Abstract
Although behavior may often be a fairly direct target of natural or sexual selection, it cannot evolve without changes in subordinate traits that cause or permit its expression. In principle, changes in endocrine function could be a common mechanism underlying behavioral evolution because they are well positioned to mediate integrated responses to behavioral selection. More specifically, hormones can influence both motivational (e.g., brain) and performance (e.g., muscles) components of behavior simultaneously and in a coordinated fashion. If the endocrine system is often “used” as a general mechanism to effect responses to selection, then correlated responses in other aspects of behavior, life history, and organismal performance (e.g., locomotor abilities) should commonly occur because any cell with appropriate receptors could be affected. Ways in which behavior coadapts with other aspects of the phenotype can be studied directly through artificial selection and experimental evolution. Several studies have targeted rodent behavior for selective breeding and reported changes in other aspects of behavior, life history, and lower-level effectors of these organismal traits, including endocrine function. One example involves selection for high levels of voluntary wheel running, one aspect of physical activity, in four replicate High Runner (HR) lines of mice. Circulating levels of several hormones (including insulin, testosterone, thyroxine, triiodothyronine) have been characterized, three of which—corticosterone, leptin, and adiponectin—differ between HR and control lines, depending on sex, age, and generation. Potential changes in circulating levels of other behaviorally and metabolically relevant hormones, as well as in other components of the endocrine system (e.g., receptors), have yet to be examined. Overall, results to date identify promising avenues for further studies on the endocrine basis of activity levels.
Introduction
Behavior may often be a fairly direct target of natural or sexual selection; however, it cannot evolve without changes in subordinate traits that cause or permit its expression. Because of their diverse effects on both neural and peripheral aspects of behavior, changes in endocrine function could be a common mechanism underlying behavioral evolution. If so, then correlated responses in other aspects of behavior, life history (Dantzer et al. 2017), and organismal performance (e.g., locomotor abilities) should commonly occur because any cell with appropriate receptors could be affected. At the same time, because hormones are likely to affect multiple traits, including through early-life parental effects (e.g., see Garland et al. (2017)), they might be “used” routinely by selection to achieve (adaptively) correlated changes (Cox et al. 2017). Nevertheless, the seminal papers in modern evolutionary physiology scarcely mentioned the endocrine system (Feder 1987; Garland and Carter 1994; Feder et al. 2000). Evolutionary endocrinology has thus developed somewhat separately from the rest of evolutionary physiology (Zera and Zhang 1995; Zera et al. 2007; Nepomnaschy et al. 2009; Adkins-Regan 2012; Cox et al. 2017; Dantzer et al. 2017). Although hybrid fields always run the risk of settling into a pattern of “choose one from column A and one from Column B,” evolutionary endocrinology can move beyond this trap by addressing emergent questions that truly integrate across disciplines and generally would not have been addressed by pure practitioners of either parental field (Table 1).
Table 1.
Evolutionary endocrinology as a hybrid field that addresses emergent questions
| Field | Evolutionary Biology | Endocrinology |
|---|---|---|
| Within-field Questions | How do populations change over time? How can we use “model organisms” to elucidate basic genetic and evolutionary principles? Relatively how important are natural selection, sexual selection, and random genetic drift in shaping biological diversity? | How does the endocrine system work? How can we use “model organisms” to elucidate basic endocrine principles? Where is the line between “normal” individual variation and pathology? How do we promote health and cure disease? |
| Emergent Questions | How does the endocrine system constrain or facilitate behavioral evolution? Which cases of pleiotropic gene action can be attributed to genetic variation in components of the endocrine system? What are the most common endocrine mechanisms that respond to selection on behavior or life-history traits? Which correlated responses to selection on behavior represent non-adaptive or maladaptive byproducts of endocrine function? | |
Ways in which behavior coadapts with other aspects of the phenotype can be investigated with various approaches, such as through phylogenetically based comparative studies (Garland et al. 2005; Rezende and Diniz-Filho 2012). Comparative studies, however, are necessarily correlational and historical in nature (Abbott et al. 2003; Goymann et al. 2004) and cannot reveal the detailed generation-to-generation changes that occur in response to selection. Quantitative-genetic analyses and studies of selection in the wild can reveal the patterns of genetic and phenotypic variation in endocrine components that form the basis for individual variation and covariation as well as the higher-level phenotypes on which natural and sexual selection act in sex-, population- and context-specific ways (Shire 1976; Storz et al. 2015; Cox et al. 2017; Dantzer et al. 2017). Beyond this, various types of phenoytpic engineering, e.g., by manipulating circulating levels of particular hormones, can help to elucidate both functional relations and selective importance (e.g., Ketterson et al. 1996; Ketterson et al. 2009; Cox et al. 2014; Dantzer et al. 2017). Finally, phenotypic evolution can be studied directly with selection experiments and experimental evolution (Swallow and Garland 2005; Garland and Rose 2009; Kawecki et al. 2012; Storz et al. 2015). Most germane to this paper, several studies have targeted rodent behavior for selective breeding and reported changes in other aspects of behavior, life history, and lower-level effectors of these organismal traits, including endocrine function (Hyde 1981; Rhodes and Kawecki 2009; Swallow et al. 2009).
In this overview, we will first consider the nature and evolution of complex traits, which generally include motivated behaviors. We then provide a very brief summary of the endocrine system and some of its more easily understood components. Next, we consider some key points about how natural selection typically acts on behavior. We then turn to examples of artificial selection on behavior, as well as one example of selection that was imposed directly on an endocrine function. We provide a summary of key endocrine findings from a long-term experiment in which mice are bred for high levels of voluntary wheel running. Finally, we draw some conclusions regarding hormones and the evolution of behavior, emphasizing what we have learned from rodent selection experiments.
Complex traits
So-called “complex traits” can be defined in various ways. First, they occur at relatively high levels of biological organization, including life-history traits (e.g., reproductive mode, such as viviparity) and most behaviors (one might exclude simple neuromuscular reflex arcs). Complex traits necessarily comprise many subordinate traits (also known as endophenotypes, e.g.: Gottesman and Gould 2003). As a behavioral example, consider ingestion (food intake). The “decision” by an animal to ingest or reject a given substance follows from sensory inputs (e.g., smell, sight, taste), rapidly integrated in the central nervous system, then leading to motor output (Berthoud 2007). The decision is influenced by a variety of external factors, such as whether the food item offers any resistance or is noxious and whether the organism perceives any predation risk while dealing with the potential food item. Internal to the organism, the endocrine systems can influence ingestion behavior at several levels, including in relation to internal states of energy balance, hunger, appetite, and affect (Magni et al. 2009; Hoskins and Volkoff 2012).
As an even more complex behavioral example, consider voluntary activity levels. In the wild, this relates to, for example, use of the home range; searching for mates, food, and other resources; patrolling territory; and migration (Feder et al. 2010). For human beings in Western industrialized societies, a large part of activity levels may relate to voluntary exercise behavior, or the lack thereof. Even (or perhaps especially) within the built environment of human societies, the control of exercise behavior is exceedingly complex and involves numerous internal and external factors, plus interactive effects (Dishman et al. 2006; Garland et al. 2011b).
A second characteristic of complex traits is that they often exhibit emergent properties, which is to say that what they do or how they work is not entirely predictable from knowledge of how their lower-level components function (Novikoff 1945; Lobo 2008). Commonly cited examples in the behavioral realm include consciousness, bird flocks, social behavior, and the functionality of bee hives.
Third, complex traits are characterized by modularity (Csete and Doyle 2002). This means that components of the overall trait can often function or evolve somewhat independently. For example, with adequate oxygenation and nutrient supply, an isolated heart or skeletal muscle can contract, generate force, and perform work. Similarly, components of ingestive behavior can function independently, and often as components of other complex behaviors (e.g., smell, motor output). The modules of complex traits are integrated in both form and function when in situ, and take on greater roles when so integrated.
Fourth, complex traits are affected by many genes and environmental factors, plus interactions of genes with genes, environmental factors with other environmental factors, and gene-by-environment interactions. Moreover, epigenetic processes can affect many components of complex traits. “Epigenetic” in modern parlance generally refers to changes caused by modification of gene expression rather than alteration of the genetic code itself. Sometimes the definition is restricted to heritable changes in gene expression that do not involve changes to the underlying DNA sequence, such as may occur by DNA methylation (Waterland and Garza 1999; Burggren and Crews 2014; Waterland 2014; Garland et al. 2017) or histone modification (Lennartsson and Ekwall 2009).
A final point about complex traits is that several of them may share specific functional components. This will obviously be true for any of the major “organ systems,” and perhaps especially true for the endocrine system, given that particular hormones and hormone receptors often play a role in multiple behaviors or physiological processes (see below; Cox et al. 2017; Schwartz and Bronikowski 2017). For example, repeated evolutionary patterns of duplication and divergence of genes for steroid hormone receptors have led to pronounced changes in the endocrine control of reproductive morphology, physiology, and behavior (e.g., see Thornton 2001; Baker 2004; Young and Crews 1995; Dean and Thornton 2007; Bridgham et al. 2009). These and other deep and diverse phylogenetic patterns of endocrine origins would lead one to expect considerable pleiotropic gene action both among the components of a given complex trait and across components—and hence across different complex traits.
Given all of the foregoing characteristics of complex traits, it should be of little surprise that their evolutionary history is generally complicated and multifactorial. A good example of this is the evolution of lactation strategies in pinnipeds, as reviewed by Schulz and Bowen (2005). They describe (e.g., see their Figure 4) complex interrelationships of environmental factors (e.g., temperature, predation regime), body size, rates of energy expenditure, fat storage, and so forth, with many of the behavioral and physiological aspects under at least partial endocrine control, although that point is not mentioned in the article.
Another example of the multifaceted evolution of a complex trait involves endothermy. In particular, the evolution of mammalian (and avian) endothermy has long been of interest in comparative, ecological, and evolutionary physiology (Garland and Carter 1994), but so far has escaped much scrutiny from evolutionary endocrinologists (Alberts and Pickler 2012; Little and Seebacher 2014). Kemp (2006) views the origin of mammalian endothermy as a paradigm for the evolution of complex traits. His Figure 3 depicts “interrelationships of the structures and functions responsible for or affected by endothermic temperature physiology of a mammal,” and also acknowledges the role of endocrine regulation. Koteja (2000) presents a model involving energy assimilation and parental care in the evolution of endothermy, but does not mention the endocrine system. On the other hand, Farmer (2000) does emphasize the potential importance of thyroid (and other) hormones in her model for the evolution of endothermy in relation to parental care. A recent review also promotes possible roles of the endocrine system, especially thyroid hormones (Nespolo et al. 2011), which have been mentioned for decades because of their role in resting metabolic rate.
In summary, the endocrine system is likely to play an essential role in the development, ontogeny, homeostatic regulation, and evolution of many, if not most, complex traits. Although natural and sexual selection are likely to act above the level of endocrine function per se, aspects of the endocrine system are highly likely to get “dragged along” during the evolution of complex traits because they affect so many parts of the phenotypic hierarchy, from gene expression and protein synthesis to organismal performance and behavior.
The endocrine system
Endocrine communication, by definition, refers to transfer of information among different tissues or organs by chemical messengers that are transported through the blood—i.e., hormones. In addition to the diffuse collection of endocrine organs and tissues, and the hormones that they synthesize and secrete, components of the endocrine system include transport proteins, receptors, and signal transduction mechanisms (for overviews of the endocrine system, see Nelson (2011); Kronenberg et al. (2016); Lazar and Birnbaum (2016)). At the coarsest level, Shire (1976) recognized an “endocrine unit” as consisting of a stimulus generator, which provides a stimulus to an endocrine tissue, whose output is carried by a transport system and then signals target organs, which may have multiple responses. Thus, functional changes in the endocrine system, on both evolutionary and ontogenetic scales, can be mediated by changes not only in hormone secretion but also in many other components.
It is worth noting that the endocrine system is far less of a “system” than some others, such as the musculoskeletal or circulatory systems, in that it subserves myriad functions and does not necessarily operate as a coordinated unit. The evolutionary origins of the vertebrate endocrine system are diverse (e.g., see Barrington (1987); Thornton (2001); Dean and Thornton (2007); Bridgham et al. (2009); Sower et al. (2009); pages R203-R204 in Storz et al. (2015); Baker (2015); Schwartz and Bronikowski (2017)), and the ways it may respond to selection are manifold. Moreover, components of the overall endocrine system may be involved in a wide array of biological functions, such as involvement of the insulin and insulin-like signaling network in cell division and growth, organismal metabolism, growth and development, reproduction, and lifespan (Schwartz and Bronikowski 2017).
Hormones fall into two major categories, hydrophilic and hydrophobic, that differ markedly in many aspects of synthesis, secretion, transport through the blood, receptors, and inactivation/clearance from the body. Hydrophilic hormones include peptides and proteins (e.g., insulin, leptin, adiponectin, growth hormone), which make up most of the hormones in the vertebrate body. Peptide and protein hormones are produced in endocrine cells via transcription, translation, and post-translational modification, packaged in membrane-bound secretory vesicles, and released into the blood by exocytosis. Hydrophilic hormones typically circulate through the body dissolved in plasma (Nelson 2011; Kronenberg et al. 2016; Lazar and Birnbaum 2016). Because they are lipophobic, hydrophilic hormones are unable to cross plasma membranes. At target cells (i.e., cells containing specific receptors for a particular hormone), therefore, these hormones bind to membrane receptors, thereby activating signal-transduction pathways within the cell. This process can have rapid (within seconds) effects on cellular function through modification of existing proteins. In addition, signal-transduction cascades sometimes lead to changes in gene expression, a much slower process. Hydrophilic hormones are rapidly inactivated by peptidases and excreted; thus, they have short half-lives, on the order of several minutes. In addition to proteins and peptides, hydrophilic hormones include the catecholamines (epinephrine, norepinephrine, and dopamine) (Nelson 2011; Kronenberg et al. 2016; Lazar and Birnbaum 2016).
Hydrophobic hormones include steroids, thyroid hormones (thyroxine, triiodothyronine), and melatonin. Steroids (e.g., estrogen, progesterone, testosterone, cortisol, corticosterone, aldosterone) are non-polar molecules consisting of three 6-carbon rings and a conjugated 5-carbon ring, synthesized from cholesterol by tissue-specific biosynthetic enzymes. Because they are lipid-soluble, steroid hormones diffuse readily across cell membranes and thus are secreted by parent cells immediately following synthesis. As a result of their limited solubility in plasma, they circulate in the blood bound, in large part, to carrier proteins, including albumin and hormone-specific carriers (Nelson 2011; Kronenberg et al. 2016; Lazar and Birnbaum 2016). Although the functions of these carrier proteins are not fully understood, they appear to prevent hydrophobic hormones from entering cells, thus maintaining a reservoir of these hormones in the circulation (Malisch and Breuner 2010). Only the unbound or “free” fraction is thought to be biologically active.
Unbound steroid molecules diffuse into all cells. In target cells, they bind to receptors located in either the cytoplasm or the nucleus. The hormone-receptor complex then binds to hormone-response elements in DNA and acts as a transcription factor, increasing or decreasing the expression of specific genes. These effects typically develop more slowly than those of hydrophilic hormones, requiring up to several hours, but persist longer. Some steroids can also act through membrane receptors to exert rapid, more transient effects on target cells (Gruber et al. 2002; Sakamoto et al. 2012). Steroid hormones are typically inactivated in the liver and excreted, and have half-lives on the order of hours. Thyroid hormones (thyroxine and triiodothyronine) and melatonin have mechanisms of transport and action similar to those of steroids (Kawata 2001).
Hormone secretion is regulated by neural inputs, other hormones, or specific physiological stimuli, and often involves negative feedback loops (Melmed et al. 2016). Insulin, for example, is secreted by the pancreas in response to high blood glucose concentrations and, correspondingly, acts on various tissues to lower blood glucose levels, thereby reducing its own secretion. As another example, adrenocorticotropic hormone (ACTH), a protein from the anterior pituitary, stimulates release of glucocorticoid hormones (cortisol and/or corticosterone, depending on the species) from the adrenal cortex. In turn, high concentrations of glucocorticoids feed back to the anterior pituitary to decrease secretion of ACTH. Simultaneously, ACTH secretion is stimulated by neuropeptides from the brain, corticotropin-releasing hormone and vasopressin. Thus, control of hormone release is often multifactorial. Expression of receptors, too, is dynamically regulated by such factors as concentrations of their ligands or of other hormones. Additionally, levels of many hormones, receptors, and even binding proteins may fluctuate systematically over both circadian and ultradian cycles (Malisch et al. 2008; Giguere et al. 2011; Nicolaides et al. 2014).
Interactions between hormones and behavior
Hormones can influence behavior through a multitude of actions on sensory systems, integrative processes in the central nervous system, motor output systems, and other peripheral organs and structures (for reviews, see Pfaff et al. (2004); Nelson (2011)). In many cases, a particular hormone exerts complementary effects on both central and peripheral components of a specific behavior—in other words, both the motivation and the ability to perform the behavior—and these effects can be acute and/or chronic (Nelson 2011). In female mammals, for example, the protein hormone prolactin, secreted by the anterior pituitary, both enhances the neural motivation to engage in maternal behavior and stimulates milk production in the mammary glands (Grattan 2002). As another example, testosterone, a steroid hormone secreted by the testes, acts both centrally to promote aggressiveness and libido and peripherally to develop and maintain weaponry and ornamentation (e.g., antlers, plumage coloration) used in inter-male competition and female courtship (Setchell et al. 2008; Nelson 2011; Pasch et al. 2011). This duality of function has evolved subject to the screening process of natural selection, and can be viewed as a possible example of genetic architecture and gene regulatory networks (acting through gene expression and downstream effects on phenotypes) evolving to match the prevailing selection regime (Pavličev and Cheverud 2015).
For some behaviors, endocrine regulation requires two or more hormones acting in concert. For example, estrogen and progesterone interact to stimulate the expression of sexual behavior in female rodents (Blaustein 2008); in male rodents, rhythmic diurnal changes of progesterone synergize with testosterone to facilitate sexual behavior (Witt et al. 1994; Phelps et al. 1998; Woolley et al. 2006). In some reptiles and amphibians, progesterone and testosterone synergize to promote aggression (Woolley et al. 2004; Crews and Moore 2005), whereas in other species these same hormones promote sexual behavior in males (Lindzey and Crews 1992; Lindzey and Crews 1993). As another example, regulation of appetite and food intake is coordinated by a large suite of hormones from the brain (e.g., corticotropin-releasing hormone), gastrointestinal tract (e.g., ghrelin, cholecystokinin), pancreas (e.g., insulin), adipocytes (e.g., leptin), and adrenal cortex (corticosterone or cortisol), in addition to other endocrine, neural and metabolic signals (Pfaff et al. 2004; Berthoud 2007; see also Alberts and Pickler (2012)).
Importantly, hormones can act at different life stages to influence behavioral phenotypes. According to the classic organizational-activational hypothesis (Phoenix et al. 1959), the expression of sexually dimorphic behaviors—including sexual, parental, and aggressive behaviors, among others—depends, first, on exposure to (in males) or absence of (in females) gonadal steroids (especially testosterone) at specific “sensitive periods” during prenatal or early postnatal development. These early-life effects more or less permanently “organize” the neural substrates of the behavior. Beginning at puberty, subsequent exposure to testicular or ovarian steroid hormones transiently “activates” the expression of the behavior (Phoenix et al. 1959; Arnold 2009). Organizational and activational effects on the brain can involve changes in numbers, morphology, size, and electrophysiological properties of neurons, as well as effects on synthesis of neurotransmitters and neuropeptides, or expression of receptors for these neurocrines or hormones (Nelson 2011).
Since its original formulation in 1959 (Phoenix et al. 1959), the organizational-activational hypothesis has been refined in important ways. First, not all sex differences in behavior are organized by sex differences in early exposure to gonadal hormones; rather, some behavioral sex differences result from direct effects of genes on the sex chromosomes, from epigenetic effects, or from exposure to hormones other than gonadal steroids (Arnold 2009; McCarthy and Arnold 2011). Second, organizational effects may be less permanent than initially thought. Third, in at least some vertebrate taxa, organizational and activational effects on the male brain are not mediated directly by testosterone but rather by estrogen, which is synthesized in neurons either de novo or via aromatization of testosterone (Sodersten 2015). Fourth, hormonal determinants of organization and activation can differ both among and within species (Crews and Moore 2005; Adkins-Regan 2012). Finally, in addition to the classic dichotomous effects of steroids in early development and adulthood, it is now becoming clear that steroids can act during puberty to exert long-term effects on the brain and behavior (Juraska et al. 2013).
A crucial point is that hormones do not directly “cause” behavior in the sense of having a deterministic effect. Instead, hormones interact with other neural and physiological systems to influence the likelihood that particular behaviors will be expressed under certain conditions (Pfaff et al. 2004; Nelson 2011). Testosterone promotes libido in many species, for example, but high circulating concentrations of testosterone do not ensure that males will engage in sexual behavior. Instead, they increase the probability that a male will seek, court, and mate with females under conditions of appropriate organismal (e.g., low stress) and environmental (e.g., presence of an attractive female, absence of a dominant male) conditions (Nelson 2011).
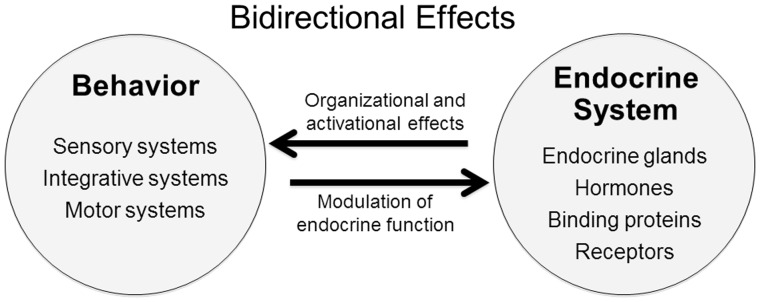
Interactions between hormones and behavior can be bi-directional (Fig. 1). Returning to the examples above, not only does prolactin enhance the expression of parental behaviors in birds and mammals, but its secretion can also be increased by engagement in these behaviors and the resulting exposure to stimuli from the young (e.g., Lea et al. 1986). In a similar fashion, testosterone promotes aggressive behavior in males, whereas engagement in competitive or aggressive interactions can feed back to increase secretion of testosterone (Wingfield et al. 1990). Other examples involve negative feedback loops between hormones and behavior. For instance, high levels of leptin, a peptide hormone secreted by adipocytes, suppress appetite, leading (in theory, at least) to a reduction in food intake and, ultimately, to a decrease in leptin secretion (Zhang and Scarpace 2006; Meek et al. 2012; Balland and Cowley 2015, references therein). In general, effects of behavior on endocrine function can be mediated by numerous mechanisms, including behaviorally induced changes in metabolism, energetics, sensory input, cognition, and mood, among others.
Fig. 1.
Interactions between hormones and behavior can be bi-directional (see text). For example, testosterone activates both sexual and aggressive behavior in males, whereas engagement in sexual or aggressive interactions can feed back to increase secretion of testosterone.
Finally, hormonal influences on behavior may be dose- and time-dependent, and relationships between hormone levels and behavior may not be linear. Across several vertebrate taxa, for example, acute elevations of glucocorticoid hormones have permissive or stimulatory effects on aggressiveness, whereas chronic glucocorticoid elevations inhibit aggression (Summers et al. 2005). In humans, excessively high concentrations of thyroid hormones can lead to nervousness, irritability, and insomnia, whereas unusually low concentrations of the same hormones may contribute to depression and cognitive impairments (Pfaff et al. 2004). Effects of hormones on behavior, and vice versa, can also differ among species and between the sexes, can change with age, and can vary among individuals with different experiential or genetic backgrounds (Pfaff et al. 2004). Among biparental rodents, for example, testosterone inhibits the expression of paternal care by fathers in some species but enhances paternal care in others (Bales and Saltzman 2016).
Selection on behavior
Behavior can be defined as what an organism does in any given situation: it is caused by motivation, but limited by ability. Most biologists who work with animals accept the general premise that selection in nature usually acts more directly on behavior than on the subordinate traits that affect behavior and determine performance abilities (e.g., see Fig. 1 in Garland and Kelly (2006), Fig. 1 in Careau and Garland (2012), Fig. 1 in Storz et al. (2015)).
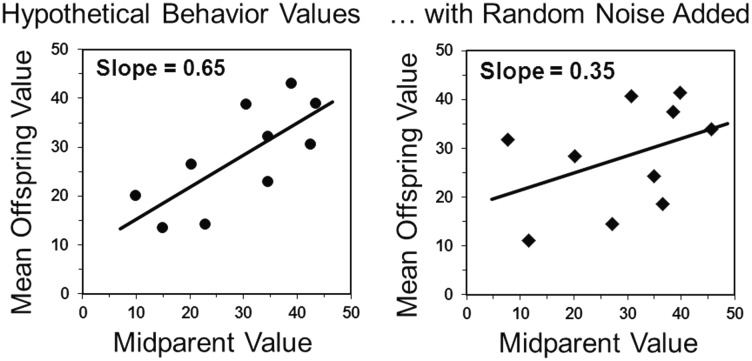
Behavior differs from morphology in that it is highly labile from moment to moment and day to day. The repeatability (consistency from day to day or over other time intervals (Boake 1989)) of behavior is often relatively low, which reduces both the intensity of selection acting on behavior (behavior is a “moving target”) and its narrow-sense heritability (Fig. 2). A reduced selection intensity and/or narrow-sense heritability (Stirling et al. 2002) will lower the possible rate of adaptive evolution for behavioral traits as compared with morphological traits. Of course, the same points could be made for circulating levels of many hormones (Cox et al. 2017).
Fig. 2.
Illustration of how low repeatability of behavior will reduce its narrow-sense heritability (ratio of additive genetic variance to total phenotypic variance in a population). On the left are hypothetical values for a behavioral trait for the average of the two parents and the average of their offspring (presuming that sex differences either do not exist for the trait in question or that they have been removed statistically). In this case, the estimate of the narrow-sense heritability is the slope of the least-squares linear regression (assuming no non-genetic parental effects and no shared-environmental effects). On the right, random noise has been added to the X and Y values for each of the 10 families, which results in a lowering of the strength of the relationship and hence a lower slope. A reduced tendency for a trait to “run in families” because of low repeatability (caused by high liability of the trait and/or high measurement error per se) will reduce the rate of response to directional selection, whether natural, sexual or artificial.
Nonetheless, behavior evolves a lot and quickly, and it is often viewed as a “driver” of evolutionary change (Sol et al. 2005; but see Huey et al. (2003)), which can be termed the “Behavior Evolves First” hypothesis (Mayr 1960; Blomberg et al. 2003; Rhodes and Kawecki 2009), or at least being at the leading edge of phenotypic evolution. Darwin (1859), for example, stated that “… the acutest observer by examining the dead body of the water-ouzel would never have suspected its sub-aquatic habits. … In such cases, and many others could be given, habits have changed without a corresponding change of structure.” In any case, behavior can and does evolve rapidly, as illustrated by substantial differences among the songs of closely related species of sparrows (Nowicki et al. 2001) or among behavior of dog breeds (Draper 1995; Careau et al. 2010).
Many studies have bred rodents and other vertebrates for particular aspects of behavior (e.g., see Supplementary Table S1). For example, various livestock breeds have been bred for behavioral traits related to production farming (e.g., see Nicol (2015) on chickens). Rodents, especially laboratory house mice and laboratory Norway rats, have been common subjects of selection experiments under controlled laboratory conditions (Hyde 1981; Rhodes and Kawecki 2009; Swallow et al. 2009). House mice have been bred for a wide range of behavioral traits, including open-field behavior (DeFries et al. 1978; Flint et al. 2004; Henderson et al. 2004), thermoregulatory nesting (Lynch 1980, 1994; Bult and Lynch 2000), female agonistic behavior (from a wild-derived starting population: Ebert and Hyde (1976); Hyde and Ebert (1976); Hyde and Sawyer (1979); Hyde and Sawyer (1980)), maternal defense (Gammie et al. 2006), voluntary wheel running (Swallow et al. 1998; Rhodes and Kawecki 2009; Swallow et al. 2009), and home-cage activity (Zombeck et al. 2011; Majdak et al. 2014).
Some interesting examples from an endocrine perspective involve experimental domestication of multiple species of mammals (foxes, river otters, mink, rats) in Siberia, begun in 1959 by Belyaev. In these studies, selection for “tameness” was viewed as a first step. The response to selection in the foxes involved many correlated responses, including some at the endocrine level (Belyaev 1979; Trut 1999; Bidau and D’Elía 2009; Kukekova et al. 2012). In pups from the unselected population, at 45 days of age the fearful response to humans appears, exploratory behavior of a new environment decreases, and there is a sharp increase in glucocorticoid content in the peripheral blood. In pups from the domesticated line, at 45 days of age the fearful response does not appear, exploratory activity is not diminished, and glucocorticoid content is not increased (Trut et al. 2004).
Domestication of rats started with 233 wild-caught Rattus norvegicus. They were tested for reaction to a human hand’s approach in their cage. The 30% that were least aggressive were bred to form a “tame” line, while the 30% most aggressive were bred to form an “aggressive” line. No non-selected control line was maintained, so in subsequent papers regarding correlated responses it is not always easy to discern which line(s) changed. In addition, no replicate selection lines were used, so random genetic factors could account for some of the correlated responses (Henderson 1989; Henderson 1997; Koch and Britton 2005; Majdak et al. 2014; Sadowska et al. 2015).
Although the rats’ responses to humans changed dramatically, conspecific aggression did not change. Several endocrine responses were observed, based on comparison of the two lines (Albert et al. 2008, and references cited therein). Serum testosterone in females was higher in the tame than in the aggressive line, although the authors downplay this result. Serum corticosterone and fecal metabolites of corticosterone were lower in the tame line, which was less reactive to restraint, pain, and injections of noradrenaline and serotonin, and had smaller adrenal glands. Brain size was larger in the tame line and brain taurine concentrations were higher, but brain serotonin and GABA concentrations were lower.
Artificial selection on endocrine function
As argued elsewhere, selection experiments can be a powerful approach for demonstrating mechanisms of behavioral or organismal-level evolution (Shire 1976; Garland 2003; Storz et al. 2015). Suppose that selection is applied to a behavioral trait, that it responds across generations, and that one or more lower-level traits are observed to change as a correlated response, consistently in replicated selection lines. One could then hypothesize that the lower-level trait (e.g., a change in the circulating levels of a particular hormone) was causally related to the change in behavior. This hypothesis could be tested with another selection experiment, directly targeting the hormone concentrations.
To our knowledge, this research approach has not yet been undertaken with any vertebrate. However, some studies have directly targeted behaviorally relevant lower-level endocrine traits. For example, laboratory mice were bidirectionally selected for iodine release rate, determined by in vivo thyroid radiation counts on the second, third, and fourth days after an injection of 5 uc I131 in adult mice (Blizard and Chai 1972). All else being equal (e.g., no variation in hormone receptor densities), a higher release rate is presumed to represent greater thyroid activity. A number of behavioral or behavior-relevant correlated responses were observed. Specifically, the low-release-rate line (= relatively hypothyroid) had increased defecation in an open-field test, had increased activity (and decreased latency to move) in the open field on only the first day, learned more quickly in a water-maze test, showed no difference in body, pituitary or testes mass, but had smaller adrenal glands and larger brains. Unfortunately, this selection experiment (like many others with rodents) included no control line and no replication, so it is not always clear which line(s) changed in response to selection.
More recently, replicate lines of zebra finches were bred for high or low corticosterone responsiveness to a mild stressor (20 min holding in a cloth bag), while two non-selected control lines were also maintained (Evans et al. 2006). After five generations of selection, an asymmetry in response was observed, with the high-selected lines evolving as would be expected but the low-selected lines apparently not diverging significantly from the control lines. Although circulating corticosterone concentrations differed two- to three-fold between high- and low-selected lines, no statistically significant differences were observed for circulating testosterone levels in adult males. Moreover, no consistent, significant differences were found for any of the life-history traits studied. A subsequent report of generations 4-5 indicated an increase in body size for birds in the low-corticosterone lines, but little effect on male sexual signal quality or dominance ranking, and no simple relationship with measures of immune function (Roberts et al. 2007).
With the wide availability of ELISAs for various hormones, we see excellent prospects for additional studies that selectively breed for endocrine function and then study correlated responses in behavior, life history, etc.
Selective breeding for wheel running
As emphasized elsewhere (Henderson 1989; Henderson 1997; Garland and Rose 2009; Rhodes and Kawecki 2009; Kawecki et al. 2012), replication and control lines are crucial for strong inference with selection experiments. Therefore, the selection experiment that our laboratory began in 1993 includes four replicate lines bred for high levels of voluntary wheel-running behavior (High Runner [HR] lines) and four non-selected control lines (Garland 2003; Swallow et al. 2009). (It does not include any lines selected for low wheel running: we did not intentionally create “couch potatoes.”) The starting (base) population was 112 male and 112 female mice of the outbred Hsd:ICR strain (Swallow et al. 1998). This strain exhibits levels of genetic variation similar to populations of wild house mice and to human populations, and has been used in other selection experiments and several quantitative-genetic analyses of various traits (e.g., Dohm et al. (2001)).
The selection criterion is the total number of revolutions run on days 5 and 6 of a 6-day period of wheel access when mice are young adults. We chose to select after a few days of familiarization with the wheels, which are attached to standard housing cages, to avoid selecting on neophobia (Kronenberger and Medioni 1985). Wheels are large (1.12 m in circumference, the size typically used for rats), reflecting an experimental design choice that anticipated possible future studies of wheel running in other species of rodents (Dewsbury 1980; Chappell and Dlugosz 2009). Further details of the selection protocols are provided elsewhere (Swallow et al. 1998; Garland 2003; Swallow et al. 2009; Careau et al. 2013).
At the outset, we had some very general hypotheses and predictions (see also Rhodes et al. (2005)). First, we expected that selective breeding for high voluntary wheel running would lead to changes in both motivation and ability. Hence, subordinate (lower-level) traits that evolved would involve both neurobiology and exercise physiology. This crudely separates into brain vs. body, so we expected that the structural genes that directly affect motivation vs. ability would be largely different. However, we also presumed that upstream regulatory control of gene expression could share some pathways.
Moreover, certain mechanisms “used” by selection mainly because they affect motivation or ability (e.g., changes in circulating levels of, or receptors for, a particular hormone) might affect numerous other traits, within or between these domains, leading to numerous correlated responses, some of which might be only tangentially related to wheel running. Some of the lower-level traits that evolved might even be somewhat detrimental to aspects of wheel running, other behaviors or reproduction.
In other words, within the general domains of motivation or ability, many of the genes whose frequencies change would likely have pleiotropic effects on other aspects of behavior or physiology. These effects might or might not be predictable. Although we do know a lot about “how organisms work,” the pathways from genes to even lower-level traits (the so-called genotype-phenotype map) are not well understood.
In addition to those general hypotheses, we also predicted changes in a number of exercise-related traits in the HR lines, including increases in endurance capacity, maximal oxygen consumption, blood hemoglobin concentration (or hematocrit), relative heart size, and changes in muscle characteristics. As outlined below, most of these predictions have turned out to be accurate.
Mice in the high-selected lines diverged rapidly from controls (Swallow et al. 1998) and reached apparent selection limits at generations 16–28, depending on sex and line (Careau et al. 2013). The gradual increase in running behavior over many generations is consistent with polygenic inheritance, as would be expected for such a high-level, complex trait. Running on days 1–4 has also increased in the HR lines, with some important changes in the additive-genetic variance-covariance matrix for running across the days (Careau et al. 2015). (An analysis of an advanced intercross population involving one of the four HR lines demonstrated some separate quantitative trait loci affecting days 1–4 versus 5–6 (Kelly et al. 2011).) Interestingly, the increase in distance run was caused mainly by an increase in average running speed, although males also show an increase in running duration (Garland et al. 2011a). Although the HR lines evolved to be smaller in body size and to have reduced body fat (Girard et al. 2007), differences in life-history traits (weaning success, litter size, total litter mass, mean offspring mass, sex ratio at weaning) had not evolved as of generations 21–22 (Girard et al. 2002).
As of the current generation (76), various aspects of exercise physiology and morphology have changed in the HR lines, including increases in endurance capacity (Meek et al. 2009), maximal oxygen consumption (Dlugosz et al. 2009; Kolb et al. 2010), heart size (Kolb et al. 2010), size of femoral head and hindlimb symmetry (Garland and Freeman 2005), along with reductions in muscle mass (Garland et al. 2002; Kelly et al. 2013) and alterations in muscle fiber type (Bilodeau et al. 2009). In addition to an increased ability for aerobically supported exercise, mice from the HR lines have alterations in the brain reward system that presumably indicate higher motivation for wheel running (Rhodes et al. 2005; Belke and Garland 2007; Rhodes and Kawecki 2009; Keeney et al. 2012). Moreover, the HR lines exhibit some evidence of being addicted to wheel running (Kolb et al. 2013).
Although the HR lines have evolved to be smaller in both body mass and length under most conditions (Swallow et al. 1999; Kelly et al. 2006; Copes et al. 2015), it is not entirely clear if this would be beneficial for sustained, endurance-type exercise. A similar question concerns their generally reduced body fat (Swallow et al. 2001; Girard et al. 2007; Nehrenberg et al. 2009; but see Vaanholt et al. (2007b); Meek et al. (2014); Acosta et al. (2015)). Studies of circulating hormones (next section) indicate that two produced by white adipose tissue (adiponectin, leptin) show altered concentrations in the HR mice, even after adjusting statistically for body fat.
The HR lines show evidence of increased adaptive plasticity for some traits (Houle-Leroy et al. 2000; Garland and Kelly 2006; Kelly et al. 2006; Middleton et al. 2008). Perhaps most dramatically, HR and C mice did not differ significantly in gastrocnemius GLUT-4 transporter protein abundance when housed without wheels, but after five days of wheel access HR mice had 2.4-fold more GLUT-4 than C mice (Gomes et al. 2009). This difference was not a simple linear function of the amount of wheel running on the previous day, which is much higher in HR mice. Therefore, this is arguably a case of the evolution of increased “self-induced adaptive plasticity” in the HR lines (Swallow et al. 2005).
Endocrine studies of the HR mice
The potential bidirectional relationships (Fig. 1) between endocrine function and exercise behavior are manifold (e.g., Radosevich et al. 1988; Coleman et al. 1998; Girard and Garland 2002; Droste et al. 2007; Sinclair et al. 2014). Accordingly, a number of studies have addressed aspects of endocrine differences between HR and control mice, with the vast majority considering circulating hormone concentrations (adiponectin, corticosterone, IGF-1, insulin, leptin, testosterone, thyroxine, triiodothyronine). A few studies have reported masses of endocrine organs (adrenal glands, ovaries, testes: Klomberg et al. 2002; Swallow et al. 2005; Malisch et al. 2007). In total, these studies are far from providing a complete picture of endocrine evolution in the HR lines, in part because they have mostly included only one sex, age, generation, and housing condition (e.g., with or without wheels). The following paragraphs highlight some of the findings. A few studies with collaborators in the Netherlands have, due to logistical constraints, included only a subset of the HR and control lines, and these will not be considered here (Vaanholt et al. 2007a; Vaanholt et al. 2008; Guidotti et al. 2016).
Mice from the HR lines have higher baseline adiponectin levels, even after accounting statistically for their lower body fat (Vaanholt et al. 2007b). Adiponectin is a protein hormone produced by adipose tissue and is the most abundant adipokine in the circulation that can access the brain. It has many functions, with more being discovered on a regular basis (Stofkova 2009; Galic et al. 2010). Adiponectin is involved in energy balance, glucose regulation, lipolysis, and some aspects of the brain reward system. In general, it is viewed as having a positive role in metabolic health, working against the metabolic syndrome and type 2 diabetes. Circulating concentrations of adiponectin are also known to respond to physical exercise (Saunders et al. 2012). Therefore, it seems possible that this hormone is involved in both motivation and ability of the HR mice.
Baseline plasma corticosterone concentrations are elevated approximately two-fold in HR mice of both sexes (Malisch et al. 2007). We have found no differences in the circadian pattern of blood corticosterone concentrations nor in levels of corticosterone-binding globulin, and so we believe that the higher corticosterone levels likely have functional significance (Malisch et al. 2008). Glucocorticoids are not just “stress hormones” in the conventional sense. They are generally involved in energy mobilization during exercise, may stimulate physical activity (Sibold et al. 2011; Malisch et al. in revision; references therein), and may interact with the brain’s reward system. Various studies of multiple species of mammals, including the HR mice, show that circulating corticosterone levels respond to physical exercise, both acutely and chronically (Coleman et al. 1998; Girard and Garland 2002; Droste et al. 2007). As with adiponectin, it seems that this hormone may be involved with both motivation and ability of the HR mice.
Although one can make a case that elevated corticosterone levels have evolved adaptively in the HR lines, we have also considered the possibility that they could be a nonadaptive or even maladaptive byproduct of changes in the brain (e.g., the hypothalamus: Malisch et al. 2007). Elevated glucocorticoids can reduce growth rates in mammals, and we have found correlational evidence that this may have occurred in the HR mice. Specifically, mean baseline corticosterone levels for each sex are negatively related to body mass across the eight lines (Malisch et al. 2007). In addition, at the level of individual variation, growth over days 26–55 of age was negatively related to mean nighttime corticosterone levels in females from both HR and control lines (Girard and Garland 2002). Most recently, we have found that adding corticosterone hemisuccinate to drinking water confirms the negative effects on growth rates from weaning to adulthood in males of both HR and control lines (J. S. Singleton and T. Garland, unpublished results).
Much as glucocorticoids have been pigeon-holed as “stress hormones,” leptin has perhaps been overemphasized as the “obesity hormone.” In fact, the endocrine effects of leptin in both peripheral tissues and the central nervous system are manifold (e.g., Zhang and Scarpace 2006; Stofkova 2009; Galic et al. 2010; Balland and Cowley 2015; Houweling et al. 2015) and should be viewed from a broader perspective. Relatively few studies have examined leptin’s effect on voluntary exercise (e.g., Choi et al. 2008; Morton et al. 2010; Meek et al. 2012; Fernandes et al. 2015); however, given its pervasive effects, it is safe to presume that leptin may influence both motivation and ability for voluntary exercise.
Consistent with their usually lower amounts of body fat, young adult female HR mice have lower baseline circulating leptin levels than do mice from the control lines. This difference is statistically significant even when using body fat as a covariate in the statistical model (Girard et al. 2007). However, not all studies have found reduced leptin levels in HR mice (Vaanholt et al. 2007b), and one report indicates dramatically different effects of early-life wheel access on adult leptin levels in the HR (decreased) and their control (increased) lines (Acosta et al. 2015).
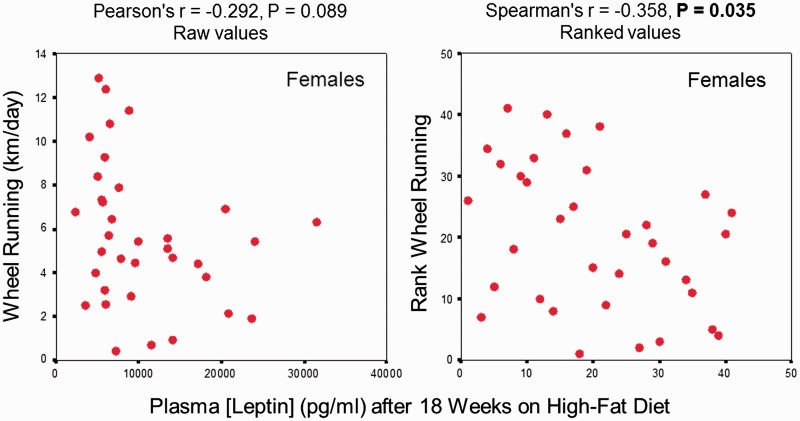
The Mouse Phenome Database (MPD; Jackson Labs) provides a wealth of tools for exploring both phenotypic and genetic associations of traits in laboratory strains of house mice (Bult 2012), which now include some wild-derived strains of other species. Perusal of this data base will make clear that mouse strains show tremendous diversity in many aspects of behavior and endocrine function (e.g., see Spearow et al. (1999); Svenson et al. (2007); Li et al. (2008); Miller et al. (2010)). We searched the MPD for information on wheel running and hormone levels for inbred strains. Among 35 inbred strains of mice, mean daily wheel-running distance is negatively related to circulating leptin levels (Svenson et al. 2007) in females (Fig. 3) but not in males (data not shown). The relationship is statistically significant for ranked values. Further support for a negative relationship between circulating leptin levels and physical activity is provided by various studies of humans (e.g., Franks et al. 2003; Holtkamp et al. 2003: see also Girard et al. (2007)). An important area for future research will be determining whether maternal variation in circulating hormone levels is affecting adult phenotypes of their offspring, possibly in a way that contributes to the selection limits observed in all four of the HR lines (Careau et al. 2013; Careau et al. 2015; Garland et al. 2017).
Fig. 3.
Among 35 inbred strains of mice, mean daily wheel-running distance is negatively related to circulating leptin levels in females, but not for males (values not shown). The relationship is statistically significant for ranked values. Data are from the Mouse Phenome Database at The Jackson Laboratory (http://phenome.jax.org/). Values for wheel running are from Lightfoot (http://phenome.jax.org/db/q?rtn=projects/details&sym=Lightfoot1), whereas leptin levels are from Naggert (http://phenome.jax.org/db/qp?rtn=views/measplot&brieflook=14306&projhint=Naggert1).
Conclusions and future directions
As demonstrated by the examples cited above, complex traits, such as most behaviors, evolve in complicated ways. Artificial selection on vertebrate behavior commonly results in correlated endocrine responses. However, few studies have demonstrated that the endocrine changes are causally related to the evolutionary changes in behavior. Subsequent studies selecting on endocrine function might provide compelling evidence for causality (Garland 2003), but this has yet to be attempted. Nevertheless, the endocrine system includes so many “moving parts” (e.g., see Shire (1976); Young and Crews (1995)) that it may often respond in diverse ways to selection at the level of behavior: “multiple solutions” (Turner et al. 2010; Losos 2011; Garland et al. 2011a) seem likely to be the rule, rather than the exception.
Endocrine-level correlated responses to selection on behavior may occur for several reasons. First, they may represent mechanisms underlying behavioral change at the levels of motivation and/or ability. In this capacity, these effects could occur early in life (i.e., organizational effects), possibly through epigenetic mechanisms, and/or later in life (activational effects). More generally, hormones have the potential to mediate suites of traits that either facilitate or constrain adaptation (or responses to sexual selection), depending on the details of multivariate selection acting on those traits (McGlothlin and Ketterson 2008; Pavličev and Cheverud 2015; see also Garland (1994)).
Second, endocrine responses to selection on behavior may represent ontogenetic or immediate effects of the altered behavior, such as acute changes in circulating corticosterone levels in response to physical activity or longer-term changes resulting from exercise training over many days or weeks (e.g., Droste et al. 2007). As an example of the former, HR mice have higher corticosterone levels compared to control mice during the scotophase, as an acute response to their increased running (Girard and Garland 2002). (The HR mice also have higher baseline circulating corticosterone levels than controls when sampled at rest, during the day (Malisch et al. 2007).)
Third, pleiotropic gene action (reflecting functional relationships) is expected to be pervasive for many aspects of the endocrine system, such that various components may get “dragged along” by selection on higher-level traits. As a consequence, some of the endocrine changes that are observed in response to selection on a particular behavior (e.g., voluntary wheel running) might be nonadaptive or even maladaptive “byproducts” of selection on the target behavior.
Although the approach of selection experiments and experimental evolution (Garland and Rose 2009) is an excellent way to study the consequences of selection on behavior (Garland 2003; Rhodes and Kawecki 2009), establishing causal evolutionary relationships between hormones and behavior remains a difficult undertaking. Nonetheless, the endocrine system is well positioned to mediate coordinated changes in multiple levels of subordinate traits underlying behavior, given the pervasive effects of hormones on sensory systems, integrative systems, motor systems, and effectors. In many cases, these effects should be general enough as to apply to vertebrates in the wild, not just the animals and conditions typically used for selection experiments (e.g., see Supplementary Table S1).
Supplementary Material
Acknowledgments
Thanks to National Science Foundation, NIH, UCR, UW-Madison, many collaborators, postdocs, graduate students, and undergrads! We also acknowledge support from the SICB (Divisions of Animal Behavior, Comparative Endocrinology, Ecology and Evolution, and Evolutionary Developmental Biology) and NSF (IOS 1539936) that facilitated everyone’s participation in the meeting. David Crews and an anonymous reviewer provided very helpful comments on the article.
Supplementary data
Supplementary data available at ICB online.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr., et al. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43:67–82. [DOI] [PubMed] [Google Scholar]
- Acosta W, Meek TH, Schutz H, Dlugosz EM, Vu KT, Garland T., Jr. 2015. Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice. Physiol Behav 149:279–86. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. 2012. Hormonal organization and activation: evolutionary implications and questions. Gen Comp Endocrinol 176:279–85. [DOI] [PubMed] [Google Scholar]
- Alberts JR, Pickler RH. 2012. Evolution and development of dual ingestion systems in mammals: notes on a new thesis and its clinical implications. Int J Pediatr 2012:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert FW, Shchepina O, Winter C, Römpler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, et al. 2008. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm Behav 53:413–21. [DOI] [PubMed] [Google Scholar]
- Arnold AP. 2009. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME. 2004. Co-evolution of steroidogenic and steroid-inactivating enzymes and adrenal and sex steroid receptors. Mol Cell Endocrinol 215:55–62. [DOI] [PubMed] [Google Scholar]
- Bales KL, Saltzman W. 2016. Fathering in rodents: neurobiological substrates and consequences for offspring. Horm Behav 77:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balland E, Cowley MA. 2015. New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol 39:59–65. [DOI] [PubMed] [Google Scholar]
- Barrington EJW. 1987. The phylogeny of the endocrine system In: Csaba GG, editor. Development of Hormone Receptors. Basel, Boston, Springer; p. 137–48. [Google Scholar]
- Belke TW, Garland T., Jr. 2007. A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. J Exp Anal Behav 88:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev DK. 1979. Destabilizing selection as a factor in domestication. J Hered 70:301–8. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. 2007. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav 91:486–98. [DOI] [PubMed] [Google Scholar]
- Bidau CJ, D’Elía G. 2009. Domestication through the centuries: Darwin’s ideas and Dmitry Belyaev’s long-term experiment in silver foxes. Gayana 73:55–72. [Google Scholar]
- Bilodeau GM, Guderley H, Joanisse DR, Garland T., Jr. 2009. Reduction of type IIb myosin and IIB fibers in tibialis anterior muscle of mini-muscle mice from high-activity lines. J Exp Zool Part Ecol Genet Physiol 311A:189–98. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. 2008. Progesterone and progestin receptors in the brain: the neglected ones. Endocrinology 149:2737–8. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Chai CK. 1972. Behavioral studies in mice selectively bred for differences in thyroid function. Behav Genet 2:301–9. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T, Jr., Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–45. [DOI] [PubMed] [Google Scholar]
- Boake CR. 1989. Repeatability: its role in evolutionary studies of mating behavior. Evol Ecol 3:173–82. [Google Scholar]
- Bridgham JT, Ortlund EA, Thornton JW. 2009. An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult CJ. 2012. Bioinformatics resources for behavior studies in the laboratory mouse. Int Rev Neurobiol 104:71–90. [DOI] [PubMed] [Google Scholar]
- Bult A, Lynch CB. 2000. Breaking through artificial selection limits of an adaptive behavior in mice and the consequences for correlated responses. Behav Genet 30:193–206. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Crews D. 2014. Epigenetics in comparative biology: why we should pay attention. Integr Comp Biol 54:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Garland T., Jr. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool 85:543–71. [DOI] [PubMed] [Google Scholar]
- Careau V, Réale D, Humphries MM, Thomas DW. 2010. The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. Am Nat 175:753–8. [DOI] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, Garland T., Jr. 2013. Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution 67:3102–19. [DOI] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, Garland T., Jr. 2015. Evolution of the additive genetic variance–covariance matrix under continuous directional selection on a complex behavioural phenotype. Proc R Soc B Biol Sci 282:20151119.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, Dlugosz EM. 2009. Aerobic capacity and running performance across a 1.6 km altitude difference in two sciurid rodents. J Exp Biol 212:610–9. [DOI] [PubMed] [Google Scholar]
- Choi Y-H, Li C, Hartzell DL, Little DE, Della-Fera MA, Baile CA. 2008. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res 188:100–8. [DOI] [PubMed] [Google Scholar]
- Coleman MA Garland T Jr., Marler CA Newton SS Swallow JG Carter PA.. 1998. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus). Physiol Behav 63:279–85. [DOI] [PubMed] [Google Scholar]
- Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T, Jr., 2015. Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: results from an artificial selection experiment. Physiol Behav 149:86–94. [DOI] [PubMed] [Google Scholar]
- Cox RM, Lovern MB, Calsbeek R. 2014. Experimentally decoupling reproductive investment from energy storage to test the functional basis of a life-history trade-off. J Anim Ecol 83:888–98. [DOI] [PubMed] [Google Scholar]
- Cox RM, McGlothlin JW, Bonier F. 2017. Hormones as mediators of phenotypic and genetic integration: an evolutionary genetics approach. Integr Comp Biol 57: in press. [DOI] [PubMed] [Google Scholar]
- Crews D, Moore MC. 2005. Historical contributions of research on reptiles to behavioral neuroendocrinology. Horm Behav 48:384–94. [DOI] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. 2002. Reverse engineering of biological complexity. Science 295:1664–9. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Westrick SE, van Kesteren F. 2017. Relationships between endocrine traits and life histories in wild animals: insights, problems, and potential pitfalls. Integr Comp Biol 57: in press. [DOI] [PubMed] [Google Scholar]
- Darwin CR. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 1st ed London: John Murray. [PMC free article] [PubMed] [Google Scholar]
- Dean AM, Thornton JW. 2007. Mechanistic approaches to the study of evolution: the functional synthesis. Nat Rev Genet 8:675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFries JC, Gervais MC, Thomas EA. 1978. Response to 30 generations of selection for open-field activity in laboratory mice. Behav Genet 8:3–13. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. 1980. Wheel-running behavior in 12 species of muroid rodents. Behav Processes 5:271–80. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud H-R, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH. 2006. Neurobiology of exercise. Obesity 14:345–56. [DOI] [PubMed] [Google Scholar]
- Dlugosz EM, Chappell MA, McGillivray DG, Syme DA, Garland T, Jr., 2009. Locomotor trade-offs in mice selectively bred for high voluntary wheel running. J Exp Biol 212:2612–8. [DOI] [PubMed] [Google Scholar]
- Dohm MR, Hayes JP, Garland T., Jr. 2001. The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper TW. 1995. Canine analogs of human personality factors. J Gen Psychol 122:241–52. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst ACE, Reul JMHM. 2007. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology 86:26–37. [DOI] [PubMed] [Google Scholar]
- Ebert PD, Hyde JS. 1976. Selection for agonistic behavior in wild female Mus musculus. Behav Genet 6:291–304. [DOI] [PubMed] [Google Scholar]
- Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. 2006. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol 19:343–52. [DOI] [PubMed] [Google Scholar]
- Farmer CG. 2000. Parental care: the key to understanding endothermy and other convergent features in birds and mammals. Am Nat 155:326–34. [DOI] [PubMed] [Google Scholar]
- Feder ME. 1987. New directions in ecological physiology. Cambridge; New York: Cambridge University Press. [Google Scholar]
- Feder ME, Bennett AF, Huey RB. 2000. Evolutionary physiology. Annu Rev Ecol Syst 31:315–41. [Google Scholar]
- Feder ME Garland T Jr Marden JH Zera AJ.. 2010. Locomotion in response to shifting climate zones: not so fast. Annual Review of Physiology 72:167–90. [DOI] [PubMed] [Google Scholar]
- Fernandes MFA, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, Fulton S. 2015. Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab 22:741–9. [DOI] [PubMed] [Google Scholar]
- Flint J, DeFries JC, Henderson ND. 2004. Little epistasis for anxiety-related measures in the DeFries strains of laboratory mice. Mamm Genome 15:77–82. [DOI] [PubMed] [Google Scholar]
- Franks PW, Farooqi S, Luan J, Wong M-Y, Halsall I, O'Rahilly S, Wareham NJ. 2003. Does physical activity energy expenditure explain the between-individual variation in plasma leptin concentrations after adjusting for differences in body composition? J Clin Endocrinol Metab 88:3258–63. [DOI] [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR. 2010. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–39. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Garland T, Jr.,, Stevenson SA. 2006. Artificial selection for increased maternal defense behavior in mice. Behav Genet 36:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T,, Jr. 1994. Quantitative genetics of locomotor behavior and physiology in a garter snake In: Boake CRB, editor. Quantitative genetic studies of behavioral evolution. Chicago: University of Chicago Press; p. 251–77. [Google Scholar]
- Garland T,, Jr. 2003. Selection experiments: an under-utilized tool in biomechanics and organismal biology In: Bels VL, Gasc J-P, Casinos A, editors. Vertebrate biomechanics and evolution. Oxford, UK: BIOS Scientific Publishers Ltd; p. 23–56. [Google Scholar]
- Garland T, Jr, Carter PA. 1994. Evolutionary physiology. Annu Rev Physiol 56:579–621. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Freeman PW. 2005. Selective breeding for high endurance running increases hindlimb symmetry. Evolution 59:1851–4. [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA. 2006. Phenotypic plasticity and experimental evolution. J Exp Biol 209:2344–61. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Bennett AF,, Rezende EL. 2005. Phylogenetic approaches in comparative physiology. J Exp Biol 208: 3015–35. [DOI] [PubMed] [Google Scholar]
- Garland T,, Jr., Cadney MD, Waterland RA. 2017. Early-life effects on adult physical activity: concepts, relevance, and experimental approaches. Physiol Biochem Zool in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr., Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. 2011a. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc R Soc B Biol Sci 278:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr., Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. 2002. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution 56:1267–75. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr., Rose MR. (eds). 2009. Experimental evolution: concepts, methods, and applications of selection experiments. Berkeley (CA: ): University of California Press. [Google Scholar]
- Garland T, Jr., Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, et al. 2011b. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Dufour CR, Eichner LJ, Deblois G, Cermakian N. 2011. Estrogen-related receptor α, the molecular clock, and transcriptional control of metabolic outputs. Cold Spring Harb Symp Quant Biol 76:57–61. [DOI] [PubMed] [Google Scholar]
- Girard I, Swallow JG, Carter PA, Koteja P, Rhodes JS, Garland T., Jr. 2002. Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J Appl Physiol 92:1553–61. [DOI] [PubMed] [Google Scholar]
- Girard I, Rezende EL, Garland T,, Jr. 2007. Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool 80:568–79. [DOI] [PubMed] [Google Scholar]
- Girard I, Swallow JG, Carter PA, Koteja P, Rhodes JS, Garland T., Jr. 2002. Maternal-care behavior and life-history traits in house mice (Mus domesticus) artificially selected for high voluntary wheel-running activity. Behav Processes 57:37–50. [DOI] [PubMed] [Google Scholar]
- Gomes FR, Rezende EL, Malisch JL, Lee SK, Rivas DA, Kelly SA, Lytle C, Yaspelkis BB, Garland T., Jr. 2009. Glycogen storage and muscle glucose transporters (GLUT-4) of mice selectively bred for high voluntary wheel running. J Exp Biol 212:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II Gould TD.. 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry 160:636–45. [DOI] [PubMed] [Google Scholar]
- Goymann W, Moore IT, Scheuerlein A, Hirschenhauser K, Grafen A, Wingfield JC. 2004. Testosterone in tropical birds: effects of environmental and social factors. Am Nat 164:327–34. [DOI] [PubMed] [Google Scholar]
- Grattan DR. 2002. Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction 123:497–506. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. 2002. Production and action of estrogens. N Engl J Med 346:340–52. [DOI] [PubMed] [Google Scholar]
- Guidotti S, Meyer N, Przybyt E, Scheurink AJW, Harmsen MC, Garland T, Jr., van Dijk G. 2016. Diet-induced obesity resistance of adult female mice selectively bred for increased wheel-running behavior is reversed by single perinatal exposure to a high-energy diet. Physiol Behav 157:246–57. [DOI] [PubMed] [Google Scholar]
- Henderson ND. 1989. Interpreting studies that compare high-and low-selected lines on new characters. Behav Genet 19:473–502. [DOI] [PubMed] [Google Scholar]
- Henderson ND. 1997. Spurious associations in unreplicated selected lines. Behav Genet 27:145–54. [DOI] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. 2004. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet 34:267–93. [DOI] [PubMed] [Google Scholar]
- Holtkamp K, Herpertz-Dahlmann B, Mika C, Heer M, Heussen N, Fichter M, Herpertz S, Senf W, Blum WF, Schweiger U, et al. 2003. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J Clin Endocrinol Metab 88:5169–74. [DOI] [PubMed] [Google Scholar]
- Hoskins LJ, Volkoff H. 2012. The comparative endocrinology of feeding in fish: insights and challenges. Gen Comp Endocrinol 176:327–35. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy P, Garland T, Jr., Swallow JG, Guderley H. 2000. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol 89:1608–16. [DOI] [PubMed] [Google Scholar]
- Houweling P, Kulkarni RN, Baldock PA. 2015. Neuronal control of bone and muscle. Bone 80:95–100. [DOI] [PubMed] [Google Scholar]
- Huey RB, Hertz PE, Sinervo B. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat 161:357–66. [DOI] [PubMed] [Google Scholar]
- Hyde JS. 1981. A review of selective breeding programs. In: McLearn GE, editor. Development of animal models as pharmacogenetic tools : proceedings of a workshop, December 4–6, 1978, Boulder, (CO): US Department of Health and Human Services Public Health Service Alcohol Drug Abuse and Mental Health Administration National Institute on Alcohol Abuse and Alcoholism, Rockville, Maryland; Washington, DC. p. 59–77.
- Hyde JS, Ebert PD. 1976. Correlated response in selection for aggressiveness in female mice. I. Male aggresiveness. Behav Genet 6:421–7. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Sawyer TF. 1979. Correlated characters in selection for aggressiveness in female mice. II. Maternal aggressiveness. Behav Genet 9:571–7. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Sawyer TF. 1980. Selection for agonistic behavior in wild female mice. Behav Genet 10:349–59. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL. 2013. Sexual differentiation of the adolescent rodent brain: Hormonal influences and developmental mechanisms. Horm Behav 64:203–10. [DOI] [PubMed] [Google Scholar]
- Kawata M. 2001. Subcellular steroid/nuclear receptor dynamics. Arch Histol Cytol 64:353–68. [DOI] [PubMed] [Google Scholar]
- Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC. 2012. Experimental evolution. Trends Ecol E 27:547–60. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T., Jr. 2012. Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior. Pharmacol Biochem Behav 101:528–37. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Bell TA, Selitsky SR, Buus RJ, Hua K, Weinstock GM, Garland T, Jr., Pardo-Manuel de Villena F, Pomp D. 2013. A novel intronic single nucleotide polymorphism in the Myosin heavy polypeptide 4 gene is responsible for the Mini-Muscle phenotype characterized by major reduction in hind-limb muscle mass in mice. Genetics 195:1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, Blank KM, Garland T., Jr. 2006. Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol 267:360–74. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Nehrenberg DL, Hua K, Garland T, Jr., Pomp D. 2011. Exercise, weight loss, and changes in body composition in mice: phenotypic relationships and genetic architecture. Physiol Genomics 43:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp TS. 2006. The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure. Zool J Linn Soc 147:473–88. [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. 2009. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol 49:365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan VAL, Cawthorn MJ, Parker PG, Ziegenfus C. 1996. Phenotypic engineering: using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis 138:70–86. [Google Scholar]
- Klomberg KF, Garland T, Jr, Swallow JG, Carter PA. 2002. Dominance, plasma testosterone levels, and testis size in house mice artificially selected for high activity levels. Physiol Behav 77:27–38. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. 2005. Divergent selection for aerobic capacity in rats as a model for complex disease. Integr Comp Biol 45:405–15. [DOI] [PubMed] [Google Scholar]
- Kolb EM, Kelly SA, Garland T,, Jr. 2013. Mice from lines selectively bred for high voluntary wheel running exhibit lower blood pressure during withdrawal from wheel access. Physiol Behav 112113:49–55. [DOI] [PubMed] [Google Scholar]
- Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, Garland T., Jr. 2010. Erythropoietin elevates VO2, max but not voluntary wheel running in mice. J Exp Biol 213:510–9. [DOI] [PubMed] [Google Scholar]
- Koteja P. 2000. Energy assimilation, parental care and the evolution of endothermy. Proc R Soc Lond B Biol Sci 267:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger J-P, Medioni J. 1985. Food neophobia in wild and laboratory mice (Mus musculus domesticus). Behav Processes 11:53–9. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM, Melmed S, Larsen PR, Polonsky KS. 2016. Principles of endocrinology In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams textbook of endocrinology, 13 ed. Elsevier; p. 2–11. [Google Scholar]
- Kukekova AV, Temnykh SV, Johnson JL, Trut LN, Acland GM. 2012. Genetics of behavior in the silver fox. Mamm Genome 23:164–77. [DOI] [PubMed] [Google Scholar]
- Lazar MA, Birnbaum MJ. 2016. Principles of hormone action In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams textbook of endocrinology, 13 ed Philadelphia. Elsevier; p. 18–48. [Google Scholar]
- Lea RW, Vowles DM, Dick HR. 1986. Factors affecting prolactin secretion during the breeding cycle of the ring dove (Streptopelia risoria) and its possible role in incubation. J Endocrinol 110:447–58. [DOI] [PubMed] [Google Scholar]
- Lennartsson A, Ekwall K. 2009. Histone modification patterns and epigenetic codes. Biochim Biophys Acta BBA 1790:863–8. [DOI] [PubMed] [Google Scholar]
- Lindzey J, Crews D. 1992. Interactions between progesterone and androgens in the stimulation of sex behaviors in male little striped whiptail lizards, Cnemidophorus inornatus. Gen Comp Endocrinol 86:52–8. [DOI] [PubMed] [Google Scholar]
- Lindzey J, Crews D. 1993. Effects of progesterone and dihydrotestosterone on stimulation of androgen-dependent sex behavior, accessory sex structures, and in vitro binding characteristics of cytosolic androgen receptors in male whiptail lizards (Cnemidophorus inornatus). Horm Behav 27:269–81. [DOI] [PubMed] [Google Scholar]
- Li R, Svenson KL, Donahue LRB, Peters LL, Churchill GA. 2008. Relationships of dietary fat, body composition, and bone mineral density in inbred mouse strain panels. Physiol Genomics 33:26–32. [DOI] [PubMed] [Google Scholar]
- Little AG, Seebacher F. 2014. The evolution of endothermy is explained by thyroid hormone-mediated responses to cold in early vertebrates. J Exp Biol 217:1642–8. [DOI] [PubMed] [Google Scholar]
- Lobo I. 2008. Biological complexity and integrative levels of organization. Nat Educ 1:141. [Google Scholar]
- Losos JB. 2011. Convergence, adaptation, and constraint. Evolution 65:1827–40. [DOI] [PubMed] [Google Scholar]
- Lynch CB. 1980. Response to divergent selection for nesting behavior in Mus musculus. Genetics 96:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CB. 1994. Evolutionary inferences from genetic analyses of cold adaptation in laboratory and wild populations of the house mouse. In: Boake CRB, editor. Quant Genet Stud Behav Evol Chicago: University of Chicago Press, 278–301. [Google Scholar]
- Magni P, Dozio E, Ruscica M, Celotti F, Masini MA, Prato P, Broccoli M, Mambro A, Morè M, Strollo F. 2009. Feeding behavior in mammals including humans. Ann N Y Acad Sci 1163:221–32. [DOI] [PubMed] [Google Scholar]
- Majdak P, Bucko PJ, Holloway AL, Bhattacharya TK, DeYoung EK, Kilby CN, Zombeck JA, Rhodes JS. 2014. Behavioral and pharmacological evaluation of a selectively bred mouse model of home cage hyperactivity. Behav Genet 44:516–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW. 2010. Steroid-binding proteins and free steroids in birds. Mol Cell Endocrinol 316:42–52. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr. 2008. Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol 156:210–7. [DOI] [PubMed] [Google Scholar]
- Malisch JL, deWolski K, Meek TH, Acosta W, Middleton KM, Crino OL, Garland T., Jr. (in revision) Acute restraint stress alters wheel-running behavior immediately following stress and up to 20 hours later in house mice. Physiol Biochem Zool. [DOI] [PubMed]
- Malisch JL, Saltzman W, Gomes FR, Rezende EL, Jeske DR, Garland T., Jr. 2007. Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol Biochem Zool 80:146–56. [DOI] [PubMed] [Google Scholar]
- Mayr E. 1960. The emergence of evolutionary novelties. In: Tax S, editor. Evolution after Darwin. Vol. I. The evolution of life, its origin, history and future. Chicago: University of Chicago Press; p. 349–80. [Google Scholar]
- McCarthy MM, Arnold AP. 2011. Reframing sexual differentiation of the brain. Nat Neurosci 14:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Philos Trans R Soc B Biol Sci 363:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek TH, Dlugosz EM, Vu KT, Garland T,, Jr. 2012. Effects of leptin treatment and Western diet on wheel running in selectively bred high runner mice. Physiol Behav 106:252–8. [DOI] [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, Keeney BK, Hannon RM, Dlugosz EM, Garland T., Jr. 2014. Effects of early-life exposure to Western diet and wheel access on metabolic syndrome profiles in mice bred for high voluntary exercise. Genes Brain Behav 13:322–32. [DOI] [PubMed] [Google Scholar]
- Meek TH, Lonquich BP, Hannon RM, Garland T,, Jr. 2009. Endurance capacity of mice selectively bred for high voluntary wheel running. J Exp Biol 212:2908–17. [DOI] [PubMed] [Google Scholar]
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. (eds) 2016. Williams textbook of endocrinology, 13 ed Philadelphia., Elsevier. [Google Scholar]
- Middleton KM, Kelly SA, Garland T,, Jr. 2008. Selective breeding as a tool to probe skeletal response to high voluntary locomotor activity in mice. Integr Comp Biol 48:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT. 2010. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS ONE 5:e14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. 2010. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. AJP Endocrinol Metab 300:E392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrenberg DL, Hua K, Estrada-Smith DGarland T, Jr Pomp D 2009. Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity 17:1402–9. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. 2011. An introduction to behavioral endocrinology. 4th ed Sunderland (MA: ): Sinauer Associates. [Google Scholar]
- Nepomnaschy PA, Vitzthum VJ, Flinn MV. 2009. Evolutionary endocrinology: Integrating proximate mechanisms, ontogeny, and evolved function. Am J Hum Biol 21:728–30. [DOI] [PubMed] [Google Scholar]
- Nespolo RF, Bacigalupe LD, Figueroa CC, Koteja P, Opazo JC. 2011. Using new tools to solve an old problem: the evolution of endothermy in vertebrates. Trends Ecol E 26:414–23. [DOI] [PubMed] [Google Scholar]
- Nicol C.J. 2015. The behavioral biology of chickens. Wallingford, U.K.: CABI. [Google Scholar]
- Nicolaides NC, Charmandari E, Chrousos GP, Kino T. 2014. Recent advances in the molecular mechanisms determining tissue sensitivity to glucocorticoids: novel mutations, circadian rhythm and ligand-induced repression of the human glucocorticoid receptor. BMC Endocr Disord 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff AB. 1945. The concept of integrative levels and biology. Science 101:209–15. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA, Hughes M, Podos J. 2001. The evolution of bird song: male and female response to song innovation in swamp sparrows. Anim Behav 62:1189–95. [Google Scholar]
- Pasch B, George AS, Hamlin HJ, Guillette LJ, Phelps SM. 2011. Androgens modulate song effort and aggression in Neotropical singing mice. Horm Behav 59:90–7. [DOI] [PubMed] [Google Scholar]
- Pavličev M, Cheverud JM. 2015. Constraints evolve: context dependency of gene effects allows evolution of pleiotropy. Annu Rev Ecol Evol Syst 46:413–34. [Google Scholar]
- Pfaff DW, Phillips MI, Rubin RT. 2004. Principles of hormone behavior relations. Amsterdam; Boston: Elsevier Academic Press. [Google Scholar]
- Phelps SM, Lydon JP, O’Malley BW, Crews D. 1998. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav 34:294–302. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–82. [DOI] [PubMed] [Google Scholar]
- Radosevich PM, Lacy DB, Brown LL, Williams PE, Abumrad NN. 1988. Effects of insulin-induced hypoglycemia on plasma and cerebrospinal fluid levels of ir-β-endorphins, ACTH, cortisol, norepinephrine, insulin and glucose in the conscious dog. Brain Res 458:325–38. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Diniz-Filho JAF. 2012. Phylogenetic analyses: comparing species to infer adaptations and physiological mechanisms. Compr Physiol 2:639–74. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr. 2005. Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol 45:438–55. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Kawecki TJ. 2009. Behavior and neurobiology In: Garland Theodore, Jr., Rose Michael R., editors. Experimental evolution: concepts, methods, and applications of selection experiments. Berkeley (CA: ): University of California Press; p. 263–300. [Google Scholar]
- Roberts ML, Buchanan KL, Hasselquist D, Bennett ATD, Evans MR. 2007. Physiological, morphological and behavioural effects of selecting zebra finches for divergent levels of corticosterone. J Exp Biol 210:4368–78. [DOI] [PubMed] [Google Scholar]
- Sadowska J, Gębczyński AK, Konarzewski M. 2015. Effect of reproduction on the consistency of the between-line type divergence in laboratory mice selected on basal metabolic rate. Physiol Biochem Zool 88:328–335. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Takahashi H, Matsuda K, Nishi M, Takanami K, Ogoshi M, Sakamoto T, Kawata M. 2012. Rapid signaling of steroid hormones in the vertebrate nervous system. Front Biosci Landmark Ed 17:996–1019. [DOI] [PubMed] [Google Scholar]
- Saunders TJ, Palombella A, McGuire KA, Janiszewski PM, Després J-P, Ross R. 2012. Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab 2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TM, Bowen WD. 2005. The evolution of lactation strategies in pinnipeds: a phylogenetic analysis. Ecol Monogr 75:159–77. [Google Scholar]
- Schwartz TS, Bronikowski AM. 2017. The insulin and insulin-like signaling network in ecothermic reptiles: more questions than answers? Integr Comp Biol 57: in press. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA. 2008. Social correlates of testosterone and ornamentation in male mandrills. Horm Behav 54:365–72. [DOI] [PubMed] [Google Scholar]
- Shire JGM. 1976. The forms, uses and significance of genetic variation in endocrine systems. Biol Rev 51:105–41. [DOI] [PubMed] [Google Scholar]
- Sibold JS, Hammack SE, Falls WA. 2011. C57 mice increase wheel-running behavior following stress: preliminary findings. Percept Mot Skills 113:605–18. [DOI] [PubMed] [Google Scholar]
- Sinclair ELE, de Souza CRN, Ward AJW, Seebacher F. 2014. Exercise changes behaviour. Funct Ecol 28:652–9. [Google Scholar]
- Sodersten P. 2015. Steinach and Young, discoverers of the effects of estrogen on male sexual behavior and the “male brain”. eNeuro November/December 2015, 2(6) e0058-15.2015 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D, Stirling DG, Lefebvre L. 2005. Behavioral drive or behavioral inhibition in evolution: subspecific diversification in holarctic passerines. Evolution 59:2669–77. [PubMed] [Google Scholar]
- Sower SA, Freamat M, Kavanaugh SI. 2009. The origins of the vertebrate hypothalamic–pituitary–gonadal (HPG) and hypothalamic–pituitary–thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol 161:20–9. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. 1999. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 285:1259–61. [DOI] [PubMed] [Google Scholar]
- Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J Evol Biol 15:277–89. [Google Scholar]
- Stofkova A. 2009. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul 43:157–68. [PubMed] [Google Scholar]
- Storz JF, Bridgham JT, Kelly SA, Garland T., Jr. 2015. Genetic approaches in comparative and evolutionary physiology. Am J Physiol - Regul Integr Comp Physiol 309:R197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Watt MJ, Ling TL, Forster GL, Carpenter RE, Korzan WJ, Lukkes JL, Øverli Ø. 2005. Glucocorticoid interaction with aggression in non-mammalian vertebrates: Reciprocal action. Eur J Pharmacol 526:21–35. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. 2007. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol 102:2369–78. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T,, Jr. 1998. Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28:227–37. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Garland T., Jr. 2005. Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits—an introduction to the symposium. Integr Comp Biol 45:387–90. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Hayes JP, Koteja P, Garland T,, Jr. 2009. Selection experiments and experimental evolution of performance and physiology In: Garland Theodore, Jr., Rose Michael R., editors. Experimental evolution: concepts, methods, and applications of selection experiments. Berkeley (CA: ): University of California Press; p. 301–51. [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T,, Jr. 1999. Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J Exp Biol 202:2513–20. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T,, Jr. 2001. Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol [B] 171:651–9. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Rhodes JS, Garland T., Jr. 2005. Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integr Comp Biol 45:426–37. [DOI] [PubMed] [Google Scholar]
- Thornton JW. 2001. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci 98:5671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trut LN. 1999. Early canid domestication: the farm-fox experiment. Am Sci 87:160–9. [Google Scholar]
- Trut LN., Plyusnina IZ, Oskina IN. 2004. An experiment on fox domestication and debatable issues of evolution of the dog. Russian Journal of Genetics 40:644–55. [PubMed] [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. 2010. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol Biol E 27:1269–1278. [DOI] [PubMed] [Google Scholar]
- Vaanholt LM, De Jong B, Garland T, Jr., Daan S, Visser GH. 2007a. Behavioural and physiological responses to increased foraging effort in male mice. J Exp Biol 210:2013–2024. [DOI] [PubMed] [Google Scholar]
- Vaanholt L, Meerlo P, Garland T, Jr., Visser G, Dijk G van. 2007b. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per sé. Horm Metab Res 39:377–383. [DOI] [PubMed] [Google Scholar]
- Vaanholt LM, Jonas I, Doornbos M, Schubert KA, Nyakas C, Garland T, Jr., Visser GH, van Dijk G. 2008. Metabolic and behavioral responses to high-fat feeding in mice selectively bred for high wheel-running activity. Int J Obes 32:1566–75. [DOI] [PubMed] [Google Scholar]
- Waterland RA. 2014. Epigenetic mechanisms affecting regulation of energy balance: many questions, few answers. Annu Rev Nutr 34:337–55. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Garza C. 1999. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr 69:179–97. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. 1990. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–46. [Google Scholar]
- Witt DM, Young LJ, Crews D. 1994. Progesterone and sexual behavior in males. Psychoneuroendocrinology 19:553–562. [DOI] [PubMed] [Google Scholar]
- Woolley S, Omalley B, Lydon J, Crews D. 2006. Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behav Brain Res 167:197–204. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D. 2004. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol Behav 83:347–60. [DOI] [PubMed] [Google Scholar]
- Young LJ, Crews D. 1995. Comparative neuroendocrinology of steroid receptor gene expression and regulation: Relationship to physiology and behavior. Trends Endocrinol Metab 6:317–23. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG, Williams TD. 2007. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu Rev Ecol Evol Syst 38:793–817. [Google Scholar]
- Zera AJ, Zhang C. 1995. Evolutionary endocrinology of juvenile hormone esterase in Gryllus assimilis: direct and correlated responses to selection. Genetics 141:1125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Scarpace PJ. 2006. The role of leptin in leptin resistance and obesity. Physiol Behav 88:249–56. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, DeYoung EK, Brzezinska WJ, Rhodes JS. 2011. Selective breeding for increased home cage physical activity in Collaborative Cross and Hsd:ICR mice. Behav Genet 41:571–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.