Abstract
Background
Recent findings suggest that transcranial direct current stimulation of the primary motor cortex may ameliorate freezing of gait. However, the effects of multitarget simultaneous stimulation of motor and cognitive networks are mostly unknown. The objective of this study was to evaluate the effects of multitarget transcranial direct current stimulation of the primary motor cortex and left dorsolateral prefrontal cortex on freezing of gait and related outcomes.
Methods
Twenty patients with Parkinson’s disease and freezing of gait received 20 minutes of transcranial direct current stimulation on 3 separate visits. Trans-cranial direct current stimulation targeted the primary motor cortex and left dorsolateral prefrontal cortex simultaneously, primary motor cortex only, or sham stimulation (order randomized and double-blinded assessments). Participants completed a freezing of gait-provoking test, the Timed Up and Go, and the Stroop test before and after each transcranial direct current stimulation session.
Results
Performance on the freezing of gait-provoking test (P = 0.010), Timed Up and Go (P = 0.006), and the Stroop test (P = 0.016) improved after simultaneous stimulation of the primary motor cortex and left dorsolateral prefrontal cortex, but not after primary motor cortex only or sham stimulation.
Conclusions
Transcranial direct current stimulation designed to simultaneously target motor and cognitive regions apparently induces immediate aftereffects in the brain that translate into reduced freezing of gait and improvements in executive function and mobility.
Keywords: freezing of gait, Parkinson’s disease, brain stimulation, transcranial direct current stimulation
Freezing of gait (FOG) is one of the most disturbing and least understood symptoms of Parkinson’s disease (PD),1 markedly decreases independence1 and increases fall risk.2 As current pharmacological and nonpharmacological treatments are not optimal, especially in the advanced stages, new therapies are needed to manage this disabling phenomena.1
There is growing interest in using transcranial direct current stimulation (tDCS) as a low-cost, noninvasive option for meeting the therapeutic gaps associated with PD and FOG.3,4 Because PD is first and foremost a motor disturbance phenomena, most studies in PD used tDCS to target the primary motor cortex (M1), reporting improvements in motor function and gait, compared with sham stimulation.3,4 In a crossover pilot study, FOG was less severe after 5 sessions of M1 stimulation, with benefits 4 weeks later.5 These results suggest that tDCS targeting M1 might be an effective tool to intervene on FOG, with immediate and perhaps longer-term effects.
Several hypotheses suggest that FOG is not only a motor problem, but also arises in part because of deficits in executive function,1,6–8 a cognitive domain mediated by the dorsolateral prefrontal cortex (DLPFC).9,10 tDCS targeting the DLPFC appears to positively affect cognition, gait, and postural control in other populations.11–18 A recent study applied trans-cranial magnetic stimulation (TMS) over the motor cortex and tDCS over the left-DLPFC (lDLPFC), observing a positive impact on gait and cognition in patients with FOG.19 We speculated that tDCS designed to simultaneously target M1 and lDLPFC may have a greater effect on FOG and related outcomes compared with tDCS targeting M1 alone. We therefore developed a novel tDCS montage using Stim-weaver technology20,21 to direct current flow to both brain regions simultaneously. We compared a single session of this “multitarget” tDCS to tDCS targeting only the M1, and to an “active” sham protocol20,22 to examine its short-term effects on FOG severity and common tests of mobility and executive function in individuals with PD. We hypothesized that multitarget tDCS of M1 and lDLPFC would reduce FOG severity and enhance motor and cognitive function, with a larger improvement than after M1 only or sham stimulation.
Methods
Participants
Twenty individuals with PD and FOG participated in this study. All subjects met the criteria for idiopathic PD according to the UK Brain Bank criteria23 and had documented FOG (see Supplementary Material for details).
Study Design
This double-blind, randomized, sham-controlled tDCS study consisted of 4 in-person visits. In visit 1, demographic, clinical, and cognitive features were assessed (see Supplementary Material). Participants also performed all assessment tests to minimize learning effects on the outcome measures from pre- to post-tDCS in visits 2-4. Because FOG is a highly variable phenomenon,1 a crossover design was conducted, with pre-/post-assessments for each condition. We focused on within-visit, pre- to post-tDCS changes to mitigate any effects because of practice or day-to-day variations.
In visits 2-4, FOG, mobility, and cognition were assessed before and immediately after a 20-minute tDCS session. tDCS condition (ie, M1 only, M1 and lDLPFC, sham) order was randomized across participants and was separated by at least a 48-hour washout period.24–26 Assessment order was fixed: gait, FOG-provoking test, Timed Up and Go (TUG), and the Stroop test.
Statistical Analyses
We first examined the within-visit effects of each tDCS condition separately on the primary outcome (change in FOG score) and secondary outcomes (TUG time, gait speed, and correct responses in the Stroop interference task) using Student paired t tests to compare pre- and post-tDCS scores. We then compared the effects of the 3 tDCS conditions using 1-way repeated-measures analysis of variance. The dependent variable of each model was the pre- to post-tDCS change in each outcome. A 2-tailed P ≤ 0.05 was considered statistically significant. Blinding efficacy and further details are in the Supplementary Material.
Results
Subject characteristics are summarized in Supplementary Table 1. As expected, motor and cognitive deficits were common, and the measures of FOG reflected a high degree of FOG.
Effects of tDCS on FOG
An example demonstrating the beneficial effect of the multitarget tDCS on FOG for 1 participant is shown in a Supplementary Material video; the FOG score decreased from 6 at baseline to 0 after the stimulation. Similar to what was seen in the video, FOG scores in 15 of the 17 subjects (88%) were lower (better) after multitarget stimulation (see Fig. 1A). On average, multitarget tDCS reduced (improved) FOG-provoking scores (P = 0.010). In contrast, significant improvements were not seen after M1 only (P = 0.576) or active-sham stimulation (P = 0.858); see Figure 1B, C.
FIG. 1.
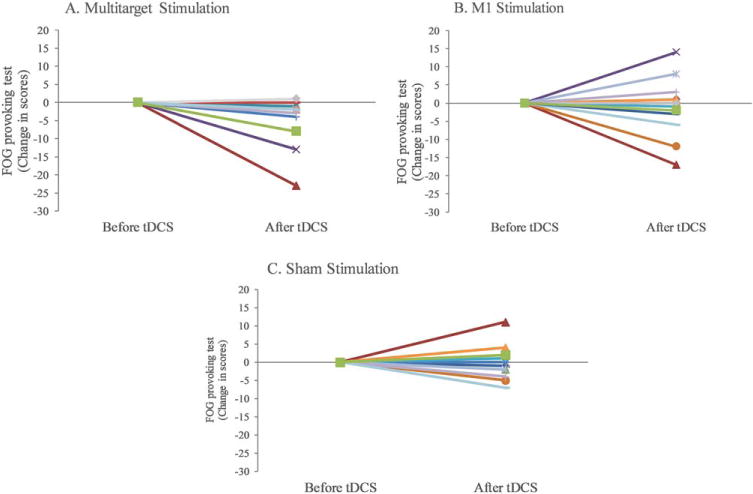
Individual changes in FOG-provoking test scores after multitarget stimulation (A), M1 stimulation (B) or sham stimulation (C). Each line represents outcome for the same subject in the three simulation conditions. Negative values indicate improvement because lower scores on this test reflect less FOG. For example, the square-green line represents an individual that after the multitarget stimulation had a reduction of 8 points, while after stimulating the motor cortex alone had a reduction of 2 points, compared with an increase of 2 points after sham stimulation. Note that even after removing the 3 subjects with the largest reductions, on a group level, the significant improvement after the multitarget stimulation still persisted (p = 0.003). [Color figure can be viewed at wileyonlinelibrary.com]
Multitarget tDCS appeared to have a greater effect on FOG severity than M1 only and active-sham stimulation (P = 0.06); see Figure 2A. Multitarget tDCS induced a larger reduction in FOG score compared with sham stimulation (P = 0.022), and a marginally-significant reduction in this score compared with M1-only stimulation (P = 0.063). The effects of M1 only and sham stimulation on FOG score were similar to each other (P = 0.391). The decrease in FOG scores following multitarget tDCS was correlated with baseline FOG scores (Pearson’s r = −0.59, P = 0.012); subjects with more severe freezing at baseline showed greater improvement following multitarget stimulation.
FIG. 2.
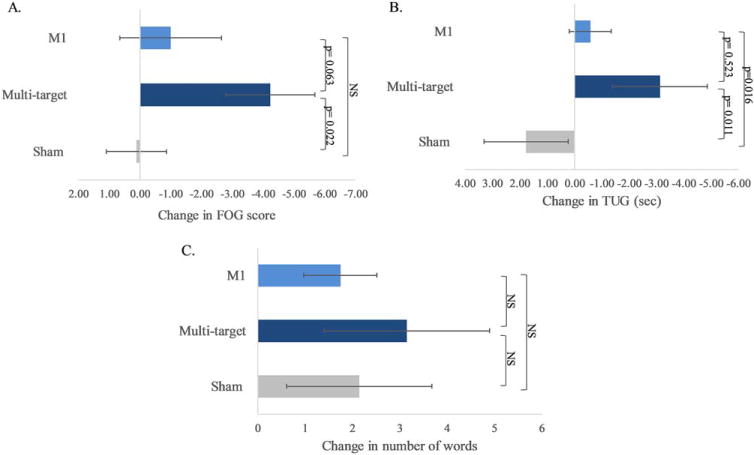
The immediate effects of tDCS on FOG, mobility, and executive function. (A) FOG-provoking test scores. (B) Time Up and Go (TUG) test. (C) Stroop interference trial. Bar graphs indicate the change (D) in performance from pre- to post-tDCS, along with standard error. For both the FOG-provoking and the TUG, lower scores indicate better performance. For Stroop interference scores, a larger number of correct words indicates better executive function (ie, response inhibition). [Color figure can be viewed at wileyonlinelibrary.com]
Effects of tDCS on Secondary Outcomes
Multitarget tDCS improved TUG performance (P = 0.006). In contrast, TUG time was not affected by M1-alone stimulation (P = 0.227) or active-sham (P = 0.260). As summarized in Figure 2B, changes in TUG performance differed across tDCS conditions (P = 0.024). Multitarget tDCS improved TUG time (Δ = −3.168 ± 7.631) more than sham (Δ = 1.9 ± 6.5; P = 0.011), but not significantly more than M1 stimulation (Δ = −0.8 ± 3.1; P = 0.523). In addition, M1 stimulation improved TUG time more than sham (P = 0.016). Multitarget tDCS also improved gait speed (111.9 ± 2.1 to 116.6 ± 2.1 cm/s; P = 0.019). In contrast, gait speed did not change after M1 (P = 0.804) or after sham stimulation (P = 0.110). Across-condition analysis revealed no differences in gait speed (P > 0.285).
Multitarget tDCS improved the correct number of words in the Stroop interference task (P = 0.016). In contrast, significant improvements were not observed after M1 only (P = 0.188) or after active-sham stimulation (P = 0.190). Across-condition change analysis did not reveal any significant difference between the 3 conditions (P = 0.759); see Figure 2C.
tDCS Blinding Efficacy
After multitarget and after M1-only stimulation, the majority of participants believed that they received real stimulation (≥70%), with similar confidence levels (see Supplementary Table 2). Following sham, 50% of the participants thought they received real stimulation, with a relatively high confidence level. When comparing the 3 stimulations, no significant difference was found in the number of subjects who reported real or sham. The confidence levels were also similar after real and sham responses.
Discussion
A single session of multitarget tDCS designed to simultaneously facilitate the excitability of both M1 and lDLPFC reduced the severity of FOG in individuals with PD immediately following stimulation. In addition, putative motor and cognitive mediators of FOG, as reflected in TUG, gait speed, and the Stroop interference test, also improved following this stimulation. Targeting M1 only was less effective, leading only to trends toward improved performance in the FOG-provoking test and TUG, with no effect on the Stroop. Sham stimulation had minimal or no effect on any of the outcomes. Most subjects did speculate that they were receiving multitarget or M1 tDCS, as in both the current intensity was higher than with sham, underscoring the importance of the active control (ie, M1) condition. Moreover, after sham stimulation, half the participants believed they received real stimulation (ie, chance level), with even higher confidence levels compared with those who thought that they received sham. Taken together, these findings suggest that the observed effects of multitarget stimulation were not because of a placebo effect and, that they were larger than those effects induced by M1 stimulation alone.
Several studies have applied noninvasive brain stimulation (tDCS or TMS) to influence FOG in PD, and the cortical target in most of these studies was M1.5,27–31 Here we have demonstrated that stimulating the lDLPFC simultaneously with M1 induced greater benefit to FOG severity, compared with M1 stimulation alone. We speculate that simultaneous facilitation of M1 and DLPFC may have reduced FOG severity via several pathways. First, perhaps multitarget stimulation facilitated dopaminergic circuits, similar to the way that TMS of the lDLPFC increased extra-striatal dopamine release.32 Second, improvements may be related to the abnormal cortical processing seen during functional connectivity in freezers.33 Compared with nonfreezers, PD freezers had functional decoupling between the basal ganglia network and the cognitive control network, which includes the DLPFC, while performing a virtual reality gait task in the MRI.33 This decoupling was also associated with freezing-like paroxysmal motor arrests. These and our findings suggest that freezing episodes may be caused in part by impaired communication between the prefrontal cortex, motor cortex, and subcortical structures. Third, the improvement observed after multitarget tDCS might be because of enhanced executive function (ie, set shifting, working memory, and response inhibition) and the influence on motor control, as previously suggested by prospective findings.34 Regardless of the exact mechanisms, simultaneous stimulation of M1 and lDLPFC apparently has a larger beneficial impact on FOG than stimulation of M1 alone, underscoring the importance of this prefrontal brain region and its related network(s).
We did not test the effects of tDCS targeting only the lDLPFC in the current study. Previous reports suggest that stimulating this area has no effect29 or a lesser effect on FOG and other motor outcomes in PD compared with stimulating M1 alone.13,19,27,35 Therefore, to minimize subject burden and to focus on the added value of combining M1 with DLPFC stimulation, we compared the combination stimulation to M1 alone and to sham. In addition, we did not assess if a cumulative effect with long-term retention could be achieved by repeated tDCS sessions. The present findings set the stage for future tDCS studies to address these and related questions. There is growing evidence that home-based and remote supervision tDCS is safe and feasible36 in other cohorts. Conceivably, this low-cost, noninvasive option could be used in the future at home as an adjunct therapy to help alleviate FOG, perhaps along with other forms of rehabilitation. The present findings should be interpreted cautiously, and additional research is needed on the use of noninvasive brain stimulation for the understanding and treatment of FOG. Nonetheless, we suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG.■
Supplementary Material
Acknowledgments
Relevant conflicts of interest/financial disclosures: Prof. Jeffrey Hausdorff is supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research. Dr. Brad Manor is supported by grants from the National Institute on Aging. Dr. Giulio Ruffini is a cofounder and shareholder of Neuroelectrics Corporation, which makes brain stimulation technologies such as the ones used in this study.
Funding Agency: The Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- 1.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson’s disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag. 2014;4:203–221. doi: 10.2217/nmt.14.22. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci R, Mameli F, Ruggiero F, Priori A. Transcranial direct current stimulation as treatment for Parkinson’s disease and other movement disorders. Basal Ganglia. 2016;6:53–61. [Google Scholar]
- 4.Broeder S, Nackaerts E, Heremans E, et al. Transcranial direct current stimulation in Parkinson’s disease: neurophysiological mechanisms and behavioral effects. Neurosci Biobehav Rev. 2015;57:105–117. doi: 10.1016/j.neubiorev.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Valentino F, Cosentino G, Brighina F, et al. Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord. 2014;29:1064–1069. doi: 10.1002/mds.25897. [DOI] [PubMed] [Google Scholar]
- 6.Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson’s disease: a review of current neurobehavioral evidence. Neurosci Biobehav Rev. 2014;43:213–227. doi: 10.1016/j.neubiorev.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Kucinski A, Albin RL, Lustig C, Sarter M. Modeling falls in Parkinson’s disease: Slow gait, freezing episodes and falls in rats with extensive striatal dopamine loss. Behav Brain Res. 2015;282:155–164. doi: 10.1016/j.bbr.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbossche J, Deroost N, Soetens E, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banich MT. Executive function: the search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. [Google Scholar]
- 10.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 11.Metuki N, Sela T, Lavidor M. Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimul. 2012;5:110–115. doi: 10.1016/j.brs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Zwissler B, Sperber C, Aigeldinger S, Schindler S, Kissler J, Plewnia C. Shaping memory accuracy by left prefrontal transcranial direct current stimulation. J Neurosci. 2014;34:4022–4026. doi: 10.1523/JNEUROSCI.5407-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 14.Boggio PS, Ferrucci R, Rigonatti SP, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Doruk D, Gray Z, Bravo GL, Pascual-Leone A, Fregni F. Effects of tDCS on executive function in Parkinson’s disease. Neurosci Lett. 2014;582:27–31. doi: 10.1016/j.neulet.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Hao Y, Wang Y, et al. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci. 2014;39:1343–1348. doi: 10.1111/ejn.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou D, Zhou J, Chen H, Manor B, Lin J, Zhang J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp Brain Res. 2015;233:2401–2409. doi: 10.1007/s00221-015-4310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manenti R, Brambilla M, Rosini S, et al. Time up and go task performance improves after transcranial direct current stimulation in patient affected by Parkinson’s disease. Neurosci Lett. 2014;580:74–77. doi: 10.1016/j.neulet.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Chang WH, Kim MS, Park E, et al. Effect of Dual-mode and dual-site noninvasive brain stimulation on freezing of gait in patients with Parkinson disease. Arch Phys Med Rehabil. 2017;98:1283–1290. doi: 10.1016/j.apmr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89:216–225. doi: 10.1016/j.neuroimage.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage. 2013;70:48–58. doi: 10.1016/j.neuroimage.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Fischer DB, Fried PJ, Ru G, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage. 2017;157:34–44. doi: 10.1016/j.neuroimage.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monte-Silva K, Kuo MF, Hessenthaler S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated noninvasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- 26.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Kim M-S, Chang WH, Cho J-W, Youn J-Y, Kim Y-H. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with parkinsonism. Restor Neurol Neurosci. 2014;32:743–753. doi: 10.3233/RNN-140397. [DOI] [PubMed] [Google Scholar]
- 28.Kim MS, Chang WH, Cho JW, et al. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson’s disease. Restor Neurol Neurosci. 2015;33:521–530. doi: 10.3233/RNN-140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rektorova I, Sedlackova S, Telecka S, Hlubocky A, Rektor I. Repetitive transcranial stimulation for freezing of gait in Parkinson’s disease. Mov Disord. 2007;22:1518–1519. doi: 10.1002/mds.21289. [DOI] [PubMed] [Google Scholar]
- 30.Dagan M, Herman T, Mirelman A, Giladi N, Hausdorff JM. The role of the prefrontal cortex in freezing of gait in Parkinson’s disease: insights from a deep repetitive transcranial magnetic stimulation exploratory study. Exp Brain Res. 2017;235(8):2463–2472. doi: 10.1007/s00221-017-4981-9. [DOI] [PubMed] [Google Scholar]
- 31.Chang WH, Kim MS, Cho JW, et al. Effect of cumulative repetitive transcranial magnetic stimulation on freezing of gait in patients with atypical parkinsonism: A pilot study. J Rehabil Med. 2016;48:824–828. doi: 10.2340/16501977-2140. [DOI] [PubMed] [Google Scholar]
- 32.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shine JM, Matar E, Ward PB, et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136:3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- 34.Amboni M, Barone P, Picillo M, et al. A two-year follow-up study of executive dysfunctions in Parkinsonian patients with freezing of gait at on-state. Mov Disord. 2010;25:800–802. doi: 10.1002/mds.23033. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, Boggio PS, Santos MC, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- 36.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


