Abstract
Objective
Investigate the association between infliximab trough levels and quality of life in inflammatory bowel disease patients in maintenance therapy.
Methods
We carried out a transversal study with inflammatory bowel disease patients in infliximab maintenance therapy. Infliximab trough levels were determined using a quantitative rapid test. Disease activity indices (partial Mayo Score and Harvey-Bradshaw Index) and endoscopic scores (endoscopic Mayo Score or Simple Endoscopic Score in Crohn's disease) were obtained. Quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ).
Results
Seventy-one consecutive subjects were included in the study (55 with Crohn's disease and 16 with ulcerative colitis). Drug levels were considered satisfactory (≥3 μg/mL) in 28 patients (39.4%) and unsatisfactory (<3 μg/mL) in 43 (60.6%). Satisfactory trough levels were associated with higher rates of clinical remission and mucosal healing. Higher trough levels were also associated with improved IBDQ scores, particularly regarding bowel symptoms, systemic function, and social function.
Conclusion
Satisfactory trough levels of infliximab were associated with higher rates of clinical remission, mucosal healing, and improved quality of life in inflammatory bowel disease patients on maintenance therapy.
1. Introduction
Inflammatory bowel diseases (IBD), such as Crohn's disease (CD) and ulcerative colitis (UC), constitute a significant burden for the patients and the society [1]. Patients with CD and UC require frequent outpatient care, hospitalizations, and surgeries; some of them need stomas. The described changes cause functional impairment and great deterioration in quality of life. IBD has a chronic character and affects mostly young people. The disease can reduce the ability to work, decrease productivity, cause interruptions in employment, and have negative effects in patients' social and emotional well-being [2].
Infliximab (IFX), an antitumor necrosis factor alpha (TNF-α) antibody, is a biologic drug used for the treatment of CD and UC. IFX is effective in inducing and maintaining remission in patients with CD and UC [3]. Despite its effectiveness in both induction and maintenance of remission, a substantial number of patients will eventually lose response. Loss of response (“flares”) is associated with hospitalization and even surgeries, decreasing the quality of life [4, 5].
There is increasing evidence that clinical remission (CR) and mucosal healing (MH) are associated with better response to treatment, less hospitalization, lower surgery rates, and, consequently, improved quality of life in patients with IBD [4–7]. IFX trough levels higher than >2–3 μg/ml are associated with higher rates of sustained clinical and biochemical remission and improved endoscopic outcomes. Patients with relapse often have lower trough serum drug levels [8, 9].
Few studies correlated IFX trough levels with quality of life. The impact of loss of quality of life and, consequently, less social function, emotional functional, and worse symptoms, has not been investigated in detail in our country. The purpose of the present study was to investigate the association between IFX trough levels, response to treatment, and quality of life in a group of Brazilian IBD patients.
2. Materials and Methods
2.1. Study Design and Participants
A transversal study was performed in 71 consecutive patients with CD and UC on IFX maintenance therapy (5 mg/kg). All of the patients signed an informed consent statement prior to their participation, and the study was approved by the hospital's ethics committee.
2.2. Determination of Infliximab Serum Levels
IFX trough levels were obtained from blood samples collected immediately before drug infusion. Quantitative determination in serum was performed with the Quantum Blue® Infliximab assay (BÜHLMANN, Schönenbuch, Switzerland), as described elsewhere [10]. Briefly, serum samples were diluted 1 : 20 and a 70 μL aliquot was loaded into the port of the test cartridge. After a 15 min reaction, the cartridge was read and the results were shown on the point-of-care QB reader display. According to the manufacturer, this kit has the following analytical characteristics: the limit of detection is 0.15 μg/mL, and the lower and upper limits of quantification are 0.4 μg/mL and 20 μg/mL, respectively.
2.3. Definition of Clinical Remission and Mucosa Healing
CR was assessed with Harvey Bradshaw Index (HBI) and partial Mayo Score for CD and UC, respectively. In CD, CR was defined as HBI ≤ 4. In UC, CR was defined as partial Mayo Score ≤ 2 (with no individual subscore > 1). Elective ileocolonoscopies/sigmoidoscopies performed at least three months before and three months after blood collection were considered eligible for endoscopic evaluation. MH was considered Simple Endoscopic Score ≤ 2 (in CD) or a Mayo Endoscopic Subscore ≤ 1 (in UC). Levels of infliximab were divided into two categories: satisfactory (≥3 μg/mL) and unsatisfactory (<3 μg/mL) in comparison with MH, CR, and quality of life.
2.4. Questionnaires
Quality of life was measured using the Inflammatory Bowel Disease Questionnaire (IBDQ). It consists of a 32-item questionnaire of four dimensions: bowel-related symptoms (e.g., loose stools and abdominal pain), systemic function (e.g., fatigue and sleep pattern), social function (e.g., ability to attend work and social events), and emotional status (e.g., anger, depression, and irritability). The response for each question ranges from 1 (significant impairment) to 7 (no impairment) [11].
The total IBDQ ranges from 32 (very poor quality of life) to 224 (perfect quality of life). Patients in symptomatic remission usually have a score of 170 or more. The validity, reliability, and responsiveness of this questionnaire have been previously established [12].
IBDQ was correlated with CR and MH and with IFX serum levels.
2.5. Statistical Analysis
Categorical variables were expressed as frequencies/percentages and continuous variables as means ± standard deviation. The one-sample Kolmogorov-Smirnov test was used to assess the normality of continuous variables. The ANOVA test was used to compare continuous variables. Fisher's exact test or χ 2 were used to compare categorical variables. The correlation between IFX trough levels, CR, MH, and quality of life was performed through a receiver operating characteristic curve (ROC) analysis. All p values were 2-sided, and a significance level of 5% was established. Statistical analysis was performed with IBM® SPSS® Statistics 20 (IBM SPSS, Costa Mesa, CA).
3. Results
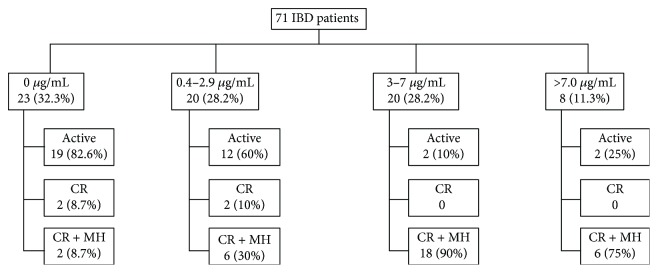
Seventy-one patients were included (55 with CD and 16 with UC) (Figure 1). Most patients were female (43/60.6%), of Caucasian ethnicity (63/88.7%), economically active (48/67.6), and with higher level of education (52/73.2%). Table 1 summarizes patients' characteristics according to IFX trough levels. The two groups (satisfactory versus unsatisfactory) were homogeneous. There was no statistical difference between the groups when we compared age, gender, ethnicity, occupation, education, condition (CD versus UC), IFX duration therapy, smoking, and use of azathioprine (mono versus combo therapy).
Figure 1.
Inflammatory bowel disease (IBD) patients according IFX trough levels and comparison between clinical remission (CR) and mucosal healing.
Table 1.
Patients' main characteristics according to infliximab levels.
| Characteristics | TL < 3 μg/mL | TL ≥ 3 μg/mL | p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
| ≤40 years | 29 | 61.7 | 18 | 38.3 | 0.803 |
| >40 years | 14 | 58.3 | 10 | 41.7 | |
| Gender | |||||
| Male | 23 | 53.5 | 20 | 46.5 | 0.146 |
| Female | 20 | 71.4 | 8 | 28.6 | |
| Ethnicity | |||||
| White | 36 | 57.1 | 27 | 42.9 | 0.135 |
| Non-white | 7 | 87.5 | 1 | 12.5 | |
| Education | |||||
| Low | 11 | 57.9 | 8 | 42.1 | 0.790 |
| High | 32 | 61.5 | 20 | 38.5 | |
| Occupation | |||||
| Unemployed | 17 | 73.9 | 6 | 26.1 | 0.128 |
| Employed | 26 | 54.2 | 22 | 45.8 | |
| Condition | |||||
| Crohn's disease | 35 | 64.8 | 19 | 35.2 | 0.257 |
| Ulcerative colitis | 8 | 47.1 | 9 | 52.9 | |
| IFX duration | |||||
| ≤2 years | 20 | 57.1 | 15 | 42.9 | 0.631 |
| >2 years | 23 | 63.9 | 13 | 36.1 | |
| Azathioprine | |||||
| No | 26 | 57.8 | 19 | 42.2 | 0.618 |
| Yes | 17 | 65.4 | 9 | 34.6 | |
| Smoking | |||||
| No | 41 | 59.4 | 28 | 40.6 | 0.515 |
| Yes | 2 | 100 | 0 | 0 | |
Of the 71 patients included, 43 (60.5%) have unsatisfactory IFX trough levels, 35 (49.3%) were with active disease, 4 (5.6%) were in CR (but not in MH), and 32 (45%) were in CR and MH (Figure 1).
Satisfactory trough level (≥3 μg/mL) was associated with CR and MH (p < 0.001). Similarly, quality of life was better if IFX the trough concentration was higher than ≥3 μg/mL (Table 2).
Table 2.
Clinical remission, mucosal healing, and quality of life scores according to infliximab levels.
| Disease control | TL ≥ 3 μg/mL | TL < 3 μg/mL | p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Clinical remission | 24 | 85.7 | 12 | 27.9 | <0.001 |
| Mucosal healing | 24 | 85.7 | 8 | 18.6 | <0.001 |
|
| |||||
| Quality-of-life score | Mean | ±SD | Mean | ±SD | |
|
| |||||
| Bowel symptoms (maximum = 70) | 59.6 | 9.3 | 52.3 | 8.5 | 0.001 |
| Systemic function (maximum = 35) | 27.3 | 5.6 | 22.7 | 5.2 | 0.001 |
| Social function (maximum = 35) | 30.8 | 5.7 | 26.7 | 7.4 | 0.015 |
| Emotional status (maximum = 84) | 65.1 | 16.5 | 60.0 | 12.9 | 0.148 |
| Global (maximum = 224) | 183.0 | 32.6 | 161.9 | 28.9 | 0.006 |
4. Discussion
In this study, we showed that IBD patients with adequate serum IFX levels have higher rates of CR and MH and improved quality of life.
This is the first Brazilian study published to compare quality of life and IFX trough levels. CD and UC have a substantial negative influence on patients' reported quality of life. Patient-reported outcomes are considered an important component of the overall evaluation of treatment effects in patients with IBD [13, 14].
Patients without clinical improvement persistently have impaired quality of life over time. On the other hand, quality of life enhances in patients who regain CR and/or achieve MH. It is known that about half of all IBD patients have relapse in disease activity during IFX maintenance therapy [8, 15]. One of the reasons for loss of response, and consequently decrease in quality of life, is reduction in serum levels of IFX in maintenance therapy. Therapeutic drug monitoring (TDM) could be a useful tool in this cases, allowing physicians to act before loss of response.
TDM is not routinely implemented in our country. However, TDM has shown to improve treatment outcomes and has important economic consequences in patients with IBD [8, 15]. Individualized therapy based on immunopharmacological evidence results in similar CR rates compared to dose intensification strategies. However, the treatment cost is higher when the physician does the “blinded” optimizations, instead of that based on the patient's IFX trough level.
The IBDQ is a validated and reproducible instrument, which can evaluate the quality of life of Brazilian inflammatory bowel disease patients. It includes 32 most frequent and important questions regarding the quality of life in IBD patients and is divided into four areas: intestinal symptoms, systemic symptoms, social aspects, and emotional aspects. This instrument provides additional information that is not evaluated by disease activity indices. Moreover, it is important to see the patient's point regarding his illness, including physical, physiological, and social performance [16–18]. Patient-reported outcomes are considered an important component of the overall evaluation of treatment effects in patients with IBD [19].
Our study showed better quality of life in patients with adequate serum levels of IFX. The patients presented improvement in the intestinal symptoms, besides improvement in the social function and systemic manifestations. Previous studies showed that the activity of CD is an important determinant of better quality of life. To our knowledge, this the first study, in our country, correlating IFX pharmacokinetics and quality of life in patients with IBD. Adequate IFX trough levels were correlated with reduction of signs and symptoms (CR and MH) and consequently better quality of life.
One of the main objectives of disease management is to achieve long-term remission (deeply sustained remission) and prevention of complications, such as fistulas, hospitalizations, surgeries, and stomas, as well as to improve quality of life [2, 19, 20]. It is important to evaluate IBD not only for the symptoms but also for the patients' perspective regarding their disease. Achieving good quality of life in chronic diseases such as CD and UC is a huge challenge for physicians.
Some important study limitations should be noted. First, despite using a validated questionnaire, only one score of quality of life was used. It may be interesting to apply other questionnaires in future studies. Second, this was a transversal study, and the IBDQ was applied in a single opportunity. It would be important to evaluate the patient's quality of life over time. Third, we did not correlate IFX trough levels with proinflammatory biomarkers (such as CRP and faecal calprotectin) as well with the patient's body mass index (BMI). Factors such as BMI and proinflammatory biomarkers may influence IFX trough levels. Our intention is to conduct a prospective study to increase the casuistry and correlate all these parameters. Finally, endoscopic evaluation was not accessed in all patients.
5. Conclusions
Adequate IFX trough levels were associated with significantly higher rates of CR, MH, and quality of life in patients with IBD. Achieving quality of life in patients with IBD is a huge challenge for physicians. IBD symptoms have a major impact on patients' lives. TDM can be a useful and important tool in patients losing response. Further prospective studies are needed to confirm our results.
Acknowledgments
The authors thank Mrs. Graziela Volpe (BÜHLMANN Laboratories AG) and Dr. Fernando Pinto (M.D., Padrão Laboratory) for sample storage and serum analysis.
Abbreviations
- IFX:
Infliximab
- IBD:
Inflammatory bowel disease
- UC:
Ulcerative colitis
- CD:
Crohn's disease
- CRP:
C-reactive protein
- CR:
Clinical remission
- MH:
Mucosal healing
- TDM:
Therapeutic drug monitoring
- IBDQ:
Inflammatory bowel disease questionnaire
- HBI:
Harvey Bradshaw Index
- TNF-α:
Antitumor necrosis factor alpha.
Data Availability
Data were collected by the computerized hospital system and medical records. All data used and analyzed during the current study can be sent, if necessary, as e-mail request (corresponding author). All data analyzed during this study are included in this published article.
Ethical Approval
The study was approved by the hospital's ethics committee.
Consent
All of the patients signed an informed consent statement prior to their participation.
Disclosure
This manuscript was presented before as poster as follows: Feitosa MR, Parra RS, Feres O and Rocha JRR. May 2017, Vol. 85, Issue 5, Suppl, Page AB262 DOI: https://doi.org/10.1016/j.gie.2017.03.593. Parra RS, Feitosa MR, Feres O and Ribeiro da Rocha JR. Journal of Crohn's and Colitis, Volume 11, Issue suppl_1, 1 February 2017, Pages S292 DOI: https://doi.org/10.1093/ecco-jcc/jjx002.549.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Rogério S. Parra conceived and designed the study, acquired the data, and wrote and reviewed the manuscript. Marley R. Feitosa acquired the data, did the statistical analysis, and reviewed the manuscript. Letícia C. H. Ribeiro acquired the data. Lais A. Castro acquired the data. José J. R. Rocha and Omar Féres were responsible for the critical revising and final approval of the version to be published.
Funding
Quantum Blue Infliximab assay was donated by BÜHLMANN Laboratories AG.
Supplementary Materials
ROC curves for (A) clinical remission and (B) mucosal healing according to infliximab trough levels.
References
- 1.Jacobs P., Bissonnette R., Guenther L. C. Socioeconomic burden of immune-mediated inflammatory diseases — focusing on work productivity and disability. 2011;88:55–61. doi: 10.3899/jrheum.110901. [DOI] [PubMed] [Google Scholar]
- 2.Holko P., Kawalec P., Mossakowska M., Pilc A. Health-related quality of life impairment and indirect cost of Crohn’s disease: a self-report study in Poland. 2016;11(12, article e0168586) doi: 10.1371/journal.pone.0168586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armuzzi A., van Assche G., Reinisch W., et al. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. 2012;6(4):492–502. doi: 10.1016/j.crohns.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Colombel J. F., Rutgeerts P., Reinisch W., et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. 2011;141(4):1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Reinisch W., Colombel J.-F., Sandborn W. J., et al. Factors associated with short- and long-term outcomes of therapy for Crohn’s disease. 2015;13(3):539–547.e2. doi: 10.1016/j.cgh.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Parra R. S., Feitosa M. R., Rocha J. J., Feres O. Inflammatory bowel diseases & disorders higher trough concentrations of infliximab are associated with clinical remission and mucosal healing in patients with inflammatory bowel disease. 2016;1:p. 104. [Google Scholar]
- 7.Beigel F., Deml M., Schnitzler F., et al. Rate and predictors of mucosal healing in patients with inflammatory bowel disease treated with anti-TNF-alpha antibodies. 2014;9(6, article e99293) doi: 10.1371/journal.pone.0099293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vande Casteele N., Ferrante M., van Assche G., et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. 2015;148(7):1320–1329.e3. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Moore C., Corbett G., Moss A. C. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. 2016;10(5):619–625. doi: 10.1093/ecco-jcc/jjw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afonso J., Lopes S., Gonçalves R., et al. Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. 2016;44(7):684–692. doi: 10.1111/apt.13757. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt G., Mitchell A., Irvine E. J., et al. A new measure of health status for clinical trials in inflammatory bowel disease. 1989;96(2):804–810. doi: 10.1016/S0016-5085(89)80080-0. [DOI] [PubMed] [Google Scholar]
- 12.Irvine E. J., Feagan B., Rochon J., et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. 1994;106(2):287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 13.Irvine E. J., Zhou Q., Thompson A. K. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. 1996;91(8):1571–1578. [PubMed] [Google Scholar]
- 14.Reilly M. C., Gerlier L., Brabant Y., Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. 2008;30(2):393–404. doi: 10.1016/j.clinthera.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Steenholdt C., Brynskov J., Thomsen O. Ø., et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. 2014;63(6):919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 16.Pontes R. M. A., Miszputen S. J., Ferreira-Filho O. F., Miranda C., Ferraz M. B. Quality of life in patients with inflammatory bowel diseases: translation to Portuguese language and validation of the “Inflammatory Bowel Disease Questionnaire” (IBDQ) 2004;41(2):137–143. doi: 10.1590/S0004-28032004000200014. [DOI] [PubMed] [Google Scholar]
- 17.van der Have M., van der Aalst K. S., Kaptein A. A., et al. Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. 2014;8(2):93–106. doi: 10.1016/j.crohns.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Khanna R., Zou G., D'Haens G., et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. 2015;41(1):77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 19.Steenholdt C., Brynskov J., Thomsen O. Ø., et al. Implications of infliximab treatment failure and influence of personalized treatment on patient-reported health-related quality of life and productivity outcomes in Crohn’s disease. 2015;9(11):1032–1042. doi: 10.1093/ecco-jcc/jjv139. [DOI] [PubMed] [Google Scholar]
- 20.Yoon J. Y., Shin J. E., Park S. H., Park D. I., Cha J. M. Disability due to Inflammatory Bowel Disease Is Correlated with Drug Compliance, Disease Activity, and Quality of Life. 2017;11(3):370–376. doi: 10.5009/gnl16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curves for (A) clinical remission and (B) mucosal healing according to infliximab trough levels.
Data Availability Statement
Data were collected by the computerized hospital system and medical records. All data used and analyzed during the current study can be sent, if necessary, as e-mail request (corresponding author). All data analyzed during this study are included in this published article.