ABSTRACT
The capacity of Candida albicans to switch reversibly between the white phenotype and the opaque phenotype is required for the fungus to mate. It also influences virulence during hematogenously disseminated candidiasis. We investigated the roles of the mating type loci (MTL) and white-opaque switching in the capacity of C. albicans to mate in the oropharynx and cause oropharyngeal candidiasis (OPC). When immunosuppressed mice were orally infected with mating-competent opaque a/a and α/α cells either alone or mixed with white cells, no detectable mating occurred, indicating that the mating frequency was less than 1.6 × 10−6. Opaque cells were also highly attenuated in virulence; they either were cleared from the oropharynx or switched to the white phenotype during OPC. Although there were strain-to-strain differences in the virulence of white cells, they were consistently more virulent than opaque cells. In vitro studies indicated that relative to white cells, opaque cells had decreased capacity to invade and damage oral epithelial cells. The reduced invasion of at least one opaque strain was due to reduced surface expression of the Als3 invasin and inability to activate the epidermal growth factor receptor, which is required to stimulate the epithelial cell endocytic machinery. These results suggest that mating is a rare event during OPC because opaque cells have reduced capacity to invade and damage the epithelial cells of the oral mucosa.
KEYWORDS: Candida albicans, OPC, mating, virulence regulation
INTRODUCTION
Candida albicans remains the most pervasive yeast pathogen that colonizes humans. In most individuals, it is a commensal, residing in the oral cavity, gastrointestinal tract, and vaginal canal in a benign state. However, in response to weakening of the host physiology, predisposing conditions, or immunosuppressing agents, the fungus can become an opportunistic pathogen, capable of causing both mucosal and invasive disease (1).
C. albicans is diploid, and in a majority of strains, the configuration of the mating type locus is a/α (2, 3). In order to mate, a/α cells must undergo homozygosis to a/a and α/α and then switch from the white to the opaque phenotype (4, 5). Only MTL-homozygous cells of alternative mating types (a/a and α/α) that exhibit the opaque phenotype are able to mate (a/a × α/α) (6, 7). Although white phase cells do not mate, they do form a sexual biofilm in vitro that facilitates mating between minority opaque a/a and α/α cells, which appear in white cell populations through the process of spontaneous switching (8, 9). Ramirez-Zavala et al. (10) found that passing white cells through the mouse gastrointestinal tract stimulates switching to the opaque phase. Pande et al. (11) then showed that deletion of WOR1, the master regulator of the opaque phenotype, decreases the fitness of a/α cells relative to wild-type a/α cells in a mouse intestinal colonization model. They further demonstrated that overexpression of WOR1 in a/α cells enhances fitness and results in a cell morphology with some of the characteristics of opaque MTL-homozygous cells. Dumitru et al. (12) used a complementation strategy to demonstrate that mating occurs in the gastrointestinal tract of mice intermittently supplied with water containing a/a or α/α cells. To date, no studies have tested whether mating-competent MTL-homozygous cells actually colonize mucosal surfaces and undergo mating, possibly in biofilms.
The oral cavity represents a distinct host niche for C. albicans colonization and pathogenicity. When C. albicans causes oropharyngeal candidiasis (OPC) in susceptible hosts, the organisms form a biofilm-like mass of cells and invade the oral epithelial cells by receptor-mediated endocytosis and active penetration (13–17). During induced endocytosis, invasins on the hyphal surface, such as Als3 and Ssa1, bind to E-cadherin and a heterodimer composed of the epidermal growth factor receptor (EGFR) and HER2 on the epithelial cell surface. This interaction activates the host cell endocytosis machinery, triggering fungal internalization (18–21). Although white-opaque switching is important for mating, it is also likely to influence the pathogenicity of these cells during OPC. For example, ALS3 is highly expressed by filaments of white cells but not filaments of opaque cells (22).
Here, we used the immunosuppressed mouse model of OPC to investigate whether C. albicans can mate in the oral cavity and to examine the roles of the mating type locus and the white-opaque transition in host cell interactions and virulence. Although we did not detect evidence of mating in vivo, we discovered that opaque cells had highly reduced virulence relative to that of white cells. Furthermore, opaque cells had diminished capacity to invade and damage oral epithelial cells in vivo. These results suggest that opaque cells have evolved to mate in anatomical sites other than the oropharynx.
RESULTS
Little to no mating occurs during OPC.
Before testing the capacity of C. albicans to mate during OPC, we verified that the strains could mate in vitro. To determine mating frequency, we constructed the hygromycin B-resistant strain 05GH from the natural MTLa/a strain P37005 and generated the nourseothricin-resistant strain WOmCh from the natural MTLα/α strain WO-1 (Table 1). Mating products of opaque 05GH (a/a) and WOmCh (α/α) cells were resistant to both hygromycin B and nourseothricin and thus could be selected on yeast extract, peptone, dextrose (YPD) agar containing these antifungal agents. Mating between the two strains was tested in vitro using two protocols. In the first protocol, cells were cross-streaked on selection YPD agar containing hygromycin B and nourseothricin. Only in the region in which 05GH and WOmCh cells were mixed did we observe cells that were resistant to both hygromycin B and nourseothricin, indicating that mating had occurred (Fig. 1).
TABLE 1.
Strains used in this studya
| Strain | Parental strain | Genotype | Phenotype | Reference or source |
|---|---|---|---|---|
| SC5314 | MTLa/α | 23 | ||
| P37005 | MTLa/a | White | 24 | |
| WO-1 | MTLα/α | White | 25 | |
| 05GH | P37005 | MTLa/a, OP4/op4::OP4p-GFP-CaHygB | Opaque | This study |
| WOmCh | WO-1 | MTLα/α, OP4/op4::OP4p-mCherry-CaSAT1 | Opaque | This study |
| SC5314 a/− | SC5314 | MTLa/−, MTLa1/mtlα2::FRT | White/opaque | This study |
| SC5314 −/α | SC5314 | MTL−/α, mtla1::FRT/MTLα2 | White/opaque | This study |
| SC5314 a/− ALS3-OE | SC5314 | MTLa/−, MTLa1/mtlα2::FRT, ADH1/adh1::ADH1p-ALS3-CaSAT1 | White/opaque | This study |
| SC5314 a/− ECE1-OE | SC5314 | MTLa/−, MTLa1/mtlα2::FRT, ADH1/adh1::ADH1p-ECE1-CaSAT1 | White/opaque | This study |
| als3Δ/Δ | DAY185 | MTLa/α, ura3::imm434::URA3-IRO1/ura3::imm434 als3::HIS1 als3::ARG4 arg4::hisG/arg4::hisG his1::hisG/his1::hisG | 26 |
White and opaque cells were obtained from colonies grown on sLee's medium for 5 days at 25°C in air.
FIG 1.

Analysis of opaque C. albicans cells mating on YPD agar. Opaque cells of a/a strain P37005 and its hygromycin B-resistant derivative 05GH were cross streaked on nonselective YPD agar with α/α strain WO-1 and its nourseothricin-resistant derivative WOmCh and incubated for 24 h at 30°C. The colonies were then replicate plated onto selective YPD agar (left) and YPD agar containing nourseothricin (ClonNAT) and hygromycin B (hyg B) (right). Images were taken after 2 days of incubation at 30°C.
In the second in vitro protocol, opaque cells of the a/a hygromycin B-resistant strain 05GH and of the α/α nourseothricin-resistant strain WOmCh were cocultured in sLee's liquid medium (Lee's medium [27] supplemented according to Bedell and Soll [28]) in suspension and then plated on both nonselective and selective YPD agar. In control cultures of opaque cells of strains P37005 (a/a) and WO-1 (α/α), no offspring that were resistant to both hygromycin B and nourseothricin were observed in two independent experiments (Table 2). Furthermore, no colonies were observed when the cells from the control mating culture listed in Table 2 were plated on YPD agar containing only 800 μg/ml hygromycin B and on YPD agar containing only 200 μg/ml nourseothricin, indicating that the frequency of cells spontaneously resistant to the drugs was <3.3 × 10−6 (data not shown). However, when opaque cells of 05GH and WOmCh were cocultured, offspring that were resistant to both hygromycin B and nourseothricin appeared in three independent experiments at a mean frequency of 8.0 × 10−5 (Table 2).
TABLE 2.
Mating in suspension cultures containing opaque 05GH cells and opaque WOmCh cells
| Straina | Expt. | No. of CFU on YPDb | Frequency of mating in suspension |
|
|---|---|---|---|---|
| No. of CFU from matingc | Frequency | |||
| op P37005 (a/a) × op WO-1 (α/α) | 1 | 2.8 × 105 | 0 | <3.6 × 10−6 |
| 2 | 3.4 × 105 | 0 | <2.9 × 10−6 | |
| Averaged data | 3.1 × 105 | 0 | <3.3 × 10−6 | |
| op 05GH (a/a) × op WOmCh (α/α) | 1 | 5.8 × 105 | 30 | 5.2 × 10−5 |
| 2 | 3.8 × 105 | 65 | 1.7 × 10−4 | |
| 3 | 5.7 × 105 | 10 | 1.8 × 10−5 | |
| Averaged data | 5.1 × 105 | 35 | 8.0 × 10−5 | |
op, opaque.
YPD is yeast extract, peptone, dextrose agar; nonselective.
CFU on YPD agar containing hygromycin B and nourseothricin; selective.
To investigate whether mating of C. albicans occurs during OPC, corticosteroid-treated mice were orally infected with the mixture of C. albicans strains listed in Table 3. To enhance the possibility of mating, mice were infected with a mixture of a/a and α/α opaque cells, either alone or in combination with a/α SC5314 or with a mixture of a/a and α/α white cells. The rationale for mixing opaque cells with either the a/α strain or white cells was to determine if the formation of a biofilm by the a/α or white cells would facilitate mating between the a/a and α/α opaque cells. Five days after inoculation in the OPC model, homogenates of the tongue samples were plated in parallel on nonselective and selective minimal medium agar to obtain total CFU and mating frequencies. No CFU were observed on selection plates for any of the five inocula (Table 4). Thus, the frequency of mating was less than 1.6 × 10−6 for all mixtures. Of note, among the 16 mice that were infected with the 100% opaque mixtures (50% 05GH, 50% WOmCh), one sample produced a small number of C. albicans colonies and only on the nonselective agar plates, suggesting that those cells were white. Therefore, the mating frequency for this mixture could not be computed. Collectively, these results indicate that in the OPC model used in these experiments, C. albicans mating occurs at a negligible frequency, if at all.
TABLE 3.
Mixture of C. albicans strains and phenotypes tested for mating during OPC
TABLE 4.
Mating frequency after 5 days of OPCb
| Strain | Avg CFU/g tissue | No. of opaque CFU | No. of CFU from matinga | Mating frequency |
|---|---|---|---|---|
| 100% a/α | 2.4 × 106 | 0 | 0 | <4.2 × 10−7 |
| 90% a/α + 10% op | 6.1 × 105 | 0 | 0 | <1.6 × 10−6 |
| 100% white | 5.7 × 105 | 0 | 0 | <1.8 × 10−6 |
| 90% white + 10% op | 4.5 × 106 | 0 | 0 | <2.2 × 10−7 |
| 100% opaque | 2.4 × 10 | 0 | 0 |
CFU on YPD agar containing hygromycin B and nourseothricin.
Data are pooled from 2 independent experiments.
The a/a and α/α strains had reduced epithelial cell invasion during OPC.
We also assessed the virulence of the various C. albicans strains and phenotypes in the OPC model. After 5 days of infection, mice infected with all of the strains grown on sLee's medium had a similar number of CFU in their tongue homogenates, with the exception of mice infected with 100% opaque cells (Fig. 2A). As described above, samples from the tongues of these mice either were sterile or contained a small number of CFU, indicating that the opaque cells had markedly impaired capacity to persist in the oropharynx and cause OPC.
FIG 2.

Virulence of white and opaque C. albicans isolates during OPC. (A) Oral fungal burden of immunosuppressed mice after 5 days of infection with the indicated mixtures of C. albicans strains. The five different mixtures that were tested are described in detail in Table 3. Results are medians ± interquartile ranges of the combined data from 2 independent experiments for a total of 14 to 16 mice per experimental group. Statistical significance was determined using the Mann-Whitney test with Bonferroni correction for multiple comparisons. ****, P < 0.0001. (B to F) Tongue histopathology after 5 days of infection. Sections of tongue were stained with periodic acid-Schiff. op, opaque; wh, white. Scale bar, 100 μm.
We next analyzed the histopathology of the tongues after 5 days of infection. On the tongues of mice infected with either 100% a/α cells or a mixture of 90% a/α cells and 10% opaque cells, the organisms formed thick mats of long hyphae that invaded the oral mucosa and disrupted the tissue architecture (Fig. 2B and C). On the tongues of mice infected with either 100% white a/a and α/α cells or 90% white a/a and α/α cells and 10% opaque cells, the fungi formed a mixture of hyphae and yeast that adhered to the troughs between the lingual papillae, with little invasion into the mucosa and minimal tissue damage (Fig. 2D and E). In the mice that were infected with 100% opaque a/a and α/α cells, no organisms were visible on histopathologic examination (Fig. 2F), as expected from the largely negative culture results. These data suggest that in the mouse model of OPC, the a/α strain was more virulent than the white a/a and α/α strains, while the opaque a/a and α/α cells were highly attenuated.
Opaque cells have reduced virulence during OPC.
In the above-described experiments, three different C. albicans strain backgrounds, SC5314, P37005, and WO-1, were used (Table 1). Recently, it has been shown that different C. albicans strains can have markedly different virulence properties in the mouse model of OPC (29). To dissect the roles of mating type and the white versus opaque phenotype in virulence, we constructed hemizygous a/− and −/α derivatives of C. albicans SC5314 (Table 1). These strains were grown as white and opaque cells on sLee's agar medium for 5 days at 25°C in air and then tested for virulence during OPC. After 2 days of infection, the oral fungal burdens of mice infected with all strains and phenotypes were not significantly different (Fig. 3A). After 5 days of infection, the oral fungal burdens of mice infected with the a/α, white a/−, white −/α, and opaque −/α cells were similar (Fig. 3B). In contrast, the oral fungal burden of mice infected with opaque a/− cells was significantly lower. To determine if phenotypic switching occurred during in vivo infection, we analyzed the phenotypes of the organisms recovered from the mice that had been infected with the opaque a/− or −/α strains. Interestingly, at both time points, all recovered colonies were composed of white cells and none retained their original opaque phenotype (see Fig. S1 in the supplemental material). Thus, at some time during the course of infection, the opaque cells had switched to white cells.
FIG 3.
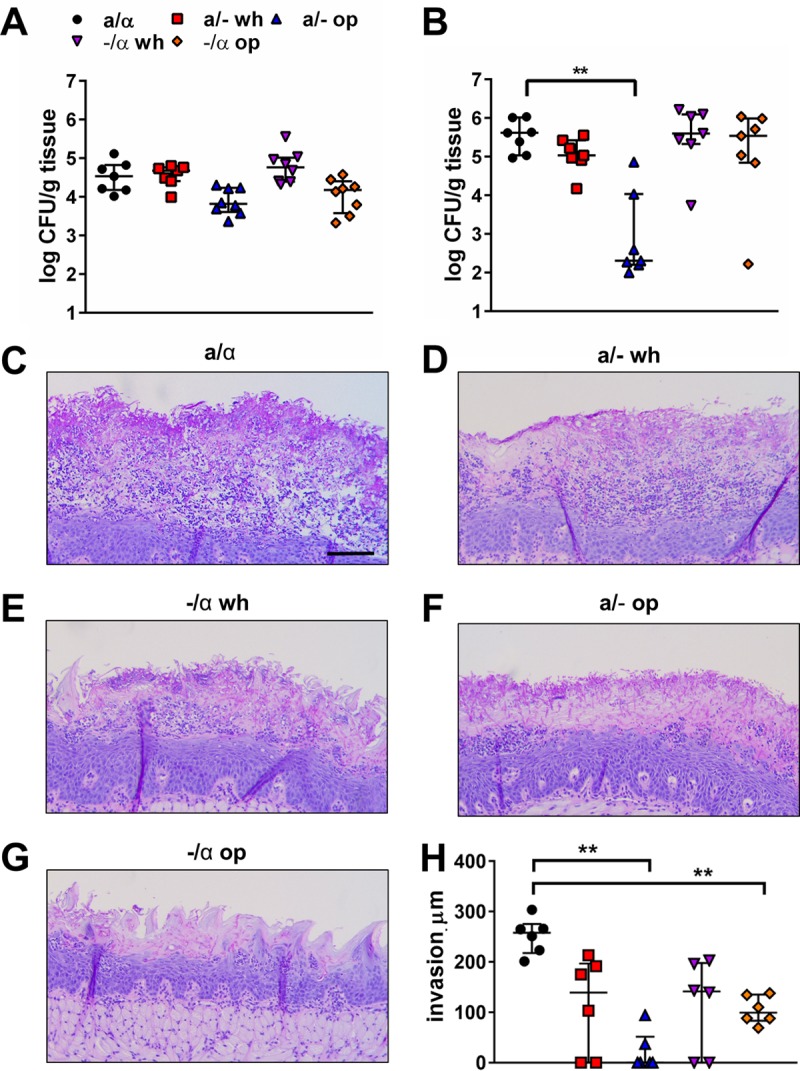
Virulence of hemizygous white and opaque cells during OPC. (A and B) Oral fungal burden of immunosuppressed mice after 2 (A) and 5 (B) days of infection with the indicated strains of C. albicans. The a/α strain was SC5314, and the hemizygous a/− and −/α strains were derived from this strain. Results are medians ± interquartile ranges from 7 mice per strain/phenotype. (C to G) Tongue histopathology after 5 days of infection. Sections of tongue were stained by periodic acid-Schiff. op, opaque; wh, white. Scale bar, 100 μm. (H) Depth of maximal fungal invasion in the fungal lesions on tongues of immunosuppressed mice after 5 days of infection. Statistical significance was determined using the Mann-Whitney test with the Bonferroni correction for multiple comparisons (**, P < 0.01).
Histopathologic examination of the infected tongues after 5 days of infection revealed that the white a/− and −/α cells acted similarly to the parent a/α cells, forming hyphae that invaded the oral mucosa and disrupted the normal architecture (Fig. 3C to E). In contrast, the opaque a/− and −/α cells formed hyphae that grew mainly on the surface of the tongue and had reduced capacity to invade the oral mucosa (Fig. 3F to G). To verify these results, we used quantitative image analysis to measure the depth of fungal invasion in the lesions of mice that had been infected with the different cell types. This analysis confirmed that in mice infected with the opaque a/− and −/α cells, the depth of fungal invasion was significantly less than that in mice infected with the parent a/α cells (Fig. 3H). Collectively, these results suggest that opaque cells have reduced virulence during OPC relative to white cells.
Opaque cells have diminished capacity to adhere to and invade oral epithelial cells in vitro.
To investigate why opaque cells had attenuated virulence, we analyzed their capacity to adhere to and invade the OKF6/TERT-2 oral epithelial cell line (30). We found that the numbers of epithelial cell-associated white cells of the a/a strain P37005 and the α/α strain WO-1 were similar to that of the a/α strain SC5314, indicating that the adherence of these strains was comparable to that of SC5314 (Fig. 4A). However, the number of endocytosed white cells of the a/a and α/α strains was significantly lower than that of SC5314, demonstrating that these strains had reduced epithelial cell invasion (Fig. 4B). In contrast, the adherence and invasion of the white a/− and −/α cells were similar to that of the parent a/α strain (Fig. 4C and D). Thus, the reduced invasion of the white a/a and α/α strains relative to the a/α strain SC5314 was likely due to factors in strains P37005 and WO-1 that were unrelated to their mating loci.
FIG 4.
Effects of mating type and white-opaque phenotype on the interactions of C. albicans with oral epithelial cells in vitro. OKF6/TERT-2 oral epithelial cells were infected for 2.5 h with the indicated C. albicans cells, which were grown on sLee's agar medium for 5 days at 25°C, after which the number of cell-associated (A and C) and endocytosed (B and D) organisms was determined by a differential fluorescence assay. The a/a strain was 05GH, the α/α strain was WOmCh, and the a/α strain was SC5314. The hemizygous a/− and −/α strains are the ones described in the legend to Fig. 3. (E) Decreased active penetration into epithelial cells of opaque cells. OKF6/TERT-2 oral epithelial cells were fixed and infected for 2.5 h with the indicated C. albicans cells, after which the number of internalized organisms was determined by a differential fluorescence assay. Results are means ± standard deviations (SD) from 3 experiments, each performed in triplicate. Statistical significance was determined by analysis of variance (ANOVA) with Bonferroni correction. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. op, opaque; wh, white.
The opaque a/a and α/α cells were markedly less adherent and invasive than both the a/α cells and the white a/a and α/α cells (Fig. 4A and B). Similarly, the opaque a/− cells and −/α cells were less adherent and invasive than the a/α cells and the white a/− and −/α cells (Fig. 4C and D). These invasion assays were performed using live epithelial cells, which C. albicans invades by both receptor-induced endocytosis and active penetration (16, 17). To compare the capacity of white and opaque cells to invade epithelial cells by active penetration, we fixed the epithelial cells with paraformaldehyde before infecting them with C. albicans; any invasion into fixed cells occurs via active penetration (21). As expected, the invasion of a/α cells into fixed epithelial cells was 50% less than invasion into live cells, indicating that C. albicans invades oral epithelial cells via both receptor-induced endocytosis and active penetration at this time point. White a/− cells invaded the fixed epithelial cells similarly to a/α cells, whereas the invasion of opaque a/− cells was significantly reduced (Fig. 4E). Taken together, these results indicate that opaque cells are somewhat less adherent to oral epithelial cells than white cells, and they are virtually unable to invade oral epithelial cells by either induced endocytosis or active penetration.
Opaque cells have reduced surface expression of Als3 and fail to induce EGFR phosphorylation.
Receptor-induced endocytosis occurs when C. albicans invasins such as Als3 interact with EGFR on the epithelial surface, activating the signaling pathways that cause the epithelial cell to produce pseudopods that engulf the organism and pull it into the cell (18, 19, 21). We investigated why the opaque a/− and −/α cells had reduced capacity to invade oral epithelial cells. First, because C. albicans invasins are expressed predominantly by hyphae, we analyzed the morphology of the white and opaque cells after they had been in contact with oral epithelial cells for 2 h. We found that white and opaque a/− and −/α cells formed hyphae of similar lengths (Fig. S2). We next analyzed the level of Als3 expression on the hyphal surface of white and opaque cells. Because the a/− and −/α cells acted similarly in the invasion assays, we selected the −/α cells for this analysis. While surface expression of Als3 by white −/α cells was similar to that of a/α cells, the surface expression of Als3 by opaque −/α cells was very low (Fig. 5A). The interaction of Als3 with EGFR activates this receptor, causing the autophosphorylation of specific tyrosine residues (17–20). We found that when oral epithelial cells were infected with the different C. albicans cells, both the a/α and the white cells induced autophosphorylation of EGFR, whereas the opaque cells actually reduced EGFR phosphorylation to below basal levels (Fig. 5B and C). Thus, the impaired capacity of the opaque cells to invade oral epithelial cells is likely due in part to their reduced surface expression of Als3, which leads to decreased activation of epithelial cell EGFR.
FIG 5.
Opaque cells have low Als3 surface expression and fail to induce epidermal growth factor receptor (EGFR) signaling in oral epithelial cells. (A) Flow-cytometric analysis of Als3 surface expression by the indicated C. albicans strains. The a/α parental strain, SC5314, was used as a positive control, and the als3Δ/Δ mutant was used as a negative control. The histogram shows a representative result from 3 independent experiments, each analyzing 10,000 cells per strain. (B) Representative immunoblot of C. albicans-induced autophosphorylation of EGFR tyrosine 1068. The oral epithelial cells were infected with the indicated C. albicans strain for 60 min, after which the phosphorylation of EGFR was determined by immunoblotting with a phosphospecific monoclonal antibody. (C) Densitometric analysis of the immunoblots such as the one shown in panel B. Results are means ± SD from 3 experiments. Statistical significance was determined using ANOVA. ctrl, control (uninfected); op, opaque; wh, white; INF, infected; UNINF, uninfected.
The capacity of C. albicans to damage oral epithelial cells is strain dependent.
C. albicans invasion of oral epithelial cells is associated with induction of epithelial cell damage, a factor that is strongly associated with virulence during OPC (17, 31, 32). We found that both white and opaque cells of the a/a and α/α strains caused very little damage to oral epithelial cells relative to the a/α strain (Fig. 6A). These results indicate that strains P37005 and WO-1 have limited capacity to damage oral epithelial cells, irrespective of whether they are growing as white or opaque cells. When the hemizygous a/− and −/α cells that were derived from the a/α strain SC5314 were tested, the extent of epithelial cell damage induced by white cells was similar to that induced by the a/α strain (Fig. 6B). In contrast, opaque a/− and −/α cells caused very little epithelial cell damage. Thus, in the SC5314 strain background that causes significant epithelial cell damage, white cells induce significantly more host cell damage than do opaque cells. However, relative to that of strain SC5314, strains P37005 and WO-1 are impaired in their capacity to damage epithelial cells, regardless of whether they are grown as white or opaque cells.
FIG 6.
Effects of mating type and white-opaque phenotype on the capacity of C. albicans to damage oral epithelial cells. Oral epithelial cells were infected with the indicated C. albicans strains for 7 h, after which the extent of host cell damage was determined by 51Cr release assay. (A) Damage by strain SC5314 and white and opaque cells of the a/a strain 05GH and the α/α strain WOmCh. (B) Damage by strains SC5314, SC5314a/−, and SC5314−/α. Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using ANOVA multiple comparison with Bonferroni correction. ****, P < 0.0001. NS, not significant.
Overexpression of ALS3 increases the endocytosis of opaque cells, while overexpression of ECE1 and ALS3 enhances the capacity of white cells to damage epithelial cells.
Many hypha-specific genes, including ALS3 and ECE1, are weakly expressed in filamentous opaque cells (22). ECE1 specifies the precursor of candidalysin, a toxin that damages oral epithelial cells (32). To determine if the low expression of ALS3 and ECE1 in opaque cells contributes to their reduced capacity to invade and damage epithelial cells, we constructed a/− strains of C. albicans in which expression of ALS3 and ECE1 was driven by the strong ADH1 promoter (Table 1). When the epithelial cell interactions of these strains were analyzed, we found that the overexpression of either ALS3 or ECE1 in white cells had no effect on the endocytosis of these cells (Fig. 7A). However, overexpression of ALS3, but not ECE1, in opaque cells significantly increased epithelial invasion (Fig. 7A). Also, overexpression of either ALS3 or ECE1 in white cells caused a modest but statistically significant increase in epithelial cell damage, whereas the overexpression of either gene in opaque cells had no detectable effect (Fig. 7B). These results suggest the impaired capacity of the opaque cells to invade oral epithelial cells is due in part to reduced expression of ALS3. However, the reduced epithelial cell damage caused by these cells is not solely the result of low expression of ECE1.
FIG 7.

Effects of overexpression of ALS3 and ECE1 on the epithelial cell interactions of white and opaque cells. Epithelial cell invasion (A) and damage (B) by strain a/− white and opaque cells overexpressing (OE) ALS3 or ECE1. Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using ANOVA with Bonferroni correction. *, P < 0.05; **, P < 0.01; NS, not significant.
DISCUSSION
In experimental mouse models, C. albicans has been found to mate in the kidneys during disseminated candidiasis (5) and in the gastrointestinal tract (12) and on the skin (33) during colonization. In contrast, we determined that in the mouse model of OPC, mating occurs at a frequency of less than 1.6 × 10−6, if it occurs at all. The likely reason for our inability to detect mating in this model is that opaque cells, which are the mating competent phenotype, were highly attenuated in their capacity to persist in the oropharynx.
A notable finding was that when the mice were infected with a mixture of opaque cells in the P37005 and WO-1 strain background, very few fungal cells were recovered from the oropharynx after 5 days of infection. Furthermore, when the mice were infected with opaque −/α and a/− cells, only white cells were recovered at the end of the infection period. Collectively, these results suggest that opaque cells have very low virulence relative to white cells during OPC. We speculate that when the mice were infected with opaque a/− and −/α cells, a small number of the opaque cells switched to the more virulent white phenotype at some time during infection. Due to their selective advantage, these white cells rapidly outcompeted the opaque cells. Previously, it was reported that the virulence of white and opaque cells differs depending on the infection model. For example, white cells are considerably more virulent than opaque cells in a mouse model of disseminated candidiasis, but opaque cells have greater capacity to infect the skin of mice (34–36).
We chose to test the mating capacity and virulence of the different phenotypes in corticosteroid-treated mice, in which the oral fungal burden remains high for many days (37). Our rationale for using immunosuppressed mice is that in the absence of an effective immune response, the organisms would persist long enough in the oropharynx for mating to occur. When strain SC5314 is used to infect immunocompetent mice, the oral fungal burden peaks at day 1 and declines to undetectable levels by day 3. Whether selective pressure from the immune system in immunocompetent mice would enhance mating is unknown, but it is conceivable that mating could occur in this model during the few days that the organism is present in the oropharynx.
When the mice were infected with white cells in the P37005 (a/a) and WO-1 (α/α) strain backgrounds, their oral fungal burden was similar to that of mice infected with strain SC5314 (a/α). However, P37005 and WO-1 white cells grew as a mixture of yeast and hyphae that adhered to the surface of the tongue, in contrast to strain SC5314, which grew predominantly as hyphae that invaded and damaged the oral mucosa. A likely explanation for the difference in virulence of these strains is the recent finding of Schonherr et al. (29), who discovered that diverse clinical isolates of C. albicans have two distinct phenotypes in the mouse model of OPC. One group, characterized by strain SC5314, forms hyphae that invade the oral mucosa, causing extensive inflammation and tissue damage. These strains also cause extensive damage to oral epithelial cells in vitro. The other group forms hyphae that adhere to the mucosal surface of the oropharynx but are weakly invasive and cause little tissue damage, either in vivo or in vitro. Our results suggest that strains P37005 and WO-1 are members of the latter group. Alternatively, it has been reported that a/a and α/α cells are less virulent during disseminated candidiasis than spontaneous a/α cells (38). Thus, it is also possible that white cells of strains P37005 and WO-1 were less virulent than those of strain SC5314 because they were homozygous at their respective mating loci.
Although white and opaque cells have been found to form filaments in response to different environmental signals (22), we did not observe a significant difference in filamentation between the white and opaque −/α and a/− cells during growth in vitro or during OPC in mice (Fig. 3D to G; see also Fig. S2 in the supplemental material). However, we did find that opaque hyphae were virtually unable to invade and damage oral epithelial cells in vitro. Consistent with previous gene expression data (22), hyphae of opaque a/− cells had very low surface expression of the Als3 invasin and were unable to activate epithelial cell EGFR. Overexpression of ALS3 in these cells partially restored their capacity to invade epithelial cells. These results indicate that the reduced expression of Als3 and perhaps other invasins in opaque hyphae render them unable to bind to epithelial cell receptors and activate the endocytic machinery. Because Als3 is also required for C. albicans to invade epithelial cells by active penetration (16), low-level expression of Als3 in the opaque hyphae likely contributed to their impaired invasion by this process as well.
Opaque hyphae were also highly defective in their capacity to damage oral epithelial cells in vitro, a result that was likely due to reduced production of candidalysin by the fungal cells (22, 32). However, we attempted to verify this hypothesis using an a/− strain of C. albicans that overexpressed ECE1 and found that white cells, but not opaque cells, caused increased epithelial cell damage. The probable explanation for these results is that the Ece1 protein must be processed by the Kex2 protease to form active candidalysin (39). Because opaque hyphae are known to have low expression of KEX2 relative to white hyphae (22), it is likely that very little Ece1 was processed into candidalysin in the opaque cells, even though there were increased levels of the precursor protein. Overall, the data indicate that the reduced expression of invasins and candidalysin by opaque cells is almost certainly the cause of their attenuated virulence during OPC. In addition, the inability of opaque cells to persist in the oropharynx prevents mating from occurring. We speculate that there is strong selection pressure that favors the switching of an opaque cell to a white cell, which further reduces the probability of mating during OPC.
Although we found no evidence of mating in the oropharynx during OPC, it is likely that this infection induces genetic diversity in C. albicans by two mechanisms. First, the stress associated with growth within the host can cause large-scale chromosomal rearrangements, as has been observed during both OPC and disseminated candidiasis (40). Second, organisms that are shed from the oropharynx can be swallowed and pass through the gastrointestinal tract, where they encounter an environment that facilitates mating (12). By these mechanisms, multiple genetic variants can be generated, enabling C. albicans to adapt to many niches within the host.
MATERIALS AND METHODS
Ethics statement.
All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) of the Los Angeles Biomedical Research Institute.
C. albicans strains and maintenance.
The C. albicans strains used in the OPC studies are described in Table 1. For all studies, these strains were maintained on nutrient agar containing Lee's medium (27) supplemented according to Bedell and Soll (28) (sLee's medium) and containing 5 μg/liter of phloxine B, which differentially stains opaque colonies red/pink (41). Prior to use for experiments, cells were spread on fresh agar containing sLee's medium and grown for 5 days at 25°C. To assess mating, we generated a nourseothricin (ClonNat)-resistant MTLα/α strain (WOmCh) from the α/α strain WO-1 (25) and a hygromycin B-resistant MTLa/a strain (05GH) from the a/a strain P37005 (24) (Table 1). To generate the WOmCh strain, the plasmid pmCherry-SAT (9) was digested with XbaI-SacI, and the resultant DNA fragment was transformed into one of the OP4 alleles of the wild-type MTLα/α WO-1 strain (Table 1). The resulting strain constitutively expressed the C. albicans-adapted nourseothricin resistance gene (CaSAT1) (42) and expressed mCherry exclusively in the opaque phase. To generate the 05GH strain, we first constructed the plasmid pGFP-HygB. The C. albicans-adapted hygromycin B resistance gene (CaHygB) was amplified with the primer pair CaHygB-1 and CaHygB-2 (see Table S1 in the supplemental material) from the plasmid pBSII-CaHygB (43) and then digested with SalI and NdeI. The resulting fragment was then ligated into the plasmid pGFP-SAT, replacing the CaSAT1 fragment. The generated plasmid pGFP-HygB was verified by sequencing. The XbaI-SacI restriction fragment from pGFP-HygB was transformed into one of the OP4 alleles of the wild-type MTLa/a strain P37005 (24), resulting in strain 05GH, which expressed the GFP gene only in the opaque phase but expressed the CaHygB gene constitutively.
MTL hemizygous strains, SC-a (MTLa/−) and SC-α (MTL−/α), were generated from the MTLa/α strain SC5314 by deleting the coding region of MTLα2 and MTLa1, respectively. To delete the genes, two plasmids were constructed, pMTLa1M for MTLa1 gene deletion and pMTLα2M for MTLα2 gene deletion. For generation of the plasmid pMTLa1M, the gene deletion cassette in the plasmid pSFS2 (42) was flanked by the 5′ and 3′ regions of the MTLa1 gene. The 5′ and 3′ DNA fragments of the MTLa1 gene were amplified by PCR with primer pairs MTLa1-1 and MTLa1-2 as well as MTLa1-3 and MTLa1-4, respectively (Table S1). The plasmid pMTLα2M was made similarly; the 5′ and 3′ DNA fragments of the MTLα2 gene were amplified by PCR with primer pairs MTLα2-1 and MTLα2-2 as well as MTLα2-3 and MTLα2-4, respectively (Table S1). To insert the DNA fragments into the plasmid, the vector pSFS2 and the PCR-amplified DNA fragments were digested with appropriate restriction enzymes indicated in Table S1, followed by ligation. To generate a/− strains overexpressing ALS3 and ECE1 under the control of the ADH1 promoter, we constructed plasmids pADH1P-ALS3 and pADH1P-ECE1, which contained a gene insertion cassette for ALS3 and ECE1, respectively (Table 1). The gene insertion cassettes were composed of a PCR-amplified open reading fame of the ALS3 or ECE1 gene, with the CaSAT1 selection marker (42), flanked by the 5′ promoter and 3′ DNA fragments of the ADH1 gene. The latter were amplified by PCR with primer pairs ADH1-5′F/−5′R and ADH1-3′F/−3′R, respectively (Table S1). In the cassettes, the ALS3 and ECE1 genes were fused to the 5′ promoter of ADH1 for expression after integration of the cassettes into one of the ADH1 alleles in the a/− strain. The coding region of ECE1 was amplified by PCR with the primer pair ECE1-F/−R (Table S1) and used for construction of pADH1P-ECE1. The ALS3 gene contained tandem repeats. Therefore, the gene was amplified by PCR in two parts with the primer pairs ALS3-1F/−1R and ALS3-2F/−2R (Table S1), and their sequences were verified prior to generation of the plasmid pADH1P-ALS3. Ligation of the two parts was mediated by insertion of a KpnI restriction site between the DNA fragments without changing the amino acid. Genomic DNA of C. albicans SC5314 served as the template for all PCR amplifications. All sequences of the amplified DNA fragments were verified by sequencing after cloning into the plasmids. The gene deletion and insertion cassettes were used to transform C. albicans SC5314 a/α and SC5314a/−, respectively.
Mouse model of oropharyngeal candidiasis.
The mating capacity and pathogenicity of the C. albicans strains were tested in the immunosuppressed mouse model of OPC as previously described (37). Male BALB/c mice were injected subcutaneously with cortisone acetate (225 mg/kg of body weight) on days −1, 1, and 3 relative to infection. For inoculation, C. albicans cells were grown overnight in sLee's medium at 25°C and then diluted and preincubated in fresh sLee's medium at 30°C for 30 min. The animals next were sedated with ketamine and xylazine, and a swab saturated with 106 C. albicans cells per ml was placed sublingually for 75 min. After 2 and 5 days of infection, the mice were sacrificed and the tongues were harvested. One-half was weighed, homogenized, and quantitatively cultured. The other one-half was fixed in zinc-buffered formalin, embedded in paraffin, sectioned, and stained with periodic acid-Schiff (PAS). To quantify the depth of fungal invasion into the tongue, images of the sections were obtained by light microscopy, after which the depth of fungal invasion relative to the surface of the adjacent, uninfected mucosa was quantified using Infinity Analysis software (Lumenera).
Assessing mating in the OPC model.
Samples from the homogenate of each tongue half were cultured on nonselective agar plates containing minimal medium (2% glucose, 0.7% yeast nitrogen base) (BD Biosciences) plus phloxine B for assessing CFU and discriminating between white and opaque colonies. In parallel, to assess for mating, the homogenates were cultured on selective agar plates containing minimal medium with phloxine B as well as 200 μg/ml nourseothricin and 500 μg/ml hygromycin B in experiment 1 and 250 μg/ml nourseothricin and 800 μg/ml hygromycin B in experiment 2. The agar plate cultures were incubated at 30°C to stabilize opaque cells. The frequency of mating was calculated by dividing the number of nourseothricin-resistant and hygromycin B-resistant colonies by total CFU in the absence of selection.
Assessing mating in vitro.
Mating between strains 05GH (a/a) and WOmCh (α/α) (Table S1) was tested in vitro both on solid agar plates and in suspension culture. For mating on agar, actively growing opaque cells from suspension cultures in sLee's medium were cross-streaked on YPD agar plates and incubated for 24 h at 30°C. The plates were then duplicate plated on nonselective YPD agar and selective YPD agar, the latter of which contained 200 μg/ml nourseothricin and 800 μg/ml hygromycin B. The plates were incubated for 2 days at 30°C and then imaged. To determine if mating occurred in suspension, tubes containing a mixture of 50% a/a and 50% α/α opaque cells at a final concentration of 1 × 106 cells per ml of sLee's medium were incubated at 25°C in a rotatory shaker at 200 rpm for 18 h. The samples were then duplicate plated onto nonselective and selective YPD agar. After 3 days of growth at 30°C, colonies were counted and analyzed for mating frequencies. Both experiments were performed twice and the data were pooled.
Measurement of C. albicans adherence to and invasion of oral epithelial cells.
The epithelial cell adherence and endocytosis of C. albicans were quantified using a differential fluorescence assay as outlined previously (17, 19). OKF6/TERT-2 cells (30) were grown to confluence on fibronectin-coated circular glass coverslips in 24-well tissue culture plates. They were infected with 2 × 105 yeast-phase C. albicans cells per well and incubated for 2.5 h, after which they were fixed, stained with fluorescently labeled anti-C. albicans antibodies, and mounted inverted on microscope slides. The coverslips were viewed with an epifluorescence microscope and the numbers of cell-associated (endocytosed plus adherent) and endocytosed organisms per high-powered field were determined, counting at least 100 organisms per coverslip. Each experiment was performed at least three times in triplicate.
To measure active penetration, we fixed the epithelial cells with paraformaldehyde prior to infection. After rinsing the cells extensively, we infected them with the live C. albicans strains for 2.5 h and then quantified the number of internalized organisms as described above.
Epithelial cell damage assay.
The extent of oral epithelial cell damage caused by C. albicans was measured using our standard 51Cr release assay (21). Briefly, 96-well tissue culture plates with detachable wells (Corning) were seeded with OKF6/TERT-2 cells in the presence of 5 μCi/ml Na251CrO4 (PerkinElmer). The next day, the unincorporated 51Cr was removed by rinsing and the epithelial cells were infected with 6 × 105 C. albicans cells per well. After a 7-h incubation, the amount of 51Cr released into the medium and retained by the cells was determined by γ-counting.
Flow cytometry.
The amount of Als3 expressed on the surface of the various C. albicans strains was determined by flow cytometry, using our previously described method (19, 44). The cells were germinated in RPMI 1640 broth at 37°C for 90 min, fixed in 3% paraformaldehyde, and then blocked with 1% goat serum. The cells next were incubated with a rabbit polyclonal antiserum raised against the recombinant N-terminal region of Als3, rinsed, and incubated with a goat anti-rabbit secondary antibody conjugated to Alexa Fluor 488 (Invitrogen). The cells were analyzed with a FACSCalibur flow cytometer (Becton, Dickinson), collecting fluorescence data for 10,000 cells of each strain.
Detection of EGFR phosphorylation.
The capacity of C. albicans to induce EGFR autophosphorylation was determined as described previously (21). OKF6/TERT-2 cells in 6-well tissue culture plates were infected with 4.5 × 106 C. albicans cells. After 60 min, the cells were rinsed with cold phosphate-buffered saline containing protease and phosphatase inhibitor cocktails and then detached from the plate with a cell scraper. The cells were collected by centrifugation and boiled in sample buffer. The lysates were separated by SDS-PAGE, and the phosphorylation of EGFR at Y1068 was detected by immunoblotting with a phosphospecific anti-EGFR antibody (3777; Cell Signaling). The blot next was stripped, and total EGFR was detected by immunoblotting with an anti-EGFR antibody (4267; Cell Signaling). Each experiment was performed at least 3 times.
Calcofluor white staining.
Chitin in the cell walls of the various C. albicans cells was stained as previously described (45). Briefly, OKF6/TERT-2 cells were infected with different C. albicans strains. After 2 h, the cells were fixed with 3% paraformaldehyde, and the fungi were stained with 0.1% calcofluor white (Sigma-Aldrich) and then imaged by confocal microscopy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Samuel W. French and Edward Veracruz, Anatomic Pathology, Harbor-UCLA Medical Center, for histopathology.
Research in the Filler laboratory was supported in part by NIH grants R01DE022600 and R01AI124566 to S.G.F. and K99DE026856 to M.S. This project was also supported by the National Center for Advancing Translational Sciences through UCLA CTSI grant UL1TR001881-01. Research in the Soll laboratory was supported by the Developmental Studies Hybridoma Bank, a National Resource created by the NIH.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00774-17.
REFERENCES
- 1.da Silva Dantas A, Lee KK, Raziunaite I, Schaefer K, Wagener J, Yadav B, Gow NA. 2016. Cell biology of Candida albicans-host interactions. Curr Opin Microbiol 34:111–118. doi: 10.1016/j.mib.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legrand M, Lephart P, Forche A, Mueller FM, Walsh T, Magee PT, Magee BB. 2004. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol Microbiol 52:1451–1462. doi: 10.1111/j.1365-2958.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 3.Rustad TR, Stevens DA, Pfaller MA, White TC. 2002. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology 148:1061–1072. doi: 10.1099/00221287-148-4-1061. [DOI] [PubMed] [Google Scholar]
- 4.Allendoerfer R, Magee DM, Smith JG, Bonewald L, Graybill JR. 1993. Induction of tumor necrosis factor-alpha in murine Candida albicans infection. J Infect Dis 167:1168–1172. doi: 10.1093/infdis/167.5.1168. [DOI] [PubMed] [Google Scholar]
- 5.Hull CM, Raisner RM, Johnson AD. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart SR, Zhao R, Daniels KJ, Soll DR. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot Cell 2:847–855. doi: 10.1128/EC.2.5.847-855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MG, Johnson AD. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302. doi: 10.1016/S0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 8.Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J 25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park YN, Daniels KJ, Pujol C, Srikantha T, Soll DR. 2013. Candida albicans forms a specialized “sexual” as well as “pathogenic” biofilm. Eukaryot Cell 12:1120–1131. doi: 10.1128/EC.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez-Zavala B, Reuss O, Park YN, Ohlsen K, Morschhauser J. 2008. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog 4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pande K, Chen C, Noble SM. 2013. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet 45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumitru R, Navarathna DH, Semighini CP, Elowsky CG, Dumitru RV, Dignard D, Whiteway M, Atkin AL, Nickerson KW. 2007. In vivo and in vitro anaerobic mating in Candida albicans. Eukaryot Cell 6:465–472. doi: 10.1128/EC.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montes LF, Wilborn WH. 1968. Ultrastructural features of host-parasite relationship in oral candidiasis. J Bacteriol 96:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. 2009. Characterization of mucosal Candida albicans biofilms. PLoS One 4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swidergall M, Solis NV, Lionakis MS, Filler SG. 2018. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat Microbiol 3:53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachtler B, Citiulo F, Jablonowski N, Forster S, Dalle F, Schaller M, Wilson D, Hube B. 2012. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 7:e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, J EE, Filler SG. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. 2012. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A 109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE Jr, Filler SG. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. 2010. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solis NV, Swidergall M, Bruno VM, Gaffen SL, Filler SG. 2017. The aryl hydrocarbon receptor governs epithelial cell invasion during oropharyngeal candidiasis. mBio 8:e00025-17. doi: 10.1128/mBio.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si H, Hernday AD, Hirakawa MP, Johnson AD, Bennett RJ. 2013. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog 9:e1003210. doi: 10.1371/journal.ppat.1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonzi WA, Irwin MY. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol 169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2:63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KL, Buckley HR, Campbell CC. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 28.Bedell GW, Soll DR. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun 26:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonherr FA, Sparber F, Kirchner FR, Guiducci E, Trautwein-Weidner K, Gladiator A, Sertour N, Hetzel U, Le GTT, Pavelka N, d'Enfert C, Bougnoux ME, Corti CF, LeibundGut-Landmann S. 2017. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol 10:1335–1350. doi: 10.1038/mi.2017.2. [DOI] [PubMed] [Google Scholar]
- 30.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20:1436–1447. doi: 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villar CC, Zhao XR. 2010. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol Oral Microbiol 25:215–225. doi: 10.1111/j.2041-1014.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 32.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Forster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachke SA, Lockhart SR, Daniels KJ, Soll DR. 2003. Skin facilitates Candida albicans mating. Infect Immun 71:4970–4976. doi: 10.1128/IAI.71.9.4970-4976.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvaal CA, Srikantha T, Soll DR. 1997. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun 65:4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie J, Tao L, Nobile CJ, Tong Y, Guan G, Sun Y, Cao C, Hernday AD, Johnson AD, Zhang L, Bai FY, Huang G. 2013. White-opaque switching in natural MTLa/alpha isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol 11:e1001525. doi: 10.1371/journal.pbio.1001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. 1999. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun 67:6652–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solis NV, Filler SG. 2012. Mouse model of oropharyngeal candidiasis. Nat Protoc 7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lockhart SR, Wu W, Radke JB, Zhao R, Soll DR. 2005. Increased virulence and competitive advantage of a/alpha over a/a or alpha/alpha offspring conserves the mating system of Candida albicans. Genetics 169:1883–1890. doi: 10.1534/genetics.104.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson JP, Mogavero S, Moyes DL, Blagojevic M, Kruger T, Verma AH, Coleman BM, De La Cruz Diaz J, Schulz D, Ponde NO, Carrano G, Kniemeyer O, Wilson D, Bader O, Enoiu SI, Ho J, Kichik N, Gaffen SL, Hube B, Naglik JR. 2018. Processing of Candida albicans Ece1p is critical for candidalysin maturation and fungal virulence. mBio 9:e02178-17. doi: 10.1128/mBio.02178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, Wang YM, Su CH, Bennett RJ, Wang Y, Berman J. 2013. The “obligate diploid” Candida albicans forms mating-competent haploids. Nature 494:55–59. doi: 10.1038/nature11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson JM, Soll DR. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol 169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Basso LR Jr, Bartiss A, Mao Y, Gast CE, Coelho PS, Snyder M, Wong B. 2010. Transformation of Candida albicans with a synthetic hygromycin B resistance gene. Yeast 27:1039–1048. doi: 10.1002/yea.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, Phan QT, Luo G, Solis NV, Liu Y, Cormack BP, Edwards JE Jr, Ibrahim AS, Filler SG. 2013. Investigation of the function of Candida albicans Als3 by heterologous expression in Candida glabrata. Infect Immun 81:2528–2535. doi: 10.1128/IAI.00013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swidergall M, van Wijlick L, Ernst JF. 2015. Signaling domains of mucin Msb2 in Candida albicans. Eukaryot Cell 14:359–370. doi: 10.1128/EC.00264-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





