Abstract
Please cite this paper as: Forshey et al. (2010) Epidemiology of influenza‐like illness in the Amazon Basin of Peru, 2008–2009. Influenza and Other Respiratory Viruses 4(4), 235–243.
Background Data addressing the incidence and epidemiology of influenza and influenza‐like illness (ILI) in tropical regions of the world is scarce, particularly for the neotropics of South America.
Methods We conducted active, population‐based surveillance for ILI across 45 city blocks within the Amazon Basin city of Iquitos, Peru. Demographic data and household characteristics were collected for all participants, and participating households were visited three times weekly to inquire about ILI (fever plus cough or sore throat) among household residents. Nasal and oropharyngeal swabs were collected from participants with ILI and tested for influenza virus infection.
Results Between May 1, 2008 and July 8, 2009, we monitored 10,341 participants for ILI for a total of 11 569·5 person‐years. We detected 459 ILI episodes, with 252 (54·9%) of the participants providing specimens. Age‐adjusted incidence of ILI was estimated to be 46·7 episodes/1000 person‐years. Influenza A and B viruses were detected in 25 (9·9%) and 62 (24·6%) specimens of ILI patients, respectively, for an estimated age‐adjusted incidence rate of 16·5 symptomatic influenza virus infections/1000 person‐years. Risk factors for ILI included age, household crowding, and use of wood as cooking fuel. For influenza virus infection specifically, age and use of wood as a cooking fuel were also identified as risk factors, but no effect of household crowding was observed.
Conclusions Our results represent the initial population‐based description of the epidemiology of ILI in the Amazon region of Peru, which will be useful for developing region‐specific strategies for reducing the burden of respiratory disease.
Keywords: Amazon, cohort, Influenza, Peru
Introduction
Influenza viruses and other pathogens that cause influenza‐like illness (ILI) are significant public health threats worldwide, causing substantial morbidity and mortality each year. While the epidemiology in temperate countries is largely well described, much less is understood about the dynamics of influenza virus transmission in the tropics, 1 particularly the neotropics of South America. In many tropical regions the health infrastructure is limited, which may lead to an increase in severe complications and a higher disease burden during seasonal outbreaks. 2 Further data is necessary to facilitate the development of locally tailored strategies for mitigating the impact of respiratory viruses, including both seasonal and pandemic strains of influenza virus.
One obstacle to studying the epidemiology of influenza and other respiratory viruses is the need for laboratory support to identify the etiologic agents associated with ILI. In Peru, recently published data from a country‐wide, clinic‐based sentinel surveillance system documented the variety of viral pathogens associated with ILI in that region. 3 Although influenza A and influenza B viruses were the dominant viral agents of ILI, together accounting for 35% of ILI cases reported, a number of other viruses were identified as well. Parainfluenza viruses, adenoviruses, respiratory syncytial virus (RSV) and enteroviruses were all isolated from patient specimens, accounting for nearly 9% of ILI cases. While these studies are useful for identifying circulating pathogens and for selecting components of the seasonal vaccine, clinic‐based surveillance generally does not permit the generation of the population‐based data necessary for unbiased measures of disease incidence and burden.
To improve our understanding of influenza epidemiology in the neotropics, we conducted a prospective cohort study of ILI in Iquitos, Peru. Our goal was to estimate the incidence of ILI and influenza in this neotropical site and to compare these rates with those calculated from an existing clinic‐based surveillance system. In addition, we addressed the hypothesis that risk factors for ILI and influenza identified in other regions of the world would also be implicated in the neotropics of South America. Specifically, we focused on participant age, 4 socioeconomic status, 5 household crowding, 6 and the presence of children living within the household. Herein, we describe the incidence and risk factors for ILI in Iquitos during the 14 months prior to the 2009 introduction of the novel pandemic H1N1 influenza A virus into the Amazon region. 7
Materials and methods
Study site
Iquitos (73·2°W, 3·7°S) is a city of approximately 400 000 inhabitants, accessible from the coast only by boat or by air, situated at 120 m above sea level at the confluence of the Nanay, Itaya, and Amazon Rivers in the Loreto Department of northeastern Peru. The climate is tropical, with an average daily temperature of 25·8°C (22·0–32·2°C). Temperature variation throughout the year is modest. The lowest average daily temperature typically occurs in June and July (25·0°C [21·0–31·1°C]), while the warmest months are typically October through December (26·2°C [22·5–33·1°C]). The average annual precipitation is 3·6 m. Precipitation occurs throughout the year, albeit typically at somewhat reduced levels from June through August.
Iquitos is composed of four distinct districts, namely Iquitos, San Juan, Belen, and Punchana. In this study, community‐based cohorts were restricted to the districts of Iquitos and Punchana, while clinics were located in all four districts. Clinic‐based surveillance was established in conjunction with the Peruvian Ministry of Health in public and military health centers located throughout the city (Figure 1), including one military clinic, one military hospital, two public hospitals, and nine public clinics. Three of the public clinics are located in rural areas outside the urban center of Iquitos (not shown).
Figure 1.
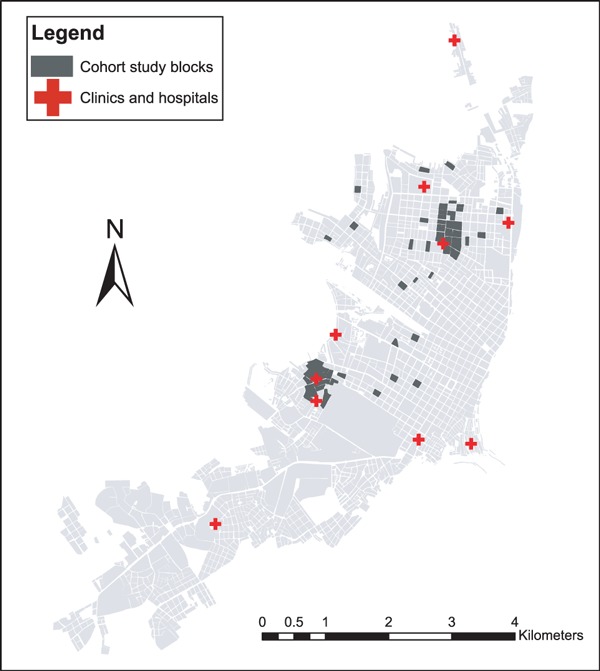
Location of study neighborhoods and participating health clinics in Iquitos, Peru.
Population‐based active surveillance was conducted within two cohorts of approximately 5000 participants each, both originally established in the context of dengue epidemiological studies. The first cohort was established during 2006 on 20 city blocks representing a geographically stratified sample of Iquitos (Figure 1), covering 10 distinct Peruvian Ministry of Health administrative zones (two blocks per zone). The second cohort was established during 2007 in two replace zones with areas. Covering approximately 12 contiguous city blocks each (Figure 1). In both studies, permission was obtained from the head of the household to monitor residents of the house for febrile illness. A census was conducted for each participating house to determine the number and age of residents. Recruitment continued until a total of approximately 5000 participants was reached for both cohorts, without regard for age or sex.
To address the possibility that socio‐economic status is related to risk for ILI, we collected data on household characteristics, including household construction, type of fuel used for cooking (gas/kerosene, electricity, wood, or a combination), sewage system, electricity in the home, and access to running water, all measured before and/or during the course of the study. In Iquitos, houses associated with higher socioeconomic index are typically constructed of brick or concrete with completely closed in walls and roofs (closed roof eaves), whereas houses associated with lower socioeconomic index tend to be constructed of wood, with significant open space between the roof and the top of the wall (open roof eaves). For cooking fuels, cooking with gas or electricity is typically associated with higher income levels, while cooking with wood is typically associated with lower income levels. Furthermore, in other studies the indoor use of wood stoves has been associated with increased risk of respiratory illness. 8 , 9 In addition to questions related to socioeconomic status, we inquired about the types of animals kept in participants’ homes.
For ILI surveillance, participating households were visited three times a week by trained health care workers. During home visits, study personnel noted if participants had moved outside the study area or were traveling for extended periods of time. In addition, families or individuals moving into the neighborhoods during the course of the study were invited to participate.
Case definition
ILI patients were identified according to the WHO case definition, which includes sudden onset of fever with cough and/or sore throat in the absence of other diagnoses. 10 For this study, fever was defined as an axillary temperature of 37·5°C or higher. Patients were also defined as febrile if they reported recent history of fever and had taken medication to relieve the fever (including ibuprofen or paracetamol) during the previous 12 hours.
Two types of samples were obtained from patients meeting the case definition with five days or fewer of illness, and who were willing to participate. A nasal swab was obtained for on‐site processing in an influenza antigen rapid test (QUICKVUE Influenza A+B test; Quidel, San Diego, CA, USA), and an oropharyngeal swab was obtained for virus cultivation. Oropharyngeal swabs were placed in transport media and stored at −70°C until they were delivered on dry ice to the NMRCD laboratory in Lima, Peru, for analysis. Demographic data and the date of illness were noted for participants in the cohort arm of the study who declined to provide a swab sample.
Laboratory analysis
As previously described, 3 patient specimens were inoculated onto four cell lines for virus isolation: Madin‐Darby canine kidney, African green monkey kidney (Vero76 and VeroE6) and Rhesus monkey kidney (LLCMK2) cells. Following the detection of cytopathic effect or after 10 days of culture, cells were spotted onto microscope slides. Cell suspensions were dried and fixed in chilled acetone for 15 minutes. A direct fluorescence assay was used for the identification of viral isolates (D3 DFA Respiratory Virus Kit; Diagnostic Hybrids, Athens, OH, USA), with antibodies specific for adenoviruses, influenza A viruses, influenza B viruses, parainfluenza viruses (types 1, 2, and 3), and RSV. Coxsackie virus was detected through the D3 IFA Enterovirus ID kit (Diagnostic Hybrids). Assays were performed according to the manufacturer’s protocols with included controls. Patients were classified as influenza virus positive in the presence of positive culture, positive rapid test, or both. In a previous study conducted by our group, 3 the sensitivity of the influenza rapid test was determined to be 72·7%, and the specificity was determined to be 93·7%, with a positive predictive value of 86·0%.
Human subjects
Study protocols (NMRCD.2000.0006, NMRCD.2002.0019, NMRCD2005.009, and NMRCD2007.007) were approved by the US Naval Medical Research Center Detachment (NMRCD) Institutional Review Board (IRB), the Peruvian Ministry of Health, and the University of California‐Davis.
Statistical analysis
Statistical analyses (Pearson’s chi‐square, Fisher’s exact test, Student’s t‐test, logistic regression) were performed in R 11 (R Foundation for Statistical Computing, Vienna, Austria), with P‐values <0·05 considered statistically significant. Multivariate logistic regression models were developed using a backward stepwise approach. Separate models were developed for ILI and influenza viruses, with influenza A and B viruses grouped together. Age‐adjusted incidence rates were calculated based on the 2007–2008 census data for urban areas of the Loreto Department. 12
Results
Participant demographics
Over the course of the study (May 1, 2008–July 8, 2009), 10 341 participants from 1570 housing units were recruited and monitored for a total of 11 569·5 person‐years. The majority of participants (9225; 89·2%) remained under surveillance for the duration of the study period. Of the full cohort, 5287 were female (50·6%), with a median age of 25, calculated at the mid‐date of the study (Table 1).
Table 1.
Age distribution of study participants, participants with influenza‐like illness (ILI), and participants with confirmed influenza virus infection
| Age (year) | Participants (% of total) | Person‐years | ILI episodes (% of total) | ILI samples (% of total) | ILI/1000 person‐years | Influenza A virus | Influenza B virus |
|---|---|---|---|---|---|---|---|
| 0–4 | 659 (6·4) | 732·3 | 90 (19·6) | 64 (25·4) | 122·9 | 6 | 13 |
| 5–14 | 1991 (19·3) | 2233·4 | 140 (30·5) | 65 (25·8) | 62·7 | 8 | 20 |
| 15–29 | 3384 (32·7) | 3756·3 | 92 (20·0) | 50 (19·8) | 24·5 | 6 | 6 |
| 30–44 | 2013 (19·5) | 2247·2 | 57 (12·4) | 34 (13·5) | 25·4 | 0 | 13 |
| 45–59 | 1399 (13·5) | 1593·5 | 50 (10·9) | 23 (9·1) | 31·4 | 2 | 7 |
| 60+ | 895 (8·7) | 1006·8 | 30 (6·5) | 16 (6·3) | 29·8 | 3 | 3 |
| Total | 10 341 (100·0) | 11 569·5 | 459 (100·0) | 252 (100·0) | 39·8 | 25 | 62 |
Data on household characteristics collected within the time period of the study was available from 1534 (97·7%) of the houses (Table 2). Attributes such as access to running water, indoor sewage systems, and electricity were common (>90%) across all households. Other attributes, including construction materials, primary cooking fuels, and household crowding were more variable (Table 2). The most common household animals were dogs (477; 31·1% of households), cats (474: 30·9%), and chickens (462; 30·1%).
Table 2.
Demographic and household characteristics of cohort participants. Numbers may not add up to the total of participants in all columns, as some data were not available for all participants or households
| All cohort participants, N (%) | Participants with influenza‐like illness, N (%) | Participants with Influenza A or B virus, N (%) | |
|---|---|---|---|
| Sex | |||
| F | 5287 (50·6) | 245 (53·1) | 41 (47·1) |
| M | 5168 (49·4) | 216 (46·9) | 46 (52·9) |
| Residents/room | |||
| <1/room | 3773 (39·1) | 136 (30·3) | 36 (42·4) |
| 1–1·9/room | 4408 (45·6) | 205 (45·7) | 29 (34·1) |
| 2–2·9/room | 1440 (15·4) | 108 (24·0) | 20 (23·5) |
| House construction | |||
| Brick/concrete | 3650 (37·6) | 138 (31·0) | 29 (34·1) |
| Wood | 1988 (20·5) | 120 (27·0) | 21 (24·7) |
| Mixed | 4072 (41·9) | 186 (41·9) | 35 (41·2) |
| Roof eaves | |||
| Open | 7571 (78·0) | 358 (80·6) | 71 (83·5) |
| Closed | 2139 (22·0) | 86 (19·3) | 14 (16·4) |
| Cooking fuel | |||
| Gas | 3362 (35·1) | 118 (26·5) | 20 (23·5) |
| Wood | 3081 (32·2) | 180 (40·3) | 36 (42·4) |
| Gas/wood | 3038 (31·7) | 146 (32·7) | 29 (33·7) |
| Electricity | 100 (1·0) | 2 (0·4) | 0 |
| Electricity | |||
| Yes | 9594 (98·8) | 440 (98·0) | 84 (98·9) |
| No | 116 (1·2) | 9 (2·0) | 1 (1·2) |
| Water source | |||
| Faucet | 8998 (92·7) | 414 (92·2) | 75 (88·2) |
| Other (Rain, well, etc) | 707 (7·3) | 35 (7·8) | 10 (11·8) |
| Sewage system | |||
| Sewer | 9053 (93·5) | 419 (93·3) | 80 (94·1) |
| Latrine | 397 (4·1) | 18 (4·0) | 3 (3·5) |
| Ditch | 231 (2·4) | 12 (2·7) | 2 (2·4) |
ILI incidence and etiology
Over the course of the study, 459 ILI cases were detected among study participants, for a crude incidence rate of 39·8 ILI episodes per 1000 person‐years (95% CI 36·2–43·4). Data from the 2007–2008 Peruvian national census was utilized to compensate for differences between the age distribution of the cohort participants (Table 1) and the overall population of Iquitos. Based on the age distribution from the 2008 census for the population of urban Loreto, the age‐adjusted incidence rate was 46·7 ILI episodes per 1000 person‐years. Swabs were obtained from 252 (54·9%) participants. Influenza B viruses (n = 62) and influenza A viruses (n = 25) were the most commonly detected viral agents, accounting for 24·6% and 9·9% of all sampled ILI episodes, respectively. Based on the number of samples collected relative to the number of ILI episodes, the crude incidence of symptomatic influenza virus infection was estimated at 13·7 cases per 1000 person‐years, with an estimated age‐adjusted incidence of 16·5 cases per 1000 person‐years. During the course of the study, adenoviruses (n = 7), RSV (n = 1), parainfluenza virus 1 (n = 3), and a Coxsackie virus (n = 1) were also isolated from patient samples, all from participants 10 years of age or younger.
Secondary ILI episodes were observed within 7 days of onset of an index case in the same household on 39 occasions, for a total of 52 secondary cases. There were 29 occasions where one additional resident presented with ILI within 7 days of the index case, seven occasions with two additional residents with ILI, and three occasions with three additional residents with ILI. For influenza A virus infection, 4·0% (7/177) of household contacts developed ILI within 7 days of onset of the index case. For influenza B virus cases, 6·2% (21/337) of household contacts developed ILI within 7 days.
Risk factors for ILI
No significant differences were observed by sex for the ILI patients compared with the study population overall (P = 0·278; Table 2). The age distribution of ILI cases was significantly younger than the overall study population (P < 0·001), with a median age of 15. The highest incidences (Table 1) and risk (Table 3) of ILI were observed among participants under the age of 5 (122·9 ILI episodes per 1000 person‐years) and between ages 5 and 14 (62·7 ILI episodes per 1000 person‐years). Incidence rates were similar when comparing all age groups older than 15 (Table 1).
Table 3.
Bivariate logistic regression analysis of influenza‐like illness (ILI) and demographic and socioeconomic factors
| All ILI | Influenza A virus or influenza B virus infection | |
|---|---|---|
| Crude OR (95% CI) | Crude OR (95% CI) | |
| Age (years) | ||
| 0–4 | 1 | 1 |
| 5–14 | 0·53 (0·40–0·72) | 0·60 (0·32–1·13) |
| 15–29 | 0·23 (0·17–0·32) | 0·16 (0·08–0·33) |
| 30–44 | 0·22 (0·16–0·32) | 0·23 (0·10–0·48) |
| 45–59 | 0·27 (0·18–0·39) | 0·25 (0·10–0·55) |
| 60+ | 0·31 (0·20–0·46) | 0·26 (0·09–0·64) |
| Residents/room | ||
| <1 | 1 | 1 |
| 1–1·9 | 1·3 (1·05–1·63) | 0·67 (0·40–1·10) |
| 2+ | 2·07 (1·59–2·68) | 1·46 (0·83–2·51) |
| Construction | ||
| Brick/concrete | 1 | 1 |
| Mixed | 1·23 (0·98–1·54) | 1·13 (0·68–1·87) |
| Wood | 1·64 (1·27–2·10) | 1·41 (0·79–2·49) |
| Roof eaves | ||
| Closed | 1 | 1 |
| Open | 1·15 (0·92–1·47) | 1·43 (0·83–2·65) |
| Cooking fuel | ||
| Gas | 1 | 1 |
| Gas/Wood | 1·32 (1·03–1·69) | 1·59 (0·90–2·86) |
| Wood | 1·69 (1·29–2·11) | 1·98 (1·16–3·50) |
Household person‐density was associated with risk for ILI (P < 0·001; Table 3), with increasing risk for greater than one person per room and greater than two people per room. This trend did not hold true for influenza virus infection (Table 3). Living in a household with a child did not increase risk for ILI or influenza virus infection (data not shown). A variety of household attributes (Table 2) were associated with increased risk for ILI in bivariate analysis, including house construction materials, roof construction, and materials used for cooking fuel (Table 3). There were no significant differences in access to electricity, household animals, running water, or indoor sewage systems between participants with ILI and the cohort at large. In the multivariate logistic regression model for ILI, age, household crowding (>2 people per room), and cooking fuel remained in the model (Table 4). For multivariate models of influenza virus infection, because of the small sample size, all participants 15 or older were collapsed into one age group. In contrast to total ILI, for influenza virus infection, household crowding was not significantly associated with increased risk for infection (Table 5). Instead, age and cooking fuel remained as significant risk factors for influenza virus infection (Table 5).
Table 4.
Adjusted odds ratios for risk factors for influenza‐like illness (ILI) in Iquitos, Peru
| All ILI episodes | |
|---|---|
| Adjusted OR (95% CI) | |
| Age (year) | |
| 0–4 | 1 |
| 5–14 | 0·52 (0·39–0·70) |
| 15–29 | 0·23 (0·17–0·31) |
| 30–44 | 0·20 (0·14–0·29) |
| 45–59 | 0·25 (0·17–0·36) |
| 60+ | 0·29 (0·19–0·44) |
| Residents/room | |
| <1 | 1 |
| 1–1·9 | 1·13 (0·90–1·43) |
| 2+ | 1·56 (1·17–2·08) |
| Cooking fuel | |
| Gas | 1 |
| Gas/wood | 1·24 (0·96–1·59) |
| Wood | 1·37 (1·06–1·77) |
Table 5.
Adjusted odds ratios for risk factors for influenza virus infection in Iquitos, Peru
| Influenza A or B virus infection | |
|---|---|
| Adjusted OR (95% CI) | |
| Age | |
| 0–4y | 1 |
| 5–14y | 0·63 (0·34–1·22) |
| 15y+ | 0·21 (0·12–0·40) |
| Cooking fuel | |
| Gas | 1 |
| Gas/wood | 1·50 (0·85–2·71) |
| Wood | 1·86 (1·08–3·29) |
Incidence of ILI in clinics in Iquitos
To further explore the epidemiology of ILI within Iquitos, we utilized data from a clinic‐based surveillance study conducted throughout the city. During the same period as our prospective cohort study (May 1, 2008 and July 8, 2009), 595 patients with ILI were enrolled in 13 clinics and hospitals located in and around Iquitos (Figure 2). The sex distribution among participants recruited in the clinics (48.7% female) was similar to that observed for cohort participants with ILI and the cohort population as a whole (P = 0.41). Participants with ILI recruited in the clinics were significantly younger overall when compared with ILI patients detected in the study neighborhoods (P < 0·001). Median age of patients with ILI enrolled in the clinics was 6; 43·4% (258/595) of the enrolled ILI population was under the age of 5% and 1·2% (7/595) was age 60 or older. Patients with ILI were detected within a similar time period following the onset of illness, with an average of 2·5 days post‐onset of disease in the neighborhoods (median = 2 days post‐onset) and an average of 2·7 days post‐onset in the clinics (median = 2 days post‐onset).
Figure 2.
Distribution of influenza‐like illness episodes and confirmed influenza virus infections enrolled in clinics or hospitals in Iquitos, Peru, May 2008–July 2009.
Although there were no significant differences in the prevalence of influenza viruses overall among ILI patients in the clinics and cohorts (P = 0·10), the distribution of influenza A viruses and influenza B viruses specifically varied dramatically. Influenza A viruses were significantly more prevalent among ILI patients recruited in the clinics (P = 0·002), while influenza B viruses were more prevalent among ILI patients in the neighborhoods (P < 0·001). Incidence rates were calculated based on the population assigned for each health center by the local health directorate (DIRESA‐Loreto). Based on data from the clinic‐based surveillance and population estimates from the DIRESA, the incidences of total ILI and specifically for influenza viruses were estimated at 416·2 cases/100 000 residents and 120·7/100 000 residents, respectively.
Temporal distribution of ILI and influenza virus in Iquitos
To explore the temporal variability of influenza virus transmission in Iquitos, we utilized data spanning 4 years of clinic‐based surveillance [July 9, 2005–July 8, 2009; including 2 years of analysis (June 2006–May 2008) previously reported elsewhere 3 ] and 14 overlapping months of population‐based surveillance (May 1, 2008–July 8, 2009). Between July 9, 2005 and July 8, 2009, nasal and oropharyngeal swabs were collected from 1864 participants with ILI. Of these, 374 (20·1%) were positive for influenza A virus, and 235 (12·6%) were positive for influenza B virus. For the 4 years included in this analysis (July 2005–July 2009), influenza A virus transmission was largely restricted to the first 5 months of the year, with 87·4% (327/374) of influenza A cases were detected between January and May. Unlike influenza A, only 52·0% of total ILI cases and 42·1% of influenza B cases were detected between January and May. Influenza B virus transmission was also marked by sharp peaks of transmission, albeit spread throughout the year (Figure 3).
Figure 3.
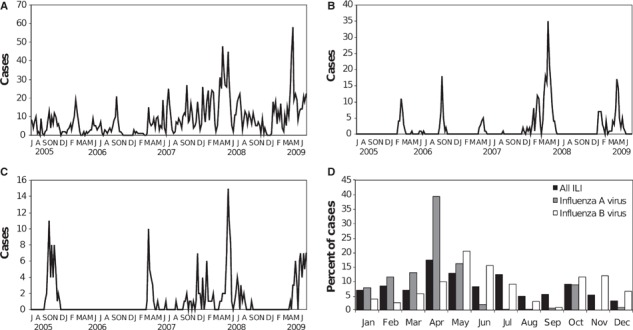
Temporal distribution of (A) influenza‐like illness (ILI) episodes and confirmed (B) influenza A virus and (C) influenza B virus cases by epidemiological week, July 9, 2005–July 8, 2009. Monthly distribution of ILI, influenza A virus infections, and influenza B virus infections during the 4 years of the study is depicted in panel (D).
Discussion
We report an incidence of ILI of approximately 5 episodes per 100 person‐years in Iquitos, with an incidence of symptomatic influenza virus infection of 1·6 per 100 person‐years. Our data indicate that ILI episodes are a significant source of morbidity in the Amazon region, similar to the incidence of dengue in the same population (approximately 2 clinically apparent dengue infections/100 person‐years covering the same time period [BMF, ACM, and TJK, unpublished results]). However, ILI episodes in general and influenza virus transmission specifically are relatively low in Iquitos as compared with other regions of the world. For example, in the United States, seasonal epidemics of influenza are suggested to attack 5–20% of the population annually, 13 and in Japan seasonal influenza has been estimated to infect 6–14% of the population annually. 14 In Singapore, the clinically apparent influenza was estimated to attack 20% of the population, 15 whereas in a rural site in Thailand, the influenza attack rate was calculated at 6% of the population. 16 From a cohort of children in Managua, Nicuaragua, Gordon et al. 4 reported incidence of ILI of approximately 35 episodes per 100 person‐years, several fold higher than observed among children in our Iquitos cohort (approximately 12 episodes per 100 for children younger than 5). Overall, however, it can be difficult to directly compare ILI and influenza incidence rates from location to location owing to differences in case capture, differences in case definitions, and year‐to‐year variability. In addition, unbiased population‐based data from many regions of the world, particularly in tropical zones, is relatively scarce.
As our cohort study covered only 14 months, it is difficult to extrapolate these results to make general statements about the yearly incidence of ILI. Data from past years of the clinic‐based surveillance system can perhaps be informative in this regard. For example, between July 2008 and July 2009, covering most of the study period reported here, 520 ILI cases were captured in Iquitos clinics, with 133 confirmed influenza virus infections. In contrast, between July 2007 and July 2008 and July 2006 and July 2007 the number of ILI cases were 645 and 219, respectively, and the number of confirmed influenza virus infections were 236 (36·6%) and 77 (35·2%), respectively. These data indicate that even though there are significant yearly fluctuations in ILI episodes and influenza virus transmission, the period covered in the current study does not appear to be an outlier relative to recent patterns of ILI in the region. It is interesting to note that during the 14 months reported here, influenza A was more common than influenza B among ILI patients recruited in the health facilities, but influenza B was more common than influenza A among ILI patients recruited in their homes. One possible explanation is highly focal transmission at the neighborhood level, such that transmission observed within the study cohort is not representative of the city as a whole. Because our study neighborhoods were geographically stratified across Iquitos, this explanation seems unlikely. Another possibility is that disease resulting from influenza B virus infection is less severe than influenza A virus, and thus influenza B patients were less likely to require attention at the local health clinic. Overall, the incidence of both ILI and influenza virus infection calculated for clinics was 10‐fold lower than calculated for study neighborhoods, suggesting that many people with ILI do not visit a health center. At least some of this discrepancy might be explained by shortcomings of patient‐capture inherent to such clinic‐based systems.
For ILI episodes overall, several risk factors were apparent. Children under the age of five had a significantly higher incidence of ILI, followed by children between the ages of 5 and 14. All age groups older than 15 had similar rates of ILI over the duration of our study. Household crowding was also found to be a risk factor for ILI, the highest risk being for participants residing in homes with more than two residents per room. Similarly, in the cohort in Nicaragua, a similar effect of household density was observed, with the effect most apparent at a high level of crowding (i.e. more than five residents per room). In our study, socioeconomic indicators, as measured by household construction attributes and the type of cooking fuel used in the home, were strong risk factors for ILI. Even though there may be some direct biological affect of these household factors, such as increased susceptibility to respiratory disease resulting from regular exposure to wood smoke in enclosed spaces, 8 , 17 it is also possible that lower socioeconomic status could be related to sanitation practices and access to hygienic products. It is interesting to note that several of these risk factors did not apply to influenza virus infections specifically, although a larger number of confirmed cases are necessary to draw definitive conclusions. In final multivariate models, household crowding was not associated with influenza virus infection. These data indicate that the mechanisms of transmission are distinct for influenza viruses relative to other etiologic agents of ILI in Iquitos and that relying on clinical classification of cases is not sufficient for understanding the epidemiology of influenza.
The seasonality of influenza virus transmission has important implications for planning vaccination and response strategies. Here, we observed that most influenza A virus infections occur during the first 5 months of the year, out of sync with winter months in the Southern Hemisphere. Thus, seasonal influenza vaccine campaigns would likely need to be timed accordingly to be effective. In tropical regions of the world, the seasonal patterns are not well understood, although the relevance of these patterns is of increasing interest. Tropical regions in Asia and South America have been suggested to serve as the source of year‐round maintenance of viral strains that then emanate out from equatorial zones in ‘traveling waves’. 18 , 19 , 20 , 21 In the tropics, several different patterns have been observed, including year‐round transmission, seasonal peaks related to the rainy season, and biannual peaks related to winter seasons in the Northern and Southern hemispheres. 22 , 23 During the four years of surveillance data presented in this study, influenza A virus circulation exhibited a bias toward transmission during the first 5 months of the year. A similar pattern was observed in Fortaleza, Brazil, which, like Iquitos, is located at approximately 4°S of the equator. 24 For the 10 years of study in Fortaleza, 94·4% of influenza infections occurred in the first half of the year, with 88·8% from February to May and significant association with the rainy season. We did not observe an obvious association between influenza transmission and rainfall in our study (data not shown), although a more complex analysis of potential climatic influences will need to be developed. For the most part, the precise drivers of influenza seasonality are still a matter of some controversy. Data from a guinea pig model suggest that variations in humidity and temperature may drive seasonal fluctuations in influenza transmission. 25 , 26 , 27 Specifically, transmission by the aerosol route, but not by the contact route, was significantly abrogated by warm or humid conditions, which could significantly impact seasonality and incidence of disease, viral load during exposure, and the ratio of subclinical disease.
Data presented herein represent some of the initial population‐based estimates of ILI and influenza incidence in the Amazon region. A notable limitation of our study is the lack of follow‐up with ILI patients recruited in the clinics and neighborhoods, thus severe outcomes subsequent to influenza infection were not measured. Important questions regarding ILI disease in the region remain unanswered. Future studies will focus on measuring the social and economic costs of ILI, as well as the range of disease outcomes. Nevertheless, our cohort and clinic‐based data provide previously unavailable baseline data for analyzing the efficacy of different intervention strategies, such as vaccination, masks, and hand washing. 28 , 29 In addition, following the recent introduction of the 2009 pandemic H1N1 influenza A virus into the region, 7 our data will serve as a basis for evaluating the impact of this novel virus on disease incidence and infection risk factors.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. This study was funded in part by the US Department of Defense Global Emerging Infections Systems Research Program, WORK UNIT NUMBER: 847705.82000.25GB.B0016. The study protocol was approved by the Ministry of Health of Peru and the Naval Medical Research Center Institutional Review Board (Protocols NMRCD.2000.0006, NMRCD.2002.0019, NMRCD.2005.009, and NMRCD.2007.007) in compliance with all applicable Federal regulations governing the protection of human subjects.
Disclosure
None of the authors has a financial or personal conflict of interest related to this study. The corresponding author had full access to all data in the study and final responsibility for the decision to submit this publication.
Copyright statement
Some of the authors are military service members (TJK) or employee of the US Government (VAL‐T). This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Acknowledgements
We thank the Dirección General de Epidemiología, the Instituto Nacional de Salud and the Ministry of Health for their support in establishing and maintaining these surveillance activities. We thank Rebeca Carrion and Regina Fernandez for management of field personnel, Wieslawa Alava, Leslye Angulo, Guadalupe Flores, Patricia Barrera, Maria Esther Gamero, Jane Rios, Josefina Garcia, Merly Sovero, Monica Nieto, Juan Perez, and Ruth Centeno for laboratory and technical support, and Jhon Ramirez for GIS support.
References
- 1. Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med 2006; 3(4):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2(1):25–32. [DOI] [PubMed] [Google Scholar]
- 3. Laguna‐Torres VA, Gomez J, Ocana V et al. Influenza‐like illness sentinel surveillance in Peru. PLoS ONE 2009; 4(7):e6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon A, Ortega O, Kuan G et al. Prevalence and seasonality of influenza‐like illness in children, Nicaragua, 2005‐2007. Emerg Infect Dis 2009; 15(3):408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumenshine P, Reingold A, Egerter S, Mockenhaupt R, Braveman P, Marks J. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis 2008; 14(5):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viboud C, Boelle PY, Cauchemez S et al. Risk factors of influenza transmission in households. Br J Gen Pract 2004; 54(506):684–689. [PMC free article] [PubMed] [Google Scholar]
- 7. Munayco CV, Gomez J, Laguna‐Torres VA et al. Epidemiological and transmissibility analysis of influenza A(H1N1)v in a southern hemisphere setting: Peru. Euro Surveill 2009; 14(32): pii=19299. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19299. [PubMed] [Google Scholar]
- 8. Ezzati M, Kammen DM. The health impacts of exposure to indoor air pollution from solid fuels in developing countries: knowledge, gaps, and data needs. Environ Health Perspect 2002; 110(11):1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chauhan AJ, Johnston SL. Air pollution and infection in respiratory illness. Br Med Bull 2003; 68:95–112. [DOI] [PubMed] [Google Scholar]
- 10. WHO . WHO Recommended Surveillance Standards, 1999. [updated 1999; cited September 22, 2009]; Available at http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdf. [Google Scholar]
- 11. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- 12. INEI (Instituto Nacional de Estadística e Informática) . Censos Nacionales 2007: XI de Población y VI de Vivienda. Lima, Peru; 2008 [updated 2008; cited September 20, 2009]; Available at http://www.inei.gob.pe/.
- 13. CDC . Influenza: the Disease, 2009. [updated 2009; cited 2009 September 22, 2009]; Available at http://www.cdc.gov/flu/about/disease/index.htm. [Google Scholar]
- 14. Kawado M, Hashimoto S, Murakami Y et al. Annual and weekly incidence rates of influenza and pediatric diseases estimated from infectious disease surveillance data in Japan, 2002–2005. J Epidemiol 2007; 17(Suppl):S32–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng TP, Pwee KH, Niti M, Goh LG. Influenza in Singapore: assessing the burden of illness in the community. Ann Acad Med Singapore 2002; 31(2):182–188. [PubMed] [Google Scholar]
- 16. Clague B, Chamany S, Burapat C et al. A household survey to assess the burden of influenza in rural Thailand. Southeast Asian J Trop Med Public Health 2006; 37(3):488–493. [PubMed] [Google Scholar]
- 17. Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure‐response study. Lancet 2001; 9282:619–624. [DOI] [PubMed] [Google Scholar]
- 18. Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008; 453(7195):615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson MI, Simonsen L, Viboud C, Miller MA, Holmes EC. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PLoS Pathog 2007; 3(9):1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS ONE 2007; 2(12):e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol 2007; 165(12):1434–1442. [DOI] [PubMed] [Google Scholar]
- 22. Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect 1998; 121(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev 2003; 4(2):105–111. [DOI] [PubMed] [Google Scholar]
- 24. Moura FE, Perdigao AC, Siqueira MM. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg 2009; 81(1):180–183. [PubMed] [Google Scholar]
- 25. Lowen AC, Steel J, Mubareka S, Carnero E, Garcia‐Sastre A, Palese P. Blocking inter‐host transmission of influenza virus by vaccination in the guinea pig model. J Virol 2009; 83(7): 2803–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J Virol 2008; 82(11):5650–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 2007; 3(10):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowling BJ, Fung RO, Cheng CK et al. Preliminary findings of a randomized trial of non‐pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 2008; 3(5):e2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacIntyre CR, Cauchemez S, Dwyer DE et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis 2009; 15(2):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


