Summary
Background
Standard approaches to estimation of losses in the HIV cascade of care are typically cross-sectional and do not include the population stages before linkage to clinical care. We used indiviual-level longitudinal cascade data, transition by transition, including population stages, both to identify the health-system losses in the cascade and to show the differences in inference between standard methods and the longitudinal approach.
Methods
We used non-parametric survival analysis to estimate a longitudinal HIV care cascade for a large population of people with HIV residing in rural KwaZulu-Natal, South Africa. We linked data from a longitudinal population health surveillance (which is maintained by the Africa Health Research Institute) with patient records from the local public-sector HIV treatment programme (contained in an electronic clinical HIV treatment and care database, ARTemis). We followed up all people who had been newly detected as having HIV between Jan 1, 2006, and Dec 31, 2011, across six cascade stages: three population stages (first positive HIV test, HIV status knowledge, and linkage to care) and three clinical stages (eligibility for antiretroviral therapy [ART], initiation of ART, and therapeutic response). We compared our estimates to cross-sectional cascades in the same population. We estimated the cumulative incidence of reaching a particular cascade stage at a specific time with Kaplan-Meier survival analysis.
Findings
Our population consisted of 5205 individuals with HIV who were followed up for 24 031 person-years. We recorded 598 deaths. 4539 individuals gained knowledge of their positive HIV status, 2818 were linked to care, 2151 became eligible for ART, 1839 began ART, and 1456 had successful responses to therapy. We used Kaplan-Meier survival analysis to adjust for censorship due to the end of data collection, and found that 8 years after testing positive in the population health surveillance, 16% had died. Among living patients, 82% knew their HIV status, 45% were linked to care, 39% were eligible for ART, 35% initiated ART, and 33% had reached therapeutic response. Median times to transition for these cascade stages were 52 months, 52 months, 20 months, 3 months, and 9 months, respectively. Compared with the population stages in the cascade, the transitions across the clinical stages were fast. Over calendar time, rates of linkage to care have decreased and patients presenting for the first time for care were, on average, healthier.
Interpretation
HIV programmes should focus on linkage to care as the most important bottleneck in the cascade. Cascade estimation should be longitudinal rather than cross-sectional and start with the population stages preceding clinical care.
Funding
Wellcome Trust, PEPFAR.
Introduction
The HIV cascade of care has been widely used to assess the scale-up of antiretroviral therapy (ART) for HIV, and has provided benchmarks for programme monitoring and highlighted opportunities for inter vention.1 The cascade describes discrete, consecutive stages through which people with HIV pass, including HIV testing, knowledge of HIV status, linkage to care, eligibility for ART,2–6 ART initiation, retention in care, and viral load suppression.3,4,6–8 Because each stage in the cascade depends on the previous stage, missing one stage will in general result in failure to benefit from ART. At the population level, failure along the cascade results in loss of life for people with HIV (an estimated 1.1 million deaths globally in 2015),9–11 increased HIV transmission,12 and substantial economic burdens.13,14 As evidence supporting earlier ART initiation15,16 and treatment as prevention17 accumulates, identification of failure points along the cascade is becoming more important. Unfortunately, the success of the approaches and strategies based on the cascade concept, including those aiming to achieve the UNAIDS 90-90-90 targets,18 is threatened if cascade data are biased or incomplete.
We previously identified four problems19 common in studies of HIV care cascades: missing the preclinical, population stages; failure to account for temporal biases; lack of longitudinal inference; and use of disparate data sources. Cross-sectional cascade analyses are likely to yield biased inference because they do not account for both the changing composition of patients between stages and the time taken to transition from one stage to the next. Although in several studies, most notably those by Nosyk and colleagues20 in 2015 and Alvarez-Uria and colleagues2 in 2013, longitudinal data have been used for cascade analysis, our analysis is the first to use longitudinal, individual-level data across all stages of the cascade, from infection to therapeutic response.
We aimed to use, for the first time, a truly longitudinal cascade, transition by transition, including population stages, both to identify the health-system losses in the cascade and to show the differences in inference between standard methods and the longitudinal approach. We apply survival analysis to data from a longitudinal population health surveillance, which has been linked to clinical HIV programme data, to assess health-system gaps in a rural region with a high prevalence of HIV infection in KwaZulu-Natal, South Africa. Whereas cross-sectional cascades focus on the proportion of people in stages, we focus on the flow between stages. Most previous studies focused on the clinical stages of the cascade, but ours includes both the population and the clinical cascade stages.
Methods
Study site and population
Our study population was individuals with HIV living within a 438 km² area in the mostly rural subdistrict of Hlabisa in KwaZulu-Natal, South Africa. The study area is located near the market town of Mtubatuba; the prevalence of HIV infection in the area is high—around 30% among adults.21,22
The Africa Health Research Institute (AHRI), one of the Wellcome Trust's five major overseas programmes, operates a comprehensive, longitudinal population health surveillance, which includes HIV testing, in the study area.23 All individuals aged 15–50 years who tested positive for HIV between Jan 1, 2006, and Dec 31, 2011, were included in the study and followed up until Jan 27, 2014. There are 17 HIV treatment and care clinics in Hlabisa subdistrict, most of which began distributing free ART between 2004 and 2007.23 The definition for individual eligibility for ART changed during the study period (table 1).9,24–26
Table 1. Definition of events that define cascade stages.
| Definition | Estimation | |
|---|---|---|
| First positive HIV test | Patient tested positive for HIV for the first time | Date of first recorded positive HIV test in the AHRI population health surveillance system |
| HIV status knowledge | Patient knows their HIV status | Annual individual surveys within the AHRI population health surveillance system cohort include a question about whether individuals know their HIV status; all who respond “yes” are recorded as knowing their status on this date; all who have a clinical event (HIV-related clinic visit, laboratory test, or initiation of ART) associated with HIV status knowledge are also recorded as knowing their status (on the date of the event) |
| Linkage to care | Patient engages with formal health-care sector for HIV-related health care | Date of first recorded HIV clinic visit, registration at an HIV clinic, clinical CD4 cell count, viral load count, or ART initiation |
| Eligibility for ART | Patient qualifies for ART, according to the South African national HIV treatment guidelines at that time | Date of first CD4 cell count that meets eligibility criteria for ART at the time of observation* |
| Initiation of ART | Patient starts to take ART | Date of the first ART prescription or dispensing; on the basis of ARTemis data |
| Therapeutic response | Virological suppression or immunological recovery, or both | Date of first instance after ART initiation of CD4 count >500 cells per μL or undetectable viral load (<200 copies per mL) |
The first denominator is “first tested positive” it is a criterion for inclusion of individuals from the AHRI population health surveillance system in the analyses. The subsequent denominators are also the events used in the survival analyses of each cascade stage to define time to event. For instance, those who enter the study analysis on the date when they “first tested positive” are then observed in their time to the event “HIV status knowledge” those included in the denominator “HIV status knowledge” are then observed in their time to the event “linkage to care”, and so on. ARTemis is an electronic clinical HIV treatment and care database. ART=antiretroviral therapy. AHRI=Africa Health Research Institute.
CD4 cell count criteria for ART eligibility varied over time: August, 2004, to March, 2010, ≤200 cells per μL;26 April, 2010, to July, 2011, CD4 count ≤200 cells per μL and ≤350 cells per μL for patients who were pregnant or patients diagnosed with active tuberculosis; after July, 2011, ≤350 cells per μL for all individuals.9,24,25
Data collection
For this analysis, we linked two longitudinal data sources: the population health surveillance, which is maintained by the AHRI, and the electronic clinical HIV treatment and care database ARTemis, which is managed by the Hlabisa Department of Health and the AHRI. The population health surveillance system is based on a series of longitudinally linked annual surveys of all individuals 15 years or older who live in the surveillance area. The surveys include HIV testing, questions about knowledge of HIV status, and questions about sexual and health-care-seeking behaviours.23 HIV test results generated in the surveys are not disclosed to participants. The ARTemis database is derived from the local Department of Health clinic-based HIV treatment and care information system, which contains data for clinic visit dates and laboratory data for all patients enrolled in either pre-ART or ART in one of the 17 clinics within the uMkhanyakude subdistrict. We linked the ARTemis data at the individual level to the data in the AHRI population health surveillance system by using a combination of individual identifers.27
Ethical approval for data collection for both the AHRI population health surveillance system and ARTemis was obtained from the Biomedical Research and Ethics Committee of the University of KwaZulu-Natal. Written consent for data collection from these studies was obtained in Zulu, and included use of collected data for anonymous research.
Statistical analysis
We used Kaplan-Meier non-parametric survival analytic methods28 to estimate the HIV cascade on the basis of the combined data from the the AHRI population health surveillance system and the ARTemis clinical database. We estimated the time to reaching the next stage of the cascade. The events defining the different cascade stages are described in table 1. Because viral load tests were not consistently done in the HIV treatment and care clinics during the analysis period, we used a composite definition of therapeutic success as having a CD4 count of more than 500 cells per μL or a single undetectable viral load (<200 copies per mL29). This definition thus incorporates, but differs from, the WHO definition of undetectable viral load (<1000 copies per mL30).
We characterised the probability of an individual starting at one stage and transitioning to a subsequent stage with a Kaplan-Meier curve for each transition stage. The competing risk of mortality was considered failure to reach a subsequent stage. Patients who reached the last date of cohort follow-up were censored. Eligibility for an event was conditional on a previous event. Whereas many other studies define retention as a binary concept (retained vs lost to follow-up), we demonstrated the full variability of retention by showing the number of clinical visits at all times before transition. Deaths and retention events were counted in each transition for each individual, if they occurred between the beginning and ending stage, so that death and retention are attributable to a specific transition.
All percentages throughout this study represent percentages located on Kaplan-Meier curves. Each point on the curves is the cumulative incidence of an event on a particular day, expressed as the percentage of all those eligible for the event on day 0 who have attained the event by that particular later day. The area above the cumulative incidence curve between the y-axis and a vertical line that is perpendicular to the x-axis and crosses the x-axis on a particular day is the expected number of person-years between the initial stage event and the subsequent event.
People were allowed to have more than one transition on the same day, and were included in the denominator for each stage transition. In an extreme example, if a person were to test positive for HIV on day 0, and their next recorded event was a CD4 count of 150 cells per μL 60 days later, they would appear as having transitioned simultaneously to HIV status knowledge, linkage to care, and ART eligibility stages 60 days after first testing positive. This updating of information conceptually preserves the conditionality of the cascade, which requires that individuals have to go through each stage to reach the next stage.
The population-based view of HIV testing includes all HIV testing sources and facilities. Our data for cascade attainment and progression from both population-based and clinical records ensure that so-called side doors31 into the cascade were captured comprehensively. Analytically, each new transition starts on the day when the event defining eligibility to this transition has occurred, irrespective of the pathway that led a person to that event. This approach correctly accounts for both side doors into the cascade and skipped and simultaneous transitions. For instance, an individual would not contribute person-time to the transition between linkage to care and eligibility, if this individual had simultaneously transitioned to linkage and to eligibility. We also quantified the magnitude of skipped transitions by allowing transitions on day 0, with the proportion who skipped through this stage shown as the y intercept in the Kaplan-Meier graphs.
The proportion of individuals reaching each stage from the time of testing positive was estimated by the Kaplan-Meier estimator, adjusting for time censorship, each day up to 8 years after first testing positive for HIV. We compared this longitudinal cascade estimate with estimated cross-sectional cascades, which were based on the last known stage status of individuals in our study population who were alive at the end of each year between 2006 and 2013.
Role of the funding source
The funders of the study had no role in study design; data collection, analysis, or interpretation; or writing of the Article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
5205 individuals who tested positive for HIV at least once in the dataset were included in the analyses and contributed a total of 24 031 person-years of observation time (table 2). We recorded 598 deaths. 4539 individuals gained knowledge of their positive HIV status, 2818 were linked to care, 2151 became eligible for ART, 1839 initiated ART, and 1456 had a successful response to therapy. 3780 patients (73%) were female; the mean age of participants was 33 years (table 2). The CD4 cell count at first presentation to the ART programme increased with calendar time, from a mean of 270 cells per μL (SD 212) in 2006–07 to a mean of 309 cells per μL (SD 220) in 2010–11 (table 2).
Table 2. Characteristics of patients, by year of first positive HIV test.
| All (2006–11) | 2006–07 | 2008–09 | 2010–11 | |
|---|---|---|---|---|
| Female sex | 73% (0.45) | 73% (045) | 71% (0.45) | 74% (0.44) |
| Age, years | 33.0 (124) | 33.2 (12.9) | 334 (12.8) | 32.4 (11.6) |
| Years of education | 8.09 (3.61) | 7.87 (3.77) | 7.91 (3.72) | 8·44 (335) |
| Married ever | 27% (0.44) | 30% (0.46) | 26% (0.44) | 25% (0.43) |
| Currently employed | 31% (0.46) | 34% (0.47) | 30% (0.46) | 29% (0.46) |
| Ever pregnant* | 83% (0.38) | 82% (0.38) | 82% (0.38) | 84% (0.36) |
| First CD4 count (cells per μL) | 299 (222) | 270 (212) | 305 (230) | 309 (220) |
| N | 5205 | 1712 | 1575 | 1918 |
Data are mean (SD). Binary variables (female, married ever, and pregnant ever) were expressed as percentages.
Percentage of female population.
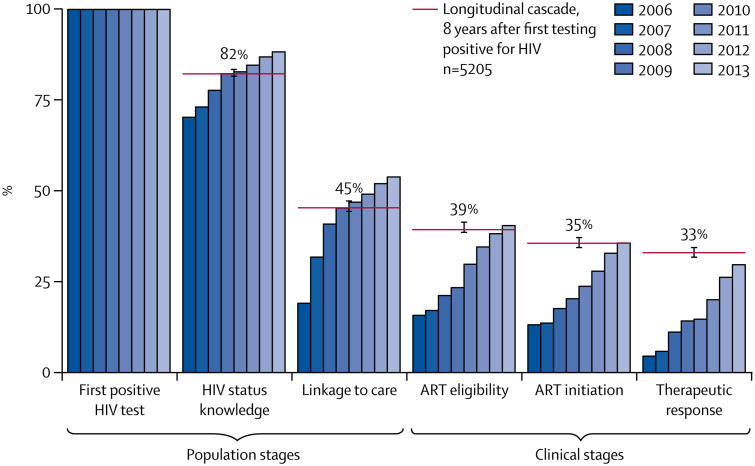
Over time, individuals transition towards downstream stages in the cascade. Because the speed of this downstream transition can vary substantially over calendar time (eg, because ART becomes available in increasingly many clinics), the cross-sectional cascade view can be misleading—for instance, it can substantially and consistently underestimate the true rate of cascade progression, because the denominator of a cascade estimate has increased rather than because transition speed to the next cascade stage has decreased (figure 1).
Figure 1. Kaplan-Meier graphs of longitudinal cascade vs annual cross-sectional cascades.
Bars represent cross-sectional cascades generated on the basis of the last known stage of cascade progression on Dec 31 of each year. The red lines show the percentage of individuals reaching each stage up to 8 years after first testing positive for HIV. 95% CIs from of the Kaplan-Meier estimates are shown as brackets on the red lines. ART=antiretroviral therapy.
At the time of initial detection in the surveillance, 67% (95% CI 65–68) of individuals knew their HIV status, 25% (24–27) had been linked to care, 15% (14–16) were eligible for ART, 13% (12–13) had begun ART, and 7% (6–8) had reached therapeutic response after initiation (appendix p 2). 2 years after first testing positive, 66% (64–68) knew their status, 25% (23–26) were linked to care, 16% (15–17) were eligible for ART, 12% (11–13) had initiated ART, and 10% (9–11) had therapeutically responded (appendix p 3). These data are a conservative estimate of time to event from initial infection, because infection must have occurred before detection. Less than 50% of individuals are expected to have transitioned to any stage in the cascade beyond knowing HIV status within 8 years of testing positive in the surveillance (among those who had not reached later stages at initial detection; appendix pp 3–4).
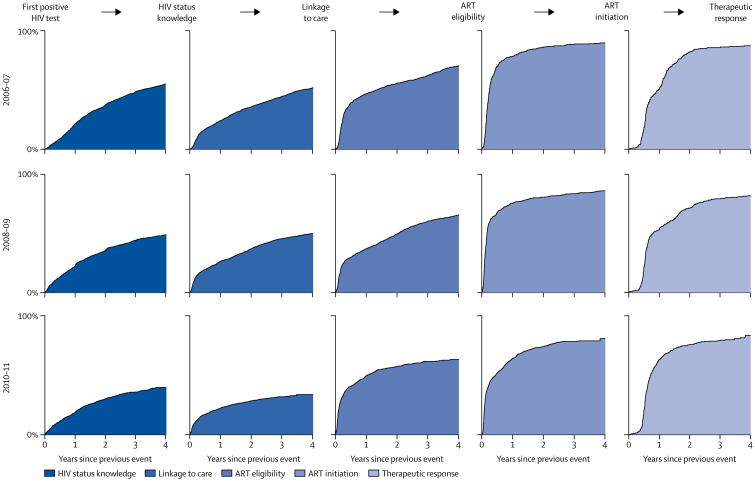
The rates of transition differ substantially across the cascade stages. Overall, the transitions in the population-based stages are slower than those across the later clinical stages (figure 2). Patients who are eligible for care typically began ART within a few months of testing eligible, with a median time to transition of about 3 months. Similarly, once initiated, the median individual reached therapeutic response within a year. Median time to transition from first testing positive for HIV test to knowing HIV status was 52.1 months (95% CI 47.6–57.9), from status knowledge to linkage to care was 51.9 months (48.4–56.4), linkage to care to ART eligibility was 19.5 months (17.0–22.3), ART eligibility to ART initiation was 3.1 months (2.8–3.4), and ART initiation to therapeutic response was 9.3 months (8.6–10.2; appendix p 5).
Figure 2. Transition to each stage by year—single state transitions.
n=5205. Columns from left to right represent cascade stage transitions; rows from top to bottom show the years in which the entry transition event occurred. For example, the chart in the fourth column, second row, is the transition from “eligible for ART” to “initiated ART” for patients who became eligible for ART between 2008 and 2009 among those who had not yet initiated ART, for up to 4 years after reaching eligibility for ART. ART=antiretroviral therapy.
Importantly, the transitions through the two population-based stages of the cascade slowed down over calendar time (figure 2). By contrast, individuals who have started treatment seemed to be responding faster in later than in earlier years, with median time to therapeutic response of 12 months (95% CI 9–13) among those who initiated ART in 2006–07 and 9 months (95% CI 8–10) among those who initiated ART in 2010–11 (appendix p 5). In the appendix, we also summarise time-to-transition data in an alternative form (p 6): we show the percentage of people who have reached the next cascade stage 2 and 4 years after reaching the present stage.
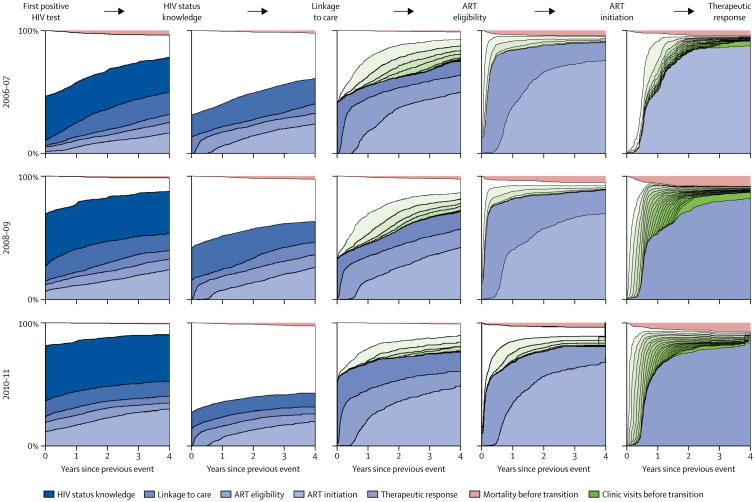
The longitudinal cascade in figure 2 is useful because it focuses on the cumulative incidence of transitioning across individual cascade stages, but does not contain detail on which stages people move to, or about sub-sequent stages. Figure 3 shows skipped transitions and simultaneous transitions across several cascade stages.
Figure 3. Transition to each stage by year, including entrance and exit stages, retention, and mortality.
n=5205. The intercept in this figure shows the percentage of people who have already transitioned across subsequent cascade stages the first time they are detected in the denominator of a particular cascade stage. These intercepts thus represent the percentages of people who skip cascade stages or simultaneously transition across several stages. Each blue-shaded striation represents the cumulative incidence of reaching a cascade stage subsequent to the one that is represented by thick black lines in each panel of this figure. The percentage of people who have had a given number of additional clinic visits before transitioning is shown in green—the darker the shade of green, the more clinic visits. The cumulative incidence of death before the next transition is shown in pink, and the white area represents the proportion of people at each timepoint who are alive, have not made any additional clinic visits, and have not transitioned to a subsequent cascade stage. ART=antiretroviral therapy.
As calendar time progressed, deaths occurred at increasingly later stages in the cascade (figure 3; appendix p 7). Retention was very high among people who were linked to care, particularly among those who began ART (figure 3). Furthermore, retention improved over calendar time, with increasingly large percentages of people having either transitioned to the next stage or making additional clinic visits without transitioning (figure 3).
To examine the overall effect of changing ART eligibility guidelines in South Africa, we constructed the HIV treatment cascade over calendar time, rather than over time since first detection of an individual with HIV (appendix p 1). Although the early stages of the cascade did not seem to be affected by changes to guidelines for ART eligibility in South Africa, we noted small increases in the proportions of people reaching later cascade stages (ART eligibility, ART initiation, and therapeutic response), which could be explained by the guideline changes.
Discussion
We used an individually linked longitudinal cascade of care from time of HIV detection in the population to clinical therapeutic response to show that linkage to care is the most important bottleneck in the HIV cascade in rural KwaZulu-Natal. Our population-based and longitudinal analysis of the cascade provides crucial insights that would not have been generated from either cross-sectional or facility-based cascade analyses.
One key finding is that the early stages of the cascade differ substantially in character from the later stages. In the early stages before treatment eligibility is known, transitions are slow and unpredictable. Individuals tend not to transition for long times, but when they do, they often do so in rapid succession across several cascade stages. Once individuals are known to be eligible for ART, transitions tend to occur rapidly and predictably.
Comparison of longitudinal and cross-sectional analyses shows how the latter can be misleading. The repeated cross-sectional cascades we constructed as an alternative to longitudinal analysis imply improvement in the cascade with time. However, much of that improvement is attributable to the passage of calendar time, rather than health-systems improvement. This insight becomes apparent only with longitudinal cascade analysis.
The longitudinal cascade contains all cross-sectional perspectives that are possible during the observation period, in addition to the cascade's development over time. Thus longitudinal cascades will always be better than otherwise-equivalent cross-sectional cascades. However, cross-sectional cascade analyses are likely to remain useful because the data needed for their construction are easier, quicker, and cheaper to collect. Data collection systems for national-level cascades might necessitate substantial financial commitment and long-term collaboration across many clinical and research sites. The data for this study came from one of Africa's largest and longest-running population-based and longitudinal public health surveillance systems. For other longitudinal cascade analyses, less resource-intensive data collection will probably be sufficient, but individual-level longitudinal data for cascade events requires substantial investment in data collection. When such investments are not feasible, cross-sectional cascades are the next-best option to inform policy and programmatic decisions.
Our findings complement and advance those of previous work on the HIV treatment cascade. In an important previous study, 32 Nsanzimana and colleagues estimated the cross-sectional cascade from multiple data sources for Rwanda. One of the main conclusions of that work—that major losses in cascade progression occur at the stage of linkage to care—also holds true in our study, which was in a very different context. Our study substantially strengthens the evidence for this finding, because we were able to estimate the cascade for the first time both longitudinally and on the basis of individually linked data across all cascade stages (rather than cross-sectional comparisons of cascade stages estimated by combining aggregate denominator and numerator data from different sources19). Our findings also complement with direct measurement the indirect evidence provided by Siedner and colleagues,33 who inferred that barriers to “presentation, diagnosis, and linkage to HIV care remain major challenges” in sub-Saharan Africa on the basis of the fact that CD4 cell count at the point of linkage to care did not increase significantly between 2002 and 2013.
Counterintuitively, as the rollout of HIV interventions expands and services improve, some measures of health-systems performance can seem to worsen. One reasonable explanation for this effect is population dynamics. Early in the rollout of ART, there is a backlog of individuals who had been ill for some time. These patients are likely to link quickly to newly available HIV care. Individuals reaching a given cascade stage in later years are more likely to have been infected more recently and, as a result, less likely to have symptoms of HIV, which could reduce the motivation to engage in care. CD4 cell counts at first presentation in our population steadily increased with time, consistent with the findings of other studies in South Africa.33 Other factors, such as underlying beliefs and psychological traits, socioeconomic status, and physical access to health services, are probably changing over time with respect to newly infected and detected individuals, and could also be contributing to the slowing of linkage over time.
Our data have several important limitations. The second stage in our analyses (ie, knowledge of HIV status) is measured on the day of a self-report of HIV status rather than on the actual day when patients became aware of their status. However, because we gathered data annually, the underestimation of the cumulative incidence of individuals who gained HIV status knowledge should not be very large. Another limitation is that some people eligible for HIV testing in the surveillance cannot be contacted in a specific year or refuse to test for HIV. However, over 5 years, more than 80% of eligible individuals tested for HIV at least once in the study population,34 ensuring that, during the observation period, most people with HIV were included in our sample. We would expect that people who do not test for HIV have even lower rates of linkage to care and other cascade progressions than those in our study, suggesting that our conclusions are likely to be conservative. A third limitation is that, although this analysis might show trends and changes, we do not quantitatively estimate the effect of the causal mechanisms underlying those changes. Future analyses should be done to understand better the determinants of the speed of cascade progression.
Interventions increasingly need to promote HIV testing in early disease stages and linkage to care among people with HIV who have not yet taken up care. Options for improvements in HIV testing uptake include financial incentives and gifts for HIV testing,35 home-based testing approaches, and testing with support from community health workers. HIV self-testing, which has become available in South Africa and several other countries in the region, could also contribute to increased knowledge of HIV status in people in early disease stages. Options for enhancement of linkage to care include interventions that address the major structural and behavioural barriers to HIV treatment uptake, including supply-side interventions (eg, subsidised transport to clinics and building of additional ART clinics) and demand-side interventions (eg, motivational counselling, financial incentives, and mobile phone reminders to link to care after a positive HIV test).
Scientists need to support efforts to improve HIV testing and linkage to care through effectiveness experiments and rigorous implementation science, and qualitative studies to discover the underlying reasons why some subpopulations are at higher risk of not testing or linking to care than others. In addition to empirical studies of interventions complementing and enhancing existing models of HIV treatment and care, research into the effect of novel models of HIV care, such as home-based ART initiation and integrated chronic disease care, is needed. Because funding for novel interventions and models of care will compete with other funding priorities, detailed (and ideally causally determined) cost data should be gathered in these studies. Importantly, as our results show, future studies of interventions and novel models of care should aim to esablish approaches to accelerate cascade progression and should be based on longitudinal outcomes data.
Supplementary Material
Research in context.
Evidence before this study
We did a targeted search based on a previous review and critique of the literature about the HIV cascade of care that we published. The primary source of evidence reviewed for this Article was the reference list for the previous paper, which includes both current and historical context for the construction of the HIV cascade. The methods review of that paper covers peer-reviewed published work, conference proceedings, and governmental institution publications. In most cascade analyses, cross-sectional designs are used to identify gaps in care, but cross-sectional analyses are likely to be biased as a result of violations of synthetic cohort and long-term steady-state assumptions. We noted a lack of comprehensive longitudinal cascade data. Of the few longitudinal cascades identified, all contained only the clinical cascade stages and therefore could not be used to identify losses in the preclinical population stages. No cascade analysis had been done that followed up individuals longitudinally over all phases of the cascade from time of infection before knowledge of status, through status knowledge, linkage to care, ART initiation, and recovery. Furthermore, of the analyses with longitudinal data, none fully leveraged serial survival analytic methods, which can provide a complete and detailed view of the cascade and reduce the need for model assumptions.
Added value of this study
In this Article, we address the limitations of previous work by presenting the first population-based longitudinal analysis of the entire HIV cascade of care. Beginning in 2006, after the initial rollout of antiretroviral therapy in South Africa's public health system, we followed up individuals from HIV infection to therapeutic response. We used survival analyses serially between all cascade stages to characterise both the simple time to transition and the shape of the survival analytic transition curve, leading to improved inference on the losses occurring at each stage. These innovations allow us to strengthen the existing evidence and to identify initial linkage to care as the most substantial loss in the cascade, which would have been biased in, or entirely missing from, other cascade-of-care analyses.
Implications of all available evidence
The major implications of our findings are twofold. First, policy makers in rural communities in South Africa and the wider region should focus their efforts on improvement of initial linkage to care, which was the most important gap identified in our study. Second, researchers should, whenever feasible, use longitudinal data and survival analytic methods in HIV cascade analyses, because inference based on cross-sectional analyses could lead to substantial bias in the identification of losses along the cascade.
Acknowledgments
Core funding supporting the AHRI population health surveillance system and ARTemis was primarily provided by the Wellcome Trust and PEPFAR. FT and TB received funding from the Wellcome Trust and from the US National Institutes of Health (NICHD R01-HD084233 and NIAID R01-AI124389). FT received additional funding from the South African MRC Flagship (MRC-RFA-UFSP-01–2013/UKZN HIVEPI) and a UK Academy of Medical Sciences Newton Advanced Fellowship (NA150161). JB received funding from the National Institutes of Health (NIH 1KO1MH105320). The contents are the responsibility of the authors and do not necessarily reflect the views of any of the funders or the US Government. TB received additional funding from the Alexander von Humboldt Foundation through the Alexander von Humboldt professor award, the European Commission, the Clinton Health Access Initiative, and the US National Institutes of Health (NIA P01-AG041710, NIAID R01-AI112339, and FIC D43-TW009775).
Funding: Wellcome Trust, PEPFAR.
Footnotes
Contributors: NH and TB developed the study concept and oversaw implementation of methods, led interpretation of analysis, and drafted the Article. FT contributed to early conceptual and technical support in the study design phase, and provided writing and input on the Article. JB contributed key insight and input to the conceptual development of the model and interpretation of results, and provided writing and input on the Article. KN and TM contributed to the dataset collection and model implementation. KH provided key support on the data collection, dataset structure, implementation, and input on the draft Article. KP contributed conceptual feedback on the development of the model and interpretation of results, and provided writing and input on the Article. DP and TB were senior authors and contributed key input and direction to all phases of the project, from model development to Article writing. TB initiated this work.
Declaration of interests: We declare no competing interests.
References
- 1.WHO. Global update on HIV treatment 2013: results, impact, and opportunities. Geneva: World Health Organization; 2013. [Google Scholar]
- 2.Alvarez-Uria G, Pakam R, Midde M, Naik PK. Entry, retention, and virological suppression in an HIV cohort study in India: description of the cascade of care and implications for reducing HIV-related mortality in low- and middle-income countries. Interdiscip Perspect Infect Dis. 2013;2013:384805. doi: 10.1155/2013/384805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;1517:383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mugglin C, Estill J, Wandeler G, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17:1509–20. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilmarx PH, Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIV AIDS. 2013;8:59–64. doi: 10.1097/COH.0b013e32835b806e. [DOI] [PubMed] [Google Scholar]
- 8.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–65. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. Fact Sheet 2016. [accessed Jan 17, 2017]; http://www.unaids.org/en/resources/fact-sheet.
- 12.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 13.Rosen S, Larson B, Brennan A, et al. Economic outcomes of patients receiving antiretroviral therapy for HIV/AIDS in South Africa are sustained through three years on treatment. PLoS One. 2010;5:e12731. doi: 10.1371/journal.pone.0012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bor J, Tanser F, Newell ML, Bärnighausen T. In a study of a population cohort in South Africa, HIV patients on antiretrovirals had nearly full recovery of employment. Health Affairs. 2012;31:1459–69. doi: 10.1377/hlthaff.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010;3:CD008272. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton JW, Johnson LF, Salomon JA, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 19.Haber N, Pillay D, Porter K, Bärnighausen T. Constructing the cascade of HIV care: methods for measurement. Curr Opin HIV AIDS. 2016;11:102–08. doi: 10.1097/COH.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 20.Nosyk B, Montaner JSG, Colley G, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14:40–49. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyirenda M, Zaba B, Bärnighausen T, Hosegood V, Newell ML. Adjusting HIV prevalence for survey non-response using mortality rates: an application of the method using surveillance data from rural South Africa. PLoS One. 2010;5:e12370. doi: 10.1371/journal.pone.0012370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27:2301–05. doi: 10.1097/QAD.0b013e328362e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanser F, Hosegood V, Bärnighausen T, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37:956–62. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.South African Department of Health. The South African Antiretroviral Treatment Guidelines 2013. Pretoria: Department of Health; 2014. [Google Scholar]
- 25.Clouse K, Pettifor A, Maskew M, et al. Initiating ART when presenting with higher CD4 counts results in reduced loss to follow-up in a resource-limited setting. AIDS. 2013;27:645–50. doi: 10.1097/QAD.0b013e32835c12f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.South African Department of Health. The South African Antiretroviral Treatment Guidelines 2010. Pretoria: Department of Health; 2010. [Google Scholar]
- 27.Houlihan CF, Bland RM, Mutevedzi PC, et al. Cohort profile: Hlabisa HIV Treatment and Care Programme. Int J Epidemiol. 2011;40:318–26. doi: 10.1093/ije/dyp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 29.Ribaudo H, Lennox J, Currier J, et al. Virologic failure endpoint definition in clinical trials: is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL? An analysis of ACTG studies. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, QC, Canada. Feb 8–11, 2009; abstr 580. [Google Scholar]
- 30.WHO. Metrics for monitoring the cascade of HIV testing, care and treatment services in Asia and the Pacific. Manila: World Health Organization Regional Office for the Western Pacific; 2014. [Google Scholar]
- 31.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune Defc Syndr. 2013;63(suppl 2):S228–32. doi: 10.1097/QAI.0b013e318298721b. [DOI] [PubMed] [Google Scholar]
- 32.Nsanzimana S, Kanters S, Remera E, et al. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. Lancet HIV. 2015;2:e208–15. doi: 10.1016/S2352-3018(15)00024-7. [DOI] [PubMed] [Google Scholar]
- 33.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60:1120–27. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larmarange J, Mossong J, Bärnighausen T, Newell ML. Participation dynamics in population-based longitudinal HIV surveillance in rural South Africa. PLoS One. 2015;10:e0123345. doi: 10.1371/journal.pone.0123345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGovern M, Herbst K, Tanser F, et al. Do gifts increase consent to home-based HIV testing? A difference-in-differences study in rural KwaZulu-Natal, South Africa. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw122. published online Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.