Introduction
External genital warts and perianal warts (EGW), also known as Condylomata acuminata, are a common clinical manifestation of human papillomavirus (HPV) infection.1 However, due to the lack of routine screening for HPV, data on prevalence and incidence of HPV infection are limited. Moreover, it is not known if a newly diagnosed infection is recently acquired or long-standing.2 HPV disease and EGW are not nationally or locally reportable (some HPV-associated conditions have to be reported in certain states or jurisdictions in the United States3), nor is reporting required by the Centers for Disease Control and Prevention (CDC). The CDC has determined that routine disease reporting is not recommended, citing the following reasons: 1) no curative treatments for patients and their sex partners or monitoring of ongoing prevention programs exist for EGW, warranting case reporting; 2) more than 90 percent of new HPV infections clear or become undetectable within two years, and the clearance generally occurs within the first six months of the post- infection period; and 3) the high prevalence of infection creates a burden for case reporting by healthcare providers, health departments, and laboratories.2-4
Estimates from the CDC demonstrate that there are a total of 110,197,000 new and existing cases of sexually transmitted diseases (STDs) in the United States; HPV infection accounts for 79,100,000 (approximately 72%) of these cases.5 There is also an apparent rise in HPV infections observed in older women.6 While this may be newly acquired infection from recent sexual activity, there is also strong evidence that the total lifetime number of sexual partners (≥5) is associated with an observed second peak in incidence/prevalence of so-called newly detected HPV infections among older women aged >45 years, suggesting that these could represent reactivations of previously latent infections.6-8 Several explanations have been provided to explain the peak in HPV prevalence in older women, including immunosenescence (natural reduction in immune function associated with aging) and hormonal changes.6,9 Lower levels of immune responsiveness may be exacerbated by the peri- and postmenopausal decline in sex hormones.8 Another explanation for the peak observed is the increased detectability of vaginal infections among older women due to the physiologic cervical changes associated with aging.6 Changes in sexual behavior during the fifth and sixth decades of life may also contribute to the higher prevalence of HPV in older women, as it may be reflective of an increase in divorce rates and establishment of new partners.8 In addition, widowhood in older men has been associated with higher rates of STDs, particularly since the availability of sildenafil (Viagra®) in 1998.10
While more than 100 HPV types have been characterized, low-risk HPV 6 and 11 are responsible for 90 percent of genital and perianal warts.11 HPV transmission is predominately associated with sexual activity, and the primary risk factor for HPV infection is the number of lifetime sexual partners.12-15 The risk factors for HPV infection are listed in Table 1.12-16 The relationship between oral contraceptive use and HPV is difficult to assess, because oral contraceptive use is strongly and consistently associated with sexual activity.14
TABLE 1.
| High number of sexual partners |
| Partner with current (or prior) multiple sexual partners |
| Uncircumcised male |
| Previous infection with herpes simplex and genital warts |
| Early age at first sexual intercourse |
| Immunocompromised |
| Young age (<25 years) |
| Smoking |
| Oral contraceptive use |
| Shorter time interval between meeting a new partner and engaging in sexual intercourse |
HPV: Transmission, Life Cycle, and Natural History
The process by which HPV infects and replicates is depicted in Figure 1. HPV requires entry into the basal epithelium, through microabrasions in the skin or mucosa, to infect basal keratinocytes.17 The most common entry points include the sites of micro- or macro trauma, where the skin splits during sexual activity, such as the posterior fourchette and the perihymenal area.18,19 Therefore, genital warts are most commonly seen in these areas in women. Areas in the epithelium that are naturally thin and immature, such as the transformation zone of the cervix or the anal verge, are most likely to develop HPV manifestations.20 HPV remains sequestered within keratinocytes and successfully evade host immune detection.17 HPV, however, does not kill its host cell and, therefore, lysis of HPV-infected epithelial cells and release of virus do not occur in the absence of trauma or treatment. HPV is shed from the surface of the epithelium in dead and dying HPV-infected epithelial cells called koilocytes.21 Koilocytes are loaded with infectious HPV genomes encased in a protein capsule.18 Depositing of these cells on the epithelium of a sexual partner may result in infection of the basal keratinocytes. Infection requires the shedding of the protein capsid before the HPV DNA can enter the cell. In the deep basal layers of the epithelium at the site of HPV infection, there is little viral replication and no cell lysis; therefore, little antigen is available and detectable by the host’s immune surveillance.22-24 After a period of latency, an accelerated viral DNA replication in differentiating cells begins.17,24 As the keratinocytes mature in the upper layers of the epithelium, viral assembly of a protein capsule surrounding the DNA core creates the infective unit. Shedding of these cells rekindles the process of transmission and releases virus without ever exposing it to immune recognition. Importantly, during this entire process, HPV remains hidden within the epithelial cells.17,24 Many HPV infections are mild and transient and, with an effective immune response, will resolve or regress spontaneously.13 Within a year, about 70 percent of women with HPV infections become HPV DNA negative, and about 91 percent of them become HPV DNA negative within two years.13 Only approximately 10 percent of women infected with HPV develop persistent infections.12,13
Figure 1.
Transmission and lifecycle of HPV17
From Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. N Engl J Med. 2009;361:271-278. Copyright © 2009 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
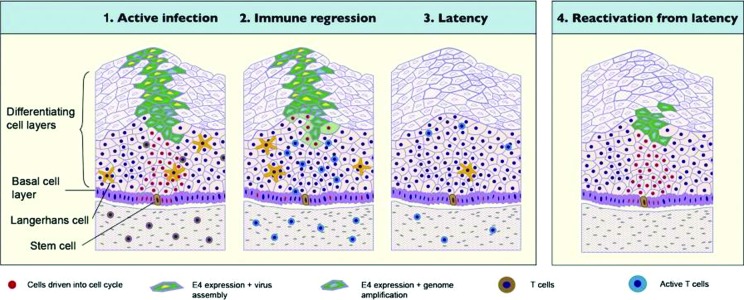
At some point, latent HPV begins to replicate in epithelial cells generated from the basal epithelium.12 In a longitudinal study of HPV infection involving female students 18 to 20 years of age, genital warts in women appeared a median of 2.9 months from exposure; some appeared within 2 to 3 weeks, while others did not occur for several months or years.25 It is estimated that 70 to 90 percent of women with HPV infections will have spontaneous remission without treatment.12,26,27 Figure 2 depicts a model of HPV latency based on evidence derived from rabbit oral papillomavirus infection (ROPV), a useful animal model of disease associated with low-risk HPV virus types in humans. In this model, each HPV-infected basal epithelial stem cell will divide with a 1:1 replication and division of the viral episome.28,29 One infected daughter cell will induce HPV viral replication.28 The other will remain quiescent in the basal epithelial stem cell pool, ensuring lifelong HPV carriage and the likelihood that the virus will remain latent in the body.28 ROPV persisted in the absence of clinical and microscopic signs of disease for up to one year after disease resolution. Viral DNA and RNA appear to be confined to basal epithelial cells and are not generally associated with completion of the viral life cycle and production of new virus particles. However, ROPV genomes are maintained in a latent state and may be capable of reactivation under certain conditions.28,29 This model, in part, explains the observed late recurrence of HPV/EGW infections.
Figure 2.
Model of HPV latency to explain recurrence of HPV infections and EGW29
From Maglennon GA, Mcintosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414(2):153—163. doi:10.1016/j.virol.2011.03.019. Copyright © 2011 Elsevier Inc. Open access under a Creative Commons Public License: http://creativecommons.org/licenses/by/3.0/legalcode
Clinical Presentation and Diagnosis of EGW
EGW usually manifest as solitary or clustered keratotic plaques and papules.12,30 They are often cauliflower-shaped, but may also be flat, papular, or frond-like.12,30 Warts may occur on the vulva, penis, scrotum, perineum, and/or anus.12,30 Typical condylomas mainly occur on keratinized squamous epithelium, and a number of lesions may join to produce large or giant condylomas.31 Genital warts are usually asymptomatic, but may be pruritic and/or cause pain or bleeding.11
Visual inspection is usually adequate for diagnosis of genital warts.11 Biopsy is not routinely recommended except to rule out malignancy in cases with sudden recent growth or increased pigmentation or fixation to underlying structures.11 Molecular testing for nucleic acids (HPV typing) is not recommended for routine diagnosis.32 Differential diagnoses for EGW consists of a number of dermatologic conditions that can affect all parts of the body, including condyloma lata, seborrheic keratosis, dysplastic and benign nevi, molluscum contagiosum, pearly penile papules, lichen sclerosis, lichen planus, lichen simplex chronicus, bowenoid papulosis, and neoplasms.32,33 These conditions must be ruled out before confirming a diagnosis of EGW.
Prevention and Treatment Guidelines
According to the 2015 CDC STD treatment guidelines, the most reliable method to avoid giving and getting HPV infection and genital warts is to abstain from sexual activity.11 While correct and consistent latex condom use by males may reduce the risk of acquiring or transmitting EGW, its use may not provide complete protection, because HPV can infect areas that are not covered by the condom.11 A quadrivalent vaccine containing HPV types 6, 11, 16, and 18, as well as a 9-valent vaccine, containing HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, are currently available in the United States. These vaccines are indicated in females and males aged 9 through 26 years for the prevention of genital warts caused by HPV types 6 and 11.34 The CDC guidelines state that the primary goal of treatment for genital warts is the amelioration of symptoms (including relieving cosmetic concerns) and, ultimately, regression of warts.11 The selection of treatment is influenced by a number of factors, including wart size, number, anatomic site of the wart, patient preference, cost of treatment, convenience, adverse events, and provider experience.11 Response to therapy is affected by factors such as immunocompetence and adherence to treatment.11
Treatments for EGW are categorized either as provider-administered or patient-applied therapies.11 Provider-administered therapies include cryotherapy; surgical therapy; trichloroacetic acid (TCA) or bichloroacetic acid (BCA), 80 to 90% (Table 2).11 Provider-administered therapies often have some unpleasant side effects. For example, cryotherapy may cause pain, necrosis, blistering, and dyspigmentation; chemical treatments (i.e., podophyllin resin, TCA, BCA) are caustic and may cause pain and local skin irritation.11 While surgical therapy may eliminate warts at a single visit, it requires substantial clinical training, additional equipment, a risk of scarring, and longer office visits.11 Patient-applied therapies include imiquimod cream, 5% and 3.75%; podofilox solution or gel, 0.5%; and sinecatechins ointment, 15% (Table 3).11 Compared with the provider-administered therapies, patient-applied treatments are preferred by some patients because they can be administered in the privacy of their home, but patients must comply with the treatment regimen and must be capable of identifying and reaching all genital warts to ensure that these treatments are effective.
TABLE 2.
Provider-administered therapies for external genital warts and perianal warts11
| TREATMENT | APPLICATION/DOSE |
|---|---|
| Cryotherapy (with liquid nitrogen or cryoprobe) | Over- and under-treatment can result in complications or low efficacy |
| Surgical removal either by tangential scissor excision, tangential shave excision, curettage, laser, or electrosurgery | Most warts can be removed at a single visit, however recurrence can occur |
| Trichloroacetic acid or bichloroacetic acid | 80%-90% solution destroy warts by chemical coagulation of proteins |
TABLE 3.
Patient-applied therapies for external genital warts and perianal warts11
| TREATMENT | APPLICATION/DOSE | DURATION OF THERAPY |
|---|---|---|
| Imiquimod cream | Apply at bedtime 3-7 nights/week Wash off after 6-10 hours | Up to 16 weeks |
| Podofilox gel/solution | Total volume of podofilox should be limited to 0.5mL per day. Total wart area treated should not exceed 10cm2 | 3 days followed by 4 days of rest Repeat for up to 4 cycles |
| Sinecatechins ointment | Apply thin layer to each wart three times daily | Up to 16 weeks or until complete clearance of warts |
Sinecatechins Ointment, 15%
Indication and description. Sinecatechins ointment, 15%, is a topical ointment indicated for the treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients aged 18 years and older.35 It is the first prescription botanical product to receive United States Food and Drug Administration (FDA) approval. This patient-applied therapy contains a proprietary, quantified mixture of catechins (85-95% by weight) and other components derived from green tea leaves (Camellia sinensis [L] O. Kuntze). Epigallocatechin gallate (EGCg) is the main catechin in sinecatechins ointment, comprising more than 55 percent of its content. Other catechin derivatives include epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECg), gallocatechin gallate (GCg), gallocatechin (GC), catechin gallate (Cg), and catechin (C). In addition to the known catechin components, it also contains gallic acid, caffeine, and theobromine, which together constitute about 2.5 percent of the drug substance, and the remaining amount of the drug substance contains undefined botanical constituents derived from green tea leaves.35 The mechanism of action of sinecatechins ointment, 15% in the clearance of genital and perianal warts is currently unknown.35 Catechins have demonstrated antioxidant activity in vitro; however, the clinical significance of this is unknown.36,37
Efficacy. Two Phase 3, randomized, double-blind, vehicle-controlled clinical studies were conducted to obtain data on the efficacy and safety of sinecatechins ointment, 15% in adult patents with 2 to 30 EGWs (total area 12-600 mm2).38,39 In each trial, the ointment was applied three times daily (TID) for up to 16 weeks, which was then followed by a 12-week, treatment-free period to assess for recurring warts.38,39 The primary efficacy-related outcome was the complete clearance of all EGWs (baseline and new) during treatment.38,39 Secondary efficacy endpoints were complete clearance of baseline EGWs, time to complete clearance of both baseline and newly emerging EGWs, partial clearance of baseline and newly emerging EGWs, and the incidence of recurrent and new EGWs during the follow-up period.38,39
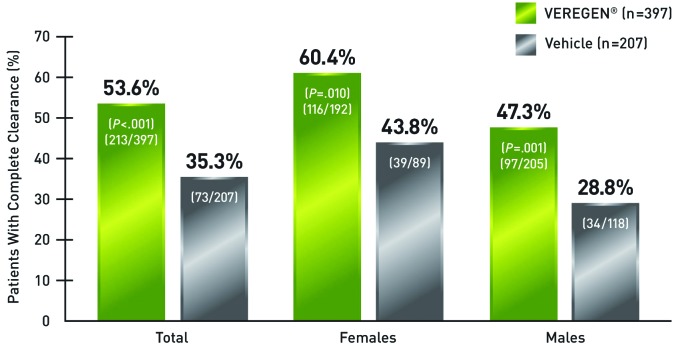
Treatment with sinecatechins ointment, 15% resulted in complete clearance of baseline and newly emerging warts in 53.6 percent of patients, or 213 out of 397, compared with 35.3 percent of patients, or 73 out of 207, in the vehicle group (Figure 3).35 Complete clearance was defined in the study as the proportion of subjects with complete clinical (visual) clearance of all external genital and perianal warts (baseline and new) by Week 16.35 When assessed by gender, females had numerically higher clearance rates following therapy compared with males; 60.4 percent of women treated with sinecatechins ointment, 15%, and 43.8 percent in the vehicle group attained complete clearance of all warts. In comparison, 47.3 percent of men treated with sinecatechins ointment, 15% and 28.8 percent in the vehicle group attained complete clearance.35
Figure 3.
Complete clearance of baseline and newly emerging warts with sinecatechins ointment, 15% (full ITT analysis).35
Treatment with sinecatechins ointment, 15% yielded low recurrence rates in patients with complete clearance. Among the 53.6 percent of patients with complete clearance, 93.2 percent experienced sustained clearance at 12 weeks post-treatment (Figure 4).35 Among the 35.3 percent of vehicle patients with complete clearance, 94.2 percent experienced sustained clearance at 12 weeks post-treatment.35 Sustained clearance refers to the 93.2 percent of subjects who did not exhibit recurrence of external genital and perianal warts 12 weeks after treatment.35 Few patients had new warts emerging during the follow-up period.39
Figure 4.
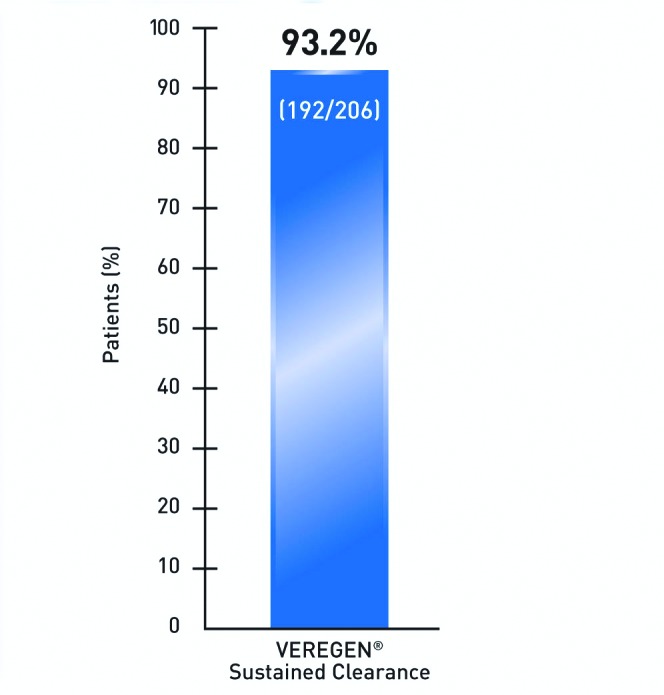
Sustained clearance rate at 12 weeks after treatment in subjects with complete clearance of all warts (pooled analysis of data from 2 randomized, double-blind, vehicle-controlled studies).35 Among the 35.3% of vehicle patients with complete clearance, 94.2% experienced sustained clearance at 12 weeks post-treatment.
Safety. The safety outcomes in the 2 Phase 3, randomized, double-blind, vehicle-controlled clinical trials included the incidence of severe local reactions (signs or symptoms) and adverse events during the treatment and follow-up periods.38,39 The incidence of patients with local adverse events leading to discontinuation was 2.3 percent. Most adverse events were mild to moderate. The most frequently reported local and regional adverse reactions, with an incidence of ≥20 percent, were erythema, pruritus, burning, pain/discomfort, erosion/ulceration, edema, induration, and vesicular rash.35 Serious local events were uncommon.
Case Studies
A roundtable meeting was held to document current perspectives on the treatment of EGW. Drawing from the extensive clinical experience of dermatology and women’s health thought leaders, simulated cases were developed for publication in this supplement to represent patients who may be seen in clinical practice. These patients do not represent any actual individual, and cases are intended to assist with clinical decision making only. The roundtable reviewed four male and two female cases with varying presentations of EGW treated with sinecatechins ointment, 15% as first-line therapy. This section provides the presentation and history, diagnosis, rationale for therapy, and clinical outcomes of the individual EGW cases.
Case 1: Treatment-naïve 21-year-old Man with No Prior History of EGW
Initial presentation and history. A 21-year-old male college student presents with a chief complaint of dry skin and spots on his penis, which he noticed over the past few weeks. He is an undergraduate student majoring in political science and prelaw and is planning to apply to law school in the fall. He is home on a break from classes and has decided to visit his physician the week before he is scheduled to return to college.
The patient is generally in good health, with no chronic medical condition and no prior history of STDs. His father and aunt have psoriasis. The patient is heterosexual and reports being sexually active with multiple partners. He only uses condoms intermittently, when his partner is not using an oral contraceptive. The patient reports smoking half a pack of cigarettes per day, on average. However, he smokes more on weekends when he is drinking or when he is stressed and studying for an exam. He is a social drinker, who reports drinking 3 to 5 drinks per night on weekend nights when he goes out to bars with his friends and fraternity brothers.
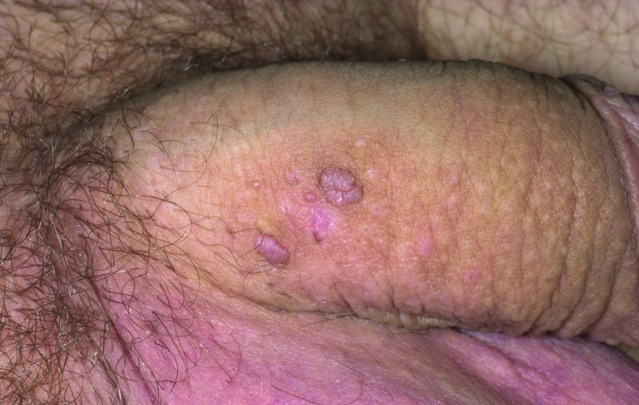
Physical examination and diagnosis. The patient’s physical examination reveals five nontender, skin-colored, keratotic, 2 to 4mm in diameter papules at the base of the penile shaft with no inguinal lymphadenopathy, ulceration, or induration (Figure 5). The diagnosis of condyloma acuminata, or EGW, is made based on the physical findings, including nontender, skin-colored, keratotic papules on the penile shaft.12,30,40 His risk factors, including smoking, young age (<25 years), and multiple current sexual partners, also support the diagnosis.13,41
Figure 5.
Clinical presentation depicting nontender lesions at the base of the penile shaft.
Skin disease: genital warts image: genital-warts-46.jpg. http://www.dermnet.com/images/Genital-Warts/picture/8021. Copyright © 2011 Dermnet.com. Reprinted from Dermnet Skin Disease Atlas with permission from Interactive Medical Media, LLC.
Treatment decision, rationale, and follow-up. The patient is prescribed sinecatechins ointment, 15% to be applied TID to all external warts.35 Sinecatechins ointment, 15% was selected because the patient is returning to college in one week, and there is the potential for loss of follow-up after his initial visit. Importantly, treatment with sinecatechins ointment, 15% does not require additional provider-administered intervention. This is a patientapplied therapy that can be kept at room temperature once dispensed. He can store the medication in his dormitory room and apply it at his convenience, in the morning, evening, and before bedtime. In addition to receiving a prescription for sinecatechins ointment, 15%, the patient is counseled about STD protection and prevention, including abstinence, mutual monogamy, consistent condom use, and vaccination.13,42 The patient is also advised that, if he were to have sexual contact, he must wash off the ointment carefully before having protected sexual contact, as the ointment may weaken condoms and vaginal diaphragms.
Case 2:Treatment-naïve 24-year-old Woman with No Prior History of EGW
Initial presentation and history. A 24-year-old financial analyst presents to the dermatologist’s office with a chief complaint of a rash in the bikini line. She is also complaining of vaginal discharge and slight bleeding. Her symptoms appeared during the past few weeks. The patient is in generally good health, with no chronic medical condition. She has been in a new relationship for the past three months and is heterosexual and monogamous. The patient was 19 years old at first intercourse and has been sexually active for the past five years. She has had three partners in the last two years, none of whom used condoms. The patient reports that she has a history of vaginal discharge and slight bleeding and has a regular gynecologist whom she sees for Pap smears and oral contraceptives. Her Pap smears have been normal in the past. However, she has not been seen recently for her current complaints and has no history of HPV vaccination.
The patient describes herself as a social drinker who likes to go out after work for happy hour with her friends and generally will have 2 to 3 drinks per night, 2 to 3 times per week—usually on Thursday and Friday nights. She recently quit smoking, but previously would smoke when offered a cigarette at parties or on breaks with a coworker. She states she smokes about 10 to 12 cigarettes per week.
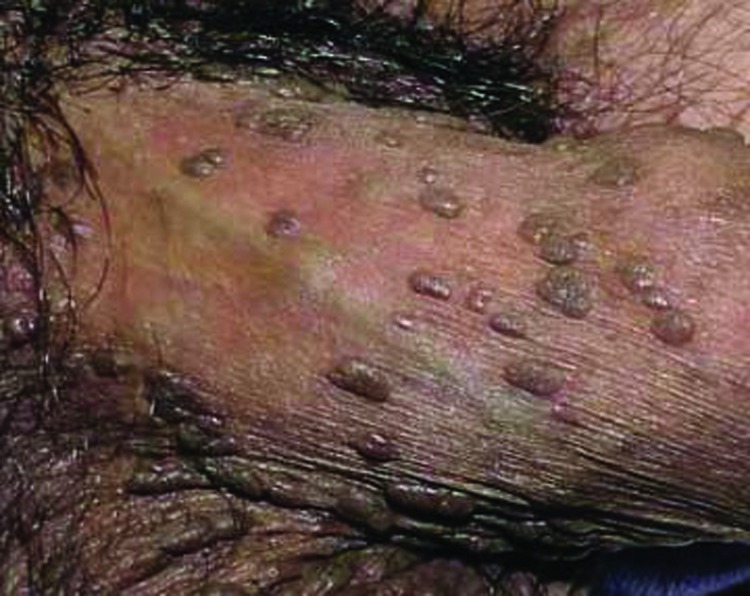
Physical examination and diagnosis. The patient’s physical examination reveals seven skin-colored, frond-like, 2 to 4mm papules on the labia majora. A brown, watery vaginal discharge is also noted. A few of the lesions appear friable, with slight bleeding (Figure 6). The diagnosis of condyloma acuminata, or EGW, is made based on the physical findings, including multiple, skin-colored, keratotic, frond-like papules with slight bleeding.12,30,31,40 Her risk factors, including young age (<25 years), prior history of smoking, and oral contractive use, having multiple partners in the past two years, as well as a shorter interval between meeting a new partner and engaging in intercourse, also support the diagnosis.13-15,41
Figure 6.
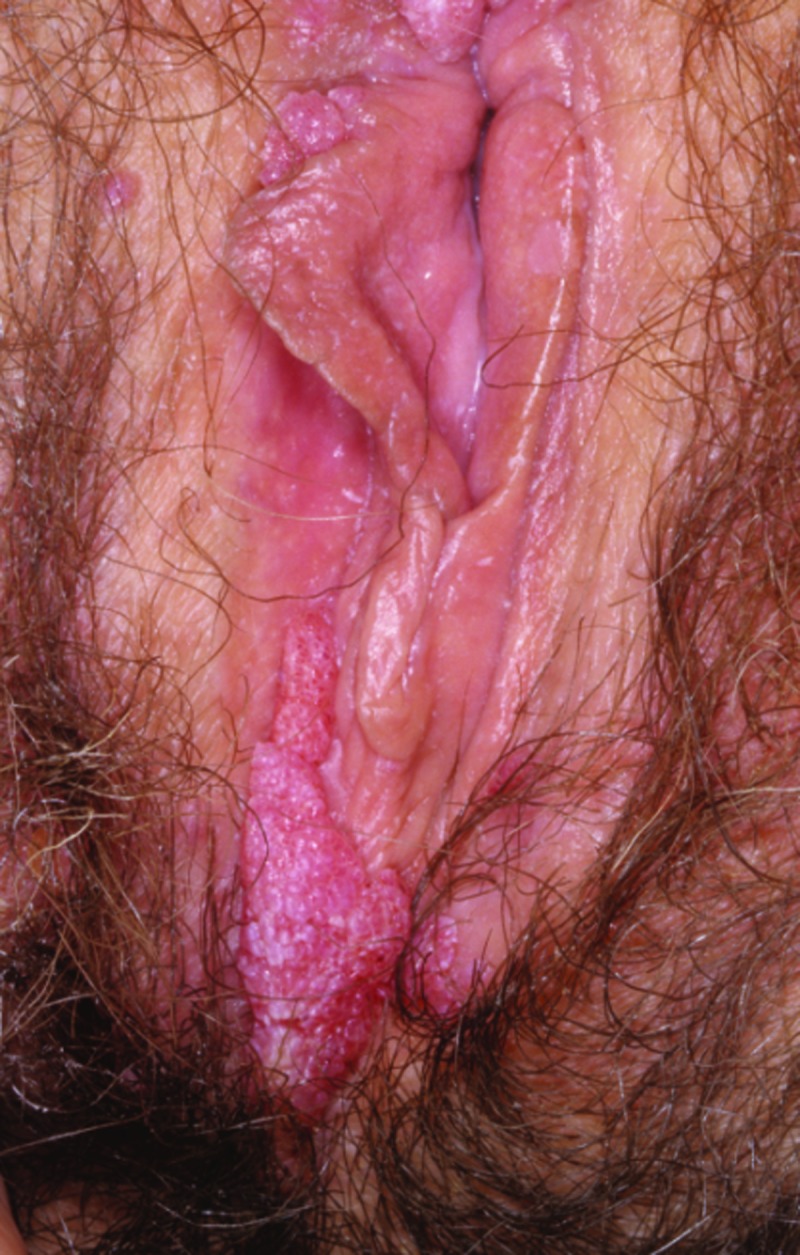
Clinical presentation depicting papules on the labia majora.
Skin disease: genital warts image: genital-warts-80.jpg. http://www.dermnet.com/images/Genital-Warts/picture/7983?imgNumber=116. Copyright © 2011 Dermnet.com. Reprinted from Dermnet Skin Disease Atlas with permission from Interactive Medical Media, LLC.
Treatment decision, rationale, and follow-up. The patient expresses concerns about provider-administered treatments. She has a busy work schedule, and it is difficult for her to take time off for doctor appointments. In addition, she has read that provider-administered therapies may have unpleasant side effects. The patient is prescribed sinecatechins ointment, 15%, to be applied TID to all external warts.35 This treatment is selected based on the patient’s desire to undergo immediate treatment with a method that has demonstrated tolerability and will not likely require frequent follow-up visits to this provider for treatment applications.11,35 In addition, sinecatechins ointment, 15% can be stored at room temperature once dispensed, and, although it is to be applied TID, sinecatechins ointment, 15% does not have to be used every eight hours. In addition to receiving a prescription for sinecatechins ointment, 15%, the patient is counseled about STD protection and prevention, including abstinence, mutual monogamy, consistent condom use, and quadrivalent HPV vaccination.13,42 The patient is also advised that, if she were to have sexual contact, she must wash off the ointment carefully before having protected sexual contact, as the ointment may weaken condoms and vaginal diaphragms. The patient is referred to her gynecologist for follow-up, including a Pap smear as needed, and for evaluation of vaginal discharge and spotting.
At her visit with the gynecologist 12 weeks after EGW diagnosis and four weeks after treatment discontinuation, the patient is tested for other STDs and is again counseled regarding STD protection and prevention. Her additional STD tests are negative and she is told that an additional Pap smear at this time is not necessary. She and her partner receive the quadrivalent vaccine to protect against high-risk strains of HPV.11
Case 3: 40-year-old Man with Refractory EGW
Initial presentation and history. A 40-year-old man presents with a chief complaint of persistent scrotal and penile warts. His lesions were previously treated, but never achieved complete clearance. He is seeking another opinion for diagnosis and treatment. He has concerns about the adverse effects of therapies, as he experienced irritation and pain in the past and because prior treatments required rest periods due to drug-related adverse events. This patient has a five-year history of persistent EGW. His prior biopsy confirmed EGW, and he has undergone treatments with podofilox and cryotherapy in the past. His last treatment course was four weeks ago. He has no evidence of immunosuppression or other STDs, and his HIV antibody testing was negative approximately a year and a half ago. The patient is heterosexual and has been in a monogamous marriage for the past four years. His wife has no visible evidence of EGW and has had normal Pap smears. The patient is employed as an insurance executive and travels frequently on business. He reports being a nonsmoker and drinks occasionally at business or social functions.
Physical examination and diagnosis. The patient’s physical examination reveals multiple hyperpigmented, brown, keratotic, 3 to 5mm papules at the base of the penile shaft and scrotum and a 1.3cm plaque composed of coalescent lesions on the scrotum (Figure 7). The diagnosis of condyloma acuminata (EGW) refractory to therapy is made based on the history and physical findings, including hyperpigmented, brown, keratotic papules and coalescent plaque-like lesions on the penis and scrotum. Other noteworthy information includes previous histologic confirmation of EGW.11,12,30,40
Figure 7.

Clinical presentation depicting papules at the base of the penile shaft and scrotum.
Source of images: Hpv-Genital-Warts-Pictures.com. http://www.hpv-genital-warts-pictures.com/genitalwartstreatment-1.html; http://www.hpv-genital-warts-pictures.com/genitalwartstreatment-3.html. Accessed on June 1, 2015.
Treatment decision, rationale, and follow-up. The patient is prescribed sinecatechins ointment, 15% to be applied TID to all external warts.35 This treatment is selected based on the patient’s desire to undergo immediate treatment with a method that likely will not require frequent follow-up visits for treatment applications or rest periods between treatments.11,35 The patient is eager to resolve his EGW in a convenient manner while managing side effects. In addition, sinecatechins ointment, 15% can be stored at room temperature once dispensed and carried with the patient when he travels on business. In clinical trials, 53.6 percent of patients treated with sinecatechins ointment, 15% demonstrated complete clearance of baseline and newly emerging EGW, and 93.2 percent of these patients who achieved complete clearance of EGW experienced sustained clearance at 12 weeks post-treatment. In the vehicle group, 35.3 percent of patients had complete clearance, and 94.2 percent of these patients experienced sustained clearance at 12 weeks post-treatment.35 The most common side effects observed with sinecatechins ointment, 15% were mild-to-moderate local skin and application-site reactions, and the incidence of patients with local adverse events leading to discontinuation was 2.3 percent.35
The patient is informed that a number of other cutaneous lesions in the genital region may resemble EGW, including neoplasms.40,43 As a result, any pigmented or other atypical lesion should be evaluated and possibly biopsied to rule out severe dysplasia, Bowen’s disease, or melanocytic lesions. Biopsy is also indicated for patients who are unresponsive to treatment.40 The patient is advised to be tested for other STDs, including HIV infection, as immunocompromised patients are generally at a higher risk of HPV infection and, therefore, genital warts are more common in individuals with impaired immunity. Wart treatment and clearance are more challenging in these patients as well.40 The patient’s last HIV antibody test was conducted more than one year ago. The patient was counseled about STD protection and prevention and told that his risk of transmission and acquisition of infection may be reduced with consistent condom use and by reducing or limiting his number of sexual partners.11,13 The biopsy of the plaque is negative for malignancy, and HPV DNA typing reveals types 6 and 11.41 Although generally not indicated for the routine diagnosis of EGW, HPV typing may be warranted in the work-up for persistent lesions or if penile carcinoma is suspected.40,44,45 The patient’s additional tests for STDs are negative and counseling regarding STD protection and prevention is reinforced.11
Case 4: 38-year-old Man with Recurrent EGW
Initial presentation and history. A 38-year-old man presents with a chief complaint of recurrent bumps on the head of his penis. The patient was diagnosed with EGW 18 months ago and treated with imiquimod cream, 5% with complete clearance. Previous work-ups for STDs also revealed that the patient had gonorrhea some years ago, which was also successfully treated. The patient is heterosexual, recently divorced, and is actively dating. He has multiple sexual partners he connects with via the internet. He reports high rates of sexual intercourse and uses condoms regularly. The patient is employed as a firefighter. He denies tobacco and alcohol use.
Physical examination and diagnosis. The patient is an uncircumcised male. There are 17 filiform lesions located on the foreskin and glans of the penis. The papules measure 1 to 3mm in diameter and no lesions are noted in the urethral meatus (Figure 8). The diagnosis of recurrent condyloma acuminata (EGW) is made based on the history and physical findings; other noteworthy information includes previous histologic confirmation of EGW.12,30,40
Figure 8.
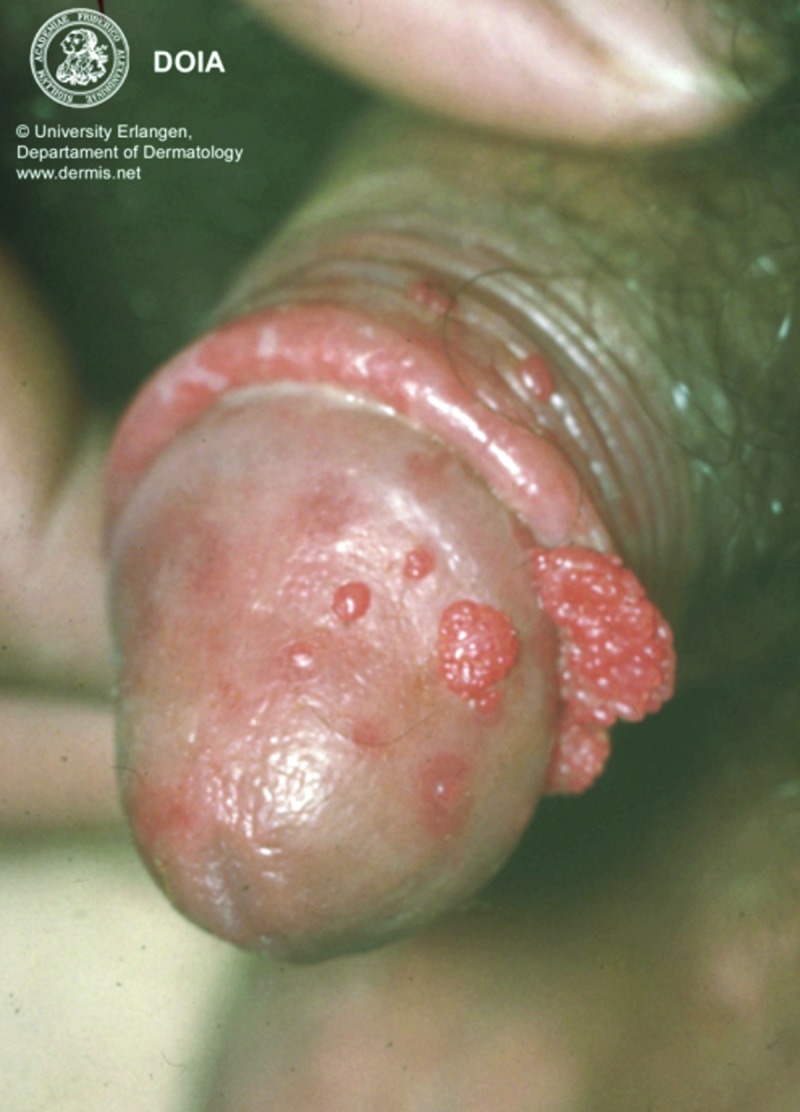
Clinical presentation depicting papules on the foreskin and glans of the penis.
Image reprinted with permission from Diepgen TL, Yihune G, et al. Dermatology Online Atlas.
Treatment decision, rationale, and follow-up. The patient is prescribed sinecatechins ointment, 15%, to be applied TID to all external warts.35 This treatment is selected based on the patient’s history of recurrent symptoms. Sinecatechins ointment, 15% is a self-administered therapy and can be stored at room temperature after dispensing. Thus, the patient can store the medication privately without the need for storage in the firehouse refrigerator. In clinical trials, 53.6 percent of patients treated with sinecatechins ointment, 15% demonstrated complete clearance of baseline and newly emerging EGW, and 93.2 percent of these patients who achieved complete clearance of EGW experienced sustained clearance at 12 weeks post-treatment. In the vehicle group, 35.3 percent of patients had complete clearance, and 94.2 percent of these patients experienced sustained clearance at 12 weeks post-treatment.35 The most common side effects observed with sinecatechins ointment, 15% were mild-to-moderate local skin and application-site reactions, and the incidence of patients with local adverse events leading to discontinuation was 2.3 percent.35
The patient is informed that a number of other cutaneous lesions in the genital region may resemble EGW, including neoplasms.40,43 As a result, any pigmented or other atypical lesion should be biopsied to rule out severe dysplasia or melanoma. Biopsy is also indicated for patients who are unresponsive to treatment.40 The patient is advised to be tested for other STDs, including HIV, as immunocompromised patients are generally at a higher risk of HPV infections and, therefore, genital warts are more common in individuals with impaired immunity. Wart treatment and clearance are more challenging in these patients as well.40 He is counseled about STD protection and prevention and also to consider circumcision, because uncircumcised men are at higher risk of EGW.11,41,46
The patient declines biopsy because he cannot afford the test and his insurance will not cover it. Additional STD tests are negative. During his follow-up visit, counseling regarding STD prevention is reinforced. The patient declines referral to a urologist for circumcision.
Case 5: 30-year-old Man with Perianal and Perineal Warts and a History of Previously Treated EGW
Initial presentation and history. A 30-year-old male physician presents with a chief complaint of warts near and around his anus with itching. The patient has a previous history of EGW treated with provider-administered therapies, including surgical destruction of anal warts via carbon dioxide laser and, on other occasions prior to that, with multiple in-office applications of podophyllin resin. The patient’s medical history is also significant for recurrent genital herpes (herpes simplex type 2), which is currently treated with an oral antiviral agent. His HIV test was negative one year ago. The patient is homosexual and has been in a monogamous relationship for six months. He and his partner have had multiple partners in the past, and he uses condoms regularly. He is a nonsmoker, but reports drinking alcohol and using intranasal cocaine on social occasions.
Physical examination and diagnosis. The physical examination is positive for approximately 15 verrucous papular lesions surrounding the anus and extending to the perineum. The lesions are nontender and measure 2 to 5mm in diameter, and no obstructive lesions are noted (Figure 9). The diagnosis of condyloma acuminata, or EGW, is made based on the history and physical findings.19,40 The patient’s risk factors for HPV also support the diagnosis. Risk factors include a male partner with prior multiple partners, current and prior infections with genital herpes and genital warts, multiple sexual partners in the past, and use of alcohol and cocaine (as possible immunomodulators that may enhance the risk of infections).41,47
Figure 9.
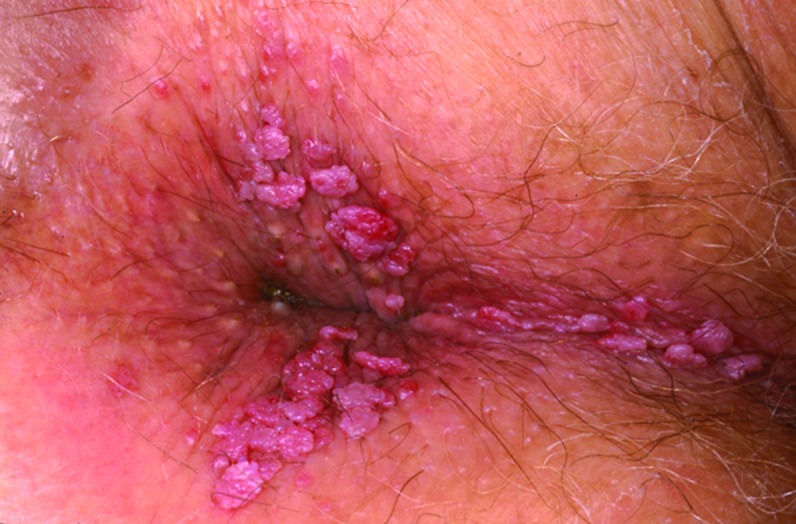
Clinical presentation depicting lesions surrounding the anus and extending to the perineum.
Skin disease: genital warts image: genital-warts-61.jpg. http://www.dermnet.com/images/Genital-Warts/picture/8005. Copyright © 2011 Dermnet.com. Reprinted from Dermnet Skin Disease Atlas with permission from Interactive Medical Media, LLC.
Treatment decision, rationale, and follow-up. The patient is prescribed sinecatechins ointment, 15% to be applied TID to all external warts.35 This treatment is selected based on the patient’s history of recurrent symptoms. Sinecatechins ointment, 15% is a self-administered therapy and can be stored at room temperature after dispensing. Thus, the patient can store the medication privately in his office. In clinical trials, 53.6 percent of patients treated with sinecatechins ointment, 15% demonstrated complete clearance of baseline and newly emerging EGW, and 93.2 percent of these patients who achieved complete clearance of EGW experienced sustained clearance at 12 weeks post-treatment. In the vehicle group, 35.3 percent of patients had complete clearance, and 94.2 percent of these patients experienced sustained clearance at 12 weeks post-treatment.35 The most common side effects observed with sinecatechins ointment, 15% were mild-to-moderate local skin and application-site reactions, and the incidence of patients with local adverse events leading to discontinuation was 2.3 percent.35
The patient is counseled to be tested again for other STDs and is advised regarding STD protection. A serologic work-up should include testing for the HIV antibody, hepatitis B and C viruses, and syphilis (to rule out condylomata lata).11 The patient is referred for anoscopy and anal Pap smear to assess for intra-anal condyloma.11,48 He is advised that his partner should be referred for STD testing and counseling.11
The patient continues antiviral suppressive therapy for recurrent genital herpes. At his follow-up visit, the counseling regarding STD prevention is reinforced. Anoscopy is negative for intra-anal warts, and anal Pap smear is not performed by the gastroenterologist.
Case 6: 60-year-old Postmenopausal Woman with a Questionable Prior History of EGW
Initial presentation and history. A 60-year-old female artist and librarian presents with a chief complaint of a small growth around her vaginal opening. Upon further questioning, she states that she also has some associated discomfort when wearing pants and during intercourse. She has also experienced some bleeding during intercourse. The patient has a questionable history of EGW when she was in her twenties; however, she does not recall how she was treated. She has had multiple abnormal Pap smears with dysplasia, including one graded as moderate dysplasia (cervical intraepithelial neoplasia [CIN 2]) treated with a loop electrosurgical excision procedure (LEEP) approximately 20 years ago. She is postmenopausal, heterosexual, and recently divorced, and she has been sexually active with a new partner for the past 12 weeks. She became sexually active at age 15.
Physical examination and diagnosis. Physical examination reveals multiple coalescing and discrete lesions at the vaginal introitus. The lesions are flat-topped, gray-pigmented papules on the posterior fourchette (between the 4:00 and 8:00 positions) ranging from 4 to 6mm in diameter. They are not obstructing the introitus, and there are no intravaginal lesions (Figure 10). A biopsy is performed, owing to the patient’s age and the increased risk of neoplasia of the vulva in this age group. The diagnosis of condyloma acuminata, or EGW, is made based on the history and physical findings, including multiple nonobstructive lesions at the vaginal introitus, which are confirmed histologically.19,40
Figure 10.
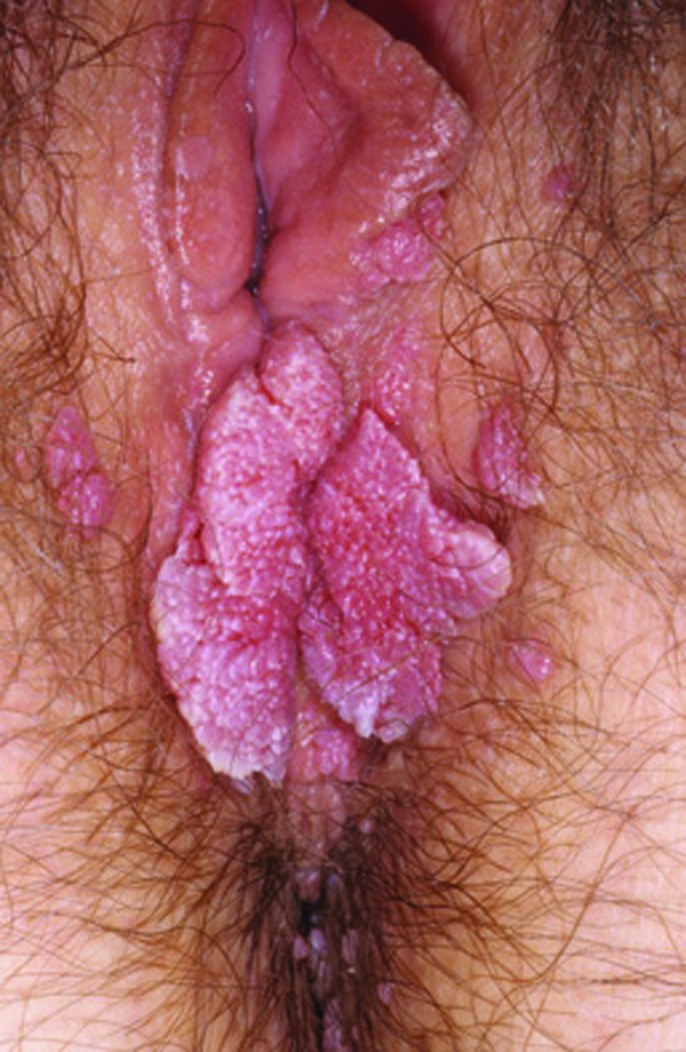
Clinical presentation depicting multiple lesions at the vaginal introitus.
Skin disease: genital warts image: genital-warts-121.jpg. http://www.dermnet.com/images/Genital-Warts/picture/8070. Copyright © 2011 Dermnet.com. Reprinted from Dermnet Skin Disease Atlas with permission from Interactive Medical Media, LLC.
Treatment decision, rationale, and follow-up. The patient is prescribed sinecatechins ointment, 15% to be applied TID to all EGW.35 Sinecatechins ointment, 15% is a self-administered therapy and can be stored at room temperature after dispensing. Thus, the patient can store the medication privately. This patient is counseled about prevention strategies, including abstinence, mutual monogamy, consistent condom use, and vaccination.13 The patient is also advised that if she were to have sexual contact, she must wash off the ointment carefully before having protected sexual contact, as the ointment may weaken condoms and vaginal diaphragms. She is recommended to follow-up with her gynecologist for a pelvic examination to assess for intravaginal EGW and/or any visible cervical lesions and for routine cervical screening, and to have her partner evaluated.11 There is no additional HPV-related disease identified upon follow-up evaluation by the patient’s gynecologist.
Summary
Sinecatechins ointment, 15% is a patient-applied prescription botanical treatment indicated for the topical treatment of external genital and perianal warts in immunocompetent patients 18 years and older. In two pivotal, randomized, double-blind, vehicle-controlled, Phase 3 studies, 53.6 percent of patients treated with sinecatechins ointment demonstrated complete clearance of baseline and newly emerging EGW, and 93.2 percent of these patients who achieved complete clearance of EGW experienced sustained clearance at 12 weeks post-treatment. In the vehicle group, 35.3 percent of patients had complete clearance, and 94.2 percent of these patients experienced sustained clearance at 12 weeks post-treatment.35 The most common side effects observed with sinecatechins ointment, 15% were mild-to-moderate local skin and application-site reactions, and the incidence of patients with local adverse events leading to discontinuation was 2.3 percent.35 The cases presented in this supplement are simulated and intended to represent patients who may be seen in clinical practice and demonstrate the role of sinecatechins ointment, 15% in the treatment of EGW. These patients do not represent any actual individual, and cases are intended to assist with clinical decision making only. The mechanism of action of sinecatechins ointment, 15% involved in the clearance of EGW is unknown and warrants further clinical and investigative studies.
Registered trademarks are the property of their respective owners.
VEREGEN® (sinecatechins) Ointment, 15% is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older. Important Safety Information: Avoid use of VEREGEN® on open wounds. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
Important Safety Information: Safety and efficacy of VEREGEN® (sinecatechins) have not been established in immunosuppressed patients or patients under 18 years of age, or pregnant women, or for the treatment of external genital and perianal warts beyond 16 weeks or for multiple treatment courses. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
Important Safety Information: The most common adverse reactions are local skin and application site reactions including (incidence ≥ 20%) erythema, pruritus, burning, pain/discomfort, erosion/ulceration, edema, induration, and vesicular rash. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
Important Safety Information: VEREGEN® (sinecatechins) has not been evaluated to treat urethral, intra-vaginal, cervical, rectal, or intra-anal human papilloma viral disease and should not be used to treat these conditions. Avoid use of VEREGEN® on open wounds. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
Important Safety Information: Safety and efficacy of VEREGEN® have not been established in immunosuppressed patients or patients under 18 years of age, or pregnant women, or for the treatment of external genital and perianal warts beyond 16 weeks or for multiple treatment courses. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
VEREGEN® (sinecatechins) Ointment, 15% is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older.
Important Safety Information: Avoid use of VEREGEN® on open wounds. Avoid exposure of VEREGEN®-treated areas to sun/UV-light because VEREGEN has not been tested under these circumstances. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement
VEREGEN® (sinecatechins) Ointment, 15% is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older.
Important Safety Information: The most common adverse reactions are local skin and application site reactions including (incidence > 20%) erythema, pruritus, burning, pain/discomfort, erosion/ulceration, edema, induration, and vesicular rash. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
VEREGEN® (sinecatechins) Ointment, 15% is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older.
Important Safety Information: Avoid exposure of VEREGEN®-treated areas to sun/UV-light because VEREGEN® has not been tested under these circumstances. See additional Important Safety Information on the following pages and Full PI at the end of the Supplement.
VEREGEN® (sinecatechins) Ointment, 15% is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older.
Important Safety Information: VEREGEN® has not been evaluated to treat urethral, intra-vaginal, cervical, rectal, or intra-anal human papilloma viral disease and should not be used to treat these conditions. Avoid use of VEREGEN® on open wounds. See additional Important Safety Information on the previous pages and Full PI at the end of the Supplement.
Biographies






Footnotes
Author disclosures: Drs. Goldenberg, Taylor, Berman, Kaufmann, Abramovits, and Zeichner are paid consultants of PharmaDerm, a division of Fougera Pharmaceuticals Inc.
Contributor Information
Gary Goldenberg, Assistant Clinical Professor, Dermatology Medical Director of the Dermatology Faculty Practice Department of Dermatology Mount Sinai Medical Center New York, New York.
Maida Taylor, Department of Obstetrics, Gynecology, and Reproductive Sciences University of California, San Francisco San Francisco, California.
Brian Berman, Department of Dermatology and Cutaneous Surgery University of Miami Miller School of Medicine Miami, Florida.
Mark Kaufmann, Associate Clinical Professor of Dermatology Department of Dermatology Mount Sinai Medical Center New York, New York.
William Abramovits, Dermatology Treatment and Research Center Dallas, Texas.
Joshua Zeichner, Director of Cosmetic and Clinical Research Department of Dermatology Mount Sinai Medical Center New York, New York.
References
- 1.Cox JT, Huh W, Mayeaux EJ, et al. Management of external genital and perianal warts (EGW): proceedings of an expert panel meeting. The Female Patient. 2011;36(11):1–16. [Google Scholar]
- 2.Gerberding JL. Atlanta, GA: Centers for Disease Control and Prevention; Centers for Disease Control and Prevention. Report to Congress: prevention of genital human papillomavirus infection; pp. 1–35. January 2004. [Google Scholar]
- 3.Hariri S, Dunne E, Saraiya 2M, et al. [August 31, 2015]. http://www.cdc.gov/vaccines/ pubs/surv-manual/chpt05-hpv.html Human papillomavirus. In: Roush SW, Baldy LM, eds. Manual for the Surveillance of Vaccine-preventable Diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
- 4. [September 2, 2015]. http://stacks.cdc.gov/view/cdc/5179/ National Center for HIV, STD, and TB Prevention, Division of STD Prevention; National Center for Infectious Diseases; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. Prevention of genital HPV infection and sequelae: report of an external consultants’ meeting. Atlanta, GA: Centers for Disease Control and Prevention; December 1999.
- 5. [August 31, 2013]. http://www.cdc.gov/std/stats/STI-Estimates-Fact-SheetFeb-2013.pdf Centers for Disease Control and Prevention. CDC fact sheet: incidence, prevalence, and cost of sexually transmitted infections in the United States. Centers for Disease Control and Prevention Website. Updated February 2013.
- 6.González P, Hildesheim A, Rodríguez AC, et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3044–3054. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rositch AF, Burke AE, Viscidi RP, et al. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012;72(23):6183–6190. doi: 10.1158/0008-5472.CAN-12-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravitt PE, Rositch AF, Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis. 2013;207(2):272–280. doi: 10.1093/infdis/jis660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DR, Weaver B. Human papillomavirus in older women: new infection or reactivation? J Infect Dis. 2013;207(2):211–212. doi: 10.1093/infdis/jis662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153(1):1–7. doi: 10.1059/0003-4819-153-1-201007060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Cox JT. Epidemiology and natural history of HPV. J Fam Pract. 2006;55(suppl):3–9. [PubMed] [Google Scholar]
- 13.Atlanta, GA: Centers for Disease Control and Prevention; Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. April 2007:1-36. [Google Scholar]
- 14.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(suppl):S16–S24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 16.Nyitray AG, Carvalho Da Silva RJ, Baggio ML, et al. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J Infect Dis. 2011;203(1):49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. N Engl J Med. 2009;361(3):271–278. doi: 10.1056/NEJMct0806938. [DOI] [PubMed] [Google Scholar]
- 18.Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23(5):458–476. doi: 10.1111/j.1529-8019.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 19.von Krogh G, Lacey CJN, Gross G, et al. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex Transm Infect. 2000;76(3):162–168. doi: 10.1136/sti.76.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayeaux EJ, Spitzer M. Preventing cervical cancer and other HPV-related diseases. Medscape Website. [August 31, 2015]. http://www.medscape.org/viewarticle/508691 Presented March 9, 2005 and June 20, 2005.
- 21.Fisher BK, Margesson LJ. Skin-colored lesions. In: Edwards L, editor. Genital Dermatology Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 149–151. [Google Scholar]
- 22.Ferris DG, Cox JT, O’Connor DM, et al. In: Modern Colposcopy Textbook and Atlas. Dubuque, IA: Kendal/Hunt Publishing Company; 2004. Management of cervical intraepithelial neoplasia; pp. 579–581. 2nd ed. [Google Scholar]
- 23.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4(1):46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 24.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2 sppl):S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191(5):731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 26.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 27.Ho GYF, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 28.Gravitt PE. Evidence and impact of human papillomavirus latency. Open Virol J. 2012;(6):98–203. doi: 10.2174/1874357901206010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414(2):153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12(3):5. [PubMed] [Google Scholar]
- 31.Gunter J. Genital and perianal warts: new treatment opportunities for human papillomavirus infection. Obstet Gynecol. 2003;189(3 Suppl):S3–S11. doi: 10.1067/s0002-9378(03)00789-0. [DOI] [PubMed] [Google Scholar]
- 32.Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210–S224. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell TX, Nathan LS, Satmary WA, Goldstein AT. Nonneoplastic epithelial disorders of the vulva. Am Fam Physician. 2008;77(3):321–326. [PubMed] [Google Scholar]
- 34.Petrosky E, Bocchini JA Jr, Hariri S, et al. Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 35. VEREGEN [package insert]. Melville, NY: PharmaDerm, a division of Fougera; 2012.
- 36.Zaveri NT. Green tea and its polyphenolic catechins : medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea— A review. J Am Coll Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 38.Stockfleth E, Beti H, Orasan R, et al. Topical Polyphenon® E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158(6):1329–1338. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 39.Tatti S, Stockfleth E, Beutner KR, et al. Polyphenon E®: a new treatment for external anogenital warts. Br J Dermatol. 2010;162(1):176–184. doi: 10.1111/j.1365-2133.2009.09375.x. [DOI] [PubMed] [Google Scholar]
- 40.Lynde C, Vender R, Bourcier M, Bhatia N. Clinical features of external genital warts. J Cutan Med Surg. 2013;17(S2):S55–S60. [PubMed] [Google Scholar]
- 41.Bhatia N, Lynde C, Vender R, Bourcier M. Understanding genital warts: epidemiology, pathogenesis, and burden of disease of human papillomavirus. J Cutan Med Surg. 2013;17(S2):S47–S54. [PubMed] [Google Scholar]
- 42.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2014;63(RR-5):1–30. [PubMed] [Google Scholar]
- 43.Bourcier M, Thomas DR. External genital warts. Skin Ther Lett. 2007;3(2):1–3. [Google Scholar]
- 44.Gross GF. Penile cancer and penile intraepithelial neoplasia. In: Pfister H, editor. Prophylaxis and early detection of HPV-related neoplasia. Monogr Virol. Vol. 28. Basel; Karger: 2012. pp. 58–71. [Google Scholar]
- 45.Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159(4):1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobian AAR, Kacker S, Quinn TC. Male circumcision: a globally relevant but under-utilized method for the prevention of HIV and other sexually transmitted infections. Annu Rev Med. 2014;65:293–306. doi: 10.1146/annurev-med-092412-090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16(2):209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortoski RA, Kell CS. Anal cancer and screening guidelines for human papillomavirus in men. J Am Osteopath Assoc. 2011;111(3 Suppl):S35–S43. [PubMed] [Google Scholar]