Abstract
Human immunodeficiency virus-acquired immunodeficiency syndrome (HIV/AIDS) remains a global health problem. Current therapeutics specifically target the viral pathogen at various stages of its life cycle, although complex interactions between HIV and other pathogenic organisms are evident. Targeting HIV and concomitant infectious pathogens simultaneously, both by therapeutic regimens and in prevention strategies, would help contain the AIDS pandemic. Lectins, a ubiquitous group of proteins that specifically bind glycosylated molecules, are interesting compounds that could be used for this purpose, with demonstrated anti-HIV properties. In addition, potential coinfecting pathogens, including other enveloped viruses, bacteria, yeasts and fungi, and protozoa, display sugar-coated macromolecules on their surfaces, making them potential targets of lectins. This review summarizes the currently available findings suggesting that lectins should be further developed to simultaneously fight the AIDS pandemic and concomitant infections in HIV infected individuals.
1. Introduction
Human immunodeficiency virus (HIV) infection remains a leading public health problem, due to the rapid mutation of the virus, resistance to existing therapies, lack of a vaccine, and inadequate use of physical prophylaxis, for example, resistance to condom use for cultural or religious reasons [1]. Despite recent advances in acquired immunodeficiency syndrome (AIDS) therapy and the available prevention methods, HIV infection continues to spread worldwide with about 5,500 new infections every day [2]. In many developing countries, notably in sub-Saharan Africa, AIDS is considered a disaster because of its devastating socioeconomic effects [3].
There have been tremendous advances over the past years in the standard use of antiretroviral drugs (ARVs) for the treatment and prevention of HIV infection [4]. Indeed, current ARVs greatly improve the clinical outcomes of HIV positive individuals and limit disease transmission with improved safety, elevated tolerability, and high potency, especially if used early in the infection [5].
One of the most recent tools for HIV prevention is preexposure prophylaxis (PrEP), whereby individuals are prescribed daily doses of antiviral drugs in anticipation of exposure, thus lowering the risk of infection. Truvada was the first FDA approved drug for HIV PrEP in an attempt to reduce and prevent HIV infection among high risk individuals; its consistent intake is highly effective in preventing HIV infection [6]. Despite the increasing awareness of health benefits associated with PrEP, its use remains low especially among women [7]. Like other preventive measures, the efficacy of PrEP is highly related to adherence, heavily relying on individuals taking pills daily for long periods of time. Even though the use of antiviral drugs has increased the lifespan of individuals with HIV, they are still more susceptible to other opportunistic infections that are potentially lethal [8]. It was shown that individuals using PrEP are more prone to other sexually transmitted diseases, mainly from unprotected sex and increased number of sexual partners [9]. In addition, opportunistic infections also accelerate AIDS progression. All things considered, the high cost of antiviral drugs and the risk of opportunistic infections in individuals with HIV demand the development of new antiviral drugs that could simultaneously target other pathogens.
Lectins are a unique and heterologous class of proteins with the ability to recognize and reversibly bind a variety of sugar structures present on the cell surface [10]. They are found in a wide range of organisms, from viruses and bacteria to animals, plants, and humans [11]. The broad distribution in nature shows that lectins have important biological functions in the corresponding organisms, including cell-cell interactions, protection from pathogens, cell adhesion, and intracellular translocation of glycoproteins, also acting as storage proteins [12–14]. Interestingly, multiple lectins have been shown to neutralize a number of viruses, including HIV, making them attractive targets for the development of novel antiviral drugs [15]. Moreover, considering their mechanisms of action, increasing evidence suggests that lectins could also target other groups of pathogens, including prokaryotic and eukaryotic organisms. This review summarizes the currently available findings demonstrating that lectins can be considered promising means to combat the HIV pandemic as well as concomitant infections in HIV infected individuals, providing affordable regimens for prevention and/or treatment.
2. Lectins Can Be Used for the Treatment and/or Prevention of HIV Infection
Even though antiretroviral therapy has successfully improved health outcomes in HIV infected individuals, especially those living in industrialized countries, a high percentage of patients still do not have access to ARVs in poor areas (e.g., sub-Saharan Africa), where the disease burden is the highest [16]. Although availability is no longer a crucial issue, lack of trained clinicians and increased prevalence of associated diseases, such as tuberculosis, have impacted the management of HIV in sub-Saharan Africa [16]. Moreover, the fact that ARVs are associated with risk of developing other infectious diseases demands the development of new antiviral drugs to include in a comprehensive package designed to contain the AIDS pandemic.
Entry inhibitors would constitute important additions to the currently available weapons against HIV-1 infection [17, 18]. Indeed, recent approaches have led to the design of active compounds interfering with entry events. For example, T-20, T-2410, and T-2429 inhibit gp41 mediated virus entry [19–21]; meanwhile, Maraviroc belongs to a separate class of antiretroviral agents that target a host protein, the chemokine coreceptor CCR5, rather than a viral molecule [22].
HIV-1 entry into target cells is mediated by binding of its envelope glycoprotein to cell surface receptors. The envelope glycoprotein gp160 is originally synthesized as a single, glycosylated, polyprotein precursor, which is then cleaved by a cellular protease to yield two subunits, including gp120 (responsible for viral binding to CD4 receptor and a coreceptor, namely, CCR5 or CXR4) and gp41, which mediate viral fusion to the cell [23]. HIV uses gp120 to bind C-type lectin receptor (CLR) on macrophages as well as DC-SIGN, a C-type mannose-specific lectin, expressed by dendritic cells [24].
Due to the glycosylated nature of viral envelope proteins, carbohydrate-binding proteins have been considered potential anti-HIV agents that would block the host-virus interaction at its earliest stage, eventually preventing the establishment of the provirus [25]. Lectins with antiretroviral activities have been identified and isolated from animals [26], plants [27], halobios, and microorganisms [28] and could serve as anti-HIV natural products.
2.1. Mechanisms of Anti-HIV Activity of Lectins
Many enveloped viruses are covered by virally encoded glycoproteins displayed on the surface. In the case of HIV-1, the envelope glycoproteins gp120 and gp41 are heavily glycosylated, and N-linked carbohydrates might make up to 50% of the total molecular weight of gp120 [29]. All the potential 24 N-linked glycosylation sites are utilized in gp120, 13 of which contain complex-type oligosaccharides while 11 primarily comprise high-mannose-type and/or hybrid-type oligosaccharide structures [30, 31]. The associated oligosaccharides contain mannose, galactose, N-acetyl-glucosamine (GlcNAc), N-acetyl-galactosamine (GalNAc), L-fucose, and sialic acid in their branches [31]. The most credible mechanism proposed for virus-cell attachment involves the interaction between positively charged regions of the viral-envelope glycoproteins and negatively charged heparan sulphate proteoglycans. The fusion process starts with binding of gp120 to the cell-surface CD4 antigen. These entry events are vulnerable to agents that specifically and strongly interact with the glycans since they may disturb the association of viral envelope proteins with host cell receptors, that is, gp120 and CD4, respectively [18, 32]. It is believed that these agents alter the efficient interaction between gp120 (or gp41) and its (co)receptors through steric hindrance, prevention of necessary conformational changes of env, and/or cross-linking of several glycans present on env and/or the target cell [33]. Lectins, which are carbohydrate-binding proteins, possess such binding properties. Indeed, sound anti-HIV effects are attributed to lectins with specific recognition for mannose (Man) and/or GlcNAc [8, 33]. A number of plant and microbial lectins have been researched in recent years, including griffithsin (GRFT), actinohivin (AH), concanavalin-A (ConA), cyanovirin-N (CV-N), microvirin (MVN), and banana lectin (BanLec). Generally speaking, these lectins contain multiple sugar-binding sites allowing them to form multivalent interactions with gp120. Such interactions confer to lectins the ability to neutralize a broad range of lab-adapted and clinically isolated strains of HIV-1 and HIV-2. The following are a few examples of lectins that have been investigated for their antiretroviral activities.
2.2. Cyanovirin-N (CV-N)
Cyanovirin-N (CV-N) is a lectin that was firstly isolated from the cyanobacterium Nostoc ellipsosporum, in an attempt to discover anti-HIV agents from natural extracts [19]. The CV-N lectin consists of 101 amino acids with a molecular weight of 11 kDa and contains two carbohydrate-binding sites with specificity towards the terminal α1,2-mannose sugars on high-mannose glycans [34]. CV-N has broad neutralization activity in the low nanomolar range against primary isolates and laboratory-adapted strains of HIV-1 and HIV-2, simian immunodeficiency virus (SIV), and feline immunodeficiency virus [35]. This broad activity of CV-N is due to high affinity of binding to gp120, essential for its anti-HIV effects. The mechanism of action of CV-N is still not quite understood, but it is believed that the antiviral activity against HIV occurs after virus-cell attachment or at a post-CD4-binding step in the entry process [36]. Unfortunately, CV-N treatment results in cell activation, mitogenicity, and increased cytokine production in human PMBC [37].
2.3. Microvirin (MVN)
Microvirin (MVN) is a lectin with 108 amino acids (14 kDa) isolated from the cyanobacterium Microcystis aeruginosa. MVN is a monomer in solution with one sugar binding site that also recognizes terminal α1,2-mannose sugars [38]. Like CV-N, MVN has the ability to neutralize a wide range of laboratory-adapted strains and clinical isolates with low nanomolar potency in most HIV-1 group M clades. Even though MNV shares 33% identity with CV-N, the former lectin is more potent and 50-fold less toxic than CV-N. Therefore, MNV is a very attractive lectin as a topical microbicide because of its ability to inhibit HIV-1 and its reduced toxicity profile [39].
2.4. Actinohivin (AH)
Actinohivin (AH) is an anti-HIV lectin isolated from the culture broth of the actinomycete Longispora albida [40]. The protein is a single polypeptide with a molecular weight of 12.5 kDa and 114 amino acids that form three sugar-binding pockets with LD-QXW motifs [28]. These three segments are highly conserved and necessary for both B- and T-tropic antisyncytium formation activity [41]. Tanaka et al. reported that AH specifically binds to high-mannose-type glycans [37]. Additionally, AH has been shown to have affinity only to glycoproteins with multiple high-mannose glycans, unlike CV-N which can bind a single high-mannose glycan attached to a protein. Also unlike CV-N, AH does not show cytopathic or mitogenic effects. Based on previous studies AH has the potential to be developed as a microbicide drug since it can effectively inhibit HIV-1 and HIV-2 with low IC50 values (2–110 nM), block syncytium formation, and display neutralization activity against laboratory-adapted strains with minimal variation in antiviral activity [42].
2.5. BanLec
BanLec is a member of the family of the jacalin-related lectins. The lectin isolated from the fruit of bananas, Musa acuminate, also has affinity towards high-mannose structures. The native lectin is a dimer composed of two identical subunits of 15 kDa containing 141 amino acids [27] and two sugar binding sites each [43]. It was shown that BanLec can inhibit various HIV-1 isolates with different tropisms in vitro, with IC50 values in the low nanomolar range. Similar to the above carbohydrate-binding proteins, BanLec inhibits HIV infection at the viral entry step by binding to high-mannose structures present on the heavily glycosylated gp120 in a concentration dependent manner, thus preventing attachment of the virus to the cell. Swanson et al. also reported that BanLec is a potent mitogen for murine T-cells, although the effects depend on the mouse strain used. Interestingly, a mutation within the sugar-binding site of BanLec notably reduces its mitogenic activity without affecting HIV neutralization. The new engineered lectin has the potential to be used as a microbicide drug [44].
2.6. Griffithsin (GRFT)
Griffithsin (GRFT) is another high-mannose-binding lectin isolated from the marine red algae Griffithsia sp. and considered the most potent HIV entry inhibitor to date [45]. The protein contains 121 amino acids and folds into a stable domain-swapped homodimer, with each subunit capable of binding three monosaccharides. GRFT is currently the leading lectin candidate for clinical use as an HIV prophylactic, neutralizing HIV with IC50 in the picomolar range [45]. Indeed, this lectin is more potent than broadly neutralizing antibodies (bNAbs), including 2G12 which also binds to high-mannose-type glycans. GRFT has potent activity against both T-tropic and M-tropic viruses and inhibits clade A, B, and C viruses. Furthermore, it is capable of preventing HIV in human cervical explant tissues with no proinflammatory cytokine production. GRFT has an excellent safety profile when tested in a rabbit vaginal irritancy model [46], with minimal toxicity when administered in single or chronic subcutaneous doses in mice and guinea pigs [47].
3. Lectins Are Active against Viruses Other Than HIV
Considering its clinical importance, the majority of studies assessing antiviral lectins have focused on HIV. However, based on their mechanisms of action carbohydrate-binding agents may target a multitude of enveloped viruses which share the feature of heavily glycosylated proteins on their surfaces. Not surprisingly, anti-HIV lectins have been extensively evaluated for their effects on other enveloped viruses (Table 1). For instance, Aspidistra elatior lectin (AEL) displays antiviral effects against the enveloped respiratory syncytial virus but also against Coxsackie virus B4, a non-enveloped virus [48], suggesting that lectins may target other viral components than surface glycoconjugates. This notion was confirmed by the protective effects of GRFT in mice infected with vaginal HPV infection, although more pronounced effects were achieved by the carrageenan-GRFT combination; such anti-HPV activity likely occurs via α6 integrin internalization as shown in vitro [49]. In addition, intraperitoneal GRFT was shown to prevent Japanese encephalitis virus (JEV) entry, both in vitro and in vivo [50]. Mechanistically, GRFT was recently reported to bind to the glycosylated proteins E and prM of JEV [51]. GRFT was assessed in parallel with other potent anti-HIV lectins from algae, namely, CV-N and scytovirin (SVN), and differential inhibitory activities were found against other enveloped viruses such as gammaretroviruses and deltaretroviruses [52]. A few envelope viruses sensitive to lectins are discussed below.
Table 1.
Lectins with neutralization activities towards different enveloped viruses.
| Virus | Antiviral lectins | EC50/IC50 | References |
|---|---|---|---|
| Hepatitis C | Cyanovirin N (CV-N) | 1.6–17.6 nM | [53] |
| Griffithsin (GRFT) | 13.9 nM | [54] | |
| Microcystis viridis (MVL) | 30.4 nM | [55] | |
| Galanthus nivalis (GNA) | 11.1 nM | [55] | |
| Cymbidium agglutinin (CA) | 10 nM | [56] | |
| Hippeastrum hybrid agglutinin (HHA) | 3 nM | [56] | |
|
| |||
| Influenza A/B | Eucheuma serra (ESA-2) | 12.4 nM | [57] |
| Kappaphycus alvarezii (KAA-2) | 12.3/1–10 nM | [58] | |
| Boodlea coasta (BCA) | 18.8–74.2 nM | [59] | |
| Narcissus tazetta (NTL) | 0.20 μg/ml–1.33 μg/ml | [60] | |
|
| |||
| Herpes simplex types 1 and 2 | Griffithsin (GRFT) | 230 nM | [61] |
| Cyanovirin N (CV-N) | Low nM | [62] | |
| Jackfruit lectin (JFL) | 2.5 μg/ml | [63] | |
| Typhonium divaricatum (L.) Decne | 3.054 μg/ml | [64] | |
| Polygonatum odoratum (POL) | 2.5 μg/ml | [65] | |
|
| |||
| Japanese encephalitis virus | Griffithsin (GRFT) | 20 nM | [50] |
|
| |||
| Coronavirus | Hippeastrum hybrid agglutinin (HHA) | 3.2 μg/ml | [66] |
| Galanthus nivalis (GNA) | 6.2 μg/ml | [66] | |
| Cymbidium agglutinin (CA) | 4.9 μg/ml | [66] | |
| Urtica dioica agglutinin (UDA) | 1.3 μM | [67] | |
|
| |||
| HIV | Griffithsin (GRFT) | 0.04–0.63 nM | [45] |
| BanLec | 0.33–4.1 nM | [44] | |
| Actinohivin (AH) | 2–110 nM | [42] | |
| Cyanovirin N (CV-N) | 0.1–36.8 | [19] | |
| Microvirin (MVN) | 2.1–167 nM | [39] | |
3.1. Lectins Targeting Hepatitis C Virus (HCV)
Presently, 20% of HIV patients are coinfected with HCV [68]. Infection with HCV causes either acute or chronic infection, which can progress to cirrhosis with the need for liver transplantation [69]. Usually, HCV-HIV coinfection is associated with a faster progression to cirrhosis and liver failure and shows poor response to treatment [70, 71]. As the most potent anti-HIV lectin described so far, GRFT has been widely assessed for its activities against other enveloped viruses. GRFT has been shown to mitigate HCV infection of mice harboring human primary hepatocytes in the liver and prevents in vitro HCV infection of Huh-7 hepatoma cells [54]. Notably, GRFT binds to the HCV envelope glycoproteins E1 and E2, blocking viral entry into human hepatocytes [72]. In addition to GRFT, other lectins, including those with anti-HIV activities, have the ability to bind and neutralize HCV. HCV is an enveloped virus, like HIV, with two heavily glycosylated glycoproteins that include E1 and E2 [73]. E1 contains 4-5 N-linked glycosylation sites and E2 up to 11, many of which are high-mannose-type glycans that ensure proper folding, attachment, and entry, protecting the virus from neutralizing antibodies [74]. Targeting viral entry with lectins could potentially be a new strategy to combat HCV. Indeed, cyanovirin-N can inhibit HCV infection by binding the N-linked glycans on its surface, preventing the interaction between E2 and the entry receptor CD81 [53]. Other studies demonstrated that CV-N, Microcystis viridis lectin (MVL), and Galanthus nivalis agglutinin (GNA) inhibit HCV in vitro, with IC50 values of 0.6 nM, 30.4 nM, and 11.1 nM, respectively, likely through distinct and complex modes of action [55, 75].
3.2. Lectins with Anti-HSV Activities
Another opportunistic viral pathogen in HIV infected individuals is herpes simplex virus type 2 (HSV-2). HSV-2 is sexually transmitted and a leading cause of genital herpes. Some individuals with the virus never show symptoms, while most experience regular outbreaks of painful sores on the genitalia [76]. It is estimated that more than 400 million people are infected with HSV-2, with a high prevalence in Africa [77]. HSV-2 is a major risk factor for acquiring HIV in both men and women. Indeed, having HSV increases the chances of HIV infection 3-fold [78], and the chances are higher in individuals with newly acquired HSV [79]. Multiple studies have reported a biological synergy between these two viruses; a number of them showed that coinfection with HSV induces HIV replication and increases HIV-1 infectiousness [80]. Coinfection with HSV has also been shown to increase genital shedding from both viruses potentially explaining the higher rate of HIV transmissibility in these individuals [81, 82]. It was reported that GRFT confers protection to mice infected with genital HSV-2 likely by preventing cell-to-cell spread, with no significant adverse effects [61]. In addition to GRFT, other lectins have been assessed for their antiherpes activities. For example, CV-N inhibits entry and cell-to-cell spread of HSV-1 with IC50s in the nanomolar range [62]. Furthermore, a mannose-binding lectin from Typhonium divaricatum (L.) Decne (family Araceae) displays antiviral effects against HSV-II, as well as Jackfruit lectin (JFL) from Artocarpus heterophyllus [63, 64]. Meanwhile, Polygonatum odoratum lectin (POL), a GNA-related mannose-binding lectin, also exerts remarkable anti-HSV-II effects [65].
3.3. Lectins Targeting Influenza Viruses
Influenza causes high morbidity in HIV positive individuals [83]. Several reports have demonstrated the anti-inhibitory activities of lectins against influenza viruses. For example, a lectin from the red alga Kappaphycus alvarezii (KAA-2) inhibits infection by multiple influenza strains with EC50 values in the low nanomolar range, by interfering with virus entry into host cells upon direct binding of hemagglutinin (HA) on the viral envelope [59]. Similarly, a lectin derived from the green alga Boodlea coacta (BCA) exerts potent anti-influenza effects by directly binding HA of multiple strains, including the pandemic H1N1-2009 virus [59]. In addition, three other lectins, including ConA, Lens culinaris agglutinin (LCA), and peanut agglutinin (PNA), were shown to suppress cell fusion and hemadsorption associated with human parainfluenza virus type 2 (hPIV-2) in vitro, mainly by preventing virus adsorption to the cells [84]. In a screen of 12 lectins, the red alga Eucheuma serra derived high-mannose binding anti-HIV lectin ESA-2 was very effective in inhibiting influenza virus infection with an EC50 of 12.4 nM [57]. Finally, the mannose-specific Narcissus tazetta lectin (NTL) strongly inhibits influenza A (H1N1, H3N2, and H5N1) and influenza B viruses with IC50 values between 0.20 μg/ml and 1.33 μg/ml and markedly reduces plaque formation by the human respiratory syncytial virus (RSV) with an IC50 of 2.30 μg/ml [60].
3.4. Lectins with Inhibitory Activities against Coronaviruses
Coronaviruses constitute an important class of human and animal pathogens, and some have been assessed for their susceptibility to lectins. Interestingly, GRFT also prevents SARS coronavirus (SARS-CoV) infection in vitro and in vivo, through specific binding to its spike glycoprotein, and shows activity against multiple other coronaviruses pathogenic to humans, other mammals, and birds; in mice, these inhibitory effects were accompanied with a specific inhibition of deleterious host immune reactions in response to SARS [85]. The Middle East respiratory syndrome coronavirus (MERS-CoV), another highly pathogenic human coronavirus, is inhibited at the entry level by GRFT to prevent infection in vitro [86]. In addition, Hippeastrum hybrid agglutinin (HHA), GNA, Cymbidium agglutinin (CA), and Urtica dioica agglutinin (UDA) demonstrate antiviral activities against coronaviruses in vitro and/or in vivo [67]. In an impressive screening of 33 plant lectins, remarkable antiviral effects on both SARS-CoV and feline infectious peritonitis virus (FIPV) with EC50 values at low μg/ml levels were observed, with strongest activities found predominantly among mannose-binding lectins [66]. Based on these data, lectins should be included in antiviral strategies to fight SARS coronavirus infections [87].
4. Lectins as Potential Candidates for Fighting Pathogenic Organisms Other Than Viruses in HIV Patients
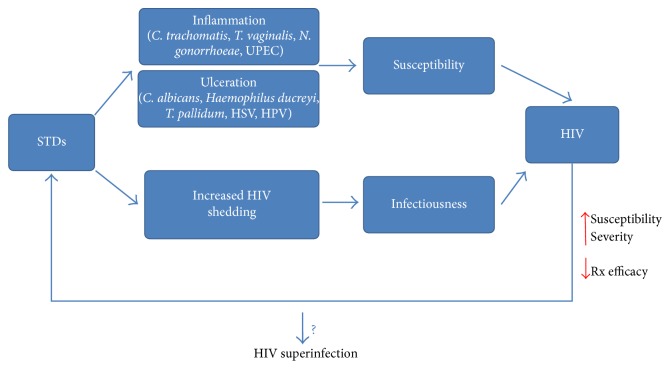
Lectins engage in nonenzymatic and noncovalent, but highly specific, interactions with carbohydrates, binding sugar moieties and oligosaccharides, for example, oligomannose N-linked glycans (NLG) displayed on the viral envelope glycoproteins. Such interactions have important biological effects, and lectins have been widely assessed for their potential activities against enveloped viruses as described above. However, among human pathogens, surface exposed oligosaccharides are not limited to viral envelopes. Indeed, it is widely admitted that glycosylation represents the most important cotranslational and posttranslational modification of proteins in virtually all living organisms [11, 88]. Thus, lectins could theoretically interfere with sugar moieties of glycans displayed on many cell types, including bacteria, yeasts, and parasites. It is known that the incidence of HIV/AIDS is exacerbated in individuals infected with other sexually transmitted pathogens. Both ulcerative (candidiasis, chancroid, syphilis, genital herpes, and genital warts) and inflammatory (chlamydia, gonorrhea, and trichomoniasis) sexually transmitted diseases (STDs) have been shown to increase susceptibility to HIV infection [89–91]. In addition, STDs increase virus shedding in HIV infected patients, rendering them even more infectious [91]. Conversely, in an immune system heavily altered by AIDS, the etiologic agents of STDs have an easier path to cause illness. Therefore, it is reasonable to assume that the biological and epidemiological synergies between HIV and STDs can also promote HIV superinfection, that is, reinfection of an individual who already has an established infection with a heterologous HIV strain (Figure 1). Although the amplitude of HIV superinfection is not entirely known, this complication certainly affects the magnitude of global HIV diversity, which is driven in large part by recombination between viruses [92]. Apart from these sexually transmissible pathogens, other microbes might also take advantage of the weakened immune system of HIV infected patients and cause various diseases and/or further alter the immune system.
Figure 1.
Bidirectional biological synergy between HIV and sexually transmitted pathogens. There are complex interactions between HIV infection and sexually transmitted diseases (STDs). Both inflammatory and ulcerative STDs increase HIV susceptibility, while enhancing virus shedding and therefore increasing patient infectiousness. This results in increased HIV burden. Meanwhile, HIV infection increases susceptibility to and the severity of STDs, decreasing also treatment efficacy. This vicious cycle might promote HIV superinfection, which is the reinfection of an individual who already has an established infection with a heterologous HIV strain. C. trachomatis: Chlamydia trachomatis; T. vaginalis: Trichomonas vaginalis; N. gonorrhoeae: Neisseria gonorrhoeae; UPEC: uropathogenic Escherichia coli; C. albicans: Candida albicans; T. pallidum: Treponema pallidum; HSV: herpes simplex virus; HPV: human papillomavirus; Rx: treatment.
Considering the above-mentioned synergy between HIV infection and STDs, in addition to the possibility of superinfection in seropositive individuals, there is an urgent need for novel anti-HIV agents, which would simultaneously target other pathogens. In the case of sexually transmitted organisms, a socioepidemiological rationale was recently proposed for the development of multipurpose prevention technologies [93], since current HIV prevention methods fall well short of needs. This would help tackle both AIDS and other STDs and likely improve the immune system of the affected patients.
4.1. Bacterial Organisms Potentially Susceptible to Lectins
Urinary tract infections (UTIs) are among the most common bacterial infections and show elevated prevalence rates in women and HIV positive individuals [94]. Glycoproteins have been shown to be involved in the transmission of bacterial pathogens. For instance, uropathogenic E. coli (UPEC), which causes the majority of urinary tract infections (UTIs), need to overcome the constant shear stress of urine flow. This is accomplished by bacterial attachment to the renal epithelium via the attachment organelles types 1 and P fimbriae [95]. More than 90% of all UPEC strains express the adhesin FimH, one of the most described mannose-specific bacterial lectins expressed on the tip of type 1 fimbriae [95–97]. Meanwhile, use of lectins to achieve attachment to host cells is widely spread in bacterial organisms. Indeed, several bacteria bind to the cell glycocalyx for colonization, as is the case for cells in contact with the environment, for example, epithelial cells [98]. Lectins safely delivered to these surfaces targeted by microbial lectins would compete for the binding of bacteria and prevent adhesion, consequently suppressing colonization and infection. Interestingly, we recently demonstrated that GRFT administered parenterally was mainly eliminated through urine [99], indicating that this molecule could help prevent colonization by competition with bacteria for the cell glycocalyx, especially those with mannose type lectins at the surface such as UPEC. Another lectin, Eutirucallin, isolated from the latex of Euphorbia tirucalli, also displays antimicrobial activity towards E. coli [100].
Chlamydia trachomatis, a critical etiologic agent of ocular and genital infections in humans, likely uses carbohydrates for attachment to host cells or entry. Three C. trachomatis glycoproteins, including the major outer membrane protein (MOMP; 40-kDa), a 32-kDa outer surface glycoprotein, and an 18-kDa molecule, have been reported [101, 102]. These surface exposed glycoproteins, especially, the MOMP, are critical for attachment and infectivity of C. trachomatis to HeLa cells, via their oligomannose-oligosaccharides [101]. It was reported that two plant lectins, including wheat germ agglutinin and Galanthus nivalis lectin, can block chlamydial attachment sites and inhibit infection in vitro [103, 104].
In Neisseria meningitides, surface glycosylated molecules, such as capsule polysaccharide, lipooligosaccharide, and O-linked glycoproteins, have been described, while N. gonorrhoeae produces both lipooligosaccharide and O-linked glycoproteins [105]. These glycans are very critical for the host pathogen interactions during infection by Neisseria spp. The wheat germ agglutinin, ricin, soybean, and peanut agglutinin have the ability to agglutinate different strains of Neisseria gonorrhoeae [106, 107].
Tuberculosis (TB) caused by the bacterium Mycobacterium tuberculosis is one of the top 10 causes of mortality worldwide according to the WHO [108]. In addition to killing over one million people yearly, TB is also the leading cause of death among HIV positive individuals [108]. Although TB is treatable, the prevalence of a multiresistant form of TB (MDR-TB) that does not respond to first-line anti-TB drugs calls for the development of new ways to cope with this infection. In M. tuberculosis, envelope mannosylated lipoarabinomannans (ManLAMs) and Mtb surface glycoproteins (glycosylated 45-kDa [Apa: alanine- and proline-rich antigenic] and 19-kDa proteins) are considered important antigenic molecules with essential roles in host protection against this pathogen; these antigens may be recognized by dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN) and other mannose-specific C-type lectins (C-TLs) [109]. Since M. tuberculosis organisms have large amounts of mannosylated cell-surface molecules, an attempt was made to assess whether lectins with potent anti-HIV activity can also inhibit these bacteria. It was reported that CV-N competes with C-type lectins DC-SIGN and mannose receptor for binding to ManLAMs and inhibits the binding of bacteria to dendritic cells. However, in vivo findings showed that this binding did not inhibit or delay infection. The authors speculated that such observation could be due to the fact that murine C-type lectins differ from human versions, which requires further investigation [110].
Recently, the novel lectin CasuL produced by Calliandra surinamensis was shown to display bacteriostatic effects and reduce biofilm formation by nonresistant and oxacillin-resistant Staphylococcus spp. [111]. The plant lectins ConBr and CFL produced by Canavalia brasiliensis and Cratylia argentea, respectively, induce antimicrobial and immunomodulatory effects in mouse peritoneal macrophages upon infection with Salmonella enterica serovar Typhimurium [112]. Despite their preliminary nature, these findings indicate that lectins could well target bacterial organisms.
4.2. Yeasts and Other Fungi Are Potential Targets of Lectins
Candida normally lives harmless in several locations of the human body, including the skin and mucosal membranes, but can overgrow and cause a disease named candidiasis in the oral cavity, the gastrointestinal tract, and the genitalia [113]. For instance, in immunocompromised individuals the selective loss of Th17 cells with the progression of HIV infection causes the decay of fungal containment [114]. Interestingly, Candida spp. possess β-1,2 mannosylated glycoproteins (e.g., Candida mannan) in addition to other sugars on their cell surface [115]. It is estimated that 80–90% of the cell wall protein mass in Candida are mannose residues added by N-glycosylation, O-glycosylation, and/or glycosylphosphatidylinositol (GPI) anchoring [116]. These sugar molecules are employed by the pathogen to initiate infection of the host cells in various mucosal surfaces. However, Candida spp. also adhere to inert abiotic surfaces, including intravascular and urinary catheters, prosthetic cardiac valves, and denture prostheses [117]. Interestingly, Punica granatum produces a chitin-binding lectin (PgTeL), with antifungal activity against Candida albicans and C. krusei, which are commonly found in immunocompromised individuals such as AIDS patients [118]. In addition, low dose of the plant lectin Con A has been shown to protect mice from a lethal dose of C. albicans by producing tumor necrosis factor α (TNFα) and activating macrophages, thus increasing the clearance of C. albicans [119]. SteLL, a chitin-binding lectin isolated from the leaves of the plant Schinus terebinthifolius, has shown antifungal activity against C. albicans at a minimal inhibitory concentration (MIC) of 6.5 μg/ml [120]. Lectins isolated from legumes have also shown antifungal activity. For instance, ConBr from Canavalia brasiliensis and DvioL isolated from Dioclea violacea have both demonstrated antifungal activity against C. albicans at a MIC of 16 μg/ml [121]. Another lectin, CasuL, alters the cell morphology and damages the cell wall in Candida krusei, indicating its potent antifungal properties [111].
Moreover, Lunatin, obtained from edible seeds of Phaseolus lunatus billb, shows potent antifungal activities against multiple fungal species, such as Sclerotium rolfsii, Physalospora piricola, Fusarium oxysporum, and Botrytis cinerea [122]. Taken together, the above findings demonstrate that lectins could interfere with the establishment of fungal diseases, though there is a need for further investigation.
4.3. Parasitic Organisms Potentially Affected by Lectins
The glycoconjugates of eukaryotic parasites, including N-glycans, O-glycans, and lipophosphoglycans, are critical to host-pathogen interactions, including adherence, invasion, and immune activation suggesting their potential importance in virulence [123].
Lipophosphoglycan associated attachment of trichomonads to the mucosal surface drive infection by Trichomonas vaginalis, commonly diagnosed in humans (170 million new infections/year) [123]. It is known that infection with T. vaginalis increases the risk of HIV acquisition [124]. Similar to HIV, T. vaginalis is sexually transmitted, meaning that using lectins with anti-HIV activity could potentially affect this parasite as well. Indeed, the well-known antiviral lectins CV-N and GRFT were recently evaluated for their effects on adherence of Trichomonas vaginalis to ectocervical cells as well as on Tritrichomonas infection in mice; treatment with the above lectins resulted in increased adherence of ricin-resistant mutants to ectocervical cells and organotypic EpiVaginal tissue cells, with decreased Tritrichomonas amounts recovered from the mouse vagina [125]. Although these effects were modest, they clearly provide a proof of concept that lectins could help tackle parasitic infections in humans.
Toxoplasma gondii is an obligate intracellular parasite that causes the disease known as toxoplasmosis. According to the CDC, more than 30 million individuals in the USA are T. gondii carriers [126]. Even though Toxoplasma gondii infection is asymptomatic in most people, pregnant women and immunocompromised individuals are at high risk of severe disease [127, 128]. Two lectins isolated from plants, including ScLL and ArtinM, have been shown to be beneficial in controlling T. gondii infection in mice [129]. The latter lectins induce the production of cytokines necessary to control T. gondii infection without any cytotoxicity. Additionally, they stimulate nitric oxide production by macrophages required for parasite killing. Eutirucallin, a plant lectin with broad antimicrobial properties, also showed antiparasitic activity in vitro against T. gondii [100].
5. Conclusion
Lectins are highly potent in neutralizing a very broad range of HIV strains and other enveloped viruses, with potential activity against multiple pathogenic prokaryotic and eukaryotic organisms that further complicate the HIV/AIDS problem. Therefore, these molecules deserve in-depth assessment for their inclusion in current efforts to end HIV infection. It should be noted that some lectins possess cytotoxicity and mitogenicity, which could potentially lead to severe adverse effects if used in humans. Meanwhile, most lectins are not monospecific and actually recognize a handful of different sugar types. Those recognizing glycans found on the surface of normal human cells have the potential to elicit off-target toxicity. However, comprehensive studies by our team and others have demonstrated the safety of GRFT. While still in preclinical development, lectins have shown tremendous potential to inhibit HIV and other pathogens and could be used to prevent multiple infections and/or improve the overall health status of HIV infected individuals.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Pool R., Hart G., Green G., Harrison S., Nyanzi S., Whitworth J. Men’s attitudes to condoms and female controlled means of protection against HIV and STDs in south-western Uganda. 2000;2(2):197–211. doi: 10.1080/136910500300804. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. How AIDS changed everything -MDG6: 15 years, 15 lessons of hope from the AIDS response. 2015.
- 3.Gökengin D., Doroudi F., Tohme J., Collins B., Madani N. HIV/AIDS: Trends in the Middle East and North Africa region. 2016;44:66–73. doi: 10.1016/j.ijid.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Volberding P. A. HIV treatment and prevention: An overview of recommendations from the 2016 IAS-USA antiretroviral guidelines panel. 2017;25(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Schuetz A., Deleage C., Sereti I., et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. 2014;10(12) doi: 10.1371/journal.ppat.1004543.e1004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima N., Davey D. J., Klausner J. D. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. 2016;30(14):2251–2252. doi: 10.1097/QAD.0000000000001185. [DOI] [PubMed] [Google Scholar]
- 7.Johnsen L. E., Thimm M. A., Singer J. M., Page K. R. P2.24Awareness and interest in pre-exposure prophylaxis (PREP) among patients receiving services at a public sexually transmitted diseases (STD) clinic in a high prevalence urban setting. Proceedings of the STI and HIV World Congress Abstracts; July 2017; Rio de Janeiro, Brazil. [DOI] [Google Scholar]
- 8.Davis J. L., Fei M., Huang L. Respiratory infection complicating HIV infection. 2008;21(2):184–190. doi: 10.1097/QCO.0b013e3282f54fff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo J. M., Dombrowski J. C., Mayer K. H. Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement. 2018;15(1):p. e1002485. doi: 10.1371/journal.pmed.1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos A. F. S., da Silva M. D. C., Napoleão T. H., Paiva P. M. G., Correia M. T. S., Coelho L. C. B. B. Lectins: Function, structure, biological properties andpotential applications. 2014;15:41–62. [Google Scholar]
- 11.Mitchell C. A., Ramessar K., O'Keefe B. R. Antiviral lectins: Selective inhibitors of viral entry. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita K., Hara-Kuge S., Ohkura T. Intracellular lectins associated with N-linked glycoprotein traffic. 1999;1473(1):147–160. doi: 10.1016/S0304-4165(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J.-F., Han Y., Xing L.-J., Xu Y.-Y., Xu Z.-H., Chong K. Cloning and expression of a novel cDNA encoding a mannose-specific jacalin-related lectin from Oryza sativa. 2006;47(1):133–139. doi: 10.1016/j.toxicon.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Song L., Li C., et al. Cloning and characterization of a novel C-type lectin from Zhikong scallop Chlamys farreri. 2007;44(5):722–731. doi: 10.1016/j.molimm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Emau P., Tian B., O'Keefe B. R., et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. 2007;36(4-5):244–253. doi: 10.1111/j.1600-0684.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 16.Taylor G. Rolling out HIV antiretroviral therapy in sub-Saharan Africa: 2003–2017. 2018;44(2):68–70. doi: 10.14745/ccdr.v44i02a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esté J. A., Telenti A. HIV entry inhibitors. 2007;370(9581):81–88. doi: 10.1016/S0140-6736(07)61052-6. [DOI] [PubMed] [Google Scholar]
- 18.Moore J. P., Doms R. W. The entry of entry inhibitors: A fusion of science and medicine. 2003;100(19):10598–10602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd M. R., Gustafson K. R., McMahon J. B., et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilby J. M., Hopkins S., Venetta T. M., et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. 1998;4(11):1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 21.Mavioso I. C. V. C., de Andrade V. C. R., Palace Carvalho A. J., Martins do Canto A. M. T. Molecular dynamics simulations of T-2410 and T-2429 HIV fusion inhibitors interacting with model membranes: Insight into peptide behavior, structure and dynamics. 2017;228:69–80. doi: 10.1016/j.bpc.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur R. D., Novak R. M. Maraviroc: The first of a new class of antiretroviral agents. 2008;47(2):236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 23.He Y., Cheng J., Li J., et al. Identification of a critical motif for the human immunodeficiency virus type 1 (HIV-1) gp41 core structure: Implications for designing novel anti-HIV fusion inhibitors. 2008;82(13):6349–6358. doi: 10.1128/JVI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balzarini J., Van Damme L. Microbicide drug candidates to prevent HIV infection. 2007;369(9563):787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang L., Zhang L., Chen C. H. Potential drug targets on the HIV-1 envelope glycoproteins, gp120 and gp41. 2003;9(18):1453–1462. doi: 10.2174/1381612033454720. [DOI] [PubMed] [Google Scholar]
- 26.Wang J.-H., Kong J., Li W., et al. A β-galactose-specific lectin isolated from the marine worm Chaetopterus variopedatus possesses anti-HIV-1 activity. 2006;142(1-2):111–117. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Peumans W. J., Zhang W., Barre A., et al. Fruit-specific lectins from banana and plantain. 2000;211(4):546–554. doi: 10.1007/s004250000307. [DOI] [PubMed] [Google Scholar]
- 28.Chiba H., Inokoshi J., Okamoto M., et al. Actinohivin, a novel anti-HIV protein from an actinomycete that inhibits syncytium formation: Isolation, characterization, and biological activities. 2001;282(2):595–601. doi: 10.1006/bbrc.2001.4495. [DOI] [PubMed] [Google Scholar]
- 29.Mizuochi T., Spellman M. W., Larkin M., Solomon J., Basa L. J., Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. 1988;254(2):599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain bisulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. 1990;265(18):10373–10382. [PubMed] [Google Scholar]
- 31.Botos I., Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. 2005;88(2):233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Mondor I., Moulard M., Ugolini S., et al. Interactions among HIV gp120, CD4, and CXCR4: Dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. 1998;248(2):394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 33.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. 2006;71(2-3):237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Bolmstedt A. J., O'Keefe B. R., Shenoy S. R., Mcmahon J. B., Boyd M. R. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. 2001;59(5):949–954. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- 35.Mori T., Boyd M. R. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. 2001;45(3):664–672. doi: 10.1128/AAC.45.3.664-672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dey B., Lerner D. L., Lusso P., Boyd M. R., Elder J. H., Berger E. A. Multiple antiviral activities of cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. 2000;74(10):4562–4569. doi: 10.1128/JVI.74.10.4562-4569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka H., Chiba H., Inokoshi J., et al. Mechanism by which the lectin actinohivin blocks HIV infection of target cells. 2009;106(37):15633–15638. doi: 10.1073/pnas.0907572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahzad-ul-Hussan S., Gustchina E., Ghirlando R., Clore G. M., Bewley C. A. Solution structure of the monovalent lectin microvirin in complex with Manα(1-2)Man provides a basis for anti-HIV activity with low toxicity. 2011;286(23):20788–20796. doi: 10.1074/jbc.M111.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huskens D., Férir G., Vermeire K., et al. Microvirin, a novel α(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. 2010;285(32):24845–24854. doi: 10.1074/jbc.m110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inokoshi J., Chiba H., Asanuma S., Takahashi A., Omura S., Tanaka H. Molecular cloning of actinohivin, a novel anti-HIV protein from an actinomycete, and its expression in escherichia coli. 2001;281(5):1261–1265. doi: 10.1006/bbrc.2001.4496. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi A., Inokoshi J., Chiba H., Omura S., Tanaka H. Essential regions for antiviral activities of actinohivin, a sugar-binding anti-human immunodeficiency virus protein from an actinomycete. 2005;437(2):233–240. doi: 10.1016/j.abb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Hoorelbeke B., Huskens D., Férir G., et al. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. 2010;54(8):3287–3301. doi: 10.1128/aac.00254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meagher J. L., Winter H. C., Ezell P., Goldstein I. J., Stuckey J. A. Crystal structure of banana lectin reveals a novel second sugar binding site. 2005;15(10):1033–1042. doi: 10.1093/glycob/cwi088. [DOI] [PubMed] [Google Scholar]
- 44.Swanson M. D., Boudreaux D. M., Salmon L., et al. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. 2015;163(3):746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori T., O'Keefe B. R., Sowder R. C., II, et al. Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 46.O'Keefe B. R., Vojdani F., Buffa V., et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. 2009;106(15):6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barton C., Kouokam J. C., Lasnik A. B., et al. Activity of and effect of subcutaneous treatment with the broad- Spectrum antiviral lectin griffithsin in two laboratory rodent models. 2014;58(1):120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X.-C., Zhang Z.-W., Chen Y.-E., Yuan M., Yuan S., Bao J.-K. Antiviral and antitumor activities of the lectin extracted from Aspidistra elatior. 2015;70(1-2):7–13. doi: 10.1515/znc-2014-4108. [DOI] [PubMed] [Google Scholar]
- 49.Levendosky K., Mizenina O., Martinelli E., et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. 2015;59(12):7290–7298. doi: 10.1128/AAC.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishag H. Z. A., Li C., Huang L., et al. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. 2013;158(2):349–358. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishag H. Z. A., Li C., Wang F., Mao X. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. 2016;215:50–54. doi: 10.1016/j.virusres.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen S. M. R., Ruscetti F. W., Rein A., et al. Differential inhibitory effects of cyanovirin-N, griffithsin, and scytovirin on entry mediated by envelopes of gammaretroviruses and deltaretroviruses. 2014;88(4):2327–2332. doi: 10.1128/JVI.02553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helle F., Wychowski C., Vu-Dac N., Gustafson K. R., Voisset C., Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. 2006;281(35):25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 54.Meuleman P., Albecka A., Belouzard S., et al. Griffithsin has antiviral activity against hepatitis C virus. 2011;55(11):5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kachko A., Loesgen S., Shahzad-Ul-Hussan S., et al. Inhibition of hepatitis C virus by the cyanobacterial protein microcystis viridis lectin: Mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. 2013;10(12):4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertaux C., Daelemans D., Meertens L., et al. Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. 2007;366(1):40–50. doi: 10.1016/j.virol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Sato Y., Morimoto K., Kubo T., et al. Entry inhibition of influenza viruses with high mannose binding lectin ESA-2 from the red alga Eucheuma serra through the recognition of viral hemagglutinin. 2015;13(6):3454–3465. doi: 10.3390/md13063454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato Y., Morimoto K., Hirayama M., Hori K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. 2011;405(2):291–296. doi: 10.1016/j.bbrc.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato Y., Hirayama M., Morimoto K., Yamamoto N., Okuyama S., Hori K. High mannose-binding lectin with preference for the cluster of α1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. 2011;286(22):19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ooi L. S. M., Ho W.-S., Ngai K. L. K., et al. Narcissus tazetta lectin shows strong inhibitory effects against respiratory syncytial virus, influenza A (H1N1, H3N2, H5N1) and B viruses. 2010;35(1):95–103. doi: 10.1007/s12038-010-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nixon B., Stefanidou M., Mesquita P. M. M., et al. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. 2013;87(11):6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiwari V., Shukla S. Y., Shukla D. A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. 2009;84(1):67–75. doi: 10.1016/j.antiviral.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wetprasit N., Threesangsri W., Klamklai N., Chulavatnatol M. Jackfruit lectin: Properties of mitogenicity and the inhibition of herpesvirus infection. 2000;53(4):156–161. [PubMed] [Google Scholar]
- 64.Luo Y., Xu X., Liu J., et al. A novel mannose-binding tuber lectin from Typhonium divaricatum (L.) Decne (family Araceae) with antiviral activity against HSV-II and anti-proliferative effect on human cancer cell lines. 2007;40(3):358–367. doi: 10.5483/bmbrep.2007.40.3.358. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y., Xu H.-L., Zhang Z.-T., et al. Characterization, molecular cloning, and in silico analysis of a novel mannose-binding lectin from Polygonatum odoratum (Mill.) with anti-HSV-II and apoptosis-inducing activities. 2011;18(8-9):748–755. doi: 10.1016/j.phymed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Keyaerts E., Vijgen L., Pannecouque C., et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumaki Y., Wandersee M. K., Smith A. J., et al. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alter M. J. Epidemiology of viral hepatitis and HIV co-infection. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Davis G. L., Alter M. J., El-Serag H., Poynard T., Jennings L. W. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. 2010;138(2):513.e6–521.e6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 70.Martín-Carbonero L., Benhamou Y., Puoti M., et al. Incidence and Predictors of Severe Liver Fibrosis in Human Immunodeficiency Virus-Infected Patients with Chronic Hepatitis C: A European Collaborative Study. 2004;38(1):128–133. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- 71.Carrat F., Bani-Sadr F., Pol S., et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. 2004;292(23):2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 72.Takebe Y., Saucedo C. J., Lund G., et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. 2013;8(5) doi: 10.1371/journal.pone.0064449.e64449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goffard A., Callens N., Bartosch B., et al. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. 2005;79(13):8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Falkowska E., Kajumo F., Garcia E., Reinus J., Dragic T. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. 2007;81(15):8072–8079. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashfaq U. A., Masoud M. S., Khaliq S., Nawaz Z., Riazuddin S. Inhibition of Hepatitis C Virus 3a genotype entry through Glanthus Nivalis Agglutinin. 2011;8, article no. 248 doi: 10.1186/1743-422X-8-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitley R. J., Roizman B. Herpes simplex virus infections. 2001;357(9267):1513–1518. doi: 10.1016/s0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 77.Looker K. J., Magaret A. S., Turner K. M. E., Vickerman P., Gottlieb S. L., Newman L. M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. 2015;10(1) doi: 10.1371/journal.pone.0114989.e0114989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freeman E. E., Weiss H. A., Glynn J. R., Cross P. L., Whitworth J. A., Hayes R. J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 79.Ramjee G., Williams B., Gouws E., Van Dyck E., De Deken B., Karim S. A. The impact of incident and prevalent herpes simplex virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. 2005;39(3):333–339. doi: 10.1097/01.qai.0000144445.44518.ea. [DOI] [PubMed] [Google Scholar]
- 80.Moriuchi M., Moriuchi H., Williams R., Straus S. E. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. 2000;278(2):534–540. doi: 10.1006/viro.2000.0667. [DOI] [PubMed] [Google Scholar]
- 81.McClelland R. S., Wang C. C., Overbaugh J., et al. Association between cervical shedding of herpes simplex virus and HIV-1. 2002;16(18):2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 82.Baeten J. M., McClelland R. S., Corey L., et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: A randomized clinical trial. 2004;189(8):1466–1471. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 83.Pickett E., Brown J., van Schalkwyk M., et al. Access to influenza immunisation services by HIV-positive patients in the UK. 2017 doi: 10.1111/irv.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uematsu J., Koyama A., Takano S., et al. Legume lectins inhibit human parainfluenza virus type 2 infection by interfering with the entry. 2012;4(7):1104–1115. doi: 10.3390/v4071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Keefe B. R., Giomarelli B., Barnard D. L., et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. 2010;84(5):2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Millet J. K., Séron K., Labitt R. N., et al. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. 2006;4(2):291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kia-Ki H., Martinage A. Post-translational chemical modification(S) of proteins. 1992;24(1):19–28. doi: 10.1016/0020-711X(92)90225-P. [DOI] [PubMed] [Google Scholar]
- 89.Sexton J., Garnett G., Røttingen J.-A. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. 2005;32(6):351–357. doi: 10.1097/01.olq.0000154504.54686.d1. [DOI] [PubMed] [Google Scholar]
- 90.Mabey D. Interactions between HIV infection and other sexually transmitted diseases. 2000;5(7):A32–A36. doi: 10.1046/j.1365-3156.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 91.Mayer K. H., Venkatesh K. K. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. 2011;65(3):308–316. doi: 10.1111/j.1600-0897.2010.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith D. M., Richman D. D., Little S. J. HIV superinfection. 2005;192(3):438–444. doi: 10.1086/431682. [DOI] [PubMed] [Google Scholar]
- 93.Boonstra H., Barot S., Lusti-Narasimhan M. Making the case for multipurpose prevention technologies: The socio-epidemiological rationale. 2014;121(5):23–26. doi: 10.1111/1471-0528.12851. [DOI] [PubMed] [Google Scholar]
- 94.Schönwald S., Begovac J., Skerk V. Urinary tract infections in HIV disease. 1999;11(3-4):309–311. doi: 10.1016/S0924-8579(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 95.Dobrindt U. Virulence factors of uropathogens U. Dobrindt. 2010;49(5):598–605. doi: 10.1007/s00120-010-2251-6. [DOI] [PubMed] [Google Scholar]
- 96.Grabosch C., Hartmann M., Schmidt-Lassen J., Lindhorst T. K. Squaric Acid Monoamide Mannosides as Ligands for the Bacterial Lectin FimH: Covalent Inhibition or Not? 2011;12(7):1066–1074. doi: 10.1002/cbic.201000774. [DOI] [PubMed] [Google Scholar]
- 97.Oelschlaeger T. A., Dobrindt U., Hacker J. Virulence factors of uropathogens. 2002;12(1):33–38. doi: 10.1097/00042307-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Hartmann M., Lindhorst T. K. The bacterial lectin FimH, a target for drug discovery—carbohydrate inhibitors of type 1 fimbriae-mediated bacterial adhesion. 2011;2011(20-21):3583–3609. doi: 10.1002/ejoc.201100407. [DOI] [Google Scholar]
- 99.Barton C., Kouokam J. C., Hurst H., Palmer K. E. Pharmacokinetics of the antiviral lectin griffithsin administered by different routes indicates multiple potential uses. 2016;8(12, article no. 331) doi: 10.3390/v8120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palharini J. G., Richter A. C., Silva M. F., et al. Eutirucallin: A lectin with antitumor and antimicrobial properties. 2017;7, article no. 136 doi: 10.3389/fcimb.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swanson A. F., Ezekowitz R. A. B., Lee A., Kuo C.-C. Human Mannose-Binding Protein Inhibits Infection of HeLa Cells by Chlamydia trachomatis. 1998;66(4):1607–1612. doi: 10.1128/iai.66.4.1607-1612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swanson A. F., Kuo C.-C. The characterization of lectin-binding proteins of Chlamydia trachomatis as glycoproteins. 1991;10(6):465–473. doi: 10.1016/0882-4010(91)90112-N. [DOI] [PubMed] [Google Scholar]
- 103.Levy N. J. Wheat germ agglutinin blockage of chlamydial attachment sites: Antagonism by N-acetyl-D-glucosamine. 1979;25(3):946–953. doi: 10.1128/iai.25.3.946-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amin K., Beillevaire D., Mahmoud E., Hammar L., Mardh P., Fröman G. Binding of Galanthus nivalis lectin to Chlamvdia trachomatis and inhibition of in vitro infection. 1995;103(7-8):714–720. doi: 10.1111/j.1699-0463.1995.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 105.Mubaiwa T. D., Hartley-Tassell L. E., Semchenko E. A., et al. The glycointeractome of serogroup B Neisseria meningitidis strain MC58. 2017;7(1, article no. 5693) doi: 10.1038/s41598-017-05894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. 1980;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- 107.Connelly M. C., Stein D. C., Young F. E., Morse S. A., Allen P. Z. Interaction with lectins and differential wheat germ agglutinin binding of pyocin 103-sensitive and -resistant Neisseria gonorrhoeae. 1981;148(3):796–803. doi: 10.1128/jb.148.3.796-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.W. H. Organization. Tuberculosis, 2018.
- 109.Ragas A., Roussel L., Puzo G., Rivière M. The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. 2007;282(8):5133–5142. doi: 10.1074/jbc.M610183200. [DOI] [PubMed] [Google Scholar]
- 110.Driessen N. N., Boshoff H. I. M., Maaskant J. J., et al. Cyanovirin-N inhibits mannose-dependent Mycobacterium-C-type lectin interactions but does not protect against murine tuberculosis. 2012;189(7):3585–3592. doi: 10.4049/jimmunol.1102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Procópio T. F., de Siqueira Patriota L. L., de Moura M. C., et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. 2017;98:419–429. doi: 10.1016/j.ijbiomac.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 112.Batista J. E. C., Ralph M. T., Vaz R. V., et al. Plant lectins ConBr and CFL modulate expression toll-like receptors, pro-inflammatory cytokines and reduce the bacterial burden in macrophages infected with Salmonella enterica serovar Typhimurium. 2017;25:52–60. doi: 10.1016/j.phymed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 113.Achkar J. M., Fries B. C. Candida infections of the genitourinary tract. 2010;23(2):253–273. doi: 10.1128/cmr.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cassone A., Cauda R. Candida and candidiasis in HIV-infected patients: Where commensalism, opportunistic behavior and frank pathogenicity lose their borders. 2012;26(12):1457–1472. doi: 10.1097/QAD.0b013e3283536ba8. [DOI] [PubMed] [Google Scholar]
- 115.Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. Candida mannan: Chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. 1991;4(1):1–19. doi: 10.1128/CMR.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Groot P. W. J., Bader O., de Boer A. D., Weig M., Chauhan N. Adhesins in human fungal pathogens: Glue with plenty of stick. 2013;12(4):470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Busscher H. J., Rinastiti M., Siswomihardjo W., Van der Mei H. C. Biofilm formation on dental restorative and implant materials. 2010;89(7):657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 118.da Silva P. M., de Moura M. C., Gomes F. S., et al. PgTeL, the lectin found in Punica granatum juice, is an antifungal agent against Candida albicans and Candida krusei. 2017;108:391–400. doi: 10.1016/j.ijbiomac.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 119.Loyola W., Gaziri D. A., Gaziri L. C. J., Felipe I. Concanavalin A enhances phagocytosis and killing of Candida albicans by mice peritoneal neutrophils and macrophages. 2002;33(3):201–208. doi: 10.1016/S0928-8244(02)00310-3. [DOI] [PubMed] [Google Scholar]
- 120.Gomes F. S., Procópio T. F., Napoleão T. H., Coelho L. C. B. B., Paiva P. M. G. Antimicrobial lectin from Schinus terebinthifolius leaf. 2013;114(3):672–679. doi: 10.1111/jam.12086. [DOI] [PubMed] [Google Scholar]
- 121.Gomes B. S., Siqueira A. B. S., Maia R. D. C. C., et al. Antifungal activity of lectins against yeast of vaginal secretion. 2012;43(2):770–778. doi: 10.1590/S1517-83822012000200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Wang J., Wang S., Rao P. Lunatin, a novel lectin with antifungal and antiproliferative bioactivities from Phaseolus lunatus billb. 2016;89:717–724. doi: 10.1016/j.ijbiomac.2016.04.092. [DOI] [PubMed] [Google Scholar]
- 123.Paschinger K., Hykollari A., Razzazi-Fazeli E., et al. The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. 2012;22(2):300–313. doi: 10.1093/glycob/cwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McClelland R. S., Sangare L., Hassan W. M., et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 125.Chatterjee A., Ratner D. M., Ryan C. M., et al. Anti-retroviral lectins have modest effects on adherence of Trichomonas vaginalis to epithelial cells in vitro and on recovery of Tritrichomonas foetus in a mouse vaginal model. 2015;10(8) doi: 10.1371/journal.pone.0135340.e0135340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prevention C. f. D. C. Toxoplasmosis. 2017.
- 127.Bal A., Dhooria S., Agarwal R., Garg M., Das A. Multiple and atypical opportunistic infections in a HIV patient with Toxoplasma myocarditis. 2014;23(6):358–362. doi: 10.1016/j.carpath.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 128.Jones J. L., Lopez A., Wilson M., Schulkin J., Gibbs R. Congenital toxoplasmosis: a review. 2001;56(5):296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- 129.de Souza L. P. F., Ramos E. L. P., Santana S. S., et al. Lectins from Synadenium carinatum (ScLL) and Artocarpus heterophyllus (ArtinM) Are Able to induce beneficial immunomodulatory effects in a murine model for treatment of Toxoplasma gondii infection. 2016;6, article no. 164 doi: 10.3389/fcimb.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]