Abstract
Exosomes are nanosized vesicles and have recently been recognized as important players in cell-to-cell communication. Exosomes contain different mediators such as proteins, nucleic acids (DNA, mRNA, miRNAs, and other ncRNAs), and lipid mediators and can shuttle their exosomal content to both neighboring and distal cells. Exosomes are very effective in orchestrating immune responses in the airways and all cell types can contribute to the systemic exosome pool. Intracellular communication between the broad range of cell types within the lung is crucial in disease emphasizing the importance of exosomes. In asthma, exosomes affect the inflammatory microenvironment which ultimately determines the development or alleviation of the pathological symptoms. Recent studies in this area have provided insight into the underlying mechanisms of disease and led to interest in using exosomes as potential novel therapeutic agents.
1. Introduction
Asthma is a heterogeneous syndrome involving inflammation and obstruction of the airways that affects 300 million people worldwide [1, 2]. Limited knowledge of the disease mechanisms is the greatest obstacle to the development of novel treatments. Although two forms of asthma have been traditionally defined in the clinic (T2 and non-T2), this ignores the broad range of phenotypes that have been described and the underlying pathophysiology of these phenotypes. As a result, asthma is increasingly recognized as a syndrome rather than a single disease [3, 4]. The goal of asthma research is to link asthma classification based on phenotypes with pathophysiological mechanism and thereby define asthma endotypes which will predict drug efficacy [4]. Several asthma phenotypes have been described such as allergic bronchopulmonary mycosis and severe late-onset hypereosinophilic asthma [4, 5]; however, a small group of patients have asthma that is uncontrolled or only partially controlled despite intensive treatment [6]. This form of asthma is commonly referred to as severe asthma [7] which is often associated with serious morbidity and even mortality [6].
The emergence of biomarkers such as blood eosinophils linked with T2-asthma targeted biologic therapies opens new hopes for patients with severe asthma. However, further research is required to understand the mechanisms underlying pathophysiology of severe non-T2 asthma and to define the optimal biological treatment. In addition to this it is important to have readily accessible biomarkers that define patient subsets to ensure that the correct drug is given to the right patient at the right time. This is essential for the patients' perspective and for the healthcare provider where the current blunt measures such as blood eosinophils do not distinguish differences in underlying pathophysiological processes.
Exosomes are small vesicles (30–100 nm in diameter) that enable cell-to-cell communication by shuttling different molecules such as nucleic acids (DNA, mRNA, and micro (mi)RNAs), lipids, proteins such as heat shock 70-kDa protein (HSP)70, and specific cell surface markers reflecting the exosome cell of origin. These would include CD9, CD63, and CD81 if the exosome was endosomal in origin [8]. Exosomes can, therefore, significantly affect target cell function resulting in the development of a pathological state [9].
Exosomes have been most extensively studied in association with the pathogenesis of diverse diseases, such as cancer [10, 11] and infectious disease [12–14] as well as in asthma [15]. Exosome biology has provided us with fundamental insights into the mechanisms of cellular crosstalk in asthma and may also act as important biomarkers of the disease. In this review we summarize recent advances regarding the roles of exosomes in the pathogenesis of severe asthma and discuss their potential as biomarkers for targeted treatments.
2. Asthma Pathogenesis
Asthma is a complex disease whose underlying pathophysiology is not completely understood [16]. As a chronic inflammatory airway disease, asthma involves many cells from the innate and adaptive immune systems which act on airway epithelial cells to trigger bronchial hyperreactivity and airway remodeling in response to environmental stimuli such as allergens, infections, or air pollutants [3, 17]. The main features of allergic asthma are increases in the numbers and activity of airway mast cells and eosinophils which are due to the pathophysiological effects of proinflammatory cytokines such as interleukin- (IL-) 4, IL-5, and IL-13 released by activated CD4+ T-cells (Th2 cells) in response to environmental allergens [3]. In addition to lymphocytes and plasma cells, a large number of eosinophils and neutrophils are observed in the bronchial tissues and mucus of asthmatic airways [18].
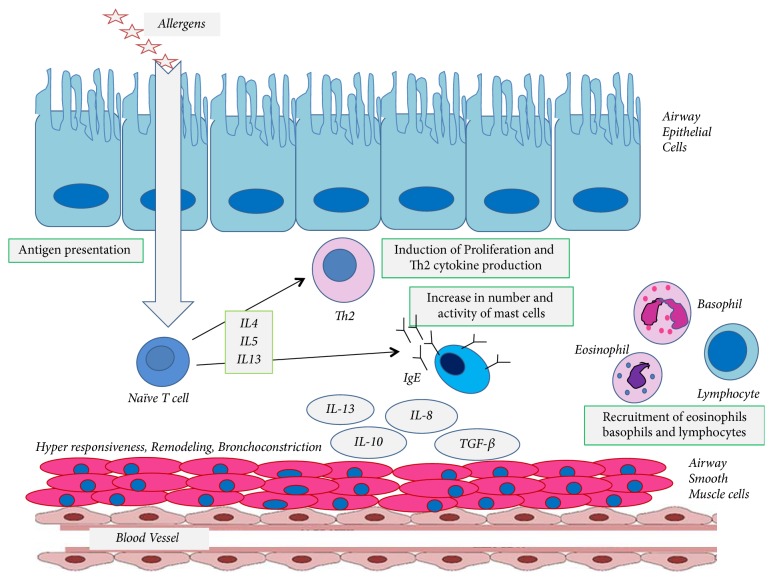
During an asthma attack, airway provocation with allergens triggers a rapid decrease in bronchial airflow with an early immunoglobulin E- (IgE-) mediated reaction that may be followed by a late-phase IgE-mediated decrease in bronchial airflow for 4–8 hours [19]. Based on our understanding of the pathophysiology of allergic asthma, activated CD4 T-lymphocytes recruit leukocytes to the airway from the bloodstream and the presence of these stimulated leukocytes results in the secretion of inflammatory mediators from eosinophils, mast cells, and lymphocytes within the airway. The expression of Th2 cytokines from activated T-lymphocytes also directs the switch from IgM to IgE antibody production [20]. Mast cell activation and degranulation are triggered following cross-linking of the membrane bound high affinity IgE receptor (FcεRI) on mast cells which causes them to release inflammatory lipid mediators such as histamine and leukotrienes (LTs). In addition, IL-5 directs the recruitment of eosinophils from the bone marrow to the site of airway inflammation [21, 22]. Chronic inflammation in the asthmatic airway leads to repeated cycles of tissue injury and repair which results in structural alterations and remodeling of the airways over time [23, 24] (Figure 1).
Figure 1.
The pathogenicity of asthma. The entry of allergens into the airway triggers the Th2 response through the antigen presenting cells and induce the differentiation of naïve CD4+ T-cells into CD4+ Th2 cells in the presence of IL-4, IL-5, and IL-13. Activated CD4 T-lymphocytes recruit leukocytes to the airway from the bloodstream which will follow with the secretion of inflammatory mediators from eosinophils, mast cells, and lymphocytes within the airway. The expression of Th2 cytokines directs the switch from IgM to IgE antibody production. Mast cell activation and degranulation are triggered following cross-linking of the membrane bound high affinity IgE receptor on mast cells. Chronic inflammation in the asthmatic airway leads to repeated cycles of tissue injury and repair which results in structural alterations and remodeling of the airways over time.
3. Exosome Properties and Function
Exosomes are small 30–100 nm membrane-enclosed vesicles. They were discovered in 1983 and initially were described as small vesicles that bud from reticulocytes during their maturation and thought to function as the cell's “garbage bin” [8]. Further studies indicated that exosomes are released from most mammalian cells and are found in nearly all biological fluids [25]. Later studies determined the biological function of exosomes [26, 27] and highlighted their involvement in many pathological conditions such as cancer and neurodegenerative and infectious diseases as well as in immune-modulatory processes [28, 29]. The watershed moment in the study of exosomes came in 2007 with the finding that exosomes contained more than 1200 mRNAs which were translated into proteins following delivery to recipient cells [29, 30]. The crucial importance of exosomes, therefore, lies in their capacity to shuttle information between cells and influence the function of recipient cells [12]. Exosomes have also recently been implicated in cell homeostasis and the removal of unwanted molecules [31].
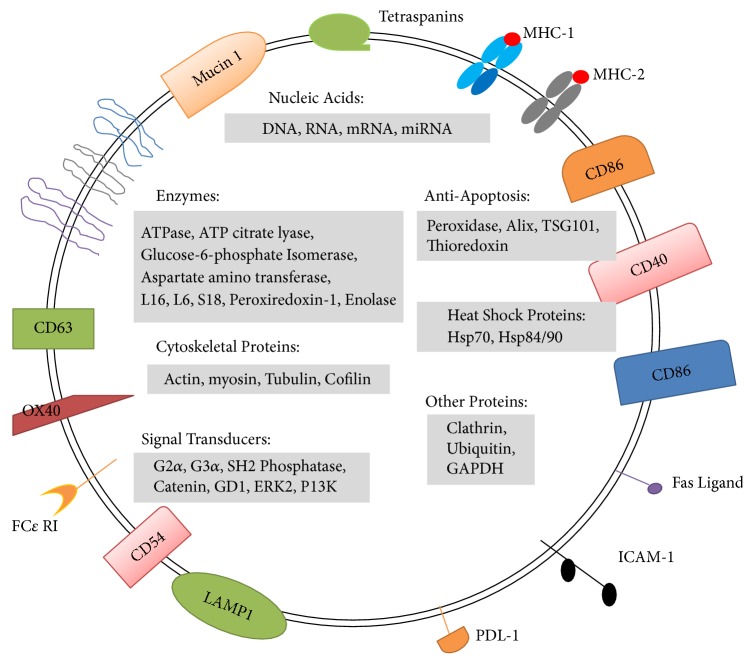
Exosome-derived signaling molecules include proteins, lipids, nucleic acids, and miRNAs whose packaging together gives an advantage of simultaneous delivery of multiple components to target cells [32]. An important feature of exosomes is that they are highly stable in biological fluids [33]. In addition, their content and composition resemble their cell of origin and these may change according to the physiological or pathological conditions the cell is exposed to [34]. Exosomes may contain many bioactive agents including prostaglandins and LTs, lipids, transmembrane receptors such as integrins β1 and β2, costimulatory molecules, membrane-localized classes I and II major histocompatibility complexes (MHC), signal transduction proteins, and nucleic acids (mRNA and miRNAs) [12] (Figure 2).
Figure 2.
Exosomes are small membrane-enclosed vesicles containing mRNA and miRNA, lipids, and a vast array of different proteins depending on their cell of origin. Generally exosomes are enriched in some of generic proteins such as proteins involved in MVB formation, tetraspanins, and membrane transports as well as a number of cytosolic proteins. In addition some compounds associated with specific pathological condition have been identified in exosomes.
Extensive investigations have elucidated the role of exosomes in intercellular communication and the regulation of physiological functions and homeostasis as well as their contribution to various pathological conditions [32]. It is within this context that we review the function of exosomes in the development of asthma with particular reference to severe disease.
4. Exosomes in Severe Asthma
The lung is a complex organ composed of a wide range of immune and structural cells within the parenchyma and airway [35]. For optimal functioning, cell-cell communication is essential and so exosomes are expected to play crucial role in lung biology and function [36]. In relation to the pathobiology of asthma, exosomes are released from the key cells implicated in disease such as mast cells, eosinophils, dendritic cells (DCs), T-cells, and bronchial epithelial cells. These in turn can trigger the activation, or repression, of other asthma-associated cells and enhance allergic responses [37].
DC-derived exosomes have costimulatory molecules on their surfaces that can activate allergen-specific Th2 cells [33, 38]. In addition, eosinophil-derived exosomes have important roles in the modulation of asthma and their numbers are increased in asthmatic patients [39, 40]. Analysis of exosomal miRNAs in patients with severe asthma compared with healthy subjects showed an altered miRNA content. The dysregulated miRNAs were involved in pathways related to airway integrity as well as being correlated with some clinical features such as eosinophil count or FEV1 [41]. In a separate study, the differential exosomal miRNAs profile in SA patients were associated with TGF-ß signaling pathway, the ErbB signaling pathway, and focal adhesion [42].
BAL exosomes from asthmatic patients express the epithelial marker mucin 1 on their surface indicating that they are derived from bronchial epithelial cells [43]. They were able to induce the production of CXCL-8 and LT C4 in target bronchial epithelial cells [44]. Whether this is a natural autocrine effect of these exosomes or whether other cells are the physiological target cell is unknown but BAL exosomal miRNAs from asthmatics were involved in IL-13-mediated events [45]. In a feedback manner, IL-13 promotes exosome production by airway epithelial cells and these exosomes subsequently enhance the proliferation of undifferentiated lung macrophages [44]. Thus, both structural and effector cells produce exosomes that modulate the chronic inflammatory processes involved in asthma [15].
4.1. Exosomes from Immune Effector Cells
Inflammation is the main pathogenic driver in asthma. Exosomes can promote inflammation via regulating the function of immune cells at the level of their recruitment, activation, or differentiation. A broad range of cells in lung are involved in asthmatic inflammation including airway epithelial cells [46–48], eosinophils [39, 49], lymphocytes [32, 46, 50], macrophages [46, 51], and DCs [48].
Eosinophils are multifunctional granulocytes that have an important role in both allergy and asthma due to their production, storage, and release of a range of inflammatory mediators. These include chemokines, lipid mediators, and cytotoxic granule proteins such as major basic protein (MBP), eosinophil peroxidase (EPX), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN), which together result in several key features of asthma.
Eosinophils from asthma patients release a greater number of exosomes in comparison with those released from cells of healthy subjects. These exosomes contain the main eosinophilic proteins such as EPO, MBP, and ECP and may, therefore, play a similar role in driving the progression of asthma as their parent cell [39]. Eosinophil-derived exosomes isolated from asthmatics may have both autocrine and paracrine functions as they increase in the production of chemokines, reactive oxygen species (ROS), and nitric oxide (NO) from target eosinophils as well as enhancing eosinophil migration by upregulating the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and integrin α2 [49] which is a critical step in asthma development [15].
Lymphocytes are key players in the inflammatory response in allergy and asthma. B-lymphocytes produce antigen specific immunoglobulins E (IgE) following Th2 cell activation and release of Th2 cytokines [52]. B-lymphocytes can also trigger an asthmatic response by acting as an antigen presetting cell (APC) without the involvement of IgE and T-lymphocytes [53]. In addition, B-lymphocytes are involved in the differentiation of naïve Th0-lymphocytes into Th1- or Th2-lymphocytes by releasing IFN-γ or IL-4, respectively [54]. Finally, IL-10-producing B-cells or Breg (B regulatory) downregulate inflammation in hyperresponsiveness airway and suppress allergic inflammation by recruitment of natural Treg (CD4+ CD25+ FoxP3+) cells to the lung [55].
B-cell-derived exosomes resemble their parent phenotype and carry MHC classes I and II and integrins β1 and β2 as well as the costimulatory molecules CD40, CD80, and CD86. As a result, they can specifically present antigenic peptides to T-cells and induce T-cell responses [56]. B-cell exosomes also contain HSP70 which is important in DC maturation [57]. B-cell-derived exosomes can also modulate the proliferation and production of Th2 cytokines from T2 cells due to the presence of exosomal antigens such as birch peptide (Bet v1) to the same degree as observed upon direct contact between B- and T-cells. This highlights the important roles of B-cell-derived exosomes in asthmatic inflammation as they can bypass the need for direct cell-to-cell contact [56].
Like other immune cells, T-lymphocytes can release exosomes [58–60]. Cytotoxic CD8+ T-cells release granules containing cytolysis mediators [60]. However, the bioactivity and potential immune-regulatory effect of T-cell-derived exosomes is not clear [61, 62]. Exosome released by T-cells is a selective and highly regulated process since T-cell receptor (TCR) activation, but not stimulation with mitogenic signals such as phorbol esters, greatly increases exosome production [58].
Exosomes released by activated CD4+ T-cells suppress the cytotoxic responses and antitumor immunity by CD8+ T-lymphocytes. These activated T-cells release 5–100 nm saucer-shaped exosomes that contain many proteins including lysosomal-associated membrane protein 1 (LAMP-1) and lymphocyte function associated antigen-1 (LFA-1) as well as CD4+ T-cell markers such as CD4, TCR, CD25, and Fas ligand [63]. Recent studies emphasize the importance of lipids in mediating T-cell-derived exosome production and function. These exosomes are enriched in sphingomyelin and cholesterol [64] and ceramide, tetraspanins, and myelin and lymphocyte (MAL) protein are important in T-cell exosome biogenesis [61]. MAL is a 17 KDa hydrophobic proteolipid located in the endoplasmic reticulum of T-cells and is involved in T-cell signal transduction. MAL was initially thought to be expressed only in T-cells but later was found also in myelin-forming cells and in polarized epithelial cells where it has a role in the apical transport of secretory proteins [65]. Activated CD3+ T-cells also release biologically active exosomes. These exosomes together with IL-2 triggered the proliferation of autologous resting CD3+ T-cells and induced a distinct cytokine profile [63].
In addition, several studies have shown that exosomes originating from other cell types can modulate T-cell function and subsequently affect the allergic asthmatic response [66–68]. For example, exosomes originating from B-cells [15], DCs [32], and epithelial-derived BALF exosomes [43] trigger T2 cytokine production along with increased proliferation and activation.
Mast cells are key immune cells in the development of allergic reactions and Th2 responses [69]. Activation of mast cells leads to the release of bioactive mediators such as histamine, prostaglandins, and LTs which subsequently trigger the allergic response. Mast cells also contribute to the secretion of proinflammatory cytokines such as TNF-α and IL-13 which drive the innate and adaptive immune responses in asthma [70, 71].
Mast cells constitutively release exosomes which have downstream effects on other immune cell types. For example, mast cell-derived exosomes induce DCs to acquire costimulatory MHC class II, CD80, CD86, and CD40 molecules enabling them to have an antigen presenting capacity for T-cells [72]. These exosomes can also modulate the activation of B- and T-lymphocytes and stimulate the production of cytokines such as IL-2, IL-12, and IFN-γ by these cells [73].
Mast cell-derived exosomes can enable target cell signaling from cell surface receptors upon contact with immune effector cells. For example, mast cell-derived exosomes trigger IgE production by B-cells in the absence of T-cells through their CD40 surface ligand [74]. Moreover, exosomes originating from bone marrow-derived mast cells (BMMCs) contain CD63 and OX40L on their surface and so can ligate with OX40 on the surface of T-cells and induce T-cell proliferation and differentiation of naïve T-cells to Th2 cells. BMMC-derived exosomes modulate the airway inflammation and remodeling responses seen in murine models of allergic asthma [75]. Mast cell-derived exosomes carry FcεRI which can bind to free IgE. This can result in decreased serum levels of IgE and limit the effects of mast cell activation. This indicates the potential of mast cell-derived exosomes as a novel anti-IgE factor in controlling the pathogenesis of severe asthma [75].
Lastly, mast cell-derived exosomes can also modulate T-cell function by donation of their contents [76] and induce the secretion of proinflammatory cytokines by human airway smooth muscle cells (ASMCs) which leads to preservation of asthmatic features [77].
Basophiles are a population of basophilic leukocytes and are like mast cells in that they are granular and are involved in allergic immune responses [78]. Basophiles comprise 0.5–1% of circulating white blood cells; however, upon inflammatory or chemotactic stimuli they increase in number and are recruited to the site of infection, for example. As with mast cells, basophils modulate the immune response by affecting other immune effector cells. Basophils can induce the proliferation and survival of naïve B-cells and direct their differentiation into antibody-producing cells. The crosstalk between these cells can be mediated via direct cell-to-cell contact as well as through soluble mediators and exosomes [79]. It was known for a long time that basophils release granules that resemble exosomes [80]; however there is limited evidence of exosome production by basophils [78].
Dendritic cells are specialized effector cells in the immune system. Acting as antigen presenting cells (APC) they process and present antigens to T-cells as well as having the capacity to phagocytose dead cells and bacteria and thereby contribute to innate immunity [81, 82]. Exosomes derived from DCs resemble their parent's morphology by possessing MHC classes I and II molecules on their surface enabling them to stimulate T-cell responses [66] or they may be captured by other APCs to induce immune responses [66]. DC-derived exosomes can present allergens and trigger the induction of Th2 responses [83]. For example, exosomes released from DCs obtained from subjects allergic to cat dander induce IL-4 responses in peripheral blood mononuclear cells (PBMCs) [38].
DC-derived exosomes contain HLA-DR, MHC, CD86, and CD54 on their surface. The presence of the costimulatory molecule CD86 indicates the potential of these exosomes to induce T-cell proliferation and differentiation whilst CD54 enables exosomes to interact with T-lymphocytes via LFA-1 [84]. These exosomes also contain enzymes that can convert LT A4 to other LTs such as LTB4 and LTC4 [84].
These exosomes also contribute to the recruitment and migration of granulocytes and leukocytes to the site of inflammation. This process is mediated by metabolites of arachidonic acid (5-keto eicosatetraenoic acid, KETE, and LTB4) that are produced following transfer of exosome-derived enzymes. These proinflammatory lipid metabolites are important in triggering asthma pathogenesis [84].
4.2. Exosomes from the Lung Structural Cells
Exosomes released from structural lung cells also contribute to fine-tuning of the immune response in asthma via managing intercellular communication [8]. Exosomes released by bronchial fibroblasts can be taken up by bronchial epithelial cells. Intriguingly, although the levels of transforming growth factor- (TGF-) β2 in exosomes derived from severe asthmatic fibroblasts were lower than that in exosomes derived from healthy subjects, fibroblast-derived exosomes from severe asthmatics induced increased proliferation of epithelial cells. The level of TGF-β2 in the fibroblast-derived exosomes was significantly related to the level in the cell of origin which controlled the exosome effect on bronchial epithelial cell proliferation. Thus, modulation of fibroblast TGF-β2 levels by overexpression or knockdown had concomitant effects on exosome levels of TGF-β2 and on epithelial cell proliferation [85].
The production of exosomes by lung cells and their protein content was higher in a mouse model of asthma. In this model IL-13 augmented the secretion of exosomes by lung epithelial cells and these exosomes enhanced the proliferation and differentiation of macrophages. Inhibition of exosome production by GW4869 alleviated the induction of asthmatic features in this model [44].
5. Exosomal miRNAs in Severe Asthma Pathogenesis
Exosomes as important mediators of cell communication can deliver miRNAs from one cell to a distinct target cell at a neighboring or distal site and subsequently affect the function of the target cell [86]. miRNAs modulate both innate and adoptive immune response with miR-21, miR-146a, and miR-155 being reported as key miRNAs in the asthmatic immune response [87]. Each miRNA can target hundreds of genes; so any changes in miRNAs level can influence many signaling pathways and have profound effects on disease pathogenesis [88].
In asthma, dysregulated miRNA expression has been observed in many cells and compartments including airway biopsies, lymphocytes, epithelial cells, and peripheral blood [89]. For example, in a murine model of asthma upregulation miR-21 was associated with altered IL-12 expression and a heightened Th2 response [90, 91]. Overexpression of miR-21 along with miR-126 was also detected in airway epithelial cells of asthmatic patients [92]. Other dysregulated miRNAs include miR-1248, miR-let7a, miR-570, miR-133a, and miR-328 which are decreased in plasma of asthmatic patient [93] whilst miR-221 was increased in ASMC from patients with severe asthma and regulated ASM proliferation and the secretion of proinflammatory mediators such as IL-6 [94].
Similarly, the exosomal miRNA content may also be altered in pathological conditions. For example, an analysis of BAL-derived exosomal miRNAs in asthma reveals the altered expression of 24 miRNAs in asthmatic patients compared to healthy subjects which are implicated in the regulation of IL-13-mediated functions [95]. In addition, CD8+ cells released exosome like vesicles that contain miR-150 and are coated with antigen specific antibody [87]. Internalization of these vesicles by the T-cells leads to antigen specific tolerance in mice [87].
Analysis of circulating exosomal miRNAs by next-generation sequencing demonstrated upregulation of miR-128, miR-140-3p, miR-196b-5p, and miR-486-5p in severe asthma patients in comparison to healthy subjects. These differentially expressed miRNAs were mostly involved in ErbB signaling pathway and focal adhesion [42]. In another study, the altered severe asthma exosomal miRNA content was associated with airway epithelial cell integrity and feature of asthma such as peripheral blood granulocyte counts [41].
6. The Therapeutic Potential of Exosomes in Asthma
Exosomes can regulate homeostasis and vital immune functions in the lung microenvironment. Exosomal contents have recently been suggested as potential diagnostic biomarkers in multiple diseases. In addition, as described above, exosomes can act as traps to prevent immune activation. Mast cell-derived exosomes possess FCεR1 on their surface which can bind free serum IgE and limit the effects of mast cell activation [75]. Furthermore, CD8+ cells release microvesicles that contain miR-150 which can suppress allergic contact dermatitis (ACD) and induce an antigen specific tolerance in mice [87].
Mesenchymal stem cells (MSC) release exosomes with the capacity to accelerate wound healing and lung tissue regeneration and this may be of use in alleviating airway remodeling in asthma [96]. These exosomes also have antiapoptotic and anti-inflammatory properties indicating that they may be effective in other lung chronic inflammatory conditions [97]. Clinical trials using exosome-based therapy in acute respiratory distress syndrome (ARDS) are being conducted [98]. In an animal model of ARDS, exosomes derived from MSC reduce lung inflammation via induction of keratinocyte growth factor (KGF) expression in the injured alveolus and thereby improve the lung protein permeability [99].
Immunotherapy for non-small-cell lung cancer (NSCLC) using dexosomes is also undergoing clinical trials. Dexosomes are DC-derived exosomes that are loaded with tumor antigen [100]. The data to date indicate that exosome therapy is feasible and safe and may represent an alternative approach to traditional therapeutic methods in inflammatory diseases such as asthma. Further studies are required to examine the effect of exosomes on the different pathological features associated with patients with distinct phenotypes of severe asthma.
7. Conclusion
In recent years, exosomes have emerged as an important area in biomedical research. Exosomes play a key role in local and distant intracellular communication and have been implicated as having a crucial role in the regulation of normal cellular function and increasingly in pathological conditions. These nanovesicles are also being increasingly recognized as potentially powerful tools for the prognosis, diagnosis, monitoring, and treatment of patients in many therapeutic areas.
Within the lung microenvironment cell-to-cell communication is of upmost importance. In asthma, exosomes can regulate immune and inflammatory responses in a beneficial and detrimental manner. The severity of asthma has been linked with distinct exosomal pools and/or content which have important roles in disease at least in primary cells and in in vivo models of disease. In addition, the unique constituents of exosomes indicate their potential as biomarkers or as novel therapeutic agents. However, there are still many unsolved problems in the area including the selectively packaging of exosomal content and the mechanisms involved in the precise delivery to target cells; these need to be elucidated.
Acknowledgments
Esmaeil Mortaz was supported by the National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran, and Division of Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of Science, Utrecht University, Utrecht, Netherlands. Sharon E. Mumby was supported by the British Heart Foundation (PG/14/27/30679) and Ian M. Adcock by the Wellcome Trust (093080/Z/10/Z).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Fireman P. Understanding asthma pathophysiology. Proceedings of the Allergy and asthma proceedings; 2003; OceanSide Publications, Inc.; [PubMed] [Google Scholar]
- 2.Subbarao P., Mandhane P. J., Sears M. R. Asthma: Epidemiology, etiology and risk factors. 2009;181(9):E181–E190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambrecht B. N., Hammad H. The immunology of asthma. 2014;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 4.Lötvall J., Akdis C. A., Bacharier L. B., et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Lin T.-Y., Poon A. H., Hamid Q. Asthma phenotypes and endotypes. 2013;19(1):18–23. doi: 10.1097/MCP.0b013e32835b10ec. [DOI] [PubMed] [Google Scholar]
- 6.Stirling R. G., Chung K. F. Severe asthma: Definition and mechanisms. 2001;56(9):825–840. doi: 10.1034/j.1398-9995.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellanti J. A., Settipane R. A. Addressing the challenges of severe asthma. 2015;36(4):237–239. doi: 10.2500/aap.2015.36.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alipoor S. D., Mortaz E., Garssen J., Movassaghi M., Mirsaeidi M., Adcock I. M. Exosomes and Exosomal miRNA in Respiratory Diseases. 2016;2016:11. doi: 10.1155/2016/5628404.5628404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alipoor S. D., Mortaz E., Tabarsi P., et al. Bovis Bacillus Calmette-Guerin (BCG) infection induces exosomal miRNA release by human macrophages. 2017;15(1):105–128. doi: 10.1186/s12967-017-1205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azmi A. S., Bao B., Sarkar F. H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. 2013;32(3-4):623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dörsam B., Reiners K. S., von Strandmann E. P. Cancer-derived extracellular vesicles: Friend and foe of tumour immunosurveillance. 2018;373(1737) doi: 10.1098/rstb.2016.0481.20160481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schorey J. S., Cheng Y., Singh P. P., Smith V. L. Exosomes and other extracellular vesicles in host-pathogen interactions. 2015;16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming A., Sampey G., Chung M.-C., et al. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. 2014;71(2):109–120. doi: 10.1111/2049-632X.12135. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini H. M., Fooladi A. A. I., Nourani M. R., Ghanezadeh F. The Role of exosomes in infectious diseases. 2013;12(1):29–37. doi: 10.2174/1871528111312010005. [DOI] [PubMed] [Google Scholar]
- 15.Sastre B., Cañas J. A., Rodrigo-Muñoz J. M., del Pozo V. Novel modulators of asthma and allergy: Exosomes and microRNAs. 2017;8:826–835. doi: 10.3389/fimmu.2017.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsicopoulos A., De Nadai P., Glineur C. Environmental and genetic contribution in airway epithelial barrier in asthma pathogenesis. 2013;13(5):495–499. doi: 10.1097/ACI.0b013e328364e9fe. [DOI] [PubMed] [Google Scholar]
- 17.Melén E., Pershagen G. Pathophysiology of asthma: Lessons from genetic research with particular focus on severe asthma. 2012;272(2):108–120. doi: 10.1111/j.1365-2796.2012.02555.x. [DOI] [PubMed] [Google Scholar]
- 18.Busse W. W., Banks-Schlegel S., Wenzel S. E. Pathophysiology of severe asthma. 2000;106(6):1033–1042. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- 19.Barrios R. J., Kheradmand F., Batts L., Corry D. B. Asthma: Pathology and pathophysiology. 2006;130(4):447–451. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- 20.Schreck D. M. Asthma pathophysiology and evidence-based treatment of severe exacerbations. 2006;63(3):S5–S13. doi: 10.2146/ajhp060127. [DOI] [PubMed] [Google Scholar]
- 21.Bradding P., Walls A. F., Holgate S. T. The role of the mast cell in the pathophysiology of asthma. 2006;117(6):1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Gosens R., Zaagsma J., Meurs H., Halayko A. J. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. 2006;7(1):73–89. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauad T., Bel E. H., Sterk P. J. Asthma therapy and airway remodeling. 2007;120(5):997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Beasley R., Page C., Lichtenstein L. Airway remodelling in asthma. 2002;2(4):109–116. doi: 10.1046/j.1472-9725.2.s4.1.x. [DOI] [Google Scholar]
- 25.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatnagar S., Schorey J. S. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. 2007;282(35):25779–25789. doi: 10.1074/jbc.m702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh P. P., Smith V. L., Karakousis P. C., Schorey J. S. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. 2012;189(2):777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim C.-H., Hong M.-J., Park S.-D., et al. Enhancement of anti-tumor immunity specific to murine glioma by vaccination with tumor cell lysate-pulsed dendritic cells engineered to produce interleukin-12. 2006;55(11):1309–1319. doi: 10.1007/s00262-006-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P. P., LeMaire C., Tan J. C., Zeng E., Schorey J. S. Exosomes released from m.tuberculosis infected cells can suppress ifn-γ mediated activation of naïve macrophages. 2011;6(4, article e18564) doi: 10.1371/journal.pone.0018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi A., Okada R., Nagao K., et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. 2017;8:15287–15298. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hough K. P., Chanda D., Duncan S. R., Thannickal V. J., Deshane J. S. Exosomes in immunoregulation of chronic lung diseases. 2017;72(4):534–544. doi: 10.1111/all.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Admyre C., Telemo E., Almqvist N., et al. Exosomes—nanovesicles with possible roles in allergic inflammation. 2008;63(4):404–408. doi: 10.1111/j.1398-9995.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 34.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. 2015;6:203–218. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita Y., Kosaka N., Araya J., Kuwano K., Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. 2015;21(9):533–542. doi: 10.1016/j.molmed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Eissa N. T. The exosome in lung diseases: message in a bottle. 2013;131(3):904–905. doi: 10.1016/j.jaci.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Fujita Y., Yoshioka Y., Ito S., Araya J., Kuwano K., Ochiya T. Intercellular communication by extracellular vesicles and their microRNAs in Asthma. 2014;36(6):873–881. doi: 10.1016/j.clinthera.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Admyre C., Grunewald J., Thyberg J., et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. 2003;22(4):578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 39.Mazzeo C., Cañas J. A., Zafra M. P., et al. Exosome secretion by eosinophils: a possible role in asthma pathogenesis. 2015;135(6):1603–1613. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Prado N., Marazuela E. G., Segura E., et al. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. 2008;181(2):1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 41.Francisco-Garcia A., Martinez-Nunez R. T., Rupani H., et al. LSC Abstract Altered small RNA cargo in severe asthma exosomes. 2016 [Google Scholar]
- 42.Suzuki M., Konno S., Makita H., et al. LSC Abstract - Altered circulating exosomal RNA profiles detected by next-generation sequencing in patients with severe asthma. 2016 [Google Scholar]
- 43.Torregrosa Paredes P., Esser J., Admyre C., et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. 2012;67(7):911–919. doi: 10.1111/j.1398-9995.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- 44.Kulshreshtha A., Ahmad T., Agrawal A., Ghosh B. Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. 2013;131(4):1194–1203.e14. doi: 10.1016/j.jaci.2012.12.1565. [DOI] [PubMed] [Google Scholar]
- 45.Levänen B., Bhakta N. R., Torregrosa Paredes P., et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. 2013;131(3):894.e8–903.e8. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes P. J. Immunology of asthma and chronic obstructive pulmonary disease. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 47.Barnes P. J. The cytokine network in asthma and chronic obstructive pulmonary disease. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammad H., Lambrecht B. N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. 2008;8(3):193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 49.Cañas J. A., Sastre B., Mazzeo C., et al. Exosomes from eosinophils autoregulate and promote eosinophil functions. 2017;101(5):1191–1199. doi: 10.1189/jlb.3AB0516-233RR. [DOI] [PubMed] [Google Scholar]
- 50.Medoff B. D., Thomas S. Y., Luster A. D. T cell trafficking in allergic asthma: The ins and outs. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 51.Anderson G. P. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. 2008;372(9643):1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 52.Lindell D. M., Berlin A. A., Schaller M. A., Lukacs N. W. B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. 2008;3(9) doi: 10.1371/journal.pone.0003129.e3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vooght V., Carlier V., Devos F. C., et al. B-lymphocytes as key players in chemical-induced asthma. 2013;8(12) doi: 10.1371/journal.pone.0083228.e83228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris D. P., Haynes L., Sayles P. C. Reciprocal regulation of polarized cytokine production by effector B and T cells. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 55.Natarajan P., Guernsey L. A., Schramm C. M. Regulatory B cells in allergic airways disease and asthma. 2014;1190:207–225. doi: 10.1007/978-1-4939-1161-5_15. [DOI] [PubMed] [Google Scholar]
- 56.Admyre C., Bohle B., Johansson S. M., et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. 2007;120(6):1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 57.Clayton A., Turkes A., Navabi H., Mason M. D., Tabi Z. Induction of heat shock proteins in B-cell exosomes. 2005;118(16):3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 58.Blanchard N., Lankar D., Faure F., et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. 2002;168(7):3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 59.Peters P. J., Geuze H. J., van der Donk H. A., et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. 1989;19(8):1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 60.Peters P. J., Borst J., Oorschot V., et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. 1991;173(5):1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ventimiglia L. N., Alonso M. A. Biogenesis and Function of T Cell-Derived Exosomes. 2016;4:90–84. doi: 10.3389/fcell.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H., Xie Y., Li W., Chibbar R., Xiong S., Xiang J. CD4 T cell-released exosomes inhibit CD8 cytotoxic T-lymphocyte responses and antitumor immunity. 2011;8(1):23–30. doi: 10.1038/cmi.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahlgren J., Karlson T. D. L., Glader P., Telemo E., Valadi H. Activated Human T Cells Secrete Exosomes That Participate in IL-2 Mediated Immune Response Signaling. 2012;7(11) doi: 10.1371/journal.pone.0049723.e49723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosque A., Dietz L., Gallego-Lleyda A., et al. Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin-containing protein. 2016;7(20):29287–29305. doi: 10.18632/oncotarget.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin-Belmonte F., Arvan P., Alonso M. A. MAL Mediates Apical Transport of Secretory Proteins in Polarized Epithelial Madin-Darby Canine Kidney Cells. 2001;276(52):49337–49342. doi: 10.1074/jbc.M106882200. [DOI] [PubMed] [Google Scholar]
- 66.Théry C., Duban L., Segura E., Væron P., Lantz O., Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 67.Clayton A., Al-Taei S., Webber J., Mason M. D., Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 68.Abusamra A. J., Zhong Z., Zheng X., et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. 2005;35(2):169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Reuter S., Stassen M., Taube C. Mast cells in allergic asthma and beyond. 2010;51(6):797–807. doi: 10.3349/ymj.2010.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bradding P., Brightling C. Mast cell infiltration of airway smooth muscle in asthma. 2007;101(5):p. 1045. doi: 10.1016/j.rmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Rossios C., Pavlidis S., Gibeon D., et al. Impaired innate immune gene profiling in airway smooth muscle cells from chronic cough patients. 2017;37(6) doi: 10.1042/BSR20171090.BSR20171090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skokos D., Botros H. G., Demeure C., et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. 2003;170(6):3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 73.Tkaczyk C., Villa I., Peronet R., David B., Chouaib S., Mécheri S. In vitro and in vivo immunostimulatory potential of bone marrow-derived mast cells on b- and T-lymphocyte activation. 2000;105(1 I):134–142. doi: 10.1016/S0091-6749(00)90188-X. [DOI] [PubMed] [Google Scholar]
- 74.Gauchat J.-F., Henchoz S., Mazzei G., et al. Induction of human IgE synthesis in B cells by mast cells and basophils. 1993;365(6444):340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 75.Xie G., Yang H., Peng X., et al. Mast cell exosomes can suppress allergic reactions by binding to IgE. 2017 doi: 10.1016/j.jaci.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 76.Li F., Wang Y., Lin L., et al. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. 2016;2016:10. doi: 10.1155/2016/3623898.3623898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Y. C., Harris T., Stewart A. G., MacKay G. A. Secreted factors from human mast cells trigger inflammatory cytokine production by human airway smooth muscle cells. 2012;160(1):75–85. doi: 10.1159/000339697. [DOI] [PubMed] [Google Scholar]
- 78.Stone K. D., Prussin C., Metcalfe D. D. IgE, mast cells, basophils, and eosinophils. 2010;125(2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merluzzi S., Betto E., Ceccaroni A. A., Magris R., Giunta M., Mion F. Mast cells, basophils and B cell connection network. 2014;63(1):94–103. doi: 10.1016/j.molimm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 80.Dvorak A. M. Degranulation and recovery from degranulation of basophils and mast cells, in Ultrastructure of Mast Cells and Basophils. 2005;85:205–251. doi: 10.1159/000086519. [DOI] [PubMed] [Google Scholar]
- 81.Gu X., Erb U., Büchler M. W., Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. 2015;136(4):E74–E84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- 82.Zitvogel L., Mayordomo J. I., Tjandrawan T., et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: Dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. 1996;183(1):87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vallhov H., Gutzeit C., Hultenby K., Valenta R., Grönlund H., Scheynius A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. 2015;70(12):1651–1655. doi: 10.1111/all.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esser J., Gehrmann U., D'Alexandri F. L., et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. 2010;126(5):1032.e4–1040.e4. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 85.Haj-Salem I., Plante S., Gounni A. S., Rouabhia M., Chakir J. Fibroblast-derived exosomes promote epithelial cell proliferation through TGF-β2 signalling pathway in severe asthma. 2018;73(1):178–186. doi: 10.1111/all.13234. [DOI] [PubMed] [Google Scholar]
- 86.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 87.Rebane A., Akdis C. A. MicroRNAs in allergy and asthma. 2014;14(4):424–438. doi: 10.1007/s11882-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 88.Alipoor S. D., Adcock I. M., Garssen J., et al. The roles of miRNAs as potential biomarkers in lung diseases. 2016;791:395–404. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang X. The emerging role of microRNAs in asthma. 2011;353(1-2):35–40. doi: 10.1007/s11010-011-0771-z. [DOI] [PubMed] [Google Scholar]
- 90.Lu T. X., Munitz A., Rothenberg M. E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. 2009;182(8):4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu T. X., Hartner J., Lim E.-J., et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-γ pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. 2011;187(6):3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu X.-B., Wang M.-Y., Zhu H.-Y., Tang S.-Q., You Y.-D., Xie Y.-Q. Overexpression of microRNA-21 and microRNA-126 in the patients of bronchial asthma. 2014;7(5):1307–1312. [PMC free article] [PubMed] [Google Scholar]
- 93.Szymczak I., Wieczfinska J., Pawliczak R. Molecular Background of miRNA Role in Asthma and COPD: an updated insight. 2016;2016:10. doi: 10.1155/2016/7802521.7802521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry M. M., Baker J. E., Gibeon D. S., Adcock I. M., Chung K. F. Airway smooth muscle hyperproliferation is regulated by MicroRNA-221 in severe asthma. 2014;50(1):7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Z., Chen J., Tian T., et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. 2008;118(7):2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porro C., Lepore S., Trotta T., et al. Isolation and characterization of microparticles in sputum from cystic fibrosis patients. 2010;11(1):94–108. doi: 10.1186/1465-9921-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang L., Ma W., Ma Y., Feng D., Chen H., Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? 2015;11(2):238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson J. G., Liu K. D., Zhuo H., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y. G., Feng X. M., Abbott J., et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morse M. A., Garst J., Osada T. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. 2005;3(1):9–25. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]