Abstract
Oxidative stress leads to alveolar epithelial cell injury and fibroblast–myofibroblast differentiation (FMD), key events in the pathobiology of pulmonary fibrosis (PF). Sirtuin 3 (SIRT3) is a mitochondrial protein deacetylase regulator of antioxidant response and mitochondrial homeostasis. Here, we demonstrate reduced SIRT3 expression in the lungs of old mice compared to young mice, as well as in two murine models of PF. The analysis of the pattern of SIRT3 expression in the lungs of patients with PF revealed low SIRT3 staining within the fibrotic regions. We also demonstrated, using murine models of PF and human lung fibroblasts, that reduced SIRT3 expression in response to transforming growth factor beta 1 (TGFβ1) promotes acetylation (inactivation) of major oxidative stress response regulators, such as SOD2 and isocitrate dehydrogenase 2. Reduction of SIRT3 in human lung fibroblasts promoted FMD. By contrast, overexpression of SIRT3 attenuated TGFβ1-mediated FMD and significantly reduced the levels of SMAD family member 3 (SMAD3). Resveratrol induced SIRT3 expression and ameliorated acetylation changes induced by TGFβ1. We demonstrated that SIRT3-deficient mice are more susceptible to PF compared to control mice, and concomitantly exhibit enhanced SMAD3 expression. Collectively, these data define a SIRT3/TGFβ1 interaction during aging that may play a significant role in the pathobiology of PF.
Keywords: Age-related pathology, Lungs/pulmonary, Mitochondria, Reactive oxygen species, SIRT3
Lung fibrogenesis is the major cause of death in systemic scleroderma (SSc) and idiopathic pulmonary fibrosis (IPF), two diseases having unknown etiology (1,2). About 75000–100000 people in the United States have SSc, most of whom are women between the ages of 30 and 50 years (3). SSc is characterized by multiorgan vasculopathy and fibrosis of the skin and internal organs (4). With regard to IPF, about 100000 people in the United States suffer from this interstitial lung disease, which has incidence and prevalence rates that are increasing with the rate of the aging population (5).
Transforming growth factor beta 1 (TGFβ1) signaling is a key player in fibrogenesis. It promotes reactive oxygen species and myofibroblast differentiation across tissues and is recognized as a critical factor in the pathogenesis of SSc and IPF. However, it is unclear whether or how TGFβ1 influences tissue aging or how biological aging promotes the fibrotic response. New evidence demonstrates that impairment of mitochondrial homeostasis and recycling, mediated in part by TGFβ1, may contribute to age-related lung diseases such as IPF (6). Nevertheless, the cell type–dependent molecular mechanisms that promote fibrogenesis remain unknown.
Sirtuins, NAD+-dependent protein deacetylases, can modulate the oxidative stress response, metabolism, and life span (7–9). Due to the preferential mitochondrial localization and association with extended life span in humans, Sirtuin 3 (SIRT3) emerged in the last few years as a protein of particular interest in aging studies and age-related diseases (10–12). SIRT3 participates in regulating multiple processes through its effects on the acetylation of proteins involved in oxidative stress response, mitochondrial dynamics, and metabolism, which are mechanisms that are altered in PF. For example, SIRT3 promotes deacetylation of forkhead box O3a (FoxO3a) leading to FoxO3a-dependent gene expression (13). SIRT3 also deacetylates several components of the mitochondrial antioxidant response and respiratory chain, including the mitochondrial matrix protein isocitrate dehydrogenase 2 (IDH2), a major source of reduced nicotinamide adenine dinucleotide phosphate (NADPH), as well as superoxide dismutase 2, mitochondrial (SOD2) (14,15). In addition, SIRT3 deacetylates and destabilizes hypoxia-inducible factor-1α, which is involved in the metabolic reprograming of mitochondria (16). Therefore, due to the myriad of roles SIRT3 plays in the regulation of mitochondrial function, metabolism, and dynamics, it is expected that changes in SIRT3 expression within the aging lung will result in an altered, and possibly compromised, response to injury.
Here, we demonstrate that there is, indeed, a deficiency in SIRT3 within the murine aging lung and even more so in the fibrotic lung and that absence of SIRT3 promotes the fibrotic response mediated by TGFβ1. Specifically, our studies revealed that lung exposed to bleomycin and normal human lung fibroblasts (NHLF) treated with TGFβ1 showed reduced SIRT3 expression, which prevented SOD2 and IDH2 deacetylation and activation resulting in an inefficient oxidative stress response, favorable to myofibroblast differentiation. By contrast, overexpression of SIRT3 reduced the effects of TGFβ1 on myofibroblast differentiation. Importantly, we found that SIRT3 deficiency promotes PF, concomitant with an increase in SMAD family member 3 (SMAD3) expression. In conclusion, we propose that reduced SIRT3 activity, whether through TGFβ1 signaling and/or age-related loss of expression, negatively impacts the mitochondrial oxidative stress response, promotes a myofibroblast phenotype, and re-enforces TGFβ1 signaling, probably contributing to the perpetuation of a fibrotic lung in the elderly adults.
Materials and Methods
Mice and Tissue Samples
All mice were obtained from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 male mice, aged 6–8 weeks, were treated with 3×108 plaque-forming units of replication-deficient adenovirus encoding either green fluorescent protein (GFP; control group, AdGFP; n = 5) or active TGFβ1 (AdTGFβ1; n = 5) administered by oropharyngeal aspiration. For age-dependent studies, C57BL/6 male mice, aged young (2 months old, n = 5 per treatment) and old (22 months old, n = 5 per treatment), received 2U/kg bleomycin (Teva Parenteral Medicines, Irvine, CA) or vehicle only (phosphate-buffered saline) for controls by oropharyngeal aspiration. 129S1/SvImJ (wild-type [WT] control) and 129-Sirt3tm1.1Fwa/J (knockout) male mice (4 months old) received 2U/kg bleomycin (n = 7 per bleomycin treatment per knockout group, n = 5 per WT group) or phosphate-buffered saline (n = 7 per phosphate-buffered saline treatment per knockout group, n = 5 per WT group) as controls by oropharyngeal aspiration. Animal procedures and cell culture reagents are described in Supplementary Material. Tissue microarray from patients with scleroderma and PF (SSc-PF; n = 16) or IPF (n = 13), and normal (control) donor lung tissues (n = 10) as well as lung fibroblasts isolated from SSc patients (n = 4) and controls (n = 4) were provided by the National Scleroderma Core Center at the Medical University of South Carolina. Isolated fibroblasts were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Mediatech, Manassas, VA), supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA) and Penicillin–Streptomycin (Gibco).
Transfections, mitochondrial isolation, Western blot (WB), RNA isolation and real-time quantitative polymerase chain reaction (qRT-PCR), histology and immunostaining, and gene expression array analysis are described in detail in Supplementary Material.
Statistical Analysis
All data are expressed as mean values ± SEM. Comparisons between two groups were made using the unpaired, two-tailed Student’s t test. Analysis of variance and Bonferroni’s multiple comparison tests were used for multiple groups. Statistical significance was assigned at a value of p < .05. All experiments were repeated at least twice.
Results
Downregulation of SIRT3 Expression in Lung Aging and PF
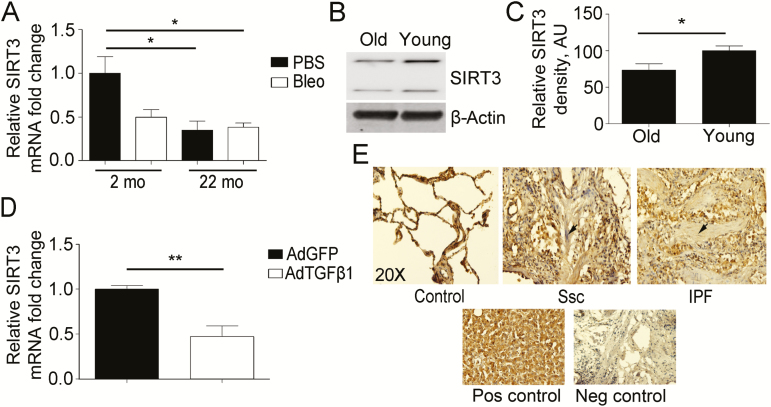
Mice lacking SIRT3 develop several diseases related to aging at an accelerated pace (17). Although it is known that the molecular mechanisms of lung aging and fibrosis are related, few studies have been undertaken to show the role of SIRT3 in age-related lung diseases, such as IPF that has an increasing incidence with age (5). Here, we aimed to determine the changes in SIRT3 expression during lung aging and PF. Young (2-month-old, n = 5) and old (22-month-old, n = 5) mice were exposed to vehicle or bleomycin by oropharyngeal aspiration to induce lung injury leading to the development of fibrosis. qRT-PCR revealed that the group of old mice has a significant reduction in SIRT3 expression compared to young mice (Figure 1A). WB analysis of lung fibroblasts isolated from young and old mice confirmed a decline in SIRT3 expression (Figure 1B and C). Bleomycin exposure resulted in reduced levels of SIRT3 transcripts in young mice, with no additional reduction by bleomycin in SIRT3 transcript levels detected in 22-month-old mice (Figure 1A).
Figure 1.
Downregulation of Sirtuin 3 (SIRT3) in aging and pulmonary fibrosis (PF). (A) Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis for SIRT3 mRNA expression in aging (22-month-old) and young (2-month-old) mice at 14 days after oropharyngeal aspiration of phosphate-buffered saline (PBS) or bleomycin (Bleo) (n = 5 per treatment). (B) Representative Western blot (WB) for SIRT3 expression in mouse lung fibroblasts isolated from old (22-month-old) and young (2-month-old) mice. β-Actin used as a loading control. (C) Densitometry analysis of WB for SIRT3 expression is shown in (B). (D) qRT-PCR analysis for SIRT3 mRNA expression in young mice at 14-day post-oropharyngeal aspiration of control Adenovirus-GFP (AdGFP) or Adenovirus-TGFβ1 (AdTGFβ1; n = 5 per treatment). (E) Immunohistochemistry in lung tissue samples from control (n = 10), systemic scleroderma (SSc; n = 16), and idiopathic PF (IPF; n = 13) patients show a differential expression pattern of SIRT3. Positive cells appear brown (diaminobenzidene stain). Nuclei counterstained with hematoxylin appear blue. The arrow shows a fibrotic lesion. Liver tissue was used as a positive control. Immunoglobulin G alone was used as a negative control. *p < .05, **p < .01. AU, arbitrary units.
Next, we evaluated the expression of SIRT3 in another model of PF that is TGFβ1 dependent. C57BL/6 young mice were exposed to a replication-deficient adenovirus encoding active TGFβ1 (AdTGFβ1). The results of qRT-PCR analyses showed a significant reduction in SIRT3 expression in the lung at 14-day postinfection compared to GFP (AdGFP) control infection (Figure 1D). The downregulation of SIRT3 in aging and the two murine models of PF suggest that SIRT3 plays a role in lung fibrogenesis during aging. Further, the expression pattern of SIRT3 in lung tissues was investigated by immunohistochemistry staining using 10 control, 16 SSc, and 13 IPF specimens. The results showed an absence of SIRT3 staining within fibrotic areas compared to adjacent areas within the same tissue (Figure 1E).
At the cellular level, we chose to determine if TGFβ1 promotes SIRT3 transcriptional changes using primary human lung fibroblasts extracted from SSc patients. We chose SSc lung fibroblasts because they have been shown to express high levels of TGFβ receptors and to be highly responsive to the effects of TGFβ1 in culture (18). Our results showed that lung fibroblasts from four SSc patients exhibited decreased levels of SIRT3 transcripts upon treatment with TGFβ1 (Supplementary Figure 1).
TGFβ1 Regulates SIRT3 Expression During Fibrogenesis, Promoting the Acetylation of SOD2 and IDH2
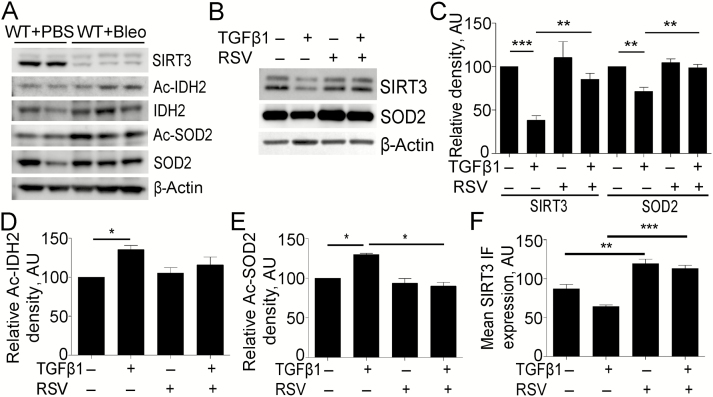
We investigated if the effects of bleomycin-induced lung injury on SIRT3 expression are concomitant with changes in the acetylation of two major SIRT3 substrates, SOD2 (MnSOD) and IDH2. WB analysis confirmed a significant reduction in SIRT3 protein levels in the lungs of mice exposed to bleomycin compared to control. The changes in SIRT3 were concomitant with an increase in acetylation of IDH2 and SOD2, components of the mitochondrial antioxidant machinery (Figure 2A).
Figure 2.
Transforming growth factor beta 1 (TGFβ1) regulates Sirtuin 3 (SIRT3) concomitant with the acetylation of major oxidative stress response regulators. (A) Representative Western blot (WB) for SIRT3, acetylated isocitrate dehydrogenase 2 (Ac-IDH2), IDH2, acetylated superoxide dismutase 2, mitochondrial (SOD2) (Ac-SOD2), and SOD2 expression from wild-type (WT) mice exposed to bleomycin (Bleo) or phosphate-buffered saline (PBS) vehicle, at 14-day postexposure. β-Actin was used as a loading control. (B) Representative WB for SIRT3 and SOD2 expression from normal human lung fibroblasts (NHLF) treated or untreated, with TGFβ1 and/or resveratrol. (C) Densitometry analysis of WB for SIRT3 and SOD2 expression is shown in (B). (D) Densitometry analysis of WB for acetylated IDH2 (K413) expression from NHLF treated or untreated, with TGFβ1 and/or resveratrol shown in Supplementary Figure 2C. (E) Densitometry analysis of WB for acetylated SOD2 (K68) expression from NHLF treated or untreated, with TGFβ1 and/or resveratrol shown in Supplementary Figure 2D. (F) Quantification of representative immunofluorescence images for SIRT3 in NHLF treated, with TGFβ1 and/or resveratrol at 24 hours, shown in Supplementary Figure 2E. *p < .05, **p < .01, ***p < .005. AU, arbitrary units; RSV, resveratrol.
TGFβ1 promotes differentiation of human lung fibroblasts into myofibroblasts; cells which are responsible for the majority of collagen deposition and stiffness during PF. In consequence, elucidation of fibroblast-specific TGFβ signaling can provide insight into potential pathogenesis of lung fibrosis. Thus, we endeavored to determine whether TGFβ1 regulates SIRT3 expression in human lung fibroblasts. WB analysis showed that TGFβ1 reduced the expression of SIRT3 and SOD2 in NHLF at 24 hours, which was restored to normal levels in the presence of resveratrol, a polyphenol compound that inhibits myofibroblast differentiation and transcriptionally induces SIRT3 expression (19–21) (Figure 2B and C, Supplementary Figure 2A). Analysis of qRT-PCR for NHLF treated with increasing doses of TGFβ1 for 24 hours demonstrated a decline in SIRT3 and SOD2 transcript levels in a dose-dependent manner (Supplementary Figure 2B).
The acetylation of IDH2 and SOD2 during fibroblast–myofibroblast differentiation (FMD) was interrogated to provide a better understanding of SIRT3-mediated deacetylase activity and the type of oxidative stress response during fibrogenesis. We observed an increase in the acetylated form of IDH2 (K413) in NHLF at 24 hours with TGFβ1 treatment compared to vehicle (Figure 2D, Supplementary Figure 2C). The WB analysis also revealed an increase in acetylated SOD2 (K68) in mitochondria, suggesting that TGFβ1-induced repression of SIRT3 results in less SIRT3-dependent deacetylase activity and, in consequence, a deficient antioxidant response mediated by IDH2 and SOD2 (Figure 2E, Supplementary Figure 2D). Immunofluorescence and subsequent quantification confirmed the reduction in SIRT3 levels post-TGFβ1 treatment (Figure 2F, Supplementary Figure 2E). Our findings correlate with the increase in reactive oxygen species during FMD, previously reported (6).
Downregulation of SIRT3 Promotes Myofibroblast Differentiation Mediated by TGFβ1
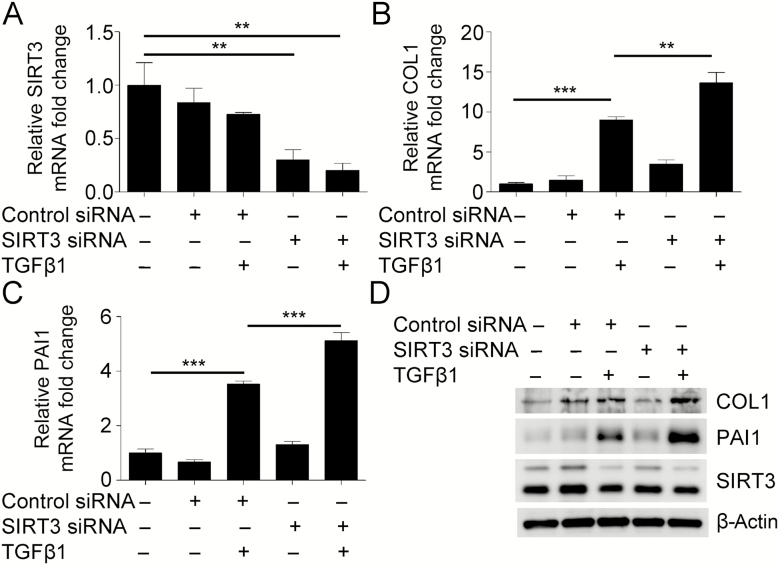
To determine the biological relevance of the repression of SIRT3 during FMD mediated by TGFβ1, we transfected NHLF with siRNA targeting SIRT3 and evaluated the expression of fibrotic markers. qRT-PCR analyses demonstrated that repression of SIRT3 promoted expression of collagen type I and plasminogen activator inhibitor type 1 (PAI1), which was significant compared to the nontargeting siRNA control after TGFβ1 treatment (Figure 3A–C). WB analyses confirmed the induction of collagen type I and PAI1 expression at the protein level (Figure 3D, Supplementary Figure 3). These results indicate that inhibition of SIRT3 may promote TGFβ1-dependent myofibroblast differentiation.
Figure 3.
Downregulation of Sirtuin 3 (SIRT3) promotes myofibroblast differentiation mediated by transforming growth factor beta 1 (TGFβ1). (A–C) Real-time quantitative reverse-transcriptase polymerase chain reaction analysis from normal human lung fibroblasts (NHLF), transfected with siRNA targeting SIRT3, treated with or without TGFβ1, to evaluate transcriptional changes in SIRT3 and fibrotic markers collagen type I (COL1) and plasminogen activator inhibitor-1 (PAI1). (D) Representative Western blot for COL1, PAI1, and SIRT3 expression in NHLF deficient in SIRT3. β-Actin was used as a loading control. **p < .01, ***p < .005.
Overexpression of SIRT3 Decreases Myofibroblast Differentiation Potential Mediated by TGFβ1
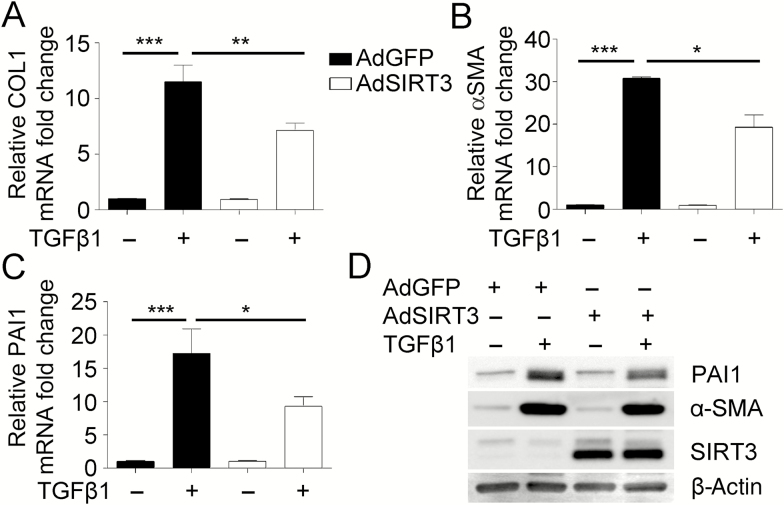
Because we demonstrated that downregulation of SIRT3 promotes myofibroblast differentiation mediated by TGFβ1, we further interrogated the possible inhibitory effect of SIRT3 on TGFβ1-induced myofibroblast differentiation. For this experiment, expression of fibrotic markers was analyzed in NHLF transfected with an adenovirus encoding either GFP (AdGFP, control) or SIRT3 (AdSIRT3) in the presence or absence of TGFβ1 and cofactor NAD+. Analyses by qRT-PCR showed a significant reduction in collagen type I, alpha-smooth muscle actin, and PAI1 expression post-TGFβ1 treatment when SIRT3 was overexpressed (AdSIRT3) compared to control treatment (AdGFP) (Figure 4A–C, Supplementary Figure 4A). We confirmed the statistically significant decrease in the expression of fibrotic markers alpha-smooth muscle actin and PAI1 by WB as presented by densitometry analysis (Figure 4D, Supplementary Figure 4B–E). Taken together, these data support the role of SIRT3 in modulating TGFβ1-mediated FMD.
Figure 4.
Overexpression of Sirtuin 3 (SIRT3) decreases myofibroblast differentiation potential mediated by transforming growth factor beta 1 (TGFβ1). (A–C) Real-time quantitative reverse-transcriptase polymerase chain reaction analysis from normal human lung fibroblasts (NHLF), transfected with adenovirus overexpressing SIRT3, treated with or without TGFβ1, to evaluate transcriptional changes in fibrotic markers collagen type I (COL1), α-smooth muscle actin (α-SMA), and plasminogen activator inhibitor-1 (PAI1). (D) Representative Western blot for PAI1, α-SMA, and SIRT3 expression in NHLF overexpressing SIRT3. β-Actin was used as a loading control. *p < .05, **p < .01, ***p < .005.
SIRT3-Deficient Mice Are Susceptible to Bleomycin-Induced PF
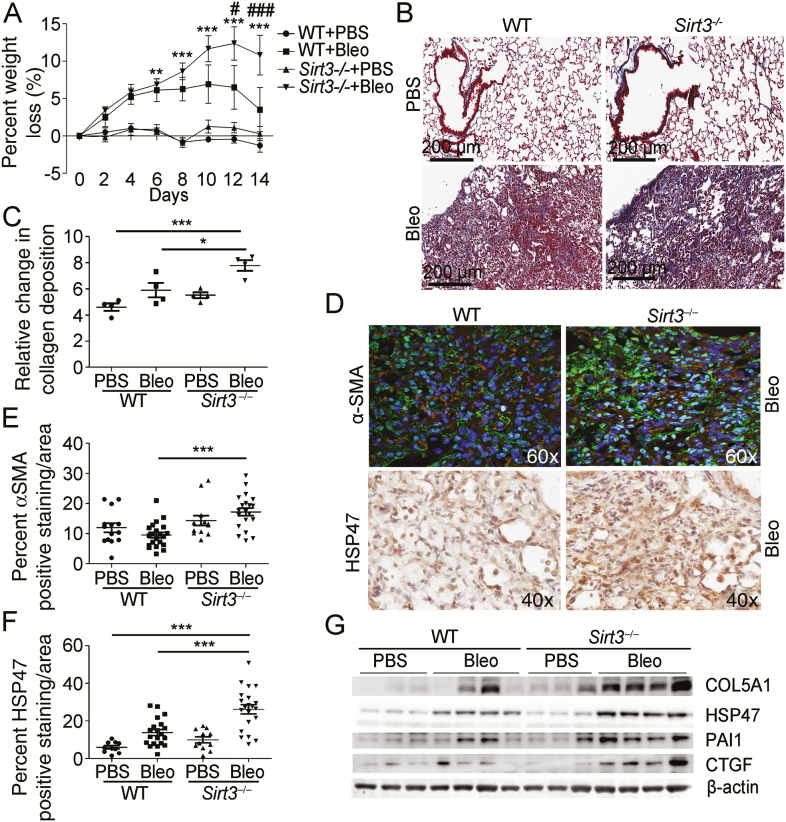
To examine further the role for SIRT3 in PF, we subjected SIRT3-deficient (Sirt3−/−) and WT control mice to lung injury induced by oropharyngeal aspiration of bleomycin (2U/kg weight). At 14-day post-bleomycin exposure lungs were analyzed for markers of fibrosis. After bleomycin exposure, we observed SIRT3-deficient mice had increased weight loss compared to WT mice and failed to recover their loss by day 14 (Figure 5A). Histological analysis of WT and Sirt3−/− lungs and quantification of Masson’s trichrome staining showed increased collagen deposition in Sirt3−/− mice compared to control after bleomycin exposure (Figure 5B and C). Similarly, quantitative confocal imaging analysis for alpha-smooth muscle actin and immunohistochemistry analysis for fibrotic marker heat shock protein 47 revealed a significant increase in the expression of these two fibrotic markers in Sirt3−/− mice compared to WT mice (Figure 5D–F). Assessment of the severity of fibrotic changes by the Ashcroft scale (22) also demonstrated an increased fibrotic burden in SIRT3-deficient mice as compared to control after bleomycin exposure (Supplementary Figure 5A). qRT-PCR analysis confirmed the lack of SIRT3 transcripts in knockout mice as well as an increase in transcript levels for the fibrotic marker PAI1 subsequent to injury (Supplementary Figure 5B and C). Similarly, the expression of fibrotic markers was confirmed by WB analysis. The results revealed that expression of collagen type 5 alpha chain 1, heat shock protein 47, PAI1, and connective tissue growth factor were increased in the Sirt3−/− mice as compared to controls following bleomycin-induced injury (Figure 5G, Supplementary Figure 5D–G). Taken together, these data suggest that SIRT3 deficiency may contribute to PF.
Figure 5.
Sirtuin 3 (SIRT3)-deficient mice are susceptible to bleomycin (Bleo)-induced pulmonary fibrosis. (A) The graph depicts weight loss as a percentage in SIRT3-deficient (Sirt3−/−; n = 7 per treatment) and wild-type (WT; n = 5 per treatment) control mice after Bleo or phosphate-buffered saline (PBS) vehicle. (B and C) Representative images and quantification of Masson’s trichrome staining to evaluate collagen deposition in SIRT3-deficient and WT mice after Bleo or PBS vehicle, at 14-day postexposure. Positive collagen deposition appears blue. (D) Representative images of immunofluorescence (IF) staining for α-smooth muscle actin (α-SMA) and immunohistochemistry (IHC) staining for heat shock protein 47 (HSP47) fibrotic marker expression in SIRT3-deficient and WT control mice after Bleo, 14-day postexposure. In IF images, positive cells appear green with nuclei counterstained with 4′,6-diamidino-2-phenylindole appearing blue. In IHC images, positive cells appear brown (diaminobenzidene stain) with nuclei counterstained with hematoxylin appearing blue. (E) Quantification of IF staining for α-SMA fibrotic marker expression in SIRT3-deficient and WT control mice after Bleo or PBS vehicle, 14-day postexposure. (F) Quantification of IHC staining for HSP47 fibrotic marker expression in SIRT3-deficient and WT control mice after Bleo or PBS vehicle, 14-day postexposure. (G) Representative Western blot for fibrotic markers collagen type 5 alpha chain 1 (COL5A1), HSP47, plasminogen activator inhibitor-1 (PAI1), and connective tissue growth factor (CTGF) expression in lung tissue extracted from SIRT3-deficient mice. β-Actin was employed as a loading control. *p < .05, **p < .01, ***p < .005. In (A), **p < .01, ***p < .005 comparing Sirt3−/− Bleo-exposed group to WT PBS control group, #p < .05, ###p < .005 comparing Sirt3−/− bleomycin-exposed group to WT bleomycin-exposed group.
Oxidative stress has been implicated in the stimulation of mucin synthesis in airways (23–25). Bleomycin-induced lung injury increases the number of airway secretory cells and their mucin production, whereas antioxidants reduce the mucus hypersecretion in the bleomycin rat model (26). As SIRT3 plays a major antioxidant role, alcian blue-PAS staining was performed to evaluate mucin expression in lung tissue sections. The results revealed that Sirt3−/− mice exposed to bleomycin have an increase in the amount of goblet cells with acid mucin staining (deep blue) and mucous cells with neutral mucin stain (magenta) compared to WT controls (Supplementary Figure 6A and B). For cells that contain a mixture of mucin types, an intermediate color between blue and magenta is present. Additionally, Sirt3−/− mice displayed high levels of interleukin-6 mRNA transcripts, suggesting an escalation of the inflammatory response after lung injury (Supplementary Figure 6C). Hematoxylin and eosin stain confirmed an increase in the inflammatory infiltrates in lungs from the Sirt3−/− mice exposed to bleomycin-induced lung injury compared to WT (Supplementary Figure 6D). The results indicate that SIRT3-deficient mice are characterized by increased mucin production and interstitial inflammation after bleomycin lung injury.
A SIRT3–TGFβ Crosstalk During Fibrogenesis
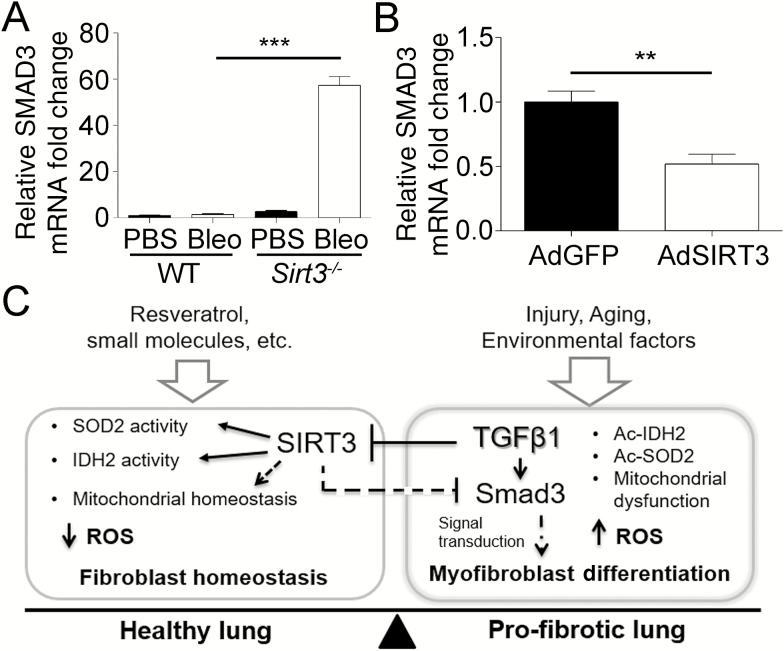
We next aimed to explore the molecular aspects of the relationship between SIRT3 and the TGFβ1 signaling pathway. qRT-PCR analysis in SIRT3-deficient (Sirt3−/−) mice exposed to bleomycin indicated a significant increase in the transcript levels of SMAD3 compared to WT control mice (Figure 6A).
Figure 6.
Interaction between Sirtuin 3 (SIRT3) and transforming growth factor beta 1 (TGFβ1) during fibrogenesis. (A) Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis for SMAD family member 3 (SMAD3) expression in SIRT3-deficient (Sirt3−/−; n = 7 per treatment) and wild-type (WT; n = 5 per treatment) control mice after bleomycin (Bleo) or phosphate-buffered saline vehicle, at 14-day postexposure. (B) qRT-PCR confirmation for SMAD3 expression in normal human lung fibroblasts (NHLF) overexpressing SIRT3. **p < .01, ***p < .005. (C) Model for the role of SIRT3/TGFβ crosstalk in pulmonary fibrosis. The proposed model describes the role of SIRT3 in the regulation of SMAD3 and the antioxidant response during fibrogenesis. Aging, lung injury, TGFβ1 expression, genetic and environmental factors that contribute to reduction of SIRT3 favor the profibrotic effects of TGFβ1. By contrast, induction of SIRT3, by resveratrol, small molecules, or genetic approaches, enhances antifibrotic effects in the healthy lung mediated through downregulation of TGFβ1 signal transduction and an enhanced antioxidant response. Taken together, we propose that SIRT3 is critical to modulating the fibrotic response to lung injury in the elderly adults.
To determine if SIRT3 plays a direct role in the TGFβ signaling pathway, we screened RNA transcripts from human lung fibroblasts exposed to adenovirus overexpressing SIRT3 (AdSIRT3) or GFP control (AdGFP) using a gene expression array for the TGFβ signaling pathway. The results indicate that transcription of multiple signaling factors and downstream targets of the TGFβ pathway were significantly reduced in human lung fibroblasts overexpressing SIRT3 compared to GFP control (Supplementary Figure 7). The screen identified SMAD3 as a transcript deregulated by induction of SIRT3. The results were confirmed by qRT-PCR using independent primers (Figure 6B). Conversely, in silico analysis, using Genomatix software suite (www.genomatix.de/matinspector.html, Genomatix GmbH, Munich, Germany) confirmed a SMAD3 binding site in the SIRT3 promoter region and supports previous studies showing a moderated binding of SMAD3 on the SIRT3 promoter by Chip on Chip (27). Taken together, our data suggest that SIRT3 deficiency may contribute to the development of PF by promotion of a deficient antioxidant response as well as promotion of the TGFβ pathway, contributing to the perpetuation of the disease and the accelerated aging phenotype of the fibrotic lung (proposed model Figure 6C).
Discussion
The aging lung is characterized by increased TGFβ1 signaling and collagen deposition (28,29). Accelerated biological aging as well as increases in TGFβ1 activity are common features of PF in IPF and SSc (30,31). Furthermore, TGFβ1 polymorphisms have been associated with human longevity (32,33). Nevertheless, it is unclear if, and how, TGFβ1 influences biological aging. In this study, we propose that SIRT3 constitutes a new link between TGFβ1 activity and aging in the progression of PF. Our study indicates that TGFβ1 reduces SIRT3 expression leading to an inefficient antioxidant response and promotion of myofibroblast differentiation. Our studies support the role of SIRT3 in the regulation of SMAD3 and the antioxidant response, limiting the effects of TGFβ1 during aging and fibrogenesis. The SIRT3/TGFβ1 crosstalk could contribute to the accelerated aging phenotype that characterizes the progression of PF.
Mitochondria play a central role in oxidative energy metabolism and age-related diseases. SIRT3, an NAD+-dependent protein deacetylase with preferential localization to the mitochondria, has previously been associated with life span (34). Mice lacking SIRT3 (Sirt3−/−) develop several diseases associated with aging at an accelerated pace. Here, we demonstrated that SIRT3 expression declines in the aging lung, murine models of PF, and fibrotic areas within the lungs of people afflicted with IPF and SSc. Our results support recent studies indicating that induction of SIRT3 prevents tissue remodeling in aging mice, albeit these prior studies did not address the lung (35,36). In the future, it will be important to determine if induction of SIRT3 is beneficial in profibrotic fibroblasts obtained from patients.
We demonstrated that deficiency in SIRT3, a gene in which single nucleotide polymorphisms have been shown to be involved in human longevity (37), promotes myofibroblast differentiation, whereas overexpression of SIRT3 reduces SMAD3 transcriptional activation leading to reduced TGFβ1 signaling in human lung fibroblasts. Importantly, lack of SMAD3 confers resistance to TGFβ, injury, or inflammation mediated renal and lung fibrosis in animal models (38–40). Our studies are consistent with recent studies showing that activation of SIRT3 blocked the TGFβ pathway in heart fibroblasts. Those studies demonstrated that SIRT3 inhibits SMAD3 signaling by deacetylating and activating glycogen synthase kinase 3β in a NAD+-dependent manner (20,41). It is possible that the crosstalk between TGFβ1/SIRT3 happens at translational and post-translational levels.
Several studies previously reported that myofibroblast differentiation, driven by TGFβ1, is characterized by an increase in reactive oxygen species due in part to unbalanced mitochondria homeostasis, deficient recycling, and deficient oxidative stress responses (6,42). Other studies have shown that increased cellular superoxide levels are characteristic of stressed SIRT3-deficient mouse embryonic fibroblasts (43). Thus, the ability of SIRT3 to protect cells from oxidative stress has been shown to be dependent on SOD2- and IDH2-induced activity (14,44–46). Importantly, our studies indicate that acetylation (deactivation) of SOD2 and IDH2 are SIRT3 dependent and a characteristic of FMD and bleomycin-induced PF. We demonstrated that resveratrol, a polyphenol known to inhibit PF in murine models, promotes SIRT3 expression and SOD2 and IDH2 deacetylation, thereby promoting cellular homeostasis and inhibition of myofibroblast differentiation (6,20). Interestingly, deficiency in SOD2 expression has been associated with premature aging (47,48). Thus, it is plausible that the deficient SIRT3-dependent antioxidant activity could contribute to accelerated biologic aging in PF (17,49).
SIRT3 has been shown to deacetylate and activate FoxO3a within mitochondria as well as increase FoxO3a-dependent gene expression (13). FoxO3a modulates mitochondrial clearance of defective mitochondria through transcriptional regulation of autophagy-related genes. We previously demonstrated that the aging and fibrotic lung is characterized by a deficient autophagy, due in part to low FoxO3a activity in lung fibroblasts and the fibrotic lung of IPF patients (50). Further studies are needed to determine the role of TGFB1/SIRT3/FoxO3a axis as a major regulator of autophagy and mitochondria dynamics in PF.
This study demonstrated that SIRT3 is a limiting factor in the fibrotic response and that reduced expression of SIRT3 promotes TGFβ1-mediated fibrosis. In the future, the effects of SIRT3 on resolution of established fibrosis need to be evaluated. Our cellular studies were fibroblast centric. Nevertheless, we demonstrated that the response to bleomycin in SIRT3-deficient mice is also characterized by increased mucin production in goblet cells and increased inflammation, supporting the need to further investigate the role of SIRT3 in a cell type–dependent manner.
The abundance of acetylated lysine residues in the mitochondria increases significantly with age in muscle and correlates with physical decline (12). The future dissection of the role of mitochondrial post-translational modifications in mitochondrial dynamics and metabolism during lung aging needs to be undertaken. Such studies could provide a pool of biomarkers and new therapeutic targets for PF. We expect that small molecules, as specific SIRT3 activators, will be developed soon as therapeutic approaches to promote inhibition and/or resolution of lung fibrosis.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institute of General Medical Sciences at the National Institutes of Health (grant number P20 GM103629-04 to C.G.S.); ATS/Scleroderma Foundation (grant number 552114G1 to C.G.S.); and the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (grant numbers P30 AR061271, K24 AR060297 to C.F.-B.).
Conflict of Interest
No conflicts of interest, financial or otherwise, are disclosed by the authors.
Author Contributions
All authors were involved in the revision of the manuscript. M.L.S. performed experiments, analyzed data, partially wrote the manuscript, and prepared the figures; R.G. performed immunofluorescence and histopathology analysis, and prepared figures; C.F.-B. provided tissue array samples from IPF, scleroderma and control patients, lung fibroblasts and contributed to the editing and revision of the manuscript; J.A.L. contributed to discussion of results and editions of the manuscript; and C.G.S. conceived the original study, analyzed the data, oversaw the project, and wrote the manuscript.
Supplementary Material
References
- 1. Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi:10.1073/pnas.83.12.4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawakami T, Ihn H, Xu W, Smith E, LeRoy C, Trojanowska M. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J Invest Dermatol. 1998;110:47–51. doi:10.1046/j.1523-1747.1998.00073.x [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8:42–54. doi:10.1038/nrrheum.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814–824. doi:10.1378/chest.12-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. doi:10.1183/13993003.01653-2015 [DOI] [PubMed] [Google Scholar]
- 6. Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell. 2015;14:774–783. doi:10.1111/acel.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao Y, Yang Y, Zhu WG. Sirtuins: nodes connecting aging, metabolism and tumorigenesis. Curr Pharm Des. 2014;20:1614–1624. doi:10.2174/13816128113199990513 [DOI] [PubMed] [Google Scholar]
- 8. Chakraborty C, Doss CG. Sirtuins family—recent development as a drug target for aging, metabolism, and age related diseases. Curr Drug Targets. 2013;14:666–675. doi:10.2174/1389450111314060008 [DOI] [PubMed] [Google Scholar]
- 9. Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi:10.1101/sqb.2011.76.010629 [DOI] [PubMed] [Google Scholar]
- 10. Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. doi:10.3389/fnagi.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sadoshima J. Sirt3 targets mPTP and prevents aging in the heart. Aging (Albany NY). 2011;3:12–13. doi:10.18632/aging.100266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hebert SL, Marquet-de Rougé P, Lanza IR, et al. Mitochondrial aging and physical decline: insights from three generations of women. J Gerontol A Biol Sci Med Sci. 2015;70:1409–1417. doi:10.1093/gerona/glv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs KM, Pennington JD, Bisht KS, et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4:291–299. doi:10.7150/ijbs.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi:10.1074/jbc.M112.355206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Park SH, Ozden O, et al. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic Biol Med. 2012;53:828–833. doi:10.1016/j.freeradbiomed.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schumacker PT. SIRT3 controls cancer metabolic reprogramming by regulating ROS and HIF. Cancer Cell. 2011;19:299–300. doi:10.1016/j.ccr.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab. 2015;26:486–492. doi:10.1016/j.tem.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamane K, Ihn H, Kubo M, Tamaki K. Increased transcriptional activities of transforming growth factor beta receptors in scleroderma fibroblasts. Arthritis Rheum. 2002;46:2421–2428. doi:10.1002/art.10477 [DOI] [PubMed] [Google Scholar]
- 19. Zhou X, Chen M, Zeng X, et al. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5:e1576. doi:10.1038/cddis.2014.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen T, Li J, Liu J, et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am J Physiol Heart Circ Physiol. 2015;308:H424–434. doi:10.1152/ajpheart.00454.2014 [DOI] [PubMed] [Google Scholar]
- 21. Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288:H1131–H1138. doi:10.1152/ajpheart.00763.2004 [DOI] [PubMed] [Google Scholar]
- 22. Hübner RH, Gitter W, El Mokhtari NE, et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44:507–511, 514. doi:10.2144/000112729 [DOI] [PubMed] [Google Scholar]
- 23. Shim JJ, Dabbagh K, Ueki IF, et al. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol. 2001;280:L134–L140. [DOI] [PubMed] [Google Scholar]
- 24. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi:10.4049/jimmunol.164.3.1546 [DOI] [PubMed] [Google Scholar]
- 25. Lou YP, Takeyama K, Grattan KM, et al. Platelet-activating factor induces goblet cell hyperplasia and mucin gene expression in airways. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1927–1934. doi:10.1164/ajrccm.157.6.9709113 [DOI] [PubMed] [Google Scholar]
- 26. Mata M, Ruíz A, Cerdá M, et al. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J. 2003;22:900–905. doi:10.1183/09031936.03.00018003 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Handley D, Kaplan T, et al. High throughput determination of TGFβ1/SMAD3 targets in A549 lung epithelial cells. PLoS One. 2011;6:e20319. doi:10.1371/journal.pone.0020319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calabresi C, Arosio B, Galimberti L, et al. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol. 2007;42:1003–1011. doi:10.1016/j.exger.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 29. Sueblinvong V, Neujahr DC, Mills ST, et al. Predisposition for disrepair in the aged lung. Am J Med Sci. 2012;344:41–51. doi:10.1097/MAJ.0b013e318234c132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res. 2013;162:156–173. doi:10.1016/j.trsl.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 31. Homer RJ, Herzog EL. Recent advances in pulmonary fibrosis: implications for scleroderma. Curr Opin Rheumatol. 2010;22:683–689. doi:10.1097/BOR.0b013e32833ddcc9 [DOI] [PubMed] [Google Scholar]
- 32. Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C elegans. Development. 2003;130:1089–1099. doi:10.1242/dev.00330 [DOI] [PubMed] [Google Scholar]
- 33. Carrieri G, Marzi E, Olivieri F, et al. The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians. Aging Cell. 2004;3:443–448. doi:10.1111/j.1474-9728.2004.00129.x [DOI] [PubMed] [Google Scholar]
- 34. Bellizzi D, Dato S, Cavalcante P, et al. Characterization of a bidirectional promoter shared between two human genes related to aging: SIRT3 and PSMD13. Genomics. 2007;89:143–150. doi:10.1016/j.ygeno.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Zhang D, Chen D. SIRT3: striking at the heart of aging. Aging (Albany NY). 2011;3:1–2. doi:10.18632/aging.100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeng H, Vaka VR, He X, Booz GW, Chen JX. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19:1847–1856. doi:10.1111/jcmm.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albani D, Ateri E, Mazzuco S, et al. Modulation of human longevity by SIRT3 single nucleotide polymorphisms in the prospective study “Treviso Longeva (TRELONG)”. Age (Dordr). 2014;36:469–478. doi:10.1007/s11357-013-9559-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–L593. doi:10.1152/ajplung.00151.2001 [DOI] [PubMed] [Google Scholar]
- 39. Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390–5395. doi:10.4049/jimmunol.175.8.5390 [DOI] [PubMed] [Google Scholar]
- 40. Inazaki K, Kanamaru Y, Kojima Y, et al. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int. 2004;66:597–604. doi:10.1111/j.1523-1755.2004.00779.x [DOI] [PubMed] [Google Scholar]
- 41. Sundaresan NR, Bindu S, Pillai VB, et al. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3 beta. Mol Cell Biol. 2015;36:678–692. doi:10.1128/mcb.00586-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi:10.1136/thx.2009.113456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim HS, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi:10.1016/j.ccr.2009.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi:10.1016/j.molcel.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ozden O, Park SH, Kim HS, et al. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging. 2011;3:102–107. doi:10.18632/aging.100291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson ML, Irving BA, Lanza IR, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol A Biol Sci Med Sci. 2015;70:1386–1393. doi:10.1093/gerona/glu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scheurmann J, Treiber N, Weber C, et al. Mice with heterozygous deficiency of manganese superoxide dismutase (SOD2) have a skin immune system with features of “inflamm-aging”. Arch Dermatol Res. 2014;306:143–155. doi:10.1007/s00403-013-1389-7 [DOI] [PubMed] [Google Scholar]
- 48. Weyemi U, Parekh PR, Redon CE, Bonner WM. SOD2 deficiency promotes aging phenotypes in mouse skin. Aging. 2012;4:116–118. doi:10.18632/aging.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luckhardt TR, Thannickal VJ. Systemic sclerosis-associated fibrosis: an accelerated aging phenotype? Curr Opin Rheumatol. 2015;27:571–576. doi:10.1097/bor.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Im J, Hergert P, Nho RS. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am J Physiol Lung Cell Mol Physiol. 2015;309:L552–L561. doi:10.1152/ajplung.00079.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.