long (11 factors); therefore, we trimmed the score down to 6 components considered to be most relevant:
Were study selection and data extraction performed by dual reviewers?
Was the literature search comprehensive?
Were the included study characteristics described?
Was the quality of the included studies assessed and reported?
Were the methods used to combine results appropriate?
Were conflicts of interest reported?
For each systematic review, each component was scored as 1 (done appropriately) or 0 (unclear or not done), and individual scores were summed for a total score, with higher scores indicating lower risk of bias. Risk-of-bias assessment was performed by 2 independent reviewers (C.R.F., D.P., J.R., J.T., C.K., M.R.K., or A.J.L.), and disagreement was recorded and resolved by consensus or a third reviewer (J.T.).
Analysis
Study characteristics were presented descriptively. As these results were unlikely to be normally distributed, we used nonparametric measures like medians and interquartile ranges (IQRs) to present descriptive summaries.
In reporting findings from systematic reviews, meta-analytic results were presented preferentially. Odds ratios will exaggerate effects in common conditions (such as studies of people suffering from pain, nausea and vomiting, or spasticity). Risk differences show the absolute effect but do not allow easy comparisons across populations or allow for estimation of benefit on populations with varying baseline risks. Therefore, for dichotomous outcomes (like ≥ 30% pain reduction), any meta-analysis presenting odds ratios or risk differences was redone using risk ratios (RR) with the same numbers used by the original authors. If heterogeneity was present (I2 statistic ≥ 25%), a random-effects meta-analysis was performed. If heterogeneity was not present (I2 statistic < 25%), a fixed-effects meta-analysis was performed.
Some of the meta-analyses included crossover studies with multiple doses or even multiple interventions, meaning that single patients could be counted multiple times. For example, a crossover RCT of 3 different doses and a placebo could count the same patient as 4 different observations. As a result, some meta-analyses reported “observations” that exceeded the total number of patients in the study. Other meta-analyses reported only the first round of the trial after randomization, and so the number of observations matched the number of patients in the study. When recalculating the meta-analyses of past authors, we did not modify how they managed the total observations.
Performing new meta-analyses
If meta-analyses from different systematic reviews used different RCTs for the same condition and outcome, we performed a new meta-analysis of all unique studies, with all duplicates removed. When the same parallel RCT was used in different meta-analyses, we selected the version of the study that included the largest number of patients, as this more likely reflects an intention-to-treat analysis. When the same crossover RCTs were used in more than 1 meta-analysis, we selected the versions that included only the original randomization (not the additional crossovers). This more accurately reflects the total number of patients as compared with the total number of observations.
If more than 10 RCTs contributed to a meta-analysis, a funnel plot was created to assess the risk of publication bias. Risk ratios were converted to odds ratios for this test (as this is the more common measure for funnel plots). Sensitivity analyses were performed for outcomes in which results suggested external factors might be influencing heterogeneity and the results. These were determined post hoc.
Last, outcomes assessed with meta-analyses were evaluated using the GRADE approach10 with a panel of 6 authors (G.M.A., C.K., A.J.L., J.T., D.P., J.R.).
SYNTHESIS
Figure A1, available in the online supplement at CFPlus,* provides details of search and study flow. The librarian search identified 241 articles and the reference list search of included systematic reviews added 844 new articles. After appropriate exclusion based on title and abstract, full review was performed on 62 articles. A total of 31 systematic reviews were included, with 27 (87%) coming from the librarian search. Agreement for study selection from the librarian search was 98%, for data extraction was 92%, and for risk-of-bias assessment was 86%.
Table 1 provides details of baseline characteristics of the 31 included systematic reviews.2,14–43 Table A2* provides reasons for exclusion of the articles that went for full review. Of the 31 included systematic reviews, 11 had 2 or more areas of focus, leading to 46 systematic reviews. Within these 46 systematic reviews, the median (IQR) number of included RCTs was 7 (5 to 18) and the median (IQR) number of included patients was 725 (305 to 1242). Fifteen (15 of 46, 33%) systematic reviews included fewer than 300 patients or the number could not be calculated. Excluding the 12 systematic reviews of adverse events (which required a meta-analysis for inclusion), meta-analyses were included in 41% (14 of 34) of the systematic reviews, with pain systematic reviews least likely to provide meta-analysis (30%, 7 of 23). On a scale of 0 to 6 (with higher scores indicating lower risk of bias, the median (IQR) modified AMSTAR risk-of-bias score for the systematic review articles was 4 (2 to 5). Complete details of the risk-of-bias assessment are provided in Table A3.* Table A4* provides details of novel meta-analyses performed in this study, including the RCTs and which meta-analyses the RCTs were drawn from, as well as the types of therapy used.
Table 1.
Characteristics of included systematic reviews
| SYSTEMATIC REVIEW | CORE TOPIC | SUBGROUP | NO. OF RCTS | NO. OF PATIENTS | META-ANALYSES | MODIFIED AMSTAR* SCORE |
|---|---|---|---|---|---|---|
| Whiting et al, 20152 | Pain | Chronic pain | 28 | 2454 | Yes | 6 |
| Spasticity | Spasticity (due to MS or paraplegia) | 14 | 2280 | Yes | ||
| Nausea and vomiting | Chemotherapy | 28 | 1772 | Yes | ||
| Adverse events | Any | 62 | NR | Yes | ||
| Martin-Sanchez et al, 200914 | Pain | Chronic pain | 18 | 809 | Yes | 5 |
| Adverse events | Chronic pain | 6 | 540 | Yes | ||
| Andreae et al, 201515 | Pain | Neuropathic pain | 5 | 178 | Yes | 6 |
| Petzke et al, 201616 | Pain | Neuropathic pain | 15 | 1619 | Yes | 5 |
| Adverse events | Neuropathic pain | 11 | 1574 | Yes | ||
| Lobos Urbina and Peña Duran, 201617 | Pain | Cancer pain | 6 | NR | Yes | 1 |
| Adverse events | Cancer | NR | NR | Yes | ||
| Iskedjian et al, 200718 | Pain | MS pain | 7 | 298 | Yes | 6 |
| Adverse events | MS | 7 | 298 | Yes | ||
| Mücke et al, 201619 | Pain | Palliative care pain | 2 | 537 | Yes | 6 |
| Nausea and vomiting | Palliative care nausea and vomiting | 5 | 635 | Yes | ||
| Adverse events | Palliative care | 6 | 1031 | Yes | ||
| Fitzcharles et al, 201620 | Pain | Rheumatologic | 4 | 160 | No | 5 |
| Fitzcharles et al, 201621 | Pain | Rheumatologic | 4 | 203 | No | 4 |
| Walitt et al, 201622 | Pain | Fibromyalgia | 2 | 72 | No | 5 |
| Stevens and Higgins, 201723 | Pain | Acute pain | 7 | 611 | No | 5 |
| Tateo, 201724 | Pain | Cancer pain | 8 | 683 | No | 3 |
| Smith et al, 201525 | Nausea and vomiting | Chemotherapy | 23 | 1326 | Yes | 6 |
| Adverse events | Chemotherapy | 11 | 1055 | Yes | ||
| Machado Rocha et al, 200826 | Nausea and vomiting | Chemotherapy | 30 | 1719 | Yes | 5 |
| Tramèr et al, 200127 | Nausea and vomiting | Chemotherapy | 30 | 1760 | Yes | 2 |
| Adverse events | Chemotherapy | 19 | 1111 | Yes | ||
| Wade et al, 201028 | Spasticity | MS spasticity | 3 | 666 | Yes | 2 |
| Adverse events | MS | 3 | 666 | Yes | ||
| Meza et al, 201729 | Pain | MS pain | 3 | 327 | No | 1 |
| Spasticity | MS spasticity | 4 | 1247 | No | ||
| Adverse events | MS | 4 | 1025 | No | ||
| Wang et al, 200830 | Adverse events | Any | 23 | 2068 | Yes | 5 |
| Koppel et al, 201431 | Spasticity | MS spasticity | 17 | NR | No | 4 |
| Adverse events | MS | 24 | 2737 | Yes | ||
| Boychuk et al, 201532 | Pain | Neuropathic pain | 13 | 771 | No | 4 |
| CADTH, 201033 | Pain | Chronic noncancer pain | 3 | 265 | No | 1 |
| CADTH, 201034 | Pain | Neuropathic pain | 7 | 444 | No | 0 |
| CADTH, 201135 | Pain | Nabilone for chronic pain | 2 | 44 | No | 2 |
| Campbell et al, 200136 | Pain | Various | 9 | 222 | No | 5 |
| Cotter, 200937 | Nausea and vomiting | Chemotherapy | 9 | 885 | No | 3 |
| Deshpande et al, 201538 | Pain | Chronic noncancer pain | 6 | 226 | No | 5 |
| Jensen et al, 201539 | Pain | Various | 22 | 1227 | No | 1 |
| Lakhan and Rowland, 200940 | Spasticity | MS spasticity | 6 | 481 | No | 5 |
| Lynch and Campbell, 201141 | Pain | Chronic noncancer pain | 18 | 766 | No | 4 |
| Lynch and Ware, 201542 | Pain | Chronic noncancer pain | 11 | 1185 | No | 4 |
| Tsang and Giudice, 201643 | Pain | Nabilone for pain | 7 | 251 | No | 2 |
CADTH—Canadian Agency for Drugs and Technologies in Health, MS—multiple sclerosis, NR—not reported, RCT—randomized controlled trial.
Possible scores range from 0 to 6, with higher scores representing lower risk of bias.
Pain
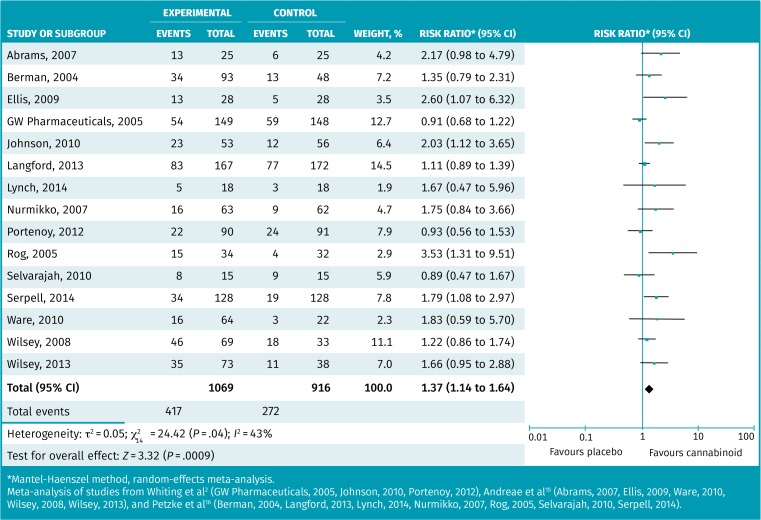
Table 2 provides the results of 7 systematic reviews that performed meta-analyses examining pain.2,14–19 The results showed that pain rating (range 0 to 10, with higher being worse pain) was statistically improved in 3 of 4 meta-analyses and, in those, improvement was approximately 0.4 to 0.8 more than placebo.2,18 Iskedjian et al provided additional data, indicating that from a baseline of about 6.3, cannabinoids improved pain 1.6 points versus 0.8 for placebo.18 Five reviews reported a 30% or more pain reduction,2,15–17,19 and although all demonstrated similar positive effects, only the results of 2 were statistically significant. Figure 1 provides the responder meta-analysis of 15 RCTs demonstrating approximately 39% of patients taking medical cannabinoids attained a 30% or better pain reduction compared with 30% of placebo patients, with an RR of 1.37 (95% CI 1.14 to 1.64) and a number needed to treat (NNT) of 11.2,15,16 Most RCTs examined neuropathic pain (13 of 15), while the remainder examined cancer pain (2 of 15). The funnel plot was relatively symmetric, suggesting a low risk of publication bias (Figure A5*).
Table 2.
Effect estimates, event rates, and NNTs for meta-analyses examining medical cannabinoids versus placebo for pain
| SYSTEMATIC REVIEW | TYPE OF PAIN | OUTCOME | NO. OF RCTS (NO. OF PARTICIPANTS) | AUTHORS’ META-ANALYSIS RESULT (95% CI), HETEROGENEITY | META-ANALYSIS RE-ANALYZED (95%CI), HETEROGENEITY | CANNABINOID EVENT RATE | CONTROL EVENT RATE | NNT |
|---|---|---|---|---|---|---|---|---|
| Whiting et al, 20152 | Chronic | ≥ 30% reduction in pain | 8 (1370) | OR = 1.41 (0.99 to 2.00), I2 = 48% | RR = 1.23 (0.98 to 1.56), I2 = 51% | 37% | 31% | NS (approximately 19)* |
| Pain score on NRS from 0–10 | 6 (948) | WMD = 0.46 (0.11 to 0.80), I2 = 59% | NA | NA | NA | NA | ||
| Score on pain inventory from 0–10 | 3 (613) | WMD = 0.17 (−0.16 to 0.50), I2 = 0% | NA | NA | NA | NA | ||
| Score on neuropathic pain scale from 0–100 | 5 (764) | WMD = 3.89 (0.47 to 7.32), I2 = 41% | NA | NA | NA | NA | ||
| Martin-Sanchez et al, 200914 | Chronic | Pain | 7 (278) | SMD = 0.61 (0.37 to 0.84), I2 = 0% | NA | NA | NA | NA |
| Andreae et al, 201515 | Neuropathic† | ≥ 30% reduction in pain | 5 (405) | OR = 3.22 (1.59 to 7.22), I2 = NR | RR = 1.62 (1.24 to 2.12), I2 = 2% | 47% | 29% | 6 |
| Petzke et al, 201616 | Neuropathic | ≥ 30% reduction in pain | 9 (1346) | RD = 0.10 (0.03 to 0.19), I2 = 38% | RR = 1.34 (1.04 to 1.74), I2 = 52% | 38% | 30% | 14 |
| ≥ 50% reduction in pain | 6 (737) | RD = 0.05 (0.0 to 0.11), I2 = 44% | RR = 1.48 (0.77 to 2.84), I2 = 44% | 19% | 16% | NS | ||
| Average pain intensity | 13 (1575) | SMD = 0.1 (0 to 0.2), I2 = 0% | NA | NA | NA | NA | ||
| Lobos Urbina and Peña Duran, 201617 | Cancer | ≥ 30% reduction in pain | 2 (290) | RR = 1.35 (0.63 to 2.09), I2 = NR | NA | NR | NR | NA |
| Iskedjian et al, 200718 | MS | Change in pain on VAS from 0–10 | 7 (298) | 0.8 more pain reduction (P = .03), I2 = 0 | NA | 6.2 baseline, improved 1.6 | 6.4 baseline, improved 0.8 | NA |
| Mücke et al, 201619 | Palliative | ≥ 30% reduction in pain | 2 (537) | RD = 0.07 (−0.01 to 0.16), I2 = 0% | RR = 1.34 (0.96 to 1.86), I2=0% | 30% | 23% | NS |
| All studies | Chronic | ≥ 30% reduction in pain | 15 (1985) | NA | RR = 1.37 (1.14 to 1.64), I2 = 43% | 39% | 30% | 11 |
MS—multiple sclerosis, NA—not applicable, NNT—number needed to treat, NR—not reported, NRS—numeric rating scale, NS—not significant, OR—odds ratio, RCT—randomized controlled trial, RD—risk difference, RR—risk ratio, SMD—standardized mean difference, VAS—visual analogue scale, WMD—weighted mean difference.
Confidence intervals suggest that benefit is likely, so estimated NNT provided.
Included only inhaled medical marijuana RCTs.
Figure 1.
Responder meta-analysis of patients attaining ≥30% reduction in pain with medical cannabinoids compared with placebo
*Mantel-Haenszel method, random-effects meta-analysis.
Meta-analysis of studies from Whiting et al2 (GW Pharmaceuticals, 2005, Johnson, 2010, Portenoy, 2012), Andreae et al15 (Abrams, 2007, Ellis, 2009, Ware, 2010, Wilsey, 2008, Wilsey, 2013), and Petzke et al16 (Berman, 2004, Langford, 2013, Lynch, 2014, Nurmikko, 2007, Rog, 2005, Selvarajah, 2010, Serpell, 2014).
We performed 3 sensitivity analyses within pain management (for ≥ 30% pain reduction) based on cannabinoid type, study size, and study duration (Figures A6a, A6b, and A6c, respectively*). Comparing types of medical cannabinoids, inhaled cannabinoids had an RR of 1.52 (95% CI 1.17 to 1.99) and an NNT of 6, while buccal-spray cannabinoids had an RR of 1.28 (95% CI 1.02 to 1.61) and an NNT of 16, but with no clear difference in subgroups (P = .34). No RCTs of oral medications were identified for the 30% or more pain reduction responder analysis. In comparing the size of studies, small studies (≤ 150 patients) had an RR of 1.56 (95% CI 1.26 to 1.92) and an NNT of 6, while large studies (> 150 patients) had a non-significant RR of 1.09 (95% CI 0.86 to 1.39), with a statistically significant difference in subgroups (P = .03). In comparing duration of studies, RCTs shorter than 1 week had an RR of 1.58 (95% CI 1.13 to 2.20) and an NNT of 5; RCTs of 2 to 5 weeks had an RR of 1.79 (95% CI 1.31 to 2.43) and an NNT of 7, and RCTs of 9 to 15 weeks had a non-significant RR of 1.07 (95% CI 0.87 to 1.32). Subgroup comparisons were statistically significant (P = .01).
Systematic reviews focusing on pain reduction in particular populations or conditions generally found inconsistent or equivocal results. Fitzcharles and colleagues and Walitt and colleagues reported insufficient evidence for benefit in rheumatologic pain and fibromyalgia, respectively.20–22 Stevens and Higgins reported on 7 RCTs for acute pain and found a decrease in pain in 1, worse pain in another, and no effect in 5, concluding that cannabinoids have no role in acute pain.23 In cancer pain, the results of the 2 systematic reviews are unclear: Tateo inconsistently reported outcomes,24 and results of the meta-analysis by Lobos Urbina and Peña Duran did not meet statistical significance (although the effect estimate suggests benefit similar to our meta-analysis results).17
Nausea and vomiting
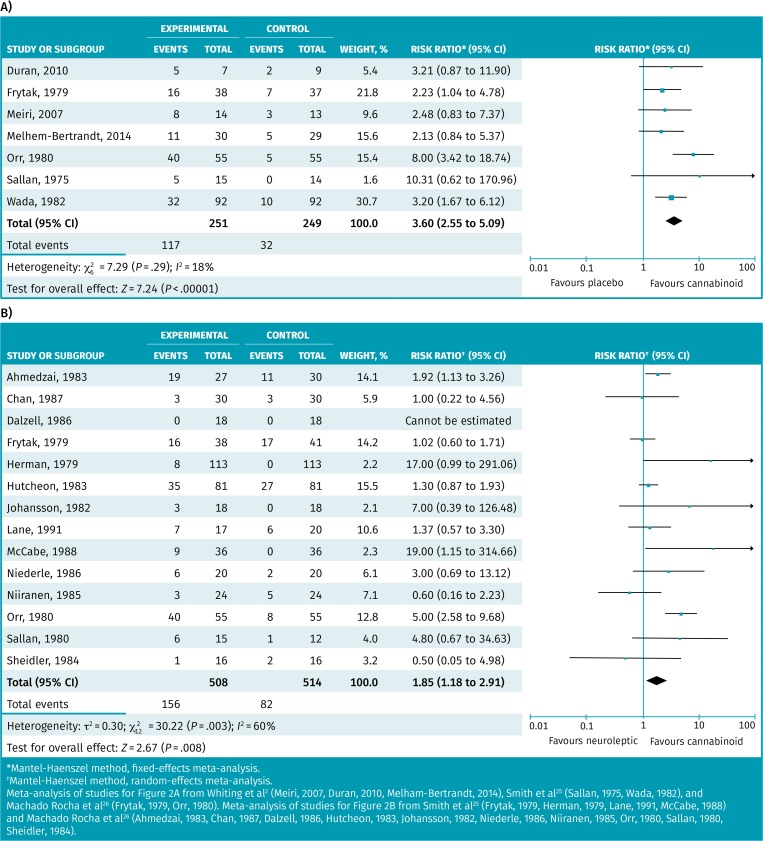
Table 3 provides the results from the 5 systematic reviews that performed meta-analyses examining medical cannabinoids versus placebo or other antiemetics for nausea and vomiting.2,19,25–27 Most of the data involve nausea and vomiting arising from chemotherapy, except the review by Mücke et al, which examined palliative patients.19 Results of the meta-analysis in palliative patients (reported in standard mean differences) did not reach statistical significance.19 The standard mean difference effect is difficult to interpret clinically but is likely trivial. Otherwise, the benefits seen in individual meta-analyses suggest or demonstrate statistically significant benefit. It should be noted that effect estimates were larger for patient preferences than for improvements in nausea and vomiting. For example, Smith et al reported an RR of 2.86 for the absence of nausea and vomiting but 4.82 for patient preference.25 The responder meta-analysis of 7 RCTs found approximately 47% of medical cannabinoid patients had control of nausea and vomiting compared with 13% taking placebo, with an RR of 3.60 (95% CI 2.55 to 5.09) and an NNT of 3 (Figure 2).2,25,26 The responder meta-analysis of 14 RCTs found approximately 31% of medical cannabinoid patients had control of nausea and vomiting compared with 16% taking neuroleptics, with an RR of 1.85 (95% CI 1.18 to 2.91) and an NNT of 7 (Figure 2).2,25,26
Table 3.
Effect estimates, event rates, and NNTs of meta-analyses examining medical cannabinoids versus placebo or other antiemetics for nausea and vomiting in chemotherapy (or in palliative patients for Mücke et al19)
| SYSTEMATIC REVIEW | OUTCOME (COMPARISON) | NO. OF RCTS (NO. OF PARTICIPANTS) | AUTHORS’ META-ANALYSIS RESULT (95% CI), HETEROGENEITY | META-ANALYSIS RE-ANALYZED (95% CI), HETEROGENEITY | CANNABINOID EVENT RATE, % | CONTROL EVENT RATE, % | NNT |
|---|---|---|---|---|---|---|---|
| Whiting et al, 20152 | Nausea and vomiting—complete response (vs placebo) | 3 (102) | OR = 3.82 (1.55 to 9.42), I2 = 0% | RR = 2.43 (1.30 to 4.52), I2 = 0% | 47 | 20 | 4 |
| Smith et al, 201525 | Absence of nausea and vomiting (vs placebo) | 3 (288) | RR = 2.86 (1.76 to 4.65), I2 = 0% | NA | 37 | 12 | 4 |
| Patient preference (vs placebo) | 2 (256) | RR = 4.82 (1.74 to 13.36), I2 = 69% | NA | 72 | 18 | 2 | |
| Absence of nausea and vomiting (vs prochlorperazine) | 4 (414) | RR = 2.00 (0.74 to 5.38), I2 = 60% | NA | 20 | 11 | NS | |
| Patient preference (vs other drugs) | 9 (799) | RR = 2.76 (1.88 to 4.03), I2 = 61% | NA | 63 | 19 | 3 | |
| Mücke et al, 201619 | Improvement in nausea and vomiting symptoms (vs placebo)* | 2 (307) | SMD = 0.20 (−0.03 to 0.44), I2 = 0% | NA | NA | NA | NA |
| Machado Rocha et al, 200826 | Nausea and vomiting within 1 d of chemotherapy (dronabinol vs placebo) | 2 (185) | RR = 0.47 (0.19 to 1.16), I2 = 91% | NA | 40 | 87 | NS |
| Nausea and vomiting within 1 d of chemotherapy (dronabinol vs neuroleptics) | 5 (325) | RR = 0.67 (0.47 to 0.96), I2 = 79% | NA | 52 | 80 | 4 | |
| Nausea and vomiting within 1 d of chemotherapy (nabilone vs neuroleptics) | 6 (277) | RR = 0.88 (0.72 to 1.08), I2 = 64% | NA | 75 | 85 | NS | |
| Tramèr et al, 200127 | Control of nausea (vs placebo) | 4 (231) | RelR = 1.21 (1.03 to 1.42), I2 = NR | NA | 70 | 57 | 8 |
| Control of vomiting (vs placebo) | 4 (231) | RelR = 1.84 (1.42 to 2.38), I2 = NR | NA | 66 | 36 | 4 | |
| Control of nausea (vs antiemetic) | 7 (422) | RelR = 1.38 (1.18 to 1.62), I2 = NR | NA | 59 | 43 | 7 | |
| Control of vomiting (vs antiemetic) | 6 (395) | RelR = 1.28 (1.08 to 1.51), I2 = NR | NA | 57 | 45 | 9 | |
| Patient preference (vs placebo) | 4 (404) | RelR = 5.67 (3.95 to 8.15), I2 = NR | NA | 76 | 13 | 2 | |
| Patient preference (vs antiemetic) | 14 (1212) | RelR = 2.39 (2.05 to 2.78), I2 = NR | NA | 61 | 26 | 3 | |
| All studies | Control of nausea and vomiting (vs placebo) | 7 (500) | NA | RR = 3.60 (2.55 to 5.09), I2 = 18% | 47 | 13 | 3 |
| Control of nausea and vomiting (vs antiemetics) | 14 (1022) | NA | RR = 1.85 (1.18 to 2.91), I2 = 60% | 31 | 16 | 7 |
NA—not applicable, NNT—number needed to treat, NR—not reported, NS—not significant, OR—odds ratio, RCT—randomized controlled trial, RelR—relative risk, RR—risk ratio, SMD—standardized mean difference.
This was for palliative patients (1 HIV RCT and 1 refractory cancer pain RCT).
Figure 2.
Responder meta-analysis of patients having control of their nausea and vomiting resulting from chemotherapy: A) Medical cannabinoid compared with placebo; B) medical cannabinoid compared with another antiemetic (neuroleptics).
*Mantel-Haenszel method, fixed-effects meta-analysis.
†Mantel-Haenszel method, random-effects meta-analysis.
Meta-analysis of studies for Figure 2A from Whiting et al2 (Meiri, 2007, Duran, 2010, Melham-Bertrandt, 2014), Smith et al25 (Sallan, 1975, Wada, 1982), and Machado Rocha et al26 (Frytak, 1979, Orr, 1980). Meta-analysis of studies for Figure 2B from Smith et al25 (Frytak, 1979, Herman, 1979, Lane, 1991, McCabe, 1988) and Machado Rocha et al26 (Ahmedzai, 1983, Chan, 1987, Dalzell, 1986, Hutcheon, 1983, Johansson, 1982, Niederle, 1986, Niiranen, 1985, Orr, 1980, Sallan, 1980, Sheidler, 1984).
The funnel plot was relatively symmetric, suggesting a low risk of publication bias (Figure A7*). The heterogeneity for medical cannabinoids versus neuroleptics was high (I2 = 60%), so we performed 2 sensitivity analyses on type of cannabinoid and study size (Figure A8*). Sensitivity analysis on duration was not performed, as studies collected data over 1 day. Analyses of type of cannabinoid and study size subgroups did not resolve the heterogeneity, and there were no differences between subgroups. There remains considerable heterogeneity that cannot be explored further via subgroup analyses. This heterogeneity includes (but is not limited to) patient type (age and sex), tumour type (blood, testicular, breast, colorectal, mixed, etc), chemotherapy regimens, and dosing of cannabinoids or neuroleptics.
Spasticity
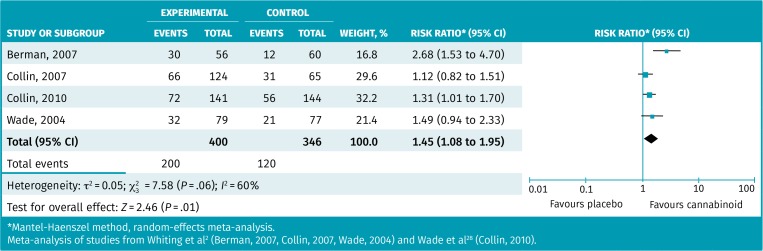
Table 4 provides the results from the 3 systematic reviews that performed meta-analyses examining medical cannabinoids versus placebo for spasticity.2,28,29 Two of 3 meta-analyses of scale score changes found statistically significant improvement in spasticity scale scores (possible range 0 to 10) varying from 0.31 to 0.76 more than for placebo.2,28 Our re-analysis of the largest meta-analysis found approximately 35% of medical cannabinoid patients achieved 30% or greater spasticity reductions compared with 25% of patients taking placebo, with an RR of 1.37 (95% CI 1.07 to 1.76) and an NNT of 10. The responder meta-analysis of 4 RCTs found that approximately 50% of patients taking medical cannabinoids reported a positive global impression of change compared with 35% of patients taking placebo, with an RR of 1.45 (95% CI 1.08 to 1.95) and an NNT of 7 (Figure 3).2,28 Most RCTs examined patients with multiple sclerosis, with only the smallest RCT in the meta-analysis examining patients with spinal cord injury.
Table 4.
Effect estimates, event rates, and NNTs of meta-analyses examining medical cannabinoids versus placebo for spasticity
| SYSTEMATIC REVIEW | OUTCOME | NO. OF RCTS (NO. OF PARTICIPANTS) | AUTHORS’ META-ANALYSIS RESULT (95% CI), HETEROGENEITY | META-ANALYSIS RE-ANALYZED (95% CI), HETEROGENEITY | CANNABINOID EVENT RATE | CONTROL EVENT RATE | NNT |
|---|---|---|---|---|---|---|---|
| Whiting et al, 20152 | ≥ 30% improvement in spasticity | 2 (519) | OR = 1.64 (0.95 to 2.83), I2 = 44% | RR = 1.43 (0.99 to 2.08), I2 = 35% | 35% | 24% | NS (approximately 10)* |
| Mean reduction on Ashworth spasticity scale | 5 (1244) | WMD = 0.12 (−0.01 to 0.24), I2 = 0% | NA | NR | NR | NA | |
| Change in spasticity (VAS-NRS scale) | 3 (698) | WMD = 0.76 (0.14 to 1.38), I2 = 73% | NA | NR | NR | NA | |
| Global impression of change | 3 (461) | OR = 2.09 (1.02 to 4.27),† I2 = 69% | RR = 1.57 (0.97 to 2.55), I2 = 73% | 49% | 32% | NS (approximately 6)* | |
| Wade et al, 201028 | ≥ 30% improvement in spasticity | 3 (652) | OR = 1.57 (1.11 to 2.23), I2 = NR | RR = 1.37 (1.07 to 1.76), I2 = 0% | 35% | 25% | 10 |
| Change in spasticity (VAS-NRS scale) | 3 (652) | Mean change in VAS-NRS of 0.31 (0.04 to 0.59), I2 = NR | NA | Started at about 6.2, decreased by 1.27 | Started at about 6.2, decreased by 0.95 | NA | |
| Global impression of change | 3 (605) | OR = 1.66 (1.19 to 2.30), I2 = NR | RR = 1.32 (1.10 to 1.58), I2 = NS | 51% | 38% | 8 | |
| Meza et al, 201729 | Spasticity (change in any scale) | 4 (1247) | SMD = 0.07 (−0.04 to 0.19), I2 = NR | NA | NR | NR | NS |
| All studies | Global impression of change | 4 (746) | NA | RR = 1.45 (1.08 to 1.95), I2 = 60% | 50% | 35% | 7 |
NA—not applicable, NNT—number needed to treat, NR—not reported, NRS—numerical rating scale, NS—not significant, OR—odds ratio, RCT—randomized controlled trial, RR—risk ratio, SMD—standardized mean difference, VAS—visual analogue scale, WMD—weighted mean difference.
Confidence intervals suggest that benefit is likely, so estimated NNT provided.
Whiting et al2 report an OR of 1.44 (95% CI 1.07 to 1.94), I2 = 0% but when we re-ran this meta-analysis we found the numbers presented in the table. We contacted the authors but did not hear back.
Figure 3.
Responder meta-analysis of patients with a positive global impression of change for spasticity with medical cannabinoids compared with placebo
*Mantel-Haenszel method, random-effects meta-analysis.
Meta-analysis of studies from Whiting et al2 (Berman, 2007, Collin, 2007, Wade, 2004) and Wade et al28 (Collin, 2010).
Adverse events
Table 5 provides the results of the 12 systematic reviews reporting adverse events of medical cannabinoids versus placebo.2,14,16–19,25,27-31 Results of all 5 meta-analyses of overall adverse events were statistically significant, demonstrating numbers needed to harm (NNH) of 5 to 8.2,17,28–30 In 1 of 4 meta-analyses, serious adverse events were statistically significant (odds ratio of 1.41, 95% CI 1.04 to 1.92); however, absolute events were not provided.2 Martin-Sanchez et al noted that psychosis, while rare, appeared to occur more frequently in RCTs enrolling cannabinoid-naïve patients compared with those enrolling patients with past cannabinoid use.14 In 5 of 8 meta-analyse, withdrawal due to adverse events was statistically significantly increased, with NNHs of 8 to 22.16,25,27,28,31 Rates of multiple specific adverse events were statistically significant, ranging from “feeling high” (NNH of 2 to 4) and sedation (NNH = 5) to disorientation and confusion (NNH = 15). In a meta-analysis of 6 RCTs (740 patients), Smith et al found that medical cannabinoids increased withdrawal due to adverse events compared with antiemetics (mostly prochlorperazine): RR of 3.16 (95% CI 1.26 to 7.93), 7% versus 1%, and an NNH of 17.25
Table 5.
Effect estimates, event rates, and NNHs of meta-analyses examining medical cannabinoids versus placebo for adverse events
| TYPE OF ADVERSE EVENT | STUDY | NO. OF RCTS (NO. OF PARTICIPANTS) | RELATIVE EFFECT ESTIMATE (95% CI), HETEROGENEITY | RE-ANALYSIS EFFECT ESTIMATE (95% CI), HETEROGENEITY | CANNABINOID EVENT RATE | PLACEBO EVENT RATE | NNH |
|---|---|---|---|---|---|---|---|
| Overall | Lobos Urbina and Peña Duran, 201617 | NR | OR = 3.03 (2.42 to 3.80), I2 = 44% | NA | 92% | 78% | 8 |
| Meza et al, 201729 | 4 (1025) | RR = 1.18 (1.10 to 1.27), I2 = NR | NA | NR | NR | NA | |
| Wade et al, 201028 | 3 (666) | NR | RR = 1.42 (1.27 to 1.59),* NA | 79% | 56% | 5 | |
| Wang et al, 200830 | 23 (2068) | Rate ratio of 1.86 (1.57 to 2.21), I2 = 87% | NA | 10.4 per patient-year | 6.9 per patient-year | NA | |
| Whiting et al, 20152 | 29 (3714) | OR = 3.03 (2.42 to 3.80), I2 = 31% | RR = 1.30 (1.21 to 1.39), I2 = 53% | 81% | 62% | 6 | |
| Serious | Mücke et al, 201619 | 6 (1031) | RR = 1.15 (0.88 to 1.49), I2 = NR | NA | 26% | 17% | NS |
| Petzke et al, 201616 | 11 (1568) | RD = 1% (−1% to 3%), I2 = NR | NA | 6.3% | 17% | NS | |
| Wang et al, 200830 | 23 (2068) | Rate ratio 1.04 (0.78 to 1.39), I2 = NR | NA | 0.37 per patient-year | 0.25 per patient-year | NA | |
| Whiting et al, 20152 | 34 (3248) | OR = 1.41 (1.04 to 1.92), I2 = 0% | NA | NR | NR | NA | |
| Withdrawal | Smith et al, 201525 | 2 (276) | RR = 6.85 (1.96 to 23.99), I2 = 0% | NA | 14% | 1% | 8 |
| Tramèr et al, 200127 | 19 (1111) | RelR = 4.67 (3.07 to 7.09), I2 = NR | NA | 11% | 2% | 11 | |
| Mücke et al, 201619 | 6 (1031) | RR = 1.20 (0.85 to 1.71), I2 = NR | NA | 15% | 11% | NS | |
| Petzke et al, 201616 | 11 (1574) | RD = 0.04 (0.01 to 0.07), I2 = 22% | RR = 2.03 (1.43 to 2.88), I2 = 0% | 11% | 5% | 19 | |
| Iskedjian et al, 200718 | 7 (508 observations) | NA | NA | 4.3% | 3.6% | NA | |
| Koppel et al, 201431 | 24 (2737) | NR | NA | 7% | 2% | 22 | |
| Wade et al, 201028 | 3 (666) | NR | RR = 3.04 (1.59 to 5.81),* NA | 11% | 4% | 14 | |
| Whiting et al, 20152 | 23 (2755) | OR = 2.94 (2.18 to 3.96), I2 = 2% | NA | NR | NR | NA | |
| Sedation | Smith et al, 201525 | 2 (139) | RR = 4.47 (0.35 to 57.81), I2 = 72% | NA | 59% | 25% | NS |
| Tramèr et al, 200127 | 15 (1373) | RelR = 1.66 (1.46 to 1.89), I2 = NR | NA | 50% | 30% | 5 | |
| Whiting et al, 20152 | 26 (3168) | OR = 2.83 (2.05 to 3.91) I2 = 27% | NA | NR | NR | NA | |
| “Feeling high” | Smith et al, 201525 | 3 (137) | RR = 31.10 (6.37 to 151.85), I2 = 0% | NA | 70% | 0% | 2 |
| Tramèr et al, 200127 | 8 (1032) | RelR = 10.6 (6.86 to 16.5), I2 = NR | NA | 35% | 3% | 4 | |
| Dysphoria | Smith et al, 201525 | 2 (96) | RR = 9.00 (0.50 to 160.59), I2 = NA | NA | 8% | 0% | NS |
| Tramèr et al, 200127 | 10 (690) | RelR = 8.06 (3.38 to −19.2), I2 = NR | NA | 13% | 0.3% | 8 | |
| Martin-Sanchez et al, 200914 | 4 (343) | OR = 2.56 (0.66 to 9.92), I2 = 0% | RR = 2.85 (0.74 to 10.93), I2 = 0% | 4% | 1% | NS | |
| Euphoria | Martin-Sanchez et al, 200914 | 4 (202) | OR = 4.11 (1.33 to 12.72), I2 = 0% | RR = 3.67 (1.02 to 13.13), I2 = 0% | 15% | 2% | 9 |
| Whiting et al, 20152 | 27 (2420) | OR = 4.08 (2.18 to 7.64), I2 = 49% | NA | NR | NR | NA | |
| Blurred vision or visual hallucination | Tramèr et al, 200127 | 10 (859) | RelR = 6.10 (2.41 to 15.4), I2 = NR | NA | 6% | 0% | 17 |
| Martin-Sanchez et al, 200914 | 5 (296) | OR = 8.34 (4.63 to 15.03), I2 = 0% | RR = 4.93 (2.54 to 9.58), I2 = 0% | 44% | 8% | 3 | |
| Whiting et al, 20152 | 10 (898) | OR = 2.19 (1.02 to 4.68), I2 = 0% | NA | NR | NR | NA | |
| Tinnitus | Martin-Sanchez et al, 200914 | 2 (152) | OR = 2.18 (0.93 to 5.11), I2 = 0% | RR = 2.11 (0.69 to 6.41), I2 = 0% | 16% | 7% | NS |
| Disorientation or confusion | Martin-Sanchez et al, 200914 | 5 (508) | OR = 3.24 (1.51 to 6.97), I2 = 0% | RR = 2.85 (1.25 to 6.47), I2 = 0% | 9% | 2% | 15 |
| Whiting et al, 20152 | 12 (1736) | OR = 5.41 (2.61 to 11.19), I2 = 0% | NA | NR | NR | NA | |
| Dissociation or acute psychosis | Tramèr et al, 200127 | 6 (571) | RelR = 8.58 (6.38 to 11.5), I2 = NR | NA | 5% | 0% | 20 |
| Martin-Sanchez et al, 200914 | 4 (277) | OR = 3.18 (0.89 to 11.33), I2 = 0% | RR = 3.96 (0.90 to 17.40), I2 = 0% | 5% | 0% | NS (20)† | |
| Whiting et al, 20152 | 2 (37) | OR = 1.09 (0.07 to 16.35), I2 = 25% | NA | NR | NR | NA | |
| Speech disorders | Martin-Sanchez et al, 200914 | 3 (200) | OR = 4.13 (2.08 to 8.20), I2 = 0% | RR = 2.91 (1.28 to 6.64), I2 = 0% | 32% | 7% | 5 |
| Ataxia or muscle twitching | Martin-Sanchez et al, 200914 | 6 (540) | OR = 3.84 (2.49 to 5.92), I2 = 39% | RR = 2.43 (1.61 to 3.67), I2 = 0% | 30% | 11% | 6 |
| Whiting et al, 20152 | 6 (920) | OR = 2.62 (1.12 to 6.13), I2 = 0% | NA | NR | NR | NA | |
| Numbness | Martin-Sanchez et al, 200914 | 4 (226) | OR = 3.98 (1.87 to 8.49), I2 = NR | RR = 3.47 (1.34 to 9.00), I2 = 0% | 21% | 4% | 6 |
| Impaired memory | Martin-Sanchez et al, 200914 | 2 (227) | OR = 3.45 (1.19 to 9.98), I2 = NR | RR = 3.41 (0.95 to 12.27), I2 = 0% | 11% | 2% | NS (12)† |
| Disturbance in attention or disconnected thoughts | Martin-Sanchez et al, 200914 | 5 (381) | OR = 5.12 (2.34 to 11.21), I2 = NR | RR = 4.29 (1.75 to 10.53), I2 = 0% | 17% | 2% | 7 |
| Dizziness | Mücke et al, 201619 | 4 (823) | RD = 3% (−2% to 8%), I2 = NR | NA | 14% | 11% | NS |
| Wade et al, 201028 | 3 (666) | NR | RR = 2.87 (2.02 to 4.08),* NA | 32% | 11% | 5 | |
| Whiting et al, 20152 | 41 (4243) | OR = 5.09 (4.10 to 6.32), I2 = 18% | NA | NR | NR | NA | |
| Nausea | Whiting et al, 20152 | 30 (3579) | OR = 2.08 (1.63 to 2.65), I2 = 0% | NA | NR | NR | NA |
| Diarrhea | Whiting et al, 20152 | 17 (2077) | OR = 1.65 (1.04 to 2.62), I2 = 15% | NA | NR | NR | NA |
| Fatigue | Whiting et al, 20152 | 20 (2171) | OR = 2.00 (1.54 to 2.62), I2 = 0% | NA | NR | NR | NA |
| Central nervous system | Petzke et al, 201616 | 9 (1304) | RD = 36% (14% to 59%), I2 = NR | NA | 60% | 27% | 4 |
| Psychiatric | Mücke et al, 201619 | 5 (763) | RD = 1% (−2% to 4%), I2 = NR | NA | 4% | 3% | NS |
| Petzke et al, 201616 | 9 (1304) | RD = 11% (6% to 16%), I2 = NR | NA | 17% | 5% | 9 | |
| Wade et al, 201028 | 3 (666) | NR | RR = 3.29 (1.98 to 5.48),* NA | 19% | 6% | 8 | |
| Dry mouth | Whiting et al, 20152 | 36 (4181) | OR = 3.50 (2.58 to 4.75), I2 = 28% | NA | NR | NR | NA |
| Depression | Whiting et al, 20152 | 15 (2353) | OR = 1.32 (0.87 to 2.01), I2 = 0% | NA | NR | NR | NA |
| Anxiety | Whiting et al, 20152 | 12 (1242) | OR = 1.98 (0.73 to 5.35), I2 = 54% | NA | NR | NR | NA |
| Vomiting | Whiting et al, 20152 | 17 (2191) | OR = 1.67 (1.13 to 2.47), I2 = 0% | NA | NR | NR | NA |
| Asthenia or weakness | Whiting et al, 20152 | 15 (1717) | OR = 2.03 (1.35 to 3.06), I2 = 0% | NA | NR | NR | NA |
| Dyspnea | Whiting et al, 20152 | 4 (375) | OR = 0.83 (0.26 to 2.63), I2 = 0% | NA | NR | NR | NA |
| Hypotension | Tramèr et al, 200127 | 13 (982) | RelR = 2.23 (1.75 to 2.83), I2 = NR | NA | 25% | 11% | 8 |
NA—not applicable, NNH—number needed to harm, NR—not reported, NS—not significant, OR—odds ratio, RCT—randomized controlled trial, RD—risk difference, RelR—relative risk, RR—risk ratio, RCT—randomized controlled trial.
Preplanned pooling of 3 studies. Combined data available, so RR was calculated without formal meta-analysis.
Confidence intervals suggest that benefit is likely, so estimated NNH provided.
GRADE evaluation
Multiple issues affecting the validity of this research are detailed in Table A9.* Using the GRADE approach,10 risk of bias was noted for RCT size, RCT duration, quality of included RCTs, lack of blinding, inconsistent RCT inclusion within systematic reviews,44 and inconsistent outcome reporting. Concerns regarding indirectness were noted for frequent use of co-analgesia (meaning medical cannabinoids could not be considered first line) and enrolment (as previous cannabinoid users were frequently enrolled in the RCTs). For example, subgroup analysis of medical cannabinoids versus antiemetics found the effect on nausea and vomiting was smaller in cannabis-naïve patients than in patients with previous use of cannabinoids.25 Concerns regarding inconsistency were noted owing to the heterogeneity of the RCT results. Dose effects were identified in some systematic reviews15 but not in others.14 The highest risk of bias was noted for RCTs of inhaled medical cannabinoids. For example, in the largest systematic review of pain,2 the median number of patient-days (a combination of duration and sample size) was 115 for RCTs of smoked cannabis compared with 1470 patient-days for oral formulations or buccal spray. Table 6 provides the summary of key findings with GRADE evaluation results.2,18,25,28
Table 6.
Summary of findings and GRADE recommendations
| OUTCOMES | COMPARATOR | RELATIVE EFFECT (95% CI) | CERTAINTY OF EVIDENCE (GRADE) |
|---|---|---|---|
| Pain | |||
| • ≥ 30% reduction of pain | Placebo | RR = 1.37 (1.14 to 1.64) |
|
| • Change in pain scale2 | Placebo | WMD = 0.5 (0.11 to 0.80)* | Overall: Very low owing to serious risk of bias, serious inconsistency, serious indirectness, and serious imprecision |
| Nausea and vomiting | |||
| • Control of nausea and vomiting | Placebo | RR = 3.60 (2.55 to 5.09) | Moderate owing to serious risk of bias and serious imprecision, but magnitude had large effect |
| Antiemetic | RR = 1.85 (1.18 to 2.91) | Low owing to serious risk of bias and serious inconsistency | |
| • Patient preference25 | Placebo | RR = 4.82 (1.74 to 13.36) | Low owing to serious risk of bias, serious inconsistency, and serious imprecision, but magnitude had large effect† |
| Antiemetic | RR = 2.76 (1.88 to 4.03) | Low owing to serious risk of bias, serious inconsistency, and serious imprecision, but magnitude had large effect† | |
| Spasticity | |||
| • ≥ 30% improvement in spasticity28 | Placebo | RR = 1.37 (1.07 to 1.76) | Low owing to serious risk of bias and serious publication bias |
| • Change in spasticity scale2,28 | WMD = 0.31 or 0.76 | Very low owing to serious risk of bias, serious inconsistency, and serious imprecision | |
| • Global impression of change | RR = 1.45 (1.08 to 1.95) | Low owing to serious risk of bias and serious inconsistency | |
| Adverse events | |||
| • Withdrawal owing to adverse events | Placebo | NNH = 8 to 22 | High owing to serious risk of bias and serious imprecision, but magnitude had large effect and plausible confounding had large effect |
GRADE—Grading of Recommendations Assessment, Development and Evaluation, NNH—number needed to harm, RR—risk ratio, WMD—weighted mean difference.
Whiting et al2 article selected because it was more general pain rather than multiple sclerosis pain as examined in the study by Iskedjian et al.18
Patient preference is inconsistent with effect on nausea and vomiting and therefore might reflect more than control of nausea and vomiting but also the euphoria or “high” received from cannabinoids.
DISCUSSION
The evidence indicates the most consistent effects of medical cannabinoids are adverse events. A variety of adverse events have a greater magnitude of effect than the potential benefits for the conditions targeted. Not only are overall adverse events far more common, so are withdrawals due to adverse events, even when compared with other active interventions. It is important to recognize that the rate of adverse events is likely underreported, as many studies enrolled cannabis users. Experienced cannabis users have a reduced risk of adverse events, as they are preselected as resistant, have developed tolerance, or perhaps even appreciate a number of the adverse events (like “feeling high,” euphoria, or sedation). Therefore, the total number and severity of adverse events is almost certainly greater than reported, particularly for those naïve to cannabinoids. For example, rare serious events like psychosis appear to be more common among naïve users,14 but confirmation will depend on much larger trials, enrolling cannabinoid-naïve patients and following them for adequate time. Other rare events that our study would likely not identify include cannabinoid hyperemesis syndrome (or cyclic vomiting)45 or amotivational syndromes.46 Research is still in its infancy in providing clarity for these conditions and their link to cannabinoid use.
Within spasticity, the benefits of medical cannabinoids likely approach clinically meaningful improvement. Change in visual analogue scale scores could be up to 0.8 (out of 10) more than placebo, with 35% of patients attaining a 30% or more improvement compared with 25% of patients taking placebo. Within nausea and vomiting, the benefits of medical cannabinoids constitute clinically meaningful improvement, with 47% avoiding nausea or vomiting within the day after chemotherapy compared with 13% taking placebo. Two areas of context are needed for these findings. First, spasticity research is primarily done in multiple sclerosis, with a small amount of positive research in paraplegic patients, and nausea and vomiting findings apply only to patients receiving chemotherapy. Second, for both spasticity and nausea and vomiting, improvements in patient preference are consistently greater than the actual effects on the conditions, such as attaining a 30% or greater reduction in spasticity or the absence of nausea and vomiting. One of the potential causes for this discrepancy could include the adverse events that some might find desirable like “feeling high,” euphoria, or even sedation.
For pain, the benefits of medical cannabinoids border on clinically meaningful. The changes on scales of 0 to 10 were improvements of approximately 0.4 to 0.8 points more than placebo, with only the higher end approaching clinical relevance. Results from a number of “30% or more reduction in pain” meta-analyses did not reach statistical significance, but those that did had widely variant magnitudes of effect. Our sensitivity analysis revealed that the type of cannabinoid studied did not lead to statistically significant differences in outcomes, but the NNT for inhaled cannabinoids was 6 compared with 16 for buccal cannabinoids. More important, study size and study duration had statistically significant influences on study results. Small studies had an NNT of 6 and shorter studies had NNTs of 4 to 7, while results from large or longer-duration studies were not statistically significant. Given that larger and longer studies are less likely to find spurious results, these findings draw into question if medical cannabinoids have a reliable effect on pain. As all inhaled cannabinoid studies are smaller and shorter duration, these effects are also likely unreliable.
Prescribing cannabinoids clearly has a number of challenges. On the one hand, experienced cannabinoid users might seek medical cannabinoids for inappropriate reasons like legalizing or attaining insurance coverage for their recreational use. On the other hand, in patients with no past cannabis use who could potentially meet reasonable criteria for a trial of therapy, the possible harms will likely be greater than present evidence suggests. To help put medical cannabinoids in context, it is important to reflect on other drugs that can provide therapeutic benefits but that also have abuse potential owing to psychotropic effects that some might find desirable. Opioids have become a national challenge, with national efforts under way to improve prescribing,47 and need no further discussion here. Alcohol is not a recognized agent for pain management, but preliminary research has begun. Meta-analysis of results from 9 studies of healthy individuals subjected to painful stimuli found that alcohol consumption statistically significantly reduced pain by 1.25 on a 0 to 10 scale, with a 5.3 pain level without alcohol and a 4.05 rating with a blood alcohol level of 0.08%.48 While these data have many validity issues and are not directly comparable to the cannabinoid research, it does suggest that pain reduction with alcohol is equivalent to, or even better than, with cannabinoids. We are by no means advocating that alcohol should be considered a reasonable treatment, and question even the place of research in the area. However, the use of cannabinoids for medical treatment requires some level of reflection before application.
Limitations
Many of the weaknesses of the included studies were identified previously in the GRADE evaluation presented in the results section. Those are likely the greatest weaknesses of this study. With our meta-analyses, like others, combining weak studies does not strengthen the quality of the original research, and this needs to be considered when interpreting the results. We did not pull all individual RCTs identified in the included systematic reviews and therefore might have missed elements of the RCTs, particularly if the details were not accurately recorded in the included systematic reviews. Because our risk-of-bias evaluation was on systematic reviews, we could not perform a sensitivity analysis based on the quality of included RCTs. Last, we report only limited results from descriptive systematic reviews. Given that RCT authors frequently selectively report outcomes49 and systematic review authors might in turn also selectively report those outcomes, we believed that any reporting of individual RCT outcomes would only compound these potential biases. However, in doing so we might have missed potentially relevant content. For example, any oral cannabinoids (nabilone or dronabinol) seem to be rarely studied for pain and did not appear in our or the other responder meta-analysis. While descriptive systematic reviews report a few RCTs of nabilone and dronabinol for pain, these were at high risk of bias and any selection of results for this report would likely be difficult to interpret.
Conclusion
There is reasonable evidence that cannabinoids improve nausea and vomiting after chemotherapy. They might improve spasticity (primarily in multiple sclerosis). There is some uncertainty about whether cannabinoids improve pain, but if they do, it is neuropathic pain and the benefit is likely small. Adverse effects are very common, meaning that benefits would need to be considerable to warrant trials of therapy. The data from this study were used to inform primary care clinical practice guideline recommendations (page 111).50
Editor’s key points
▸ Although cannabinoids have been promoted for an array of medical conditions, the evidence base is challenged by bias and a lack of high-level research. Two large evidence synopses suggested that only 3 conditions have an adequate volume of evidence to inform prescribing recommendations: chronic pain, nausea and vomiting after chemotherapy, and spasticity.
▸ The authors conducted a systematic review of systematic reviews focusing on these conditions, for which medical cannabinoids have the best evidence base and the highest likelihood of having medical advantages, and on adverse events.
▸ These data were used to inform the development of a simplified primary care medical cannabinoid prescribing guideline.
Points de repère du rédacteur
▸ Bien qu’on ait préconisé l’usage du cannabis pour différentes conditions médicales, les preuves sur lesquelles on s’est appuyé sont souvent teintés de partialité et ne reposent pas sur des études de qualité supérieure. Deux vastes synthèses des données probantes donnent à penser qu’il n’y a que 3 problèmes de santé pour lesquels il existe suffisamment de données pour conclure qu’on peut utiliser le cannabis : la douleur chronique, les nausées et les vomissements causés par la chimiothérapie, et la spasticité.
▸ Les auteurs ont effectué une revue systématique de revues systématiques portant sur les conditions pour lesquelles on possède les meilleures données probantes et sur les possibilités que le cannabis médical puisse être avantageux dans ces cas, de même que sur les effets indésirables de cette substance.
▸ Les données tirées de notre étude ont servi à élaborer une directive simplifiée concernant la prescription de cannabis à des fins médicales dans un milieu de soins primaires.
Footnotes
Contributors
All authors made substantial contributions to the design of the study, conducting the study, and writing and editing the manuscript.
Competing interests
No author has a conflict of interest or disclosure. The project received no external funding.
The online supplement, including Figures A1, A5, A6, A7, and A8 and Tables A2, A3, A4 and A9, is available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.National Academies of Sciences, Engineering, and Medicine . The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 2.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. doi: 10.1001/jama.2015.6358. Errata in: JAMA 2016;315(14):1522, JAMA 2015;314(21):2308, JAMA 2015;314(5):520, JAMA 2015;314(8):837. [DOI] [PubMed] [Google Scholar]
- 3.Montané E, Duran M, Capellà D, Figueras A. Scientific drug information in newspapers: sensationalism and low quality. The example of therapeutic use of cannabinoids. Eur J Clin Pharmacol. 2005;61(5–6):475–7. doi: 10.1007/s00228-005-0916-7. Epub 2005 Jun 28. [DOI] [PubMed] [Google Scholar]
- 4.Lake S, Kerr T, Montaner J. Prescribing medical cannabis in Canada: are we being too cautious? Can J Public Health. 2015;106(5):e328–30. doi: 10.17269/cjph.106.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel L. Cautious first guidance for prescribing pot. CMAJ. 2014;186(16):E595. doi: 10.1503/cmaj.109-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Amant H, Ware MA, Julien N, Lacasse A. Prevalence and determinants of cannabinoid prescription for the management of chronic noncancer pain: a postal survey of physicians in the Abitibi-Témiscamingue region of Quebec. CMAJ Open. 2015;3(2):E251–7. doi: 10.9778/cmajo.20140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziemianski D, Capler R, Tekanoff R, Lacasse A, Luconi F, Ware MA. Cannabis in medicine: a national educational needs assessment among Canadian physicians. BMC Med Educ. 2015;15:52. doi: 10.1186/s12909-015-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.College of Family Physicians of Canada . Authorizing dried cannabis for chronic pain or anxiety. Preliminary guidance from the College of Family Physicians of Canada. Mississauga, ON: College of Family Physicians of Canada; 2014. Available from: www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed 2017 Apr 1. [Google Scholar]
- 9.Health Canada . Consumer information—cannabis. (Marihuana, marijuana) Ottawa, ON: Health Canada; 2016. Available from: www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/marihuana/info/cons-eng.pdf. Accessed 2017 Apr 1. [Google Scholar]
- 10.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Sanchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10(8):1353–68. doi: 10.1111/j.1526-4637.2009.00703.x. Epub 2009 Sep 1. [DOI] [PubMed] [Google Scholar]
- 15.Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221–32. doi: 10.1016/j.jpain.2015.07.009. Epub 2015 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids in neuropathic pain syndromes [article in German] Schmerz. 2016;30(1):62–88. doi: 10.1007/s00482-015-0089-y. [DOI] [PubMed] [Google Scholar]
- 17.Lobos Urbina D, Peña Duran J. Are cannabinoids effective for treatment of pain in patients with active cancer? Medwave. 2016;16(Suppl 3):e6539. doi: 10.5867/medwave.2016.6539. [DOI] [PubMed] [Google Scholar]
- 18.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23(1):17–24. doi: 10.1185/030079906x158066. [DOI] [PubMed] [Google Scholar]
- 19.Mücke M, Carter C, Cuhls H, Prüẞ M, Radbruch L, Häuser W. Cannabinoids in palliative care: systematic review and meta-analysis of efficacy, tolerability and safety [article in German] Schmerz. 2016;30(1):25–36. doi: 10.1007/s00482-015-0085-2. [DOI] [PubMed] [Google Scholar]
- 20.Fitzcharles MA, Baerwald C, Ablin J, Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz. 2016;30(1):47–61. doi: 10.1007/s00482-015-0084-3. [DOI] [PubMed] [Google Scholar]
- 21.Fitzcharles MA, Ste-Marie PA, Häuser W, Clauw DJ, Jamal S, Karsh J, et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthrit Care Res. 2016;68(5):681–8. doi: 10.1002/acr.22727. [DOI] [PubMed] [Google Scholar]
- 22.Walitt B, Klose P, Fitzcharles MA, Phillips T, Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;(7):CD011694. doi: 10.1002/14651858.CD011694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaseth Scand. 2017;61(3):268–80. doi: 10.1111/aas.12851. Epub 2017 Jan 16. [DOI] [PubMed] [Google Scholar]
- 24.Tateo S. State of the evidence: cannabinoids and cancer pain—a systematic review. J Am Assoc Nurse Pract. 2017;29(2):94–103. doi: 10.1002/2327-6924.12422. Epub 2016 Nov 10. [DOI] [PubMed] [Google Scholar]
- 25.Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464. doi: 10.1002/14651858.CD009464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado Rocha FC, Stéfano SC, De Cássia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl) 2008;17(5):431–43. doi: 10.1111/j.1365-2354.2008.00917.x. Epub 2008 Jul 9. [DOI] [PubMed] [Google Scholar]
- 27.Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010;16(6):707–14. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- 29.Meza R, Peña J, Garcia K, Corsi O, Rada G. Are cannabinoids effective in multiple sclerosis? Medwave. 2017;17(Suppl 1):e6865. doi: 10.5867/medwave.2017.6865. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178(13):1669–78. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–63. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- 33.Canadian Agency for Drugs and Technologies in Health . Cannabinoids as co-analgesics: review of clinical effectiveness. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2010. [Google Scholar]
- 34.Canadian Agency for Drugs and Technologies in Health . Cannabinoids for the management of neuropathic pain: review of clinical effectiveness. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2010. [Google Scholar]
- 35.Canadian Agency for Drugs and Technologies in Health . Nabilone for chronic pain management: a review of clinical effectiveness, safety, and guidelines. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2011. [Google Scholar]
- 36.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323(7303):13–6. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotter J. Efficacy of crude marijuana and synthetic delta-9-tetrahydrocannabinol as treatment for chemotherapy-induced nausea and vomiting: a systematic literature review. Oncol Nurs Forum. 2009;36(3):345–52. doi: 10.1188/09.ONF.345-352. [DOI] [PubMed] [Google Scholar]
- 38.Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain. Systematic review of randomized controlled trials. Can Fam Physician. 2015;61:e372–81. Available from: www.cfp.ca/content/cfp/61/8/e372.full.pdf. Accessed 2018 Jan 5. [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep. 2015;19(10):50. doi: 10.1007/s11916-015-0524-x. [DOI] [PubMed] [Google Scholar]
- 40.Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009;9:59. doi: 10.1186/1471-2377-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–44. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharm. 2015;10(2):293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- 43.Tsang CC, Giudice MG. Nabilone for the management of pain. Pharmacotherapy. 2016;36(3):273–86. doi: 10.1002/phar.1709. Epub 2016 Feb 29. [DOI] [PubMed] [Google Scholar]
- 44.Tafelski S, Häuser W, Schäfer M. Efficacy, tolerability, and safety of cannabinoids for chemotherapy-induced nausea and vomiting—a systematic review of systematic reviews. Schmerz. 2016;30(1):14–24. doi: 10.1007/s00482-015-0092-3. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J Med Toxicol. 2017;13(1):71–87. doi: 10.1007/s13181-016-0595-z. Epub 2016 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawn W, Freeman TP, Pope RA, Joye A, Harvey L, Hindocha C, et al. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology (Berl) 2016 Oct;233(19–20):3537–52. doi: 10.1007/s00213-016-4383-x. Epub 2016 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659–66. doi: 10.1503/cmaj.170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B. Analgesic effects of alcohol: a systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain. 2017;18(5):499–510. doi: 10.1016/j.jpain.2016.11.009. Epub 2016 Dec 2. [DOI] [PubMed] [Google Scholar]
- 49.Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias Group Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PLoS One. 2013;8(7):e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allan GM, Ramji J, Perry D, Ton J, Beahm NP, Crisp N, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician. 2018;64:111–20. (Eng), e64–75 (Fr). [PMC free article] [PubMed] [Google Scholar]