ABSTRACT
Giardiasis is one of the most common human intestinal diseases worldwide. Several experimental animal models have been used to evaluate Giardia infections, with gerbils (Meriones unguiculatus) being the most valuable model due to their high susceptibility to Giardia infection, abundant shedding of cysts, and pathophysiological alterations and signs of disease similar to those observed in humans. Here, we report cytokine and antibody profiles both during the course of Giardia infection in gerbils and after immunization with a novel oral vaccine comprising a mixture of purified variant-specific surface proteins (VSPs). Transcript levels of representative cytokines of different immune profiles as well as macro- and microtissue alterations were assessed in Peyer's patches, mesenteric lymph nodes, and spleens. During infection, cytokine responses showed a biphasic profile: an early induction of Th1 (gamma interferon [IFN-γ], interleukin-1β [IL-1β], IL-6, and tumor necrosis factor [TNF]), Th17 (IL-17), and Th2 (IL-4) cytokines, together with intestinal alterations typical of inflammation, followed by a shift toward a predominant Th2 (IL-5) response, likely associated with a counterregulatory mechanism. Conversely, immunization with an oral vaccine comprising the entire repertoire of VSPs specifically showed high levels of IL-17, IL-6, IL-4, and IL-5, without obvious signs of inflammation. Both immunized and infected animals developed local (intestinal secretory IgA [S-IgA]) and systemic (serum IgG) humoral immune responses against VSPs; however, only infected animals showed evident signs of giardiasis. This is the first comprehensive report of cytokine expression and anti-Giardia antibody production during infection and VSP vaccination in gerbils, a reliable model of the human disease.
KEYWORDS: giardiasis, animal models, cytokines, immunization
INTRODUCTION
Giardia lamblia infection is one of the most common intestinal diseases worldwide (1). Giardia has a simple life cycle consisting of infective cysts and vegetative trophozoites. Infection is transmitted by ingestion of cysts, which are passed in the feces. Trophozoites are responsible for the clinical manifestations associated with the disease, which vary from asymptomatic infections to acute or chronic diarrhea. Symptoms in humans typically occur 2 weeks after infection and are usually mild, such as cramps and mild chronic diarrhea, even though severe complications associated with malabsorption are also frequent (2). The mechanisms by which G. lamblia causes disease are poorly understood. The parasite is suspected to be noninvasive, and little or no mucosal inflammation has been reported during acute infection in humans (3). Disease severity may depend on multiple parasite- and host-related factors (4). In some cases, particular variant-specific surface proteins (VSPs) have been suggested to be involved in pathogenicity, but antigenic variation is, undoubtedly, responsible for the chronicity of Giardia infections (5). Nevertheless, the stimulus causing VSP switching remains elusive.
Although Giardia species inhabit the intestinal tracts of almost all classes of vertebrates, G. lamblia is the only recognized species found in humans and most other mammals (1). Several experimental models have been used to evaluate the clinical signs and the pathology of giardiasis as well as the course of antigenic variation during Giardia infections (6). Mice are not infected with Giardia assemblage A (represented by the human WB isolate) but can be naturally infected with Giardia muris. In contrast to most human isolates of G. lamblia, G. muris and most isolates from the other assemblages do not cause infection in humans and cannot proliferate in culture. Moreover, the murine model of giardiasis does not reproduce all the symptoms of the disease found in humans (7). Conversely, Meriones unguiculatus rodents (gerbils) are one of the most useful animal models since they present high susceptibility to infections by oral inoculation of either cysts or trophozoites, abundant cyst elimination in the feces of infected animals, and pathophysiological alterations similar to those observed in humans (6, 8, 9). In this animal model, infections cause disease symptoms, including diarrhea and loss of weight (10, 11), making it a suitable model for the study of the course of Giardia infections. However, the immune response of these rodents to Giardia infections is poorly known due to the lack of genetically modified animals and gerbil-specific immunological tools (8, 9). Giardia undergoes surface antigenic variation, a mechanism by which parasitic microorganisms can evade the host's immune response (12). Antigenic variation in Giardia involves variant-specific surface proteins (VSPs). VSPs are cysteine-rich integral membrane proteins that form a dense coat on the parasite. Of a repertoire of over 200 homolog genes encoded in the parasite genome, only one VSP is expressed on the surface of every single trophozoite at any given moment (12); however, a switch in expression to an antigenically distinct VSP has been reported to occur spontaneously (13, 14).
In a previous work, we reported that a mechanism similar to RNA interference (RNAi) is involved in the control of the expression of surface antigens and that knocking down the expression of key enzymes of the RNAi pathway produced a change from expression of single to multiple VSPs in individual Giardia trophozoites (15). Then, we hypothesized that trophozoites expressing the complete VSP repertoire on their surfaces would confer protection against future infections. Therefore, we performed experiments using altered parasites of the Giardia WB isolate in the gerbil model of giardiasis. Our results showed that the animals initially infected with cells expressing all of the VSPs encoded in their genome are largely protected from infection by Giardia clones expressing a unique VSP on their surface or by cysts obtained from infected individuals. Additionally, immunization with the entire repertoire of VSPs purified from these transgenic cells also provided protection against subsequent infections (5). Similar results to those observed in gerbils were obtained in young and adult dogs and cats (16). However, the gerbil immunological basis of Giardia infection compared to VSP-based vaccine protection has still not been studied.
The aims of this work were to determine cytokine profiles, histopathological and macromorphological alterations of relevant tissues (intestine, spleens [SPL], Peyer's patches [PPs], and mesenteric lymph nodes [MLNs]), and production of anti-VSP-specific antibodies after gerbil infection or vaccination by using new reagents and techniques developed for this animal model.
RESULTS AND DISCUSSION
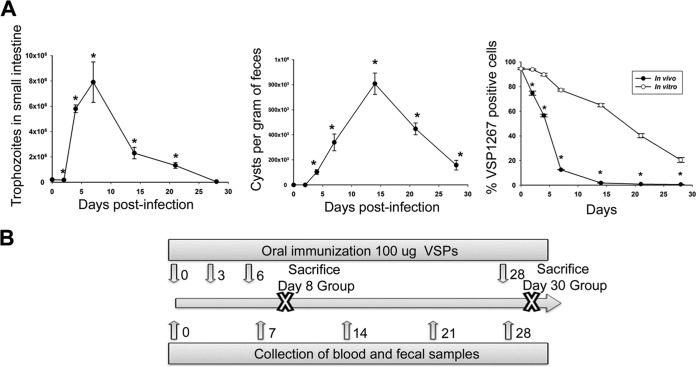
To describe the pathology by which G. lamblia causes disease in our model, the course of the infection in gerbils inoculated with WB1267 trophozoites (parasites from the WB isolate expressing the VSP1267 on their surface) was followed by quantifying trophozoite load in the small intestine and cysts in stool samples. Trophozoites reached their maximum number at 7 days postinfection (dpi) (Fig. 1A), followed by a rapid decrease but persistence up to 28 dpi. The rapid decrease in the number of parasites is very consistent among all gerbil infections reported to date (5, 9, 17) and varies only slightly from decreases reported in mice, dogs, cats, and humans (16, 18, 19), suggesting that the course of Giardia infection is similar in all host species.
FIG 1.
Infection and immunization. (A) Gerbil infection kinetics. Animals were infected intragastrically with 2 × 105 G. lamblia trophozoites resuspended in PBS–5 mM cysteine. Cysts were quantified by immunofluorescence using an anti-CWP2-specific MAb. Trophozoites were counted from small intestine at the indicated time points. Clonal expression of VSP1267 was evaluated by immunofluorescence assays with the MAb 5C1. Each point represents the mean value ± standard error of the mean of the results obtained in three independent experiments (n = 12). *, P < 0.01 compared to results in uninfected animals or to in vitro culture. (B) Immunization scheme. Immunization was performed with VSPs as described in Materials and Methods.
The expression of VSP1267 evaluated in trophozoites recovered from the infected animals showed a marked decrease of the original VSP level at as early as 2 dpi (Fig. 1A), indicating early antigenic switching after infection establishment. The occurrence of in vivo switching during the first stages of infection and prior to the formation of specific antibodies has been previously described (20). However, the reason for this fast switching is not clear. Selection of a VSP, rather than switching, has been suggested for VSPH7 of the assemblage B isolate GS-M in the mouse model of infection (21); however, the large number of trophozoites recovered at 4 dpi indicated that VSP switching is more likely to occur than selection of a different VSP in surviving trophozoites. Several stimuli other than antibodies have been proposed to induce antigenic variation (22, 23). Thus, it is likely that environmental changes (culture versus intestinal conditions) could trigger VSP switching. It is also possible that mechanisms of the innate immune system, such as oxidative stress (reactive nitric and oxygen species) and/or soluble intestinal factors (22, 23) might favor this accelerated change in VSP expression over in vitro switching (Fig. 1A). In contrast to trophozoite load, the kinetics observed in cyst elimination in stool samples showed a peak between 14 and 21 dpi (Fig. 1A), following a pattern previously described for Giardia infections in gerbils (5, 9).
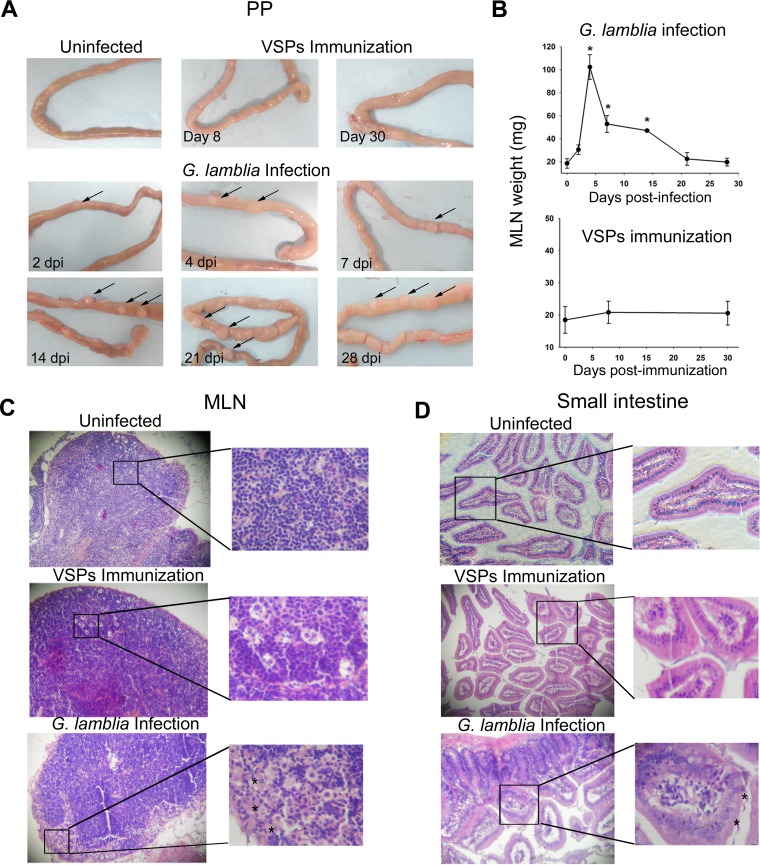
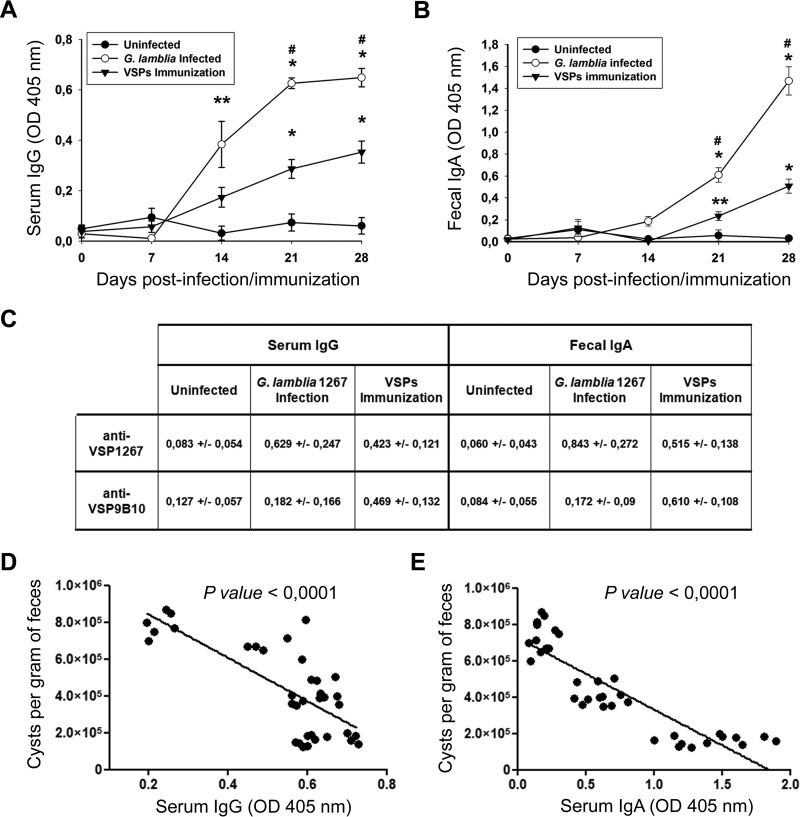
The visual analysis of PPs showed a progressive size increase from 2 dpi, with PPs reaching their largest size at 21 dpi (Fig. 2A). The histopathological analysis of infected animals also showed a clear weight gain of MLNs from 2 dpi, with a peak at 4 dpi; after that point, MLN weight began to decrease until it reached normal levels at 21 dpi (Fig. 2B). Histological sections of MLNs from infected animals (Fig. 2C) showed reactive lymphadenitis with lymphoid hyperplasia and abundant eosinophilic areas enriched in histiocytes. Both lymphoid hyperplasia and histiocyte accumulation occurred at the expense of the three ganglion compartments (cortex, paracortex, and medulla); however, histiocytosis was more marked at the medullar sinus (Fig. 2C). Histopathological analysis of the small intestine revealed marked changes in the intestinal mucosa during Giardia infection, including a generalized inflammatory process with pronounced alterations of the gut architecture, such as flattening of villi, crypts, and goblet cell hyperplasia, inflammatory infiltrate in mucosa and submucosa, and increases in the number of intraepithelial leukocytes and in mucus secretion (Fig. 2D). These intestinal alterations have also been observed by previous reports in different models of giardiasis including in gerbils (11, 17). No morphological alterations were observed at this level in vaccinated or uninfected animals. All infected gerbils were able to develop a systemic humoral immune response, as evidenced by specific IgG, and an intestinal secretory response (secretory IgA [S-IgA]) (Fig. 3). Notably, the development of the humoral response correlated with the decrease in cyst number (Fig. 3D and E), indicating that these systemic and local humoral immune responses could be related to the control of giardiasis. Not only innate defense mechanisms but also both acquired humoral and cell-mediated immune responses protect against Giardia infections (7). In fact, several studies have demonstrated that a variety of immunological factors participate in the control of primary infection by G. lamblia, including CD4+ T cells and their cytokines as well as B cells and their antibodies (24, 25). Previous research has suggested that humoral immunity is important for parasite elimination since hypogammaglobulinemic individuals (7) and mice genetically deficient in functional B cells or specifically lacking IgA-expressing B cells tend to present prolonged infections (26). Accordingly, antibodies produced in the intestinal mucosa may directly act in giardiasis control by binding to surface antigens and, thereby, interfering with their adherence to the intestinal mucosa as well as promoting their immobilization and agglutination (5). In this study, experimental infection by G. lamblia stimulated an intestinal secretory-specific immune response. The increase in the sizes of both PPs and MLNs would indicate an active response of these immune compartments.
FIG 2.
Histopathological analysis. (A) Macroscopic examination of upper small intestines. Arrows indicate an increase in size of the Peyer's patches in the infected versus uninfected gerbils used as controls. (B) Mesenteric lymph node size. The MLN size from experimental gerbils was determined by weighing at the indicated day postinfection. Each point represents the mean value ± standard error of the mean of the results obtained in three independent experiments (n = 12). *, P < 0.05 compared to results in uninfected animals (0 dpi). (C and D) Light microscopy images. Representative images (magnification, ×20) from MLN and upper small intestine from uninfected, VSP-immunized, or infected gerbils are shown. Slides were stained by hematoxylin-eosin. Asterisks indicate some Giardia trophozoites in the intestinal lumen from infected gerbils (7 dpi) and eosinophilic areas enriched with histiocytes in an MLN section (4 dpi).
FIG 3.
Systemic and intestinal humoral immune responses. Blood and stool samples were collected at 0, 7, 14, 21, and 28 days after infection or immunization. Serum IgG (A) and secretory IgA (B) levels were determined by ELISA using a polyclonal mouse anti-gerbil IgG and IgA developed in our laboratory (see Materials and Methods). Plates were sensitized with G. lamblia WB1267 soluble extract. Each point represents the mean value ± standard error of the mean of the results obtained in three independent experiments (n = 12). *, P < 0.01 compared to results in uninfected animals; **, P < 0.05 compared to results in uninfected animals; #, P < 0.01 for results in infected animals versus those in animals immunized with VSPs (Tukey's test between the groups). (C) Levels of specific serum IgG and fecal IgA anti-VSP1267 or anti-VSP9B10. Samples of blood and stool from 28 dpi or immunizations were tested. Plates were sensitized with immunopurified VSP1267 or VSP9B10, respectively. Values are the means ± standard errors of the means (n = 12). (D and E) Linear correlation between cyst numbers and either serum IgG or fecal IgA response. Data from 14, 21, and 28 dpi were included in the analysis.
The evaluation of the vaccine outcome revealed that immunization with VSPs was also able to induce a marked humoral response, showing significant levels of serum IgG and fecal S-IgA against both the whole parasite and individual VSPs (Fig. 3A to C). The persistence of Giardia at the site of infection provoking a constant stimulation of the immune system, the presence of other antigens different from VSPs, and the adjuvant effect of the inflammatory reaction present during the course of infection could be the reasons for the higher antibody levels present in the natural infection than after immunization with the multi-VSP vaccine. In previous works we have shown that both infection with the homologous VSP-expressing Giardia clone (i.e., primary VSP1267 infection and secondary VSP1267 infection) and immunization with VSP and infection with G. lamblia expressing either VSP1267 or VSP9B10 are protective; therefore, although the vaccine humoral responses were lower than responses seen in natural infection, these antibody levels would be sufficient to provide protection. Remarkably, no histopathological changes were observed in the vaccinated animals (Fig. 2A to C), indicating that the inflammatory process may be causing the signs of the disease since oral vaccination with purified VSPs did not induce signs of giardiasis.
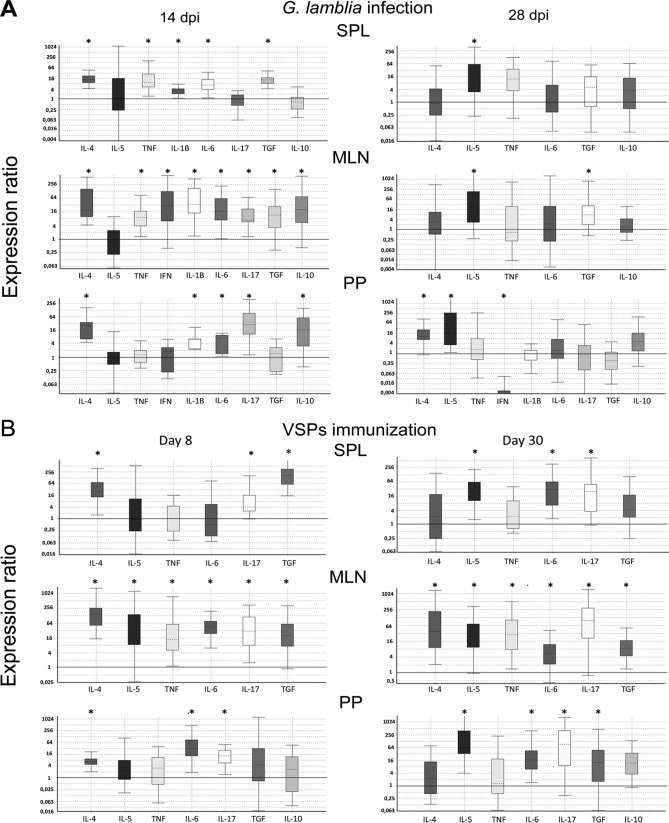
With respect to the mechanisms involved in immune response induction in humans or experimental hosts infected with G. lamblia, previous studies have shown that the parasite and/or its components induce a mixed Th1/Th2 response (24, 27, 28), and, more recently, reports have described a key role of a Th17 response in the control of Giardia infections. Interleukin-17 (IL-17) appears to be essential for the control of these infections in diverse hosts such as mice, cattle, and humans (29–32). In the present work, cytokines that characterize Th1 proinflammatory events (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], IL-1β, and IL-6), Th2 mediator induction (IL-4 and IL-5), Th17 (IL-17), and immune regulation (transforming growth factor β [TGF-β] and IL-10) were chosen to characterize the immune response induced by parasite infection and VSP vaccination. Cytokine mRNA levels were quantified in the SPL, PPs, and MLNs from gerbils at 14 and 28 dpi or at 8 and 30 days after immunization (Fig. 4A). Cytokine profiles showed marked differences during the course of both processes, and only P values of <0.05 (Fig. 4, asterisks) were considered in the analysis.
FIG 4.
Transcript expression of different cytokines in infected or VSP-immunized gerbils. At 14 and 28 days postinfection (A) or at 8 and 30 days postimmunization (B), the animals were sacrificed, and cells were obtained from PPs, MLNs, and SPL. After total RNA extraction (TRIzol) and cDNA synthesis, cytokine transcript levels were determined via quantitative PCR. Data represent the increase rate compared to the levels in uninfected animals. All data were normalized to the corresponding reference gene using the ΔCT method and analyzed by REST software. Only those cytokines with detectable transcript levels are shown in the graphs. A P value of <0.05 (*) was considered significant. Data are representative of three independent experiments with four animals per group.
The infection analysis showed an induction of a mixed Th1/Th2/Th17 response at 14 dpi, including high levels of the specific proinflammatory IL-1β. At 28 dpi, IL-5 mRNA levels were significantly increased in all tissues. In PPs, the IL-5 increase was accompanied by a rise in IL-4 and a decrease in IFN-γ transcripts, whereas in MLNs only TGF-β transcript levels were increased. No significant differences were observed in the other studied cytokines. The increase in IL-4 and IL-5 mRNA levels and a decrease in IFN-γ expression observed at 28 dpi in PPs (Fig. 4A) suggest the development of a classic Th2 response. The higher levels of IL-4 produced by PP cells might have inhibited the development of the Th1 T cell subset (33), causing inhibition of IFN-γ production. This switch from an initial Th1/proinflammatory response to a marked Th2 response in the late infection likely is a regulatory mechanism induced by the parasite to evade the deleterious effects associated with the effector molecules produced during this type of immune response, namely, nitric oxide, among others (34–36). The differences observed in cytokines among the three tissues are likely due to the fact that Giardia trophozoites do not invade the mucosa and to immune response compartmentalization of the peripheral lymphoid organs, especially in PPs and MLNs.
We have previously shown that a vaccine based on a cocktail of Giardia immunogenic VSPs was able to induce a protective immune response in gerbils (5) and domestic animals (16). In this work, we also evaluated the immune response generated by immunization with this protein mix. To assess the possible toxicity of repeated doses, which could induce a local inflammation, and to further assay the vaccine's early immunostimulatory activity, histopathological changes and cytokine levels were measured 2 days after the application of the last vaccine dose (day 8). Furthermore, to characterize the type of response generated by this protective vaccine, a boost with an additional oral administration of the VSP mix was performed 28 days following the immunization protocol, and 2 days later (day 30), different analyses were performed. In agreement with previous findings, immunization with VSPs did not induce any obvious inflammation, nor did it produce signs of the disease (5). PPs and MLNs did not show increased size, and no significant histopathological alterations in the small intestine of vaccinated gerbils were observed (Fig. 2A to C).
With respect to the cytokine profile developed by the vaccine, the early response was characterized by a marked increase in IL-4, IL-6, IL-17, and TG-β, mainly in MLNs and PPs and, to a lesser extent, in the SPL (Fig. 4B). In MLNs, increased levels of IL-5 and TNF-α were also observed. The responses at day 30 maintained high levels of IL-6, IL-17, and TGF-β whereas IL-4 remained high only in MLNs and SPL. Importantly, at this immunization stage, in addition to the high levels of IL-17, a strong increase in IL-5 was observed (Fig. 4B).
The possible protective role of Th1-, Th2- or Th17-type immunity in Giardia infection was not clear. Evidence from different experimental models has been controversial. In humans, for example, it has been shown that Giardia can stimulate the production of IFN-γ from human CD4+ T cells in vitro although these cells do not trigger cytotoxicity or migration (37). A recent study has shown that increased levels of IFN-γ, IL-4, and IL-5 in fecal samples from infected individuals are associated with a prolonged course of G. lamblia infection, suggesting that these cytokines may favor parasite persistence (28). Moreover, increased levels of IFN-γ, IL-4, and IL-5 were also associated with increased length of G. lamblia infections (24). In particular, the participation of IL-6 in the control of Giardia infection in mice has been suggested to be important (38, 39). IL-6 participates in both T and B cell responses and has been involved in the promotion of T cell differentiation toward Th17 cells (40). In our study, increased levels of IL-6 transcripts were observed early during the infection (14 dpi) as well as after immunization (days 8 and 30) (Fig. 4). In MLNs, this increase was accompanied by an increase in TGF-β, a cytokine also involved in Th17 development. In fact, very high levels of IL-17 were found in the immunized animals, particularly at day 30, when 80- and 100-fold increases in IL-17 in PPs and MLNs, respectively, were reached (Fig. 4B). Moreover, a significant increase in IL-17 levels during Giardia infection, particularly at 14 dpi, was observed in PPs and MLNs (Fig. 4A). Accordingly, although several studies have described an important role of IL-17 in different models of giardiasis in recent years (41), this is the first report that describes this immune response in gerbil infections. In addition, a Th17 response to immunization with VSPs, consisting of an increase in IL-6, TGF-β, and IL-17, shows the importance of these proteins as relevant protective immunogens of Giardia.
The influence of Th2-mediated responses on mucosal IgA immunoregulation is also well known. For instance, IL-5 promotes switching to the IgA isotype (7), an antibody that is important in eradicating both G. muris and G. lamblia infection (26). In this case, the high levels of IL-4 and IL-5 present in PP cells would be in agreement with the S-IgA detected in fecal samples. The pronounced Th2-type profile observed in PPs at 14 and 28 dpi was also observed in gerbils (9), which showed the specific increase of serum IgG1 and IgG2, as well as in G. muris-infected mice (42). Moreover, it has been demonstrated that TGF-β induces an IgA class switch on B lymphocytes (43), suggesting that the increase in mRNA levels of this cytokine observed both during infection and after immunization with VSPs could contribute to the production of intestinal S-IgA. The protective role of this cytokine has also been reported in humans. For instance, Taherkhani et al. (44) determined the association between TGF-β1 polymorphism and susceptibility to giardiasis, showing that individuals with symptomatic disease have lower levels of S-IgA than asymptomatic and control groups. In addition, the frequency of the TGF-β1 polymorphisms was significantly higher in symptomatic patients than in asymptomatic ones. TGF-β has also been identified as a critical cytokine for commitment to Th17 development, upregulating IL-23R expression (45). Reports have shown that Giardia antigens trigger a Th2-mediated response (44), which might not provide protection and may also even be harmful. Therefore, the relative success of Giardia in completing its life cycle during a primary infection might also be due to the capacity of the parasite to trigger a Th2-type response.
A strong Th1 response is also important in reducing Giardia trophozoite load and fecal cyst counts (34, 35). It appears that the association of Giardia with macrophages elicits mainly an oxidative response, with nitric oxide having an important role (36). In the context of a Th1 response, a protective role has also been described for TNF-α. Accordingly, a significant increase in this cytokine was observed in MLNs and SPL at 14 dpi, as well as in MLNs of VSP-immunized animals at 8 and 30 dpi (Fig. 4).
In conclusion, the VSP-based vaccine was able to induce high levels of IL-17, IL-6, IL-4, and IL-5, stimulating the production of intestinal S-IgA and serum IgG, without obvious signs of inflammation.
Here, we also describe a very extensive cytokine profile in the gerbil model of giardiasis for the first time. In recent years, the induction of a Th17 immune response in mouse models has been strongly associated with protection against giardiasis. Here, in a more valuable model we have demonstrated that a strong IL-17 response can be detected during infection and VSP-based vaccination. Although the gerbil model presents high similarities to human giardiasis, the species has been little explored due to the previous lack of immunological tools. The results presented in this study greatly contribute to the understanding of the immune response in this suitable experimental model of giardiasis and provide conclusive explanations of the involvement of the immune system during both symptomatic infections and protective vaccination.
MATERIALS AND METHODS
Ethics.
All procedures performed on animals were conducted according to protocols approved by the Institutional Committee for Care and Use of Experimental Animals. These protocols adhere to the U.S. Public Health Service (PHS) guidelines for animal research. No animals were harmed during the collection of blood and fecal samples.
Parasites.
Giardia lamblia WB (ATCC 50803) was cultured in TYI-S-33 medium. Clones expressing VSP1267 were obtained by limiting dilution in 96-well culture plates and selected by immunofluorescence with a specific monoclonal antibody (MAb), 5C1. Reactive clones were then expanded overnight in culture medium and tested for homogeneity before use.
Vaccine preparation.
The generation of transgenic trophozoites expressing the whole repertoire of VSPs has been previously reported (15). Briefly, the complementary sequences of the gene encoding Giardia Dicer were cloned into plasmid pTubHA.pac. Antisense fragments were amplified by PCR from genomic DNA (gDNA) of clone WB/9B10 and then cloned into the vector. Giardia was transfected by electroporation, and transgenic trophozoites were selected with puromycin. Silencing of Dicer was verified by quantitative reverse transcription-PCR (qRT-PCR), and expression of multiple VSPs was determined by immunofluorescence assays using a panel of MAbs directed to different VSPs (5, 15). The entire repertoire of VSPs expressed in transgenic trophozoites was then purified by immuno-affinity using MAb 12F1 generated against the conserved 5-amino-acid tail of VSPs (5, 15). Animals were immunized by three oral administrations of 100 μg of VSPs every 3 days (5). The animals from the day 30 group received a booster dose 2 days before sacrifice.
Gerbils.
Specific-pathogen-free, 6-week-old outbred gerbil (Meriones unguiculatus) males and females (Animal Facility, Catholic University of Cordoba) were used; they were housed in air-conditioned (18 to 22°C, 40 to 50% humidity) racks with a 12-h light-dark cycle. Gerbils were fed ad libitum with autoclaved food and sterile water supplemented with a mixture of filter-sterilized vitamin solution. Before infection, gerbils were tested for the presence of serum antibodies against Giardia antigens by enzyme-linked immunosorbent assay (ELISA) and for Giardia cysts in stool samples by light and immunofluorescence microscopy using cyst-specific MAb 7D6. In both infection and immunization experiments, four animals per group were used, and three independent assays were performed.
Infections.
Gerbil infection was induced by orogastric inoculation of 2 × 105 trophozoites resuspended in 0.5 ml of 1× phosphate-buffered saline (PBS) (5). Control gerbils received 0.5 ml of PBS by the same route. Feces were collected weekly from week 0 to week 4. Cysts were identified visually by light microscopy and immunofluorescence assays with MAb 7D6. Giardia cysts excreted by gerbils were quantified by collecting stool pellets from individually housed gerbils over a 24-h period. The stool samples were weighed, resuspended in 2 ml of PBS, and filtered. The filtrate was centrifuged at 250 × g for 10 min. After three washes, the pellet was suspended in 2 ml of PBS, and cysts were stained and counted in a hemacytometer (5). Gerbils were considered uninfected if no cysts were found in the feces.
Trophozoite recovery.
At the indicated times postinfection (Fig. 1A), the gerbils were euthanized, and the first portion of small intestine (10 cm) was removed and placed in TSY-S-33 medium on ice. The intestine pieces were minced after 30 min on ice, and parasites were counted with a hemacytometer. The percentage of trophozoites expressing VSP1267 was determined by immunofluorescence assays using the specific MAb 5C1.
Blood samples.
Blood samples from gerbils were collected weekly to detect the presence of circulating gerbil antibodies to Giardia antigens.
Fecal extracts.
Fecal samples were obtained from each animal on the same day of blood sample collection. Samples of feces (0.1 g) were placed in polystyrene microtubes containing 0.2 ml of PBS, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktail tablets (Complete, EDTA-free; Roche Diagnostics). After incubation at 4°C for 1 h, the tubes were centrifuged (10,000 × g, 4°C, 10 min) to remove insoluble materials. The supernatant (fecal extract) was collected and stored at −70°C until use (46).
Total protein extraction from G. lamblia trophozoites.
Trophozoites were washed twice with PBS and resuspended in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (Complete, EDTA-free; Roche Diagnostics). After incubation at 4°C for 1 h, samples were centrifuged at 10,000 × g at 4°C for 10 min, and the supernatant was collected. Total protein concentration was determined by the bicinchoninic assay (BCA) method (Pierce BCA protein assay kit; Thermo Fisher Scientific).
Immunoenzymatic assay (ELISA).
An ELISA was performed to determine the levels of IgG and IgA antibodies in serum and fecal samples, respectively, from infected and immunized gerbils. Mouse anti-gerbil IgG and IgA antibodies were developed in our laboratory (see below). Microtiter plates (96-well; Greiner Bio-One, Germany) were coated with G. lamblia WB1267 soluble extract (5 μg/ml) in carbonate buffer (50 mM, pH 9) and incubated overnight at 4°C. Plates were washed twice with PBS and blocked with PBS plus 10% bovine serum albumin (PBS-BSA) at room temperature (RT) for 1 h. Serum samples were diluted 1:2 in PBS-BSA and incubated at RT for 1 h. Positive- and negative-control sera were included in each plate for all assays. After plates were washed, the anti-gerbil IgG and anti-gerbil IgA polyclonal antibodies were added (1:500 in PBS–1% BSA) and incubated at RT for 1 h. Finally, an alkaline phosphatase-labeled secondary antibody was used (1:2,000 in PBS–1% BSA). After 1 h at RT, plates were washed, and the reaction product was revealed with 1 mg/ml p-nitrophenyl phosphate (Sigma Chemical Co., USA) diluted in glycine buffer (0.1 M, pH 10.4). The reaction was stopped with 50 μl of 3 M NaOH. Optical density (OD) was measured using a microplate reader at 405 nm (Multiskan; Thermo Scientific). For determination of anti-VSP1267 and anti-VSP9B10 humoral responses, the ELISA plates were coated with the corresponding immunopurified proteins using the specific MAbs 5C1 to VSP1267 and anti-9B10 to VSP9B10.
Production of mouse anti-gerbil IgG and IgA polyclonal antibodies.
Mouse anti-gerbil IgG and IgA antibodies were developed by immunization of BALB/c mice with gamma and alpha heavy chains, respectively. First, total Igs were purified from normal gerbil serum by affinity chromatography using a thiophilic adsorption kit (Pierce). After SDS-PAGE, the two specific heavy chains were successfully traced using commercial antibodies, anti-human alpha chain and gamma chain (Sigma-Aldrich) (which cross-reacted with gerbil Igs), and purified from the polyacrylamide gel. The immunization protocol consisted of four weekly doses with 50 μg of gerbil alpha or gamma chain; the former dose was emulsified with complete Freud adjuvant, and the latter was emulsified with incomplete Freud adjuvant. Ten days after the last immunization, mice were bled, and the polyclonal serum was stored for later use.
RNA extraction and cDNA synthesis.
Total RNA was extracted from gerbil spleens (SPL), Peyer's patches (PPs), and mesenteric lymph nodes (MLNs) using TRIzol reagent (Thermo Fisher Scientific), according to manufacturer's instructions. After DNase I (Roche) treatment (2 h at 37°C), total RNA was spectrophotometrically quantified, and several PCRs were performed to check for the presence of genomic DNA. When no DNA was detected, cDNA synthesis was performed using Superscript IV reverse transcriptase (Invitrogen), according to the manufacturer's instructions (20-μl final volume), and stored at −70°C for subsequent quantitative PCRs (qPCRs).
Real-time RT-PCR.
cDNA (8 μl) was used in a final volume of 10 μl; a triplicate for each gene was performed. Each PCR cycle consisted of an initial incubation at 95°C for 15 min, a denaturation step (94°C, 1 min), an annealing step (58°C, 30 s), and an elongation step (72°C, 30 s). There were a total of 40 cycles and a final incubation at 72°C for 10 min (additional extension step). The melting curve was performed from 50°C to 90°C, with readings taken every 1°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene. All data were normalized to the corresponding reference gene transcription level using the comparative ΔCT (where CT is threshold cycle) method and analyzed using REST 2009 software (Relative Expression Software Tool, version 2.0.13; Qiagen). The primers used in the reaction mixtures are described in Table 1 (47–50).
TABLE 1.
Oligonucleotide sequences used in qPCR analysis
| Primer target | Directiona | Primer sequence | Product size (bp) | Reference |
|---|---|---|---|---|
| IL-5 | Fw | 5′-ATTCTAACTCTCGCCTGGGTCTGG-3′ | 315 | 47 |
| Rv | 5′-GAACTGCCGTGCTCTCCGTCTC-3′ | |||
| IL-4 | Fw | 5′-CAGGGTGCTCCGCAAATTT-3′ | 67 | 48 |
| Rv | 5′-GACCCCGGAGTTGTTCTTCA-3′ | |||
| IL-6 | Fw | 5′-AGGATCCAGGTCAAATAGTCTTTCC-3′ | 77 | 48 |
| Rv | 5′-TTCCGTCTGTGACTCCAGTTTCT-3′ | |||
| GAPDH | Fw | 5′-CATGGCCTTCCGAGTTCCT-3′ | 60 | 47 |
| Rv | 5′-TTCTGCAGTCGGCATGTCA-3′ | |||
| IL-17 | Fw | 5′-AGCTCCAGAGGCCCTCGGAC-3′ | 236 | 49 |
| Rv | 5′-AGGACCAGGATCTCTTGCTG-3′ | |||
| TNF-α | Fw | 5′-GCTCCCCCAGAAGTCGGCG-3′ | 254 | 49 |
| Rv | 5′-CTTGGTGGTTGGGTACGACA-3′ | |||
| IL-1β | Fw | 5′-GGCAGGTGGTATCGATVATC-3′ | 494 | 49 |
| Rv | 5′-CACCTTGGATTTGACTTCTA-3′ | |||
| IL-10 | Fw | 5′-CATGGGTCTTGGGAAGAGAA-3′ | 196 | 50 |
| Rv | 5′-CCATTCCCAAAGGAATTGAA-3′ | |||
| TGFβ | Fw | 5′-GCTACCACGCCAACTTCTGT-3′ | 197 | 50 |
| Rv | 5′-GTTGGACAACTGCTCCACCT-3′ | |||
| IFNγ | Fw | 5′-CCATGAACGCTACACACTGCATC-3′ | 224 | 50 |
| Rv | 5′-GAAGTAGAAAGAGACAATCTGG-3′ |
Fw, forward; Rv, reverse.
Histological studies.
At the indicated times postinfection or postimmunization (Fig. 2), gerbils were euthanized, and the MLNs and the first portion of the small intestine (10 cm) were removed and fixed by immersion in 4% paraformaldehyde. Fixed tissues were dehydrated, embedded in paraffin, cut, and stained with hematoxylin-eosin for morphological analysis.
ACKNOWLEDGMENTS
This work was supported by grants from FONCYT (PICT-E 0234 and PICT-2116), CONICET (D4408), and UCC (80020150200144CC) of Argentina and from a Georg Forster Award of the Alexander von Humboldt Foundation, Germany, to H.D.L.
REFERENCES
- 1.Adam RD. 2001. Biology of Giardia lamblia. Clin Microbiol Rev 14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buret AG. 2008. Pathophysiology of enteric infections with Giardia duodenalius. Parasite 15:261–265. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- 3.Gottstein B, Stocks NI, Shearer GM, Nash TE. 1991. Human cellular immune response to Giardia lamblia. Infection 19:421–426. doi: 10.1007/BF01726454. [DOI] [PubMed] [Google Scholar]
- 4.Roxstrom-Lindquist K, Palm D, Reiner D, Ringqvist E, Svard SG. 2006. Giardia immunity—an update. Trends Parasitol 22:26–31. doi: 10.1016/j.pt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, Lujan HD. 2010. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat Med 16:551–557. doi: 10.1038/nm.2141. [DOI] [PubMed] [Google Scholar]
- 6.Belosevic M, Faubert GM, MacLean JD, Law C, Croll NA. 1983. Giardia lamblia infections in Mongolian gerbils: an animal model. J Infect Dis 147:222–226. doi: 10.1093/infdis/147.2.222. [DOI] [PubMed] [Google Scholar]
- 7.Faubert G. 2000. Immune response to Giardia duodenalis. Clin Microbiol Rev 13:35–54. doi: 10.1128/CMR.13.1.35-54.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arguello-Garcia R, Ortega-Pierres MG. 1997. Giardia duodenalis: analysis of humoral immune response in experimentally infected gerbils (Meriones unguiculatus). Arch Med Res 28:171–178. [PubMed] [Google Scholar]
- 9.Amorim RM, Silva DA, Taketomi EA, Morato MG, Mundim MJ, Ribeiro DP, Oliveira TC, Viana JC, Gomes MA, Cury MC. 2010. Giardia duodenalis: kinetics of cyst elimination and the systemic humoral and intestinal secretory immune responses in gerbils (Meriones unguiculatus) experimentally infected. Exp Parasitol 125:297–303. doi: 10.1016/j.exppara.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Deselliers LP, Tan DT, Scott RB, Olson ME. 1997. Effects of Giardia lamblia infection on gastrointestinal transit and contractility in Mongolian gerbils. Dig Dis Sci 42:2411–2419. doi: 10.1023/A:1018879621272. [DOI] [PubMed] [Google Scholar]
- 11.Buret A, Hardin JA, Olson ME, Gall DG. 1992. Pathophysiology of small intestinal malabsorption in gerbils infected with Giardia lamblia. Gastroenterology 103:506–513. doi: 10.1016/0016-5085(92)90840-U. [DOI] [PubMed] [Google Scholar]
- 12.Nash TE. 1997. Antigenic variation in Giardia lamblia and the host's immune response. Philos Trans R Soc Lond B Biol Sci 352:1369–1375. doi: 10.1098/rstb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash TE. 2002. Surface antigenic variation in Giardia lamblia. Mol Microbiol 45:585–590. doi: 10.1046/j.1365-2958.2002.03029.x. [DOI] [PubMed] [Google Scholar]
- 14.Nash TE, Banks SM, Alling DW, Merritt JW Jr, Conrad JT. 1990. Frequency of variant antigens in Giardia lamblia. Exp Parasitol 71:415–421. doi: 10.1016/0014-4894(90)90067-M. [DOI] [PubMed] [Google Scholar]
- 15.Prucca CG, Slavin I, Quiroga R, Elias EV, Rivero FD, Saura A, Carranza PG, Lujan HD. 2008. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456:750–754. doi: 10.1038/nature07585. [DOI] [PubMed] [Google Scholar]
- 16.Serradell MC, Saura A, Rupil LL, Gargantini PR, Faya MI, Furlan PJ, Lujan HD. 2016. Vaccination of domestic animals with a novel oral vaccine prevents Giardia infections, alleviates signs of giardiasis and reduces transmission to humans. NPJ Vaccines 1:16018. doi: 10.1038/npjvaccines.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araujo NS, Mundim MJ, Gomes MA, Amorim RM, Viana JC, Queiroz RP, Rossi MA, Cury MC. 2008. Giardia duodenalis: pathological alterations in gerbils, Meriones unguiculatus, infected with different dosages of trophozoites. Exp Parasitol 118:449–457. doi: 10.1016/j.exppara.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Hewlett EL, Andrews JS Jr, Ruffier J, Schaefer FW III. 1982. Experimental infection of mongrel dogs with Giardia lamblia cysts and cultured trophozoites. J Infect Dis 145:89–93. doi: 10.1093/infdis/145.1.89. [DOI] [PubMed] [Google Scholar]
- 19.Byrd LG, Conrad JT, Nash TE. 1994. Giardia lamblia infections in adult mice. Infect Immun 62:3583–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal A, Nash TE. 1988. Antigenic variation of Giardia lamblia in vivo. Infect Immun 56:1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash TE, Aggarwal A, Adam RD, Conrad JT, Merritt JW Jr. 1988. Antigenic variation in Giardia lamblia. J Immunol 141:636–641. [PubMed] [Google Scholar]
- 22.Emery SJ, Mirzaei M, Vuong D, Pascovici D, Chick JM, Lacey E, Haynes PA. 2016. Induction of virulence factors in Giardia duodenalis independent of host attachment. Sci Rep 6:20765. doi: 10.1038/srep20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gargantini PR, Serradell Mdel C, Rios DN, Tenaglia AH, Lujan HD. 2016. Antigenic variation in the intestinal parasite Giardia lamblia. Curr Opin Microbiol 32:52–58. doi: 10.1016/j.mib.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Wahid A, Faubert G. 2008. Characterization of the local immune response to cyst antigens during the acute and elimination phases of primary murine giardiasis. Int J Parasitol 38:691–703. doi: 10.1016/j.ijpara.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Singer SM, Nash TE. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun 68:170–175. doi: 10.1128/IAI.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford TD, Housley MP, Boes M, Chen J, Kagnoff MF, Gillin FD, Eckmann L. 2002. Central importance of immunoglobulin A in host defense against Giardia spp. Infect Immun 70:11–18. doi: 10.1128/IAI.70.1.11-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagel I, Cabrera M, Puccio F, Santaella C, Buvat E, Infante B, Zabala M, Cordero R, Di Prisco MC. 2011. Co-infection with Ascaris lumbricoides modulates protective immune responses against Giardia duodenalis in school Venezuelan rural children. Acta Trop 117:189–195. doi: 10.1016/j.actatropica.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Long KZ, Rosado JL, Santos JI, Haas M, Al Mamun A, DuPont HL, Nanthakumar NN, Estrada-Garcia T. 2010. Associations between mucosal innate and adaptive immune responses and resolution of diarrheal pathogen infections. Infect Immun 78:1221–1228. doi: 10.1128/IAI.00767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dann SM, Manthey CF, Le C, Miyamoto Y, Gima L, Abrahim A, Cao AT, Hanson EM, Kolls JK, Raz E, Cong Y, Eckmann L. 2015. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp Parasitol 156:68–78. doi: 10.1016/j.exppara.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grit GH, Van Coppernolle S, Devriendt B, Geurden T, Dreesen L, Hope J, Vercruysse J, Cox E, Geldhof P, Claerebout E. 2014. Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet Parasitol 202:145–155. doi: 10.1016/j.vetpar.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Saghaug CS, Sornes S, Peirasmaki D, Svard S, Langeland N, Hanevik K. 2016. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin Vaccine Immunol 23:11–18. doi: 10.1128/CVI.00419-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreesen L, De Bosscher K, Grit G, Staels B, Lubberts E, Bauge E, Geldhof P. 2014. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect Immun 82:3333–3340. doi: 10.1128/IAI.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. 1996. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol 70:7103–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatesan P, Finch RG, Wakelin D. 1996. Comparison of antibody and cytokine responses to primary Giardia muris infection in H-2 congenic strains of mice. Infect Immun 64:4525–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatesan P, Finch RG, Wakelin D. 1997. A comparison of mucosal inflammatory responses to Giardia muris in resistant B10 and susceptible BALB/c mice. Parasite Immunol 19:137–143. doi: 10.1046/j.1365-3024.1997.d01-189.x. [DOI] [PubMed] [Google Scholar]
- 36.Pavanelli WR, Gutierrez FR, Silva JJ, Costa IC, Menezes MC, Oliveira FJ, Itano EN, Watanabe MA. 2010. The effects of nitric oxide on the immune response during giardiasis. Braz J Infect Dis 14:606–612. doi: 10.1016/S1413-8670(10)70119-7. [DOI] [PubMed] [Google Scholar]
- 37.Ebert EC. 1999. Giardia induces proliferation and interferon gamma production by intestinal lymphocytes. Gut 44:342–346. doi: 10.1136/gut.44.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou P, Li E, Zhu N, Robertson J, Nash T, Singer SM. 2003. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infect Immun 71:1566–1568. doi: 10.1128/IAI.71.3.1566-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamda JD, Nash TE, Singer SM. 2012. Giardia duodenalis: dendritic cell defects in IL-6 deficient mice contribute to susceptibility to intestinal infection. Exp Parasitol 130:288–291. doi: 10.1016/j.exppara.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura A, Naka T, Kishimoto T. 2007. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A 104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer SM. 2016. Control of giardiasis by interleukin-17 in humans and mice—are the questions all answered? Clin Vaccine Immunol 23:2–5. doi: 10.1128/CVI.00648-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djamiatun K, Faubert GM. 1998. Exogenous cytokines released by spleen and Peyer's patch cells removed from mice infected with Giardia muris. Parasite Immunol 20:27–36. doi: 10.1046/j.1365-3024.1998.t01-1-00122.x. [DOI] [PubMed] [Google Scholar]
- 43.Stavnezer J, Kang J. 2009. The surprising discovery that TGF beta specifically induces the IgA class switch. J Immunol 182:5–7. doi: 10.4049/jimmunol.182.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Taherkhani H, Hajilooi M, Fallah M, Khyabanchi O, Haidari M. 2009. Gene polymorphism in transforming growth factor-beta codon 10 is associated with susceptibility to giardiasis. Int J Immunogenet 36:345–349. doi: 10.1111/j.1744-313X.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 45.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 46.Zitomersky NL, Coyne MJ, Comstock LE. 2011. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect Immun 79:2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, Reddy MV, Kaliraj P. 2007. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol 116:483–491. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaoka Y, Yamauchi K, Ota H, Sugiyama A, Ishizone S, Graham DY, Maruta F, Murakami M, Katsuyama T. 2005. Natural history of gastric mucosal cytokine expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect Immun 73:2205–2212. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. 2009. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci 100:2152–2159. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. 2004. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol 202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]