Abstract
Background:
Age-related peripheral hearing impairment (HI) is prevalent, treatable, and may be a risk factor for dementia in older adults. In prospective analysis, we quantified the association of HI with incident dementia and with domain-specific cognitive decline in memory, perceptual speed, and processing speed.
Methods:
Data were from the Health, Aging and Body Composition (Health ABC) study, a biracial cohort of well-functioning adults aged 70–79 years. Dementia was defined using a prespecified algorithm incorporating medication use, hospital records, and neurocognitive test scores. A pure-tone average in decibels hearing level (dBHL) was calculated in the better hearing ear using thresholds from 0.5 to 4kHz, and HI was defined as normal hearing (≤25 dBHL), mild (26–40 dBHL), and moderate/severe (>40 dBHL). Associations between HI and incident dementia and between HI and cognitive change were modeled using Cox proportional hazards models and linear mixed models, respectively.
Results:
Three-hundred eighty seven (20%) participants had moderate/severe HI, and 716 (38%) had mild HI. After adjustment for demographic and cardiovascular factors, moderate/severe audiometric HI (vs. normal hearing) was associated with increased risk of incident dementia over 9 years (hazard ratio: 1.55, 95% confidence interval [CI]: 1.10, 2.19). Other than poorer baseline memory performance (difference of −0.24 SDs, 95% CI: −0.44, −0.04), no associations were observed between HI and rates of domain-specific cognitive change during 7 years of follow-up.
Conclusions:
HI is associated with increased risk of developing dementia in older adults. Randomized trials are needed to determine whether treatment of hearing loss could postpone dementia onset in older adults.
Keywords: Cognition, Cognitive aging, Epidemiology, Sensory
Age-related peripheral hearing impairment (HI) may be a risk factor for incident dementia in older adults. A small number of epidemiologic studies support this hypothesis (1–3); however, limitations of these studies include reliance on self-reported hearing loss (vs. objectively measured hearing using audiometry) (3) or lack of adjustment for cardiovascular factors (2). HI may also be a risk factor for cognitive impairment (4,5) and accelerated cognitive decline over time (6,7), although some studies have shown no association (8), and the specificity of this association with different cognitive domains is unknown. Proposed biological mechanisms suggest that the relationship between HI and cognition may be causal (9). Because HI is both highly prevalent (10) and treatable, the question of whether HI is a risk factor for dementia has major public health implications for the possible design of interventions to postpone dementia in older adults.
A previous study in the Health, Aging and Body Composition (Health ABC) study, a biracial cohort of well-functioning adults aged 70–79 years, reported associations between HI and faster decline in global cognition and in executive function over 6 years (7). Here we expand upon that work to test the hypothesis that participants with HI have increased risk of incident dementia over 9 years compared with participants having normal hearing, independent of cardiovascular disease and other risk factors. Additionally, in complementary analysis, we used data from the Health ABC Cognitive Vitality Substudy (CVS) to test that HI is associated with faster rates of 7-year cognitive decline in three domains: memory, perceptual speed, and processing speed.
Methods
Study Population
Health ABC is a prospective cohort study of 3,075 community-dwelling black (42%) and white (58%) older adults aged 70–79 years in 1997–1998 randomly sampled from Medicare enrollees living in Memphis, Tennessee or Pittsburgh, Pennsylvania. Eligibility criteria included no self-reported mobility difficulty (walking a quarter mile and climbing 10 steps without resting) or disability (difficulty performing activities of daily living), no known life-threatening cancers, and no plans to leave the area within 3 years (11).
All Health ABC participants signed written informed consent, and the study was approved by the institutional review boards of both study sites.
HI and dementia: Analytic population
In total, 2,034 participants were dementia-free in 1997–1998 (Year 1, dementia definition published previously (12)) and also underwent audiometric testing in 2001–2002 (Year 5). Of these participants, 145 were missing covariate data, yielding a final analytic sample of 1,889. Compared with participants included in the analysis, excluded participants were older (74.5 vs. 73.9 years), scored lower on the Modified Mini-Mental State (3MS) exam at Year 1 (85.8 vs. 92.7 points) and were more likely to be black (55.3% vs. 33.1%) and to report current smoking (14.6% vs. 7.7%).
HI and cognitive decline: Analytic population
The CVS is a subcohort of 929 participants aged 72–81 years in 1999–2000 (Year 3). By design, half of the CVS participants were exceptionally well functioning (in the sex-, race- and site-specific 85th percentile of the fastest times on the Long Distance Corridor Walk (11) in Year 2) and half were a random sample of remaining participants. Compared with all Health ABC participants, CVS participants were more likely to be women (54% vs. 50%), of white race (65% vs. 55%), and have less education (30% with <12 years vs. 23%) (13). In all, 789 CVS participants completed neuropsychological testing at Year 3 (1999–2000) and at up to three additional visits during 7 years of follow-up (1999–2006) and also underwent hearing testing in Year 5. Participants with dementia (n = 55) and with missing covariate data (n = 13) were excluded, yielding a final analytic sample of 721. Relative to all CVS participants, participants excluded from analyses were older, more likely to be from the Memphis study site and have lower baseline 3MS (14) scores.
Dementia
Consistent with previous work in HealthABC (12), incident dementia was defined as the use of a prescribed dementia medication (galantamine, rivastigmine, memantine, donepezil, or tacrine), dementia diagnosis from adjudicated hospital records, or a race-stratified 3MS decline more than 1.5 SDs from the baseline mean. Dementia medications and hospital records were assessed annually and the 3MS measured in Years 1, 3, 5, 8, 10, and 11.
CVS Neurocognitive Assessments
The Buschke Selective Reminding Test (SRT) (15) tests verbal memory; an examiner presents in writing and reads aloud a list of 12 unrelated words. Words not recalled are repeated at the start of the next trial, for a total of six trials. The Boxes Test (16) and Digit Copying Test (16) are tests of psychomotor speed in which participants complete as many boxes or copy as many digits as possible in 30 seconds. The Pattern Comparison Test (PCT) (16) and Letter Comparison Test (LCT) (16) are 30-second tests of attention and speed in which participants identify whether a pair of patterns of lines (PCT) or two strings of letters (LCT) is the “same” or “different.”.
To facilitate comparisons of decline across tests, tests were standardized to z-scores. Three a priori domain-specific z-scores were created by averaging test-specific z-scores: verbal memory (Buschke SRT total score), psychomotor speed (Boxes Test and Digit Copying Test), and perceptual speed (PCT and LCT).
Hearing Assessment
Pure-tone air conduction audiometry was conducted in 2001–2002 (Year 5). Air conduction thresholds in each ear were obtained with a portable audiometer (Maico MA40) and supra-aural earphones (TDH 39). Testing was completed in a sound-attenuating booth meeting ANSI standards, and thresholds were measured in decibels hearing level (dBHL). A pure-tone average (PTA) in the better hearing ear was calculated using thresholds for 0.5, 1, 2, and 4kHz (17). For analysis, we categorized PTA using clinically defined cut points (normal hearing: ≤25 dBHL, mild: 26–40 dBHL, and moderate/severe: >40 dBHL). In secondary analyses, PTA was scaled to 10 dB and modeled continuously.
Hearing aid use was self-reported at Year 5.
Other Independent Variables
Demographic information including age (years), race (white or black), sex, and education (less than high school, high school, or postsecondary) was collected in 1997–1998. Self-reported smoking status (never, former, or current) was collected at each study visit. Hypertension was considered present if prevalent at Year 1 (systolic blood pressure ≥140 mmHg, or diastolic blood pressure >90 mmHg, or by participant self-report of a diagnosis by a physician with or without antihypertensive medication use). Physician-diagnosed hypertension (reported by the participant) was used to update hypertension status at each subsequent visit. Diabetes was considered present if prevalent at Year 1, defined as physician-diagnosed diabetes (reported by the participant), use of diabetes drug, or fasting glucose ≥126mg/dL. Physician diagnosis of diabetes (reported by the participant) was used to update diabetes status at each subsequent visit. History of stroke was assessed in Years 2–14 by the question, “Since we last spoke to you about 6 months ago, has a doctor told you that you had a stroke, mini-stroke, or TIA?”
Statistical Analysis
The association between HI and incident dementia was modeled using Cox proportional hazards models. Because most participants were not at risk of a dementia diagnosis until the second administration of the 3MS, the origin was modeled at Year 3 (1999–2000). Participants with prevalent dementia at Year 3 (n = 149) were excluded. The final administration of the 3MS to the entire Health ABC cohort was in 2007–2008; outcomes were administratively censored for this analysis at that time, resulting in up to 9 years of follow-up. Because hearing was not measured until Year 5, in an additional analysis, we restricted incident dementia to after Year 5. The assumption of proportional hazards was verified by assessing correlation between scaled Schoenfeld residuals and transformed survival times (18). Because of nonproportionality, models were stratified by postsecondary education. Ties were handled using the exact partial likelihood method. The assumption of linearity was assessed using smoothed residual plots. Penalized splines were used to allow for smooth, nonlinear effects in regression models.
Persons with HI, particularly those with moderate/severe HI, may benefit from using a hearing aid, although the effect of hearing aid use on incident dementia is unknown. To assess this relationship, analyses were repeated restricted to 387 participants with moderate/severe HI and including self-reported hearing aid use as a covariate.
In the CVS, linear mixed effects models were used to estimate average differences in rates of cognitive change over 7 years (1999–2006) by HI status measured in 2000–2001. An interaction term between HI and time was used to test whether rates of cognitive change differed by HI status. Time on study was the time scale.
Confounding variables were selected based on known associations between the confounding factor, HI and dementia/cognitive decline. Model 1 adjusted for demographic characteristics (age, race, sex, education, and study site). Model 2 adjusted for Model 1 covariates and cardiovascular factors (smoking status, hypertension, and diabetes). Time-varying covariates were measured at Year 3.
Linear mixed effects models were estimated using PROC MIXED in SAS 9.3 and Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13, College Station, Texas, StataCorp LP). Survival analyses were conducted using R version 3.0.2 (September 25, 2013) (19), using the survival package (20) and in Stata 13.
Results
Of the 1,889 participants, 1,103 (58%) had HI and 387 (35%) of participants with HI had moderate/severe hearing loss. Compared with participants without HI, participants with moderate/severe HI were older (mean age 76 years vs. 75 years), scored lower on the 3MS (92 vs. 93 points), and were more likely to be men (65% vs. 37%) and White (78% vs. 58%), have less than high school education (23% vs. 16%), diabetes (22% vs. 16%) and be a current or former smoker (64% vs. 49%; Table1). CVS results were similar (Table 1).
Table 1.
Characteristics of Analytic Samples by HI Status, Health ABC Study
| Health ABC Study, N = 1,889 | CVS Substudy, N = 721 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cohort (N = 1,889) | HI Status | p Value* | Total Cohort (N = 721) | HI Status | p Value* | |||||
| Moderate/Severe (n = 387) | Mild (n = 716) | Normal Hearing (n = 786) | Moderate/Severe (n = 123) | Mild (n = 263) | Normal Hearing (n = 335) | |||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Age (y)† | 75.5 (3) | 76.4 (3) | 75.6 (3) | 74.9 (3) | <.01 | 75.1 (3) | 76.2 (3) | 75.4 (3) | 74.5 (3) | <.01 |
| Female | 996 (53) | 137 (35) | 361 (50) | 498 (63) | <.01 | 372 (52) | 35 (29) | 137 (52) | 200 (60) | <.01 |
| Black race | 625 (33) | 85 (22) | 208 (29) | 332 (42) | <.01 | 314 (44) | 39 (32) | 97 (37) | 178 (53) | <.01 |
| Education | ||||||||||
| <High school | 331 (18) | 89 (23) | 114 (16) | 128 (16) | .03 | 127 (18) | 35 (29) | 40 (15) | 53 (16) | .04 |
| High school | 638 (34) | 123 (32) | 251 (35) | 264 (34) | 239 (33) | 35 (29) | 93 (35) | 111 (33) | ||
| Postsecondary | 920 (49) | 175 (45) | 351 (49) | 394 (50) | 355 (49) | 54 (44) | 130 (49) | 171 (51) | ||
| Smoking | ||||||||||
| Current | 145 (8) | 38 (10) | 46 (6) | 61 (8) | <.01 | 45 (6) | 10 (8) | 13 (5) | 22 (7) | .19 |
| Former | 888 (47) | 208 (54) | 355 (50) | 325 (41) | 324 (45) | 64 (52) | 118 (45) | 139 (42) | ||
| Never | 856 (45) | 141 (36) | 315 (44) | 400 (51) | 352 (49) | 50 (41) | 128 (49) | 174 (52) | ||
| Diabetes | 335 (18) | 86 (22) | 123 (17) | 126 (16) | .03 | 129 (18) | 30 (24) | 45 (17) | 54 (16) | .11 |
| Hypertension | 1228 (65) | 249 (65) | 465 (65) | 514 (66) | .93 | 449 (63) | 78 (64) | 170 (65) | 201 (60) | .53 |
| Stroke | 67 (4) | 13 (3) | 28 (4) | 26 (3) | .81 | 26 (4) | 3 (2) | 9 (3) | 14 (4) | .66 |
| 3MSE score† | 93 (5) | 92 (5) | 93 (5) | 93 (5) | .01 | 93 (5) | 92 (5) | 93 (5) | 93 (5) | .33 |
| Hearing aid use | 243 (13) | 176 (21) | 64 (9) | 3 (0.4) | <.01 | 67 (9) | 49 (40) | 15 (6) | 3 (1) | <.01 |
Note. Health ABC = Health, Aging and Body Composition; HI = hearing impairment; 3MSE = Modified Mini-Mental State Exam.
*p Value from the Kruskal–Wallis test (continuous variables) or Fisher exact test (categorical variables).
†Values are expressed as mean (standard deviation).
Dementia
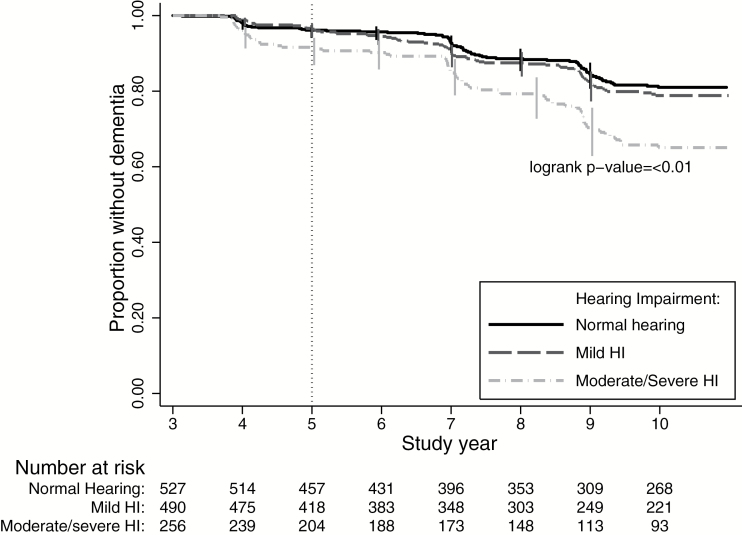
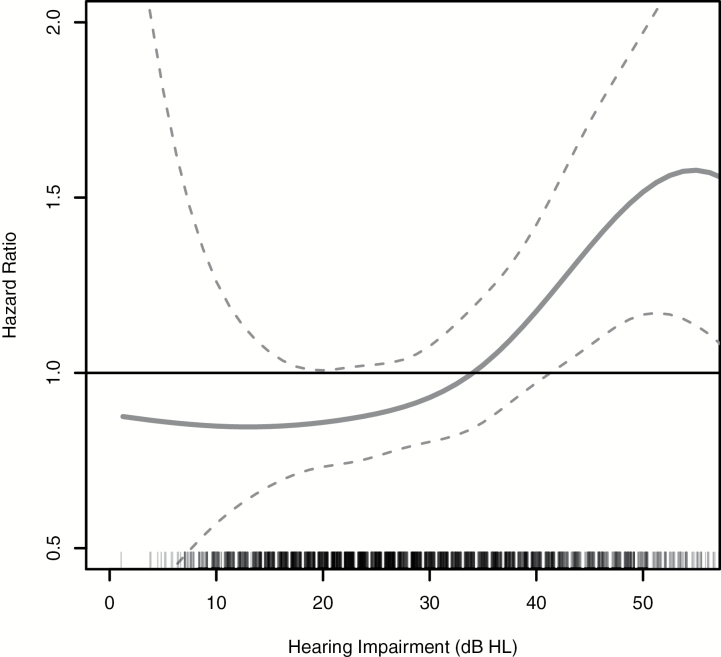
Over 9 years, 229 participants developed incident dementia, with the largest proportion of cases among those participants with the greatest level of HI (18%; Table 2, Figure 1). After full adjustment, the hazard ratio (HR) for incident dementia comparing participants with moderate/severe HI to participants with normal hearing was 1.55 (95% confidence interval [CI]: 1.10, 2.19; Table 2). This association was robust, persisting in a sensitivity analysis restricted to incident dementia measured from 2001 to 2008 (HR: 1.55, 95% CI: 1.09, 2.18) and modeling PTA continuously (HR: 1.14, 95% CI: 1.03, 1.25) per every 10-dB increase in hearing loss. Figure 2 shows the multivariable-adjusted results when PTA was modeled continuously using penalized splines. A linear trend between increasing PTA and an increased log hazard of incident dementia was observed between 10 and 50 dBHL.
Table 2.
Multivariable-Adjusted HRs and 95% CIs of the Association Between HI and Incident Dementia, Health ABC Study, N = 1889, 1999–2008
| Incident Dementia | Model 1* | Model 2† | |||
|---|---|---|---|---|---|
| N incident/Ntotal (%) | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Follow-up from 1999–2008 (primary analysis)‡ | |||||
| Normal hearing | 80/786 (10) | Referent | — | Referent | — |
| Mild HI | 79/716 (11) | 1.03 (0.75, 1.42) | .84 | 1.02 (0.75, 1.40) | .89 |
| Moderate/severe HI | 70/387 (18) | 1.64 (1.16, 2.30) | .01 | 1.55 (1.10, 2.19) | .01 |
| P-trend | — | — | .01 | — | .02 |
| PTA continuous (per 10 dB increase) | — | 1.15 (1.05, 1.27) | <.01 | 1.14 (1.03, 1.26) | .01 |
| Follow-up from 2001–2008 (sensitivity analysis)§ | |||||
| Normal hearing | 80/786 (10) | Referent | — | Referent | — |
| Mild HI | 79/716 (11) | 1.03 (0.75, 1.41) | .86 | 1.02 (0.74, 1.40) | .91 |
| Moderate/severe HI | 70/387 (18) | 1.63 (1.16, 2.30) | .01 | 1.55 (1.09, 2.18) | .01 |
| P-trend | — | — | .01 | — | .02 |
| PTA continuous (per 10 dB increase) | — | 1.15 (1.04, 1.27) | <.01 | 1.14 (1.03, 1.25) | .01 |
Note. dB, decibel; Health ABC = Health, Aging and Body Composition; HI = hearing impairment; HR: hazard ratio; PTA, pure-tone average; 3MSE = Modified Mini-Mental State Exam.
*Model 1 adjusted for age, sex, race, education, and study site.
†Model 2 adjusted for Model 1 characteristics and smoking status, hypertension, diabetes, and stroke.
‡Hearing from Year 5; Dementia from Years 3–11 (1999–2008).
§Hearing from Year 5; Dementia from Years 5–11 (2001–2008).
Figure 1.
Kaplan–Meier survival estimate of time to dementia by hearing impairment status, Health ABC Study, N = 1,889, 1999–2008. Probability of remaining dementia-free estimated with the Kaplan–Meier method. The origin was defined as Year 3 of the study, with hearing assessed at Year 5. Health ABC = Health, Aging and Body Composition.
Figure 2.
Multivariable-adjusted association between PTA and incident dementia, Health ABC Study, N = 1,889, 1999–2008. HR of incident dementia associated with PTA (in dBHL) when modeled continuously using penalized splines (used to allow for smooth, nonlinear effects in regression models). Adjusted for age (year), sex, race, education (less than high school/highs school/postsecondary), study site (Memphis or Pittsburgh), smoking status (never/former/current), hypertension, diabetes, and history of stroke. dBHL = decibels hearing level; Health ABC = Health, Aging and Body Composition; HI = hearing impairment; HR: hazard ratio; PTA, pure-tone average.
Of the participants with moderate or severe HI, 21% (n = 176) used a hearing aid at Year 5. In analyses restricted to this group, the estimated effect of hearing aid use was in the anticipated direction (ie, reduced dementia risk, HR: 0.84, 95% CI: 0.51, 1.39) but did not achieve statistical significance and inference is limited, given the wide CI.
Cognitive Decline
We conducted additional analyses to investigate the association between HI and specific cognitive domains (memory, perceptual speed, and processing speed). At baseline (Year 3), participants with HI had lower mean total recall scores on the Buschke SRT (p = 0.001, Table 1), but mean scores in psychomotor speed and perceptual speed did not differ by HI status (Table 1). The baseline difference in memory score persisted after full adjustment; participants with moderate or severe HI scored an average of −0.24 SD (p = 0.02) lower than participants with normal hearing. When PTA was modeled continuously, we estimated an average difference of −0.05 SD (p = 0.04) for every 10 dB decrease (Table 3). No differences in rates of cognitive change for any cognitive domains were observed by HI status (Table 3).
Table 3.
Multivariable-Adjusted*, Population-Average Differences in Estimates of Annual Rates of Standardized Cognitive Change by Baseline HI Status, Health ABC Cognitive Vitality Substudy, N = 721, 1999–2006
| Baseline Performance | Annual Rate of Change | |||
|---|---|---|---|---|
| Estimate† (95% CI) | p Value | Estimate‡ (95% CI) | p Value | |
| Memory | ||||
| Mild HI (vs. normal hearing) | −0.10 (−0.26, 0.04) | .16 | −0.00 (−0.03, 0.02) | .85 |
| Moderate/severe HI (vs. normal hearing) | −0.24 (−0.44, −0.04) | .02 | −0.02 (−0.06, 0.01) | .21 |
| Per every 10 dB decrease | −0.05 (−0.11, −0.00) | .04 | −0.00 (−0.01, 0.01) | .46 |
| Psychomotor speed | ||||
| Mild HI (vs. normal hearing) | −0.08 (−0.22, 0.06) | .25 | 0.00 (−0.02, 0.02) | .85 |
| Moderate/severe HI (vs. normal hearing) | −0.15 (−0.33, 0.04) | .13 | −0.02 (−0.05, 0.00) | .07 |
| Per every 10 dB decrease | −0.04 (−0.09, 0.02) | .17 | −0.01 (−0.01, 0.00) | .09 |
| Perceptual speed | ||||
| Mild HI (vs. normal hearing) | −0.02 (−0.15, 0.12) | .82 | −0.01 (−0.03, 0.01) | .36 |
| Moderate/severe HI (vs. normal hearing) | −0.09 (−0.27, 0.09) | .32 | −0.02 (−0.05, 0.00) | .11 |
| Per every 10 dB decrease | −0.02 (−0.07, 0.03) | .46 | −0.01 (−0.01, 0.00) | .08 |
Note. dB, decibel; Health ABC = Health, Aging and Body Composition; HI = hearing impairment; HR: hazard ratio; PTA, pure-tone average; 3MSE = Modified Mini-Mental State Exam.
*Adjusted for age, sex, race, education, study site, smoking status, hypertension, diabetes, and history of stroke.
†Difference in baseline (Year 3) standardized cognitive performance comparing persons with HI to persons with normal hearing.
‡Difference in annual rates of cognitive change comparing persons with HI to persons with normal hearing.
Discussion
In a biracial cohort of well-functioning older adults (mean age 76 years in 1999–2000), our results demonstrate that moderate/severe peripheral HI (>40 dBHL) is associated with greater risk of incident dementia over 9 years (HR: 1.55, 95% CI: 1.10, 2.19), compared with participants having normal hearing after adjustment for multiple demographic, health behavior, and disease covariates. Except for poorer baseline memory performance (difference of −0.24 SD, 95% CI: −0.44, −0.04), no associations were observed between HI and rates of 7-year cognitive change in the domains of memory, perceptual speed, or processing speed.
Our results are consistent with previous longitudinal studies of audiometric HI and dementia (1,2) and global cognitive change (2,6,7) over time. In 639 participants aged 36–90 years, baseline HI was associated with increased risk of all-cause dementia over 12 years (HR: 1.27 per every 10-dB loss, 95% CI: 1.06, 1.50) (1). In 1,057 men (mean age 56 years), each 10-dB increase in hearing level at baseline was associated with an increased odds of all-cause dementia measured 17 years later (odds ratio: 2.7, 95% CI: 1.4, 5.2) (2).
Although we documented significantly poorer baseline performance in memory in participants with HI, we did not find differences in rates of cognitive decline by HI status in memory or speed. These results differ from previous studies that have found faster rates of cognitive decline over time in persons with HI in memory (2,6,21,22) and executive function (7,21). The cognitive assessments used in this analysis were administered only to a healthy subset of all Health ABC participants. Although on average, cognitive function in this group declined over time, the decline observed was about half the average rate of change estimated in nationally representative studies in older adults (23,24). Given this relative lack of decline and the smaller sample size, we may have been underpowered to detect a change in domain-specific cognitive function over time.
Whether HI is a marker or a cause of dementia and cognitive impairment has yet to be determined. It may be that both HI and dementia are caused by a common underlying pathology such as vascular disease. Even though we adjusted for cardiovascular risk factors, we cannot rule out that the observed association stems from other possible common etiologies (eg, unmeasured genetic factors). HI may also be a marker of socioeconomic disadvantage and/or poorer health; however, our analysis adjusted for education and other demographic factors known to influence dementia diagnosis, and additional adjustment for self-reported health status did not alter the findings (data not shown).
Alternatively, we hypothesize that HI may be causally associated with dementia and cognitive decline through three possible mechanisms: increased cognitive load, changes in brain structure/function, and increased social isolation (9). Poor or impaired encoding of sound by the cochlea may require extra cognitive processing effort, limiting effort available for encoding the content of speech into memory and resulting in increased cognitive load, or effortful listening (25,26). Neuroimaging studies suggest that HI may influence brain structure outside of the auditory cortex (27,28). In 126 participants aged 56–86 years, HI was longitudinally associated with accelerated atrophy in the right temporal lobe (difference in annual rate of change comparing participants with and without HI: −0.29cm3; 95% CI: −0.54, −0.04) as well as with whole brain atrophy (difference in annual rate of change associated with HI: 1.20cm3, 95% CI: −2.17, −0.22) (27). An additional mechanism by which HI may cause dementia or reduced cognitive function is through increased social isolation. Social isolation is associated with physiologic changes, such as increased inflammation and glucocorticosteroid levels, which may alter brain structure and has been associated with accelerated cognitive decline and dementia (29), although epidemiologic evidence is mixed (30,31).
The dementia diagnosis used in this study is consistent with previous studies in this cohort but may be less sensitive than diagnosis through a structured clinical interview, and we were unable to assess the association between HI and dementia type. Our dementia diagnosis was based on prescribed dementia medication, dementia diagnosis from adjudicated hospital records, or an observed race-stratified 3MS decline more than 1.5 SDs from the baseline mean. A possible concern when utilizing community-diagnosed dementia (eg, medication or hospital record data) is that reduced audibility can produce behaviors consistent with dementia in cognitively normal individuals when assessed with largely auditory–verbal assessment tools. However, in our study, estimates of the association between HI and dementia were similar whether diagnosis incorporated observed cognitive change data or was limited to community-based diagnosis (HR: 1.69, 95% CI: 1.03, 2.70; n = 114 vs. HR: 1.57, 95% CI: 0.95, 2.59; n = 115; data not shown).
Audiometric data were collected only at one point in time, and so we were unable to assess time-related changes in hearing status with cognitive decline and dementia. Because HI prevalence increases with advancing age (10), it is likely, however, that this limitation would conservatively result in an underestimate of the association.
This study adds to the small body of epidemiologic literature demonstrating a longitudinal relationship between HI and incident dementia and extends this observation to a more racially diverse population.
In summary, this study found that moderate or severe HI was associated with a 55% increase in the risk of incident dementia during 9 years of follow-up in a biracial cohort of 1,889 men and women aged 70–79 years at baseline (HR: 1.55, 95% CI: 1.10, 2.19). These findings lend support to the hypothesis that HI may be a risk factor for dementia in older adults. With approximately two of the three adults aged older than 70 years in the United States affected by hearing impairment (10), HI is an attractive target for intervention in order to prevent or delay dementia in older adults. Randomized clinical trials are needed to determine whether aural rehabilitation can stem the oncoming tide of dementia in our aging population.
Funding
This work was supported by the National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; and N01-AG-6-2106, NIA grant R01-AG028050, and NINR grant R01-NR012459.
References
- 1. Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi:10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi:10.1212/WNL.0b013e31826e263d [DOI] [PubMed] [Google Scholar]
- 3. Gurgel RK, Ward PD, Schwartz S, et al. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35(5):775–781. doi:10.1097/MAO.0000000000000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi:10.1037/a0024238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–1136. doi:10.1093/gerona/glr115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deal JA, Sharrett AR, Albert MS, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. 2015;181:680–690. doi:10.1093/aje/kwu333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299. doi:10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol. 2015;11:166–175. doi:10.1038/nrneurol.2015 [DOI] [PubMed] [Google Scholar]
- 9. Lin FR, Albert M. Hearing loss and dementia—who is listening? Aging Ment Health. 2014;18:671–673. doi:10.1080/13607863.2014.915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(5):582–590. doi:10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. [DOI] [PubMed] [Google Scholar]
- 12. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051 doi:10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi:10.1093/gerona/glq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 15. Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. [DOI] [PubMed] [Google Scholar]
- 16. Salthouse TA. General and specific speed mediation of adult age differences in memory. J Gerontol B Psychol Sci Soc Sci. 1996;51:P30–P42. [DOI] [PubMed] [Google Scholar]
- 17. Prevention of Blindness and Deafness Grades of Hearing Impairment. World Health Organization Web site. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed November 29, 2015.
- 18. Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika; 1994;81;515–526. [Google Scholar]
- 19. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 20. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer, 2000. [Google Scholar]
- 21. Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc. 2005;53:374–380. [DOI] [PubMed] [Google Scholar]
- 22. Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve analysis of late-life sensory and cognitive function over 8 years: evidence for specific and common factors underlying change. Psychol Aging. 2003;18:714–726. [DOI] [PubMed] [Google Scholar]
- 23. Salthouse TA. Major issues in cognitive aging. New York, NY: Oxford University Press, 2010. [Google Scholar]
- 24. Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40:684–689. doi:10.1093/ageing/afr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20:241–248. [DOI] [PubMed] [Google Scholar]
- 26. Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24:761–766. doi:10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi:10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peelle JE, Troiani V, Grossman M, et al. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638–12643. doi:10.1523/JNEUROSCI.2559-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40:218–227. doi:10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mick P, Kawachi I, Lin FR. The Association between Hearing Loss and Social Isolation in Older Adults. Otolaryngol Head Neck Surg. 2014; 150:378–384. doi:10.1177/0194599813518021 [DOI] [PubMed] [Google Scholar]
- 31. Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10:e0119616 doi:10.1371/journal.pone.0119616 [DOI] [PMC free article] [PubMed] [Google Scholar]