Abstract
Obesity prevalence continues to increase worldwide, as do the numerous chronic diseases associated with obesity, including diabetes, non-alcoholic fatty liver disease, dyslipidemia, and hypertension. The prevalence of bariatric surgery also continues to increase and remains the most effective and sustainable treatment for obesity. Over the last several years, numerous prospective and longitudinal studies have demonstrated the benefits of bariatric surgery on weight loss, mortality, and other chronic diseases. Even though the mechanisms underlying many of these beneficial effects remain poorly understood, surgical management of obesity continues to increase given its unmatched efficacy. In this commentary, we discuss recent clinical advancements as well as several areas needed for future research, including indications for bariatric and metabolic surgery, determination of responders and non-responders, metabolic surgery in non-obese individuals, and the evolving role of bariatric surgery in adolescents.
Keywords: metabolic surgery, bariatric surgery, diabetes, weight loss
Introduction
Obesity prevalence continues to rise and has become the most significant disease affecting health care worldwide 1. Not only does obesity have close associations with diabetes and cardiovascular disease 1– 3 but it is also a risk factor for cancer 4 and non-alcoholic fatty liver disease (NAFLD) that can progress to cirrhosis and liver failure 5, 6. The burden of obesity on quality of life as well as the economy 7– 9 has spurred the development of numerous weight loss therapies that range from behavioral to pharmacologic to surgical.
The management of obesity has become considerably more complex as our understanding of weight regulation has also increased. Genetic studies suggest that body weight is at least partially heritable, with heritability estimates ranging from 40% to 70% and differing significantly between the sexes 10. Clearly, there are some well-defined monogenic forms of obesity 11, but for the great majority of overweight or obese individuals, environment drives the accumulation and maintenance of body weight over time. Diet, exercise, and other lifestyle interventions have failed to lead to robust and sustainable weight loss 12– 14. Moreover, isolated pharmacologic therapies targeting body weight regulation have insufficient effect sizes. To date, bariatric surgery is the only effective therapy that leads to marked and sustained body weight loss.
Why is bariatric surgery so effective against obesity? How does bariatric surgery lead to these sustained effects? These questions remain despite an increasingly complex understanding of bariatric surgery and its postoperative physiology 15, 16. A likely explanation is that body weight regulation is such a highly regulated process that targeting an isolated hormonal or neural pathway pharmacologically is easily overridden by a multitude of other factors contributing to weight maintenance. This physiology means that lifestyle interventions (for example, exercise and dietary modifications) and other pharmacologic approaches undoubtedly fail with time. If one is able to lose weight in the short term, then he or she is continually fighting the natural homeostatic processes attempting to counteract that degree of weight loss. Unlike non-surgical interventions, however, bariatric surgery concurrently affects multiple anatomic and physiologic processes that are arguably impossible to collectively target pharmacologically. Numerous basic and clinical studies have identified a variety of observations, including augmented secretion of satiety factors from the gastrointestinal tract 17– 19, altered neural circuitry in the gut and brain 15, 20– 23, remodeling of the gut microbiome 24– 27, altered gastric emptying 28, 29, rapid intestinal nutrient delivery 30, and (probably) more 31. Overall, bariatric surgery targets a variety of pathways involved in body weight regulation which enable it to exert powerful and sustained effects.
As bariatric surgery continues to grow and surgical treatment of obesity and other chronic illnesses (for example, diabetes, dyslipidemia, and hypertension) continues to rise, understanding the short- and long-term effects and outcomes of these operations will become even more important. In the following, we briefly review the current surgical treatment of obesity and recent evidence demonstrating its efficacy, not only for obesity but also for other chronic illnesses, and the future directions and questions the field will face in the coming decade.
Bariatric surgery
The prevalence of bariatric surgery continues to increase across the globe 32, 33, although the rate of increase is slowing in North America. The types of bariatric operations and other procedures being performed are also continuing to rise. Aside from evolving experimental operations (for example, one-anastomosis/single-anastomosis gastric bypass and gastric plication), there are several novel types of endoscopic interventions in preclinical or clinical testing (for example, gastric balloons and duodenal mucosal resurfacing). Obesity treatment is an active area of research, and, given the breadth of emerging devices, we are focusing on only the most common operations herein. Regardless, experimental operations and devices do not yet contribute to a significant number of procedures worldwide, but these evolving tools may play an increasing role in obesity management in the future.
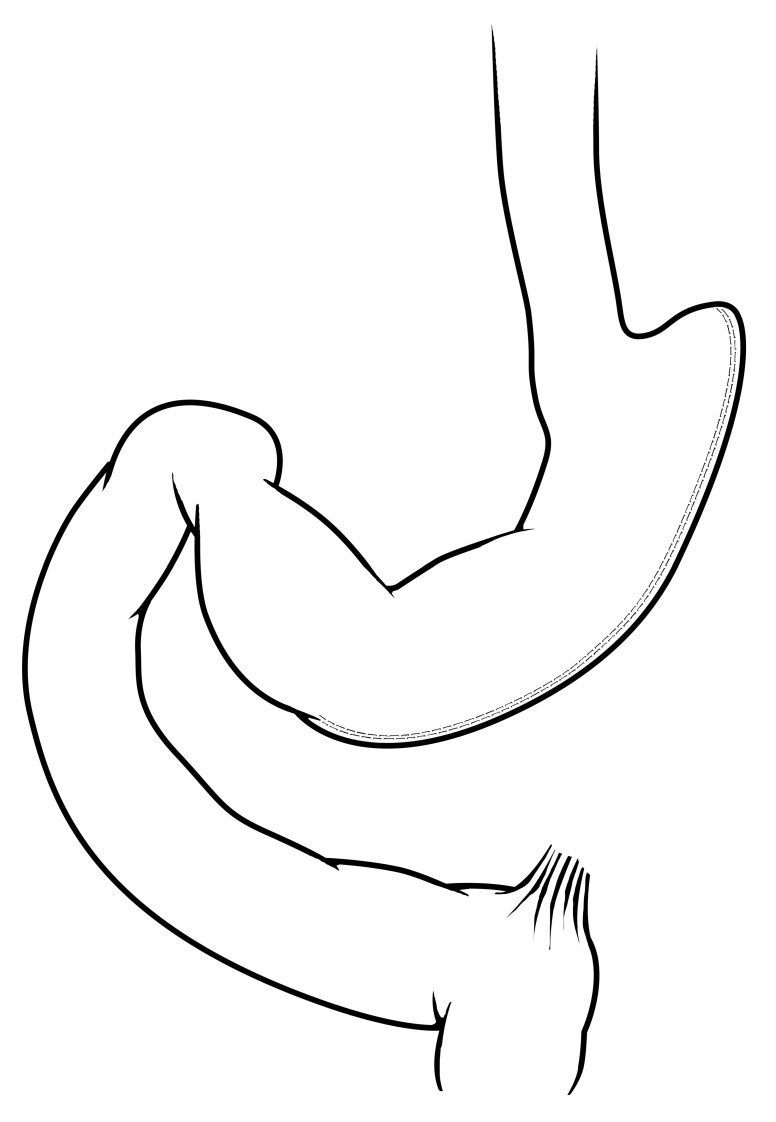
In terms of surgery, three operations make up the overwhelming majority of bariatric surgical volume worldwide. These include the vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB), and adjustable gastric banding (AGB). The VSG ( Figure 1) is the most popular bariatric operation worldwide and is estimated to account for nearly 50% of all operations 32. VSG is performed by using a cutting/sealing tissue stapler to create a long stomach tube that resembles a “sleeve”, irreversibly removing the greater curvature of the stomach. The greater curvature of the stomach is a known site of secretion for ghrelin and other gastrointestinal hormones 34. There is no other gastrointestinal rearrangement with the VSG operation. In general, VSG is well tolerated and, like all bariatric operations, has a very low rate of perioperative complications (<1%) in experienced hands. Studies typically report a weight loss of between 50% and 60% of excess body weight 36, 37, and excess body weight is calculated from ideal body weight 38.
Figure 1. Vertical sleeve gastrectomy.
A majority of the greater curvature of the stomach is excised in this operation, creating a tube-like stomach with a marked reduction in gastric capacity. Reprinted with permission 35.
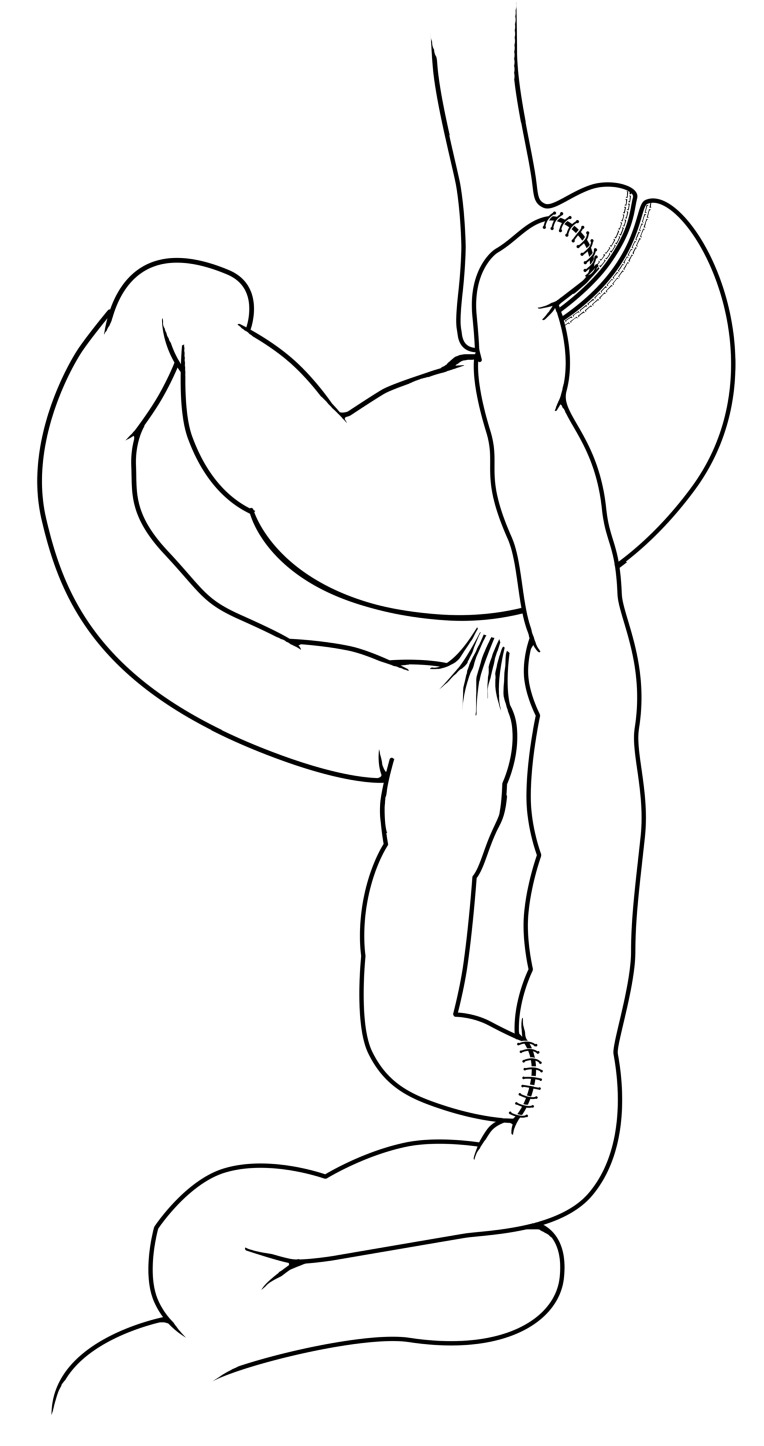
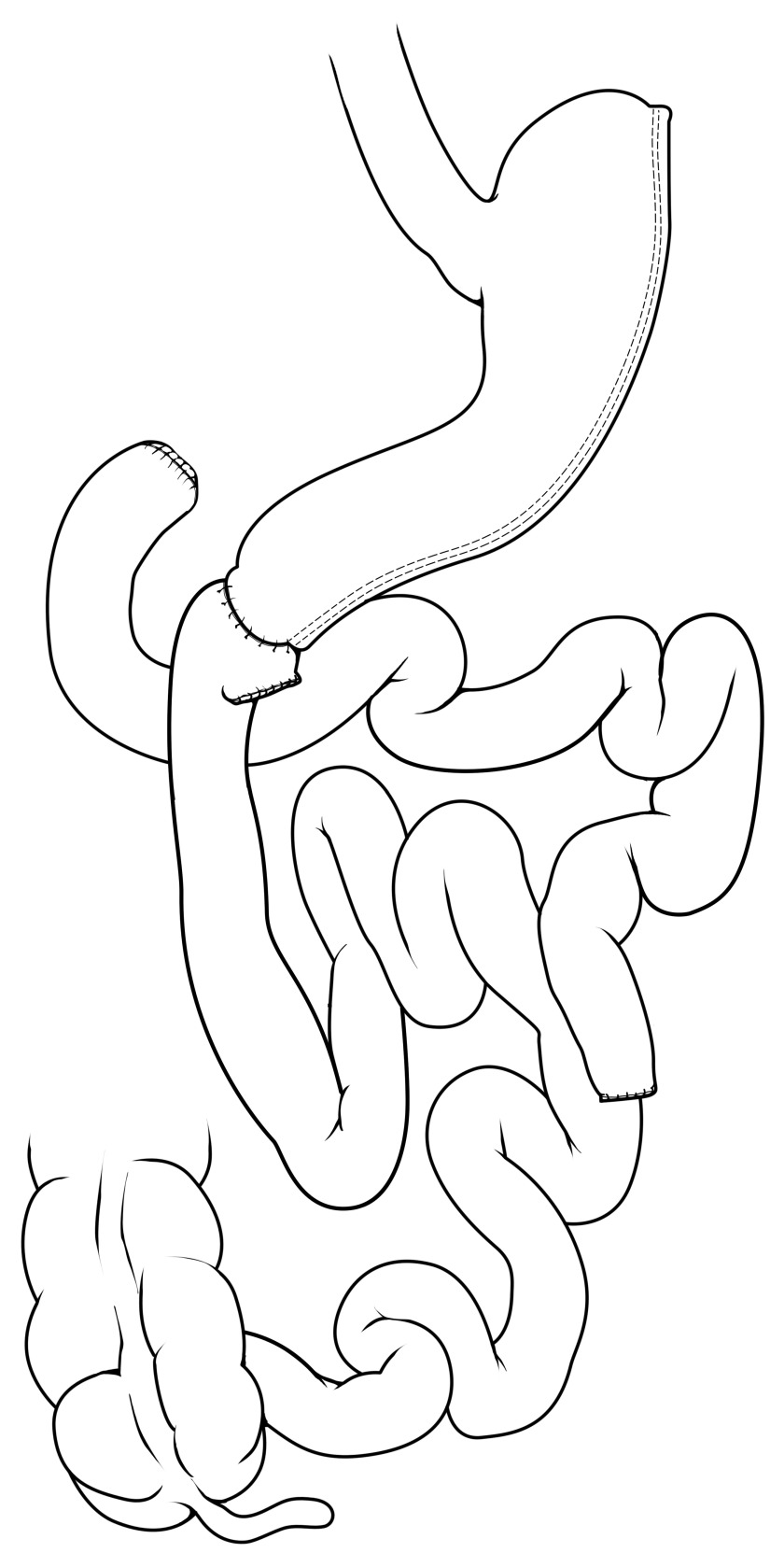
RYGB ( Figure 2) is the second most prevalent bariatric operation worldwide and is estimated to contribute to about 40% of bariatric operations 32. RYGB was formerly the most prevalent operation until recently surpassed by VSG. Surgically, RYGB is technically more challenging, as it involves creating a small stomach pouch (typically about 30 mL) that is connected to an end of the more distal small intestine (that is, jejunum), which creates a “Roux” limb (about 100–150 cm). This Roux limb is sometimes referred to as the alimentary limb, the limb by which foodstuffs travel after transit through the stomach pouch. In order to re-establish the flow of biliary and pancreatic digestive secretions from the liver and pancreas, respectively, the excluded limb of bowel is connected downstream to meet the Roux limb. This limb carrying bile and pancreatic enzymes is referred to as the biliopancreatic limb (about 50–75 cm). The convergence of the Roux and biliopancreatic limbs is connected at the jejunojejunostomy and forms a Y-configuration. The two limbs join at this site, and the remaining distal small bowel is known as the common channel. The common channel is the only site for mixing of digestive enzymes/secretions from the biliopancreatic limb with foodstuffs of the Roux limb. Unlike VSG that has one long staple line forming the sleeve-like stomach, the RYGB has two anastomoses or “connections” created during the operation as well as the stomach remnant that remains in place to drain gastric secretions into the biliopancreatic limb. Even despite these two connections, the risk of intestinal leak or bleeding occurs infrequently in the perioperative setting (<1%) 39, 40. In terms of weight loss, studies have demonstrated that weight loss of RYGB is similar to VSG, reaching about 50–60% of excess body weight loss 36, 37.
Figure 2. Roux-en-Y gastric bypass.
The stomach is divided, creating a small gastric pouch (about 30 mL) that is connected via a gastrojejunostomy to a distal segment of jejunum, which forms the Roux limb of the procedure. The remainder of the stomach is referred to as the “gastric remnant” and drains into the bypassed portion of bowel, referred to as the “biliopancreatic limb”. Bowel continuity is restored for the biliopancreatic limb by a jejunojejunostomy, creating the “Y” configuration of the operation. Thus, ingested nutrients proceed rapidly through the stomach pouch and move immediately into the jejunal Roux limb in the absence of bile and pancreatic secretions. Bile and pancreatic secretions drain via the biliopancreatic limb and then mix with the chyme/nutrients at the point of the jejunojejunostomy. Reprinted with permission 35.
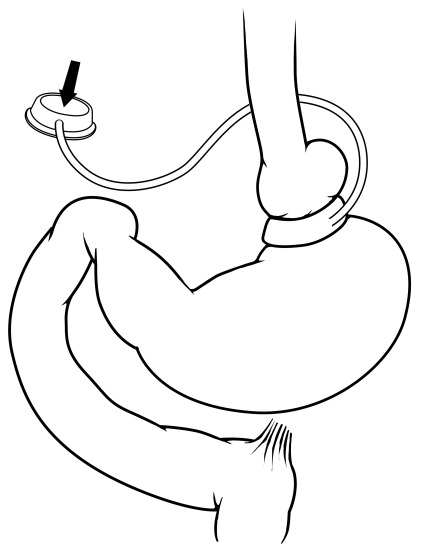
An operation that has fallen out of favor for obesity management is the AGB ( Figure 3), although it is estimated to continue to contribute to approximately 7% of bariatric operations 32. The banding operation involves placing an externally compressive device on the upper portion of the stomach, which can be inflated or deflated with a subcutaneous port, permitting adjustment of the degree of gastric compression to limit stomach distention and food intake. Even though this is the third most common procedure and has the benefit of being completely reversible, its efficacy pales in comparison with other bariatric operations. Originally, the promise of the AGB was fewer complications but preserved weight loss efficacy. Even though perioperative complications associated with AGB are also rare (<1%), the lack of efficacy and the advent of newer and more effective options in the surgical armamentarium have led to increasingly fewer individuals choosing AGB. The weight loss response with AGB is highly variable, and prospective studies show, on average, a body weight loss of about 20% 41. Given the trends in AGB over the last decade, its use will likely continue to fall, especially with the increasing use of other non-surgical procedures.
Figure 3. Adjustable gastric banding.
In this operation, an external ring is placed around the proximal portion of the stomach and has a balloon that lines the inside portion of the ring. The inflatable balloon is connected to a port in the subcutaneous tissue of the upper abdomen that allows the balloon volume, and therefore the amount of external gastric restriction, to be adjusted. Reprinted with permission 35.
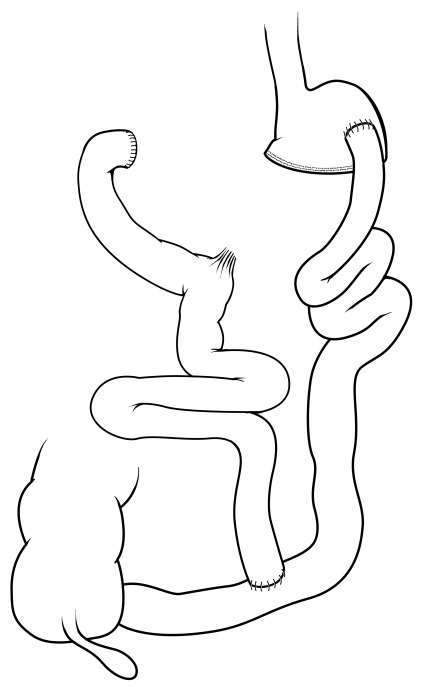
Biliopancreatic diversion (BPD) and BPD with duodenal switch (BPD/DS) are two less commonly performed operations worldwide (~1% overall). However, similar to RYGB, these operations involve significant rearrangement of the small intestines with a gastric resection that leaves either a smaller stomach pouch (about 300–400 mL) with the BPD ( Figure 4) or a sleeve-like stomach with the BPD/DS ( Figure 5). These are similar operations, but in each case the biliopancreatic secretions are diverted far distal (typically about 100–150 cm proximal to the colon). Compared with RYGB, BPD and BPD/DS have improved weight loss efficacy 42, and estimates are around 60–70% of excess body weight loss. Even though this has not been well studied, in limited larger studies this efficacy is at the expense of increased perioperative morbidity 43 as well as nutritional complications (for example, vitamin and mineral deficiencies) that are associated with a small but significant increase in daily bowel movements consistent with fast intestinal transit and malabsorption 42.
Figure 4. Biliopancreatic diversion.
This operation essentially diverts bile and pancreatic secretions to the distal bowel for mixing with nutrients/chyme, typically much further distal than a Roux-en-Y gastric bypass. Traditional biliopancreatic diversion consists of a modest reduction in stomach volume, typically about 300–400 mL, as well as the diversion of bile and pancreatic secretions. Reprinted with permission 35.
Figure 5. Biliopancreatic diversion with duodenal switch.
As in traditional biliopancreatic diversion, bile and pancreatic secretions are diverted to the distal bowel for mixing with nutrients/chyme. The duodenal switch component, however, is accompanied by a vertical sleeve gastrectomy for reduction of gastric volume.
Bariatric surgery and clinical outcomes
Weight loss and mortality benefits
Even though bariatric surgery dates to the 1960s 44, the last decade has seen a number of rigorous, high-quality studies focused on the effects of surgical weight loss and associated chronic medical conditions. The first and largest prospective case controlled study examining surgical weight loss was, and remains, the Swedish Obesity Subjects (SOS) study 45, 46. Despite being overrepresented with vertical banded gastroplasty, an operation that has since fallen out of favor, the SOS study was the first prospective study demonstrating superior weight loss with bariatric surgery (all operations grouped together) compared with non-surgical alternatives 47. More recently, other long-term studies have confirmed that bariatric surgery leads to significantly greater weight loss compared with non-surgical interventions. A retrospective cohort of Veterans Affairs patients (n = 1,787) showed that RYGB led to significantly greater and sustained weight loss compared with VSG or AGB. RYGB led to about 10% more weight loss (relative to baseline body weight) compared with VSG and about 17% more compared with AGB 48. Similar results were seen in observational studies by Adams and colleagues, showing about 28% weight loss (relative to baseline body weight) compared with 0.2% and 0% in non-surgical patients 49. Longer observational studies (>10 years) have corroborated these estimates and demonstrated superior weight loss with surgery at 10 and 12 years 50, 51 that is unmatched by non-surgical therapy.
Even though retrospective epidemiological studies suggested that weight loss might be associated with increased mortality 52– 54, the prospective and randomized Look AHEAD (Action for Health in Diabetes) trial demonstrated that weight loss secondary to intensive lifestyle intervention was not associated with increased mortality. However, the weight loss in the intensive lifestyle group compared with controls at the end of the study (6% versus 3.5%) was also not associated with decreased mortality 55. Unlike weight loss secondary to intensive lifestyle intervention, weight loss secondary to bariatric surgery has been shown to be associated with decreased mortality. Ten-year estimates from the SOS study 56 show significant decreases in mortality risk (adjusted hazard ratio of 0.71). These prospective, observational findings from the SOS study have also been demonstrated in a retrospective cohort of RYGB with cause-specific mortality rates decreased by 56% for coronary artery disease, 92% for diabetes, and 60% for cancer 57. Even though the majority of bariatric surgery is done in women, similar mortality and cardiovascular benefits are also observed in men 58 but with lower cardiovascular events and cardiovascular-related death 59. RYGB and VSG have the best-studied effects overall for weight loss, although AGB has also shown decreased 5- and 10-year all-cause mortality, a benefit that is observed in both sexes 58.
Perioperative and long-term safety
Although surgical treatment of obesity is the most effective therapy, it is also the most invasive with perioperative risk related to the operation itself, including general anesthesia and postoperative recovery. As bariatric surgery has continued to become more prevalent, there have been concerns raised about the perioperative safety of these procedures. Improved anesthetic and surgical management of obese patients undergoing bariatric surgery, however, has been shown to have no greater risk than other elective operations routinely performed in adults and pediatric patients 60– 62, including laparoscopic cholecystectomy and laparoscopic hysterectomy 63. Estimates for perioperative 90-day mortality for bariatric surgery are approximately 0.3%, and quality improvement programs are continuing to work to make these low numbers even lower.
Aside from perioperative safety, which has been well studied, a number of smaller prospective, randomized trials demonstrated that bariatric surgery appears to be safe overall at least up to five years postoperatively 40, 64, although these trials are not without limitations. Studies to date 40, 64 have shown that surgery is associated with adverse events that one would expect to be more prevalent following surgery (for example, bowel obstruction). Given the difficulties of longer-term follow-up (>5 years), complications associated with surgical management (for example, recurrent bowel obstruction, bleeding, perforation, and marginal ulceration) are not well studied and represent an important area for future study. Recent retrospective studies in smaller cohorts suggest that these types of adverse events related to surgery are not insignificant and the long-term benefits and risks associated with surgery should be considered given the degree of obesity and other comorbidities when a patient is considered for surgery 65.
Resolution of metabolic diseases
In addition to the clear benefits associated with weight loss, one of the most intriguing effects of bariatric surgery is its tendency to resolve other chronic metabolic diseases (for example, diabetes and dyslipidemia) prior to weight loss. A substantial portion of these changes preceding significant weight loss are driven by caloric restriction perioperatively, as the effects of dietary restriction are well known 66, 67 and have been examined in patients following bariatric surgery 68– 71. Regardless, there are other detectable metabolic effects that occur independently of caloric restriction (for example, 72– 74). The role of these metabolic effects in the short- and long-term metabolic outcomes of these operations is not well understood.
Even though these effects occur prior to significant weight loss (reviewed in 16, 35, 75), postoperative weight loss undoubtedly further improves these chronic disease states that are exacerbated by obesity. To better understand these effects related to bariatric surgery, several randomized and prospective studies to date have targeted these effects on metabolic diseases (for example, diabetes and dyslipidemia) and their response over time. Surgical Treatment And Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) is a prospective, randomized trial demonstrating that bariatric surgery (RYGB and VSG) is more effective than intensive lifestyle therapy alone for diabetes treatment and has sustained benefits, including weight loss up to 5 years 76. To date, both RYGB and VSG have similarly improved diabetes efficacy, even though RYGB tends to have slightly increased weight loss. The Diabetes Surgery Study (DSS) is another 5-year, randomized, observational study examining RYGB added to intensive lifestyle therapy and medical management of type 2 diabetes. The DSS has the additional benefits of examining a triple endpoint—that is, systolic blood pressure of less than 130 mmHg, hemoglobin A1C of less than 7%, and low-density lipoprotein (LDL) cholesterol of less than 100 mg/dL—based on optimal diabetes management guidelines as well as having multiple, including international, study sites 64. Similar to STAMPEDE, the DSS showed a significant benefit of added RYGB to intensive lifestyle and medical management to the triple endpoint at 5 years, although the effect appeared to wane over time. Both DSS and STAMPEDE are similar to a third trial—conducted by Mingrone and colleagues—examining RYGB and BPD for weight loss and diabetes management, demonstrating that degree of weight loss is not necessarily predictive of which patients will have diabetes resolution 77. This third trial has the smallest sample size—three groups of 20 subjects per group (that is, intensive medical treatment, RYGB, BPD)—a clear limitation compared with STAMPEDE that allocated 50 subjects to three groups (that is, intensive lifestyle/medical, RYGB, and VSG) and the DSS with 120 subjects overall in two equal groups at randomization (that is, intensive lifestyle/medical and RYGB).
Overall, it is important to note that the clinical trials mentioned above 40, 64, 77 as well as other studies demonstrate that the benefits of RYGB on diabetes resolution are not limited to class III obese subjections (that is, body mass index [BMI] of more than 40 kg/m 2) 78. Even in mild to moderate obesity (BMI of 30–39.9 kg/m 2), RYGB not only leads to superior diabetes resolution or improvement compared with medical therapy (28% versus 0%) but also helps patients meet other biochemical goals of diabetes management (for example, hemoglobin A1C, LDL cholesterol, and systolic blood pressure) 64. Even though most studies focus on the resolution of insulin resistance/diabetes, effects on other cardiovascular markers (that is, LDL cholesterol and blood pressure) are important and, though less commonly observed, represent an area for further study. Aside from using bariatric surgery as a treatment for diabetes, the SOS study 79 examined the role of surgery for the prevention of diabetes with impressive results (adjusted hazard ratio of 0.17).
Even though all patients may not have complete resolution of diabetes, data suggest that diabetes and insulin resistance are significantly ameliorated. Aside from the trials discussed above, a single-center trial (n = 69 overall) comparing RYGB, AGB, and lifestyle intervention for diabetes showed 3-year follow-up data with either partial or complete resolution of diabetes in 40% of RYGB and 29% of AGB compared with zero in a lifestyle intervention group. Consistent with these rates, even in those without complete resolution, the use of diabetes medications decreased in the surgical group (−65% with RYGB and −33% with AGB) compared with none in the lifestyle weight loss group 80. Overall, evidence indicates that these operations have significant effects on weight loss as well as benefits to metabolic disease.
Cancer
Unlike the seemingly more direct relationship between obesity and atherosclerosis or diabetes, the relationship between cancer and obesity remains intriguing. Obesity is a bona fide risk factor for cancer 3, 4, with protective effects conferred by bariatric surgery, an effect that is presumably due to weight loss over time associated with surgery 57, 81, 82. It should be noted that the mortality benefit of bariatric surgery from the SOS study mentioned above 56 is driven primarily by decreased cancer-related death, more so than major cardiovascular outcomes. The links between bariatric surgery and cancer are strong, and weight management and obesity treatment using bariatric surgery to decrease cancer risk as well as cancer recurrence are gaining popularity among oncologists 83. The interaction of bariatric surgery and cancer is an intense area of investigation, from not only an epidemiological perspective but also a basic scientific perspective. The mechanism of why bariatric surgery confers protection from cancer is unknown, but whether this occurs solely from weight loss or other intrinsic changes of the operations remains to be determined 84.
Unanswered questions and future investigation
Bariatric surgery as a treatment for obesity, as well as its benefits on associated chronic medical conditions, continues to gain acceptance and popularity worldwide. Obesity-associated type 2 diabetes as an indication for bariatric surgery is a clear paradigm shift in diabetes management in recent years 85, 86. With these clinical changes, a number of important questions continue to arise that are shaping the current and future research landscape. These areas are ripe for investigation and need to be addressed in the coming years for the field of metabolic and bariatric surgery to continue to grow and optimally benefit this increasing patient population.
Can responders and non-responders be identified preoperatively?
As with many therapies, patient response can vary considerably and this is perhaps most obvious with weight loss following bariatric surgery. From the perspective of weight loss, being able to identify those individuals who will or will not respond is critically important, especially as surgery becomes increasingly used for the treatment of obesity and other diseases. The largest barrier to determining whether we can predict which individuals will respond is the lack of sufficiently large and diverse patient cohorts to be able to construct accurate predictive models. Given the numerous clinical variables of interest (for example, sex, race, baseline body weight, comorbid medical conditions, and operation type), this would require tens of thousands of patients at a minimum. Regardless, smaller retrospective studies have attempted to identify characteristics that suggest success, but these are limited. For example, the higher the baseline BMI, the greater the amount of absolute weight loss in adolescents 87 and adults 88. Even though this makes practical sense, as individuals with a higher preoperative body weight have much more excess body weight to lose, this finding has not been conclusively demonstrated in adults 89.
The largest retrospective analysis (about 27,000 patients), from the Michigan Bariatric Surgery Collaborative 90, demonstrated that patients most likely to achieve a BMI of less than 30 kg/m 2 were patients who had a preoperative BMI of less than 40 kg/m 2. Moreover, these patients had the greatest resolution rates for comorbidities. With respect to diabetes resolution, several studies have similarly suggested that shorter duration of type 2 diabetes and higher preoperative C-peptide concentration are associated with greater diabetes resolution postoperatively 91. Along with younger age, which has also been shown to be a positive predictor of better weight loss success 91, 92, this suggests that obese diabetic patients benefit from earlier intervention. These findings give credence to the argument that delaying bariatric surgery until individuals reach a BMI of more than 40 kg/m 2 may be counterproductive and actually be hurting more people in the end. The effects of withholding effective obesity treatment need to be better examined for the short- and long-term consequences on the patient as well as the health care system 93.
The problem with predicting weight loss over time is not straightforward, and being able to predict who will or will not be successful would allow more patient-focused treatment to optimize outcomes for all individuals. However, identifying the patients who will be resistant to surgical weight loss or those individuals who will regain a significant amount of their lost weight over time would be immensely important if those outcomes could be predicted at the initial preoperative consultation. As a corollary, once patients exhibit some degree of weight regain, there is no consensus on how those individuals should be treated 94. In most instances, the problem is multifactorial and the solution requires a multidisciplinary approach, although the best strategies for these patients remain unknown. One particularly complicating factor of identifying responders and non-responders is that weight regain may not always be associated with worsening of metabolic endpoints 95, 96. Thus, how “failure” of bariatric surgery is defined is critically important to the approach to the patient and overall clinical care.
Is there a role for metabolic surgery in non-obese patients?
As mentioned, examination of the predictors of who will and will not respond to bariatric surgery has suggested that younger individuals with fewer comorbid medical conditions experience the greatest benefit of bariatric surgery. This raises the question of whether individuals should be receiving bariatric surgery before they develop morbid obesity and become generally sicker overall. Bariatric surgery is overall safe and effective at treating diabetes in lower-BMI (<35 kg/m 2) individuals 97. In fact, clinical data demonstrate significant efficacy of bariatric operations in ethnic groups susceptible to diabetes at lower BMI ranges (<35 kg/m 2) 98– 100 as well as diabetics without significant obesity 101– 103. Clinical and experimental evidence strongly suggests the existence of factors altered by bariatric surgery that drive body weight-independent changes in these patients that are not completely linked to weight loss. The indications for bariatric surgery have changed significantly over the last two decades and paralleled this clinical and experimental evidence. At one time, bariatric surgery was offered only to individuals with a BMI of more than 40 kg/m 2. As the benefits of surgical weight loss on obesity-related comorbid diseases became evident, the BMI threshold fell to more than 35 kg/m 2 for patients with at least one obesity-related comorbidity. Again, that BMI threshold has fallen, and numerous clinical and professional societies have recently endorsed the consideration of metabolic and bariatric surgery for the treatment of type 2 diabetes in individuals with a BMI in the 30–34.9 kg/m 2 range 85. Thus, it is reasonable to ask whether or not we should be operating on individuals primarily for intractable diabetes in the absence of obesity.
There is currently insufficient evidence to justify bariatric surgery for non-obese patients, although increasing reports suggest that non-obese diabetics may benefit from bariatric surgery with improved control or resolution of diabetes. Many of these reports are in ethnic groups in which type 2 diabetes develops at a much lower BMI 104– 106. We speculate that operating in individuals at lower body weight (25–30 kg/m 2) who are at high risk for weight gain and metabolic illness over time may become more commonplace in the coming years. The new clinical guidelines that recommend consideration of “metabolic surgery” in patients with a BMI of less than 35 kg/m 2 with intractable diabetes 85, 86 are a direct extension of this rationale. Regardless, changes in clinical practice to include individuals with a BMI of less than 30 kg/m 2 are not currently supported by any randomized or controlled trials, and any further changes in clinical practice will require further studies.
Aside from the potential health benefits of operating on patients prior to significant weight gain or metabolic illness, bariatric surgery may be associated with decreased health care costs 107. However, these cost savings may be easier to realize if individuals undergo bariatric surgery at younger ages when they are less sick and have a better chance of making a full metabolic recovery. Further studies examining these endpoints are needed, but we anticipate that the indications for bariatric and metabolic surgery will continue to broaden with time.
What is the role of bariatric surgery in adolescent obesity?
The role of bariatric surgery in adolescent obesity is an increasingly debated topic, as the long-term effects of these operations in pediatric patients are largely unknown and understudied. Regardless, pediatric obesity continues to worsen and contributes to the adult obesity epidemic, and it shows no sign of slowing. Even though studies have suggested that treating patients who are younger and not quite as ill from a metabolic standpoint makes sense, the interactions of surgical weight loss with the normal developmental processes in adolescents are unknown. On the contrary, many argue that not offering bariatric surgery is withholding the most effective treatment to a group of adolescents and young adults despite knowing that medical or lifestyle interventions are largely ineffective. Long-term follow-up studies (10–20 years) with close monitoring are necessary in adolescent patients. One such study is under way as part of the Teen-LABS study (Teen-Longitudinal Assessment of Bariatric Surgery), the first observational study of bariatric surgery in adolescents. Teen-LABS enrolled 242 patients undergoing bariatric surgery, which included 161 RYGB and 67 VSG. The 3-year data are promising, showing that weight loss and dyslipidemia markers were improved in all groups 108. With the currently unknown long-term effects of bariatric surgery, it is likely that the adolescent population will be targets of increasing obesity treatments in the coming years. Thus, determining the efficacy of these emerging treatments alongside that of bariatric surgery is of utmost importance.
Similar to the adult obesity epidemic, but perhaps more worrisome, is the rise in NAFLD in obese adolescents. NAFLD is expected to only worsen in the coming decade, paralleling the childhood obesity epidemic. Previously, it was thought that pediatric and adolescent patients were protected from developing NAFLD; however, studies have demonstrated that its prevalence is rising. Of the adolescents followed as part of the Teen-LABS study, the largest prospective observational study of adolescent bariatric surgery to date, about 60% had NAFLD at the time of surgery 109. Aside from the detrimental medical implications of cirrhosis in an ever-enlarging group of adolescents and young adults 110, 111, the economic implications of NAFLD and adolescent obesity are frightening, and these individuals desperately need effective therapies. Longitudinal evaluation of adolescent bariatric surgery is critical in order to identify its efficacy in this population, as surgical management of obesity will likely continue to lag compared with adults.
Conclusions
Despite the long-held beliefs that obesity is merely a failure of willpower or a character flaw, recent years have proven that body weight regulation, which includes powerful neural controls on appetite and energy expenditure, is much more complex than could ever have been imagined. Although many diseases associated with obesity include those directly related to excess adiposity, including sleep apnea, osteoarthritis, and stress incontinence, other diseases like insulin resistance/diabetes, NAFLD, dyslipidemia, and hypertension appear to be secondary diseases that develop in the chronic inflammatory milieu associated with obesity 112, 113.
Metabolic and bariatric surgery for the treatment of obesity and its associated medical conditions is safe overall, and its prevalence undoubtedly will continue to increase in the coming years. With limitations of effective, non-surgical treatment options and continued worsening of the childhood and adult obesity epidemics, it remains to be seen how prevalent obesity surgery may become in younger and less overweight individuals. We speculate that, in the coming decades, the indications for metabolic and bariatric surgery will not only continue to broaden to treat obesity but also preclude its development in high-risk individuals. Further study of the science of bariatric surgery and its profound metabolic effects is critical to increasing the quality of care to this growing patient population as well as combating the economic costs associated with obesity and other metabolic diseases.
Abbreviations
AGB, adjustable gastric banding; BMI, body mass index; BPD, biliopancreatic diversion; BPD/DS, biliopancreatic diversion with duodenal switch; DSS, Diabetes Surgery Study; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; RYGB, Roux-en-Y gastric bypass; SOS, Swedish Obesity Subjects; STAMPEDE, Surgical Treatment And Medications Potentially Eradicate Diabetes Efficiently; Teen-LABS, Teen-Longitudinal Assessment of Bariatric Surgery; VSG, vertical sleeve gastrectomy
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Alfonso Torquati, Center for Weight Loss and Bariatric Surgery, Department of General Surgery, Rush University Medical Center, Chicago, Illinois, USA
Robert Cooney, Department of Surgery, SUNY Upstate Medical University, Syracuse, New York, USA
Ali Aminian, Department of General Surgery, Cleveland Clinic, Cleveland, Ohio, USA
Charles J. Billington, Division of Endocrinology & Diabetes, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA
Funding Statement
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, specifically grants DK059637 (Vanderbilt Mouse Metabolic Phenotyping Center), DK020593 (Vanderbilt Diabetes Research and Training Center), DK058404 (Vanderbilt Digestive Disease Research Center), 1UL1TR002243 (The Vanderbilt Institute for Clinical and Translational Research), F32 DK103474 (VLA), U24 DK076169 MicroMouse sub-project 30835-22 (VLA), R01 DK105847 (NNA), and R01 DK1070860 (NNA). This work was also supported by a research grant from the Society of American Gastrointestinal and Endoscopic Surgeons (VLA).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. : Health Effects of Overweight and Obesity in 195 Countries over 25 Years. 2017;377(1):13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Lavie CJ, Milani RV, Ventura HO: Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. 2009;53(21):1925–32. 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
- 3. Guh DP, Zhang W, Bansback N, et al. : The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. 2009;9:88. 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calle EE, Rodriguez C, Walker-Thurmond K, et al. : Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. 2003;348(17):1625–38. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 5. Nobili V, Day C: Childhood NAFLD: a ticking time-bomb? 2009;58(11):1442. 10.1136/gut.2009.184465 [DOI] [PubMed] [Google Scholar]
- 6. Younossi Z, Anstee QM, Marietti M, et al. : Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. 2018;15(1):11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Specchia ML, Veneziano MA, Cadeddu C, et al. : Economic impact of adult obesity on health systems: a systematic review. 2015;25(2):255–62. 10.1093/eurpub/cku170 [DOI] [PubMed] [Google Scholar]
- 8. Goettler A, Grosse A, Sonntag D: Productivity loss due to overweight and obesity: a systematic review of indirect costs. 2017;7(10):e014632. 10.1136/bmjopen-2016-014632 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Grieve E, Fenwick E, Yang HC, et al. : The disproportionate economic burden associated with severe and complicated obesity: a systematic review. 2013;14(11):883–94. 10.1111/obr.12059 [DOI] [PubMed] [Google Scholar]
- 10. Bray MS, Loos RJ, McCaffery JM, et al. : NIH working group report-using genomic information to guide weight management: From universal to precision treatment. 2016;24(1):14–22. 10.1002/oby.21381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Heymsfield SB, Wadden TA: Mechanisms, Pathophysiology, and Management of Obesity. 2017;376(3):254–66. 10.1056/NEJMra1514009 [DOI] [PubMed] [Google Scholar]
- 12. Caudwell P, Hopkins M, King NA, et al. : Exercise alone is not enough: weight loss also needs a healthy (Mediterranean) diet? 2009;12(9A):1663–6. 10.1017/S1368980009990528 [DOI] [PubMed] [Google Scholar]
- 13. Look AHEAD Research Group: Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. 2014;22(1):5–13. 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sacks FM, Bray GA, Carey VJ, et al. : Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. 2009;360(9):859–73. 10.1056/NEJMoa0804748 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Seeley RJ, Chambers AP, Sandoval DA: The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. 2015;21(3):369–78. 10.1016/j.cmet.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albaugh VL, Banan B, Ajouz H, et al. : Bile acids and bariatric surgery. 2017;56:75–89. 10.1016/j.mam.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. : Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. 2013;62(9):3044–52. 10.2337/db13-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salehi M, Prigeon RL, D'Alessio DA: Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. 2011;60(9):2308–14. 10.2337/db11-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jørgensen NB, Jacobsen SH, Dirksen C, et al. : Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. 2012;303(1):E122–31. 10.1152/ajpendo.00073.2012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Hajnal A, Kovacs P, Ahmed T, et al. : Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. 2010;299(4):G967–79. 10.1152/ajpgi.00070.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajnal A, Zharikov A, Polston JE, et al. : Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. 2012;7(11):e49121. 10.1371/journal.pone.0049121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thanos PK, Michaelides M, Subrize M, et al. : Roux-en-Y Gastric Bypass Alters Brain Activity in Regions that Underlie Reward and Taste Perception. 2015;10(6):e0125570. 10.1371/journal.pone.0125570 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Browning KN, Fortna SR, Hajnal A: Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. 2013;591(9):2357–72. 10.1113/jphysiol.2012.249268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kong LC, Tap J, Aron-Wisnewsky J, et al. : Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. 2013;98(1):16–24. 10.3945/ajcn.113.058743 [DOI] [PubMed] [Google Scholar]
- 25. Tremaroli V, Karlsson F, Werling M, et al. : Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. 2015;22(2):228–38. 10.1016/j.cmet.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Flynn CR, Albaugh VL, Cai S, et al. : Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. 2015;6:7715. 10.1038/ncomms8715 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Palleja A, Kashani A, Allin KH, et al. : Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. 2016;8(1):67. 10.1186/s13073-016-0312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Dirksen C, Damgaard M, Bojsen-Møller KN, et al. : Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. 2013;25(4):346–e255. 10.1111/nmo.12087 [DOI] [PubMed] [Google Scholar]
- 29. Carswell KA, Vincent RP, Belgaumkar AP, et al. : The effect of bariatric surgery on intestinal absorption and transit time. 2014;24(5):796–805. 10.1007/s11695-013-1166-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Holst JJ, Gribble F, Horowitz M, et al. : Roles of the Gut in Glucose Homeostasis. 2016;39(6):884–92. 10.2337/dc16-0351 [DOI] [PubMed] [Google Scholar]
- 31. Cavin JB, Bado A, Le Gall M: Intestinal Adaptations after Bariatric Surgery: Consequences on Glucose Homeostasis. 2017;28(5):354–64. 10.1016/j.tem.2017.01.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Angrisani L, Santonicola A, Iovino P, et al. : Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. 2017;27(9):2279–89. 10.1007/s11695-017-2666-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. American Society for Metabolic and Bariatric Surgery: Estimate of Bariatric Surgery Numbers, 2011–2016 [Internet].asmbs.org.2016; [cited 2018 Apr 8]. Reference Source [Google Scholar]
- 34. Choi E, Roland JT, Barlow BJ, et al. : Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. 2014;63(11):1711–20. 10.1136/gutjnl-2013-305964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Albaugh VL, Flynn CR, Tamboli RA, et al. : Recent advances in metabolic and bariatric surgery [version 1; referees: 2 approved]. 2016;5: pii: F1000 Faculty Rev-978. 10.12688/f1000research.7240.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peterli R, Wölnerhanssen BK, Peters T, et al. : Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. 2018;319(3):255–65. 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Salminen P, Helmiö M, Ovaska J, et al. : Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. 2018;319(3):241–54. 10.1001/jama.2017.20313 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Shah B, Sucher K, Hollenbeck CB: Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. 2006;21(3):312–9. 10.1177/0115426506021003312 [DOI] [PubMed] [Google Scholar]
- 39. Ikramuddin S, Korner J, Lee WJ, et al. : Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. 2013;309(21):2240–9. 10.1001/jama.2013.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schauer PR, Bhatt DL, Kirwan JP, et al. : Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. 2014;370(21):2002–13. 10.1056/NEJMoa1401329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dixon JB, O'Brien PE, Playfair J, et al. : Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. 2008;299(3):316–23. 10.1001/jama.299.3.316 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Risstad H, Søvik TT, Engström M, et al. : Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. 2015;150(4):352–61. 10.1001/jamasurg.2014.3579 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Hedberg J, Sundström J, Sundbom M: Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. 2014;15(7):555–63. 10.1111/obr.12169 [DOI] [PubMed] [Google Scholar]
- 44. Mason EE, Ito C: Gastric bypass. 1969;170(3):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sjöström CD, Lissner L, Wedel H, et al. : Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. 1999;7(5):477–84. 10.1002/j.1550-8528.1999.tb00436.x [DOI] [PubMed] [Google Scholar]
- 46. Karlsson J, Taft C, Rydén A, et al. : Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. 2007;31(8):1248–61. 10.1038/sj.ijo.0803573 [DOI] [PubMed] [Google Scholar]
- 47. Sjöström L: Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. 2013;273(3):219–34. 10.1111/joim.12012 [DOI] [PubMed] [Google Scholar]
- 48. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. : Bariatric Surgery and Long-term Durability of Weight Loss. 2016;151(11):1046–55. 10.1001/jamasurg.2016.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Adams TD, Davidson LE, Litwin SE, et al. : Health benefits of gastric bypass surgery after 6 years. 2012;308(11):1122–31. 10.1001/2012.jama.11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams TD, Davidson LE, Litwin SE, et al. : Weight and Metabolic Outcomes 12 Years after Gastric Bypass. 2017;377(12):1143–55. 10.1056/NEJMoa1700459 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Mehaffey JH, LaPar DJ, Clement KC, et al. : 10-Year Outcomes After Roux-en-Y Gastric Bypass. 2016;264(1):121–6. 10.1097/SLA.0000000000001544 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Williamson DF, Thompson TJ, Thun M, et al. : Intentional weight loss and mortality among overweight individuals with diabetes. 2000;23(10):1499–504. 10.2337/diacare.23.10.1499 [DOI] [PubMed] [Google Scholar]
- 53. Williamson DF, Pamuk E, Thun M, et al. : Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. 1995;141(12):1128–41. 10.1093/oxfordjournals.aje.a117386 [DOI] [PubMed] [Google Scholar]
- 54. Williamson DF, Pamuk E, Thun M, et al. : Prospective study of intentional weight loss and mortality in overweight white men aged 40-64 years. 1999;149(6):491–503. 10.1093/oxfordjournals.aje.a009843 [DOI] [PubMed] [Google Scholar]
- 55. Look AHEAD Research Group, . Wing RR, Bolin P, et al. : Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. 2013;369(2):145–54. 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Sjöström L, Narbro K, Sjöström CD, et al. : Effects of bariatric surgery on mortality in Swedish obese subjects. 2007;357(8):741–52. 10.1056/NEJMoa066254 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Adams TD, Gress RE, Smith SC, et al. : Long-term mortality after gastric bypass surgery. 2007;357(8):753–61. 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 58. Arterburn DE, Olsen MK, Smith VA, et al. : Association between bariatric surgery and long-term survival. 2015;313(1):62–70. 10.1001/jama.2014.16968 [DOI] [PubMed] [Google Scholar]
- 59. Sjöström L, Peltonen M, Jacobson P, et al. : Bariatric surgery and long-term cardiovascular events. 2012;307(1):56–65. 10.1001/jama.2011.1914 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Courcoulas AP, Yanovski SZ, Bonds D, et al. : Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. 2014;149(12):1323–9. 10.1001/jamasurg.2014.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inge TH, Zeller MH, Jenkins TM, et al. : Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. 2014;168(1):47–53. 10.1001/jamapediatrics.2013.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, et al. : Perioperative safety in the longitudinal assessment of bariatric surgery. 2009;361(5):445–54. 10.1056/NEJMoa0901836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aminian A, Brethauer SA, Kirwan JP, et al. : How safe is metabolic/diabetes surgery? 2015;17(2):198–201. 10.1111/dom.12405 [DOI] [PubMed] [Google Scholar]
- 64. Ikramuddin S, Korner J, Lee W, et al. : Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A 1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. 2018;319(3):266–78. 10.1001/jama.2017.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Jakobsen GS, Småstuen MC, Sandbu R, et al. : Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. 2018;319(3):291–301. 10.1001/jama.2017.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Nuttall FQ, Almokayyad RM, Gannon MC: Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. 2015;64(2):253–62. 10.1016/j.metabol.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 67. Henry RR, Wiest-Kent TA, Scheaffer L, et al. : Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. 1986;35(2):155–64. 10.2337/diab.35.2.155 [DOI] [PubMed] [Google Scholar]
- 68. Lips MA, de Groot GH, van Klinken JB, et al. : Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. 2014;80(6):834–42. 10.1111/cen.12254 [DOI] [PubMed] [Google Scholar]
- 69. Jackness C, Karmally W, Febres G, et al. : Very low–calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients. 2013;62(9):3027–32. 10.2337/db12-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Isbell JM, Tamboli RA, Hansen EN, et al. : The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. 2010;33(7):1438–42. 10.2337/dc09-2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lingvay I, Guth E, Islam A, et al. : Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? 2013;36(9):2741–7. 10.2337/dc12-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schmidt JB, Pedersen SD, Gregersen NT, et al. : Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. 2016;40(2):281–90. 10.1038/ijo.2015.162 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Lips MA, de Groot GH, Berends FJ, et al. : Calorie restriction and Roux-en-Y gastric bypass have opposing effects on circulating FGF21 in morbidly obese subjects. 2014;81(6):862–70. 10.1111/cen.12496 [DOI] [PubMed] [Google Scholar]
- 74. Lips MA, Van Klinken JB, van Harmelen V, et al. : Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. 2014;37(2):3150–6. 10.2337/dc14-0195 [DOI] [PubMed] [Google Scholar]
- 75. Mulla CM, Middelbeek RJ, Patti M: Mechanisms of weight loss and improved metabolism following bariatric surgery. 2018;1411(1):53–64. 10.1111/nyas.13409 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Schauer PR, Bhatt DL, Kirwan JP, et al. : Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. 2017;376(7):641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Mingrone G, Panunzi S, De Gaetano A, et al. : Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. 2015;386(9997):964–73. 10.1016/S0140-6736(15)00075-6 [DOI] [PubMed] [Google Scholar]
- 78. Cummings DE, Arterburn DE, Westbrook EO, et al. : Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. 2016;59(5):945–53. 10.1007/s00125-016-3903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carlsson LM, Peltonen M, Ahlin S, et al. : Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. 2012;367(8):695–704. 10.1056/NEJMoa1112082 [DOI] [PubMed] [Google Scholar]
- 80. Courcoulas AP, Belle SH, Neiberg RH, et al. : Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. 2015;150(10):931–40. 10.1001/jamasurg.2015.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sjöström L, Gummesson A, Sjöström CD, et al. : Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. 2009;10(7):653–62. 10.1016/S1470-2045(09)70159-7 [DOI] [PubMed] [Google Scholar]
- 82. Anveden Å, Taube M, Peltonen M, et al. : Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. 2017;145(2):224–9. 10.1016/j.ygyno.2017.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Alamuddin N, Bakizada Z, Wadden TA: Management of Obesity. 2016;34(35):4295–305. 10.1200/JCO.2016.66.8806 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Schauer DP, Feigelson HS, Koebnick C, et al. : Association Between Weight Loss and the Risk of Cancer after Bariatric Surgery. 2017;25(Suppl 2):S52–S57. 10.1002/oby.22002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rubino F, Nathan DM, Eckel RH, et al. : Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. 2016;39(6):861–77. 10.2337/dc16-0236 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Brito JP, Montori VM, Davis AM: Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. 2017;317(6):635–6. 10.1001/jama.2016.20563 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Inge TH, Jenkins TM, Zeller M, et al. : Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. 2010;156(1):103–108.e1. 10.1016/j.jpeds.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wood GC, Benotti PN, Lee CJ, et al. : Evaluation of the Association Between Preoperative Clinical Factors and Long-term Weight Loss After Roux-en-Y Gastric Bypass. 2016;151(11):1056–62. 10.1001/jamasurg.2016.2334 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Panunzi S, De Gaetano A, Carnicelli A, et al. : Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. 2015;261(3):459–67. 10.1097/SLA.0000000000000863 [DOI] [PubMed] [Google Scholar]
- 90. Varban OA, Cassidy RB, Bonham A, et al. : Factors Associated With Achieving a Body Mass Index of Less Than 30 After Bariatric Surgery. 2017;152(11):1058–64. 10.1001/jamasurg.2017.2348 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Aung L, Lee WJ, Chen SC, et al. : Bariatric Surgery for Patients With Early-Onset vs Late-Onset Type 2 Diabetes. 2016;151(9):798–805. 10.1001/jamasurg.2016.1130 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Valera-Mora ME, Simeoni B, Gagliardi L, et al. : Predictors of weight loss and reversal of comorbidities in malabsorptive bariatric surgery. 2005;81(6):1292–7. 10.1093/ajcn/81.6.1292 [DOI] [PubMed] [Google Scholar]
- 93. Cohen RV, Luque A, Junqueira S, et al. : What is the impact on the healthcare system if access to bariatric surgery is delayed? 2017;13(9):1619–27. 10.1016/j.soard.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 94. Karmali S, Brar B, Shi X, et al. : Weight recidivism post-bariatric surgery: a systematic review. 2013;23(11):1922–33. 10.1007/s11695-013-1070-4 [DOI] [PubMed] [Google Scholar]
- 95. Aminian A, Jamal M, Augustin T, et al. : Failed Surgical Weight Loss Does Not Necessarily Mean Failed Metabolic Effects. 2015;17(10):682–4. 10.1089/dia.2015.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tamboli RA, Breitman I, Marks-Shulman PA, et al. : Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. 2014;22(7):1617–22. 10.1002/oby.20776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li Q, Chen L, Yang Z, et al. : Metabolic effects of bariatric surgery in type 2 diabetic patients with body mass index < 35 kg/m 2. 2012;14(3):262–70. 10.1111/j.1463-1326.2011.01524.x [DOI] [PubMed] [Google Scholar]
- 98. Malapan K, Goel R, Tai CM, et al. : Laparoscopic Roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients. 2014;10(5):834–40. 10.1016/j.soard.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 99. Shah SS, Todkar JS, Shah PS, et al. : Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index < 35 kg/m 2. 2010;6(4):332–8. 10.1016/j.soard.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee WJ, Ser K, Chong KH, et al. : Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. 2010;147(5):664–9. 10.1016/j.surg.2009.10.059 [DOI] [PubMed] [Google Scholar]
- 101. Cohen R, Pinheiro JS, Correa JL, et al. : Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m 2: a tailored approach. 2006;2(3):401–4, discussion 404. 10.1016/j.soard.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 102. DePaula AL, Macedo AL, Schraibman V, et al. : Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20-34. 2009;23(8):1724–32. 10.1007/s00464-008-0168-6 [DOI] [PubMed] [Google Scholar]
- 103. DePaula AL, Macedo AL, Mota BR, et al. : Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21-29. 2009;23(6):1313–20. 10.1007/s00464-008-0156-x [DOI] [PubMed] [Google Scholar]
- 104. Du X, Fu X, Shi L, et al. : Effects of Laparoscopic Roux-en-Y Gastric Bypass on Chinese Type 2 Diabetes Mellitus Patients with Different Levels of Obesity: Outcomes After 3 Years' Follow-Up. 2018;28(3):702–11. 10.1007/s11695-017-2903-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Baskota A, Li S, Dhakal N, et al. : Bariatric Surgery for Type 2 Diabetes Mellitus in Patients with BMI <30 kg/m 2: A Systematic Review and Meta-Analysis. 2015;10(7):e0132335. 10.1371/journal.pone.0132335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang G, Zhu L, Li W, et al. : Can low BMI Chinese patients with type 2 diabetes benefit from laparoscopic Roux-en-Y gastric bypass surgery? 2016;12(10):1890–5. 10.1016/j.soard.2016.06.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Neovius M, Narbro K, Keating C, et al. : Health care use during 20 years following bariatric surgery. 2012;308(11):1132–41. 10.1001/2012.jama.11792 [DOI] [PubMed] [Google Scholar]
- 108. Inge TH, Courcoulas AP, Jenkins TM, et al. : Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. 2016;374(2):113–23. 10.1056/NEJMoa1506699 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Xanthakos SA, Jenkins TM, Kleiner DE, et al. : High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. 2015;149(3):623–34.e8. 10.1053/j.gastro.2015.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Doycheva I, Watt KD, Alkhouri N: Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. 2017;65(6):2100–9. 10.1002/hep.29068 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. : The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. 2009;58(11):1538–44. 10.1136/gut.2008.171280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Beamish AJ, Olbers T, Kelly AS, et al. : Cardiovascular effects of bariatric surgery. 2016;13(12):730–43. 10.1038/nrcardio.2016.162 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 113. Dixon JB: Surgical management of obesity in patients with morbid obesity and nonalcoholic fatty liver disease. 2014;18(1):129–46. 10.1016/j.cld.2013.09.011 [DOI] [PubMed] [Google Scholar]