SUMMARY
Toxoplasma gondii is an obligate intracellular protozoan parasite of the phylum Apicomplexa, and toxoplasmosis is an important disease of both humans and economically important animals. With a limited array of drugs available there is a need to identify new therapeutic compounds. Aureobasidin A (AbA) is an antifungal that targets the essential inositol phosphorylceramide (IPC, sphingolipid) synthase in pathogenic fungi. This natural cyclic depsipeptide also inhibits Toxoplasma proliforation, with the protozoan IPC synthase orthologue proposed as the target. The data presented here show that neither AbA nor an analogue (Compound 20), target the protozoan IPC synthase orthologue or total parasite sphingolipid synthesis. However, further analyses confirm that AbA exhibits significant activity against the proliferative tachyzoite form of Toxoplasma, and Compound 20, whilst effective, has reduced efficacy. This difference was more evident on analyses of the direct effect of these compounds against isolated Toxoplasma, indicating that AbA is rapidly microbicidal. Importantly, the possibility of targeting the encysted, bradyzoite, form of the parasite with AbA and Compound 20 was demonstrated, indicating that this class of compounds may provide the basis for the first effective treatment for chronic toxoplasmosis.
Key words: Toxoplasma, sphingolipid biosynthesis, Aureobasidin A, bradyzoite
INTRODUCTION
Aureobasidin A (AbA; Fig. 1) is a cyclic depsipeptide antifungal antibiotic isolated from the fungus Aureobasidium pullulans R106 (Ikai et al. 1991; Takesako et al. 1991). Resistance in Saccharomycin cerevisiae was found to be conferred by dominant negative mutations in the Aureobasidin resistance (AUR1) gene (Heidler and Radding, 1995). Subsequently, AUR1 was identified as encoding the essential inositol phosphorylceramide (IPC) synthase activity in fungi (Nagiec et al. 1997). AbA has been shown to be an irreversible inhibitor of the S. cerevisiae IPC synthase, acting in a time dependant manner (Aeed et al. 2009), with the toxic effects associated with both a build up of the bioactive substrate ceramide and the deprivation of IPC (Cerantola et al. 2009). Recent efforts have utilized a semi-synthetic approach to generate analogues of AbA which demonstrate improved activity against some pathogenic fungal species, for example Aspirgillus fumigatus (Wuts et al. 2015).
Fig. 1.
The structures of the cyclic depsipeptide compounds Aureobasidin A and its analogue Compound 20 (Wuts et al. 2015).
IPC is an essential sphingolipid found in fungi, plants and some protozoa (Young et al. 2012). In contrast, mammals lack IPC and instead synthesize sphingomyelin (SM) as their major sphingolipid species using SM synthase (Huitema et al. 2004). Complex sphingolipids, such as IPC and SM, are major components of the outer leaflet of eukaryotic plasma membranes that are thought to be involved, together with sterols, in the formation of micro-domains known as lipid rafts. These rafts have been proposed to function in a diverse array of processes from the polarised trafficking of lipid-modified proteins, to the assembly and activation of signal transduction complexes (Simons and Ikonen, 1997). The non-mammalian nature of IPC synthase makes it an attractive drug target, and it has been validated as such in both the pathogenic fungi and the kinetoplastid protozoa (Georgopapadakou, 2000; Hanada, 2005; Mina et al. 2009, 2010).
Toxoplasma gondii is an obligate, intracellular protozoan parasite, able to invade and colonize a wide variety of nucleated vertebrate cells. It is a member of the Apicomplexa, a diverse phylum including important pathogens of domestic animals and humans such as Eimeria (the etiological agent of coccidiosis in poultry), Theileria (East Coast Fever in Cattle), Cryptosporidium (diarrhoea) and Plasmodium (malaria). In common with other apicomplexans Toxoplasma has a complex lifecycle, involving a definitive, feline, host; and both rapidly proliferative, tachyzoite forms (all tissues in acute disease) and slowly dividing, bradyzoite forms (muscle and brain tissue cysts in chronic disease) (Dubey, 1977). Toxoplasma is an opportunistic pathogen and is a significant cause of disease (toxoplasmosis) in the immunocompromised: particularly organ transplant recipients, those receiving anti-cancer chemotherapy and AIDS patients (Chowdhury, 1986). In utero toxoplasmosis is also a significant cause of congenital defects in humans (Chowdhury, 1986) and spontaneous abortion in economically important domestic animals (Dubey, 1977). The diseases listed above are associated with rapidly dividing, tachyzoite Toxoplasma, either directly acquired or the result of the reactivation of a chronic infection. However, in addition, bradyzoite, chronic, toxoplasmosis has been associated with psychiatric disorders, including schizophrenia (Webster et al. 2013). The drugs available for acute toxoplasmosis (tachyzoite stage) have various problems with efficacy and safety, furthermore no treatments are available for chronic disease (encysted bradyzoite stage) therefore new therapies are urgently required (Antczak et al. 2016).
The synthesis of IPC by Toxoplasma was first reported on the basis of metabolic labelling experiments (Sonda et al. 2005) and subsequently confirmed using directed mass spectrometry (Pratt et al. 2013). In addition, inhibition of parasite IPC synthesis by AbA was indicated and the tractability of this natural compound as a new lead proposed (Sonda et al. 2005; Coppens, 2013). Utilising AbA and the availability of a well characterized orthologue with improved pharmacokinetic properties, Compound 20 (Fig. 1, modified with a pyridyl group at AbA position 4; Wuts et al. 2015), here we examine the effect of these compounds on the Toxoplasma AUR1 orthologue (TgSLS; (Pratt et al. 2013) and total sphingolipid biosynthesis; and on the proliferation of both tachyzoite and bradyzoite form parasites. The results demonstrate that whilst both compounds inhibit the proliferation of Toxoplasma, neither inhibits TgSLS nor total sphingolipid biosynthesis as previously proposed (Sonda et al. 2005; Coppens, 2013). However, despite uncertainty regarding the mode of action, the ability of this class of cyclic depsipeptides to clear encysted bradyzoite-like form Toxoplasma from infected tissue culture cells marks them as a possibly unique therapy for chronic toxoplasmosis.
MATERIALS AND METHODS
Cell culture
Toxoplasma gondii (strains RH-TATi-1 (Meissner et al. 2002), RH-HX-KO-YFP2-DHFR (Gubbels et al. 2003) and Pru-GRA2-GFP-DHFR (Kim et al. 2007) were maintained in Vero, Human Foreskin Fibroblast (HFF) or Chinese Hamster Ovary (CHO) cells grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS) at 37 °C and 5% CO2. Type II Toxoplasma (Pru strain) tachyzoites were differentiated to the bradyzoite-like form in HFF cells via an alkaline shift to pH8 as previously described (Soete et al. 1994).
Metabolic labelling
Saccharomoyces cerevisiae and Vero cells were labelled with 5 µm of NBD C6-ceramide complexed with Bovine Serum Albumin (BSA) (Invitrogen) for use as controls as previously (Denny et al. 2006). Toxoplasma tachyzoites were separated from host cell material by filtration through 3 and 5 mm polycarbonate filters (Millipore) after disruption by passage through a narrow gauge needle. Released parasites were then isolated by centrifugation at 1430 g for 15 min at room temperature, washed and resuspended in serum-free DMEM (Invitrogen) at 107 mL−1 and incubated for 1 h at 37 °C before the addition of NBD C6-ceramide complexed with BSA to 5 µm, and a further 1 h at 37 °C. For the inhibitor studies, AbA or Compound 20 were added to isolated Toxoplasma at 10 µg mL−1 and incubated at 37 °C for 1, 4 or 7 h, before the addition of NBD C6-ceramide complexed with BSA to 5 µm and a further incubation at 37 °C for 1 h. Lipids were extracted and analysed by HPTLC as previously described (Mina et al. 2009).
Toxoplasma susceptibility assay
HFF cells were seeded at 104 cells per well in 96 well microtitre plates (Nunc). After 18 h at 37 °C isolated Toxoplasma RH-HX-KO-YFP2-DHFR (Gubbels et al. 2003) were inoculated into the host cells at 6250 parasites per well. Following a further 20 h incubation compounds were added at the appropriate concentrations. In an additional experiment, isolated tachyzoite parasites were pre-treated with compounds for 2 and 8 h, then washed, prior to infection of HFF cells. For bradyzoite assays, the Toxoplasma Pru-GRA2-GFP-DHFR (Kim et al. 2007) tachyzoites were added at the same concentration but then transformed as described (Soete et al. 1994) before the addition of the compounds. Plates were washed after 2 or 8 h, or not, as described in text. The plates were read using a Biotek Synergy H4 plate reader (Ex 510 nm; Em 540 nm) after 6 or 3 days, respectively.
Yeast susceptibility assay
YPH499-HIS-GAL-AUR1 (a yeast strain in which expression of the essential IPC synthase, AUR1p, is under the control of a galactose promoter) complemented with TgSLS or AUR1 (Denny et al. 2006; Pratt et al. 2013) were assayed for susceptibility to AbA and Compound 20. The transgenic yeast strains were maintained on SD -HIS -URA agar (0·17% Bacto yeast nitrogen base, 0·5% ammonium sulphate, 2% glucose, containing the appropriate nutritional supplements) at 30 °C. To analyse susceptibility to AbA and Compound 20 plates containing 5 or 10 µg mL−1 of the compound were prepared and 10 µL of an aqueous suspension of yeast spotted onto the surface before incubation at 30 °C.
Transcript analyses
For the mRNA analyses, total RNA from equivalent numbers of CHO cells infected for 72 h with Toxoplasma RH-TATi parasites, or non-infected, was extracted using the RNeasy kit (Qiagen) according to the manufacturer's protocol. Following DNase treatment (RQ1, Promega) cDNA was synthesized using the ImProm-II Reverse Transcription System (Promega) according to manufacturer's protocol. Quantitative PCR (qPCR) was performed in a RotorGene® RG3000 (Corbett Research) using SYBR Green Jump-Start Taq Ready Mix (Sigma Aldrich) according to the manufacturer's instructions. The hamster, Cricetulus griseus, CgLcb2 (encoding subunit 2 of SPT) was amplified using the primer pair – 5′CAGACAACTTTGTTTTCGG3′ and 5′GGGTGGCATTGTAGGGC3′. The reference gene, CgβTub, was amplified using the primer pair – 5′TAAAACGACGGCCAGTGAGC3′ and 5′TCTCCTGGCGAGTGCTGC3′. The qPCR was carried out in triplicate on 3 replicates with an annealing temperature 55°C for CgLcb2 and CgβTub.
RESULTS
Comparing the effect of AbA and its analogue Compound 20 on the proliferation of the Toxoplasma tachyzoite form
AbA has previously been shown to inhibit the proliferation of the rapidly dividing, tachyzoite form of Toxoplasma. The effective concentration of compound reducing proliferation by 50% (ED50) was calculated as 0·3 µg mL−1 by cell counting 48 h post infection and 46 h post addition of AbA (Sonda et al. 2005). In order to gain a more rapid and robust dataset to facilitate comparative analyses of the efficacy of AbA and Compound 20 we utilised the availability of the yellow fluorescent protein labelled Toxoplasma, RH-strain (Gubbels et al. 2003). Gubbels et al. demonstrated the tractability of this system by comparison with β-galactosidase producing parasites. Following validation and parameter setting (data not shown), HFF cells were plated onto 96-well plates and infected with 6250 Toxoplasma per well as described in the section Materials and Methods. After 20 h the compounds were added and, without washing, the plate incubated for 144 h (6 days) before fluorescent readings were taken. Following data analyses the ED50 was calculated as described (Fig. 2). As can been seen both AbA and Compound 20 showed activity against Toxoplasma RH tachyzoites. However, the parent compound (ED50 of 0·75, 95% CI 0·60 to 0·93 µg mL−1) was slightly more efficacious than its derivative (ED50 of 1·49, 95% CI 1·20 to 1·85 µg mL−1). This differential activity was even more evident on further analyses. Previously, using an indirect assay (vacuole formation), it has been indicated that the efficacy of AbA against Toxoplasma is partially reversible after 24, but not 48 h, exposure (Sonda et al. 2005). To further analyse the reversibility of the efficacy of cyclic depsipeptides, the infected HFF cells were washed following 2 and 8 h of compound treatment and proliferation then followed for 6 days as previously (Fig. 2). In keeping with Sonda et al. (2005) efficacy was partially reversible, but Toxoplasma were clearly susceptible to AbA in an 8 h treatment (ED50 of 4·82, 95% CI 3·73 to 6·22 µg mL−1), and even 2 h exposure demonstrated some activity (ED50 of 9·58, 95% CI 6·66 to 13·76 µg mL−1). However, in contrast, the activity of Compound 20 was demonstrated to be almost completely reversible under the conditions employed.
Fig. 2.
ED50 of Aureobasidin A (AbA, A-D) or Compound 20 (Cpmd 20, E-H) – μg mL−1; (95% Confidence Interval) – against the Toxoplasma RH tachyzoite form in HFF cells. 6 days post addition of the compounds. In agreement with Sonda et al. (2005), both compounds were non-toxic to HHF cells under the conditions employed. A and B: no wash out post-compound addition; C and D: wash out 2 h post-compound addition; E and F: wash out 8 h post-compound addition; G and H: 2 h pre-treatment of isolated parasites pre-infection. Calculated using GraphPad Prism 7, log(inhibitor) vs normalized response – Variable slope. >10 µg mL−1 – ED50 could not be determined. Representative in triplicate dataset.
Interestingly, the unrelated kinetoplastid protozoa, Trypanosoma cruzi (the causative agent of American Trypanosomiasis or Chagas disease) has also been shown to be sensitive to AbA, with the IPC synthase again proposed as the target (Salto et al. 2003). However, enzyme analyses did not confirm this and it was suggested that the compound acts on the host to promote clearance of the parasite (Figueiredo et al. 2005). In order to test this hypothesis in Toxoplasma infection, tachyzoite parasites were isolated from infected cells as described in the section Materials and Methods and then treated with various concentrations of AbA and Compound 20 prior to washing and infecting host HFF cells. A 2 h treatment again demonstrated that AbA was effective (ED50 of 4·78, 95% CI 3·95 to 5·79 µg mL−1), whilst the analogue was inactive (Fig. 2). Longer periods post-isolation (8 h) lead to untreated parasites losing infectivity.
The sensitivity of the Toxoplasma gondii sphingolipid synthase and sphingolipid synthesis per se to AbA and its analogue Compound 20
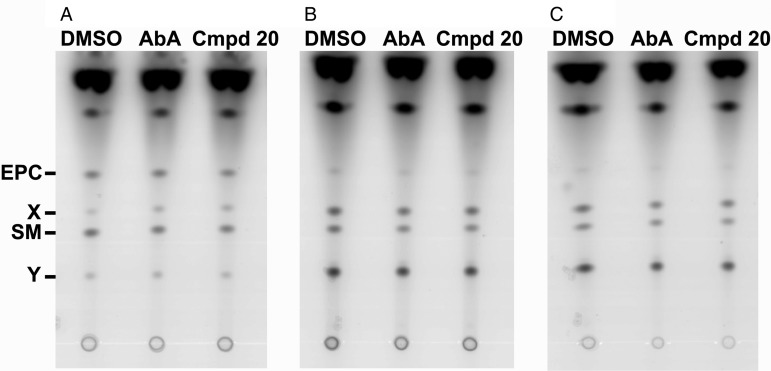
Host sphingolipid biosynthesis is unaffected by (Fig. S1) and non-essential for (Pratt et al. 2013; Romano et al. 2013), Toxoplasma proliferation. Therefore, de novo synthesis of sphingolipids is an attractive target for new antiprotozoal drug leads. The antifungal sphingolipid (IPC) synthase inhibitor AbA has been proposed to inhibit the Toxoplasma orthologue (Sonda et al. 2005; Coppens, 2013). However, analyses of an enzyme isolated from Toxoplasma demonstrating IPC synthase activity (TgSLS) did not support this conclusion (Pratt et al. 2013). Utilizing the previously constructed, auxotropic, TgSLS complemented yeast strains (YPH499-HIS-GAL-AUR1 pRS426 TgSLS, with YPH499-HIS-GAL-AUR1 pRS426 AUR1 as a control), the sensitivity of the protozoan enzyme to AbA and Compound 20 was analysed (Fig. 3). The results clearly demonstrated that the Toxoplasma enzyme conferred resistance to yeast against both cyclic depsipeptides at concentrations lethal to yeast reliant on AUR1 activity (5 and 10 µg mL−1). However, whilst TgSLS clearly functions as an IPC synthase in yeast and in vitro, Toxoplasma have also been demonstrated, by the incorporation of tritiated serine, to synthesize sphingomyelin (SM) and glycosphingolipids (GSLs) (Gerold and Schwarz, 2001). The presence of SM and GSLs in isolated Toxoplasma was subsequently confirmed using mass spectrometry (Welti et al. 2007; Pratt et al. 2013). In addition, relatively high levels of ethanolamine phosphorylceramide (EPC), a non-abundant species in mammalian cells, were also reported (Welti et al. 2007; Pratt et al. 2013). In light of this synthetic complexity, and the potential of enzymatic diversity, the effect of AbA and Compound 20 on total sphingolipid biosynthesis in Toxoplasma was investigated. Labelling isolated Toxoplasma with NBD-C6−ceramide as described in the section Materials and Methods demonstrated that the parasite synthesized a complex of sphingolipid species, including SM and EPC (co-migrating with mammalian equivalents; Vacaru et al. 2013). However, IPC was not evident and 2 other species (X and Y) remain unassigned (Fig. 4). The addition of AbA and Compound 20 at 10 µg mL−1 for 1, 4 and 7 h, before 1 h NBD-C6−ceramide labelling, had no effect on the synthesis of the sphingolipids compared with controls (Fig. 5). This demonstrated that this class of cyclic depsipeptides do not exert their activity through inhibition or dysregulation of sphingolipid biosynthesis. However, it is notable that the complex sphingolipid profile produced does change as the time post parasite isolation increases, with the levels of labelled lipids X and Y increased at 4 and 7 h, EPC levels decreased and SM levels unchanged (Fig. 5). This indicated that the stress of isolation from the host cell leads to the modification sphingolipid biosynthesis or to catabolism.
Fig. 3.
Yeast dependent on the expression of the Toxoplasma AUR1p orthologue TgSLS (YPH499-HIS-GAL-AUR1 pRS426 TgSLS) are resistant to Aureobasidin A (AbA) and Compound 20 (Cmpd 20) at 5 and 10 µg mL−1. This contrasts to the sensitivity of yeast dependent on AUR1 expression (YPH499-HIS-GAL-AUR1 pRS426 AUR1).
Fig. 4.
Vero cells (Host), isolated Toxoplasma tachyzoites (Toxo) and Saccharomyces cerevisiae (Yeast), labelled for 1 h with NBD-C6-ceramide and complex sphingolipids then fractionated by HPTLC. Like the host cells, Toxoplasma parasites synthesize sphingomyelin (SM) and ethanolamine phosphorylceramide (EPC), two unique sphingolipids are also produced (X and Y). However, unlike in S. cerevisiae, no labelled inositol phosphorylceramide (IPC) is evident from either host or Toxoplasma cells. Representative dataset.
Fig. 5.
Isolated Toxoplasma tachyzoites treated with Aureobasidin A (AbA) and Compound 20 (Cmpd 20) at 10 µg mL−1 for 1 (A), 4 (B) and 7 (C) hours before labelling with NBD-C6-ceramide for 1 h. Neither compound affected the complex sphingolipid profile synthesized at any time point when compared with the vehicle control (DMSO). SM – Sphingomyelin (SM); EPC – Ethanolamine PhosphorylCeramide; X and Y – Unclassified sphingolipids. Representative dataset.
Comparing the efficacy of AbA and its analogue Compound 20 against the encysted Toxoplasma bradyzoite form
With a complete lack of treatments available for chronic disease, in which Toxoplasma has reached the encysted bradyzoite stage, new therapies are urgently needed (Antczak et al. 2016). Therefore, although the mode of action of the cyclic depsipeptides remains unclear, the efficacy of these compounds against the encysted form of the parasite was analysed. Utilizing the Type II Pru strain of Toxoplasma modified to express GFP (Kim et al. 2007) we analysed the efficacy of AbA and Compound 20 against HFF cells infected with parasites transformed into a bradyzoite-like stage using an established protocol (Soete et al. 1994). Following 3 days of exposure, both compounds demonstrated promising activity against the encysted Toxoplasma (Fig. 6), again AbA demonstrated slightly higher efficacy (ED50 of 2·51, 95% CI 1·96 to 3·23 µg mL−1) than Compound 20 (ED50 of 3·74, 95% CI 3·13 to 4·47 µg mL−1). This showed that the cyclic depsipeptides may represent promising candidates for therapies to treat both acute and chronic toxoplasmosis.
Fig. 6.
ED50 of Aureobasidin A (A, AbA) or Compound 20 (B, Cpmd 20) – μg mL−1 (95% Confidence Interval) – against the Toxoplasma Pru bradyzoite form in Human Foreskin Fibroblast (HFF) cells. Three days post addition of the compounds. In agreement with Sonda et al. (2005), both compounds were non-toxic to HHF cells under the conditions employed. Calculated using GraphPad Prism 7, log(inhibitor) vs normalized response – Variable slope. Representative in triplicate dataset.
DISCUSSION
Toxoplasma is an important cause of disease in humans and domestic animals. Whilst there are several drugs available to treat acute (tachyzoite stage) toxoplasmosis, there is a complete absence of effective therapies for chronic disease (encysted bradyzoite stage; Antczak et al. 2016). It has been demonstrated that Toxoplasma remain able to replicate in CHO cells where the activity of the first and rate limiting step in sphingolipid biosythesis, serine palmitoyltransferase (SPT), was greatly reduced and complex sphinglipid levels consequently lowered (Hanada et al. 1992; Pratt et al. 2013). In addition, in this study we showed that key enzymes in host (CHO) sphingolipid biosynthesis are unaffected by Toxoplasma infection (Fig. S1). Together, these data indicated that targeting the de novo Toxoplasma sphingolipid biosynthetic pathway could represent a viable strategy towards the identification of new antiprotozoals. A strategy that could also be applicable to other apicomplexan parasites such as Plasmodium spp. (Lauer et al. 1995), and one that has is already being investigated for kinetoplastid protozoan pathogens (Denny et al. 2006; Mina et al. 2009, 2010, 2011).
To these ends it has been suggested that the antifungal cyclic depsipeptide, AbA exerts its effect on Toxoplasma by inhibiting a sphinglipid (IPC) synthase, an orthologue of its validated target in yeast (Nagiec et al. 1997; Sonda et al. 2005). Given the status of the fungal and kinetoplastid IPC synthases as promising drug targets (Young et al. 2012), the identification of the Toxoplasma orthologue (Pratt et al. 2013) led to its consideration as a target for anti-apicomplexan drugs. TgSLS functions as an IPC synthase and the product was identified in parasite extracts using directed mass spectrometry. However, AbA was demonstrated to be non-active against the enzyme activity in vitro (Pratt et al. 2013).
To investigate this compound class further, here we utilized the availability of AbA and a synthetically modified analogue, Compound 20 (Wuts et al. 2015), to test the efficacy and mode of action of these cyclic depsipeptides against Toxoplasma. As expected, neither compound inhibited the growth of transgenic yeast dependent on the expression of TgSLS (Fig. 3). Furthermore, the compounds also exhibited no effect on the synthesis of complex sphingolipids in Toxoplasma (Fig. 5). Interestingly, no IPC synthesis was apparent indicating that this activity may be low, in tachyzoites at least. However, both SM and EPC (Azzouz et al. 2002; Welti et al. 2007) were clearly produced, as well as 2 uncharacterised complex sphingolipids (Fig. 4). However, despite this lack of dysregulation of sphingolipid biosythesis, both AbA and Compound 20 are active against the tachyzoite form of the parasite in infected HHF cells. AbA exhibited greater efficacy and, unlike Compound 20, demonstrated a rapid and direct ‘cidal activity against the Toxoplasma parasite (Fig. 2). Furthermore, and importantly, both AbA and Compound 20 clear encysted bradyzoite-like form Toxoplasma from infected tissue culture at low concentrations (Fig. 6). Given the well established lack of toxicity of these compounds to mammalian cells, coupled with the promising pharmacokinetic properties of Compound 20 (Wuts et al. 2015), this class of cyclic depsipeptides may form the basis of a unique therapy for chronic toxoplasmosis and perhaps, some psychiatric disorders.
ACKNOWLEDGMENTS
We thank Ian Edwards (Durham University) for technical support and John Mina for helpful discussions. We are also indebted to Dominique Soldati-Favre (University of Geneva), Markus Meissner (University of Glasgow) and Boris Striepen (University of Georgia) for provision of the cell lines utilized and Aureogen Inc for providing the compounds.
FINANCIAL SUPPORT
This work was supported by the Biotechnology and Biological Research Council (BB/M024156/1 to PWD). AQIA was funded by the Government of Iraq. AJM and MBL were funded by BBSRC Impact and NPRONET awards to PWD.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0031182017000506.
click here to view supplementary material
References
- Aeed P. A., Young C. L., Nagiec M. M. and Elhammer A. P. (2009). Inhibition of inositol phosphorylceramide synthase by the cyclic peptide Aureobasidin A. Antimicrobial Agents and Chemotherapy 53, 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak M., Dzitko K. and Dlugonska H. (2016). Human toxoplasmosis-Searching for novel chemotherapeutics. Biomedicine and Pharmacotherapy 82, 677–684. [DOI] [PubMed] [Google Scholar]
- Azzouz N., Rauscher B., Gerold P., Cesbron-Delauw M. F., Dubremetz J. F. and Schwarz R. T. (2002). Evidence for de novo sphingolipid biosynthesis in Toxoplasma gondii. International Journal for Parasitology 32, 677–684. [DOI] [PubMed] [Google Scholar]
- Cerantola V., Guillas I., Roubaty C., Vionnet C., Uldry D., Knudsen J. and Conzelmann A. (2009). Aureobasidin A arrests growth of yeast cells through both ceramide intoxication and deprivation of essential inositolphosphorylceramides. Molecular Microbiology 71, 1523–1537. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. N. (1986). Toxoplasmosis: a review. Journal of Medicine 17, 373–396. [PubMed] [Google Scholar]
- Coppens I. (2013). Targeting lipid biosynthesis and salvage in apicomplexan parasites for improved chemotherapies. Nature Reviews Microbiology 11, 823–835. [DOI] [PubMed] [Google Scholar]
- Denny P. W., Shams-Eldin H., Price H. P., Smith D. F. and Schwarz R. T. (2006). The protozoan inositol phosphorylceramide synthase: a novel drug target which defines a new class of sphingolipid synthase. Journal of Biological Chemistry 281, 28200–28209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J. P. (1977). Toxoplasma, Hammondia, Besnotia, Sarcocystis, and other cyst-forming coccidia of man and animals In Parasitic Protozoa (ed. Kreier J. P.), pp. 101–237. Academic Press, New York. [Google Scholar]
- Figueiredo J. M., Dias W. B., Mendonca-Previato L., Previato J. O. and Heise N. (2005). Characterization of the inositol phosphorylceramide synthase activity from Trypanosoma cruzi. Biochemical Journal 387, 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H. (2000). Antifungals targeted to sphingolipid synthesis: focus on inositol phosphorylceramide synthase. Expert Opinions on Investigative Drugs 9, 1787–1796. [DOI] [PubMed] [Google Scholar]
- Gerold P. and Schwarz R. T. (2001). Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Molecular and Biochemical Parasitology 112, 29–37. [DOI] [PubMed] [Google Scholar]
- Gubbels M. J., Li C. and Striepen B. (2003). High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrobial Agents and Chemotherapy 47, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K. (2005). Sphingolipids in infectious diseases. Japanese Journal of Infectious Diseases 58, 131–148. [PubMed] [Google Scholar]
- Hanada K., Nishijima M., Kiso M., Hasegawa A., Fujita S., Ogawa T. and Akamatsu Y. (1992). Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. Journal of Biological Chemistry 267, 23527–23533. [PubMed] [Google Scholar]
- Heidler S. A. and Radding J. A. (1995). The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent Aureobasidin A (LY295337). Antimicrobial Agents and Chemotherapy 39, 2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema K., Van Den Dikkenberg J., Brouwers J. F. and Holthuis J. C. (2004). Identification of a family of animal sphingomyelin synthases. EMBO Journal 23, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai K., Takesako K., Shiomi K., Moriguchi M., Umeda Y., Yamamoto J., Kato I. and Naganawa H. (1991). Structure of Aureobasidin A. Journal of Antibiotics (Tokyo) 44, 925–933. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Fouts A. E. and Boothroyd J. C. (2007). Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. Journal of Immunology 178, 5154–5165. [DOI] [PubMed] [Google Scholar]
- Lauer S. A., Ghori N. and Haldar K. (1995). Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proceeds of the National Academy of Sciences USA 92, 9181–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M., Schluter D. and Soldati D. (2002). Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837–840. [DOI] [PubMed] [Google Scholar]
- Mina J. G., Pan S. Y., Wansadhipathi N. K., Bruce C. R., Shams-Eldin H., Schwarz R. T., Steel P. G. and Denny P. W. (2009). The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug target. Molecular and Biochemical Parasitology 168, 16–23. [DOI] [PubMed] [Google Scholar]
- Mina J. G., Mosely J. A., Ali H. Z., Shams-Eldin H., Schwarz R. T., Steel P. G. and Denny P. W. (2010). A plate-based assay system for analyses and screening of the Leishmania major inositol phosphorylceramide synthase. International Journal of Biochemistry and Cell Biology 42, 1553–1561. [DOI] [PubMed] [Google Scholar]
- Mina J. G., Mosely J. A., Ali H. Z., Denny P. W. and Steel P. G. (2011). Exploring Leishmania major inositol phosphorylceramide synthase (LmjIPCS): insights into the ceramide binding domain. Organic and Biomolecular Chemistry 9, 1823–1830. [DOI] [PubMed] [Google Scholar]
- Nagiec M. M., Nagiec E. E., Baltisberger J. A., Wells G. B., Lester R. L. and Dickson R. C. (1997). Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. Journal of Biological Chemistry 272, 9809–9817. [DOI] [PubMed] [Google Scholar]
- Pratt S., Wansadhipathi-Kannangara N. K., Bruce C. R., Mina J. G., Shams-Eldin H., Casas J., Hanada K., Schwarz R. T., Sonda S. and Denny P. W. (2013). Sphingolipid synthesis and scavenging in the intracellular apicomplexan parasite, Toxoplasma gondii. Molecular and Biochemical Parasitology 187, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J. D., Sonda S., Bergbower E., Smith M. E. and Coppens I. (2013). Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole. Molecular Biology of the Cell 24, 1974–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salto M. L., Bertello L. E., Vieira M., Docampo R., Moreno S. N. and de Lederkremer R. M. (2003). Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryotic Cell 2, 756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K. and Ikonen E. (1997). Functional rafts in cell membranes. Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Soete M., Camus D. and Dubremetz J. F. (1994). Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Experimental Parasitology 78, 361–370. [DOI] [PubMed] [Google Scholar]
- Sonda S., Sala G., Ghidoni R., Hemphill A. and Pieters J. (2005). Inhibitory effect of Aureobasidin A on Toxoplasma gondii. Antimicrobial Agents and Chemotherapy 49, 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesako K., Ikai K., Haruna F., Endo M., Shimanaka K., Sono E., Nakamura T., Kato I. and Yamaguchi H. (1991). Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. Journal of Antibiotics (Tokyo) 44, 919–924. [DOI] [PubMed] [Google Scholar]
- Vacaru A. M., van den Dikkenberg J., Ternes P. and Holthuis J. C. (2013). Ceramide phosphoethanolamine biosynthesis in Drosophila is mediated by a unique ethanolamine phosphotransferase in the Golgi lumen. Journal of Biological Chemistry 288, 11520–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. P., Kaushik M., Bristow G. C. and McConkey G. A. (2013). Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? Journal of Experimental Biology 216, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R., Mui E., Sparks A., Wernimont S., Isaac G., Kirisits M., Roth M., Roberts C. W., Botte C., Marechal E. and McLeod R. (2007). Lipidomic analysis of Toxoplasma gondii reveals unusual polar lipids. Biochemistry 46, 13882–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuts P. G., Simons L. J., Metzger B. P., Sterling R. C., Slightom J. L. and Elhammer A. P. (2015). Generation of broad-spectrum antifungal drug candidates from the natural product compound Aureobasidin A. ACS Medical Chemistry Letters 6, 645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Mina J. G., Denny P. W. and Smith T. K. (2012). Sphingolipid and ceramide homeostasis: potential therapeutic targets. Biochemistry Research International 2012, 248135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0031182017000506.
click here to view supplementary material