Abstract
Impaired consciousness occurs suddenly and unpredictably in people with epilepsy, markedly worsening quality of life and increasing risk of mortality. Focal seizures with impaired consciousness are the most common form of epilepsy and are refractory to all current medical and surgical therapies in about one-sixth of cases. Restoring consciousness during and following seizures would be potentially transformative for these individuals. Here, we investigate deep brain stimulation to improve level of conscious arousal in a rat model of focal limbic seizures. We found that dual-site stimulation of the central lateral nucleus of the intralaminar thalamus (CL) and the pontine nucleus oralis (PnO) bilaterally during focal limbic seizures restored normal-appearing cortical electrophysiology and markedly improved behavioral arousal. In contrast, single-site bilateral stimulation of CL or PnO alone was insufficient to achieve the same result. These findings support the “network inhibition hypothesis” that focal limbic seizures impair consciousness through widespread inhibition of subcortical arousal. Driving subcortical arousal function would be a novel therapeutic approach to some forms of refractory epilepsy and may be compatible with devices already in use for responsive neurostimulation. Multisite deep brain stimulation of subcortical arousal structures may benefit not only patients with epilepsy but also those with other disorders of consciousness.
Keywords: consciousness, epilepsy, intralaminar thalamus, neurostimulation, pontine reticular formation
Introduction
Impaired consciousness associated with refractory seizures severely impacts the productivity, safety, and quality of life of patients suffering from epilepsy (Vickrey et al. 2000; Sander 2003; Blumenfeld 2012). In addition, impaired consciousness during seizures is associated with a crippling social stigma that is only mitigated by complete seizure control, something that is out of reach for nearly a quarter of all patients with epilepsy. Neurostimulation has grown in recent years as a treatment for refractory epilepsy, but still leaves many patients without complete seizure freedom (Englot et al. 2011; Heck et al. 2014; Salanova et al. 2015). For these patients, a strategy aimed at improving quality of life, morbidity, and mortality despite ongoing seizures could be highly beneficial.
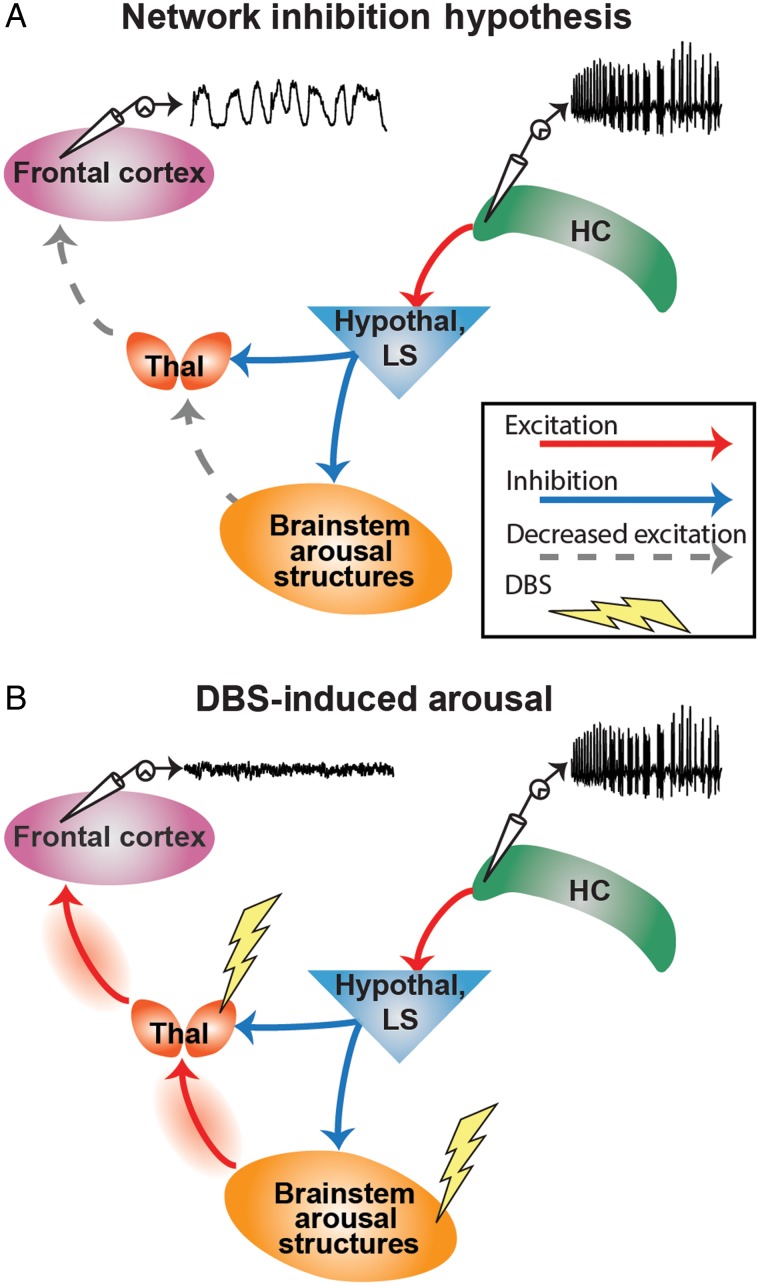
Consciousness depends on several functions including: 1) specific sensorimotor and other systems constituting the content of consciousness; and 2) systems regulating the overall level of consciousness manifested as awareness, attention, and basic conscious arousal (Posner et al. 2007; Schiff and Laureys 2009; Blumenfeld 2010, 2012, 2015; Giacino et al. 2014). Importantly, conscious arousal, which distinguishes coma or deep sleep from the alert state, can be assessed by relatively simple behavioral and physiological observations and forms the crucial necessary first step enabling other higher order aspects of consciousness to occur (Posner et al. 2007). The mechanism by which consciousness is lost during and after temporal lobe, focal limbic seizures has been explored and consolidated in the “network inhibition hypothesis” (Norden and Blumenfeld 2002; Blumenfeld 2012) (Fig. 1A). Recent animal studies (Englot et al. 2008, 2009; Gummadavelli, Motelow, et al. 2015; Li et al. 2015; Motelow et al. 2015) and human data (Blumenfeld, McNally, et al. 2004; Blumenfeld, Rivera, et al. 2004; Englot et al. 2010; Cunningham et al. 2014) point to functional deafferentation of the cortex as central to the loss of conscious arousal (Brown et al. 2010; Giacino et al. 2014). Inhibition of subcortical arousal structures including the upper brainstem reticular formation and one of its direct targets, the intralaminar thalamus during focal limbic seizures, may result in cortical states resembling deep sleep or coma (Blumenfeld 2012) (Fig. 1A). Thus, therapeutic activation of these subcortical arousal structures could attenuate the loss of conscious arousal experienced in focal limbic seizures (Fig. 1B).
Figure 1.
The network inhibition hypothesis and proposed therapeutic intervention. (A) Schematized representation of focal hippocampal seizure causing cortical slow waves and loss of consciousness. Seizure activity propagates via excitatory projections to the hypothalamus (Hypothal) and lateral septum (LS). This activates neurons with inhibitory projections to numerous subcortical arousal structures including the midbrain and pontine reticular formation in the upper brainstem and the intralaminar thalamus (Thal). Inactivating these arousal structures has the effect of decreasing excitatory input to the cortex (frontal cortex, as well as other cortical areas), thus allowing it to fall into a sleep-like state. This is electrophysiologically characterized by large-amplitude, low-frequency “slow waves.” (B) Dual-site deep brain stimulation (DBS) in the inhibited brainstem arousal structures and thalamus during focal hippocampal seizures restores excitatory input to the cortex, restoring awake-like cortical fast activity and improving conscious arousal.
Previous studies investigating the intralaminar thalamus and pontine reticular formation provide a basis for further exploration. The general role of intralaminar thalamus in attention, arousal, and consciousness has been repeatedly described over many years (Penfield 1936; McLardy et al. 1968; Hassler et al. 1969; Sturm et al. 1979; Van der Werf et al. 2002). More recently, electrical stimulation of this region in animal models and humans has yielded promising results regarding its capacity to improve arousal during pathologic states of decreased consciousness (Schiff et al. 2007; Gummadavelli, Motelow, et al. 2015). Likewise, early stimulation studies in the upper brainstem reticular formation also showed electrophysiological wake-like cortical activity in animals under anesthesia (Morison et al. 1941; Moruzzi and Magoun 1949). However, the use of electrical stimulation of these subcortical arousal regions to maintain or improve consciousness during a seizure has not previously been described.
Relative to more common applications of therapeutic neurostimulation for movement disorders or aborting/preventing seizures, targets and methods for promoting consciousness are poorly developed (Gummadavelli, Kundishora, et al. 2015). Therefore, we propose a new strategy for addressing the loss of consciousness in focal limbic seizures based on the current proof-of-principle preclinical study. We electrically stimulated both the central lateral nucleus of the intralaminar thalamus (CL) and the pontine nucleus oralis (PnO, a member of the pontine reticular formation) in anesthetized and awake, behaving animals during focal limbic seizures. By assessing electrophysiological and behavioral metrics of arousal, we provide evidence that deep brain stimulation can reduce cortical slow-wave activity, increase multiunit cortical activity, and increase behavioral arousal during seizures.
Materials and Methods
Animals
The Yale University Institutional Animal Care and Use Committee approved all procedures. Adult female Sprague-Dawley rats weighing 200–280 g (Charles River Laboratories) were used.
Surgery and Electrode Implantation
The animal model of focal limbic seizures was prepared as described previously (Englot et al. 2008) with the modifications described below. Animals were deeply anesthetized with an intramuscular injection of 80–90 mg/kg ketamine (Henry Schein Animal Health, Ashburn, VA, USA) and 15 mg/kg xylazine (AnaSed; Lloyd Laboratories, Quezon City, Philippines). Craniotomies were placed stereotactically over sites of planned electrode placement, as well as for 4–5 stainless-steel anchoring screws (0–80 × 3/32; PlasticsOne, Roanoke, VA, USA) and a ground screw (E363-20; PlasticsOne) (Supplementary Fig. 1A).
To monitor changes in cortical physiology with focal limbic seizures and deep brain stimulation, we chose to record from lateral orbital frontal cortex (LOFC). This region demonstrates typical ictal and postictal slow-wave activity as well as changes in functional neuroimaging, which are also present in other widespread cortical regions in both animal models and human studies (Blumenfeld, Rivera, et al. 2004; Englot et al. 2008, 2009, 2010; Gummadavelli, Motelow, et al. 2015; Motelow et al. 2015).
All coordinates are reported as final electrode tip locations in reference to bregma (Paxinos and Watson 1998). For “acute preparations,” a single tungsten monopolar microelectrode (UEWMGGSEDNNM; FHC, Bowdoin, ME, USA) with an impedance of 2–4 MΩ was implanted at an approach angle of 20° from vertical to record local field potentials (LFPs) in the right lateral orbitofrontal cortex (LOFC); anterior–posterior (AP) +4.2 mm, medial–lateral (ML) −2.2 mm, superior–inferior (SI) −4.2 mm (Paxinos and Watson 1998). To overcome the stimulus-induced artifact that obscures multiunit data, a second differential recording electrode was custom modified by affixing 2 tungsten monopolar electrodes (UEWMGGSEDNNM; FHC) with impedances of 3–4 MΩ together with bonding cement (Krazy glue, Elmer's Products, Inc., Westerville, OH, USA) such that their tips were separated by <0.5 mm and it was implanted at an approach angle of 20° from vertical in the left lateral orbitofrontal cortex; AP +4.2, ML +2.2, SI −4.2 mm. This unique configuration of 2 high impedance electrodes in close proximity had the effect of subtracting out the large shared noise signal while preserving the independent neuronal signals close to each electrode tip. For “chronic preparations,” a stainless-steel twisted-pair bipolar electrode (E363-2-2TW; PlasticsOne) cut to 20 mm and with electrode tips separated by 1 mm in the coronal plane was implanted at an angle of 20° into the right lateral orbitofrontal cortex (AP +4.2, ML −2.2, SI −4.2 mm). Hippocampal electrodes were bilaterally implanted for acute preparations to mitigate hippocampal fatigue whereas, in chronic preparations, a unilateral electrode was placed, secondary to space limitations. Twisted-pair bipolar electrodes with tips separated by 1 mm, insulation shaved to 0.5 mm, and electrode tips in the coronal plane were implanted into the dorsal hippocampus (HC) (AP −3.8, ML ± 3.4, SI −3.6 mm). For acute and chronic preparations involving the CL, 2 twisted-pair bipolar electrodes with electrode tips in the sagittal plane were implanted in bilateral CL (AP −2.8; ML ± 1.4; SI −5.2 mm). For acute and chronic preparations involving the PnO, 2 twisted-pair bipolar electrodes with electrode tips in the sagittal plane were implanted at an approach angle of 20° in bilateral PnO (AP −8.0; ML ± 1.4; SI −8.0 mm).
In chronic experiments, electrodes were firmly cemented to nearby anchoring screws with dental cement (Lang Dental Manufacturing, Wheeling, IL, USA). All electrode pins except for the ground pin were carefully bent and placed into two, 6-pin pedestals (MS363; PlasticsOne). The ground pin was bonded to the outside of the caudal-most pedestal (Krazy glue; Elmer's Products, Inc.) and the whole assembly was sealed with silicon sealant for insulation (Kwik-cast; World Precision Instruments, Inc., Sarasota, FL, USA). Skin was reapproximated with interrupted silk sutures (Surgical Specialties Corp., Vancouver, BC, Canada) around each pedestal. Chronic recordings began at least 48 h postoperatively.
Electrophysiology and Video Recording Procedures
We performed electrophysiological recordings under the following conditions: 1) Acute experiments under deep anesthesia were performed with ketamine/xylazine 80–90/15 mg/kg anesthesia, injected intramuscularly, resulting in the presence of continuous cortical slow waves during which no seizures were triggered; 2) acute experiments under light anesthesia were done after animals had recovered from deep anesthesia to a state whereby slow waves occurred at approximately <3 waves per 10 s of recordings as described previously (Englot et al. 2008); 3) experiments in chronically implanted, behaving animals were performed in the awake state, under deep anesthesia, under anesthesia-induced sleep with intramuscular injection of intramuscular injection of a low-dose of ketamine (15 mg/kg) and xylazine (3 mg/kg), or during naturally occurring sleep.
Stimulations of CL and/or PnO were generated by independent isolated pulse stimulators (Model 2100, A-M systems, Sequim, WA, USA). For all experiments, CL and PnO stimulus parameters were titrated per animal in the acute setting under deep anesthesia, unilaterally on each side to determine effective stimulus parameters to produce physiologic cortical arousal. Stimulations in acute animals under deep anesthesia and in chronically implanted animals under anesthesia or during sleep were 20 s duration. Stimulations during focal limbic seizures were 120 s. This duration was chosen to include the majority of the seizure in most cases (mean seizure duration = 57.9 ± 8.4 s) and often included part of the postictal period. Stimulus frequencies were chosen based on prior work (Gummadavelli, Motelow, et al. 2015) and pilot experiments (not shown) demonstrating that a frequency of approximately 100 Hz was most effective in CL and approximately 50 Hz in PnO to produce cortical physiological arousal. Stimulus current amplitudes were then titrated individually per animal. CL was initially stimulated with a square biphasic pulse (0.5 ms/phase) at 100 Hz (Gummadavelli, Motelow, et al. 2015) in a range from 150 to 400 µA per side during titration. The eventual acute experimental amplitudes of stimulation fell between 150 and 400 µA split bilaterally (ca. 75–200 µA per side); the higher amperages during titration were tested to ensure a lack of afterdischarges or seizure induction from the stimulation. PnO was stimulated in acute experiments with a square biphasic pulse (0.5 ms/phase) at 50 Hz and titration was in the same fashion as CL (range 35–200 µA unilaterally, or 70–400 µA split bilaterally). Sham stimulation of CL and PnO was performed with 0.2 µA split bilaterally.
For chronic experiments, a secondary titration was performed at least 48 h postoperatively to account for any change in electrode impedance secondary to gliosis at the intraparenchymal electrode tip. Stimulus titration was again performed under deep anesthesia without seizures. Final ranges of stimulation delivered bilaterally in all experiments were as follows: acute PnO only 70–400 µA; acute CL only 400 µA; chronic PnO only 70–400 µA; dual-site CL: 150–350 µA, PnO 50–300 µA. For these stimuli delivered bilaterally, the current reaching each side was approximately 50% of the total.
Focal limbic seizures in acute and chronic experiments were induced with a 2-s biphasic square pulse at 60 Hz in the unilateral HC (1 ms/phase) generated by a WPI linear stimulus isolator (Model A395, World Precision Instruments, Inc.) with current ranging from 100 µA to 1.5 mA, adjusted to obtain focal hippocampal seizures of at least 15 s duration, without generalization of poly-spike discharges to LOFC. Stimulations were manually triggered by keyboard input via custom Spike2 (version 5.2, CED, Cambridge, UK) scripts running through a digital-to-analog converter (Power 1401; CED). Seizures in acute experiments were induced from a state of light anesthesia as described previously (Englot et al. 2008; Motelow et al. 2015). Seizures in chronically implanted animals were induced in the awake state. In one animal, seizures were induced 15 min after intramuscular administration of 0.9 mg/kg diazepam to prevent seizure generalization as described previously (Englot et al. 2009).
CL and PnO stimulations done under low-dose anesthesia without seizures in chronically implanted animals were performed after intramuscular injection of approximately 15 mg/kg ketamine and 3 mg/kg xylazine. This dose was selected to attain sufficient anesthetic depth to produce a state of slow-wave activity and behavioral arrest without deepening anesthesia to surgical depth. CL only, PnO only, and dual-site stimulation were also performed during periods of naturally occurring sleep. In all cases, sham stimulations (0.2 µA split bilaterally) of CL and PnO were alternated with true stimulations.
Recordings of cortical, HC, CL, and PnO signals were done on separate channels of microelectrode amplifiers (A-M systems Model 1800). In acute recordings from LOFC where high impedance FHC electrodes were used, LFPs were recorded via the monopolar electrode and multiunit activity (MUA) was recorded via the custom-assembled differential recording electrode described above. MUA was recorded with 1000× amplifier gain and then high pass filtered at 400–10 000 Hz with an additional 2.0 dB gain using a digital filter (Model 3364; Krohn-Hite, Brocton, MA, USA). LFP signals for acute recordings were recorded with 1000× amplifier gain and filtered at 1–500 Hz. Cortical LFP were further low-pass filtered at 100 Hz with an additional 1.5 dB gain using a digital filter (Model 3364; Krohn-Hite).
In chronically implanted animals, in which only bipolar LFP electrodes were used for recording and stimulation, two, 6-pin connector wires (PlasticsOne) were connected to a single 12-pin commutator (PlasticOne). The commutator was then connected to the head stages of the A-M Systems amplifiers using 2 customized 6-pin connectors (363SL-6; PlasticsOne). The ground wire was connected separately to a common ground inside the Faraday cage, which in turn was connected to the building ground. HC, CL, PnO, and cortical signals were filtered at 1–500 Hz on the recording amplifiers with 1000× gain. Cortical LFP activity was then further filtered with a 50-Hz low-pass filter with an additional 1.5 dB gain via a digital filter (Model 3364; Krohn-Hite).
All signals in both acute and chronic experiments were digitized with an analog-to-digital converter (Power 1401; CED) using Spike2 (Version 5.2; CED). Cortical and HC LFP were sampled at 1000 Hz; cortical MUA was sampled at 20 000 Hz. For chronic recordings, electrophysiology-synchronized video was captured with a digital camera (Hercules, La Gacilly, France) and recorded with Spike2 Video Recorder (CED).
After completing all electrophysiological recordings, animals were sacrificed with a 0.5-mL intraperitoneal injection of pentobarbital sodium and phenytoin sodium solution (Euthasol; Virbac, Fort Worth, TX, USA.) for histologic localization of electrodes.
Histology
Animals were perfused transcardially with 0.1% heparinized phosphate-buffered saline (PBS) (APP Pharmaceuticals, Lake Zurich, IL, USA) followed by 4% paraformaldehyde (PFA; JT Baker, Center Valley, PA, USA) in PBS. The brain was then removed and post-fixed for at least 48 h in 4% PFA in PBS at 4°C. Brains were washed 3 times in PBS in preparation for slicing, placed in 2% agarose gel (American Bioanalytical, Natick, MA, USA) and cut at 100 µm on a Vibratome (Leica Microsystems, Wetzlar, Germany). Slices were mounted on polarized slides (ThermoScientific, Waltham, MA, USA) and dried for at least 72 h. Slides were stained with cresyl violet using a manufacturer-recommended protocol for reagents (FD NeuroTechnologies, Columbia, MD, USA) to confirm electrode location. Slides were then cover-slipped with Permount (Fisher Chemicals, Pittsburg, PA, USA). Images of slices were taken at ×40 magnification on a compound light microscope (Carl Zeiss, Oberkochen, Germany), imaged with a digital camera (Motic, Hong Kong), and digitally stitched together (Microsoft Image Composite Editor, Redmond, WA, USA). Electrode locations for PnO were confirmed when they fell ventral to the superior cerebellar peduncle, lateral to the paramedian raphe nucleus, medial to the ventral nucleus of the lateral lemniscus, and dorsal to both the ventrolateral tegmental area and the reticulotegmental nucleus of the pons (Supplementary Fig. 1B). Electrode locations for CL were confirmed when they fell no more than 1.5 mm ventral to the HC, in the dorsal thalamus, and were just lateral to the stria medullaris/lateral habenula complex or the mediodorsal thalamic nucleus (Supplementary Fig. 1C).
Data Analysis and Statistics
Data were analyzed in Spike2 (CED) and Microsoft Excel (Microsoft, Redmond, WA, USA).
Analysis epochs were defined as follows: During experiments under deep anesthesia, the pre-DBS epoch was defined as the 20 s immediately prior to stimulation; intra-DBS was defined as the whole 20 s stimulation; post-DBS was defined as the 20 s immediately following stimulation. For time-course analysis following DBS, we also used 10-s epochs extending from 0 to 60 s after DBS. During all other experiments, the baseline epoch was defined as the 30 s immediately preceding hippocampal stimulation; ictal pre-DBS was defined as the time immediately following hippocampal stimulation to the time immediately preceding PnO or CL stimulation; ictal DBS was defined as the time during which DBS and seizure were occurring simultaneously; postictal DBS was defined as the time immediately following seizure, during DBS; postictal post-DBS was defined as the 30 s immediately following the termination of DBS.
Any seizures exhibiting secondary generalization based on poly-spike discharges in the frontal cortical LFP during the analysis epochs were excluded (n = 13 trials) (Englot et al. 2009). For each epoch, cortical LFP power was determined by fast Fourier transform (bin size 1/1.024 Hz, nonwindowed). In all trials, power was then normalized to the maximum power in the baseline epoch. Multiunit data were first spike sorted in Spike2 (CED) using the template-matching method to identify up to 3 units per recording session. To distinguish periodic unit firing versus more continuous tonic firing, autocorrelograms were generated in Spike2 (see Fig. 4B for examples) and further analyzed in Excel. Periodicity was quantified from the autocorrelograms by taking the average amplitudes (absolute value) of the first minimum (greatest anticorrelation) and first maximum (greatest correlation) moving away from time 0. During epochs where there was no periodic firing (typically the baseline and ictal DBS epochs, see Fig. 4B), the maximum and minimum times of the ictal pre-DBS epoch from the same trial were applied to the other epochs for purposes of direct comparison. Average spike rate within each epoch was calculated using Spike2 (CED).
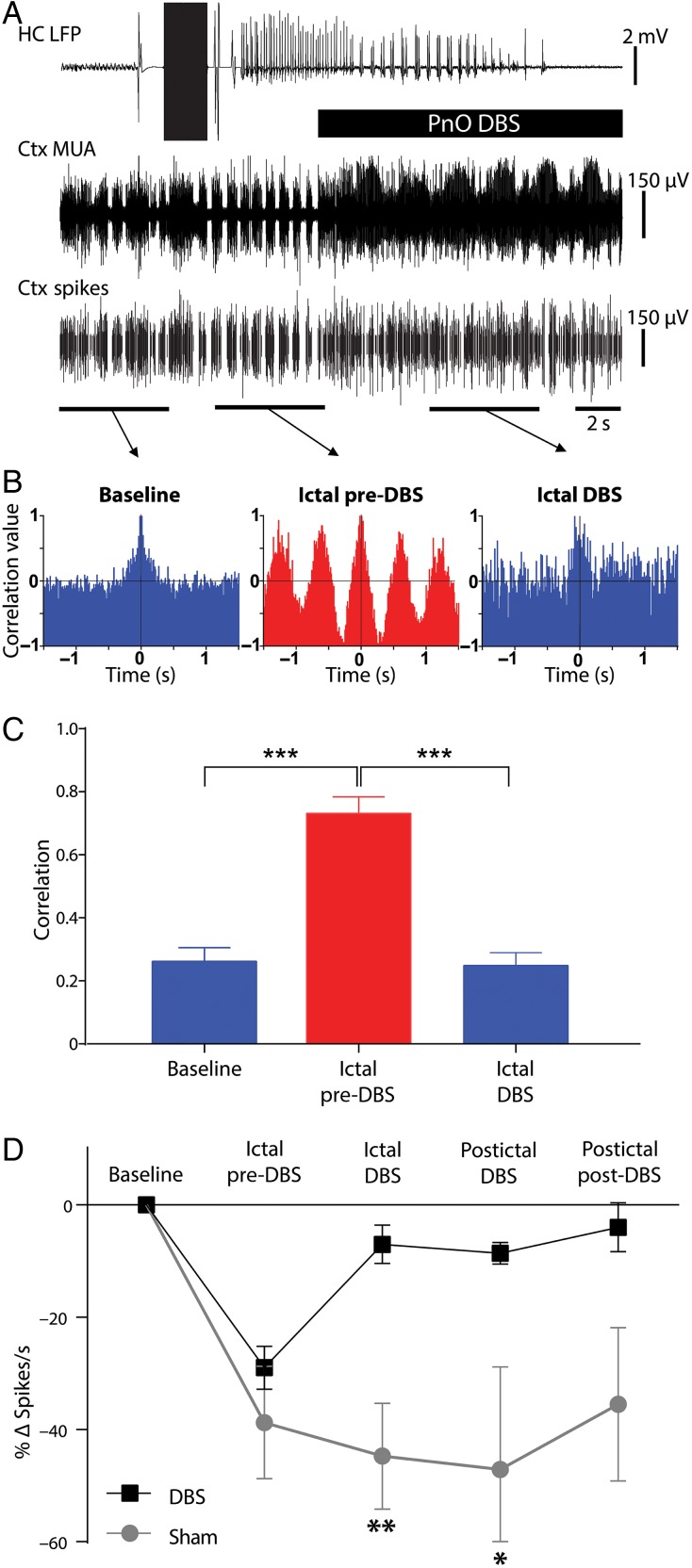
Figure 4.
Transition of cortical multiunit activity from phasic to tonic firing with DBS during focal limbic seizures. (A) Example of PnO stimulation during focal limbic seizure induced by 2 s, 60 Hz stimulation of the hippocampus (HC) under light anesthesia. DBS reverts the phasic UP and DOWN states seen in lateral orbital frontal cortical multiunit activity (Ctx MUA) during the ictal pre-DBS epoch back to tonic desynchronized firing. The bottom-most trace (Ctx Spikes) represents spike-sorted Ctx MUA data. (B) Autocorrelograms of the marked epochs demonstrated desynchronized firing in the baseline and ictal DBS epochs and highly phasic firing in the ictal pre-DBS epoch. (C) Group average peak correlation for the first period (see Materials and Methods) revealed a significant difference between ictal pre-DBS and both the baseline or ictal DBS epochs. (D) Group average spikes per second data demonstrate a large drop in spike rate during focal limbic seizures that is reversed in the DBS case but not in the sham case. There is a significant difference between DBS and sham condition change in spike rate during the ictal DBS and postictal DBS epochs. For C and D, all results are mean ± SEM; n = 8 animals. Same animals as Figure 3 except for one animal that did not have usable MUA data. *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni–Holm corrected.
During trials in chronically implanted animals, behavioral level of arousal was measured using a modified version of a scale previously developed and used in this animal model (Englot et al. 2009; Gummadavelli, Motelow, et al. 2015). A score of 0–4 was assigned for each epoch: 0 for no exploratory behavior with the animal lying on its side or lying asleep; 1 for being upright/lying awake with its ventral surface on the floor of the cage (righting reflex intact); 2 for forepaw exploratory movements on the floor of the cage or actively sniffing the floor of the cage; 3 for forepaws used to explore the sides of the cage; 4 for locomotion requiring movement of both forelimbs and hind limbs. The highest behavioral score achieved in an epoch was assigned as the score for that epoch. Scoring of all epochs was performed by each of two observers, one of whom was blinded to epoch identity and stimulation (sham or true) conditions. Discrepancies of one point between observations were averaged. No discrepancies of more than one point were assigned during the scoring for any epoch, suggesting relatively good interobserver reliability.
Between 2 and 9 focal limbic seizures or stimulations were obtained during experiments in each animal. In all analyses, intra-animal data were averaged before performing group analysis so that the sample size equaled the number of animals. This approach provides a more conservative estimate of sample size; however, similar results were obtained in all cases if group analyses were instead performed by total numbers of seizures or stimulations (data not shown). Data are reported as group mean ± standard error of mean (SEM). Statistical significance was assessed by two-tailed Student's t-test with threshold P = 0.05, corrected for multiple comparisons using the Bonferroni–Holm method.
Results
Cortical Physiological Arousal with PnO Stimulation Under Deep Anesthesia
We electrically stimulated bilateral PnO in animals under deep anesthesia to determine stimulus parameters (see Materials and Methods) effective in producing physiological cortical arousal as measured by increased cortical desynchronization and increased cortical tonic spiking rates.
The large-amplitude 1- to 3-Hz slow waves that are prominent and continuous in the cortical LFP during deep anesthesia were eliminated during and after PnO DBS (Supplementary Fig. 2). Persistent cortical desynchronization during and after the stimulus was associated with a sustained increase in neuronal activity as measured by cortical MUA (Supplementary Fig. 2B, middle and right trace). No afterdischarges or seizure-like activity was measured in the PnO or hippocampal electrodes during or after stimulation with the current ranges used in our experiments.
To quantify loss of low-frequency cortical activity, LFP power (normalized to the maximum prestimulus power within animal) was measured in epochs immediately prior to, during, and after DBS. We observed decreases in low-frequency power during and after DBS, especially in the delta-band frequencies (Supplementary Fig. 2C; n = 7 animals). This decrease persisted for over 60 s after stimulation (Supplementary Fig. 2D), and was not present with PnO sham DBS (Supplementary Fig. 2D; n = 7) or in animals in which the electrodes fell outside the target area (data not shown; n = 4). Average delta-band power (0–4 Hz) was significantly decreased (−73.5 ± 5.1%) during DBS compared with pre-DBS (Supplementary Fig. 2E; n = 7; P < 0.0001, Bonferroni–Holm corrected). No significant change in delta-band power was measured with PnO sham DBS compared with the pre-DBS epoch (Supplementary Fig. 2E; n = 7; P = 0.58).
Cortical Physiological Arousal with PnO Stimulation During Focal Limbic Seizures
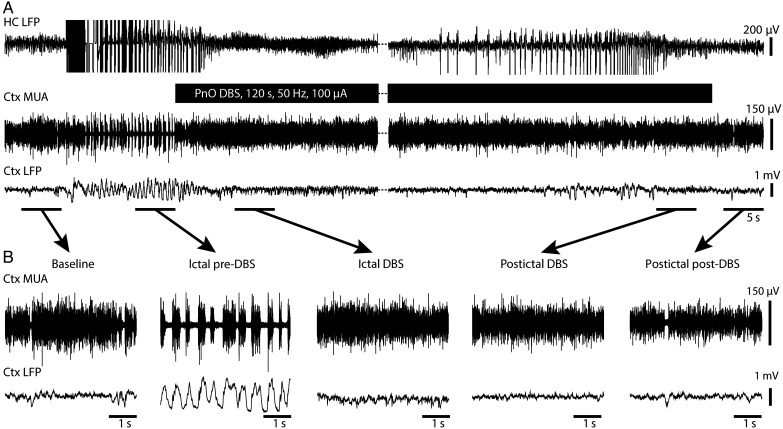
Animals that were initially anesthetized to surgical depth were allowed to recover to a level of light anesthesia indicated by a predominance of cortical fast activity on LFP as described previously (Englot et al. 2008, 2009). Focal limbic seizures were then induced with a 2-s hippocampal stimulation. Approximately 10 s after seizure onset, during which time it was determined that there was no secondary generalization of the seizure, bilateral PnO were again stimulated at an amplitude between 70 and 400 µA, titrated per animal or 0.2-µA sham stimulation. During seizures, prior to PnO DBS, we observed cortical slow waves (Fig. 2A,B), previously known to occur during and after focal limbic seizures (Blumenfeld, Rivera, et al. 2004; Englot et al. 2008, 2009). PnO DBS during the ictal and postictal states abolished cortical slow waves both during and after stimulation (Fig. 2A,B). PnO DBS was also associated with persistently increased cortical activity during and after stimulation as indicated by sustained MUA increases and persistently desynchronized cortical activity as indicated by a shift from phasic to tonic firing states (Fig. 2A,B).
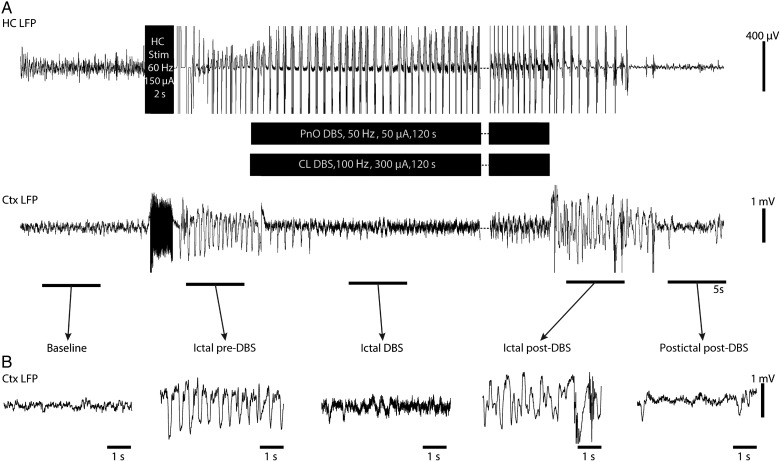
Figure 2.
Cortical physiological arousal with PnO DBS in a focal limbic seizure. (A) Pontine nucleus oralis DBS during focal limbic seizure in a lightly anesthetized animal decreases slowing in lateral orbital frontal cortex. Seizure was induced by 2-s, 60-Hz hippocampal (HC) stimulation. Break in recording of 51 s, during which seizure and DBS continue, enables display of the post-DBS time period. Total DBS duration was 120 s. (B) Five-second-long magnified insets of marked baseline, ictal pre-DBS, ictal DBS, ictal post-DBS, and postictal post-DBS epochs exemplify desynchronized lateral orbital frontal cortical local field potentials (Ctx LFP) intra- and post-DBS. Lateral orbital frontal cortical multiunit activity (MUA) transitions from phasic firing in the ictal pre-DBS period to tonic firing in response to DBS. After PnO stimulation, cortex remains in desynchronized state and no postictal state ensues.
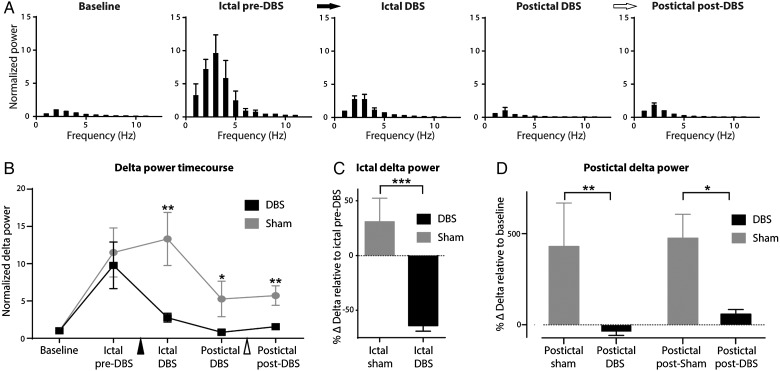
Group analysis of cortical LFP showed decreases in low-frequency power during and after PnO stimulation both ictally and postictally (Fig. 3A,B; n = 9). These changes appeared very similar to those seen under deep anesthesia without seizures (Supplementary Fig. 2C). Delta-band power during the ictal DBS epoch was significantly decreased when compared with the ictal pre-DBS epoch (−64.1 ± 5.0%) (n = 9; P < 0.001, Bonferroni–Holm corrected) (Fig. 3B,C). This was in contrast to the lack of a decrease seen with sham DBS (n = 7; P < 0.17). Cortical delta-band power during and after PnO DBS remained similar to baseline delta power, and significantly lower than sham stimulation delta-band power throughout all DBS and post-DBS time epochs (Fig. 3B; n = 9 for DBS; n = 7 for sham controls; ictal DBS P < 0.01; postictal DBS P < 0.02; postictal post-DBS P < 0.006, Bonferroni–Holm corrected). In contrast, sham–DBS controls showed elevated cortical delta power throughout the ictal and postictal periods (Fig. 3B). Although DBS significantly reduced cortical delta power, seizure duration, as defined by the hippocampal recording, was not significantly changed, lasting 72.2 ± 15.4 s with DBS and 83.6 ± 16.9 s for sham controls (n = 9, 7 respectively; P = 0.64).
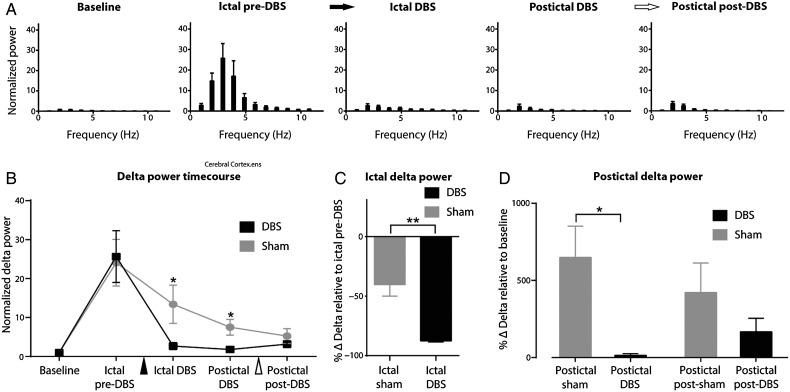
Figure 3.
Group local field potential data for PnO DBS during focal limbic seizures under light anesthesia. (A) Ictal pre-DBS low-frequency power is increased. PnO DBS decreases this low-frequency power during ictal DBS, postictal DBS, and postictal post-DBS epochs. Black arrow indicates onset and white arrow indicates end of PnO DBS. (B) Time course of delta-band (0–4 Hz) power during each epoch compared with sham stimulation. There is a significant difference between DBS and sham conditions during the ictal DBS, postictal DBS, and postictal post-DBS epochs. Black arrowhead indicates onset and white arrowhead end of PnO stimulation. (C) Ictal DBS delta-band power relative to the ictal pre-DBS delta-band power is significantly decreased compared with sham controls. (D) Postictal DBS and postictal post-DBS delta-band power relative to baseline delta power remains elevated in sham conditions but are returned to near baseline levels in the DBS conditions; n = 9 (DBS), n = 7 (sham) animals. All results are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni–Holm corrected.
In acute experiments under light anesthesia, we also investigated the effects of PnO stimulation on cortical neuronal firing to confirm that the observed LFP changes during focal limbic seizures indeed represented increased cortical arousal. During seizures, prior to PnO DBS, the ictal slow waves were accompanied by alternating UP and DOWN states of neuronal firing (Fig. 2), similar to those seen under deep anesthesia and sleep (Steriade et al. 1993). During and after PnO DBS, tonic, desynchronized MUA was seen (Figs 2 and 4A), similar to activity described previously for lightly anesthetized or awake states (Englot et al. 2008). Autocorrelograms for the baseline epoch exhibited desynchronous characteristics (Fig. 4B left), whereas autocorrelograms for the ictal pre-DBS epoch showed periodic firing (Fig. 4B middle). PnO DBS was associated with a reversion of the autocorrelogram to a desynchronous state (Fig. 4B right). Group analysis revealed that mean peak correlation values for the first period (see Materials and Methods) were significantly higher for the ictal pre-DBS epoch compared with both the baseline (n = 8; P < 0.0001) and the ictal DBS (n = 8; P < 0.0001) epochs (P-values Bonferroni–Holm corrected; Fig. 4C). PnO DBS effectively decreased the degree of correlation to baseline levels such that there was no statistically significant difference between those 2 epochs (n = 8; P = 0.83).
Mean spiking rate was also decreased during the transition from tonic firing at baseline to phasic firing during focal limbic seizures (Fig. 4D, ictal pre-DBS). PnO DBS restored the cortical spiking rate to near baseline levels in the ictal and postictal periods, with significantly higher spiking rates than sham in the ictal and postictal DBS epochs (n = 8; P = 0.005, P = 0.03 respectively, Bonferroni–Holm corrected; Fig. 4D), and a nonsignificant trend to persistent increased firing in the postictal post-DBS epoch as well (Fig. 4D).
Cortical Physiological Arousal with PnO Stimulation During Focal Limbic Seizures in Awake Animals Led to Minimal Improvement in Behavior
We next aimed to determine if PnO DBS in the ictal and postictal states would translate to behavioral signs of arousal. In awake, behaving animals, we again used the 2-s, 60-Hz hippocampal stimulation to induce focal limbic seizures, accompanied by cortical slow waves as well as behavioral arrest (Supplementary Fig. 3A,B). These slow waves were similar to those observed in the LOFC as the animal was naturally sleeping or under deep ketamine/xylazine anesthesia. At least 5 s after seizure onset, during which time it was determined that there was no secondary generalization of the seizure, we stimulated bilateral PnO (current titrated individually per animal; range 70–400 µA) or delivered a 0.2-µA sham stimulus. The decreases in low-frequency power during PnO DBS in the awake chronically implanted animals (Supplementary Fig. 3A,B) resembled the effect of stimulation in the acute preparation. Group analysis demonstrated decreased low-frequency power during and after PnO stimulation (Supplementary Fig. 3C), which remained significantly lower than sham controls during the ictal DBS, postictal DBS, and postictal post-DBS epoch (n = 8; Supplementary Fig. 3D). While we observed that stimulation abolished ictal slow waves in the cortical LFP during and after DBS, the degree of behavioral recovery, as measured by spontaneous exploratory behavior, was not significant (n = 8, P = 0.17). We noted that application of similar PnO DBS stimulus amplitudes (up to 400 µA) to awake animals without seizures was capable of interrupting motor activity (data not shown). Therefore, we sought an approach to augment the cortical physiological arousal produced by PnO DBS, while using lower PnO stimulus amplitudes in the hopes of also providing behavioral benefit.
Dual-Site CL and PnO Stimulation Improves Cortical Physiological Arousal During Focal Limbic Seizures
As prior work had suggested an important role for the CL in modulating arousal following focal limbic and secondarily generalized seizures (Gummadavelli, Motelow, et al. 2015; Motelow et al. 2015), we decided to stimulate this region. CL stimulation alone at 400 µA did not significantly decrease cortical slowing during the ictal period (Supplementary Fig. 4). However, combined CL and PnO stimulation enabled us to reduce the PnO stimulation current by 47% (mean PnO current = 150 ± 25 µA in PnO alone vs. 79 ± 9 µA in dual-site stimulation; P = 0.01) while robustly abolishing slow waves during the ictal and postictal periods (Fig. 5).
Figure 5.
Dual-site stimulation of CL + PnO produces cortical physiological arousal during focal limic seizure in an awake, behaving animal. (A) Combined central lateral thalamic and pontine nucleus oralis DBS during focal limbic seizure in an awake, behaving animal decreases slowing in lateral orbital frontal cortical local field potentials (Ctx LFP). Seizure was induced by 2-s, 60-Hz (HC) hippocampal stimulation. Break in recording of 90 s, during which seizure and DBS continue, enables display of the post-DBS time period. Total DBS duration was 120 s. (B) Five-second-long magnified insets of marked baseline, ictal pre-DBS, ictal DBS, ictal post-DBS, and postictal post-DBS epochs exemplify desynchronized Ctx LFP intrastimulus. Slowing returns after stimulation ceases, while the seizure continues (total seizure duration in this example was longer than the DBS). Postictally, Ctx LFP returns to desynchronized state.
In group analyses, dual-site CL and PnO DBS resembled the effects of stimulating PnO alone by markedly reducing both ictal and postictal cortical slow waves in awake, behaving animals (Fig. 6A,B). Delta-band power in the ictal DBS epoch was significantly decreased compared with the ictal pre-DBS epoch (−87.59 ± 0.87%; n = 6; P < 0.0001, Bonferroni–Holm corrected) as well as compared with sham DBS (Fig. 6B,C). Postictal cortical slowing remained elevated in sham controls but was significantly reduced with dual-site DBS, restoring cortical activity to near baseline levels (Fig. 6B,D). Notably, seizure duration remained unchanged between DBS (52.6 ± 13.5 s; n = 6) and sham controls (44.8 ± 8.9 s; n = 7; P = 0.63).
Figure 6.
Group data for dual-site CL and PnO stimulation during focal limbic seizures in awake, behaving animals. (A) Dual-site CL + PnO DBS decreases low-frequency power during and following focal limbic seizures induced in the awake state. Black arrow indicates onset and white arrow termination of PnO DBS. (B) Time course of delta-band (0–4 Hz) power during each time epoch when compared with sham stimulation. There is a significant difference between the DBS and sham conditions during the ictal DBS and postictal DBS epochs. Black arrowhead indicates onset and white arrowhead indicates end of PnO DBS. (C) Ictal DBS delta-band power relative to the ictal pre-DBS delta power is significantly decreased compared with sham stimulation. (D) Postictal DBS delta-band power relative to baseline delta power remains elevated in the sham case and is returned to near baseline levels in the simulation case. All results are mean ± SEM; n = 6 animals. *P < 0.05, **P < 0.01, Bonferroni–Holm corrected.
We noted comparable decreases in cortical slow waves with dual-site stimulation without seizures, under anesthesia and from naturally occurring sleep (data not shown).
Dual-Site CL and PnO Stimulation Markedly Improves Behavioral Arousal During Focal Limbic Seizures
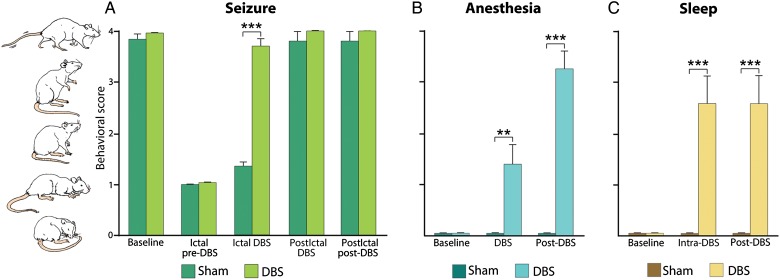
Focal limbic seizures were characterized by behavioral arrest with appearance of minor automatisms as previously described (Englot et al. 2008). However, with dual-site bilateral DBS in awake animals during focal limbic seizures, we observed a marked improvement in normal exploratory behaviors (Fig. 7A; see also Supplementary Video 1). Ratings were performed by 2 observers with high interobserver reliability (κ = 0.97). Dual-site CL + PnO DBS restored normal cortical function and enabled animals to exhibit normal awake behaviors including relaxed forepaw and hindpaw ambulation and exploration of the sides of the cage despite ongoing hippocampal seizure activity (Supplementary Video 1). Normal exploratory behaviors were significantly increased during ictal compared with sham DBS (n = 6; P < 0.0001, Bonferroni–Holm corrected; Fig. 7A) and were not significantly different from preseizure baseline. Of note, the dual-site DBS did not interfere with normal exploratory behaviors in the postictal period (Fig. 7A) and also did not interfere with normal behavior during nonseizure periods (data not shown).
Figure 7.
Behavioral arousal with dual-site CL+ PnO DBS during focal limbic seizures, anesthesia-induced sleep, and natural sleep. (A) CL + PnO DBS significantly increased spontaneous exploratory behavior during focal limbic seizures when compared with sham stimulation; n = 6 animals. (B) CL + PnO DBS significantly increased spontaneous exploratory behavior during the intra- and post-DBS epochs under low-dose anesthesia-induced sleep; n = 6 animals. (C) CL + PnO stimulation significantly increased spontaneous exploratory behavior during the intra- and poststimulation epochs during physiological sleep; n = 6 animals (same animals as in Fig. 6). All data (A–C) are shown as mean ± SEM. **P < 0.01, ***P < 0.001, Bonferroni–Holm corrected.
In addition to behavioral arrest during seizures, we often also observed postictal “wet-dog shakes” which have been previously described in focal rodent limbic seizures (Dyer et al. 1979) accompanied by transient poly-spike discharges on electroencephalogram (EEG). Postictal wet-dog shakes were also significantly decreased in frequency with dual-site CL + PnO DBS, occurring in only 11.7% of trials when compared with 91.7% of sham-DBS control trials (n = 6 animals, P < 0.0001) (Supplementary Video 1).
For comparison, we also studied effects of dual-site CL + PnO DBS on other states of cortical slow waves and hypoarousal aside from focal limbic seizures, including anesthesia-induced sleep and naturally occurring sleep. We found that dual-site DBS significantly increased exploratory activity ratings when compared with sham DBS during anesthesia (n = 6; intra-DBS P < 0.01; post-DBS P < 0.0001, Bonferroni–Holm corrected; Fig. 7B) and naturally occurring sleep (n = 6; intra-DBS P < 0.01; post-DBS P < 0.0001, Bonferroni–Holm corrected; Fig. 7C). Animals also showed a significant reduction of slow-wave activity in response to the DBS, demonstrating a physiological transition to an awake state during their exploratory behaviors, before eventually returning to sleep or falling back under anesthesia (data not shown).
Discussion
We have found that dual-site stimulation of subcortical arousal in the thalamus and rostral pons can reverse the adverse effects of focal limbic seizures on conscious arousal, increasing cortical physiological arousal and behavioral responsiveness in the ictal and postictal periods. Stimulation of PnO alone restored an awake-like cortical state during and after DBS under deep anesthesia, as well as ictally and postictally. This same PnO stimulation in awake, behaving animals yielded similar electrophysiological results; however, there was no evidence of behavioral improvement. Stimulation of thalamic CL alone produced cortical physiological arousal under deep anesthesia and postictally (Gummadavelli, Motelow, et al. 2015) but was ineffective in preventing cortical slow waves during focal limbic seizures. By combining these 2 targets through dual-site CL + PnO stimulation, we achieved both electrophysiological and behavioral improvement during focal limbic seizures. To our knowledge, these results are the first preclinical data of deep brain stimulation improving ictal level of conscious arousal.
The findings reported here support a model in which serial and/or parallel subcortical arousal systems are depressed during focal limbic seizures (Fig. 1A). The arousal function of these systems can be restored by multisite stimulation, providing a more potent synergistic effect on cortical circuits than stimulation at a single node of the network (Fig. 1B). This strategy for multisite stimulation of subcortical arousal may benefit not only epilepsy but also other disorders of consciousness including traumatic brain injury, stroke, or other states of chronically depressed level of consciousness.
The specific sites stimulated in this study are integral nodes in subcortical arousal; however, additional targets could be considered in future investigations (Gummadavelli, Kundishora, et al. 2015). The pontine reticular formation and, in particular, PnO have been implicated in the modulation of spontaneous activity, rapid eye movement sleep, and wakefulness with sometimes disparate conclusions as to its role. Early studies concluded that the pontine reticular formation had no role in spontaneous activity (Lynch 1971), while some recent evidence suggests that it definitively contributes to behavioral state and cortical slow-wave regulation (Giber et al. 2015). Multiple drug infusion studies have solidified its role as a sleep regulatory region (Lopez-Rodriguez et al. 1994; Bourgin et al. 1995; Marks and Birabil 1998; Ahnaou et al. 1999). More recently, stimulation of the PnO has been shown to induce cortical desynchronization in lightly anesthetized rats as well as increase functional connectivity to basal forebrain-paralimbic structures such as the nucleus basalis of Meynert (Pillay et al. 2014).
Likewise, the intralaminar thalamus has also been implicated in the modulation of sleep–wake state, anesthesia, level of awareness, and attention (Steriade et al. 1993, 1996; Kinomura et al. 1996; Jones 2001; Van der Werf et al. 2002). Thalamic CL stimulation in an exemplary patient suffering chronic posttraumatic encephalopathy with minimally conscious state yielded stimulation-dependent arousal and improved functional outcomes including arousal (Schiff et al. 2007). Stimulation of CL has been previously shown by our group to induce cortical desynchronization under anesthesia and to improve arousal in freely behaving animals in the postictal period (Gummadavelli, Motelow, et al. 2015). Here, we demonstrate its insufficiency at producing cortical desynchronization during focal limbic seizures when stimulated alone and feel that this reflects a more widespread multipathway inhibition of subcortical arousal ictally than that is present postictally. Further evidence of the multipathway subcortical arousal network can be seen when contrasting the effects of single-site with dual-site stimulation; cortical desynchronization was more robust and required lower amplitudes in both loci with dual stimulation when compared with single-site stimulation. With stimulation of either locus alone being insufficient, but stimulation of both being necessary to produce the electrophysiological and behavioral correlates of restored conscious arousal, this supports the idea of multiple, complexly overlapping arousal pathways that have differential effects on consciousness depending on the combination of active nodes and the states during which they become active. In the context of previous studies and rapidly improving responsive neurostimulation and DBS technology, our results offer a feasible new strategy for addressing the loss of consciousness in medically refractory focal epilepsy, with clear implications for other disorders of consciousness.
Further studies are needed to explore optimal stimulation paradigms and parameters. For example, future investigation may include stimulation concurrent with, or even before, seizure onset, which was not done during the present studies to allow for assessment of secondary generalization prior to therapeutic stimulus delivery. The present study specifically investigated focal limbic seizures without generalization, although in previous work we found a beneficial effect on arousal by stimulating CL in the postictal period after secondarily generalized seizures (Gummadavelli, Motelow, et al. 2015). In focal limbic seizures without generalization, we also found a beneficial effect on cortical physiological arousal in the postictal period, although we did not observe significant postictal deficits. Thus, behavioral benefits could not be observed in the postictal period. Further studies using more detailed behavioral testing with stimulus/response paradigms may enable a more nuanced understanding of any cognitive improvements provided by DBS in the ictal and postictal periods. Other stimulation parameters such as frequency, pulse-width, amplitude, duration, and stimulation pattern (e.g., intermittent vs. continuous), also require further investigation. Furthermore, although gamma band activity was partly obscured by the stimulus frequency ranges used in the present study, it could be of interest to investigate changes in high-frequency gamma band cortical activity as an additional marker of cortical activation in future investigations. Of particular interest are recent data showing that optogenetic activation of brainstem cholinergic neurons in the pedunculopontine tegmental nucleus of rats during focal limbic seizures is capable of improving cortical physiological arousal (Furman et al. 2015). In addition, optogenetic stimulation of GABAergic and glycinergic axon fibers from the pontine reticular formation has been shown to cause intralaminar thalamic inhibition, leading to behavioral arrest and interruption of awake-like cortical activity in freely moving mice (Giber et al. 2015) further solidifying a role for subcortical interactions in modulation of arousal. Whereas optogenetic's mechanism-of-action provides an elegant dissection of specific neuronal contributions to network function, the complex and potentially nonspecific mechanisms of electrical stimulation prohibits us from fully identifying which neuronal populations are being activated or deactivated. Furthermore, en passant fibers, perhaps from other subcortical arousal systems, are surely affected in an unknown, possibly contributory way. On the other hand, the relatively broad and potentially robust effects of electrical stimulation, a mode of neuromodulation already in wide clinical use, clearly allow rapid practical clinical translation especially when coupled with current responsive neurostimulation paradigms (Fountas et al. 2005; Anderson et al. 2008; Al-Otaibi et al. 2011; Morrell and Group RNSSiES 2011; Ben-Menachem and Krauss 2014).
With regard to alternative targets, other subcortical arousal structures have been investigated within the context of sleep, anesthesia, Parkinson's, and other disorders and have shown varying effects on level of arousal (Gummadavelli, Kundishora, et al. 2015). While therapeutic electrodes have been implanted in infratentorial targets very near PnO in humans, such as the pedunculopontine nucleus (Mazzone et al. 2005; Plaha and Gill 2005), a supratentorial target may offer a decreased surgical risk profile. Of note, the nucleus basalis is supratentorial, is in the ascending projection pathway of PnO, and has widespread cholinergic and noncholinergic arousal connections with the neocortex (Englot and Blumenfeld 2009; Englot et al. 2009; Pillay et al. 2014; Motelow et al. 2015) making it a promising potential future target for stimulation.
Impairment from loss of consciousness during seizures is a major contributor to the morbidity and mortality associated with the epilepsies (Vickrey et al. 2000; Sander 2003; Charidimou and Selai 2011; Chen et al. 2014). The mechanisms of subcortical impairment causing loss of consciousness during seizures are also implicated in the cardiorespiratory dysfunction leading to sudden unexpected death in epilepsy (Devinsky 2011; Massey et al. 2014). Furthermore, the decreased productivity, decreased quality of life, and social stigma associated with loss of consciousness highlight it as a clinical attribute worthy of being addressed. Continuous DBS of the anterior nucleus of the thalamus as well as NeuroPace-based responsive neuromodulation of epileptogenic foci have thus far been focused solely on decreasing seizure frequency (Salanova et al. 2015; Morrell and Halpern 2016). For patients lacking complete seizure control, that is, patients with medically and surgically refractory epilepsy, epileptic foci in eloquent areas, or health conditions which contraindicate a large resective surgery, improving their level of consciousness during seizures with DBS could have a dramatic impact on their quality of life as well as their overall mortality risk. Current stereotactic surgery on brain areas in the same or very close proximity to those discussed here have been proven safe and effective in other neurological disorders (Mazzone et al. 2005; Plaha and Gill 2005; Schiff et al. 2007; Giacino et al. 2012). We expect the present results to set the stage for rapid translation of these preclinical data into a new therapeutic strategy to improve quality of life for people with epilepsy.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by Howard Hughes Medical Institute —Citizens United for Research in Epilepsy Medical Student Research Fellowships (A.J.K. and A.G.), and by National Institutes of Health R01 NS066974, R21 NS083783, and P30 NS052519 (H.B.).
Supplementary Material
Notes
We thank Dr James O. McNamara for helpful comments and for making us aware of the behavior and physiology of postictal wet-dog shakes in rodent limbic seizures. We also thank Abbie Kundishora for illustrations used in the figures. Conflict of Interest: Dr Willie is a consultant for Monteris Medical and MRI Interventions. Dr Gerrard is a consultant for Medtronic.
References
- Ahnaou A, Basille M, Gonzalez B, Vaudry H, Hamon M, Adrien J, Bourgin P. 1999. Long-term enhancement of REM sleep by the pituitary adenylyl cyclase-activating polypeptide (PACAP) in the pontine reticular formation of the rat. Eur J Neurosci. 11:4051–4058. [DOI] [PubMed] [Google Scholar]
- Al-Otaibi FA, Hamani C, Lozano AM. 2011. Neuromodulation in epilepsy. Neurosurgery. 69:957–979; discussion 979. [DOI] [PubMed] [Google Scholar]
- Anderson WS, Kossoff EH, Bergey GK, Jallo GI. 2008. Implantation of a responsive neurostimulator device in patients with refractory epilepsy. Neurosurg Focus. 25:E12. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Krauss GL. 2014. Epilepsy: responsive neurostimulation-modulating the epileptic brain. Nat Rev Neurol. 10:247–248. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, editor. 2010. Higher Order Cerebral Function. In: Neuroanatomy through Clinical Cases, 2nd ed., Chapter 19. Sunderland, MA: Sinauer Assoc Publ. [Google Scholar]
- Blumenfeld H. 2012. Impaired consciousness in epilepsy. Lancet Neurol. 11:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. 2015. Neuroanatomical Basis of Consciousness. In: Gosseries O, Laureys S, Tononi G, editors. The Neurology of Consciousness. Chapter 1. NY: Elsevier, Academic Press. [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ et al. 2004. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 14:892–902. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. 2004. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 63:1015–1021. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, Gaultier C, Adrien J. 1995. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 6:532–536. [DOI] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. 2010. General anesthesia, sleep, and coma. N Engl J Med. 363:2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A, Selai C. 2011. The effect of alterations in consciousness on quality of life (QoL) in epilepsy: searching for evidence. Behav Neurol. 24:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Chen EY, Gebre RZ, Johnson MR, Li N, Vitkovskiy P, Blumenfeld H. 2014. Epilepsy and driving: potential impact of transient impaired consciousness. Epilepsy Behav. 30:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Chen WC, Shorten A, McClurkin M, Choezom T, Schmidt CP, Chu V, Bozik A, Best C, Chapman M et al. 2014. Impaired consciousness in partial seizures is bimodally distributed. Neurology. 82:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O. 2011. Sudden, unexpected death in epilepsy. N Engl J Med. 365:1801–1811. [DOI] [PubMed] [Google Scholar]
- Dyer RS, Swartzwelder HS, Eccles CU, Annau Z. 1979. Hippocampal after discharges and their post-ictal sequelae in rats: a potential tool for assessment of CNS neurotoxicity. Neurobehav Toxicol. 1:5–19. [PubMed] [Google Scholar]
- Englot DJ, Blumenfeld H. 2009. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res. 177:147–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Chang EF, Auguste KI. 2011. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 115:1248–1255. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. 2008. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 28:9066–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. 2009. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 29:13006–13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, Yu L, Gordon A, Purcaro MJ, Motelow JE et al. 2010. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 133:3764–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, Jenkins PD. 2005. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 83:153–158. [DOI] [PubMed] [Google Scholar]
- Furman M, Zhan Q, McCafferty C, Lerner BA, Motelow JE, Meng J, Ma C, Buchanan GF, Witten IB, Deisseroth K et al. 2015. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: effects on cortical physiology. Epilepsia. 56(12):e198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino J, Fins JJ, Machado A, Schiff ND. 2012. Central thalamic deep brain stimulation to promote recovery from chronic posttraumatic minimally conscious state: challenges and opportunities. Neuromodulation. 15:339–349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Fins JJ, Laureys S, Schiff ND. 2014. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 10:99–114. [DOI] [PubMed] [Google Scholar]
- Giber K, Diana MA, Plattner VM, Dugue GP, Bokor H, Rousseau CV, Magloczky Z, Havas L, Hangya B, Wildner H et al. 2015. A subcortical inhibitory signal for behavioral arrest in the thalamus. Nat Neurosci. 18:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadavelli A, Kundishora AJ, Willie JT, Andrews JP, Gerrard JL, Spencer DD, Blumenfeld H. 2015. Neurostimulation to improve level of consciousness in patients with epilepsy. Neurosurg Focus. 38:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H. 2015. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 56:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler R, Ore GD, Dieckmann G, Bricolo A, Dolce G. 1969. Behavioural and EEG arousal induced by stimulation of unspecific projection systems in a patient with post-traumatic apallic syndrome. Electroencephalogr Clin Neurophysiol. 27:306–310. [DOI] [PubMed] [Google Scholar]
- Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP et al. 2014. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 55:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 2001. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24:595–601. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. 1996. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 271:512–515. [DOI] [PubMed] [Google Scholar]
- Li W, Motelow JE, Zhan Q, Hu YC, Kim R, Chen WC, Blumenfeld H. 2015. Cortical network switching: possible role of the lateral septum and cholinergic arousal. Brain Stimul. 8:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Kohlmeier K, Morales FR, Chase MH. 1994. State dependency of the effects of microinjection of cholinergic drugs into the nucleus pontis oralis. Brain Res. 649:271–281. [DOI] [PubMed] [Google Scholar]
- Lynch G. 1971. Behavioral excitability and the giant-celled pontine reticular formation (nucleus reticularis pontis oralis). Brain Res. 32:449–453. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. 1998. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 86:29–37. [DOI] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. 2014. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 10:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. 2005. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 16:1877–1881. [DOI] [PubMed] [Google Scholar]
- McLardy T, Ervin F, Mark V, Scoville W, Sweet W. 1968. Attempted inset-electrodes-arousal from traumatic coma: neuropathological findings. Trans Am Neurol Assoc. 93:25–30. [PubMed] [Google Scholar]
- Morison RS, Dempsey EW, Morison BR. 1941. Cortical responses from electrical stimulation of the brain stem. Am J Physiol. 131:0732–0743. [Google Scholar]
- Morrell MJ, Group RNSSiES. 2011. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 77:1295–1304. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Halpern C. 2016. Responsive direct brain stimulation for epilepsy. Neurosurg Clin N Am. 27:111–121. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. 1949. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1:455–473. [PubMed] [Google Scholar]
- Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, Gummadavelli A, Zayyad Z, Lee HS, Chu V et al. 2015. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 85:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. 2002. The role of subcortical structures in human epilepsy. Epilepsy Behav. 3:219–231. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Penfield W. 1936. Epilepsy and surgical therapy. Arch Neuro Psychiatr. 36:449–484. [Google Scholar]
- Pillay S, Liu X, Baracskay P, Hudetz AG. 2014. Brainstem stimulation increases functional connectivity of basal forebrain-paralimbic network in isoflurane-anesthetized rats. Brain Connect. 4:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaha P, Gill SS. 2005. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 16:1883–1887. [DOI] [PubMed] [Google Scholar]
- Posner JBSC, Schiff ND, Plum F. 2007. Plum and Posner's Diagnosis of Stupor and Coma. USA: Oxford University Press. [Google Scholar]
- Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E et al. 2015. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 84:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JW. 2003. The epidemiology of epilepsy revisited. Curr Opin Neurol. 16:165–170. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O'Connor J et al. 2007. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 448:600–603. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Laureys S. 2009. Disorders of consciousness. Preface. Ann N Y Acad Sci. 1157:ix–xi. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. 1996. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 16:2788–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. 1993. Thalamocortical oscillations in the sleeping and aroused brain. Science. 262:679–685. [DOI] [PubMed] [Google Scholar]
- Sturm V, Kuhner A, Schmitt HP, Assmus H, Stock G. 1979. Chronic electrical stimulation of the thalamic unspecific activating system in a patient with coma due to midbrain and upper brain stem infarction. Acta Neurochir (Wien). 47:235–244. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. 2002. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 39:107–140. [DOI] [PubMed] [Google Scholar]
- Vickrey BG, Berg AT, Sperling MR, Shinnar S, Langfitt JT, Bazil CW, Walczak TS, Pacia S, Kim S, Spencer SS. 2000. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia. 41:760–764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.