Abstract
Background
Osteoarthritis (OA) is the most common joint disorder worldwide and one of the leading causes of disability in the elderly. We have investigated the novel sodium hyaluronate derivative chemically linked with diclofenac (DF), diclofenac etalhyaluronate (SI-613), which is a potentially safer and more effective treatment for OA knee pain. In this study, we evaluated the pharmacological effects of SI-613 in experimental arthritis models.
Methods
We compared the analgesic and anti-inflammatory effects of intra-articularly administered SI-613, hyaluronic acid (HA), and of orally administered diclofenac sodium (DF-Na) in rat silver nitrate-induced arthritis model and rabbit antigen-induced arthritis model.
Results
A single intra-articular (IA) administration of SI-613 significantly suppressed pain responses in rats in a dose-dependent manner. The analgesic effects were greater than those of HA, a mixture of DF-Na and HA, or an oral once-daily administration of DF-Na. In the rabbit arthritis model, SI-613 significantly reduced knee joint swelling compared with that in the control group on day 1 after a single IA injection. This significant anti-inflammatory effect was observed until day 28. In the pharmacokinetic study, the DF concentration in the synovium after SI-613 administration reached its maximum concentration of 311.6 ng/g on day 1, and gradually declined to 10 ng/g by day 28. It fell below the lower limit of quantification on day 35. Thus, a clear correlation was found between pharmacokinetics and pharmacodynamics. These results demonstrate that SI-613 exerts its long-lasting and potent anti-inflammatory effect by sustainable release of DF in the knee joint tissues.
Conclusion
A single IA injection of SI-613 was shown to exert analgesic and anti-inflammatory effects for 28 days in non-clinical pharmacological studies, suggesting that SI-613 will be a promising candidate in the treatment of osteoarthritis pain.
Keywords: Osteoarthritis, Hyaluronan, Diclofenac etalhyaluronate, Sustained-release, Conjugate technology
Background
Osteoarthritis (OA) is the most common joint disorder worldwide and one of the leading causes of disability in the elderly [1]. Treatment for knee OA aims to relieve pain and improve function, in order to mitigate reductions in physical activity. The mainstay of pharmacological therapy for OA includes acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) (oral and topical), cyclooxygenase-2 (COX-2) inhibitors, and IA therapies such as intra-articular sodium hyaluronate (IA-HA) injections and intra-articular-steroid (IA-steroid) injections.
Oral NSAIDs are widely prescribed for the treatment of OA pain. Nevertheless, upper gastrointestinal tract complications have been reported in patients who received long-term oral NSAIDs [2]. NSAIDs are also considered to have limited efficacy for OA pain relief. In the 1990s, a number of selective COX-2 inhibitors were developed to reduce the adverse events of NSAIDs. However, most of them were withdrawn from the market after the cardiovascular adverse effects of COX-2 inhibitors were reported in 2004 [3]. Meanwhile, oral NSAIDs including diclofenac sodium (DF-Na) were also reported to have the same concerns as COX-2 inhibitors [4, 5]. Thereafter, a few topical NSAIDs formulations were developed and launched for the relief of OA pain. Topical NSAIDs formulations, such as diclofenac sodium 1% gel, have equivalent efficacy and fewer adverse events compared with oral NSAIDs [6–8]. The intra-articular (IA) injection of hyaluronic acid (HA) is a recognized treatment for pain associated with symptomatic knee OA [9–11]. The pain relief afforded by IA-HA injections is long lasting and often lasts longer than 13 weeks [12, 13]. However, the efficacy of IA-HA injections is moderate compared to that of IA-steroids or oral NSAIDs. Therefore, the profiles of the next generation of OA therapeutics should be potent and longer lasting with higher safety, which will improve the quality of life for OA patients.
In the pursuit of the next generation of OA therapeutics, we developed a novel conjugated compound, SI-613. It is a novel derivative of high-molecular-weight fermented HA (600,000 to 1,200,000 Da), which tethers the NSAID diclofenac (DF) via a 2-aminoethanol linker extended from glucuronic acid moieties (Fig. 1). SI-613 gradually releases DF by hydrolytic cleavage of the ester linkage in a pH-dependent manner. Furthermore, SI-613 releases DF locally in a sustained manner and remains in the joint for a long period, similar to the existing IA-HA injection. It is expected to be more advantageous compared to IA-HA injections and NSAIDs in terms of efficacy and duration.
Fig. 1.
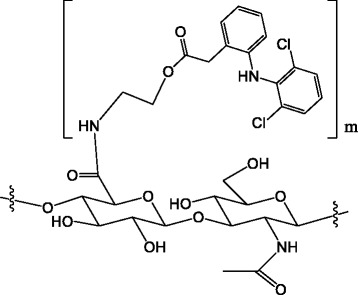
Chemical structure of N-[2-[[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]ethyl]hyaluronamide (diclofenac etalhyaluronate, SI-613)
In the present study, we investigated the pharmacological effects of the IA administration of SI-613 and compared them with those of p.o. DF-Na and IA-HA administration. Furthermore, we investigated the pharmacokinetics of intra-articularly administered SI-613.
Methods
Animals
Male Sprague-Dawley (SD) rats (5 weeks old) were obtained from Charles River Laboratories Japan Inc. (Tokyo, Japan). Male New Zealand White rabbits (12–15 weeks old) were obtained from Oriental Yeast Co., Ltd. (Tokyo, Japan). Animals were maintained under specific pathogen free conditions at a room temperature of 23 ± 3 °C and air humidity of 50 ± 20% on a 12-h/12-h light/dark cycle. Animals were quarantined and acclimatized to the environmental conditions for 1 week.
Rat model of silver nitrate-induced arthritic pain
The silver nitrate-induced arthritic pain model is known as a subacute arthritis model, in which the inflammatory response and pain last for at least 3 days following injury. This model involves the activation of prostaglandin pathways and has been used for evaluating the analgesic effects of various NSAIDs or kappa-opioid receptor agonist [14–18]. Overall, 136 male Sprague-Dawley (SD) rats were obtained from Charles River Laboratories Japan Inc. General anesthesia was maintained by the inhalation of isoflurane (Forane; Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan). Silver nitrate solution (1%, Wako Pure Chemical Industries, Ltd., Osaka, Japan) at a volume of 50 μL/joint was injected in the knee joint cavity of the left hindlimbs of rats. Animals with no abnormalities were allocated to four groups in each study based on the weight-bearing rate of the inflamed joints and pain score at the time of allocation, by using stratified continuous randomization. Test substances were administered on the day following the arthritis induction. In the first study, SI-613 (contents of DF; 11.8% (w/w), manufactured by Seikagaku Corporation, Tokyo, Japan) at doses of 0.05, 0.15, and 0.5 mg/50 μL/joint (5.9, 17.7, and 59 μg/joint in DF equivalent) or phosphate-buffered saline (PBS) was once injected into the joint cavities of the left hindlimbs for the dose-response assessments.
In the second study, SI-613 (0.5 mg/joint, 59 μg/joint in DF equivalent), HA (0.5 mg/joint, Seikagaku Corporation), a mixture of DF-Na (59 μg/joint, Wako Pure Chemical Industries, Ltd.) and HA (0.5 mg/joint) (DF-Na + HA), or PBS was administered in the same manner for the proof of concept. The doses of DF-Na and HA were set at those of respective components in SI-613 formulation. The DF-Na solution (1 mg/mL) was prepared using the Water for Injection (Otsuka Pharmaceutical Factory, Inc., Tokyo, Japan) and administered orally at a dose of 2 mg/kg (approximately 0.3 mg/body) once daily for 3 days. The oral DF-Na dose, 2 mg/kg, was set in accordance with the dose for adult humans with a body weight of 50 kg, on the assumption that the maximum daily dose in clinical practice administered to patients with OA or rheumatoid arthritis would be 100 mg. It has been reported that DF-Na exerted anti-inflammatory effects in rats when administered at this dose [19].
Pain was assessed under blinded conditions by scoring pain-related behaviors based on the following criteria and measuring the weight-bearing rates of hindlimbs with a load-measuring device (Tokken Inc., Chiba, Japan) at a same time each day for 3 days after the injection of the test materials.
The criteria for assigning pain scores were as follows: 0; normal, 1; mild claudication with lifting the foot, 2; severe claudication with completely closing the toe, 3; walking on three legs. The weight-bearing rate was calculated using the following formula:
Weight-bearing rate (%) = Mean weight-bearing load on inflamed leg (g) / Body weight (g) × 100.
In addition, the prostaglandin E2 (PGE2) content, which plays a critical role in inducing inflammation and pain associated with arthritis, was determined in the synovial fluid (SF). Briefly, SI-613 (0.5 mg/joint) or PBS was administered into the joint cavity of the left hindlimb. After assessing the severity of pain, animals were sacrificed by exsanguination under 2% isoflurane anesthesia 1, 2 and 3 days after administration, respectively. The SF was collected by washing the joint cavity with saline (Otsuka Pharmaceutical Factory, Inc.) containing indomethacin (Indacin, MSD K. K., Tokyo, Japan), which was effective to prevent joint puncturing-induced production of PGE2 (unpublished result). The PGE2 content in the SF was measured with a High Sensitivity PGE2 Correlate-EIA kit (Assay Designs Inc., Ann Arbor, MI).
Effect of SI-613 on the PGE2 content in the SF of rabbits with antigen-induced arthritis
The anti-inflammatory effects of SI-613 were evaluated in a rabbit arthritis model induced by ovalbumin (OVA) [20–22], and compared with those of orally-administered DF-Na or the active chemical compositions of SI-613. To prepare the anesthetic, saline (2 mL), midazolam (1 mL, 5 mg/mL, Astellas Pharma Inc., Tokyo, Japan), xylazine (2 mL, 0.02 g/mL, Bayer Medical Ltd., Tokyo, Japan), and butorphanol tartrate (1 mL, 5 mg/mL, Meiji Seika Kaisha, Ltd., Tokyo, Japan) were mixed. The anesthetic was administered intravenously to each animal at a volume of 1 mL/body. OVA (Sigma-Aldrich Co., St. Louis, MO) emulsion with Freund’s complete adjuvant (FCA; CAPPEL Laboratories Inc., Cochranville, PA) was injected intradermally into the backs of 80 male rabbits at a dose of 5 mg/animal twice at an interval of 13 or 14 days. Twenty-three days after the first immunization, 1% OVA solution was injected in the joint cavities of the left hindlimb at a volume of 500 μL/joint to induce arthritis. Two days after the induction of arthritis, test materials of 5 mg/joint SI-613, a mixture of 0.59 mg/joint DF-Na and 5 mg/joint HA (DF-Na + HA), or PBS (control) were administered at a volume of 500 μL/joint in the knee joint cavity. DF-Na was orally administered at a dose of 2 mg/kg. Animals were sacrificed by exsanguination under 2% isoflurane anesthesia 3 and 72 h after administration, respectively. The SF was collected by washing the joint cavity twice with saline (Otsuka Pharmaceutical Factory, Inc.) containing 20 μg/mL of indomethacin (Indacin, MSD K. K.) at 3 or 72 h after the administration of the test materials. The PGE2 content in the SF was determined as a mechanism biomarker for the anti-inflammatory effect using a High Sensitivity Prostaglandin E2 Enzyme Immunoassay Kit (Assay Designs Inc.).
Long-lasting anti-inflammatory effect of SI-613 on antigen-induced arthritis in rabbits
The duration and efficacy of the anti-inflammatory effect of SI-613 was studied in OVA-induced arthritis rabbits [20–22]. Two days after the arthritis induction, the knee joint diameter in all 60 rabbits was measured with a digital thickness gauge (Teclock Corp., Nagano, Japan). The joint swelling was expressed as the difference in millimeters between the inflamed (left) and non-inflamed (right) knee joint diameters. The same researcher evaluated the knee joint swelling on all groups on all days. Fifteen animals (knee joint swelling was not more than 7.30 mm) and 5 animals (knee joint swelling was not less than 10.40 mm) were excluded. Forty animals were divided into two groups of 20 animals each by the stratified random sampling method based on the knee joint swelling and body weight of the day, and were administered with 5 mg/joint SI-613 or PBS at a volume of 500 μL/joint into the joint cavities. The knee joint swelling was evaluated the day before (day 0), and on days 1, 3, 7, 14, 21, 28, 35, and 42 after a single injection of the test materials. The knee joint swelling was expressed as the difference in the width of the right and left knee joint.
Distribution of DF in knee tissues after a single IA administration of SI-613 and its chemical compositions in rabbits: Short-term study
Arthritis was induced in the 12 rabbits as described above. Two days after the induction of arthritis, 5 mg/joint SI-613, a mixture of 0.59 mg/joint DF-Na and 5 mg/joint HA (DF-Na + HA), or PBS was administered to the knee joint cavity at a volume of 500 μL/joint. DF-Na was orally administered at a dose of 2 mg/kg. The concentrations of free DF in the synovium and synovial lavage fluid were measured by high performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) at 3 and 72 h after a single IA administration and after a single oral administration of the test materials, respectively. Moreover, plasma concentrations of DF were determined to compare the systemic exposure of DF after the administration of SI-613 with that of the other compounds. The synovium was homogenized in 40-fold volume (40 mL for 1 g tissue) of 10 mM ammonium formate (pH 6.0)/methanol (3:2, v/v) on an ice bath. Then, free DF in the homogenate was extracted with tert-butyl methyl ether-1% acetic acid (6:1, v/v). Free DF in the synovial fluid or plasma was adsorbed to an Oasis HLB cartridge (30 mg/1 cm3, Waters Corporation, Milford, MA) and eluted with methanol. The internal standard of deuterium-labeled diclofenac (diclofenac-d7) was added to each sample. The extract was loaded onto a CAPCELL PAK C18 MG HPLC column (Shiseido Co. Ltd., Tokyo, Japan, column size: 4.6 mm × 35 mm, particle size: 5 μm) at 40 °C, and eluted with 10 mM ammonium formate (pH 6.0)/methanol (2:3, v/v) at a flow rate of 0.5 mL/minute. For mass detection, we used a QTRAP® 5500 System (AB SCIEX, Framingham, MA) equipped with an electrospray ionization (ESI) source in positive ions in multiple reaction monitoring (MRM) mode. Linear calibration (r > 0.999) was attained at 5–1000 ng/g for the synovium and at 1–200 ng/mL for the synovial fluid and plasma. The extraction efficiencies of diclofenac were 84.2–92.9% for the synovium, 95.2–100.3% for the synovial fluid, and 94.2–96.5% for the plasma.
Evaluation of DF in knee tissues after a single IA administration of SI-613 in rabbits: long-term study
Arthritis was induced in the 24 rabbits as described above. Two days after the induction of arthritis in the rabbits [20–22], 5 mg/joint SI-613 or PBS was administered at a volume of 500 μL/joint into the knee joint cavity. Concentrations of DF in the synovium and synovial lavage fluid were measured by LC-MS/MS on days 1, 3, 7, 14, 21, 28, 35, and 42 after a single IA administration.
Statistical analyses
Statistical analyses were performed using the Statistical Analysis System, SAS (SAS Institute Inc., Cary, NC). Direct effects from treatment with SI-613 were assessed using two-way analyses of variance followed by Williams’ or Tukey’s test for the analgesic assessment of the joint pain model in rats. A Student’s t-test, Welch’s t-test, or Tukey’s test was performed for the assessment of the PGE2 content in the SF. A Student’s t-test with Holm’s correction was used for the assessment of long-lasting anti-inflammatory effects. Results of the measurements in each group were represented as the mean and 95% confidence intervals (CI) for the pharmacological study or standard deviations (SD) for the pharmacokinetic study; p values of < 0.05 were considered statistically significant.
Results
Analgesic effects of SI-613 on silver nitrate-induced arthritic pain in rats
SI-613 improved the pain behavioral scores significantly at doses of 0.05, 0.15, and 0.5 mg/joint in a dose-dependent manner (Fig. 2a and c). In addition, SI-613 at 0.15 and 0.5, but not at 0.05 mg/joint significantly increased the weight-bearing rates in a dose-dependent manner compared with those in the control group (Fig. 2b and d). Furthermore, compared with PBS, HA, or DF-Na + HA, or the repeated oral administration of DF-Na, SI-613 significantly improved the pain behavioral score (Fig. 3a and c) and increased the weight-bearing rate (Fig. 3b and d). On day 1, the pain score in the DF-Na + HA group was lower than that in the control group; however, this analgesic effect was not observed on and after day 2. It is concluded that a single IA administration of SI-613 exerts a more efficacious and longer-lasting analgesic effect for arthritic pain than that exerted by individual chemical compositions.
Fig. 2.
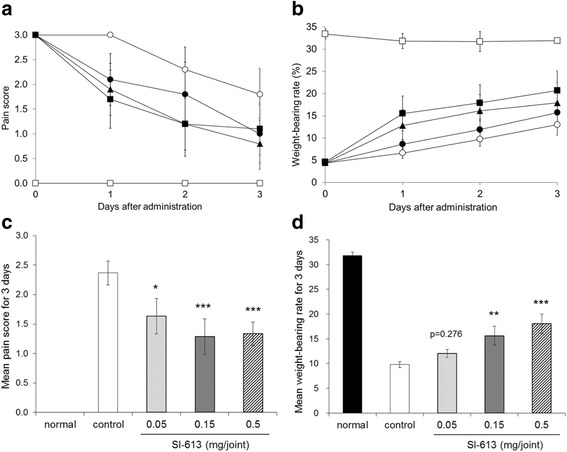
Analgesic effect of SI-613 in silver nitrate-induced arthritic pain model in rats. Silver nitrate-induced arthritic rats given 0.05 mg (closed circle), 0.15 mg (closed triangle), 0.5 mg (closed square) SI-613, or vehicle (open circle) intra-articularly, and non-treated normal rats (open square) were evaluated for pain score (a) and weight-bearing rate (b) over time. Mean values of pain scores (c) and weight-bearing rate (d) for 3 days were calculated and subjected to statistical analysis: two-way analyses of variance followed by Williams’ test. ***p < 0.005, **p < 0.01, *p < 0.05 (vs. control, significant level at 5%, two-tailed). Values represent the means ±95% confidence intervals (n = 9 per group, except for the normal group n = 3)
Fig. 3.
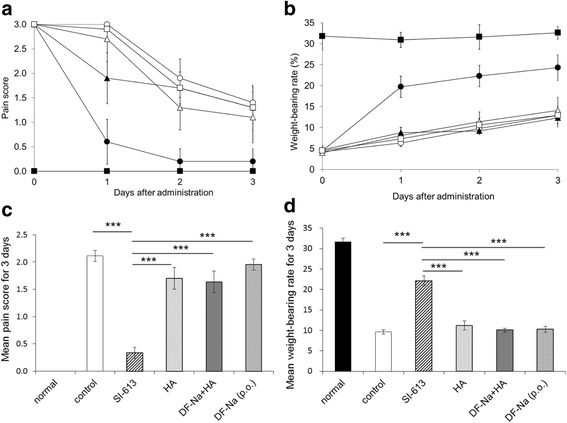
Analgesic effects of SI-613 and its chemical compositions in silver nitrate-induced arthritic pain model in rats. Silver nitrate-induced arthritic rats given SI-613 (closed circle), HA (open triangle), DF-Na + HA (closed triangle), or vehicle (open circle) intra-articularly; those given DF-Na orally once daily for 3 days (open square); and non-treated normal rats (closed square) were evaluated for pain score (a) and weight-bearing rate (b) over time. Mean values of pain scores (c) and weight-bearing rate (d) for 3 days were calculated and subjected to statistical analysis: two-way analyses of variance followed by Tukey’s test. ***p < 0.001 (vs. SI-613, significant level at 5%, two-tailed). Values represent the means ±95% confidence intervals (n = 9 per group, except for the normal group n = 3)
In addition, the anti-inflammatory effect of SI-613 was assessed by measuring the PGE2 content in the SF. The PGE2 content decreased over time, but high values were maintained for 3 days in this animal model. SI-613 group showed lower PGE2 content than the control group (Fig. 4b). The analgesic effect was confirmed each day (Fig. 4a).
Fig. 4.
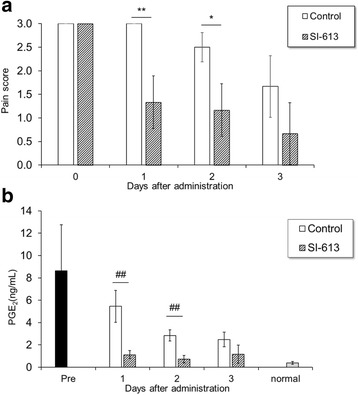
Effects of SI-613 on PGE2 content in the synovial fluid of silver nitrate-induced arthritic rats. Silver nitrate-induced arthritic rats given 0.5 mg SI-613 or vehicle intra-articularly were evaluated for pain score (a), and sacrificed for measurement of the prostaglandin E2 (PGE2) content in the SF (b) on days 1, 2, and 3. For statistical analysis of pain score, Wilcoxon test was used. **p < 0.01, *p < 0.05 (vs. control, significant level at 5%, two-tailed). For statistical analysis of the PGE2 content, Welch’s t-test was used. ##p < 0.01 (vs. control, significant level at 5%, two-tailed). Values represent the means ±95% confidence intervals [n = 6 per group, except for the normal group (PGE2 content) n = 7]
Effect of SI-613 on the PGE2 content in the SF of rabbits with antigen-induced arthritis
At 3 h after administration, the mean PGE2 content in the SF was 27,651 pg/joint (95% CI = 17,844–37,458 pg/joint) in the control group, whereas that in the DF-Na group was 3767 pg/joint (95% CI = 847–6687 pg/joint) (Fig. 5a). Orally administered DF-Na significantly suppressed the production of PGE2 in the SF compared with the control group, suggesting that this model was appropriate for measuring PGE2. At 72 h after the injection, the mean PGE2 content in the SF was 8267 pg/joint (95% CI = 6535–9999 pg/joint) in the control group. In the DF-Na, DF-Na + HA, and SI-613 groups, the PGE2 content was 8873 pg/joint (95% CI = 6464–11,282 pg/joint), 6378 pg/joint (95% CI = 4319–8437 pg/joint), and 106 pg/joint (95% CI = 70–142 pg/joint), respectively. SI-613 significantly suppressed the production of PGE2 in the SF compared with that of DF-Na, DF-Na + HA, and the control group (Fig. 5b). No significant differences were observed between the DF-Na, DF-Na + HA, and control groups. A single IA administration of SI-613 was shown to exert a longer-lasting effect compared with DF-Na or DF-Na + HA.
Fig. 5.

Effects of SI-613 on PGE2 content in the synovial fluid of antigen-induced arthritis in rabbits. Two days after the induction of arthritis, the test materials were administered. The synovial fluid (SF) was collected at 3 (a) or 72 (b) hours after the administration of the test materials. The prostaglandin E2 (PGE2) content in the SF was measured with a PGE2 enzyme-linked immunosorbent assay (ELISA) kit. For statistical analysis, a Student’s t-test and Tukey’s test were used for the 3 h and 72 h data, respectively. a ***p < 0.001 (vs. control, significant level at 5%, two-tailed). Values represent the means ±95% confidence intervals. (n = 10 per group, except for the normal group n = 5) (b) ***p < 0.001 (vs. SI-613, significant level at 5%, two-tailed). Values represent the means ±95% confidence intervals. (n = 10 per group, except for the normal group n = 5)
Long-lasting anti-inflammatory effect of SI-613 on antigen-induced arthritis in rabbits
SI-613 significantly decreased the knee joint swelling compared with the control on day 1 after the injection, and exerted an anti-inflammatory effect continuously until day 28 (Fig. 6). However, on day 35 and day 42, there was no significant difference in the joint swelling of SI-613-treated and control animals.
Fig. 6.
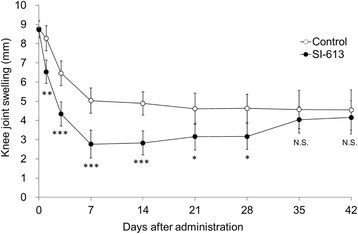
Long-lasting anti-inflammatory effect of SI-613 on the knee joint swelling of antigen-induced arthritic rabbits. Two days after the induction of arthritis, 1% SI-613 or PBS was administered at a volume of 500 μL/joint into the joint cavities. The knee joint swelling was evaluated on the day before (day 0), and on days 1, 3, 7, 14, 21, 28, 35, and 42 after the injection of test materials. For statistical analysis, a Student’s t-test with Holm’s correction was used. ***p < 0.001, **p < 0.01, *p < 0.05 (vs. control, significant level at 5%, two-tailed). N.S., not significant. Values represent the means ±95% confidence intervals. (n = 20 per group)
Distribution of DF in knee tissues after a single administration of SI-613 and its chemical compositions in rabbits: short-term study
In the antigen-induced arthritis model, the DF concentrations in the synovium and synovial lavage fluid were determined at 72 h after a single IA administration of SI-613 or a mixture of DF-Na and HA. In addition, the DF concentrations at 3 and 72 h after the oral administration of DF-Na were determined. As shown in Table 1, the SI-613-treated group showed higher DF concentrations in the synovium and synovial lavage fluid than the other groups at 72 h after the injection.
Table 1.
DF concentrations in the synovium and synovial joint cavity of rabbits with antigen-induced arthritis
| Group | Sampling point | Animal Number | Concentration of DF | |
|---|---|---|---|---|
| Synovium (ng/g tissue) | Synovial lavage fluid (ng/mL fluid) | |||
| DF-Na (oral) | 3 h | 1 | 155.8 | 124.6 |
| 2 | 52.88 | 58.23 | ||
| 3 | 75.83 | 45.66 | ||
| 72 h | 4 | BLQ1 | BLQ2 | |
| 5 | BLQ1 | BLQ2 | ||
| 6 | BLQ1 | BLQ2 | ||
| A mixture of DF-Na and HA (IA) | 72 h | 1 | BLQ1 | BLQ2 |
| 2 | BLQ1 | BLQ2 | ||
| 3 | 48.02 | 21.70 | ||
| SI-613 (IA) | 72 h | 1 | 15.23 | 60.64 |
| 2 | 45.47 | 38.82 | ||
| 3 | 351.1 | 1540 | ||
BLQ1: below limit of quantification, < 5 ng/g
BLQ2: < 1 ng of DF per mL of synovial lavage fluid
Time for calculation of area under the DF plasma concentration-time curve (AUC0-t), t (day), was the latest time point at which DF was quantifiable. The half-life (T1/2) was determined by semi-log plotting the data of at least three time points after Tmax. T1/2, AUC0-∞, and AUC0-t were not obtained for one animal in the DF-Na + HA group and all animals in the SI-613 group, both of which did not provide a required number of effective time points after Tmax, and shown as NC (not calculated). The maximum plasma concentration (Cmax) of DF in the SI-613 group (IA) was 462 and 94 times lower than those of the DF-Na group (oral) and DF-Na + HA group (IA), respectively. Similarly, the AUC0-t of the SI-613 group (IA) was 187 and 16 times smaller than that of the DF-Na group (oral) and DF-Na + HA group (IA), respectively (Table 2).
Table 2.
Plasma DF concentrations of rabbits with antigen-induced arthritis
| Group | Cmax (ng/mL) |
Tmax (h) |
AUC0-t (ng·h/mL) |
AUC0-∞ (ng·h/mL) |
t1/2 (h) |
|---|---|---|---|---|---|
| DF-Na (oral) | 621.0 ± 371.6 | 2.4 ± 3.2 | 4693 ± 1905 | 4132b | 12.8b |
| Mixturea | 125.9 ± 68.5 | 0.39 ± 0.53 | 393.4 ± 167.6 | 423.2 ± 154.5 | 3.4 ± 2.0 |
| SI-613 (IA) | 1.343 ± 0.050 | 24 ± 0 | 25.11b | NC | NC |
Mean ± SD (n = 3 or n = 2) calculated from the individual PK parameters
NC Not calculated.
BLQ: < 1 ng/mL
a a mixture of DF-Na and HA (IA)
b n = 2
Evaluation of DF in knee tissues after a single IA administration of SI-613 in rabbits: long-term study
Concentrations of DF in the synovium and synovial lavage fluid of antigen-induced arthritis rabbits were determined after a single IA injection of 5 mg of SI-613 by LC-MS/MS. The mean concentration in the synovium was 9.754 ng/g on day 28 and decreased below the lower limit of quantification (< 5 ng/g) on day 35 (Fig. 7a). The mean amount of DF in the joint cavity lavage fluid was 5.940 ng/joint on day 21 and decreased below the lower limit of quantification (< 1 ng of DF per mL of synovial lavage fluid) on day 28 (Fig. 7b). The pharmacokinetic parameters of DF are listed in Table 3.
Fig. 7.
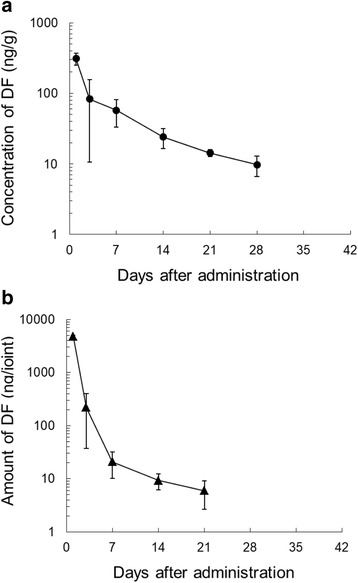
a Profile of diclofenac sodium (DF) concentrations in the synovium after a single IA administration of SI-613 in rabbits with antigen-induced arthritis. SI-613 was intra-articularly injected at a dose of 5 mg/joint, and each value shows the mean ± standard deviation (S.D.) of 3 animals. The lower limit of quantification was 5 ng/g. b Profile of DF concentrations in the joint cavity after a single IA administration of SI-613 in rabbits with antigen-induced arthritis. SI-613 was intra-articularly injected at a dose of 5 mg/joint, and each value shows the mean ± S.D. of 3 animals. The lower limit of quantification was 1 ng of DF per mL of synovial lavage fluid
Table 3.
PK parameters of DF after single IA administration of SI-613 in rabbits with antigen-induced arthritis
| Cmax (ng/g) |
Tmax (day) |
AUC0-28day (ng∙day/g) |
AUC0-∞ (ng∙day/g) |
t1/2 (day) |
|
| Synovium | 311.6 | 1 | 1336 | 1487 | 10.8a |
| Cmax (ng/joint) |
Tmax (day) |
AUC0-21day (ng∙day/joint) |
AUC0-∞ (ng∙day/joint) |
t1/2 (day) |
|
| Synovial lavage fluid | 4726 | 1 | 7956 | 8018 | 7.7b |
SI-613 was intra-articularly injected at a dose of 5 mg/joint. The PK parameters were calculated from mean concentrations of DF in synovium or mean amounts of DF in joint cavity (n = 3)
a, Calculated from 14 to 28 days after administration
b, Calculated from 7 to 21 days after administration
Discussion
We have investigated the novel HA derivative chemically linked with DF, SI-613, which is a potentially safer and more effective treatment for OA knee pain. In the present study, the pharmacological effects of SI-613 were comprehensively evaluated by comparing the pain response, weight-bearing rate on the inflamed legs, joint swelling, and PGE2 content in the SF as an indicator of hydrarthrosis using rat and rabbit arthritis models. In the arthritic pain model, it was shown that a single IA injection of SI-613 exerted an analgesic effect more effectively than orally administered DF-Na in a dose-dependent manner. The analgesic effect of SI-613 was most probably due to inhibition of PGE2 production, although the effect on it was slightly varying depending on the model. The PGE2-inhibitory effect was statistically significant at 72 h after SI-613 administration in the rabbit model but not in the rat model. This can be attributed to the difference of the dynamic range between the models. In the rabbit models, the difference of normal and control groups was sufficiently large even at 72 h. On the other hand, the PGE2 level of the control group in the rat model was only about 6 times larger than that of the normal group, and then there was no statistically significant difference between the SI-613 and control groups. Moreover, the effect of SI-613 was long-lasting, which was never achieved with the DF-Na + HA mixture. The unstable analgesic effect of uncombined-DF-Na (without HA) is probably due to the fact that it is not well retained in the synovium, which is a therapeutic target tissue for OA. In contrast, it is most likely that intra-articularly administered SI-613 is retained in the synovium for a prolonged period and sustainably releases DF in the inflamed region. The relevance of this notion is supported by other studies that found that HA administered intra-articularly to the joint cavity penetrated into the synovium and remained in the synovium for a longer period [23, 24]. Furthermore, this efficient retention in the synovium might benefits from the high affinity of HA and its cell surface receptor, CD44 [25], expressed in the synovium [26]. SI-613, thus, delivered efficacious concentrations of DF to synoviocytes. Therefore, we consider that HA is an indispensable component for the analgesic effect of SI-613.
The superiority of SI-613 was supported by its inhibitory effect on the production of PGE2. This effect resulted from DF being sustainably released from SI-613. The relationship between the pharmacological effects and pharmacokinetics of SI-613 was investigated using a rabbit antigen-induced arthritis model. SI-613 showed a sustainable anti-inflammatory effect for 72 h after administration, whereas the mixture of DF and HA or oral DF-Na did not. The concentration of DF in the synovium and SF at 72 h after the administration of SI-613 was higher than that after the administration of the mixture of DF and HA or oral DF-Na. SI-613 exerted a long-lasting analgesic effect, for 28 days. However, no significant difference was observed on day 35 and 42. The DF concentration in the synovium reached its maximum level of 311.6 ng/g on day 1 after the injection and this gradually declined to 10 ng/g by day 28. It fell below the lower limit of quantification (< 5 ng/g) on day 35. Therefore, there is a clear correlation between the analgesic effect and the retention period of DF. The DF concentrations at 28 days after the SI-613 administration were at comparable levels with those in humans after repeated administration of DF preparations. In the clinical studies of current DF preparations, the DF concentrations in the synovium ranged from 5 to 35 ng/g after repeated oral administration of DF tablets and hard capsules or repeated topical administration of DF gel ointments and cataplasms [27, 28]. These findings suggest that SI-613 exerted an analgesic effect via the sustained release of DF, and this pharmacological effect lasted for at least 28 days.
It has been reported that HA inhibits the phosphorylation of p38 mitogen-activated protein kinase (MAPK) via its principal receptor CD44 and exerts an anti-inflammatory effect [29]. Furthermore, HA inhibits the production of PGE2, and pretreatment with OS/37, a monoclonal antibody specific for the hyaluronate-binding epitope on CD44, reversed the inhibitory effects of HA [30]. The inhibition of the production of PGE2 by HA was also confirmed in a clinical study [31]. In addition, it was reported that HA exerted an analgesic effect by covering free nerve endings in articular tissues such as the synovial membranes, menisci, and ligaments [32]. This suggests that SI-613 exerts a clearly superior analgesic effect than DF or HA, or co-administered DF + HA.
Oral NSAIDs are widely prescribed for the relief of OA pain, however, upper gastrointestinal tract complications have been reported in patients who received long-term oral NSAIDs [2]. NSAIDs are also considered to have limited efficacy for OA pain relief. In 1990s, a number of selective COX-2 inhibitors were developed to reduce the adverse events of NSAIDs. However, most of them were withdrawn from the market after the cardiovascular adverse effects of COX-2 inhibitor were reported in 2004 [3]. Meanwhile, oral NSAIDs including DF-Na were also reported to have the same concerns as COX-2 inhibitors [4, 5]. In the present study, the Cmax of DF (1.343 ng/mL) in the animals given a single IA effective dose of SI-613 was 462 times lower than that in the animals given a single oral effective dose of DF-Na. The AUC0-t (25.11 ng·h/mL) of the SI-613-treated group was 187 times lower than that of the oral DF-Na-treated group. Moreover, the DF concentrations after the SI-613 injection were lower than the reported values of DF-Na after oral administration to humans at the clinical dose (3 × 50 mg/day) [33]. Additionally, the pharmacokinetics of Voltaren® Gel (1% diclofenac sodium topical gel) has been assessed in healthy volunteers following repeated applications to 1 knee [4 × 4 g per day (= 160 mg DF-Na per day)] for 7 days [33]. The Cmax of DF was 15 ± 7.3 ng/mL, and the value is comparable with those of SI-613-treated animals. These results indicate that systemic toxicities are unlikely to be attributable to DF after a single IA administration of SI-613 to the knees.
OA is characterized by gradual cartilage degeneration. Although NSAIDs are effective in relieving OA pain and have been in use for decades, it remains controversial as to what effects NSAIDs have on the progression of OA. Reijman et al. made the observation that the chronic use of DF, but not ibuprofen, naproxen, or piroxicam, accelerated the progression of knee and hip OA in subjects aged over 55 years [34]. In another paper, Huskisson et al. reported that indomethacin increases the rate of radiological deterioration in the knee joint space of patients with OA [35]. However, the beneficial or neutral effects of NSAIDs have been reported in in vitro and in vivo studies [36–39]. de Boer et al. reported that celecoxib, a selective COX-2 inhibitor, has a chondroprotective effect [40]. Therefore, it is still unclear whether NSAIDs cause cartilage degeneration. IA-HA can protect against articular cartilage degeneration of the knee accelerated by NSAIDs (loxoprofen monosodium and indomethacin) via the inhibition of matrix metalloproteinase (MMP) production [41, 42]. MMPs are upregulated in the chondrocytes of human OA [43–45] and play a critical role in cartilage destruction by degrading collagen and aggrecan, the main proteoglycan of chondrocyte. SI-613, containing HA as a component, may inhibit the interleukin-1β-stimulated production of MMP-1, − 3 and − 13 in human chondrocytes as well as HA. Future research agendas on SI-613 should involve clarifying the pharmacological effect of HA moiety for SI-613.
Conclusion
In conclusion, our results show that a single IA administration of SI-613 provides an efficacious and safe treatment for OA pain with a potent and long-lasting analgesic effect compared to existing IA-HA injections or oral NSAIDs.
Acknowledgments
We wish to thank Dr. Tetsuya Ohtaki, Dr. Yoshitaka Tanaka and Takatoshi Kubo in Seikagaku Corporation for study support and assistance in the preparation of the manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article is stored in Seikagaku Corporation, Tokyo, Japan. Further inquiries on the data may be submitted to Keiji Yoshioka (keiji.yoshioka@seikagaku.co.jp).
Abbreviations
- CI
Confidence intervals
- COX-2
Cyclooxygenase-2
- DF
Diclofenac
- FCA
Freund’s complete adjuvant
- HA
Hyaluronic acid
- IA
Intra-articular
- LC-MS/MS
High performance liquid chromatography coupled with tandem mass spectrometry
- MAPK
Mitogen-activated protein kinase
- MMP
Matrix metalloproteinase
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- OA
Osteoarthritis
- OVA
Ovalbumin
- PBS
Phosphate-buffered saline
- PGE2
Prostaglandin E2
- SD
Standard deviations
- SF
Synovial fluid
Authors’ contributions
KY, TK, RZ, and YY performed most experiments. KY, TK, RZ, and KM designed the study, interpreted the findings, and wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval
The animal studies were reviewed and approved by the Animal Experiment Ethics Committee of Seikagaku Corporation and performed under the animal husbandry/management system in an appropriate environment with animal protection/welfare in mind.
Competing interests
All authors of this paper are employees of Seikagaku Corporation. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Keiji Yoshioka, Email: keiji.yoshioka@seikagaku.co.jp.
Tomochika Kisukeda, Email: tomochika.kisukeda@seikagaku.co.jp.
Ryoji Zuinen, Email: ryoji.zuinen@seikagaku.co.jp.
Yosuke Yasuda, Email: yosuke.yasuda@seikagaku.co.jp.
Kenji Miyamoto, Email: kenji.miyamoto@seikagaku.co.jp.
References
- 1.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, et al. Risk of serious NSAID-related gastrointestinal events during long-term exposure: a systematic review. Med J Aust. 2006;185:501–506. doi: 10.5694/j.1326-5377.2006.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 3.Tarone RE, Blot WJ, McLaughlin JK. Nonselective nonaspirin nonsteroidal anti-inflammatory drugs and gastrointestinal bleeding: relative and absolute risk estimates from recent epidemiologic studies. Am J Ther. 2004;11:17–25. doi: 10.1097/00045391-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 5.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;c7086:342. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peniston JH, Gold MS, Wieman MS, Alwine LK. Long-term tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Clin Interv Aging. 2012;7:517–523. doi: 10.2147/CIA.S35416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efe T, Sagnak E, Roessler PP, Getgood A, Patzer T, Fuchs-Winkelmann S, et al. Penetration of topical diclofenac sodium 4% spray gel into the synovial tissue and synovial fluid of the knee: a randomised clinical trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:345–350. doi: 10.1007/s00167-013-2408-0. [DOI] [PubMed] [Google Scholar]
- 8.Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2%topical solution for osteoarthritis of the knee: a randomized, double-blind,vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241–250. doi: 10.1185/03007995.2015.1113400. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartil. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on osteoarthritis guidelines. Arthritis Rheum. 2000;43:1905–15. [DOI] [PubMed]
- 12.Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2012;20:350–356. doi: 10.1016/j.joca.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Day R, Brooks P, Conaghan PG, Petersen M. Multicenter trial group. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31:775–782. [PubMed] [Google Scholar]
- 14.Belle AL, Tislow R. A method of evaluating analgesics of the antiarthralgic type in the laboratory animal. J Pharm Exp Ther. 1950;98:19. [Google Scholar]
- 15.Nakamura H, Yokoyama Y, Motoyoshi S, Seto Y, Ishii K, Imazu C, et al. Anti-inflammatory and analgesic activities of a trans-cutaneous non-steroidal anti-inflammatory agent, etofenamate gel, in experimental animals.(in Japanese) Nippon Yakurigaku Zasshi. 1982;80:183–194. doi: 10.1254/fpj.80.183. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura H, Yokoyama Y, Ishii K, Motoyoshi S, Seto Y, Shimizu M. Effect of a non-steroidal anti-inflammatory drug, tolmetin sodium on arthritic pain, traumatic edema and LPS-induced fever. (in Japanese) Yakugaku Zasshi. 1981;101:649–656. doi: 10.1248/yakushi1947.101.7_649. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Yokoyama Y, Motoyoshi S, Ishii K, Imazu C, Seto Y, et al. The pharmacological profile of 2-(8-methyl-10,11-dihydro-11-oxodibenz[b, f]oxepin-2-yl)propionic acid (AD-1590), a new non-steroidal anti-inflammatory agent with potent antipyretic activity. Arzneimittelforschung. 1983;33(11):1555e69. [PubMed] [Google Scholar]
- 18.Tsukahara-Ohsumi Y, Tsuji F, Niwa M, Nakamura M, Mizutani K, Inagaki N, et al. SA14867, a newly synthesized kappa-opioid receptor agonist with antinociceptive and antipruritic effects. Eur J Pharmacol. 2010;647:62–67. doi: 10.1016/j.ejphar.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Tsurumi K, Hiramatsu Y, Yamaguchi A, Hayashi M, Shibuya T, Fujimura H. Anti-inflammatory action of N-(2,6-dichlorophenyl)-o-aminophenylacetic acid, its sodium salt, N-(2,6-dichlorophenyl)-anthranilic acid and its sodium salt. 2. On subacute inflammation. (in Japanese) Nippon Yakurigaku Zasshi. 1973;69:319–334. doi: 10.1254/fpj.69.319. [DOI] [PubMed] [Google Scholar]
- 20.Kiniwa M, Yamamoto N, Hashimoto Y, Miyake H, Masuda H. Anti-inflammatory effect of THS-201, a new intra-articular steroid. (in Japanese) Nippon Yakurigaku Zasshi. 1986;87:89–97. doi: 10.1254/fpj.87.89. [DOI] [PubMed] [Google Scholar]
- 21.Pettipher ER, Henderson B, Edwards JC, Higgs GA. Effect of indomethacin on swelling, lymphocyte influx, and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis. 1989;48:623–627. doi: 10.1136/ard.48.8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green KL, Foong WC. Treatment of antigen-induced arthritis in rabbits by the intra-articular injection of methylprednisolone, 90Y or chlorambucil. J Pharm Pharmacol. 1993;45:815–820. doi: 10.1111/j.2042-7158.1993.tb05692.x. [DOI] [PubMed] [Google Scholar]
- 23.Antonas KN, Fraser JR, Muirden KD. Distribution of biologically labelled radioactive hyaluronic acid injected into joints. Ann Rheum Dis. 1973;32:103–111. doi: 10.1136/ard.32.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson DW, Simon TM. Intra-articular distribution and residence time of Hylan a and B: a study in the goat knee. Osteoarthr Cartil. 2006;14:1248–1257. doi: 10.1016/j.joca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 26.Hale LP, Haynes BF, McCachren SS. Expression of CD44 variants in human inflammatory synovitis. J Clin Immunol. 1995;15:300–311. doi: 10.1007/BF01541320. [DOI] [PubMed] [Google Scholar]
- 27.Miyatake S, Ichiyama H, Kondo E, Yasuda K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol. 2009;67:125–129. doi: 10.1111/j.1365-2125.2008.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mashima T, Kondo M, Sakiyama N. Concentrations of Voltaren (diclofenac sodium) SR capsule in serum and tissues of patients with rheumatoid arthritis. Japanese journal of inflammation (Japan) 1995;15:255–259. [Google Scholar]
- 29.Julovi SM, Ito H, Hiramitsu T, Yasuda T, Nakamura T. Hyaluronan inhibits IL-1β-stimulated collagenase production via down-regulation of phosphorylated p38 in SW-1353 human chondrosarcoma cells. Mod Rheumatol. 2008;18:263–270. doi: 10.3109/s10165-008-0067-7. [DOI] [PubMed] [Google Scholar]
- 30.Mitsui Y, Gotoh M, Nakamura K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26:1032–1037. doi: 10.1002/jor.20558. [DOI] [PubMed] [Google Scholar]
- 31.Goto M, Hanyu T, Yoshio T, Matsuno H, Shimizu M, Murata N, et al. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin Exp Rheumatol. 2001;19:377–383. [PubMed] [Google Scholar]
- 32.Gotoh S, Miyazaki K, Onaya J, Sakamoto T, Tokuyasu K, Namiki O. Experimental knee pain model in rats and analgesic effect of sodium hyaluronate (SPH). (in Japanese) Nippon Yakurigaku Zasshi. 1988;92:17–27. doi: 10.1254/fpj.92.17. [DOI] [PubMed] [Google Scholar]
- 33.Prescribing Information for Voltaren 1% Gel (diclofenac sodium topical gel). (revised: Nov. 2011, NDA No. 022 122).
- 34.Reijman M, Bierma-Zeinstra SM, Pols HA, Koes BW, Stricker BH, Hazes JM. Is there an association between the use of different types of nonsteroidal antiinflammatory drugs and radiologic progression of osteoarthritis? The Rotterdam study. Arthritis Rheum. 2005;52:3137–3142. doi: 10.1002/art.21357. [DOI] [PubMed] [Google Scholar]
- 35.Huskisson EC, Berry H, Gishen P, Jubb RW, Whitehead J. Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK study group. Longitudinal investigation of nonsteroidal Antiinflammatory drugs in knee osteoarthritis. J Rheumatol. 1995;22:1941–1946. [PubMed] [Google Scholar]
- 36.Ding C. Do NSAIDs affect the progression of osteoarthritis? Inflammation. 2002;26:139–142. doi: 10.1023/A:1015504632021. [DOI] [PubMed] [Google Scholar]
- 37.Dingle JT. The effects of NSAID on the matrix of human articular cartilages. Z Rheumatol. 1999;58:125–129. doi: 10.1007/s003930050161. [DOI] [PubMed] [Google Scholar]
- 38.Smith RL, Kajiyama G, Lane NE. Nonsteroidal antiinflammatory drugs: effects on normal and interleukin 1 treated human articular chondrocyte metabolism in vitro. J Rheumatol. 1995;22:1130–1137. [PubMed] [Google Scholar]
- 39.Dieppe P, Cushnaghan J, Jasani MK, McCrae F, Watt I. A two-year, placebo-controlled trial of non-steroidal anti-inflammatory therapy in osteoarthritis of the knee joint. Br J Rheumatol. 1993;32:595–600. doi: 10.1093/rheumatology/32.7.595. [DOI] [PubMed] [Google Scholar]
- 40.de Boer TN, Huisman AM, Polak AA, Niehoff AG, van Rinsum AC, Saris D, et al. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthr Cartil. 2009;17:482–488. doi: 10.1016/j.joca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Mihara M, Higo S, Uchiyama Y, Tanabe K, Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthr Cartil. 2007;15:543–549. doi: 10.1016/j.joca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthr Cartil. 2009;17:1513–1518. doi: 10.1016/j.joca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Okada A, Okada Y. Progress of research in osteoarthritis. Metalloproteinases in osteoarthritis. Clin Calcium. 2009;19:1593–1601. [PubMed] [Google Scholar]
- 44.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takaishi H, Kimura T, Dalal S, Okada Y, D'Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is stored in Seikagaku Corporation, Tokyo, Japan. Further inquiries on the data may be submitted to Keiji Yoshioka (keiji.yoshioka@seikagaku.co.jp).


