ABSTRACT
Kingella kingae is a Gram-negative coccobacillus that is increasingly being recognized as an important cause of invasive disease in young children. The pathogenesis of K. kingae disease begins with colonization of the oropharynx, followed by invasion of the bloodstream, survival in the intravascular space, and dissemination to distant sites. Recent studies have revealed that K. kingae produces a number of surface factors that may contribute to the pathogenic process, including a polysaccharide capsule and an exopolysaccharide. In this study, we observed that K. kingae was highly resistant to the bactericidal effects of human serum complement. Using mutant strains deficient in expression of capsule, exopolysaccharide, or both in assays with human serum, we found that elimination of both capsule and exopolysaccharide was required for efficient binding of IgG, IgM, C4b, and C3b to the bacterial surface and for complement-mediated killing. Abrogation of the classical complement pathway using EGTA-treated human serum restored survival to wild-type levels by the mutant lacking both capsule and exopolysaccharide, demonstrating that capsule and exopolysaccharide promote resistance to the classical complement pathway. Consistent with these results, loss of both capsule and exopolysaccharide eliminated invasive disease in juvenile rats with an intact complement system but not in rats lacking complement. Based on these observations, we conclude that the capsule and the exopolysaccharide have important redundant roles in promoting survival of K. kingae in human serum. Each of these surface factors is sufficient by itself to fully prevent serum opsonin deposition and complement-mediated killing of K. kingae, ultimately facilitating intravascular survival and promoting K. kingae invasive disease.
KEYWORDS: Kingella kingae, capsule, exopolysaccharide, serum resistance
INTRODUCTION
The encapsulated Gram-negative coccobacillus Kingella kingae is a member of the commensal flora in the oropharynx in young children and is emerging as an important pathogen in the pediatric population (1). Recent epidemiological studies using sensitive PCR-based diagnostics have revealed that K. kingae is a leading cause of osteoarticular infections in young children between 6 and 36 months of age (2–4). In addition, K. kingae is a known cause of bacteremia and endocarditis in this population (2, 3). Following asymptomatic colonization of the upper respiratory tract, K. kingae can breach the epithelium, enter the bloodstream, and spread to distant sites to produce disease (1, 5–8). The mechanism by which K. kingae evades host innate immune responses during oropharyngeal colonization, in the bloodstream, and at sites of invasive disease is currently poorly understood.
Survival of bacteria in the bloodstream involves a complex interplay between the organism and the innate and adaptive immune systems. The innate immune system provides a rapid and immediate response to infection and plays an especially important role in children, who have a relatively naive adaptive immune system. A key component of innate immunity in the bloodstream is the complement system, a highly regulated and multifunctional group of circulating proteins that promote recognition of pathogens by immune cells through chemotaxis and opsonization and that are capable of direct killing of bacteria (9, 10). Complement is activated via the classical, the alternative, and the lectin pathways; all three of these pathways converge on the deposition of the protein fragment C3b on the bacterial surface. C3b promotes opsonization and formation of the membrane attack complex (MAC), which mediates direct lysis of Gram-negative bacteria (9, 10). Invasive bacterial pathogens express a variety of extracellular factors that mediate resistance to complement-mediated opsonin deposition and bacterial lysis.
Bacterial pathogens commonly express surface polysaccharides, which serve a multitude of functions and often allow the organism to tolerate environmental stressors, evade host immune mechanisms, and, ultimately, survive within the host. Capsular polysaccharides are lipidated, surface-anchored carbohydrate chains that have been widely shown to protect bacteria against mucosal and intravascular inflammatory responses by preventing phagocytosis and complement-mediated lysis (11–14). The polysaccharide capsules of Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, and a variety of other organisms have been extensively studied due to their importance as virulence factors and their effective use as vaccine antigens (15–18). In K. kingae, four distinct polysaccharide capsules, designated types a, b, c, and d, have been identified (19). Capsule types a and b account for greater than 95% of invasive disease isolates (19, 20). Previous work has demonstrated that the capsule is required for full K. kingae virulence in a juvenile rat model of invasive disease (21, 22).
Bacteria can also express additional or alternative surface polysaccharides, known as exopolysaccharides, which are secreted carbohydrate polymers that are not covalently anchored to the bacterial membrane and, hence, are different from polysaccharide capsules (23, 24). To date, exopolysaccharides have been studied largely in the context of bacterial biofilm formation and dispersal. In addition to expressing a capsular polysaccharide, K. kingae produces a galactofuranose homopolymer exopolysaccharide called the PAM galactan, which has been previously shown to have antibiofilm properties (21, 25). While a number of bacterial polysaccharide capsules have been studied for their ability to promote evasion of complement-mediated and neutrophil-mediated killing, understanding of the role of exopolysaccharides in these functions is limited (26–29).
In this study, we found that K. kingae is highly resistant to serum killing, resulting from the overlapping ability of the polysaccharide capsule and the exopolysaccharide to prevent opsonin deposition and complement-mediated lysis via the classical pathway. Elimination of both the capsular polysaccharide and the exopolysaccharide resulted in a complete loss of virulence in juvenile rats, highlighting the critical roles of these surface polysaccharides in K. kingae virulence.
RESULTS
K. kingae is highly resistant to complement-mediated lysis.
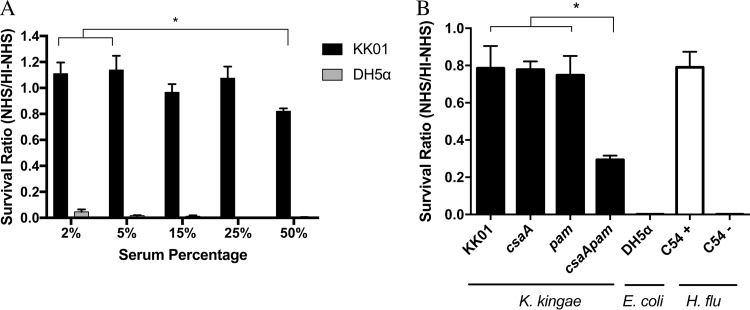
The ability of K. kingae to survive in the bloodstream in young children suggests that the organism is capable of evading the host innate immune response. To test the capacity of K. kingae to evade complement-mediated lysis, we performed serum bactericidal assays using normal human serum (NHS) as a source of active complement or heat-inactivated NHS (HI-NHS) as a source of inactive complement. K. kingae strain KK01 at an inoculum of 1.0 × 103 CFU was incubated for 1 h with serum concentrations ranging from 2% to 50% (Fig. 1A), using Escherichia coli strain DH5α as a serum-sensitive control. To assess serum sensitivity, a survival ratio was calculated by dividing the number of CFU recovered from NHS by the number of CFU recovered from HI-NHS. The limit of detection for plating was 20 CFU, which enabled detection of up to a 98% reduction in bacterial survival. At low NHS concentrations, there was no killing of strain KK01 but complete killing of strain DH5α (Fig. 1A). At an NHS concentration of 50%, strain KK01 had a survival ratio of approximately 0.80 (Fig. 1A). As expected, HI-NHS had no effect on the survival of strain KK01 or strain DH5α at any serum concentration. These results demonstrate that K. kingae exhibits high-level resistance to complement-mediated lysis.
FIG 1.
K. kingae is highly resistant to the bactericidal effects of complement present in pooled normal human serum, and elimination of both surface polysaccharides decreases serum resistance in K. kingae. (A) K. kingae strain KK01 and E. coli strain DH5α (∼103 CFU) were incubated with 2%, 5%, 15%, 25%, or 50% NHS or HI-NHS for 1 h. (B) K. kingae strains KK01, KK01 csaA, KK01 pam, and KK01 csaA pam, E. coli strain DH5α, and H. influenzae (H. flu) strains C54+ (encapsulated) and C54− (nonencapsulated) were incubated with 50% NHS or 50% HI-NHS for 1 h. The survival ratio was calculated by dividing the NHS CFU counts by the HI-NHS CFU counts. Abbreviations: csaA, KK01 csaA; pam, KK01 pam; csaApam, KK01 csaA pam. A total of three biological replicates were performed (n = 3). Statistical significance was determined with an unpaired Student's t test, and the error bars represent the standard error of the mean. *, P < 0.05.
Surface polysaccharides prevent complement-mediated lysis of K. kingae.
To determine the role of the K. kingae surface polysaccharides (the polysaccharide capsule and the exopolysaccharide) in serum resistance, we performed serum bactericidal assays using strain KK01 mutants deficient in expression of the polysaccharide capsule, the exopolysaccharide, or both, using strain KK01 csaA (a capsule-deficient mutant lacking the csaA capsule synthesis gene) (22), strain KK01 pam (an exopolysaccharide-deficient mutant lacking the pamABCDE galactan exopolysaccharide synthesis operon) (21), and strain KK01 csaA pam (a double mutant lacking both the capsule and the exopolysaccharide synthesis genes). Serum bactericidal assays were performed as described above using 50% serum for 1 h and using E. coli strain DH5α as a serum-sensitive control. In addition, isogenic encapsulated and nonencapsulated derivatives of H. influenzae strain C54 (designated + for encapsulated strain and − for nonencapsulated strain) were included as controls to demonstrate the importance of the polysaccharide capsule in serum resistance for another Gram-negative bacterium. Deletion of csaA, pamABCDE, or both csaA and pamABCDE resulted in no growth defects on solid agar and had no effect on survival in the presence of 50% HI-NHS. KK01 and the mutant derivatives maintained stable bacterial counts over the course of the 1-h assay in HI-NHS (data not shown). Survival in NHS by the capsule mutant (strain KK01 csaA) or the exopolysaccharide mutant (strain KK01 pam) was not affected and was similar to the survival of wild-type strain KK01 (Fig. 1B). In contrast, survival in NHS by the double mutant lacking both capsule and exopolysaccharide (strain KK01 csaA pam) was markedly reduced, with a survival ratio of less than 0.35 (P < 0.05) (Fig. 1B). While the absence of both the capsule and the exopolysaccharide resulted in reduced resistance to serum, survival of strain KK01 csaA pam was not reduced to undetectable levels, as observed with the serum-sensitive controls E. coli DH5α and H. influenzae C54 b−.
These results establish that elimination of both capsule and exopolysaccharide is critical for increased complement-mediated lysis and demonstrate the potent ability of K. kingae to evade complement-mediated lysis.
Opsonin deposition is increased in the absence of surface polysaccharides.
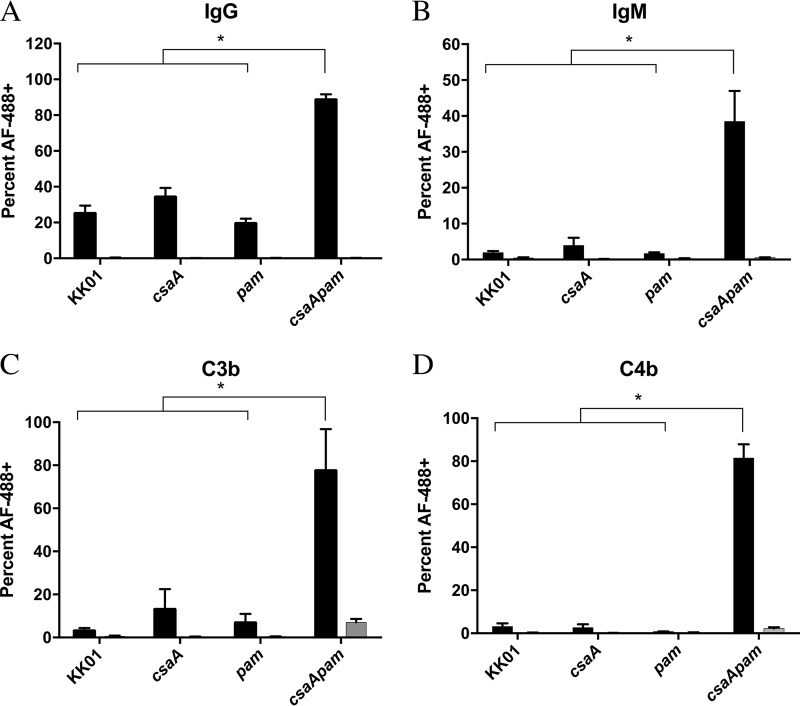
Elimination of the polysaccharide capsule in other organisms has been shown to expose bacterial surface factors and promote antibody recognition and, thus, activation of complement via the classical pathway (30–32). To determine whether K. kingae-specific antibodies were present in HI-NHS, Western blot analyses of whole-cell lysates and outer membranes were performed using HI-NHS as the primary antibody. Human serum immunoglobulins reacted with proteins present in K. kingae whole-cell and outer membrane fractions (data not shown). To further investigate whether the capsule and/or the exopolysaccharide prevents antibody binding, we performed flow cytometry assays to determine the relative levels of IgG and IgM deposition on the K. kingae surface after incubation with HI-NHS as the source of antibodies or with secondary antibody alone as a control. There was negligible fluorescence after the incubation with the secondary antibody alone (Fig. 2A and B, gray bars), thus demonstrating the absence of nonspecific binding of the secondary antibody. No significant difference in deposition was observed between strain KK01 and the single mutant strains KK01 csaA and KK01 pam after incubation with HI-NHS (Fig. 2A and B). In contrast, there was a statistically significant increase in the deposition of both IgG and IgM on the surface of strain KK01 csaA pam, consistent with our observations regarding serum sensitivity (Fig. 2A and B).
FIG 2.
Opsonin deposition increases in the surface polysaccharide-deficient mutant. Binding of IgG (A), IgM (B), C3b (C), or C4b (D) to the bacterial surface was determined using flow cytometry. Cells were stained with propidium iodine (PI) prior to analysis; 30,000 propidium iodine-positive events per biological replicate were analyzed, and a total of three biological replicates were performed (n = 3). The percentages represent events that registered as Alexa Fluor 488 positive (AF-488+). Black bars, HI-NHS (A and B) or NHS (C and D); gray bars, secondary antibody-only controls (these bars are negligible in size due to the low signal). Abbreviations: csaA, KK01 csaA; pam, KK01 pam; csaApam, KK01 csaA pam. Statistical significance was determined with an unpaired Student's t test, and the error bars represent the standard error of the mean. *, P < 0.05.
The constant region of IgG and IgM provides a platform for the initiation of the complement cascade (33–36). To determine whether antibody deposition activates the classical pathway and leads to activation of the terminal pathway, we performed flow cytometry assays to measure the deposition of C4b and C3b, two well-documented opsonins and products of complement activation, on the bacterial surface. As shown in Fig. 2C and D, there was no significant difference in the binding of C4b and C3b to strain KK01 and the single mutant strains KK01 csaA and KK01 pam. In contrast, there was a statistically significant increase in C4b and C3b deposition on strain KK01 csaA pam, consistent with the increase in antibody deposition and serum sensitivity observed with this strain (Fig. 2C and D). These data suggest that the survival of strains KK01, KK01 csaA, and KK01 pam in NHS is due in part to reduced antibody deposition and the resultant absence of activating complement fragments on the bacterial surface. Elimination of both the polysaccharide capsule and the exopolysaccharide on the bacterial surface results in increased opsonin deposition, promoting complement activation.
Inhibition of the classical pathway when surface polysaccharides are absent restores serum survival and decreases opsonin deposition.
The deposition of IgG and IgM on the bacterial surface prompts complement activation via the classical pathway. Ca2+ is considered necessary to maintain the integrity of the C1 complex, which associates with the Fc region of antigen-bound IgG or IgM and initiates the complement cascade (36, 37). The presence of EGTA in serum resistance assays chelates Ca2+ and thereby inhibits classical pathway activation, and the concomitant addition of Mg2+ preserves the alternative pathway (38).
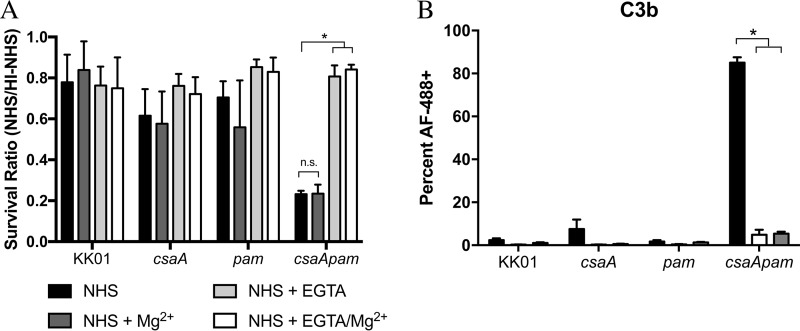
To elucidate the importance of classical pathway activation in the serum killing of strain KK01 csaA pam, we performed serum bactericidal assays in the presence of EGTA plus Mg2+. As expected, the survival of strains KK01, KK01 csaA, and KK01 pam was not influenced by the introduction of 9 mM Mg2+ alone, 5 mM EGTA alone, or 9 mM Mg2+ plus 5 mM EGTA (Fig. 3A). Conversely, the survival ratio of strain KK01 csaA pam in the presence of either EGTA alone or EGTA plus Mg2+ was restored to levels similar to the survival ratios of strains KK01, KK01 csaA, and KK01 pam (Fig. 3A). As anticipated, the presence of Mg2+ alone had no effect on the survival ratio of strain KK01 csaA pam (Fig. 3A).
FIG 3.
Inhibition of the classical pathway restores survival and reduces C3b deposition in the surface polysaccharide-deficient mutant. (A) Serum survival was assessed with either 50% NHS alone, 50% NHS plus 9 mM Mg2+, 50% NHS plus 5 mM EGTA, 50% NHS plus 9 mM Mg2+ and 5 mM EGTA, or 50% HI-NHS for 1 h. The survival ratio was calculated by dividing the NHS CFU counts by the HI-NHS CFU counts. (B) K. kingae strains were fixed and incubated for 15 min in medium alone, in medium containing 20% NHS, or in medium containing 20% NHS plus 9 mM Mg2+ and 5 mM EGTA. Binding of C3b (n = 3) to the bacterial surface was determined using flow cytometry, and 30,000 propidium iodine-positive events per experiment were analyzed. The percentages represent events that registered as Alexa Fluor 488 positive. Black bars, NHS; white bars, NHS plus 9 mM Mg2+ and 5 mM EGTA; gray bars, secondary antibody-only control. Abbreviations: csaA, KK01 csaA; pam, KK01 pam; csaApam, KK01 csaA pam. Statistical significance was determined with an unpaired Student's t test, and the error bars represent the standard error of the mean. n.s., not significant; *, P < 0.05.
In order to confirm that chemical inhibition of the classical pathway via EGTA prevents complement activation, flow cytometry assays were performed to determine the percentage of C3b deposition on the surface of K. kingae in the presence of EGTA plus Mg2+. As shown in Fig. 3B, no significant differences in C3b deposition were observed between strains KK01, KK01 csaA, and KK01 pam in the presence or absence of EGTA plus Mg2+. In contrast, there was a significant decrease in C3b deposition on strain KK01 csaA pam when this strain was incubated in NHS containing EGTA plus Mg2+ (Fig. 3B). The C3b deposition on strain KK01 csaA pam in NHS containing EGTA plus Mg2+ mirrored the C3b deposition on strain KK01 csaA pam in medium alone (Fig. 3B). Taken together, these data provide further support that the classical complement pathway is activated in the absence of K. kingae surface polysaccharides.
The four distinct capsule types of K. kingae promote serum survival in isogenic strains.
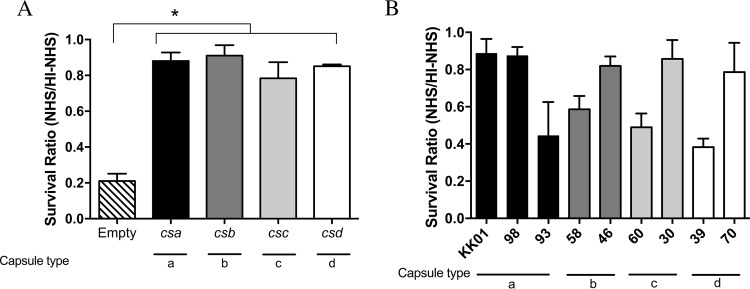
To date, a total of four distinct capsule types have been identified in diverse collections of K. kingae clinical isolates, including type a ([3)-β-GalpNAc-(1→5)-β-Kdop-(2→]), type b ([6)-α-d-GlcpNAc-(1→5)-β-(8-OAc)Kdop-(2→]), type c ([3)-β-d-Ribf-(1→2)-β-d-Ribf-(1→2)-β-d-Ribf-(1→4)-β-Kdop-(2→]), and type d ([P-(O→3)[β-d-Galp-(1→4)]-β-d-GlcpNAc-(1→3)-α-d-GlcpNAc-1-]) (19–22). The prototype strain KK01 used in this study expresses the type a capsule. To assess whether the four capsule types have different abilities to protect the organism against complement-mediated lysis, we performed bactericidal assays on isogenic derivatives of strain KK01 pam expressing either capsule type a (strain KK01Swap csa), capsule type b (strain KK01Swap csb), capsule type c (strain KK01Swap csc), or capsule type d (strain KK01Swap csd) (19). As shown in Fig. 4A, all four capsule types were associated with a survival ratio of 0.80, similar to the survival ratio of strain KK01. These data suggest that the presence of any of the four K. kingae capsule types is protective and is adequate for promoting serum resistance in the absence of exopolysaccharide in vitro.
FIG 4.
Specific capsule types do not dictate serum sensitivity in K. kingae clinical isolates. K. kingae strains (∼103 CFU) were incubated with either 50% NHS or 50% HI-NHS for 1 h. The survival ratio was calculated by dividing the CFU counts in NHS by the CFU counts in HI-NHS for isogenic derivatives of strain KK01 pam (A) or clinical isolates of K. kingae (B). Abbreviations: Empty, nonencapsulated strain KK01SwapEmpty pam; csa, capsule type a KK01Swap csa pam; csb, type b KK01Swap csb pam; csc, type c KK01Swap csc pam; csd, type d KK01Swap csd pam; 98, K. kingae clinical isolate PYKK98; 93, K. kingae clinical isolate PYKK93; 58, K. kingae clinical isolate PYKK58; 46, K. kingae clinical isolate KK146; 60, K. kingae clinical isolate PYKK60; 30, K. kingae clinical isolate ATCC 23330; 39, K. kingae clinical isolate E3339; 70, K. kingae clinical isolate BB270. A total of three biological replicates were performed (n = 3). Statistical significance was determined with an unpaired Student's t test, and the error bars represent the standard error of the mean. *, P < 0.05.
K. kingae clinical isolates show moderate to high levels of serum resistance.
Serum resistance plays an important role in pathogenicity and has been shown to be variable from strain to strain in other bacterial species. To determine the variability of serum resistance across K. kingae clinical isolates, we performed serum bactericidal assays on eight clinical isolates that represented all four capsular groups and included both invasive and healthy carrier isolates (Fig. 4B; Table 1). Additionally, the eight clinical isolates contain the pam locus and produce exopolysaccharide. K. kingae strains PYKK98, KK146, ATCC 23330, and BB270 showed high levels of serum resistance, with a survival ratio of approximately 0.80 (Fig. 4B), while strains PYKK93, PYKK58, PYKK60, and E3339 showed moderate levels of serum resistance, with survival ratios of between 0.40 and 0.60 (Fig. 4B). The results demonstrate that the clinical isolates tested were resistant to human serum; however, there was no consistent distinction in the level of susceptibility in relation to capsule type or clinical site of isolation.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| Kingella kingae | ||
| KK01 | Nonspreading/noncorroding derivative of 269-492 | 8 |
| PYKK98 | Clonal group B isolate from a case of bacteremia, capsule type a | P. Yagupsky |
| PYKK93 | Clonal group P isolate from a case of bacteremia, capsule type a | P. Yagupsky |
| PYKK58 | Clonal group N isolate from a case of septic arthritis, capsule type b | P. Yagupsky |
| KK146 | Clonal group N isolate from a case of bacteremia, capsule type b | P. Yagupsky |
| PYKK60 | Clonal group D isolate from a case of endocarditis, capsule type c | P. Yagupsky |
| ATCC 23330 | Clonal group D isolate from a healthy carrier, capsule type c | ATCC |
| E3339 | Clonal group F isolate from a healthy carrier, capsule type d | P. Yagupsky |
| BB270 | Clonal group U isolate from a healthy carrier, capsule type d | P. Yagupsky |
| KK01 csaA | Contains csaA deletion | 22 |
| KK01 pam | Contains pamABCDE deletion | 21 |
| KK01 csaA pam | Contains csaA deletion and pamABCDE deletion | This study |
| KK01SwapEmpty | Contains the capsule synthesis locus flanking genes and a deletion of the csaA region | 19 |
| KK01Swap csa | KK01SwapEmpty with csa locus recombined | 19 |
| KK01Swap csb | KK01SwapEmpty with csb locus recombined | 19 |
| KK01Swap csc | KK01SwapEmpty with csc locus recombined | 19 |
| KK01Swap csd | KK01SwapEmpty with csd locus recombined | 19 |
| KK01SwapEmpty pam | KK01SwapEmpty with pamABCDE deletion | This study |
| KK01Swap csa pam | KK01Swap csa with pamABCDE deletion | This study |
| KK01Swap csb pam | KK01Swap csb with pamABCDE deletion | This study |
| KK01Swap csc pam | KK01Swap csc with pamABCDE deletion | This study |
| KK01Swap csd pam | KK01Swap csd with pamABCDE deletion | This study |
| E. coli DH5α | E. coli F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE441 thi-1 gyrA96 relA1 | 64 |
| H. influenzae | ||
| C54 | Haemophilus influenzae serotype b isolate | 65 |
| C54 b− | Spontaneous capsule-deficient derivative of strain C54 | 66 |
| E. coli plasmid pUC19pam::ermC | pUC19 with ermC erythromycin cassette flanked by surrounding 5′ and 3′ regions of the pamABCDE locus | 21 |
Capsule and exopolysaccharide are required for full K. kingae virulence in a juvenile rat infection model.
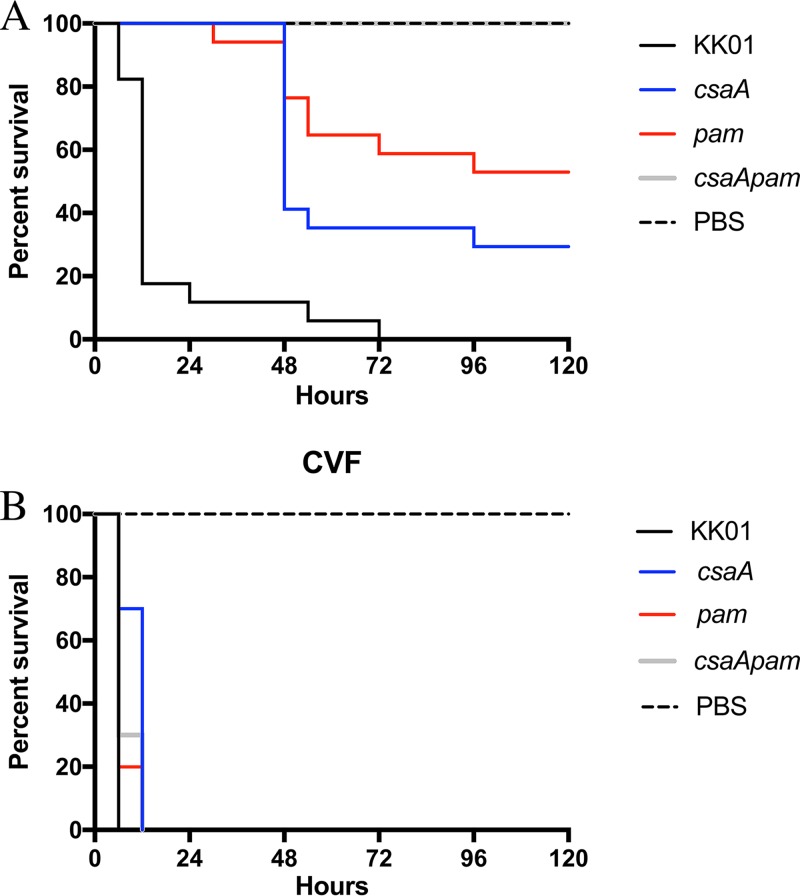
Given the importance of the capsular polysaccharide and exopolysaccharide in protecting K. kingae from the bactericidal effects of human serum in vitro, we sought to elucidate the role of these factors in vivo. To examine the role of the K. kingae surface polysaccharides in virulence and to determine the influence of complement in limiting infection in vivo, we modified a previously described juvenile rat infection protocol (22). Briefly, we injected 5-day-old Sprague-Dawley rat pups via the intraperitoneal (i.p.) route with either phosphate-buffered saline (PBS) alone or PBS with 10 µg of cobra venom factor (CVF), which forms a complex with complement components factor B and factor D and creates a convertase that depletes C3 and C5 to undetectable levels in the serum (39, 40). CVF has been used previously in different animal models, including juvenile rats, to successfully deplete complement in vivo (40–42). At 3 h postinjection with PBS alone or PBS with CVF, the rat pups were injected i.p. with 1 × 108 CFU of strain KK01, strain KK01 csaA, strain KK01 pam, or strain KK01 csaA pam, using PBS as a control (Fig. 5A and B).
FIG 5.
Surface polysaccharides are required for full K. kingae virulence in a rat infection model. Five-day-old Sprague-Dawley rats were injected via the intraperitoneal (i.p.) route with either 0.1 ml PBS (A) or 10 μg CVF in 0.1 ml PBS (B). The graphs plot Kaplan-Meyer survival curves for rats subsequently i.p. injected with 0.1 ml PBS or 1 × 108 CFU of the KK01 strains in PBS. Data are for 17 (A) and 10 (B) animals infected with each strain and 15 (A) and 9 (B) animals injected with PBS. Abbreviations: csaA, KK01 csaA; pam, KK01 pam; csaApam, KK01 csaA pam.
Among the rat pups that did not receive pretreatment with CVF, all that were infected with strain KK01 succumbed to infection, with a median survival of 12 h (Fig. 5A). At 5 days postinfection, survival among pups infected with strain KK01 csaA was 29% (P < 0.0001) (Fig. 5A), and survival among pups infected with strain KK01 pam was 53% (P < 0.0001) (Fig. 5A). In contrast, all pups injected with strain KK01 csaA pam were alive and healthy at 5 days postinfection, similar to the results for pups injected with PBS alone (P < 0.0001) (Fig. 5A). These results suggest that elimination of either capsule or exopolysaccharide results in reduced virulence of K. kingae and that elimination of both the capsule and the exopolysaccharide results in an avirulent strain.
Among pups that received CVF pretreatment, all infected with strain KK01, strain KK01 csaA, strain KK01 pam, or strain KK01 csaA pam succumbed to infection within 12 h (Fig. 5B). All pups injected with PBS alone after CVF pretreatment survived at 5 days postinfection (Fig. 5B). These results suggest that resistance to complement via the polysaccharide capsule and the exopolysaccharide is critical for K. kingae virulence in juvenile rats.
DISCUSSION
Kingella kingae is a common commensal organism in the oropharynx and is an emerging pediatric pathogen that represents a leading etiology of joint and bone infections in young children (2–4). This organism causes disease by breaching the respiratory epithelium, invading the bloodstream, and disseminating to distant sites (1, 5–8). In order to survive in the intravascular environment and then disseminate, the organism must evade innate immune mechanisms.
Our initial findings revealed that K. kingae is highly resistant to the bactericidal effects of complement present in human serum. Further examination revealed that the presence of either the capsule or the exopolysaccharide alone was sufficient to fully prevent opsonin deposition and completely protect K. kingae against complement-mediated killing, a novel observation. Elimination of both the capsule and the exopolysaccharide was required for efficient binding of opsonins (IgG, IgM, C4b, and C3b) to the bacterial surface and for significant complement-mediated killing in vitro. Abrogation of the classical complement pathway in vitro restored survival of the capsule/exopolysaccharide double mutant, clarifying the mechanism by which these surface polysaccharides promote survival in human serum. In agreement with our in vitro data, elimination of both the capsule and the exopolysaccharide rendered K. kingae completely avirulent, abolishing morbidity and mortality in the juvenile rat infection model. To confirm that the attenuation of strain KK01 csaA pam in vivo was a consequence of efficient activation of complement, pups were injected with CVF to eliminate complement. As predicted, the resulting complement deficiency was associated with an increase in virulence of all K. kingae strains, including strain KK01 csaA pam.
In recent years, a number of reports have described bacterial factors associated with evasion of complement activity in nonencapsulated organisms (43–47). However, bacterial polysaccharide capsules generally predominate as the most potent mechanism of bacterial resistance to complement-mediated serum killing, as highlighted by studies of H. influenzae, N. meningitidis, and E. coli. In N. meningitidis, transposon mutagenesis and large-scale analysis of the genome searching for genes contributing to serum resistance identified only genes involved in polysaccharide capsule or lipooligosaccharide synthesis (14). Consistent with the conclusion that the polysaccharide capsule is important for N. meningitidis serum resistance, almost all meningococcal isolates recovered from blood are encapsulated, while 30 to 70% of carrier isolates are nonencapsulated (48–50). Similarly, in H. influenzae, loss of capsule reduces or eliminates serum resistance, and overexpression of the serotype b capsule synthesis genes due to duplication events promotes greater serum resistance (13, 51, 52). In this study, we observed that nonencapsulated K. kingae exhibits high levels of serum resistance in vitro, similar to the serum resistance of wild-type K. kingae, underscoring the importance of the exopolysaccharide.
In considering our observation that the K. kingae capsule and exopolysaccharide play crucial and apparently redundant roles in serving to prevent IgG, IgM, C4b, and C3b deposition on the bacterial surface and resultant bacterial lysis, it is noteworthy that the capsule is tethered to the bacterial surface via a lipid anchor and the exopolysaccharide is unanchored. We presume that the exopolysaccharide is loosely associated with the bacterial surface, blocking access of opsonins. In earlier work, we observed that the exopolysaccharide can be released from the bacterial surface by resuspending bacteria in PBS and vigorously shaking, consistent with a noncovalent mechanism of association (21). The nature of the association and the balance between surface-associated and released exopolysaccharide during natural infection are currently unknown.
Exopolysaccharides in other bacterial species have been studied primarily as major contributors to the formation and dispersal of biofilms (24, 53–55). Exopolysaccharide function depends on the polymer structure and composition and has been studied in relation to adherence, bacterial cell aggregation, water retention, and nutrition (24, 55, 56). Few studies have characterized exopolysaccharides in the context of inhibiting complement deposition and subsequent complement and/or leukocyte evasion (26–29). In Pseudomonas aeruginosa, expression of the Psl exopolysaccharide serves to protect the organism from neutrophil antimicrobial functions; however, Psl does not play a role in evasion of complement-mediated lysis in vitro and has been studied only in the context of capsule-deficient organisms, thus contrasting with the findings of our studies of K. kingae (26). Like K. kingae, extraintestinal pathogenic Escherichia coli (ExPEC) produces two surface polysaccharides that contribute to serum resistance, namely, a group 2 polysaccharide capsule and an exopolysaccharide called colanic acid (27). However, while colanic acid is important for evasion of complement-mediated killing, the absence of the polysaccharide capsule renders ExPEC sensitive to serum even in the presence of colanic acid, suggesting that the exopolysaccharide alone is not sufficient for mediating serum resistance and thus again contrasting with the findings of studies of K. kingae (27). In K. kingae, the level of protection against complement activation and complement-mediated killing provided by the exopolysaccharide mimics the level of protection provided by the polysaccharide capsule. Thus far, two exopolysaccharide structures have been described in K. kingae: →3)-β-Galf-(1→5)-β-Galf-(1→ and →5)-β-Galf-(1→ (21, 25). It has been reported that biofilm formation and the dispersal of K. kingae clinical strain PYKK181 are dependent on the PAM galactan exopolysaccharide (25). In this study, we have shown that the PAM galactan produced by K. kingae strain KK01 prevents opsonin deposition and complement activation, providing evidence for a novel function of the K. kingae exopolysaccharide.
In K. kingae, four distinct polysaccharide capsules have been identified, designated types a, b, c, and d. Using isogenic derivatives of strain KK01 pam, all four capsule types were associated with a survival ratio similar to the survival ratio for strain KK01. These data suggest that the presence of any naturally occurring K. kingae capsule type is adequate to protect KK01 pam against serum killing under our in vitro conditions. In addition, eight clinical isolates representing all four capsular groups and including both invasive and healthy carrier isolates were assessed for serum resistance. Interestingly, these isolates exhibited moderate to high levels of serum resistance, with no consistent distinctions in level of susceptibility in relation to capsule type or clinical site of isolation. The variability in the serum survival ratio among the different clinical isolates could be due to strain-to-strain variation in the quantity of capsule and/or exopolysaccharide. Despite the contributions of capsule and exopolysaccharide to serum resistance, the capsule and exopolysaccharide double mutant remained relatively resistant to human serum, with a survival ratio of 0.40 (Fig. 1B). This observation highlights the presence of additional factors deployed by K. kingae for innate immune evasion and may be another explanation for the variability of serum resistance observed in the clinical isolates.
While our in vitro serum resistance experiments suggest no differences in resistance between the wild-type strain and the single mutant strains lacking capsule or exopolysaccharide, our in vivo studies established that each surface polysaccharide plays a role in promoting virulence in juvenile rats. Elimination of either capsule or exopolysaccharide resulted in reduced virulence, including a lower mortality rate and delayed mortality. This finding suggests that perhaps the capsule and/or the exopolysaccharide plays a role in virulence beyond protecting against humoral immunity in vivo; alternatively, serum sensitivity in vitro may be unobservable in the absence of a single surface polysaccharide. Along these lines, polysaccharide capsules and exopolysaccharides have been implicated in both promoting and dampening host inflammatory responses. The zwitterionic capsular polysaccharide A from Bacteroides fragilis, the gut microfloral commensal, is implicated in mediating development of the host immune system through induction of CD4+ maturation and by promotion of a balanced Th1/Th2 response in mice (57). Interestingly, Totté et al. have reported that a galactan homopolysaccharide in Mycoplasma mycoides subsp. mycoides similar to the K. kingae exopolysaccharide binds Toll-like receptor 2 and promotes the production of the anti-inflammatory cytokine interleukin-10 (58). Further work is necessary to elucidate the immunoregulatory potential of the K. kingae surface polysaccharides both in vitro and in vivo.
In conclusion, both the polysaccharide capsule and the exopolysaccharide of K. kingae play a crucial role in preventing opsonin deposition and complement-mediated killing, presumably facilitating intravascular survival and resulting in enhanced virulence in vivo. These results highlight an underrecognized function of a bacterial exopolysaccharide and provide important information that may facilitate development of a polysaccharide-based vaccine against K. kingae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. The parent K. kingae strain 269-492 (8) grows on solid agar as two phenotypically stable colony types, designated KK01 and KK03. As described in our earlier work, the KK01 and KK03 colony types differ in their density of piliation. K. kingae KK01 was used as the wild-type strain throughout this study. K. kingae strains were stored at −80°C in brain heart infusion (BHI) broth with 20% glycerol.
E. coli strains were stored at −80°C in Luria-Bertani (LB) broth with 15% glycerol. K. kingae strains were grown at 37°C with 5% CO2 on chocolate agar plates supplemented with 50 μg/ml kanamycin or 1 μg/ml erythromycin, as appropriate. E. coli strains were grown at 37°C on LB agar or in LB broth supplemented with 100 μg/ml ampicillin, as appropriate.
Strain construction.
The csaA mutant, the pam mutant, and the capsule swap strains were generated as previously described (19, 21, 22). To generate the KK01 csaA pam double mutant and the capsule swap strains containing the pam locus deletion, strains KK01 csaA, KK01Swap csa, KK01Swap csb, KK01Swap csc, KK01Swap csd, and KK01SwapEmpty were transformed with linearized plasmid pUC19pam::ermC. Briefly, the plasmid was purified from E. coli, linearized with NdeI, and introduced into the parental strains (the csaA mutant or swap strains) via natural transformation. Transformants were recovered on chocolate agar plates containing 1.0 μg/ml erythromycin. Correct insertion of the gene disruption construct was confirmed via PCR and sequencing of the deletion site. The absence of the capsule and the exopolysaccharide in strain KK01 csaA pam and the capsule swap pam deletion strains was confirmed using alcian blue and silver staining as previously described (21, 59), with the parental strains and strain KK01 being used as controls.
Polysaccharide extraction and staining.
For capsule extractions, bacteria were washed and resuspended in Tris acetate (pH 5) for 1 h. Cells were removed by centrifugation, and the extracts were treated with proteinase K for 1 h and then concentrated as previously described (59). For exopolysaccharide extractions, bacteria were resuspended in 5 ml PBS and vortexed. Cells were removed by centrifugation, and the bacterial supernatants were treated with proteinase K for 1 h and then concentrated as previously described (59).
Aliquots of supernatant and purified capsule from K. kingae derivatives were separated on 7.5% or 10% SDS-polyacrylamide gels, respectively. For supernatant samples, the SDS-polyacrylamide gels were treated first with silver staining and subsequently with alcian blue staining. For purified capsule samples, the SDS-polyacrylamide gels were treated with alcian blue staining. For silver staining, the gels were treated as previously described (60). For alcian blue staining, the gels were stained with 0.125% alcian blue as previously described (59).
Serum bactericidal assays.
K. kingae strains and E. coli strain DH5α were grown on chocolate agar plates and then resuspended in PBS containing 0.1% gelatin (PBS-G). H. influenzae strains were grown on chocolate agar plates overnight, resuspended in BHI with 3.5 μg/ml NAD and 0.1% lysed horse blood to an optical density at 600 nm (OD600) of 0.2, and grown to an OD600 of between 0.6 and 0.8 with shaking at 250 rpm. Samples were diluted in PBS-G to obtain a final inoculum of approximately 4.0 × 103 CFU/0.1 ml. The respective inocula were mixed with pooled NHS (Immucor, Norcross, GA) or HI-NHS (prepared by incubating NHS at 56°C for 20 min) diluted in PBS-G, as appropriate, and incubated for various times at 37°C with 5% CO2. Complement activity was assessed and confirmed using a total hemolytic assay as previously described (61). Serial dilutions of the inoculum and reaction samples were plated on chocolate agar plates and incubated overnight at 37°C with 5% CO2 to determine the CFU counts (limit of detection for plating, 20 CFU). To perform classical pathway inhibition assays, samples were incubated with EGTA at a final concentration of 5 mM and supplemental MgCl2 at a final concentration of 9 mM prior to introduction of NHS.
Flow cytometry analysis.
K. kingae strains were grown on chocolate agar plates, resuspended to an OD600 of 0.8 in PBS, pelleted, and resuspended in 4% paraformaldehyde in PBS for fixation. After incubation for 20 min at room temperature (RT), bacteria were washed with Tris-buffered saline (TBS) and resuspended in PBS. We modified previously described protocols to detect bacterial opsonization (26, 62). Briefly, fixed bacteria were incubated with 20% NHS as the source of C3b or 1% NHS as the source of C4b. Samples were incubated with gentle agitation for 15 min at RT, washed with PBS, and resuspended in TBS containing 50 mM EDTA and 0.1% bovine serum albumin (BSA). Fixed bacteria were incubated with or without a 1:250 dilution of a mouse anti-human C3b monoclonal antibody (Thermo Fisher, Rockford, IL) or a 1:250 dilution of a rabbit anti-human C4b monoclonal antibody (Abcam, Cambridge, MA) for 1 h at RT. Samples were washed twice in PBS, resuspended in PBS containing 0.1% BSA, and incubated with a 1:200 dilution of a rabbit anti-mouse IgG DyLight 488-conjugated antibody (Rockland, Limerick, PA) or a 1:200 dilution of a goat anti-rabbit IgG DyLight 488-conjugated antibody (Rockland) for 45 min at RT, as appropriate. Bacteria were washed, resuspended in PBS, and stained with propidium iodide (Biotium, Fremont, CA) for flow cytometry analysis. For classical pathway inhibition assays, samples were incubated with EGTA at a final concentration of 5 mM and supplemental MgCl2 at a final concentration of 9 mM prior to introduction of NHS.
For human IgG and human IgM surface deposition analysis, bacteria were fixed, resuspended in TBS containing 50 mM EDTA and 0.1% BSA, and then incubated with PBS as a control or 1% HI-NHS as a source of human IgG and human IgM. After gentle agitation for 1 h at RT, samples were washed twice with PBS, resuspended in PBS containing 0.1% BSA, and then incubated with a 1:200 dilution of a goat anti-human IgG (H&L) DyLight 488-conjugated antibody (Rockland) or a 1:200 dilution of a goat anti-human IgM (mu chain) DyLight 488-conjugated antibody (Rockland) for 45 min at RT. Bacteria were washed, resuspended in PBS, and stained with propidium iodide (Biotium) for flow cytometry analysis. Flow cytometry assays were performed using an Accuri C6 instrument (BD Biosciences, San Jose, CA).
K. kingae-specific serum antibody detection.
Serum antibodies reactive to K. kingae whole-cell and outer membrane fractions were determined by Western blotting. Total membranes were recovered by centrifugation of cleared bacterial sonicates, and outer membrane fractions were isolated on the basis of Sarkosyl insolubility. The whole-cell and outer membrane fractions were separated on a 7.5% SDS-polyacrylamide gel, transferred to nitrocellulose, and probed with 2% HI-NHS, followed by an anti-human Ig horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma, St. Louis, MO) at a dilution of 1:5,000. The blot was developed by using a chemiluminescent substrate.
Juvenile rat infection model.
Nursing 5-day-old Sprague-Dawley rat pups (Charles River Laboratories, Wilmington, MA) were injected via the intraperitoneal route with 0.1 ml PBS alone (control) or 10 μg of CVF (Complement Technology, Tyler, TX) in 0.1 ml PBS. K. kingae strains were grown on chocolate agar for 18 h, and the bacteria were swabbed from plates and resuspended in PBS to a final density of 1 × 108 CFU/0.1 ml. While the in vitro serum bactericidal assays used 1 × 103 CFU to conserve human serum, 1 × 108 CFU was the necessary infectious dose in vivo to consistently generate invasive disease in rat pups with strain KK01. At 3 h after CVF injection, rat pups were injected via the intraperitoneal route with 0.1 ml of the appropriate bacterial suspension or 0.1 ml of PBS alone (control). Rat pups were injected using a 27 1/2-gauge needle and then returned to their cage with a lactating dam. The experimental and control groups were housed separately with a lactating dam and were monitored for mortality and signs of illness every 6 h for the first 30 h and then twice daily for a total of 5 days. Animals found to be moribund were euthanized by using CO2 inhalation followed by secondary decapitation.
Ethics statement.
All animal experiments described within were conducted in accordance with the Animal Welfare Act, the Public Health Service policy on the humane care and use of laboratory animals from the U.S. Department of Health and Human Services, and the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (63). The Children's Hospital of Philadelphia animal research facilities have full accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The animal procedures were approved by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee (IACUC) under protocol IAC 16-001050.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism (version 7.0a) software for Mac (GraphPad Software, San Diego, CA), where a P value of <0.05 was considered statistically significant. Unpaired Student's t test was performed as appropriate. Mortality rates were calculated using the log-rank (Mantel-Cox) test for significance.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation Graduate Research Fellowship under award DGE-1321851 (to V.L.M.) and the National Institute of Allergy and Infectious Diseases under award 1R01AI121015 (to J.W.S.).
We thank Pablo Yagupsky at the Soroka University Medical Center for providing us with the K. kingae clinical isolates used in this study.
REFERENCES
- 1.Yagupsky P, Porat N, Pinco E. 2009. Pharyngeal colonization by Kingella kingae in children with invasive disease. Pediatr Infect Dis J 28:155–157. doi: 10.1097/INF.0b013e318184dbb8. [DOI] [PubMed] [Google Scholar]
- 2.Yagupsky P, Porsch EA, St Geme JW III. 2011. Kingella kingae: an emerging pathogen in young children. Pediatrics 127:557–565. doi: 10.1542/peds.2010-1867. [DOI] [PubMed] [Google Scholar]
- 3.Yagupsky P. 2014. Outbreaks of Kingella kingae infections in daycare facilities. Emerg Infect Dis 20:746–753. doi: 10.3201/eid2005.131633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, Vandenesch F, Freydiere AM. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 26:377–381. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]
- 5.Bidet P, Collin E, Basmaci R, Courroux C, Prisse V, Dufour V, Bingen E, Grimprel E, Bonacorsi S. 2013. Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr Infect Dis J 32:558–560. doi: 10.1097/INF.0b013e3182867f5e. [DOI] [PubMed] [Google Scholar]
- 6.Amit U, Dagan R, Yagupsky P. 2013. Prevalence of pharyngeal carriage of Kingella kingae in young children and risk factors for colonization. Pediatr Infect Dis J 32:191–193. doi: 10.1097/INF.0b013e3182755779. [DOI] [PubMed] [Google Scholar]
- 7.Yagupsky P, Erlich Y, Ariela S, Trefler R, Porat N. 2006. Outbreak of Kingella kingae skeletal system infections in children in daycare. Pediatr Infect Dis J 25:526–532. doi: 10.1097/01.inf.0000215243.42501.4f. [DOI] [PubMed] [Google Scholar]
- 8.Kehl-Fie TE, St Geme JW III. 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J Bacteriol 189:430–436. doi: 10.1128/JB.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarma JV, Ward PA. 2011. The complement system. Cell Tissue Res 343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson PK, Wilkinson BJ, Kim Y, Schmeling D, Quie PG. 1978. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun 19:943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz MA, Silverstein SC. 1980. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest 65:82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noel GJ, Hoiseth SK, Edelson PJ. 1992. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J Infect Dis 166:178–182. doi: 10.1093/infdis/166.1.178. [DOI] [PubMed] [Google Scholar]
- 14.Geoffroy MC, Floquet S, Métais A, Nassif X, Pelicic V. 2003. Large-scale analysis of the meningococcus genome by gene disruption: resistance to complement-mediated lysis. Genome Res 13:391–398. doi: 10.1101/gr.664303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, Wenger JD, Stephens DS. 1997. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A 94:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moxon ER, Kroll JS. 1988. Type b capsular polysaccharide as a virulence factor of Haemophilus influenzae. Vaccine 6:113–115. doi: 10.1016/S0264-410X(88)80011-2. [DOI] [PubMed] [Google Scholar]
- 17.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblatt D. 2000. Conjugate vaccines. Clin Exp Immunol 119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starr KF, Porsch EA, Seed PC, Heiss C, Naran R, Forsberg LS, Amit U, Yagupsky P, Azadi P, St Geme JW. 2016. Kingella kingae expresses four structurally distinct polysaccharide capsules that differ in their correlation with invasive disease. PLoS Pathog 12:e1005944. doi: 10.1371/journal.ppat.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porsch EA, Starr KF, Yagupsky P, St Geme JW III. 2017. The type a and type b polysaccharide capsules predominate in an international collection of invasive Kingella kingae isolates. mSphere 2:e00060-. doi: 10.1128/mSphere.00060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starr KF, Porsch EA, Heiss C, Black I, Azadi P, St Geme JW. 2013. Characterization of the Kingella kingae polysaccharide capsule and exopolysaccharide. PLoS One 8:e75409. doi: 10.1371/journal.pone.0075409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starr KF, Porsch EA, Seed PC, St Geme JW III. 2016. Genetic and molecular basis of Kingella kingae encapsulation. Infect Immun 84:1775–1784. doi: 10.1128/IAI.00128-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland IW. 1982. Biosynthesis of microbial exopolysaccharides. Adv Microb Physiol 23:79–150. doi: 10.1016/S0065-2911(08)60336-7. [DOI] [PubMed] [Google Scholar]
- 24.Nwodo UU, Green E, Okoh AI. 2012. Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra M, Byrd MS, Sergeant S, Azad AK, Parsek MR, McPhail L, Schlesinger LS, Wozniak DJ. 2012. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol 14:95–106. doi: 10.1111/j.1462-5822.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miajlovic H, Cooke NM, Moran GP, Rogers TRF, Smith SG. 2014. Response of extraintestinal pathogenic Escherichia coli to human serum reveals a protective role for Rcs-regulated exopolysaccharide colanic acid. Infect Immun 82:298–305. doi: 10.1128/IAI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganguly T, Johnson JB, Kock ND, Parks GD, Deora R. 2014. The Bordetella pertussis Bps polysaccharide enhances lung colonization by conferring protection from complement-mediated killing. Cell Microbiol 16:1105–1118. doi: 10.1111/cmi.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones CJ, Wozniak DJ. 2017. Psl produced by mucoid Pseudomonas aeruginosa contributes to the establishment of biofilms and immune evasion. mBio 8:e00864-. doi: 10.1128/mBio.00864-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard CJ, Glynn AA. 1971. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology 20:767–777. [PMC free article] [PubMed] [Google Scholar]
- 31.Tomás JM, Camprubi S, Merino S, Davey MR, Williams P. 1991. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun 59:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domenico P, Tomas JM, Merino S, Rubires X, Cunha BA. 1999. Surface antigen exposure by bismuth dimercaprol suppression of Klebsiella pneumoniae capsular polysaccharide. Infect Immun 67:664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan AR, Winter G. 1988. The binding site for C1q on IgG. Nature 332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 34.Perkins SJ, Nealis AS, Sutton BJ, Feinstein A. 1991. Solution structure of human and mouse immunoglobulin M by synchrotron X-ray scattering and molecular graphics modelling. A possible mechanism for complement activation. J Mol Biol 221:1345–1366. [DOI] [PubMed] [Google Scholar]
- 35.Zlatarova AS, Rouseva M, Roumenina LT, Gadjeva M, Kolev M, Dobrev I, Olova N, Ghai R, Jensenius JC, Reid KBM, Kishore U, Kojouharova MS. 2006. Existence of different but overlapping IgG- and IgM-binding sites on the globular domain of human C1q. Biochemistry 45:9979–9988. doi: 10.1021/bi060539v. [DOI] [PubMed] [Google Scholar]
- 36.Gadjeva MG, Rouseva MM, Zlatarova AS, Reid KBM, Kishore U, Kojouharova MS. 2008. Interaction of human C1q with IgG and IgM: revisited. Biochemistry 47:13093–13102. doi: 10.1021/bi801131h. [DOI] [PubMed] [Google Scholar]
- 37.Bryant RE, Jenkins DE Jr.. 1968. Calcium requirements for complement dependent hemolytic reactions. J Immunol 101:664–668. [PubMed] [Google Scholar]
- 38.Des Prez RM, Bryan CS, Hawiger J, Colley DG. 1975. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2 ethylene glycol tetraacetic acid. Infect Immun 11:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauman N. 1978. Lack of C5 convertase-generating activity in Naja haje cobra factor. J Immunol 120:1763–1764. [Google Scholar]
- 40.Van den Berg CW, Aerts PC, Van Dijk H. 1991. In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J Immunol Methods 136:287–294. doi: 10.1016/0022-1759(91)90015-8. [DOI] [PubMed] [Google Scholar]
- 41.Kauppi-Korkeila M, van Alphen L, Madore D, Saarinen L, Käyhty H. 1996. Mechanism of antibody-mediated reduction of nasopharyngeal colonization by Haemophilus influenzae type b studied in an infant rat model. J Infect Dis 174:1337–1340. doi: 10.1093/infdis/174.6.1337. [DOI] [PubMed] [Google Scholar]
- 42.Till GO, Morganroth ML, Kunkel R, Ward PA. 1987. Activation of C5 by cobra venom factor is required in neutrophil-mediated lung injury in the rat. Am J Pathol 129:44–53. [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy TF, Kirkham C, Lesse AJ. 2006. Construction of a mutant and characterization of the role of the vaccine antigen P6 in outer membrane integrity of nontypeable Haemophilus influenzae. Infect Immun 74:5169–5176. doi: 10.1128/IAI.00692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho DK, Ram S, Nelson KL, Bonthuis PJ, Smith AL. 2007. lgtC expression modulates resistance to C4b deposition on an invasive nontypeable Haemophilus influenzae. J Immunol 178:1002–1012. doi: 10.4049/jimmunol.178.2.1002. [DOI] [PubMed] [Google Scholar]
- 45.Hallström T, Blom AM, Zipfel PF, Riesbeck K. 2009. Nontypeable Haemophilus influenzae protein E binds vitronectin and is important for serum resistance. J Immunol 183:2593–2601. doi: 10.4049/jimmunol.0803226. [DOI] [PubMed] [Google Scholar]
- 46.Griffiths NJ, Hill DJ, Borodina E, Sessions RB, Devos NI, Feron CM, Poolman JT, Virji M. 2011. Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance. Mol Microbiol 82:1129–1149. doi: 10.1111/j.1365-2958.2011.07876.x. [DOI] [PubMed] [Google Scholar]
- 47.Del Tordello E, Vacca I, Ram S, Rappuoli R, Serruto D. 2014. Neisseria meningitidis NalP cleaves human complement C3, facilitating degradation of C3b and survival in human serum. Proc Natl Acad Sci U S A 111:427–432. doi: 10.1073/pnas.1321556111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kugelberg E, Gollan B, Tang CM. 2008. Mechanisms in Neisseria meningitidis for resistance against complement-mediated killing. Vaccine 26:I34–I39. doi: 10.1016/j.vaccine.2008.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ala'Aldeen DA, Neal KR, Ait-Tahar K, Nguyen-Van-Tam JS, English A, Falla TJ, Hawkey PM, Slack RCB. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol 38:2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Deuren M, Brandtzaeg P, van der Meer JW. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 13:144–166. doi: 10.1128/CMR.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swift AJ, Moxon ER, Zwahlen A, Winkelstein JA. 1991. Complement-mediated serum activities against genetically defined capsular transformants of Haemophilus influenzae. Microb Pathog 10:261–269. doi: 10.1016/0882-4010(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 52.Noel GJ, Brittingham A, Granato AA, Mosser DM. 1996. Effect of amplification of the Cap b locus on complement-mediated bacteriolysis and opsonization of type b Haemophilus influenzae. Infect Immun 64:4769–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerning J. 1991. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Lett 87:113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 54.De Vuyst L, Degeest B. 1999. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev 23:153–177. [DOI] [PubMed] [Google Scholar]
- 55.Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS matrix: the “house of biofilm cells.” J Bacteriol 189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flemming H, Wingender J. 2002. Extracellular polymeric substances: structure, ecological functions, technical relevance, p 1223–1231. In Encyclopedia of environmental microbiology. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 57.Surana NK, Kasper DL. 2012. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev 245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Totté P, Puech C, Rodrigues V, Bertin C, Manso-Silvan L, Thiaucourt F. 2015. Free exopolysaccharide from Mycoplasma mycoides subsp. mycoides possesses anti-inflammatory properties. Vet Res 46:122. doi: 10.1186/s13567-015-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porsch EA, Kehl-Fie TE, St Geme JW III. 2012. Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3:e00372-12. doi: 10.1128/mBio.00372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JS, Reuhs BL, Rahman MM, Ridley B, Carlson RW. 1996. Separation of bacterial capsular and lipopolysaccharides by preparative electrophoresis. Glycobiology 6:433–437. doi: 10.1093/glycob/6.4.433. [DOI] [PubMed] [Google Scholar]
- 61.Costabile M. 2010. Measuring the 50% haemolytic complement (CH50) activity of serum. J Vis Exp 2010:1923. doi: 10.3791/1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal S, Vasudhev S, DeOliveira RB, Ram S. 2014. Inhibition of the classical pathway of complement by meningococcal capsular polysaccharides. J Immunol 193:1855–1863. doi: 10.4049/jimmunol.1303177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 64.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 65.Pichichero ME, Loeb M, Anderson Smith DH. 1982. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet ii:960–962. [DOI] [PubMed] [Google Scholar]
- 66.St Geme JW, Falkow S. 1991. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun 59:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]