ABSTRACT
Staphylococcus aureus nasal carriage is a common condition affecting both healthy and immunocompromised populations and provides a reservoir for dissemination of potentially infectious strains by casual contact. The factors regulating the onset and duration of nasal S. aureus colonization are mostly unknown, and a human-relevant animal model is needed. Here, we screened 17 pig-tailed macaques (Macaca nemestrina) for S. aureus carriage, and 14 of 17 animals tested positive in the nose at one or both screening sessions (8 weeks apart), while the other 3 animals were negative in the nose but positive in the pharynx at least once. As in humans, S. aureus colonization was densest in the nose, and treatment of the nostrils with mupirocin ointment effectively cleared the nostrils and 6 extranasal body sites. Experimental nasal S. aureus colonization was established with 104 CFU/nostril, and both autologous and nonautologous strains survived over 40 days without any apparent adverse effects. A human nasal S. aureus isolate (strain D579, sequence type 398) was carried in 4 of 6 animals for over 3 weeks. Nostrils that did eradicate experimentally applied S. aureus exhibited neutrophilic innate immunity marked by elevated nasal interleukin-1β (IL-1β), IL-8, and monocyte chemotactic protein 1 levels and a 10-fold decreased IL-1 receptor antagonist/IL-1β ratio within 7 days postinoculation, analogous to the human condition. Taken together, pig-tailed macaques represent a physiological model of human S. aureus nasal carriage that may be utilized for testing natural colonization and decolonization mechanisms as well as novel classes of anti-S. aureus therapeutics.
KEYWORDS: Staphylococcus aureus, human, mucosa, nasal carriage, nonhuman primate, pig-tailed macaque
INTRODUCTION
Staphylococcus aureus colonizes the moist mucosa of the anterior nares of 20 to 30% of healthy adults on any given day, and most people and large domesticated mammals carry S. aureus transiently throughout their lifetimes (1, 2). Prevalence is not consistent between all demographics and is impacted by age, obesity, living in close quarters, HIV infection and type 2 diabetes, and lengthy hospital stays (3–5). S. aureus nasal carriage (SANC) is typically asymptomatic but presents a risk for autoinfection and dissemination of both drug-susceptible and multidrug-resistant S. aureus strains by common, nonintimate contact (6–8). S. aureus skin, soft tissue, and surgical site infections in humans place an enormous burden on health care. S. aureus infection of cows, pigs, and industrial farm workers is costly and problematic for agriculture and food processing.
Combinations of in vitro and in vivo approaches have been implemented to define host and bacterial determinants of human SANC; however, many limitations, both ethical and procedural, exist in human experimentation. As human SANC is nearly always symptomless and thus does not require clinic visits, recruitment and retention are difficult and thwart large longitudinal studies of natural carriage and clearance. Some European studies which used experimental inoculation of human noses with diverse S. aureus strains were conducted (2, 9, 10). However, institutional review board approval for experimental inoculation of human subjects in the United States has been limited to strains isolated from the subject's own nostrils (11, 12), without the possibility of testing potentially virulent or antibiotic-resistant strains. Rodent models have been useful for studying S. aureus-induced cutaneous infections, lung injury, sepsis, endocarditis, and more (13), and the cotton rat (Sigmodon hispidus) model was used for identifying bacterial determinants of SANC (14–16). However, murine and cotton rat noses do not appear to be naturally colonized with S. aureus, and inoculation with upwards of 107 to 109 CFU/nostril does not result in sustained carriage, even when providing streptomycin-containing drinking water to reduce the natural nasal flora (14, 15, 17). To monitor S. aureus carriage in these models, animals must be sacrificed so that the entire noses may be processed, precluding longitudinal analyses of host responses and S. aureus evolution within the nasal environment. Furthermore, mice are obligate nose breathers whose nasal mucosa has anatomical and resident cell type differences from the human nasal mucosa (18, 19). Whereas clearance of S. aureus from the human nose and skin is heavily reliant on a robust neutrophilic response (20), murine neutrophils play a different if not less dominant role in host immunity: murine neutrophils lack defensins and make up a much smaller percentage of circulating leukocytes than human neutrophils (19, 21). Humans and mice also differ in expression of cell differentiation markers, immunoglobulin classes, Toll-like receptors, and T cell subsets in the skin and mucosa (19); thus, translation of research findings from murine-S. aureus studies to the human SANC condition might be difficult.
Considering the experimental limitations that exist in studying SANC in humans, paired with the lack of a reproducible human-relevant model, we sought to determine whether pig-tailed macaques (Macaca nemestrina) are natural carriers of S. aureus, whether they may be experimentally colonized with S. aureus strains relevant to human S. aureus carriage, and whether their innate response to S. aureus models the human response. Macaca nemestrina pig-tailed macaques have previously been shown to be able to be used as a model of the human condition for airway development, immune function, hormonal regulation, and microbiome complexity (22–26). Here, we screened 17 pig-tailed macaques living in the Washington National Primate Research Center (WaNPRC) for nasal or pharyngeal S. aureus, and 14 were nasal S. aureus positive at one or both screening sessions (8 weeks apart). Similar to the human condition (27, 28), treatment of macaque nostrils with topical mupirocin ointment effectively cleared the nostrils and 6 extranasal sites (pharynx, left and right axillae and hands, and vagina), demonstrating that the nasal vestibule is the likely reservoir for colonizing S. aureus. We experimentally colonized macaque noses with both autologous and nonautologous S. aureus strains for over 40 days each, utilizing a physiologically relevant inoculum of 104 CFU/nostril. Experimentally inoculated animals exhibited an S. aureus-associated host defense that was similar to the human nasal defense, in that the clearance of nostril S. aureus was associated with rapid elevation of interleukin-1β (IL-1β), IL-8, and monocyte chemotactic protein 1 (MCP-1) levels and a decreased IL-1 receptor antagonist (IL-1RA)/IL-1β ratio in nasal secretions. Collectively, pig-tailed macaques represent a physiologically relevant and reproducible model of human S. aureus nasal carriage.
RESULTS
Pig-tailed macaques (Macaca nemestrina) are natural nasal carriers of S. aureus strains.
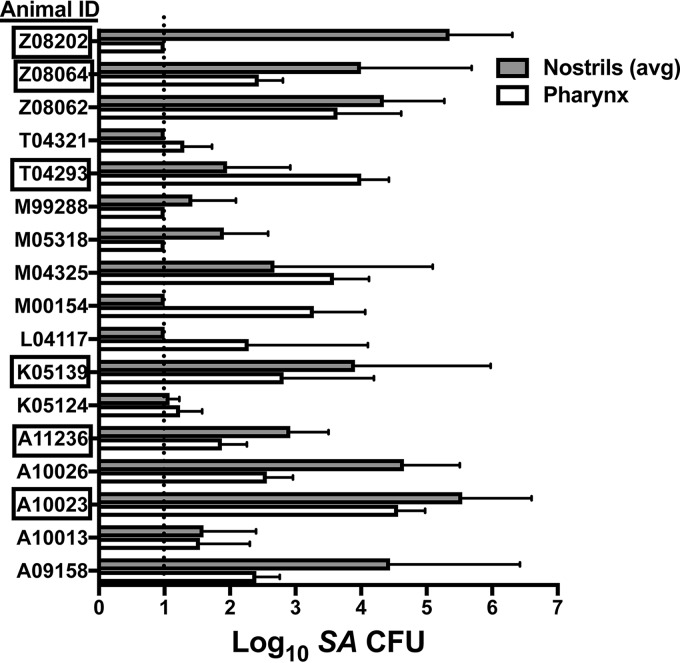
Seventeen pig-tailed macaques were surveyed for S. aureus carriage by swabbing the nasal vestibule (both nostrils) and pharynx at two study visits that occurred 8 weeks apart. Thirteen macaques were colonized with S. aureus in at least one nostril at both visits, and all 17 animals were S. aureus positive in either the nose or the pharynx at least once (Fig. 1). Six macaques were assigned to participate in nasal inoculation studies (the animals whose identification codes are boxed in Fig. 1), and the nasal isolates collected from these animals were genotyped by multilocus sequence type (MLST) analysis and spa typing. Animals A10023, K05139, T04293, and Z08064 all carried sequence type 3813 (ST3813), and animals A11236 and Z08202 hosted distinct STs, ST3814 and ST3815, respectively (Table 1). Like the majority of human nasal strains (29, 30), the macaque nasal S. aureus strains were devoid of the mecA gene, which confers methicillin resistance, and were mupirocin sensitive (Table 1).
FIG 1.
Pig-tailed macaques are natural carriers of S. aureus (SA). Seventeen macaques at WaNPRC were screened for S. aureus in the nostrils and pharynx at two visits that occurred 8 weeks apart. The mean and SD are shown for each animal. The dotted line indicates the limit of detection (10 CFU/swab). Boxed animal identification (ID) codes indicate animals that were utilized for inoculation studies.
TABLE 1.
Nasal S. aureus strains utilized in experimental inoculation of Macaca nemestrina pig-tailed macaquesc
| S. aureus strain designation | Host | MLST | spa type |
|---|---|---|---|
| ST3813 | Pig-tailed macaque | 3813a | t15866b |
| ST3814 | Pig-tailed macaque | 3814a | t15867b |
| ST3815 | Pig-tailed macaque | 3815a | t15868b |
| D579 | Human | 398 | t571 |
Newly assigned MLST.
Newly assigned spa type.
MLST, multilocus sequence type; spa type, staphylococcal protein A gene Ridom type. The mecA gene and mupirocin resistance were absent in all strains.
Clearance and recolonization of pig-tailed macaque noses with diverse S. aureus strains.
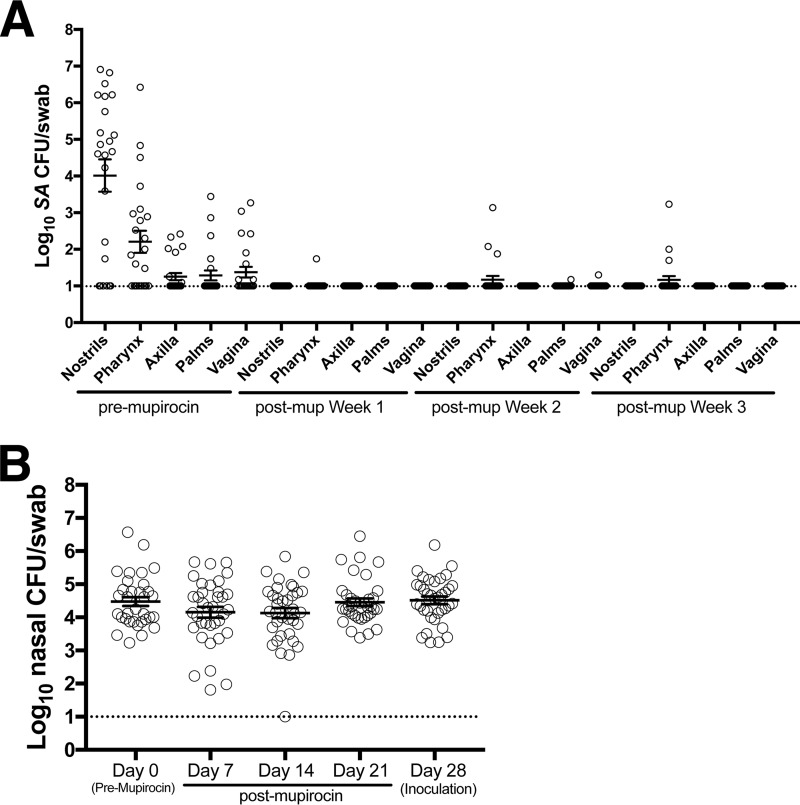
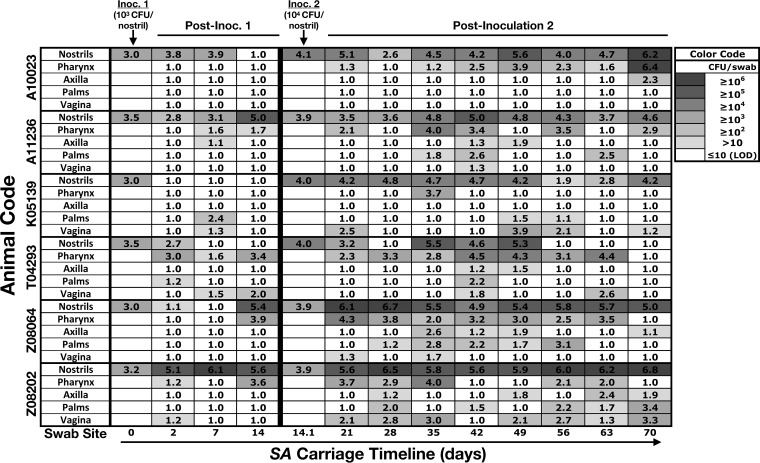
To create a model in which several animals could be nasally inoculated with S. aureus and followed up together, a nasal S. aureus decolonization protocol was established with topical nasal mupirocin, and the necessary post-mupirocin-treatment recovery period for restoration of non-S. aureus commensal microflora was evaluated. Following a 5-day, twice-daily nasal mupirocin treatment course, swabs were collected once per week for the next 4 weeks. In four independent experiments, each using 6 animals, nasal S. aureus was eradicated and the extranasal swab sites were cleared of S. aureus in 21 or more of the 24 sample collections (Fig. 2A). Commensal bacterial levels, as determined by enumerating all colonies cultured on blood agar, were fully restored by week 3 (Fig. 2B), and subsequent experimental S. aureus inoculations were administered during the 4th week following the completion of mupirocin treatment. Nasal inoculations with each macaque's own (autologous) S. aureus isolate were performed to determine an inoculum that would colonize the majority of animals during the first few weeks of follow-up. Animals were first inoculated with 103 S. aureus CFU, placed into each nostril, and followed for 2 weeks by swabbing nostrils and extranasal sites (pharynx, left and right axillae and palms, and vagina). At day 2 postinoculation, 5 out of the 6 animals' noses were S. aureus positive in at least one nostril, but by day 14, only 3 of 6 animals were colonized nasally and only 9 of 48 total body sites presented detectable S. aureus (Fig. 3). After sample collection on day 14, animals were nasally inoculated with 104 CFU/nostril, and animals were followed up for another 8 weeks (through day 70 overall) before mupirocin treatment to clear S. aureus. Colonization was established in the noses of all 6 animals and in the extremities of animals with high nasal S. aureus densities (most notably, animals Z08064 and Z08202; Fig. 3).
FIG 2.
Nasal mupirocin treatment effectively clears pig-tailed macaque nostrils and external sites. (A) The S. aureus load before and after nasal mupirocin (mup) application for each body site (average for the left and right for nostrils, axilla, and palms). Error bars indicate the mean and SEM (n = 24; 6 animals, 4 experiments each). (B) The animals' nostrils were swabbed prior to the first topical application of mupirocin and then weekly for 4 weeks, and the number of non-S. aureus CFU was enumerated. Error bars indicate the mean and SEM. The dotted horizontal lines indicate the limit of S. aureus detection (10 CFU/swab).
FIG 3.
Nasal inoculation with 104 CFU/nostril establishes long-term S. aureus carriage at nasal and extranasal body sites. Six pig-tailed macaques (the animal identification is shown at the left) were cleared of nasal S. aureus with mupirocin (5 days, twice daily) and recolonized with 103 CFU/nostril (inoculation 1 [Inoc. 1] was on day 0) of their own S. aureus strain 4 weeks later. S. aureus values are shown as the number of log10 CFU per swab and color coded in the style of a heatmap according to the S. aureus load (see the key at the upper right). On day 14 after inoculation 1, when it was evident that S. aureus carriage had not been established in the majority of animals, 104 CFU/nostril (inoculation 2 [Inoc. 2]) was administered after swab samples and nasal secretions were collected. The average for the left and right swab sites is displayed for the nostrils, axilla, and palms. Experimental inoculations 1 and 2 were designed to administer 103 and 104 S. aureus CFU/nostril, respectively, and actual values (log10) are shown. The limit of detection (LOD) was 10 S. aureus CFU/swab.
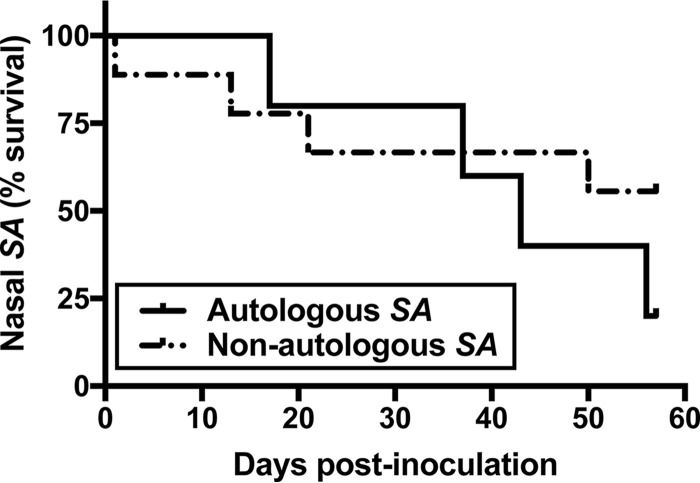
Three subsequent decolonization/recovery/experimental inoculation cycles (6 animals treated identically, 18 total inoculations) were carried out using 104 CFU/nostril. Two macaque S. aureus isolates (ST3813 and ST3814) were administered, such that in total, there were 5 nasal inoculations with autologous MLST and spa types and 7 inoculations with nonautologous strains. Swab samples were collected at day 2 or 3 postinoculation and weekly thereafter, and S. aureus survival curves were generated. Detectable S. aureus in at least one nostril was considered survival. Both autologous and nonautologous S. aureus strains survived over 40 days (median survival times, 43 days for autologous S. aureus strains and over 57 days for nonautologous S. aureus strains; Fig. 4), and the survival curves were statistically similar (P = 0.37). A human S. aureus isolate (D579, whose characteristics are shown in Table 1) was carried in the nose (range, 8.50 × 103 to 1.02 × 106 CFU/swab) and some extranasal sites (range, 5.20 × 102 to 8.75 × 103 CFU/swab) for at least 22 days in 4 of 6 animals. Mupirocin treatment was initiated at day 22 to clear the animals of S. aureus prior to return to the colony, and all sites were clear when checked at day 28 postinoculation.
FIG 4.
Macaque noses may be experimentally colonized with autologous and nonautologous S. aureus strains. Each animal's nostrils were experimentally inoculated with 104 CFU of an S. aureus strain either genetically indistinct from that animal's screening isolate (autologous S. aureus; n = 5 noses) or a nonautologous S. aureus isolate (n = 7 noses). Collection of nostril swab specimens was performed at day 2 or 3 and then weekly postinoculation through day 56. Detectable S. aureus in at least one nostril was considered survival.
To confirm that the detected S. aureus strain matched the inoculated strain, MLST and spa typing were performed on S. aureus colonies collected from (i) extranasal swab sites, (ii) nostrils that carried S. aureus continuously for several weeks, and (iii) nostrils that had apparently cleared S. aureus and then exhibited S. aureus positivity again at a subsequent visit. Acquisition of an S. aureus strain genetically distinct from the inoculated strain was rare but did occur for animal T04293 in the nose at day 35 and for animal K05139 in the nose at day 63 of the pilot 104-CFU/nostril inoculation study (Fig. 3). The S. aureus strains from the extranasal sites matched the inoculated nasal S. aureus strains in 105 out of 110 (95.5%) swab samples that were evaluated. Taken together, the presented protocol for clearing and recolonizing pig-tailed macaques with S. aureus appears to be feasible and reproducible and pig-tailed macaque SANC appears to be analogous to human SANC (2, 11, 31, 32).
Evaluation of natural S. aureus load by anatomical site.
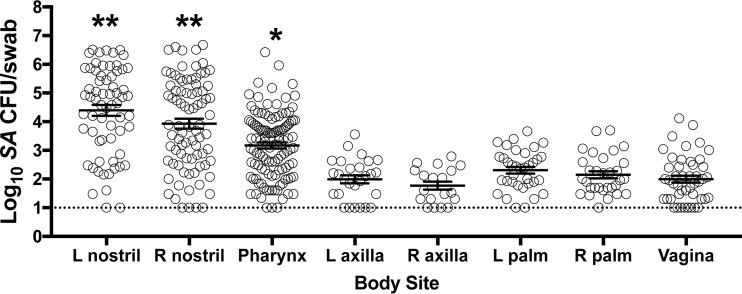
To further determine the suitability of the pig-tailed macaque as a model of human SANC, we evaluated the natural S. aureus load at eight anatomical sites: left and right nostrils, pharynx, left and right axillae, left and right palms, and vagina. Figure 5 presents the S. aureus-positive body sites, excluding nostrils positive directly due to experimental inoculation (i.e., nostrils were considered positive only from screening or preinoculation samplings or when clearance of experimentally inoculated S. aureus was evident at two consecutive prior visits). S. aureus-positive nasal swabs far outnumbered S. aureus-positive axilla, palm, and vagina swabs; and nasal swabs contained the highest density of S. aureus compared to all other sites (Fig. 5). Pharyngeal swab specimens contained more S. aureus bacteria than axilla, palm, and vagina swab specimens but a lower S. aureus load than nostril swab specimens (Fig. 5). Taken together with the results in Fig. 2A, which showed nasal mupirocin's effectiveness at clearing nasal and extranasal sites of S. aureus, these data suggest that the S. aureus reservoir in pig-tailed macaques is the nasal vestibule.
FIG 5.
The nasal vestibule represents the predominant reservoir of S. aureus during colonization. The results for naturally S. aureus-positive macaque body sites (i.e., sites positive not due to experimental inoculation of the nostrils) collected over an 18-month period are shown. **, elevated S. aureus load compared to all other sites (P < 0.0001); *, S. aureus load greater than that in the axilla, hands, and vagina (P < 0.005). Error bars indicate the mean and SEM. The dotted horizontal line indicates the limit of S. aureus detection (10 CFU/swab). L, left; R, right.
The pig-tailed macaque nasal mucosal response to S. aureus inoculation models the human nasal host defense against S. aureus.
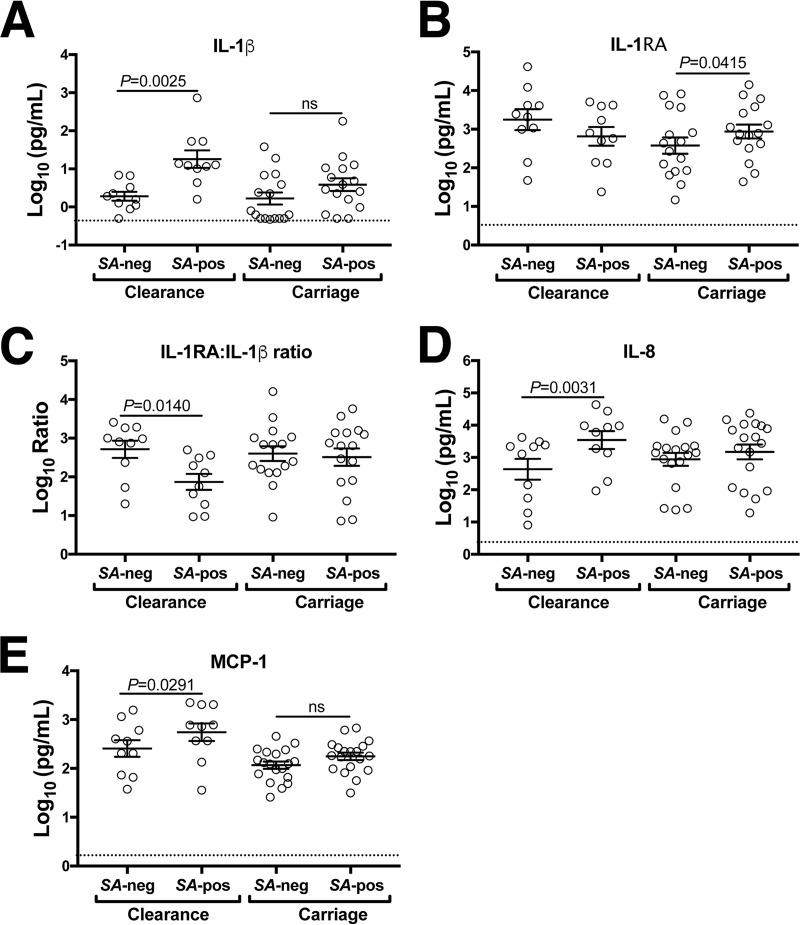
Following swab sample collections during each of the pre- and postinoculation follow-up periods, blood was collected for serum storage and nasal fluid was collected from each nostril by twice placing Whatman 540 low-ash filter paper strips into the lumen and massaging the nose for 30 s. Adsorbed proteins were extracted, concentrated, and reconstituted in neutral-pH buffer suitable for total protein quantification and Luminex-based detection of cytokines, chemokines, and growth factors. Macaque nasal secretions averaged 9.9 ± 3.1 mg/ml total protein (mean ± standard error of the mean [SEM]), whereas human nasal fluid self-collected by suction catheter averaged 5.5 ± 2.5 mg/ml (6 macaques [an average of 14 collections from each macaque] versus 14 humans [1 nasal fluid sample from each person]). Macaque nasal fluid collected pre- and postexperimental nasal inoculation with S. aureus was assayed for IL-1β, IL-1RA, IL-8, macrophage inflammatory protein 1α (MIP-1β), MCP-1, granulocyte colony-stimulating factor (G-CSF), IL-6, tumor necrosis factor alpha (TNF-α), IL-17A, and vascular endothelial growth factor (VEGF) using nonhuman primate-specific antibody sets. These analytes were chosen on the basis of the findings of previous experiments in human subjects that showed associations between increased IL-1β, IL-8, MIP-1β, MCP-1, and G-CSF expression and nasal S. aureus clearance (11, 12). IL-6 and TNF-α are hallmark inflammatory cytokines, IL-17A is associated with S. aureus host defense in mice (33, 34), and VEGF is important for mucosal barrier homeostasis and remodeling and is highly expressed in mucosal fluids (11, 35, 36). We assessed cytokine levels as the nostrils transitioned from S. aureus negativity to S. aureus positivity (paired comparisons for each subject, with one nostril analyzed per animal) and further stratified the data according to whether the nostril eventually cleared S. aureus or exhibited extended carriage. Similar to the findings for human subjects (11), S. aureus-associated elevation of nasal IL-1β, IL-8, and MCP-1 corresponded with S. aureus clearance (Fig. 6). Furthermore, macaque nostrils that eventually cleared S. aureus demonstrated a 10-fold decrease in the IL-1RA/IL-1β ratio postinoculation (Fig. 6C), while nostrils that carried S. aureus exhibited elevated IL-1RA and no change in the IL-1RA/IL-1β ratio (Fig. 6B and C). Increased expression of MIP-1β was observed in S. aureus-positive nostrils compared to negative nostrils, regardless of whether clearance was achieved (data not shown). The levels of TNF-α and IL-17A were low (<10 pg/ml) in most samples and near the lower limit of detection in the utilized assay, similar to other observations in human subjects (11, 12, 37). The serum levels of all 10 analytes were unchanged by experimental nasal S. aureus inoculation (data not shown). The animals' body weight and temperature were measured and documented each time that the animals were sedated for sample collections. Protein biscuits provided to the animals were counted and reported by the husbandry staff daily. No discernible changes in appetite, weight, or behavior of the animals were noted throughout the course of the experiments. Body temperatures remained within the normal range. Collectively, these observations suggest that the pig-tailed macaque inflammatory response to nasal S. aureus was not systemic in nature.
FIG 6.
Clearance of experimentally inoculated nasal S. aureus associates with augmented innate nasal mucosal defense. Six macaques participated in four S. aureus decolonization/experimental S. aureus recolonization protocols and had their noses sampled as described in Materials and Methods. Based on nasal swab testing results that indicated each nostril's S. aureus carriage pattern, either left or right nostril secretions (the same for all macaques in each experiment) were assessed for cytokines using a Luminex assay. The levels of IL-1β (A) and IL-1RA (B), the IL-1RA/Il-1β ratio (C), and the levels of IL-8 (D) and MCP-1 (E) in nasal secretions are shown for each nostril that transitioned from S. aureus negativity (SA-neg) to S. aureus positivity (SA-pos) either upon experimental inoculation or after testing negative for S. aureus and then positive again during the follow-up period. Cytokine data were further stratified according to whether the nostrils eventually cleared inoculated S. aureus (Clearance) or stayed colonized with inoculated S. aureus (Carriage). Error bars represent the mean and SEM. Dotted horizontal lines indicate the limit of detection.
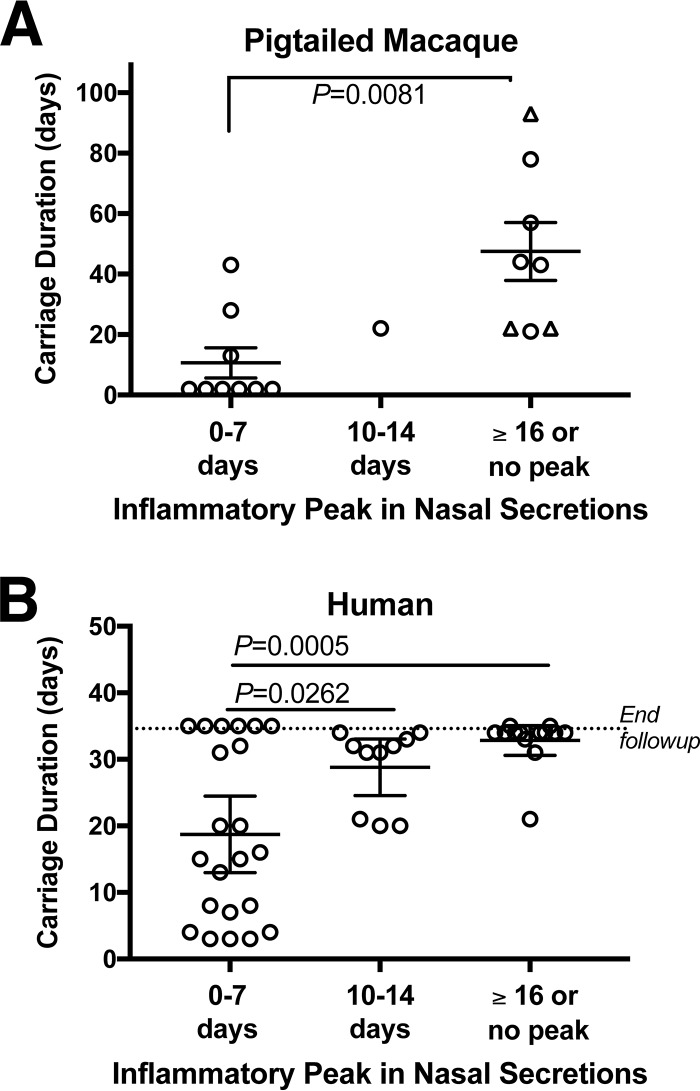
Our laboratory (A. M. Cole) has conducted 43 experimental nasal S. aureus inoculations in which healthy human subjects self-applied their previously isolated S. aureus strains and then returned for swab and nasal fluid collections for a month of follow-up to evaluate S. aureus carriage duration and mucosal host defense characteristics (11, 12). Both human subjects and macaques presented a trend that a rapid innate nasal inflammatory response, typified by peak IL-1β and IL-8 expression within 7 days of S. aureus inoculation, was associated with shortened carriage duration (Fig. 7). For macaques, nostrils that demonstrated peak nasal host defense by day 7 averaged 11 ± 5 days (mean ± SEM) of S. aureus carriage, while nostrils that exhibited a late peak (on or after day 16) or no peak averaged over 40 days of S. aureus carriage (Fig. 7A). Similarly, human noses that presented peak IL-1β and IL-8 expression by day 7 cleared S. aureus within 19 ± 3 days, while later responses to S. aureus inoculation resulted in carriage that extended beyond the month of follow-up for the majority of subjects (Fig. 7B). Taken together, these data suggest that the host effector molecules that mediate nasal S. aureus clearance in humans are present and function similarly in the pig-tailed macaque.
FIG 7.
Similar association between the nasal inflammatory peak and the S. aureus carriage duration in pig-tailed macaques and humans. The peak nasal inflammatory response to experimentally inoculated S. aureus was assessed by Luminex measurements of IL-1β and IL-8 in collected nasal secretions. For each of 18 macaque inoculations (A) and 43 human inoculations (B), the occurrence of the peak inflammatory response postinoculation (x axis) was plotted according to the duration of S. aureus carriage (y axis). Error bars indicate the mean and SEM. The triangles in panel A represent animals that were carrying S. aureus nasally on the last day of follow-up (thus, the carriage duration was actually longer).
DISCUSSION
Complications from S. aureus-induced infections burden most, if not all, medical specialties. Patients with HIV infection, with diabetes, and on dialysis, postoperative patients, and populations living in close quarters with less than optimal sanitary conditions (populations living on military bases or in prisons or nursing homes or attending day care centers) demonstrate enhanced susceptibility (1, 5). Although the reservoir for human-hosted S. aureus, the moist squamous epithelium of the nasal mucosa, has been appreciated for over 2 decades, the research community lacks an appropriate animal model for studying early nasal colonization events, asymptomatic carriage/immune tolerance, and clearance mechanisms. This presents a challenge against efforts to develop novel decolonization strategies and antibiotics.
We evaluated whether the pig-tailed macaque (Macaca nemestrina) is a natural host of nasal S. aureus. We determined that 13 of 17 animals carried S. aureus in at least one nostril at the first screening visit, and 14 of 17 animals were nasal S. aureus positive 8 weeks later (Fig. 1). Three animals (L04117, M00154, T04321) screened negative for nasal S. aureus at both sampling times but had detectable S. aureus in one or both pharyngeal swab specimens. Since we did not have access to any pig-tailed macaques living in the wild, it cannot be ruled out that macaque S. aureus carriage at the WaNPRC is the result of captivity and human handlers. However, caregiver personal protective equipment (PPE) entails full coverage during animal handling, and review of the animal housing map indicated that the three nasal S. aureus-negative animals cohabited with the nasal S. aureus-positive animals. Animal Z08202 carried >104 S. aureus CFU/nasal swab at both screening visits and was housed in the same room as animal T04321, while animal Z08064 (S. aureus positive at both screening visits) was caged immediately adjacent to animal L04117 in a separate room. Thus, these animals' negative nasal S. aureus carriage status was not likely a result of different environmental or animal caregiver factors. Moreover, new MLST and spa types were assigned to the macaque strains (Table 1), suggesting that carriage owed only to human intervention is unlikely. Rhesus macaques from the Biomedical Primate Research Center in The Netherlands were found to host and easily exchange S. aureus within the colony (38). Although our study was limited to one primate research center, it appears that research Macaca nemestrina pig-tailed macaques carry S. aureus at least intermittently, with the nose containing the highest S. aureus density for nearly all animals (Fig. 1 and 5). Importantly, this corresponds with the prevailing paradigm that human SANC involves persistent carriers and others who host S. aureus transiently throughout life (2).
The high rate of carriage in macaques (14 of 17 animals in our study and 82% of 48 rhesus macaques sampled longitudinally [38]) exceeds that in humans and warrants further study. Cattle and pig farms report nasal S. aureus carriage rates in the 50 to 90% range (39, 40), suggesting that living in close quarters and hygiene differences support S. aureus colonization. The finding that individuals living in prisons and on military bases present elevated carriage rates (41, 42) lends support to this reasoning. Given the obvious hygiene differences between macaques and humans, it will still be important to determine potential host immune differences that might permit an elevated yet asymptomatic S. aureus load in the nasal mucosa. Future studies that should be feasible in pig-tailed macaques include the determination of anti-S. aureus antibody profiles, intracellular S. aureus levels in the nasal epidermis of macaque noses (43), and identification of infiltrating immune cell populations during S. aureus carriage and upon its resolution.
An important finding of this study is that all macaques were successfully decolonized using a 5-day course of topical nasal mupirocin. Consistent with the human SANC condition (2, 11, 12, 31, 32), nasal mupirocin application eradicated S. aureus not only in the nostrils but also in the axillae, hands, and vagina, with pharyngeal S. aureus being cleared in 21 of 24 tests (Fig. 2A). This suggests that the anterior nasal vestibule of pig-tailed macaques is the reservoir for colonizing S. aureus and might be as attractive an environment to S. aureus as the human anterior nares. It should be noted that we did not test how long animals stayed clear of S. aureus following mupirocin treatment, though we did observe that all 6 animals generally stayed clear of S. aureus at all sites for at least 4 weeks. Decolonization of humans with mupirocin nasal ointment is considered beneficial for preventing postoperative infection (27, 44), but S. aureus carriage is recurrent in many subjects (45). More studies that investigate the impact of mupirocin treatment on microbial community dynamics as well as new agents to be used for S. aureus decolonization are needed, and the pig-tailed macaque might be useful in this regard. Our main objective was to develop a decolonization and inoculation protocol similar to what we previously reported for human subjects (11, 12). Importantly, recolonization of the macaques was established with a physiologically relevant inoculum of 104 S. aureus CFU/nostril. Nasal swab collections indicated that macaque nostrils averaged approximately 104 S. aureus CFU/nostril swab naturally (e.g., independent of experimental nasal colonization; Fig. 5), consistent with the findings for human noses, which carry S. aureus in the range of 101 to 106 CFU/nostril swab (median, 9.8 × 103 S. aureus CFU/swab for 30 healthy human subjects surveyed by our group in 2016 [12]).
A second indication that Macaca nemestrina pig-tailed macaques present a physiologically relevant model of human SANC is that both autologous and nonautologous strains colonized the macaque noses (at least one nostril) for over 40 days (Fig. 4), and 5 out of 6 macaques were nasally colonized with a human nasal S. aureus isolate for at least 14 days. One animal swabbed S. aureus negative at all body sites on day 14 postinoculation with human ST398 strain D579, while another was clear at all sites on day 22. Due to time and funding constraints, follow-up was restricted to 22 days following experimental inoculation with the human donor isolate, but 4 of the 6 inoculated animals carried an average of 2.7 × 105 S. aureus CFU per nostril swab on day 22 prior to mupirocin treatment to successfully clear the animals for return to the colony. Keeping in mind that the inoculum was only 104 S. aureus CFU per nostril, this indicates that, like the human nasal mucosa, the macaque nasal mucosa provides favorable conditions for S. aureus survival. Colonization of the majority of animals with this common lineage, known to affect humans, cows, and pigs and to have the ability to acquire methicillin resistance (46–49), suggests that other clinically relevant S. aureus strains will be testable in pig-tailed macaques.
Consistent with human SANC (11, 12, 50), innate host defense against nasal S. aureus in macaque noses appears to be predominantly neutrophilic in nature, with the observed induction of IL-1β and IL-8 and a 10-fold decreased IL-1RA/IL-1β ratio in nostrils that eventually cleared S. aureus (Fig. 6). Modeling of the neutrophilic response of humans to nasal S. aureus with Macaca nemestrina macaques will undoubtedly prove useful in elucidating the specific cellular mechanisms that result in clearance in certain hosts but sustained asymptomatic carriage in others. Notably, this aspect of nasal host defense cannot be adequately modeled in mice, since mice do not make IL-8 and murine neutrophils lack defensins and thus have different means of degranulating and damaging S. aureus and forming extracellular traps. While we have not yet identified which immune cells populate the macaque nasal subepithelium, previous reports showed that the pig-tailed macaque perhaps represents the best-known model for the human host defense at mucosal surfaces. It has been used to model human rectal and reproductive mucosae in studies of sexually transmitted pathogens, as well as in studies of infection-induced preterm birth and impaired fetal lung development (23, 24, 51–53). Furthermore, Macaca nemestrina macaques are colonized with a mucosal flora similar to that in humans, including with endogenous lactobacilli in the reproductive tract (54, 55). We have not yet fully evaluated the nasal microbiome of macaques at the DNA/metagenomic level, but the species in both human and macaque nares mainly comprised staphylococcal species in our initial evaluation of culturable microbes from swab fluid specimens. Importantly, S. epidermidis, S. aureus, S. hominis, S. capitis, S. haemolyticus, and S. warneri all had similar relative abundances in each host (unpublished observations). These preliminary data, combined with the known similarity between the human and pig-tailed macaque vaginal microbiome (54, 55), support the notion that the macaque nasal mucosa is preferable to rodents for studying microbial community dynamics with respect to S. aureus carriage.
In summary, the presented study demonstrates that Macaca nemestrina macaques are natural hosts of S. aureus, with the nasal mucosa containing the highest S. aureus density. This is similar to the human condition and not observed in current rodent models. Macaques were successfully cleared of S. aureus with the same topical mupirocin treatment used by humans, and 4 of 6 macaques carried an experimentally inoculated human nasal S. aureus isolate for over 3 weeks. Given that both humans and Macaca nemestrina macaques exhibit a neutrophilic response to inoculated S. aureus in the nostrils that successfully clears the bacterium, there is the potential for studying host-S. aureus interactions and new decolonization strategies with this model of human S. aureus nasal carriage.
MATERIALS AND METHODS
Study participants, animal care, and compliance.
This study was performed with pig-tailed macaques (Macaca nemestrina) housed at the Washington National Primate Research Center (WaNPRC) in accordance with all institutional and federal guidelines for the care and use of laboratory animals. Housing and care conditions at the WaNPRC facilities meet AAALAC accreditation standards for nonhuman primates and follow NIH guidelines for animal biosafety level 2 containment facilities. The study-specific protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Washington. On sample collection days (collection of swab, nasal secretion, and blood samples), animals were sedated with 1.2 mg/kg of body weight ketamine and moved to a procedure room. On days in which the only intervention was to apply mupirocin nasal ointment, the animals were sedated with a light dose of ketamine (0.8 mg/kg) and treated while remaining in their cages. In all, six healthy sexually mature female pig-tailed macaques were selected from a cohort of 17 healthy individuals for monitoring of nasal and pharyngeal S. aureus carriage status for 2 months. Their ages ranged from 9 to 13 years, and they were born to different sets of parents and thus considered unique in terms of host genetics. The subjects were chosen on the basis of the individual's ability to naturally acquire and carry S. aureus as well as their availability for participation for the entire study duration (∼18 months). There were no clinical adverse effects of nasal S. aureus inoculation reported during the duration of the study. The human nasal carrier studies referred to here were previously described (11, 12) and were performed under the guidelines of the Institutional Review Board of the University of Central Florida.
Animal screening and sample collection.
To screen pig-tailed macaques (Macaca nemestrina) for S. aureus carriage, the left and right nostrils and pharynx of each animal were sampled using a flocked Copan Diagnostics Eswab minitip and immediately stored in 1 ml of Eswab (liquid Amies) medium in transport tubes (catalog number 23600901; Fisher Scientific). Samples were shipped overnight on ice packs to the A. M. Cole laboratory. Upon arrival, the transport tubes were vortexed vigorously and the swabs were swirled in transport medium and then streaked onto CHROMagar plates for colorimetric identification of S. aureus. The swab/transport liquid was dilution plated onto tryptic soy agar II (TSAII) containing 5% sheep blood (catalog number B21261X; Fisher Scientific) for enumeration of the S. aureus and non-S. aureus CFU. S. aureus colonies were confirmed using a BD Staphyloslide latex test (catalog number B4340953; Fisher Scientific) and subcultured overnight to make S. aureus colony glycerol stocks. The remaining swab fluid was prepared as an early glycerol stock (0.5 ml sample plus 0.5 ml Bacto tryptic soy broth [TSB], 30% glycerol, stored at −80°C) and also cultured overnight (250 rpm, 37°C, 18 h) in a total volume of 2 ml TSB prior for preparation of a cultured late glycerol stock.
Experimental nasal inoculation of pig-tailed macaques with S. aureus.
Animals were decolonized of endogenous S. aureus by applying mupirocin nasal ointment (Bactroban; GlaxoSmithKline, Philadelphia, PA) twice daily for 5 days. For 4 weeks following the last mupirocin application, nostrils and extranasal sites (left and right axillae, left and right hands, pharynx, vagina) were swabbed and samples were shipped and processed as described above to confirm the clearance of S. aureus and recovery of the commensal nasal microflora. S. aureus strains were prepared for experimental inoculation during the 4th week after mupirocin treatment as follows: S. aureus colonies were grown in 50 ml of TSB for 18 h (250 rpm, 37°C), and then 1 ml of culture was added to 50 ml TSB and cultured for another 2 h. The liquid culture was transferred to a conical tube and centrifuged at 3,000 × g for 5 min, and the supernatant was discarded. The bacterial pellet was suspended in 35 ml of Hanks' balanced salt solution (HBSS) containing 1% bovine serum albumin (BSA), and then centrifugation and suspension in HBSS–1% BSA were repeated. The resulting liquid was portioned to microtubes and snap-frozen by placing the tubes in liquid nitrogen for 3 h, followed by transfer to a −80°C freezer. The concentration (the number of CFU per milliliter) for each strain's snap-frozen stock was calculated by dilution plating each of 5 aliquots on TSAII–5% blood agar. This information was used to determine the dilution scheme and final volume (≤20 μl) needed to achieve an inoculum of either 103 or 104 CFU/nostril. Inocula were administered using a micropipette and gently pinching/massaging the outside of the nose for 30 s. The first experimental inoculation tested whether 103 CFU/nostril would colonize the majority of animals (it did not), and then 104 CFU/nostril was tested and the animals were followed up at day 2 postinoculation, followed by weekly swab, nasal secretion, and blood collections until day 70. In the next 3 studies, the animals were decolonized with mupirocin as described above and then inoculated with either macaque nasal isolate ST3813 or ST3814 or human ST398 nasal isolate D579. The follow-up periods (day 2 or 3 and then weekly swab, nasal secretion, and blood collections) were 63, 93, and 22 days, respectively.
S. aureus genotyping and assessment of mupirocin sensitivity.
Strain USA300-0114 (NRS384) was obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program. Human nasal S. aureus isolates were collected as described previously (11), and macaque S. aureus isolates were collected as described above. Genomic S. aureus DNA was extracted using an UltraClean microbial DNA isolation kit (Mo Bio Laboratories, West Carlsbad, CA, USA) according to the manufacturer's instructions. DNA was placed under PCR conditions with primers to amplify the seven MLST genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL), spa, and mecA as described previously (56–58). PCR amplicons were sequenced by Eton Bioscience, Inc., and the Mega (version 7) program was used for analysis. The S. aureus MLST database (http://pubmlst.org/saureus/) was utilized to assign allele numbers and sequence types (STs) for each S. aureus isolate's seven alleles. Staphylococcal protein A (spa) gene typing was performed as previously described (57, 59) using Ridom StaphType software (http://www.spaserver.ridom.de/). Newly discovered spa types were synchronized with the Ridom server. For antibiotic resistance assessment, the presence of a 1,339-bp product or no amplicon indicated, respectively, the presence or absence of the mecA gene, which encodes the penicillin-binding protein (PBP2A) that confers resistance to β-lactam antibiotics. The results were confirmed by spreading bacterial cultures (the same ones used for DNA extraction) onto MRSASelect agar plates (Bio-Rad, Hercules, CA, USA).
Donor S. aureus strains were also tested for functional mupirocin resistance by performing turbidity (growth) assays (60). S. aureus isolates were grown to log phase in TSB, diluted to 104 CFU/90 μl in Mueller-Hinton broth (Sigma-Aldrich, St. Louis, MO) containing 5% sucrose, and loaded to a 96W culture plate. Ten microliters of 2-fold serial dilutions of either mupirocin (catalog number M-7694; Sigma-Aldrich) or vehicle (volume-matched dimethyl sulfoxide) was added to the diluted S. aureus culture so that the final concentration of mupirocin ranged from 1 to 8 μg/ml. The culture plate was covered with ThermoSeal A film (catalog number TSA-100; Excel Scientific, Inc.) and placed into a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA) programmed for an 18-h kinetic assay at 37°C. Turbidity measurements (optical density at 550 nm [OD550]) were taken every 5 min following 15 s of agitation. Growth curves (OD550 plotted against time) were generated for all wells, and both the input and 18-h incubations were plated on TSAII–5% sheep blood agar for enumeration. All strains grew to ∼108 CFU/0.1 ml in antibiotic-free medium. All strains were killed in the presence of ≤1 μg/ml mupirocin.
Blood and nasal secretion collection and processing.
Each week, blood (3 to 5 ml) was collected into serum-separating tubes (SST) to separate 1 to 2 ml serum, which was stored at −80°C until use. Nasal secretions were collected by twice inserting Whatman 540 low-ash filter paper strips (0.3 by 2.5 cm) into each nostril and massaging the nose for approximately 30 s. The strips used to sample the left and right nostrils were stored in separate tubes at −80°C. Frozen serum samples and nasal strips were batch shipped on dry ice to the A. L. Cole lab for processing and analysis. Nasal fluid proteins were extracted by vortexing for 20 min in 900 μl of 10% glacial acetic acid (in molecular-grade water), transferring the supernatant containing soluble proteins to a fresh tube (on ice), and then vortexing the strips with an additional 500 μl of acetic acid solution for 10 min. The supernatants were combined (per animal nostril), balanced in a SPD1010 SpeedVac apparatus (Thermo Fisher), and vacuum dried to ≤100 μl. Two washes were performed by adding molecular-grade water up to 1 ml and reevaporating the sample to near dryness. Samples were resuspended in nine parts Dulbecco's modified Eagle medium (DMEM) and one part radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) and then neutralized (if necessary) with a scant volume of 1 N or 10 N sodium hydroxide. Sample final volumes were standardized to 130 μl per nasal strip extract (left nostril or right nostril), and the extracts were clarified of insoluble debris by centrifugation (10,000 × g, 1 min) and stored in aliquots at −80°C until analysis. Total protein was quantified, and macaque nasal secretion extracts averaged 9 mg/ml, consistent between animals. Six extracts out of 188 were eliminated from Luminex analysis due to low protein levels.
Detection of pig-tailed macaque cytokines.
Nostril secretion protein extracts and the corresponding serum aliquots (collected on the same day) were thawed on wet ice prior to multiplex bead assay. A ProcartaPlex nonhuman primate custom 10-plex cytokine assay (Life Technologies Corporation, Carlsbad, CA) was performed for detection of G-CSF, IL-1β, IL-1RA, IL-6, IL-8, IL-17α, MCP-1, MIP-1β, TNF-α, and VEGF. Company instructions were followed step by step for standard curve generation, serum sample preparation, and instrument settings. Left nostril secretion extracts were analyzed for 3 of the 4 inoculation experiments (with 6 animals in each experiment), and right nostril extracts were run for the other inoculation experiment, as these were the nostrils in which the most transitions from S. aureus negative to S. aureus positive were observed. Nasal fluid extracts (54 μl) were mixed with 6 μl of DMEM containing 5% BSA, such that both standards and samples were diluted in a nearly equivalent mixture of DMEM, RIPA buffer, and 0.5% BSA, which acted as a background protein to help prevent target protein aggregation and nonspecific adherence to labware. A volume of 50 μl/well of the prepared standards and samples was mixed with antibody-conjugated beads to begin the assay. A Bio-Rad Bio-Plex Pro II wash system and Bio-Plex 200 reader with high-throughput fluidics (Luminex xMAP technology) were used to complete the wash steps and sample readings. Each standard curve demonstrated a good fit down to 0.5 to 4 pg/ml, and these detection limits are shown in the figures where appropriate. Data are presented as the number of picograms per milliliter or the log-transformed number of picograms per milliliter for each serum or nasal fluid extract, which was processed by equal volume throughout the work flow.
Statistical analysis.
Data were analyzed using GraphPad Prism (version 7) software (GraphPad Software, La Jolla, CA). Survival curves for autologous and nonautologous S. aureus strains in macaque noses (Fig. 4) were compared and determined to be not significantly different by both the log-rank (Mantel-Cox) test and the Gehan-Breslow-Wilcoxon test. Body site S. aureus levels (Fig. 5) were log transformed and compared using ordinary one-way analysis of variance (ANOVA) with Tukey's multiple-comparison test. Cytokine comparisons (an S. aureus-negative to S. aureus-positive transition for individual animal nostrils; Fig. 6) were made using paired t tests (two-tailed). Carriage durations for noses with different inflammatory peak patterns (Fig. 7) were compared using ordinary one-way ANOVA with Tukey's multiple-comparison test.
ACKNOWLEDGMENTS
This research was supported by funds from NIH grant AI119835 to A.M.C. and the Office of Research Infrastructure Programs (ORIP) of the NIH through WaNPRC grant P51 OD010425. The Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program is supported under NIAID, NIH, contract no. HHSN272200700055C.
We declare no conflicts of interest.
This collaborative project was designed by A.M.C., D.L.P., Y.C.S., and A.L.C. Technical work with animals was performed by S.V.H. and A.B. Microbiology procedures were performed by A.G.L., A.C.B., J.M.G., and C.F.C. Analysis of nasal secretions was the work of A.G.L. and A.L.C. Statistical analysis was performed by A.L.C. The manuscript was written by A.L.C.
REFERENCES
- 1.Mulcahy ME, McLoughlin RM. 2016. Host-bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends Microbiol 24:872–886. doi: 10.1016/j.tim.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 2.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 3.Botelho-Nevers E, Berthelot P, Verhoeven PO, Grattard F, Cazorla C, Farizon F, Pozzetto B, Lucht F. 2014. Are the risk factors associated with Staphylococcus aureus nasal carriage in patients the same than in healthy volunteers? Data from a cohort of patients scheduled for orthopedic material implantation. Am J Infect Control 42:1121–1123. doi: 10.1016/j.ajic.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Olsen K, Danielsen K, Wilsgaard T, Sangvik M, Sollid JU, Thune I, Eggen AE, Simonsen GS, Furberg AS. 2013. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 8:e63716. doi: 10.1371/journal.pone.0063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock SJ, de Silva I, Lowy FD. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol 9:605–610. doi: 10.1016/S0966-842X(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 6.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 7.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 8.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 9.Holtfreter S, Nguyen TT, Wertheim H, Steil L, Kusch H, Truong QP, Engelmann S, Hecker M, Volker U, van Belkum A, Broker BM. 2009. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol 16:1607–1614. doi: 10.1128/CVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole AL, Muthukrishnan G, Chong C, Beavis A, Eade CR, Wood MP, Deichen MG, Cole AM. 2016. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol 9:1537–1548. doi: 10.1038/mi.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole AL, Schmidt-Owens M, Beavis AC, Chong CF, Tarwater PM, Schaus J, Deichen MG, Cole AM. 8 January 2018. Cessation from smoking improves innate host defense and clearance of experimentally inoculated nasal S. aureus Infect Immun. doi: 10.1128/IAI.00912-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HK, Missiakas D, Schneewind O. 2014. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods 410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winstel V, Kuhner P, Salomon F, Larsen J, Skov R, Hoffmann W, Peschel A, Weidenmaier C. 2015. Wall teichoic acid glycosylation governs Staphylococcus aureus nasal colonization. mBio 6:e00632-15. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokai-Kun JF, Walsh SM, Chanturiya T, Mond JJ. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob Agents Chemother 47:1589–1597. doi: 10.1128/AAC.47.5.1589-1597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulcahy ME, Leech JM, Renauld JC, Mills KH, McLoughlin RM. 2016. Interleukin-22 regulates antimicrobial peptide expression and keratinocyte differentiation to control Staphylococcus aureus colonization of the nasal mucosa. Mucosal Immunol 9:1429–1441. doi: 10.1038/mi.2016.24. [DOI] [PubMed] [Google Scholar]
- 18.Treuting PM, Clifford CB, Sellers RS, Brayton CF. 2012. Of mice and microflora: considerations for genetically engineered mice. Vet Pathol 49:44–63. doi: 10.1177/0300985811431446. [DOI] [PubMed] [Google Scholar]
- 19.Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 20.Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. 2014. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun 6:639–649. doi: 10.1159/000360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer PB, Lehrer RI. 1992. Mouse neutrophils lack defensins. Infect Immun 60:3446–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.Supplement_4.S273. [DOI] [PubMed] [Google Scholar]
- 23.McAdams RM, Bierle CJ, Boldenow E, Weed S, Tsai J, Beyer RP, MacDonald JW, Bammler TK, Liggitt HD, Farin FM, Vanderhoeven J, Rajagopal L, Adams Waldorf KM. 2015. Choriodecidual group B streptococcal infection induces miR-155-5p in the fetal lung in Macaca nemestrina. Infect Immun 83:3909–3917. doi: 10.1128/IAI.00695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kersh EN, Ritter J, Butler K, Ostergaard SD, Hanson D, Ellis S, Zaki S, McNicholl JM. 2015. Relationship of estimated SHIV acquisition time points during the menstrual cycle and thinning of vaginal epithelial layers in pigtail macaques. Sex Transm Dis 42:694–701. doi: 10.1097/OLQ.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant KS, Worlein JM, Meyer JS, Novak MA, Kroeker R, Rosenberg K, Kenney C, Burbacher TM. 2017. A longitudinal study of hair cortisol concentrations in Macaca nemestrina mothers and infants. Am J Primatol 79:1–9. doi: 10.1002/ajp.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchman SA, Moss JA, Baum MM. 2016. Accurate measurement of female genital tract fluid dilution in cervicovaginal lavage samples. J Chromatogr B Analyt Technol Biomed Life Sci 1017-1018:75–81. doi: 10.1016/j.jchromb.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botelho-Nevers E, Gagnaire J, Verhoeven PO, Cazorla C, Grattard F, Pozzetto B, Berthelot P, Lucht F. 2017. Decolonization of Staphylococcus aureus carriage in 2016. Med Mal Infect 47:305–310. doi: 10.1016/j.medmal.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, van der Tweel I, van Belkum A, Verbrugh HA, Vos MC. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 29.Van Balen JC, Landers T, Nutt E, Dent A, Hoet AE. 2017. Molecular epidemiological analysis to assess the influence of pet-ownership in the biodiversity of Staphylococcus aureus and MRSA in dog- and non-dog-owning healthy households. Epidemiol Infect 145:1135–1147. doi: 10.1017/S0950268816003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker K, Schaumburg F, Fegeler C, Friedrich AW, Kock R, Prevalence of Multiresistant Microorganisms PMM Study. 2017. Staphylococcus aureus from the German general population is highly diverse. Int J Med Microbiol 307:21–27. doi: 10.1016/j.ijmm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Reagan DR, Doebbeling BN, Pfaller MA, Sheetz CT, Houston AK, Hollis RJ, Wenzel RP. 1991. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann Intern Med 114:101–106. doi: 10.7326/0003-4819-114-2-101. [DOI] [PubMed] [Google Scholar]
- 32.Parras F, Guerrero MC, Bouza E, Blazquez MJ, Moreno S, Menarguez MC, Cercenado E. 1995. Comparative study of mupirocin and oral co-trimoxazole plus topical fusidic acid in eradication of nasal carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 39:175–179. doi: 10.1128/AAC.39.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer NK, Adappa ND, Palmer JN, Cohen NA, Harro JM, Lee SK, Miller LS, Shirtliff ME. 2016. Interleukin-17A (IL-17A) and IL-17F are critical for antimicrobial peptide production and clearance of Staphylococcus aureus nasal colonization. Infect Immun 84:3575–3583. doi: 10.1128/IAI.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archer NK, Harro JM, Shirtliff ME. 2013. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun 81:2070–2075. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear GT, Kendrick SR, Chen HY, Thomas TT, Bahk M, Balderas R, Ghosh S, Weinberg A, Landay AL. 2011. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PLoS One 6:e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi GS, Park HJ, Hur GY, Choi SJ, Shin SY, Ye YM, Park HS. 2009. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin Exp Allergy 39:655–661. doi: 10.1111/j.1365-2222.2009.03216.x. [DOI] [PubMed] [Google Scholar]
- 37.Konig K, Klemens C, Haack M, Nicolo MS, Becker S, Kramer MF, Groger M. 2016. Cytokine patterns in nasal secretion of non-atopic patients distinguish between chronic rhinosinusitis with or without nasal polys. Allergy Asthma Clin Immunol 12:19. doi: 10.1186/s13223-016-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg S, van Wamel WJ, Snijders SV, Ouwerling B, de Vogel CP, Boelens HA, Willems RJ, Huijsdens XW, Verreck FA, Kondova I, Heidt PJ, Verbrugh HA, van Belkum A. 2011. Rhesus macaques (Macaca mulatta) are natural hosts of specific Staphylococcus aureus lineages. PLoS One 6:e26170. doi: 10.1371/journal.pone.0026170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mroczkowska A, Zmudzki J, Marszalek N, Orczykowska-Kotyna M, Komorowska I, Nowak A, Grzesiak A, Czyzewska-Dors E, Dors A, Pejsak Z, Hryniewicz W, Wyszomirski T, Empel J. 2017. Livestock-associated Staphylococcus aureus on Polish pig farms. PLoS One 12:e0170745. doi: 10.1371/journal.pone.0170745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dierikx CM, Hengeveld PD, Veldman KT, de Haan A, van der Voorde S, Dop PY, Bosch T, van Duijkeren E. 2016. Ten years later: still a high prevalence of MRSA in slaughter pigs despite a significant reduction in antimicrobial usage in pigs the Netherlands. J Antimicrob Chemother 71:2414–2418. doi: 10.1093/jac/dkw190. [DOI] [PubMed] [Google Scholar]
- 41.Miko BA, Befus M, Herzig CT, Mukherjee DV, Apa ZL, Bai RY, Tanner JP, Gage D, Genovese M, Koenigsmann CJ, Larson EL, Lowy FD. 2015. Epidemiological and biological determinants of Staphylococcus aureus clinical infection in New York State maximum security prisons. Clin Infect Dis 61:203–210. doi: 10.1093/cid/civ242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh J, Johnson RC, Schlett CD, Elassal EM, Crawford KB, Mor D, Lanier JB, Law NN, Walters WA, Teneza-Mora N, Bennett JW, Hall ER, Millar EV, Ellis MW, Merrell DS. 2016. Multi-body-site microbiome and culture profiling of military trainees suffering from skin and soft tissue infections at Fort Benning, Georgia. mSphere 1:e00232-16. doi: 10.1128/mSphere.00232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanssen AM, Kindlund B, Stenklev NC, Furberg AS, Fismen S, Olsen RS, Johannessen M, Sollid JU. 2017. Localization of Staphylococcus aureus in tissue from the nasal vestibule in healthy carriers. BMC Microbiol 17:89. doi: 10.1186/s12866-017-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefebvre J, Buffet-Bataillon S, Henaux PL, Riffaud L, Morandi X, Haegelen C. 2017. Staphylococcus aureus screening and decolonization reduces the risk of surgical site infections in patients undergoing deep brain stimulation surgery. J Hosp Infect 95:144–147. doi: 10.1016/j.jhin.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Economedes DM, Deirmengian GK, Deirmengian CA. 2013. Staphylococcus aureus colonization among arthroplasty patients previously treated by a decolonization protocol: a pilot study. Clin Orthop Relat Res 471:3128–3132. doi: 10.1007/s11999-013-2856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrel M, Goto M, Schweizer ML, David MZ, Livorsi D, Perencevich EN. 2017. Diffusion of clindamycin-resistant and erythromycin-resistant methicillin-susceptible Staphylococcus aureus (MSSA), potential ST398, in United States Veterans Health Administration hospitals, 2003-2014. Antimicrob Resist Infect Control 6:55. doi: 10.1186/s13756-017-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wardyn SE, Stegger M, Price LB, Smith TC. 2018. Whole-genome analysis of recurrent Staphylococcus aureus t571/ST398 infection in Farmer, Iowa, USA. Emerg Infect Dis 24:153–154. doi: 10.3201/eid2401.161184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diene SM, Corvaglia AR, Francois P, van der Mee-Marquet N, Regional Infection Control Group of the Centre Region. 2017. Prophages and adaptation of Staphylococcus aureus ST398 to the human clinic. BMC Genomics 18:133. doi: 10.1186/s12864-017-3516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uhlemann AC, McAdam PR, Sullivan SB, Knox JR, Khiabanian H, Rabadan R, Davies PR, Fitzgerald JR, Lowy FD. 2017. Evolutionary dynamics of pandemic methicillin-sensitive Staphylococcus aureus ST398 and its international spread via routes of human migration. mBio 8:e01375-16. doi: 10.1128/mBio.01375-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Belkum A, Emonts M, Wertheim H, de Jongh C, Nouwen J, Bartels H, Cole A, Cole A, Hermans P, Boelens H, Toom NL, Snijders S, Verbrugh H, van Leeuwen W. 2007. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes Infect 9:1471–1477. doi: 10.1016/j.micinf.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Lichtenwalner AB, Patton DL, Klebanoff SJ, Headley CM, Hillier SL. 2000. Vaginal myeloperoxidase and flora in the pig-tailed macaque. J Med Primatol 29:36–41. doi: 10.1034/j.1600-0684.2000.290105.x. [DOI] [PubMed] [Google Scholar]
- 52.Patton DL, Cosgrove Sweeney YT, Paul KJ. 2008. A summary of preclinical topical microbicide vaginal safety and chlamydial efficacy evaluations in a pigtailed macaque model. Sex Transm Dis 35:889–897. doi: 10.1097/OLQ.0b013e31817dfdb8. [DOI] [PubMed] [Google Scholar]
- 53.Patton DL, Cosgrove-Sweeney YT, Rabe LK, Hillier SL. 2001. The pig-tailed macaque rectal model: microflora and chlamydial infection. Sex Transm Dis 28:363–366. doi: 10.1097/00007435-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Patton DL, Sweeney YC, Rabe LK, Hillier SL. 1996. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex Transm Dis 23:489–493. doi: 10.1097/00007435-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Patton DL, Sweeney YC, Tsai CC, Hillier SL. 2004. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol 33:105–108. [DOI] [PubMed] [Google Scholar]
- 56.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muthukrishnan G, Lamers RP, Ellis A, Paramanandam V, Persaud AB, Tafur S, Parkinson CL, Cole AM. 2013. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect Dis 13:221. doi: 10.1186/1471-2334-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. 1992. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 36:6–9. doi: 10.1128/AAC.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37:3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamers RP, Eade CR, Waring AJ, Cole AL, Cole AM. 2011. Characterization of the retrocyclin analogue RC-101 as a preventative of Staphylococcus aureus nasal colonization. Antimicrob Agents Chemother 55:5338–5346. doi: 10.1128/AAC.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]