Abstract
Background:
The clinical significance of co-infections with high-risk (HR) and low-risk (LR) human papillomavirus (HPV) in the etiology of cervical cancer is debated, as prospective evidence on this issue is limited. However, the question is of increasing relevance in relation to HPV-based cancer prevention.
Methods:
In two population-based nested case-control studies among women participating in cervical screening with baseline normal smears, we collected 4659 smears from women who later developed cancer in situ (CIS; n = 524) or squamous cervical cancer (SCC; n = 378) and individually matched control subjects who remained free of disease during study follow-up. The median follow-up until diagnosis was 6.4 to 7.8 years. All smears were tested for HPV. We used conditional logistic regression models with two-way interaction terms to estimate relative risks (RRs) for CIS and SCC, respectively. All statistical tests were two-sided.
Results:
Compared with women who were infected with HRHPV only, women who were also infected with LRHPV had a lower risk for SCC (RR = 0.2, 95% confidence interval [CI] = 0.04 to 0.99, P = .049). This interaction was not shown for CIS (RR = 1.1, 95% CI = 0.4 to 3.6). Women who were positive for both HRHPV and LRHPV had, on average, a 4.8 year longer time to diagnosis of SCC than women who were positive for HRHPV only (P = .006). Results were highly robust in sensitivity analyses.
Conclusion:
Co-infection with LRHPV is associated with a lower risk of future invasive disease and longer time to diagnosis than infection with HRHPV alone. We propose that co-infection with LRHPV interferes with the rate of progression to invasive cervical cancer.
Infection with carcinogenic “high-risk” human papillomavirus types (HRHPV) is a necessary but not sufficient cause of cervical cancer worldwide (1). Low-risk HPV types (LRHPV) have negligible carcinogenic potential in the cervix uteri, but can cause condyloma (external genital warts), which also constitutes a large disease burden (2). Condyloma has an incidence peak similar to other sexually transmitted infections, ie, at age 15 to 24 years (3), and has been linked to increased subsequent risks for HPV-related cancers in several studies (4,5). Clearly, this risk association could be confounded by several unmeasured factors, above all concurrent infection with HRHPV types, the status of which is unknown in register-based studies. Also, women with manifest condyloma development may not be representative of all women infected with genital LRHPV, thus serving as an imperfect proxy for inference on any putative relationship between LRHPV and cancer.
Indeed, seropositivity for both LR HPV6/11 and HRHPV16 has been shown to lead to a statistically significant antagonistic interaction effect, ie, a reduced risk for cervical cancer, compared with expected (6–9). However, seropositivity is an imperfect measure of HPV status, not the least because it cannot distinguish between past and present infection. There is a distinct paucity of HPV DNA-based longitudinal data concerning interaction effects between HPV types in invasive cervical cancer. Yet with the advent of HPV-based screening and vaccination, additional understanding is important. This would concern both interactions between LRHPV and HRHPV, as well as between current HPV vaccine and nonvaccine types of HRHPV (ie, 16/18 vs non-16/18 HRHPV). We therefore investigated interactions between HPV types in a large, well-established population-based longitudinal study.
Methods
Participants
The source population was defined by using the Swedish National Cervical Screening Register (NCSR) to extract records on all Swedish women (1 459 258) who participated in cervical screening within one of 10 county laboratories in six Swedish counties during the period of 1969 to 2002. We then selected a cohort of 1 431 724 women whose first registered smear (defining the entry to our study) was classified as cytologically normal, as previously described (10,11). Records from this cohort were then linked—using individual personal identification numbers—to the Swedish National Cancer Register (NCR) to identify all women with a first diagnosis of cancer in situ (CIS) or invasive squamous cell carcinoma (SCC) after entry in our study. A diagnosis of CIS in the NCR translates internationally to a diagnosis of cervical intraepithelial neoplasia grade 3 (CIN3).
One control subject—matched on county laboratory, date of entry into cohort (+/-3 months), and age at first normal smear (+/-1 year)—was randomly selected for each CIS and SCC case subject. All available smears from the case subject and the control subject taken prior to the date of diagnosis of the case were retrieved from biobank archives.
Smear Analyses
Matched case subjects and control subjects were analyzed in a strictly blinded fashion, in the same batches at the same calendar time. DNA extraction was performed by validated methods (12). All smears were analyzed for the presence of seven low-risk HPV types (HPV 6, 7, 11, 42, 43, 70, and 90) and 16 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82).
The polymerase chain reaction (PCR) amplification of a consensus region using GP5+/6+ primers (13) was followed by HPV type detection (14) through detection of biotinylated HPV amplicons by a multiplex fluorescent bead-based assay (15). The presence of amplifiable DNA in the samples was determined by real-time polymerase chain reaction (PCR) for the housekeeping β-globin gene. In HPV16-positive samples, we further quantified the viral load, measured as an absolute number of viral copies of the E7 gene per microliter, using the validated Taqman real-time quantitative PCR method (16). HPV analyses were performed in the World Health Organization HPV LabNet Global Reference Laboratory (Malmö, Sweden). These methods have been previously described in detail (10,11). All available histologies from case subjects were rereviewed by a senior pathologist.
Initially, 5336 smears were eligible for statistical analyses. We subsequently excluded 125 smears with negative β-globin value, 30 smears that were taken on the date of diagnosis, 237 smears that were part of the diagnostic work-up of the case and 285 smears from incomplete case-control pairs (because either the case subject or the control subject did not have any eligible Pap smears). Hence 4659 smears belonging to 524 complete case-control pairs of CIS and 378 cases of SCC remained. For case-only analyses, we used 1625 smears from 573 CIS case subjects and 1239 smears from 418 SCC case subjects.
Statistical Analyses
Because of the matched design, conditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). These were interpreted as estimates of relative risk (RR) of CIS or SCC in HPV-exposed women. Pooled risk estimates were calculated for all high-risk types (HRHPV), for HPV16 and/or 18 (HPV16/18), for all high-risk HPV types excluding HPV16 and 18 (non-16/18 HRHPV), and for all low-risk HPV types (LRHPV).
We analyzed separately the risk associated with HPV infection in the first and in the last smear prior to the case subject’s diagnosis. We defined exposure categories as follows: 1) HPV16/18—the first/last smear being positive for HPV16 and/or 18; 2) non-16/18 HRHPV—the first/last smear being positive for one or more high-risk HPV types other than HPV16 or 18; 3) LRHPV—the first/last smear being positive for one or more low-risk HPV types. We further included interaction terms for each category 1–3 in our regression model as follows:
This model includes both main effects for the separate categories (β1, β2, and β3), as well as the interaction effects for combinations of exposure categories (parameters β4, β5, and β6). We modeled only two-way interactions (ie, the risk in women positive for two categories in the same smear), as there were too few women positive for all three categories in the same smear for reliable estimation.
In order to enable investigation interaction effects between all HRHPV and all LRHPV, we also performed an estimation using a collapsed model, where categories 1 and 2 above were combined into one exposure.
In these models, we can conveniently compare relative risks (RRs) between different exposures and exposure combinations as sums and differences of the parameters (contrasts), depending on the focus of the investigation. In our initial analysis, we estimated the risk for CIS and SCC in women exposed to any HPV compared with women unexposed to any HPV (HPV positive in any combinations vs an HPV-negative reference group).
In a second analysis, we estimated the risk for CIS and SCC in women with a double category infection, compared with single category HPV infection (eg, HRHPV+LRHPV vs an HRHPV-only reference group).
In a case-only analysis, we used linear regression to model time to diagnosis (as a continuous variable in years) and viral load of HPV16 (as a continuous measure in log copies/microliter), in case subjects with a double category infection compared with case subjects with a single category infection (ie, HRHPV+LRHPV vs HRHPV only). These analyses were adjusted for age at first smear in years by including a natural spline term.
In sensitivity analyses, we included 1) only cases that were confirmed on histological rereview (ie, double-confirmed cases) and 2) only smears that were cytologically benign from case subjects and control subjects.
All data were de-identified, and calculations were performed using R version 3.1.0 (17). All P values were two-sided and derived from the Wald chi-square test and t test, respectively. A P value under .05 was considered statistically significant. All P values and 95% confidence intervals (CIs) presented are adjusted for multiple hypothesis testing within each model (18). The study was approved by the Karolinska Institutet Ethics Review Board, (Stockholm, Sweden), which also determined that informed consent from the participants was not required.
Results
Study Population
The median age at entry was 25 years for CIS and 32 years for SCC, respectively. The median time from the first smear to diagnosis of the case subject was 6.4 to 7.8 years, yielding a median age at diagnosis of 32 years for CIS and 41 years for SCC (Table 1). The time from the last smear to diagnosis was around a year for CIS and around three years for SCC. Infection with HRHPV was prevalent in the smears before CIS (49%-67%) and before SCC (50%-57%), respectively. The proportion of smears only positive for LRHPV was universally low, at 1% to 3%. The proportion of smears positive for both HRHPV and LRHPV was slightly higher, at 1% to 5%. The most prevalent LRHPV type was HPV42 (Supplementary Tables 1 and 2, available online). HRHPV+LRHPV co-infected smears contained between one to six high-risk HPV types. Most HRHPV+LRHPV co-infected smears contained only one low-risk HPV type, but in rare cases two LRHPV infections were observed (1/69 HRHPV+LRHPV smears in CIS and 2/25 HRHPV+LRHPV smears in SCC).
Table 1.
Characteristics of the study participants*
| Cancer in situ | Squamous cell carcinoma | |||
|---|---|---|---|---|
| Characteristic | Case subjects | Control subjects | Case subjects | Control subjects |
| Cohort | ||||
| Subjects, No. | 524 | 523† | 378 | 378 |
| Age at diagnosis, y‡ | 32 (18 - 74) | 32 (18 - 74) | 41 (22 - 86) | 41 (23 - 85) |
| Age at entry, y | 25 (14 - 60) | 25 (14 - 60) | 32 (15 - 86) | 31 (15 - 84) |
| Time first smear to diagnosis, y | 6.4 (0 - 26.5) | 6.3 (0 - 26.5) | 7.8 (0 - 27.6) | 8.2 (0.1 - 27.7) |
| Time last smear to diagnosis, y | 1.5 (0 - 23.3) | 2.9 (0 - 20.7) | 3.0 (0 - 22.4) | 3.4 (0 - 24) |
| Smears, No. | 1333 | 1261 | 1060 | 1006 |
| First smear | ||||
| HRHPV pos, No. (%) | 258 (49.2) | 67 (12.8) | 187 (49.5) | 45 (11.9) |
| LRHPV pos, No. (%) | 12 (2.3) | 13 (2.5) | 7 (1.9) | 10 (2.6) |
| HR + LR pos, No. (%) | 27 (5.2) | 10 (1.9) | 8 (2.1) | 5 (1.3) |
| HPV16 VL, No. (%) | 128 (24.4) | 23 (4.4) | 95 (25.1) | 12 (3.2) |
| log(HPV16 VL) | 1.8 (-2.3 - 4.3) | 1.3 (-2.3 - 3.8) | 1.5 (-2.3 - 4.1) | 1.6 (-2.3 - 3.9) |
| Last smear | ||||
| HRHPV pos, No. (%) | 349 (66.6) | 52 (9.9) | 215 (56.9) | 30 (7.9) |
| LRHPV pos, No. (%) | 12 (2.3) | 14 (2.7) | 3 (0.8) | 10 (2.6) |
| HR + LR pos, No. (%) | 27 (5.2) | 5 (1.0) | 8 (2.1) | 4 (1.1) |
| HPV16 VL, No. (%) | 178 (34.0) | 17 (3.2) | 102 (27.0) | 9 (2.4) |
| log(HPV16 VL) | 2 (-2.3 - 4.5) | 1.5 (-2.3 - 2.9) | 1.7 (-1.2 - 5.1) | 1.8 (-2.3 - 3.9) |
* Ages, time intervals, and viral loads are reported as median (range). HRHPV = high-risk human papillomavirus, LRHPV = low-risk human papillomavirus; VL = viral load.
† In one instance, one randomly sampled cancer in situ (CIS) control subject was matched to two case subjects as per the nested case-control design, hence we included 524 CIS case subjects and 523 matched CIS control subjects.
‡ For control subjects, median age at diagnosis of the matched case subject.
Risk for CIS and SCC Compared With HPV-Negative Women
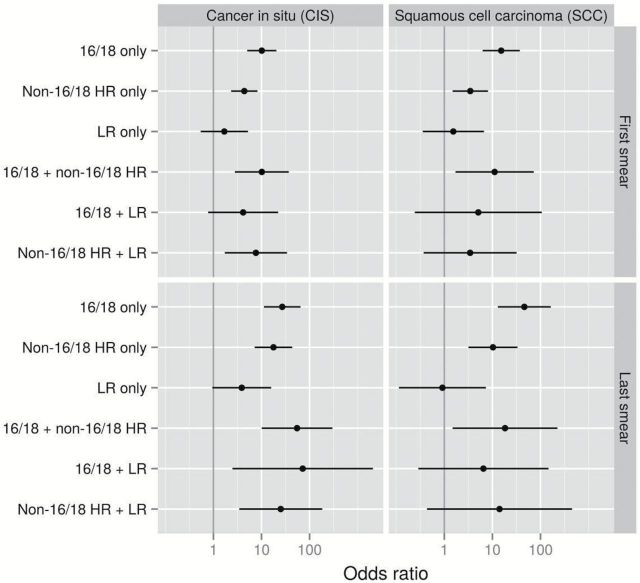
In the baseline first smear during follow-up, women infected with only HPV16/18 had the highest risk estimates for both CIS and SCC compared with HPV-negative women (RR for CIS = 10.1, 95% CI = 5.0 to 20.4, RR for SCC = 15.2, 95% CI = 6.3 to 37.1). Women positive for only non16/18HRHPV also had statistically significantly increased risks (RR for CIS = 4.4, 95% CI = 2.3 to 8.2, RR for SCC = 3.5, 95% CI = 1.5 to 8.1, P < .001), whereas there was no risk associated with LRHPV alone. Women infected with both HPV16/18 and non16/18HRHPV had 10-fold higher risks for CIS (RR = 10.2, 95% CI = 2.8 to 37.0) and SCC (RR = 11.1, 95% CI = 1.7 to 72.6) than HPV-negative women (Supplementary Table 3, available online). For combinations of HRHPV and LRHPV, risks were likewise increased compared with HPV-negative women. The same pattern was seen in the last smear before diagnosis, although more pronounced (Figure 1; Supplementary Table 3, available online).
Figure 1.
Relative risks for cancer in situ and squamous cervical cancer for different human papillomavirus (HPV) category infection patterns of 16/18, non-16/18 high-risk and low-risk positivity, singly and in combination, observed in first/last smear before diagnosis, obtained using conditional logistic regression modeling. All statistical tests were two-sided. Reference level: HPV-negative women. LR = low-risk; HR = high-risk.
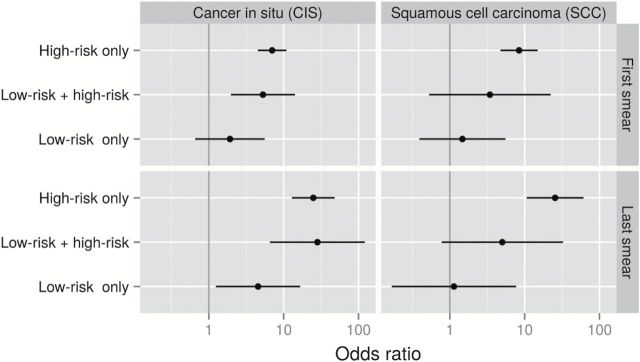
When considering a single HRHPV exposure category, women infected with any HRHPV in the first smear had seven- to eight-fold increased risks for CIS and SCC compared with HPV-negative women. These risks increased to about 25-fold if found in the last smear. Women with LRHPV only had a statistically significantly increased risk in the short term for CIS detection compared with HPV-negative women (RR in the last smear = 4.5, 95% CI = 1.2 to 16.6, P = .016). Women with LRHPV only had no increased risk of SCC. Being positive for both HRHPV and LRHPV was associated with almost the same risk as that for HRHPV alone when it came to CIS. In contrast, women infected with both HRHPV and LRHPV had a substantially lower risk estimate for SCC than those with only HRHPV, although precision was low (Figure 2; Supplementary Table 4, available online).
Figure 2.
Relative risks for cancer in situ and squamous cervical cancer for different human papillomavirus (HPV) category infection patterns of high-risk and low-risk positivity, singly or in combination, observed in first/last smear before diagnosis, obtained using conditional logistic regression modeling. All statistical tests were two-sided. Reference level: HPV-negative women. LR = low-risk; HR = high-risk.
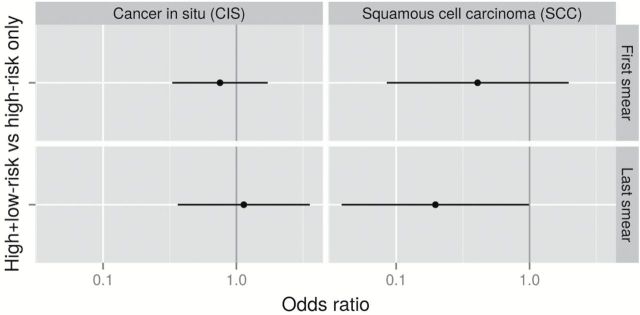
Risk for CIS and SCC in Women With Double- Compared With Single-Category HPV Infection
In order to focus on risk among HPV-positive women, the model was reparameterized to estimate the risk in women with a double-category HPV infection (HRHPV+LRHPV) compared with that in women with a single-category HPV infection (ie, HRHPV only) (Figure 3).We found that women with both HRHPV and LRHPV infection had the same risks for CIS as women with only HRHPV (RR first smear = 0.8, 95% CI = 0.3 to 1.7; RR last smear = 1.10, 95% CI = 0.4 to 3.6). However, women with both HRHPV and LRHPV infection had a tendency for a decreased risk for SCC if found in the first smear (RR = 0.4, 95% CI = 0.10 to 2.0) and a statistically significantly decreased risk for SCC if found in the last smear (RR = 0.2, 95% CI = 0.04 to 0.99, P = .049) (Supplementary Table 5, available online), compared with women with HRHPV only.
Figure 3.
Relative risks for cancer in situ and squamous cervical cancer for women infected with both high-risk human papillomavirus (HRHPV) and low-risk human papillomavirus observed in first/last smear before diagnosis, obtained using conditional logistic regression modeling. All statistical tests were two-sided. Reference level: women with only HRHPV.
In a similar fashion, we investigated whether the combination of HPV16/18 and non16/18HRHPV was associated with any different risk for CIS or SCC than being positive for HPV16/18 only. None of these estimates differed statistically (data not shown).
Time to Diagnosis in Case Subjects With Double- Compared With Single-Category HPV Infection
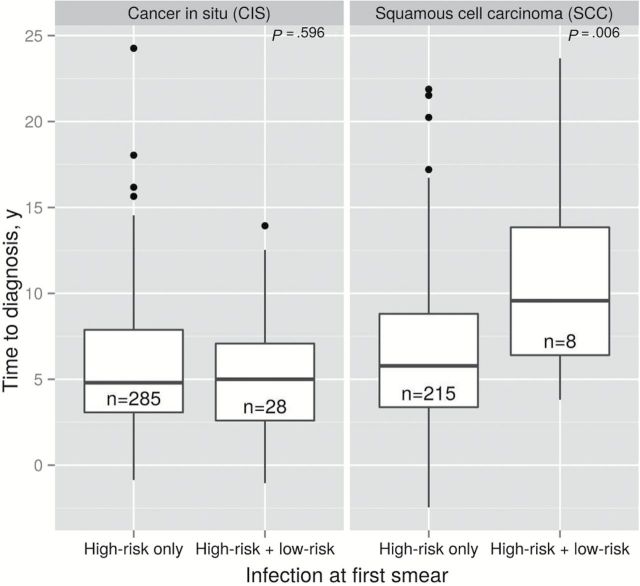
We further investigated whether time to diagnosis (in years) differed in women with a double- compared with single-category HPV infection. We found that the time to diagnosis was on average 4.8 years longer in women with double-category infection (HRHPV+LRHPV) compared with HRHPV only. The difference was highly statistically significant (P = .006) (Figure 4).
Figure 4.
Time to diagnosis in years in cancer in situ and squamous cervical cancer case subjects by human papillomavirus category infection status observed in the first smear. Results obtained through linear regression modeling adjusting for age at first smear as a continuous measure in years. All statistical tests were two-sided.
HPV16 Viral Load in Case Subjects With Double- Compared With Single-Category HPV Infection
HPV16 viral load was similar in case subjects whether positive for both HRHPV and LRHPV, or HRHPV only (data not shown).
Sensitivity Analyses
When considering histologically reconfirmed cases only, the results from the above analyses remained unchanged. Specifically, in the model of risk for SCC in women with both HRHPV and LRHPV compared with HRHPV only, the core result was highly robust (RR for SCC in the first smear = 0.1, 95% CI = 0.01 to 0.92, P = .041 and RR for SCC in last smear = 0.07, 95% CI = 0.01 to 0.6, P = .014). When restricting to cytologically benign smears, the result was similarly robust: (RR for SCC in first smear = 0.4, 95% CI = 0.08 to 1.65, P = .19, RR for SCC in last smear = 0.2, 95% CI = 0.03 to 0.70, P = .017). (Data for other categories not shown.)
Discussion
We found that women co-infected with both high-risk HPV and low-risk HPV have a lower risk for future invasive squamous cervical cancer than women infected with HRHPV alone. The reasons for this interaction are not clear and our finding may merit further replication in other cervical DNA-based studies and investigation of potential underlying mechanisms. We further show that the women in this group who did progress to invasive disease did so after a substantially longer time to diagnosis than women with HRHPV infection alone. This finding has to our knowledge not been previously demonstrated. The presence of LRHPV did not appear to alter the risk of cancer in situ (CIS/CIN3), either in the short- or the long-term perspective. We therefore propose that the presence of LRHPV interferes with the rate of HRHPV-related progression from in situ to invasive cervical disease.
We have previously shown in seroepidemiological studies that low-risk types HPV6 and HPV11 appear to act in an antagonistic fashion to infection with the high-risk type HPV16, rendering the risk for invasive cervical cancer to be lower than expected (6,8,9). However, to our knowledge, no evidence for this relationship has been available from cervical HPV DNA-based studies until now. Furthermore, serology is unable to discriminate between past or present infections, whereas in the current study we simultaneously could determine HPV exposure in the same cervical sample and in (on average) two smears from each woman. Therefore, we hold the case for a defined negative interaction effect between LRHPV and HRHPV to be strengthened through the current study. In contrast, we found no evidence for such a negative interaction effect between the combination of current vaccine HR types (ie, HPV16/18) and nonvaccine HR types (ie, non16/18HRHPV) on the risk of invasive cancer.
The strengths of this study consist of the national population-based approach and the stringency in design, laboratory analyses, and biostatistical efforts. Importantly, our study was nested in a national cervical screening program with high attendance (19), and our incidence density–based sampling design preserves the validity of the underlying cohort. We have already described major findings from this nested case-control study in a series of publications, and previous findings have been well in line with established HPV research (10,11,20), which shows that our external validity is high.
Limitations of this study include that, because of the sampling scheme and archival nature of smears, our case subjects were not uniformly HPV positive even in the smear closest to diagnosis and that, because of few cases of double-category infection, some of our estimates have limited precision. Nevertheless, we did see a statistically significant difference between risks and time to diagnosis for CIS and SCC, with the stronger findings in SCC despite the number of cases being smaller. Furthermore, these findings were highly robust and strengthened in sensitivity analyses. We lack information on potential confounders such as smoking and oral contraceptive use, factors that may conceivably alter both the likelihood to be positive for several HPV types and the risk of subsequent cervical disease. However, smoking and oral contraceptive use have been clearly shown as associated with risk for CIS (CIN3) (21–23), whereas we only saw a statistically significant effect because of multiple HPV infections in invasive cancer. Therefore, confounding by these factors cannot explain our findings. It could also be posited that LRHPV infections are of short duration and might act as a marker of recently acquired HRHPV infection, thus by definition leading to longer time to diagnosis. However, the median age at first smear of women with double-category infection was 31 years, well past the peak in HPV infection incidence, which reduces the likelihood of recent acquisition. In addition, these women’s median time to diagnosis was longer than even that of women who were HPV negative in their first smear, at 11 vs nine years, respectively (data not shown). Thus, recency of infection cannot explain our findings either.
Finally, we did not test for all known LRHPV types, meaning that some smears categorized as “HR-positive only” may in fact also contain undetected LRHPV. However, such misclassification is nondifferential between case subjects and control subjects and can thus only have biased our results towards the null. Thus, our estimates for the risk reduction associated with double-category infection are likely conservative.
Co-infection issues in HPV epidemiology are usually seen mainly as a complication factor in the context of attributing the proportion of cancer-causing HPV types. Our main interest was to investigate natural history aspects associated with the state of co-infections in prediagnostic cervical smears and to potentially inform future HPV-based cancer prevention practices of the risk stratification that may be relevant even within the known risk group of HRHPV-infected women. It should be noted that a comparison with the HPV typing of tumors was performed in the seroepidemiological context, and, indeed, the antagonistic interaction effect seen between HPV6 and HPV16 remained robust when restricting to only HPV16 DNA–containing cancers (9). With regard to the longer time to diagnosis, it has been suggested that past infection with HPV6 could shorten the duration of persistence and/or interfere with the oncogenic action of HPV16 (9). If this is correct, it could mean that women with co-infection might constitute a subgroup where there is more time to diagnose infection and act before progression takes place.
In conclusion, antagonistic interaction effects between high-risk and low-risk types of HPV have now been shown in several independent cohorts, using both differing biological material and timing in relation to diagnosis of invasive cancer. We propose that this rare yet important event interferes with the rate of progression to invasive disease.
Funding
The project described was supported by award number 1R01CA093378-01A1 and 5R01CA111720-03 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Supplementary Material
We would like to thank Ms. Ninoa Malki for database management; Dr. Anthony Gunnell for data collection; Dr. Anders Lindgren for histological review; Ms. Carina Eklund, Ms. Christina Cavala, Ms. Aline Marshall, and Ms. Kia Sjölin for laboratory analyses.
The funders had no role in study design, data collection or analysis, preparation of the manuscript, or decision to publish the manuscript.
Drs. Ploner, Eloranta, Palmgren, Ylitalo Helm, Sparén, and Adami have nothing to disclose. Dr. Sundström is the national PI for a register-based follow-up study on human papillomavirus (HPV) vaccines in Sweden, funded by MSD. Dr. Dillner has previously received grants for studies on HPV vaccines from Merck/SPMSD. Dr. Arnheim-Dahlström has previously received grants for studies on HPV vaccines from SPMSD, MSD, and GlaxoSmithKline.
References
- 1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 2. Leval A, Herweijer E, Ploner A, et al. Quadrivalent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst. 2013;105(7):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. [DOI] [PubMed] [Google Scholar]
- 4. Blomberg M, Friis S, Munk C, Bautz A, Kjaer SK. Genital warts and risk of cancer: a Danish study of nearly 50 000 patients with genital warts. J Infect Dis. 2012;205(10):1544–1553. [DOI] [PubMed] [Google Scholar]
- 5. Nordenvall C, Chang ET, Adami HO, Ye W. Cancer risk among patients with condylomata acuminata. Int J Cancer. 2006;119(4):888–893. [DOI] [PubMed] [Google Scholar]
- 6. Luostarinen T, af Geijersstam V, Bjorge T, et al. No excess risk of cervical carcinoma among women seropositive for both HPV16 and HPV6/11. Int J Cancer. 1999;80(6):818–822. [DOI] [PubMed] [Google Scholar]
- 7. Silins I, Wang Z, Avall-Lundqvist E, et al. Serological evidence for protection by human papillomavirus (HPV) type 6 infection against HPV type 16 cervical carcinogenesis. J Gen Virol. 1999;80 (Pt 11):2931–2936. [DOI] [PubMed] [Google Scholar]
- 8. Naucler P, Chen HC, Persson K, et al. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case-control study. J Gen Virol. 2007;88(Pt 3):814–822. [DOI] [PubMed] [Google Scholar]
- 9. Arnheim Dahlstrom L, Andersson K, Luostarinen T, et al. Prospective seroepidemiologic study of human papillomavirus and other risk factors in cervical cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2541–2550. [DOI] [PubMed] [Google Scholar]
- 10. Sundström K, Eloranta S, Sparen P, et al. Prospective study of human papillomavirus (HPV) types, HPV persistence, and risk of squamous cell carcinoma of the cervix. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundström K, Ploner A, Arnheim Dahlström L, et al. Prospective study of HPV16 viral load and risk of in situ and invasive squamous cervical cancer. Cancer Epidemiol Biomarkers Prev. 2012;22(1):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chua KL, Hjerpe A. Polymerase chain reaction analysis of human papillomavirus in archival cervical cytologic smears. Anal Quant Cytol Histol. 1995;17(4):221–229. [PubMed] [Google Scholar]
- 13. de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76 (Pt 4):1057–1062. [DOI] [PubMed] [Google Scholar]
- 14. Soderlund-Strand A, Rymark P, Andersson P, Dillner J, Dillner L. Comparison between the Hybrid Capture II test and a PCR-based human papillomavirus detection method for diagnosis and posttreatment follow-up of cervical intraepithelial neoplasia. J Clin Microbiol. 2005;43(7):3260–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soderlund-Strand A, Carlson J, Dillner J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J Clin Microbiol. 2009;47(3):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moberg M, Gustavsson I, Gyllensten U. Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer. J Clin Microbiol. 2003;41(7):3221–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R.: R core development team. 2014. [Google Scholar]
- 18. Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biometrica. 2008;50(3):346–363. [DOI] [PubMed] [Google Scholar]
- 19. The Swedish National Quality Register for Cervical Cancer Prevention. Annual Report for 2013 with data until 2012. In Swedish. Center for Cervical Cancer Prevention. Karolinska University Hospital Huddinge, Stockholm, Sweden: 2013. [Google Scholar]
- 20. Dahlstrom LA, Ylitalo N, Sundstrom K, et al. Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer. 2010;127(8):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunnell AS, Tran TN, Torrang A, et al. Synergy between cigarette smoking and human papillomavirus type 16 in cervical cancer in situ development. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2141–2147. [DOI] [PubMed] [Google Scholar]
- 22. Appleby P, Beral V, Berrington de Gonzalez A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621. [DOI] [PubMed] [Google Scholar]
- 23. Appleby P, Beral V, Berrington de Gonzalez A, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.