Abstract
Background
Eicosapentaenoic acid (EPA) is thought to have many beneficial effects, such as anti-atherosclerogenic and anti-inflammatory properties. However, few studies have reported its effects of endothelial dysfunction in diabetes and its direct effects on the aorta. Here, we investigated the effects of EPA treatment on impaired endothelium-dependent relaxation of the aorta in KKAy mice, a model of type 2 diabetes.
Methods
Male KKAy mice were fed a high-fat (HF) diet for 8 weeks to induce diabetes, after which they were divided into two groups. One group was fed a HF diet, and the other group was fed a HF diet containing EPA ethyl ester (EPA-E, 10 mg/day) for 4 weeks. Then, the vascular reactivities of prepared aortic rings were measured in an organ bath to determine if EPA-E administration changed vascular function in these diabetic mice. In addition, we examined effect of EPA-E and its metabolites to vascular action using aorta separated from C57BL/6 J mice.
Results
Although EPA-E administration did not change the plasma glucose and insulin levels in diabetic mice, total cholesterol levels were significantly decreased. The aorta extracted from EPA-E untreated diabetic mice showed impaired endothelium-dependent relaxation in response to acetylcholine (ACh). However, EPA-E administration improved the relaxation response to ACh to the control levels observed in non-diabetic C57BL/6 J mice. On the other hand, endothelium-independent relaxation in response to sodium nitroprusside did not significantly differ among these three groups. The enhanced contractile response by phenylephrine in diabetic mice was not altered by the administration of EPA-E. In addition, the direct administration of EPA-E metabolites such as EPA, docosahexaenoic acid, and docosapentaenoic acid led to vasodilation in the aortic rings of C57BL/6 J mice.
Conclusion
These results showed that chronic EPA-E administration prevented the development of endothelial dysfunction in KKAy mice, partly via the direct action of EPA-E metabolites on the aorta.
Keywords: Diabetes, Endothelium, Eicosapentaenoic acid, Thoracic aorta
Background
Eicosapentaenoic acid (EPA) is an n-3 polyunsaturated fatty acid (n-3 PUFA) that is abundant in fish oils. Epidemiological and clinical trials have shown that n-3 PUFA, including EPA, reduces cardiovascular disease risk [1–3] and delays the progression of atherosclerosis in patients with coronary disease [4]. Many studies have demonstrated that n-3 PUFAs have a variety of bioactive actions such as anti-inflammatory properties [5, 6], antioxidant effects [7, 8], and improvement of endothelial function [9, 10], which explains their anti-atherogenic effects. In a previous experiment, we reported that fish oil feeding combined with food restriction improved dyslipidemia and serum levels of adiponectin [11] and decreased lipid contents in the liver [12]. However, the effects of EPA-associated cardiovascular protection in diabetes mellitus are not completely understood.
Type 2 diabetes mellitus (T2DM) is a dominant risk factor for the development and progression of atherosclerosis. Usually, endothelial dysfunction precedes the onset of atherosclerosis and occurrence of cardiovascular complications, and patients with long-term T2DM have pronounced endothelial dysfunction, leading to increased cardiovascular disease risk [13, 14]. Endothelial dysfunction is defined as reduced endothelium-dependent vasodilator function, and decreased levels of bioavailable nitric oxide (NO) in the arteries. It has been suggested that the improvement of endothelial dysfunction prevents atherosclerosis [15]. KKAy mice develop obesity, elevated plasma glucose, and insulin resistance, all of which are characteristics of T2DM [16, 17].
In the present study, we investigated the effects of EPA on the aorta of KKAy mice fed a high-fat (HF) diet.
Methods
Reagents
EPA, docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), N-ω-nitro-L-arginine (L-NNA), and phenylephrine hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sodium nitroprusside dehydrate (SNP) was from Wako Chemical Company (Osaka, Japan). Acetylcholine chloride (ACh) was from Daiichi Pharmaceuticals (Tokyo, Japan). Eicosapentaenoic acid ethyl ester (EPA-E) was from Tokyo Chemistry Industry (Tokyo, Japan). EPA, DPA, and DHA were dissolved in methanol, and the other reagents were dissolved in saline. All concentrations are expressed as the final molar concentration in the organ bath.
Experimental design
Male KKAy and C57BL/6 J mice were obtained from Tokyo Laboratory Animals Science (Tokyo, Japan) at 6 weeks of age and fed a standard pelleted diet (CE2; CLEA, Tokyo, Japan) for 1 week. Mice were exposed to a 12-h light–dark cycle and maintained at a constant temperature of 22 ± 2 °C and humidity of 55 ± 10%. KKAy mice were fed a HF diet consisting of 38.9 energy% lard oil as the fat source for 8 weeks. These mice with HF diet-induced obesity were subsequently divided into two groups (n = 6 in each group). For another 4 weeks, one group was fed a HF diet (KKAy-HF), and the other group was fed a HF diet containing EPA-E (10 mg/day; KKAy-HF + EPA-E). C57BL/6 J mice were fed a Normal diet. These Normal, HF and HF + EPA diets were made based on the modified version of the AIN-93G [18]. The compositions of these diets are indicated in Table 1. The mice used for the experiments of Fig. 4 were C57BL/6 J male mice fed a CE2 at 6–8 weeks of age. The animal experiments were approved by the Institutional Animal Care and Use Committee of Josai University (Saitama, Japan).
Table 1.
Compositions of experimental diets
| Ingredients | Normal | HF | HF + EPA-E |
|---|---|---|---|
| Corn starch (g) | 479.5 | 339.5 | 339.5 |
| Sucrose (g) | 150 | 150 | 150 |
| Casein (g) | 200 | 200 | 200 |
| L-Cystein (g) | 3 | 3 | 3 |
| Cellulose (g) | 50 | 50 | 50 |
| Soybean oil (g) | 40 | 40 | 40 |
| Lard (g) | 30 | 160 | 158 |
| Cholesterol (g) | 0 | 10 | 10 |
| Mineral mix (AIN-93G) (g) | 35 | 35 | 35 |
| Vitamin mix (AIN-93G) (g) | 10 | 10 | 10 |
| Choline bitartrate (g) | 2.5 | 2.5 | 2.5 |
| Tert-Butylhydroquinone (g) | 0.01 | 0.01 | 0.01 |
| Eicosapentaenoic ethyl ester (g) | 0 | 0 | 2 |
| Total (g) | 1000 | 1000 | 1000 |
| kcal | 4159.9 | 4859.9 | 4859.9 |
| Fat % (Calories) | 15.1 | 38.9 | 38.9 |
Fig. 4.
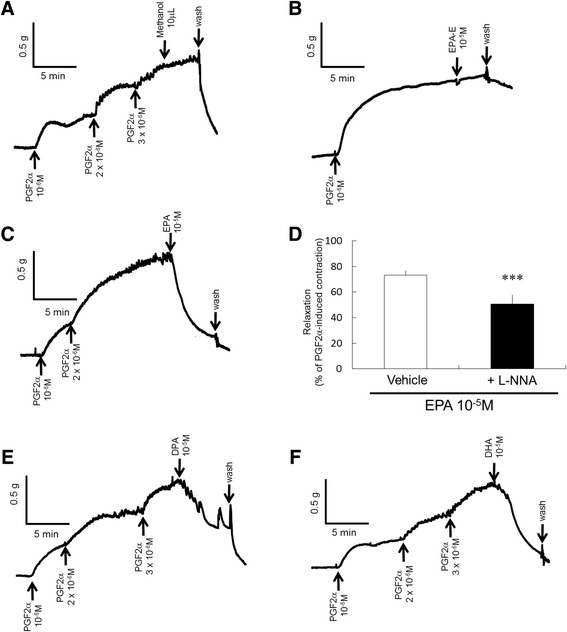
Effects of EPA-E, the metabolites and the solvent, methanol on the aortic rings extracted from C57BL/6 J mice in vitro. Original tracings showing that these reagents stimulated relaxation of the aorta after precontraction with prostaglandin F2α. EPA-E and methanol (a solvent of all fatty acids) did not relax the C57BL/6 J aortic rings (a, b). The EPA-E metabolites EPA, DPA, and DHA induced vasodilation in the aortic rings (c, e, f). In the presence of L-NNA, the relaxation induced by EPA was decreased in the aortic rings (d). Data are expressed as mean ± SEM; n = 8; ***P < 0.001 vs. Vehicle
Measurement of plasma levels of glucose, insulin, and cholesterol
Plasma parameters were measured as previously described [19]. Blood samples were centrifuged (1000 g for 20 min at 4 °C), and the plasma was stored at −20 °C until subsequent assays. Briefly, plasma levels of glucose, triglyceride and total cholesterol were determined with each commercially available enzyme kit (Wako Chemical Company, Osaka, Japan). Plasma insulin was measured by enzyme-linked immunoassay kit (Shibayagi, Gunma, Japan).
Measurement of isometric force
Each aorta was separated from the surrounding connective tissue and cut into rings of 3 mm long, as previously described [19–21]. For the vasorelaxation studies, aortic rings were precontracted with an equieffective concentration of prostaglandin F2α (PGF2α) (1 × 10− 6–3 × 10− 6 M). When the PGF2α-induced contraction had reached a plateau level, ACh (10− 9–10− 5 M) or SNP (10− 10–10− 5 M) was added in a cumulative manner, then EPA (10− 5 M), DPA (10− 5 M), or DHA (10− 5 M) were administered in a single dose. To elucidate the effects of NO, a NO synthase (NOS) inhibitor (L-NNA) (10− 4 M) was added to the bath 30 min before vascular contraction reaction in the same experiments.
Statistical analysis
All results are expressed as the mean ± standard error of the mean (SEM). Plasma parameters and body weight were compared by analysis of variance (ANOVA) followed by Scheffe’s post hoc test. Statistical comparisons of concentration–response curves were performed using two-way ANOVA with the Bonferroni post hoc test to correct for multiple comparisons. These statistical analyses were performed using the StatView program (SAS institute, Cary, NC, USA). A P value < 0.05 was deemed statistically significant.
Results
Plasma levels of glucose, insulin, cholesterol, and triglyceride and body weight
As shown in Fig. 1, non-fasting plasma levels of glucose, insulin, and total cholesterol and body weight were significantly elevated in KKAy-HF diabetic mice compared to age-matched C57BL/6 J mice. The increase in total cholesterol was decreased by administration of EPA-E for 4 weeks (Fig. 1c). However, plasma levels of glucose and insulin and body weight were not affected by EPA-E (Fig. 1a, b, e). In addition, plasma levels of triglycerides were not significantly different among the three groups (Fig. 1d).
Fig. 1.
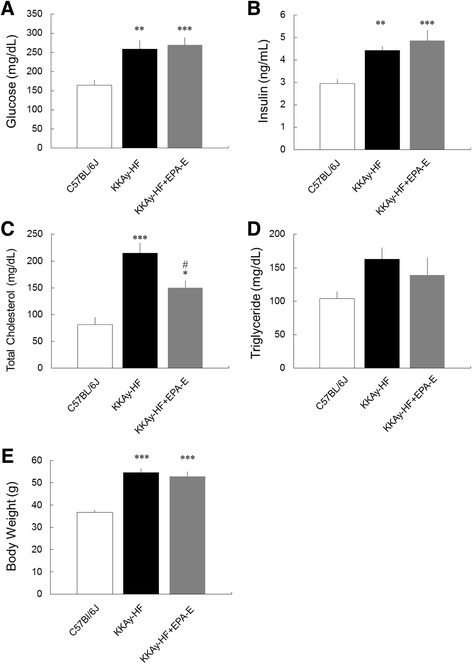
Plasma glucose (a), insulin (b), total cholesterol (c), and triglyceride (d) levels and body weight (e) in the three experimental groups. The increase in total plasma levels of cholesterol was significantly decreased by the administration of EPA-E (c). However, other parameters were not affected by the administration of EPA-E (a, b, d, e). Data are expressed as mean ± SEM for eight mice. *P < 0.05 **P < 0.01, ***P < 0.001 vs. C57BL/6 J, #P < 0.05 ##P < 0.01 vs. KKAy-HF mice
Vascular reactivity in aorta
The ACh-induced relaxation of aortic rings extracted from KKAy mice was significantly weaker than that of C57BL/6 J mice (Fig. 2a). This attenuated relaxation was significantly improved by chronic EPA-E treatment (Fig. 2a). The relaxation induced by SNP was not significantly different among the three groups (Fig. 2b). Phenylephrine caused concentration-dependent contractions that reached a maximum at 10− 5 M in aortic rings. Phenylephrine-induced contraction was significantly greater in KKAy-HF mice than in C57BL/6 J mice (Fig. 3a). The increase in contraction in KKAy-HF mice was not affected by the chronic administration of EPA-E (Fig. 3a). Next, the effects of a NOS inhibitor (L-NNA) (10− 5 M) on the response to phenylephrine were examined. The contraction induced by phenylephrine was increased in C57BL/6 J mice and reached the levels observed in KKAy-HF and KKAy-HF + EPA-E mice (Fig. 3b). Then we observed the direct actions of EPA-E and its metabolites on the aorta. When each metabolite was administered to the organ bath in a single dose, as shown by the representative tracings in Fig. 4a and b, EPA-E and methanol (a solvent of all fatty acids) did not relax the C57BL/6 J aortic ring precontraction with PGF2α. Interestingly, EPA, DPA, and DHA induced vasodilation in the aortic rings (Fig. 4c, e, f). Furthermore, the vasodilation induced by EPA was suppressed in the presence of L-NNA (Fig. 4d).
Fig. 2.
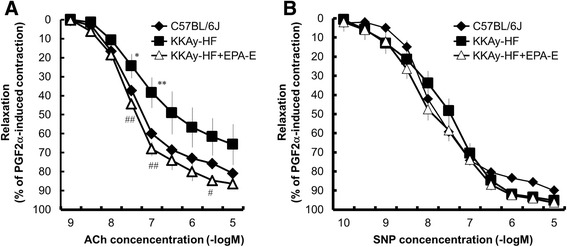
Concentration–response curves following ACh (a) and SNP (b) treatments of mice aortic rings. a Compared to C57BL/6 J mice, ACh-induced relaxation of aortic rings extracted from KKAy-HF mice was significantly weaker. This attenuated relaxation was significantly improved by chronic EPA-E treatment. b The relaxation induced by SNP was not significantly different among the three groups. Data are expressed as mean ± SEM; n = 6; *P < 0.05 **P < 0.01 vs. C57BL/6 J, #P < 0.05 ##P < 0.01 vs. KKAy-HF mice
Fig. 3.
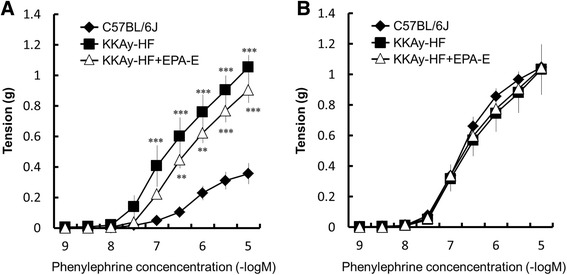
Concentration–response curves of phenylephrine-induced contraction of aortic rings (a) and contraction in the presence of L-NNA (b). a The increase in contractions in KKAy-HF mice was not affected by chronic administration of EPA-E. b In the presence of L-NNA, the contractions induced by phenylephrine were increased in C57BL/6 J mice and reached levels found in KKAy-HF and KKAy-HF + EPA-E mice. Data are expressed as mean ± SEM; n = 6–8; **P < 0.01 ***P < 0.001 vs. C57BL/6 J
Discussion
The results of the present study showed that chronic dietary EPA-E supplementation improved the endothelial dysfunction seen in type 2 diabetic mice fed a HF diet. Furthermore, EPA-E metabolites (including EPA, DHA, and DPA), but not EPA-E itself, had beneficial effects on vascular function in mice aortic rings.
Metabolic disorders including obesity, hyperglycemia, dyslipidemia, and hyperinsulinemia were seen in KKAy mice (Fig. 1), indicating that these mice are suitable models for T2DM with obesity. Among these unusual parameters, the increase in total plasma levels of cholesterol was significantly decreased by the chronic administration of EPA-E.
Although the measurement of flow-mediated dilation (FMD) is a standard method for measuring endothelial function in humans, the measurement of isolated vascular reactivity in the organ bath is used in animals because of technical limitations of doing these experiments in mice [22]. To examine the endothelium-dependent and endothelium-independent relaxation of the isolated aorta, we used ACh and SNP (Fig. 2). We found that the ACh-induced endothelium-dependent relaxation was impaired in KKAy-HF compared with wild type C57BL/6 J mice (Fig. 2a). Because the endothelium-independent relaxation of aortic rings induced by SNP was not different between C57BL/6 J and KKAy-HF mice (Fig. 2b), the activity of soluble guanylate cyclase in the smooth muscle of the aorta was not altered in KKAy-HF mice. Clinical studies have demonstrated that endothelial impairment in diabetes is the initial step of atherosclerosis [3]. Our results indicated that EPA-E can effectively improve endothelial function in the thoracic aortas of diabetic mice. Previous FMD experiments demonstrated that n-3 PUFA (including EPA and DHA) supplementation improved vascular function or coronary vasomotion [23–25]. However, pure EPA in the diet is very unstable and very easily oxidized. Therefore, the more stable ethyl-esters EPA is usually administered to patients in clinical studies [26]. Thus, it is possible that EPA-E administration reduces the progression of atherosclerotic disorders in humans.
In a previous report, dietary supplementation with fish oil or EPA was shown to reduce contractile responses evoked by noradrenaline and arachidonic acid in the rat aorta [27]. However, the enhanced contractile response by phenylephrine in KKAy-HF was not altered by the administration of EPA-E in our experiments, and contractions induced by phenylephrine in the presence of L-NNA did not significantly differ among the three groups (Fig. 3b). Phenylephrine, an α-adrenergic receptor agonist, stimulates Ca2+ entry through voltage-gated and store-operated Ca2+ channels [28], and activates Ca2+-sensitization pathways such as protein kinase C (PKC) and Rho-kinase [29, 30]. NO reduces Ca2+ entry into vascular smooth muscle [31] and c-GMP-dependent protein kinase causes phosphorylation and inactivation of myosin light-chain kinase leading to inactivation of Ca2+-sensitive pathways such as PKC and Rho-kinase [29]. In these experiments, although chronic EPA-E administration improved endothelial dysfunction in KKAy-HF mice, the vascular contractile response in KKAy-HF + EPA-E mice showed no change compared with KKAy-HF mice. Further investigations on the vascular contractile response are needed.
EPA-E administration did not change plasma glucose or insulin levels in our experiments. Our data showed that chronic EPA-E administration improved endothelial dysfunction independently of glucose homeostasis. It has been speculated that the vascular protective effects of EPA-E administration are attributable, at least in part, to the enhanced endothelial function of vasoprotective factors such as endothelium-derived relaxation factor including NO and prostacyclin. To clarify the vasoprotective effects of EPA-E, the relaxation of aortic rings following the direct administration of EPA-E in an organ bath was observed. Despite the beneficial effects of endothelial function in a previous in vivo study, EPA-E did not directly affect the vascular reaction. It has been postulated that the improvement of endothelial dysfunction in type 2 diabetic mice by chronic EPA-E administration is partly due to a decrease in total cholesterol levels and anti-platelet actions [32]. In addition, we speculated that EPA-E metabolites may have vasoprotective effects. In an EPA-E metabolism study, it was reported that when labeled 14C-EPA-E was orally administered to rats, EPA, DPA and DHA were identified as metabolites of EPA-E [33]. In this study, we found that these metabolites directly affected vascular reactivity.
Our present observations clarified that EPA, DPA, and DHA as EPA-E metabolites caused vasodilation in C57BL/6 J mice aortic rings. It has been reported that EPA induces vascular relaxation in both endothelium-dependent [10] and endothelium-independent [34] manners. Here, we found that EPA-induced relaxation partly occurred via eNOS activation because relaxation was attenuated in the presence of a NOS inhibitor. Further investigations are needed to clarify the mechanisms underlying vascular relaxation by these EPA-E metabolites.
Conclusions
We demonstrated that impairment of endothelium-dependent relaxation in aortas from type 2 diabetic mice was prevented by chronic EPA-E administration partly by the direct vasodilative action of EPA-E metabolites.
Acknowledgments
We would like to thank Yasuo Okamoto and Kazuhito Tsuboi for their advice during the preparation of the manuscript. The authors would also like to thank FORTE for their language review of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant No. JP26350904) and the KAWASAKI Foundation for Medical Science and Medical Welfare.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACh
Acetylcholine
- ANOVA
Analysis of variance
- DHA
Docosahexaenoic acid
- DPA
Docosapentaenoic acid
- EPA
Eicosapentaenoic acid
- EPA-E
Eicosapentaenoic acid ethyl ester
- HF
High fat
- L-NNA
N-ω-nitro-L-arginine
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PGF2α
Prostaglandin F2α
- PKC
Protein kinase C
- SEM
Standard error of the mean
- SNP
Sodium nitroprusside
Authors’ contributions
YT designed and performed the experiments, analyzed the data, interpreted the results, and wrote the manuscript. KO and KN interpreted the results. KK designed the experiments, interpreted the results, and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval
All experiments were performed in accordance with the Guidelines for the Institutional Animal Care and Use Committee of Josai University.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Kris-Etherton P, Goldberg IJ, Kotchen TA, et al. AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the nutrition Committee of the American Heart Association. Circulation. 2000(102):2284–99. [DOI] [PubMed]
- 2.De Caterina R. N-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 3.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Harris WS, Appel LJ, Committee AHAN. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 5.De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–1836. doi: 10.1161/01.ATV.14.11.1829. [DOI] [PubMed] [Google Scholar]
- 6.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995;15:622–628. doi: 10.1161/01.ATV.15.5.622. [DOI] [PubMed] [Google Scholar]
- 7.Casós K, Zaragozá MC, Zarkovic N, Zarkovic K, Andrisic L, Portero-Otín M, Cacabelos D, Mitjavila MT. A fish-oil-rich diet reduces vascular oxidative stress in apoE(−/−) mice. Free Radic Res. 2010;44:821–829. doi: 10.3109/10715762.2010.485992. [DOI] [PubMed] [Google Scholar]
- 8.Ober MD, Hart CM. Attenuation of oxidant-mediated endothelial cell injury with docosahexaenoic acid: the role of intracellular iron. Prostaglandins Leukot Essent Fatty Acids. 1998;59:127–135. doi: 10.1016/S0952-3278(98)90091-6. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Hossain S, Yamasaki H, Yazawa K, Masumura S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids. 1999;34:1297–1304. doi: 10.1007/s11745-999-0481-6. [DOI] [PubMed] [Google Scholar]
- 10.Omura M, Kobayashi S, Mizukami Y, Mogami K, Todoroki-Ikeda N, Miyake T, Matsuzaki M. Eicosapentaenoic acid (EPA) induces ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001;487:361–366. doi: 10.1016/S0014-5793(00)02351-6. [DOI] [PubMed] [Google Scholar]
- 11.Wakutsu M, Tsunoda N, Mochi Y, Numajiri M, Shiba S, Muraki E, Kasono K. Improvement in the high-fat diet-induced dyslipidemia and adiponectin levels by fish oil feeding combined with food restriction in obese KKAy mice. Biosci Biotechnol Biochem. 2012;76:1011–1014. doi: 10.1271/bbb.110743. [DOI] [PubMed] [Google Scholar]
- 12.Shiba S, Tsunoda N, Wakutsu M, Muraki E, Sonoda M, Tam PS, Fujiwara Y, Ikemoto S, Kasono K. Regulation of lipid metabolism by palmitoleate and eicosapentaenoic acid (EPA) in mice fed a high-fat diet. Biosci Biotechnol Biochem. 2011;75:2401–2403. doi: 10.1271/bbb.110509. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Li J, Lv X, Zhang M, Song Y, Chen L, Liu Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620:131–137. doi: 10.1016/j.ejphar.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Lam TY, Seto SW, Lau YM, Au LS, Kwan YW, Ngai SM, Tsui KW. Impairment of the vascular relaxation and differential expression of caveolin-1 of the aorta of diabetic +db/+db mice. Eur J Pharmacol. 2006;546:134–141. doi: 10.1016/j.ejphar.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 16.Castle CK, Colca JR, Melchior GW. Lipoprotein profile characterization of the KKA(y) mouse, a rodent model of type II diabetes, before and after treatment with the insulin-sensitizing agent pioglitazone. Arterioscler Thromb. 1993;13:302–309. doi: 10.1161/01.ATV.13.2.302. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Takenouchi Y, Kobayashi T, Matsumoto T, Kamata K. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis. 2009;206:397–404. doi: 10.1016/j.atherosclerosis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Takenouchi Y, Kobayashi T, Matsumoto T, Kamata K. Possible involvement of Akt activity in endothelial dysfunction in type 2 diabetic mice. J Pharmacol Sci. 2008;106:600–608. doi: 10.1254/jphs.FP0071684. [DOI] [PubMed] [Google Scholar]
- 21.Takenouchi Y, Kobayashi T, Taguchi K, Matsumoto T, Kamata K. Gender differences in vascular reactivity of aortas from streptozotocin-induced diabetic mice. Biol Pharm Bull. 2010;33:1692–1697. doi: 10.1248/bpb.33.1692. [DOI] [PubMed] [Google Scholar]
- 22.Schuler D, Sansone R, Freudenberger T, Rodriguez-Mateos A, Weber G, Momma TY, Goy C, Altschmied J, Haendeler J, Fischer JW, et al. Measurement of endothelium-dependent vasodilation in mice--brief report. Arterioscler Thromb Vasc Biol. 2014;34:2651–2657. doi: 10.1161/ATVBAHA.114.304699. [DOI] [PubMed] [Google Scholar]
- 23.Stirban A, Nandrean S, Götting C, Tamler R, Pop A, Negrean M, Gawlowski T, Stratmann B, Tschoepe D. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr. 2010;91:808–813. doi: 10.3945/ajcn.2009.28374. [DOI] [PubMed] [Google Scholar]
- 24.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/S0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto H, Yoshimura H, Noma M, Suzuki S, Kai H, Tajimi T, Sugihara M, Kikuchi Y. Improvement of coronary vasomotion with eicosapentaenoic acid does not inhibit acetylcholine-induced coronary vasospasm in patients with variant angina. Jpn Circ J. 1995;59:608–616. doi: 10.1253/jcj.59.608. [DOI] [PubMed] [Google Scholar]
- 26.Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of Eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed]
- 27.Lockette WE, Webb RC, Culp BR, Pitt B. Vascular reactivity and high dietary eicosapentaenoic acid. Prostaglandins. 1982;24:631–639. doi: 10.1016/0090-6980(82)90033-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobe K, Takenouchi Y, Kasono K, Hashimoto T, Honda K. Two types of overcontraction are involved in intrarenal artery dysfunction in type II diabetic mouse. J Pharmacol Exp Ther. 2014;351:77–86. doi: 10.1124/jpet.114.216747. [DOI] [PubMed] [Google Scholar]
- 31.Cornwell TL, Lincoln TM. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989;264:1146–1155. [PubMed] [Google Scholar]
- 32.Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242:357–366. doi: 10.1016/j.atherosclerosis.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Ishiguro J, Tada T, Ogihara T, Mizota M, Mizuguchi K, Ohzawa N, Kosuzume H, Aizawa N. Metabolism of ethyl eicosapentaenoate (EPA-E) in rats and effect of its metabolites on ellagic acid-induced thrombus formation in the stenosed femoral artery of rabbits. Chem Pharm Bull (Tokyo) 1988;36:2158–2167. doi: 10.1248/cpb.36.2158. [DOI] [PubMed] [Google Scholar]
- 34.Engler MB, Engler MM, Browne A, Sun YP, Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 2000;131:1793–1799. doi: 10.1038/sj.bjp.0703754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


