Abstract
Please cite this paper as: Dixon et al. (2010) Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory‐confirmed influenza in hospitalised children aged 6–59 months. Influenza and Other Respiratory Viruses 4(4), 231–234.
Background Influenza is major cause of paediatric hospitalisation. Influenza vaccine was offered to all children aged 6–59 months resident in Western Australia in 2008, and we wished to evaluate the effectiveness of this immunisation programme.
Objectives To assess the practicalities of a nested matched case–control design to estimate the protective effect of inactivated influenza vaccination in hospitalised children aged 6–59 months.
Methods Cases were hospitalised children with laboratory‐confirmed influenza, while matched controls were recruited from children admitted for an acute non‐respiratory illness. We estimated influenza vaccine effectiveness (VE) against influenza as 1 – the adjusted odds ratio from multivariate logistic regression.
Results The 2008 influenza season was characterised by a late peak and a predominance of influenza virus B. We recruited 26 hospitalised patients with laboratory‐confirmed influenza and 50 matched controls. The proportion of cases who were fully vaccinated was 7% versus 30% of controls giving an adjusted VE of 83% (95% CI −54 to 98).
Conclusions Recruiting sufficient controls was problematic and in the future, we will select controls hospitalised for an influenza‐like‐illness but influenza negative by laboratory PCR testing. The VE estimate was high but non‐significant, reflecting the low number of cases.
Keywords: children, hospitalisation, influenza, vaccine effectiveness
Introduction
Influenza is a significant cause of paediatric morbidity and mortality. Between 2002 and 2005, the annual rate of hospitalisations coded as influenza among Australian children aged 0–4 years was 82·1/100,000, higher than any other age group. 1 In Western Australia (WA) using population‐based data linkage for the period 1990–2000, we reported an annual influenza hospitalisation rate of 160/100 000 for non‐Aboriginal children and 640/100 000 for Aboriginal children aged <2 years. 2
A systematic review has shown influenza vaccine effectiveness (VE) was 59% (95% CI 41–71%) in preventing confirmed influenza in healthy children aged 2–16 years, 3 but there is a paucity of data of VE in children under of 2 years of age. Reflecting the burden of influenza and the accepted protective effect of vaccination, the Advisory Committee on Immunisation Practices in the United States of America recommended the use of influenza vaccine in children aged 6–23 months in 2002 4 and expanded the recommendation to those aged 6–59 months in late 2006. 5
In response to a cluster of childhood deaths related to influenza virus A infection in WA in 2007 6 and the burden of influenza in young children, influenza vaccine was provided free of charge for all WA children aged 6–59 months in 2008. We commenced the Western Australian Influenza Vaccine Effectiveness (WAIVE) study in 2008 to determine whether there is a direct protective effect of inactivated influenza vaccine in young children.
The WAIVE study is a series of observational studies measuring VE in three different patient settings: general practice, the emergency department and hospital inpatients. Here, we report on a nested case–control study using prospective incidence density case sampling and matched control sampling for hospital inpatients where controls were hospitalised for a non‐respiratory diagnosis. We discuss the methodological issues of conducting such a study and report interim VE point estimates following the first year of the programme.
Methods
We estimated influenza VE against laboratory‐confirmed influenza in children aged 6–59 months admitted to five metropolitan Perth hospitals. We defined all children admitted to hospital with laboratory‐confirmed influenza as cases and selected children admitted to hospital for an acute non‐respiratory illness during the influenza season as controls. Controls were matched for age (6–12 months, 1–2 years, 3–4 years and 4 to <5 years) and Aboriginal/Torres Strait Islander status. Cases admitted with laboratory‐confirmed influenza were identified from hospital admission lists, laboratory reports and from notifications to the Communicable Disease Control Directorate of the WA Department of Health. Screening for cases occurred at Princess Margaret Hospital for Children, WA’s tertiary paediatric hospital and five peripheral hospitals within metropolitan Perth. It is a routine procedure for all paediatric inpatients presenting with a respiratory and/or febrile illness to have a per‐nasal aspirate for virus identification testing.
Potential controls with a non‐respiratory illness were identified from hospital admission lists. For each case, we aimed at recruiting two controls admitted to hospital within a week of the case’s admission. Following informed consent, parents were given a questionnaire to complete, which included their child’s demographic data, influenza vaccinations received in 2008 and previous years and any underlying chronic illnesses. Vaccine status was validated for 87% of all participants with the child’s vaccine provider or via the Australian Childhood Immunisation Register. Children were excluded if they had a known contraindication to influenza vaccine, 7 a known immunodeficiency disorder (including human immunodeficiency virus (HIV) infection), current or recent immunosuppressive treatment or administration of immunoglobulins in the previous 3 months.
CSL Biotherapies provided Fluvax™ and Sanofi Pasteur Ltd provided Vaxigrip™, both trivalent inactivated influenza vaccines (TIV), formulated as 0·5‐ml pre‐filled syringes containing 15‐μg haemagglutinin of each of the three recommended vaccine strains for children ≥ aged 3 years to ≤5 years. Fluvax Junior™ and Vaxigrip Junior™, both formulated as 0·25‐ml pre‐filled syringes containing 7·5‐μg haemagglutinin of each of the vaccine strains, were provided for children ≥ aged 6 months to <3 years. Children who received other licensed influenza vaccines were eligible for inclusion in the study.
Cases and controls were defined as fully vaccinated according to the National Health and Medical Research Council Immunisation Handbook 8 if they had received 2 age‐appropriate doses of TIV at least 21 days apart and more than 14 days prior to ILI onset in 2008. Children who had received at least two previous doses of influenza vaccine in any year were also defined as fully vaccinated if they received one dose of the age‐appropriate vaccine in 2008. Children who received one or more doses of vaccine in any previous year but no vaccine in 2008 were counted as unvaccinated.
Per‐nasal aspirates collected from hospital inpatients and were tested for antigens to influenza and other common respiratory viruses using a standard direct immunofluorescence method and were cultured on LLC‐MK2 cells using centrifuge‐enhanced inoculation and detection by monoclonal antibodies. Samples negative for influenza by these tests were then tested by a real‐time polymerase chain reaction (PCR) assay directed at the matrix gene of influenza A or influenza B using previously published primers. 9 Samples that were culture positive were referred to the World Health Organization Collaborating Centre for Influenza Reference and Research in Melbourne, where detailed antigenic characterisation was performed.
Differences in categorical variables were tested by the Chi‐squared test or Fisher’s exact test. Using laboratory‐confirmed influenza as the primary outcome and vaccination status as the primary exposure, adjusted ORs and 95% confidence intervals (CIs) were calculated using multivariate conditional logistic regression models comparing fully vaccinated children with unvaccinated children including, sex, presence of comorbidities (yes/no) and pre‐term birth as covariates. VE was calculated as 1 – OR. Data analysis was conducted in SAS version 9 (SAS Institute Inc., Cary, NC, USA). Ethical approval for the study was obtained from the ethics committees of Princess Margaret Hospital for Children, the Joondalup Health Campus, the South Metropolitan Area Heath Service and the Western Australian Aboriginal Health Information and Ethics Committee.
Results
The influenza season in 2008 commenced at the end of July and continued through mid November and was dominated by influenza virus B with an unusually late peak. Antigenic typing at the World Health Organisation Collaborating Centre identified the circulating strains in WA as A/Solomon Islands/3/2006‐like (H1N1), A/Brisbane/59/2007‐like (H1N1), A/Brisbane/10/2007‐like (H3N2) and a mixture of viruses from the two B lineages, B/Yamagata and B/Victoria. Circulating strains matched the vaccine strains for both influenza A subtypes and for viruses of the B/Yamagata lineage, represented in the vaccine by B/Florida.
Thirty‐five hospitalised children with laboratory‐confirmed influenza were deemed eligible. Of these, 26 children (74%) were included, eight were unable to be contacted and one with a hospital acquired infection was excluded (Figure 1). There were 10 cases of influenza A infection (five H1N1, three H3N2 and two untyped) and 16 cases with influenza B. All but two cases were admitted to Princess Margaret Hospital for Children. We recruited 50 matched controls. Eight children (11%) identified as Aboriginal and/or Torres Strait Islander. Compared with controls, a greater proportion of cases were boys (P = 0·04), but there were no other significant differences between cases and controls for any other covariates (Table 1). The proportion of children in the study who were fully vaccinated was 22%, with a higher proportion of controls (30%) vaccinated compared to cases (7%). The adjusted estimate of VE comparing fully vaccinated with unvaccinated children was 83% (95% CI −54 to 98). The VE estimate was similar prior to adjusting for covariates (VE 87%, 95% CI −11 to 98). All cases of influenza A were unvaccinated, and the two cases of influenza B in fully vaccinated children were caused by strains of the mismatched B/Victoria lineage. One child admitted with laboratory‐confirmed influenza had only 1 dose of TIV and was excluded from the analysis.
Figure 1.
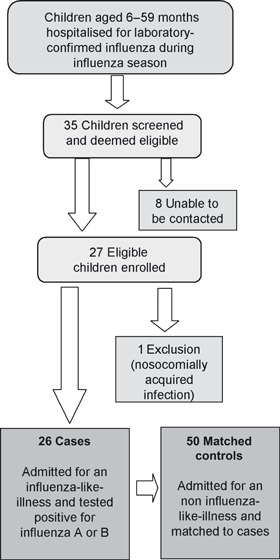
Schematic representation of WAIVE study design for hospitalised patients.
Table 1.
Case and control comparison from 76 hospital inpatients
| Co‐variate | Cases (%) | Controls (%) | P | % fully vaccinated | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≤2 | 12 (46) | 24 (48) | 0·88 | 20 | 0·60 |
| >2 | 14 (54) | 26 (52) | 25 | ||
| Sex | |||||
| Female | 8 (31) | 28 (56) | 0·04 | 25 | 0·60 |
| Male | 18 (69) | 22 (44) | 20 | ||
| ATSI* | |||||
| Yes | 3 (12) | 5 (10) | 0·84 | 13 | 0·48 |
| No | 23 (88) | 45 (90) | 24 | ||
| Gestation (weeks) | |||||
| <38 | 6 (23) | 12 (24) | 0·93 | 11 | 0·19 |
| ≥38 | 20 (77) | 38 (76) | 26 | ||
| Comorbid condition** | |||||
| Present | 3 (12) | 6 (12) | 0·95 | 23 | 0·95 |
| Absent | 23 (88) | 44 (88) | 22 | ||
*Identification as Aboriginal and/or Torres Strait Islander.
**Comorbid conditions included all chronic diseases for which influenza vaccine is recommended in Australia: cardiac disease, asthma, chronic respiratory conditions, chronic neurological disorders or other chronic illnesses requiring regular medical follow‐up or hospitalisation.
Discussion
For the first time in any Australian state, influenza vaccine was recommended and provided free of charge for all children in WA aged 6–59 months in 2008. We estimated the protection from hospitalised laboratory‐confirmed influenza afforded to vaccinated children through a case–control study with controls hospitalised for a non‐respiratory illness. Our interim VE estimate was high, although patient numbers were too low for this estimate to be statistically significant. In this first year of the study, we identified important methodological issues with control selection.
We chose a nested case–control design with controls having no respiratory symptoms. However, matching cases to controls was problematic. We found it difficult to recruit sufficient numbers of matched controls in a timely manner as very few hospitalised children had no respiratory symptoms during the peak of the influenza season. Moreover, young children are admitted for only a few days so that many potential controls had been discharged before they could be recruited, and it was necessary to complete enrolment and data collection by telephone. For future years, we will use an alternative approach to control selection where children admitted to hospital with respiratory symptoms but found to be influenza negative by laboratory testing. Matching of cases and controls will not be undertaken. This will dramatically increase the pool of patients from which controls can be selected. This so‐called test‐negative design has been used elsewhere for community‐based studies of influenza VE 10 , 11 and has recently been modelled by Orenstein and colleagues. 12 As far as we are aware, application of this approach to estimating influenza VE in hospitalised patients would be novel.
We will concentrate on recruiting cases and controls only from Princess Margaret Hospital for Children. Screening for cases in peripheral metropolitan hospitals was labour intensive and only yielded two cases. Despite a low incidence of influenza in hospitalised young children in the first year of the study, we have demonstrated a non‐significant protective effect of vaccination. Improvement to the methodology of control selection should allow us to improve the accuracy of VE estimates in future years.
Author Contributions
All authors contributed to developing WAIVE study protocol. GA Dixon, D Carcione S Williams and P Richmond managed the data collection. D Smith, AD Keil and S Williams were involved in the laboratory testing of samples and interpretation of results. P Jacoby and D Carcione were involved in data analysis. GA Dixon, H Kelly and HC Moore led the writing of the manuscript. All authors revised the manuscript critically and approved the final version for submission.
Acknowledgements
We thank all research assistants who recruited children for this study as well as all the study participants and their parents. We also thank staff of the Microbiology Department of Princess Margaret Hospital for Children, Perth, WA. We acknowledge the invaluable contribution of staff at the Vaccine Trials Group, Telethon Institute of Child Health Research, with particular thanks to Helen Shirley (study co‐ordinator), Larissa Rhind, Jan Adams, and Dr Christine Oosterhuis.
The Western Australian Influenza Vaccine Effectiveness (WAIVE) study team: Revle Bangor‐Jones, Gabriela Dixon, Paul Effler, Gary Geelhoed, Marie Hobson, Peter Jacoby, Anthony Keil, Heath Kelly, Alan Leeb, Hannah Moore, Dale Carcione, Larissa Rhind, Peter Richmond, Helen Shirley, David Smith, Paul van Buynder, Simon Williams
References
- 1. Brotherton J, Wang H, Schaffer A et al. Vaccine preventable diseases and vaccination coverage in Australia, 2003 to 2005. Commun Dis Intell 2007; 31(Suppl):S1–S152. [DOI] [PubMed] [Google Scholar]
- 2. Moore H, Burgner D, Carville K, Jacoby P, Richmond P, Lehmann D. Diverging trends for lower respiratory infections in non‐Aboriginal and Aboriginal children. J Paediatr Child Health 2007; 43(6):451–457. [DOI] [PubMed] [Google Scholar]
- 3. Smith S, Demicheli V, Di Pietrantonj C et al. Vaccines for preventing influenza in healthy children.[see comment]. Cochrane Database Syst Rev 2006; 1:CD004879. [DOI] [PubMed] [Google Scholar]
- 4. Bridges C, Fukuda K, Uyeki T, Cox N, Singleton J. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2002; 51(RR03):1–31. [PubMed] [Google Scholar]
- 5. Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56 (RR‐6):1–54. [PubMed] [Google Scholar]
- 6. Department of Health . Paediatric Immunisation 2008. Disease Watch 2008;12: 1–4. [Google Scholar]
- 7. Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook. 9th edn Canberra, Australia: Australian Government Department of Health and Ageing; 2008. [Google Scholar]
- 8. National Health and Medical Research Council . The Australian Immunisation Handbook. 9th edn Canberra: Australian Government Department of Health and Aging, 2008. [Google Scholar]
- 9. Zitterkopf NL, Leekha S, Espy MJ, Wood CM, Sampathkumar P, Smith TF. Relevance of influenza a virus detection by PCR, shell vial assay, and tube cell culture to rapid reporting procedures. J Clin Microbiol 2006; 44(9):3366–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belongia EA, Kieke BA, Donahue JG et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004‐2005 season to the 2006‐2007 season. J Infect Dis 2009; 199(2):159–167. [DOI] [PubMed] [Google Scholar]
- 11. Skowronski DM, Masaro C, Kwindt TL et al. Estimating vaccine effectiveness against laboratory‐confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007; 25(15):2842–2851. Epub 006 Oct 16. [DOI] [PubMed] [Google Scholar]
- 12. Orenstein EW, De Serres G, Haber MJ et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness.[see comment]. Int J Epidemiol 2007; 36(3):623–631. [DOI] [PubMed] [Google Scholar]


