Abstract
Introduction
Sleep disturbances are common non-motor symptoms in Parkinson's disease (PD). Experimental studies suggest involvement of the serotonergic system in the regulation of sleep and arousal. Using [11C]DASB positron emission tomography, a marker of serotonin transporter availability, we investigated whether sleep dysfunction is associated with serotonergic dysfunction in PD.
Methods
We studied 14 PD patients with sleep dysfunction, 14 PD without sleep dysfunction, and 12 healthy controls. Groups were matched for age, disease duration, severity of motor symptoms, daily intake of levodopa equivalent units, body-mass-index, depression and fatigue. [11C]DASB non-displaceable binding potential (BPND) was calculated for regions with a role in the regulation of sleep and arousal.
Results
[11C]DASB BPND was reduced by 32–49% in PD patients with sleep dysfunction, and 14–25% in PD without sleep dysfunction, compared to healthy controls. PD patients with sleep dysfunction had lower [11C]DASB BPND in caudate (P < 0.01), putamen (P < 0.001), ventral striatum (P < 0.001), thalamus (P < 0.05), hypothalamus (P < 0.001) and raphe nuclei (P < 0.01), compared to PD without sleep dysfunction. Higher severity of sleep symptoms (assessed with Parkinson Disease Sleep Scale) correlated with lower [11C]DASB binding in caudate (r = 0.77; P < 0.001), putamen (r = 0.84; P < 0.001), ventral striatum (r = 0.86; P < 0.001), thalamus (r = 0.79; P < 0.001), hypothalamus (r = 0.90; P < 0.001) and raphe nuclei (r = 0.83; P < 0.001).
Conclusions
Our findings demonstrate that sleep dysfunction in PD is associated with reduced serotonergic function in the midbrain raphe, basal ganglia and hypothalamus. Strategies to increase serotonin levels in the brain could be a promising approach to treat sleep dysfunction in PD, and may also have relevance in other neurodegenerative disorders.
Abbreviations: 5-HT, hydroxytryptophan (serotonin); BPND, non-displaceable binding potential; DASB, [N,N-dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine]; EDS, excessive daytime somnolence; ROI, region of interest; RBD, rapid eye movement behaviours disorders; SERT, serotonin transporter
Keywords: Parkinson's disease, Sleep disturbances, Serotonin, DASB, PET
Highlights
-
•
Reduced PET [11C]DASB binding in Parkinson patients with sleep disturbances
-
•
PD with sleep disturbances shows loss of serotonin in the striatum, raphe and hypothalamus.
-
•
Loss of serotonin correlated with severity of sleep symptoms in PD patients.
-
•
Serotonergic dysfunction could contribute to pathophysiology of sleep disturbances.
1. Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterised by the cardinal motor symptoms of bradykinesia, rigidity and tremor, and a range of frequent non-motor symptoms such as sleep disturbances, depression and chronic fatigue. Sleep disturbances are estimated to affect between 15 and 96% of patients with PD, constituting a significant source of complaint and affecting quality of life (Selvaraj and Keshavamurthy, 2016; Menza et al., 2010). Sleep disturbances in PD are typically divided to insomnia, parasomnias including rapid eye movement (REM) behaviours disorders (RBD), excessive daytime sleepiness (EDS) and drug induced sleep dysfunctions (Jahan et al., 2009).
Neurochemical systems including acetylcholine, noradrenaline, dopamine, histamine, orexin/hypocretin and serotonin interact to regulate the sleep-wake cycle (Saper et al., 2005). Mechanisms underlying the pathophysiology, and high prevalence, of sleep dysfunction in PD remain poorly understood. The occurrence of sleep dysfunction predating motor symptoms in PD raises the possibility that Lewy body pathology could play a role. Pre-clinical Parkinson's stages reflect the spread of Lewy body pathology to the raphe nucleus (Braak et al., 2003), the primary source of serotonin (5-HT) neurotransmitter. Serotonergic projections innervate brain regions involved in the regulation of sleep, wakefulness and arousal including the forebrain, hypothalamus, thalamus, basal ganglia and preoptic area (Wallman et al., 2011).
Post-mortem studies revealed up to 50% loss of serotonergic neurons in the brains of patients with PD (Halliday et al., 1990). Biochemical studies confirmed these findings demonstrating decreased levels of serotonin transporter (SERT) protein in striatal and extra-striatal areas (Kish, 2003; Kish et al., 2008). Positron emission tomography (PET) molecular imaging studies measuring SERT availability have revealed reductions of striatal and extra-striatal 5-HT including raphe nuclei, thalamus and hypothalamus (Albin et al., 2008; Guttman et al., 2007; Politis et al., 2010a). Furthermore, PET studies have linked serotonergic dysfunction with the development of dyskinesias (Politis et al., 2014), tremor (Loane et al., 2013), depression (Politis et al., 2010b), apathy and anxiety (Maillet et al., 2016), fatigue (Pavese et al., 2010), weight loss (Politis et al., 2011) and visual hallucinations (Ballanger et al., 2010) in PD. Therefore, there is compelling evidence from preclinical, post-mortem and human imaging studies for the role of serotonergic dysfunction in PD.
The serotonergic system plays a critical role in the sleep-wake cycle and its dysfunction could have relevance to the development of sleep disturbances in PD. Animal studies have demonstrated that lesions in the raphe nucleus result in reduced sleep which is associated with lower levels of serotonin (Jouvet, 1972). Changes in raphe nuclei following sleep deprivation included increases in neuronal size, increased firing during wake and downregulation of 5-HT receptors (Hipolide et al., 2005). Preclinical PET studies with [11C]DASB demonstrated decreases in SERT binding in rats deprived of sleep (Hipolide et al., 2005). Furthermore, PET studies in patients with PD have revealed reductions in SERT availability in key brain regions involved in the regulation of sleep and wakefulness (Politis et al., 2010a).
In this study, we hypothesized that PD patients with sleep dysfunction, as clinically identified using the original version of Parkinson Disease Sleep Scale (PDSS) (Chaudhuri et al., 2002), would show reduced serotonergic function in brain areas modulating sleep and arousal. The PDSS is widely validated and recommended by the MDS task force for clinical assessment of a range of sleep disorders and severity in PD (Hogl et al., 2010). We sought to investigate this in vivo using [11C]DASB PET molecular imaging, as a marker of SERT availability.
2. Material and methods
2.1. Participants
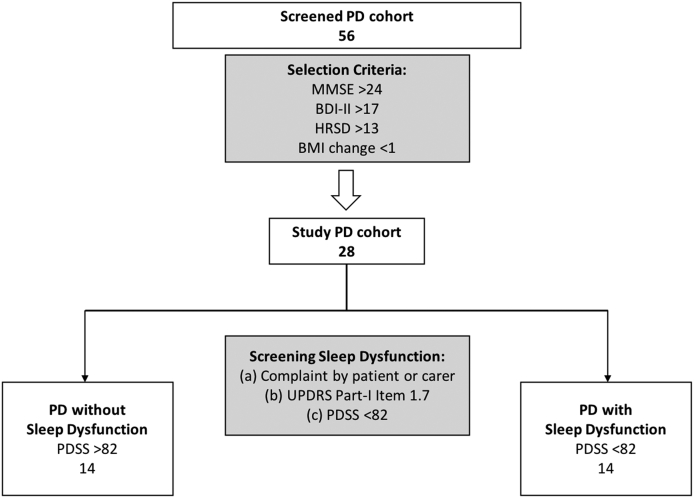
Patient selection was carried out from a cohort of 56 patients with a clinical diagnosis of idiopathic PD (Fig. 1), according to the UK Brain Bank criteria, recruited from specialist Movement Disorders clinics at King's College Hospital. Based on study selection criteria, Parkinson's patients with dementia [Mini-Mental State Examination (MMSE) < 24], depression [Hamilton Rating Scale for Depression (HRSD) > 13 and Beck Depression Inventory-II (BDI-II) > 17], fatigue [Non-Motor Symptom Scale (NMSS) fatigue sub-item], and abnormal weight gain or loss [Body Mass Index (BMI) change > 1 unit in the last 12 months] were excluded from the study. Twelve age-matched healthy individuals, with no history of neurological or psychiatric disorders and no family history of PD, served as the control group (Table 1).
Fig. 1.
Summary flow-chat depicting the inclusion criteria and selection process of Parkinson's patients with and without sleep dysfunction. PD = Parkinson's disease; PDSS = Parkinson's disease Sleep Scale; UPDRS-III = Unified Parkinson's Disease Rating Scale part-III; H&Y = Hoehn & Yahr; BDI-II = Beck Depression Inventory-II; HRSD = Hamilton Rating Scale for Depression; MMSE = Mini Mental State Examination.
Table 1.
Clinical characteristics of patients with Parkinson's disease and healthy controls.
| Healthy controls | PD without sleep dysfunction | PD with sleep dysfunction | |
|---|---|---|---|
| Number of subjects | 12 | 14 | 14 |
| Age (years ± SD) | 63.4 (±7.1) | 67.4 (±4.2) | 65.9 (±8.8) |
| BMI (mean ± SD) | 25.9 (±3.5) | 25.5 (±3.6) | 24.9 (±4.2) |
| MMSE (mean ± SD) | 29.4 (±0.7) | 29.7 (±0.6) | 28.9 (±1.9) |
| BDI-II (mean ± SD) | 3.1 (±2.6) | 7.4 (±3.5) | 8.4 (±3.1) |
| HRSD (mean ± SD) | 2.6 (±2.7) | 6.2 (±2.6) | 7.1 (±2.3) |
| NMSS (fatigue) | No | No | No |
| Disease duration (years ± SD) | – | 8.1 (±3.6) | 8.7 (±3.1) |
| H&Y OFF (mean ± SD) | – | 2.4 (±1.0) | 3.1 (±0.6) |
| UPDRS-III OFF (mean ± SD) | – | 38.6 (±11.7) | 44.1 (±9.7) |
| Daily LED (mean ± SD) | – | 792.1 (±760.5) | 835.4 (±315.4) |
| PDSS (mean ± SD) | 114.0 (±3.8) | 114.7 (±18.8) | 58.2 (±17.2)⁎⁎⁎ |
Lower PDSS represents worse score (best = 150/worst = 0). PD = Parkinson's disease; PDSS = Parkinson's disease Sleep Scale; UPDRS-III = Unified Parkinson's Disease Rating Scale part-III; H&Y = Hoehn & Yahr; BDI-II = Beck Depression Inventory-II; HRSD = Hamilton Rating Scale for Depression; NMSS = Non-Motor Symptom Scale; MMSE = Mini Mental State Examination.
P < 0.001.
Twenty-eight Parkinson's patients, who met the selection criteria, were divided into two groups according to the presence (n = 14) or absence (n = 14) of sleep dysfunction (Fig. 1; Table 1). We screened for sleep dysfunction based on a three-layer approach: (a) Complaint by the patient or carer; (b) Unified PD Rating Scale (UPDRS) Part-I, Item 1.7 (sleep dysfunction): >2 Mild, Moderate or Severe Sleep dysfunction as opposed to ‘0’ no sleep dysfunction. Patients who scored ‘1’ slight sleep dysfunction were excluded; and (c) by using the PDSS with a validated cut-off of <82 to define sleep dysfunction in patients with Parkinson Disease (Martinez-Martin et al., 2008). The PDSS, cut-off of scores <82, was used for the final classification of the presence of sleep dysfunction.
PDSS was also used to quantify severity of sleep dysfunction, and for interrogating correlations with the PET data. The PDSS is a validated 15-item scale measuring commonly reported symptoms associated with sleep dysfunction (Chaudhuri et al., 2002). Each item is rated from worst (0) to best state (10); the total score is calculated as the sum of the item scores with a maximum score of 150. Lower PDSS scores imply higher burden of sleep symptoms. The PD groups were matched for age, disease duration, severity of motor symptoms [Unified Parkinson's Disease Rating Scale part-III (UPDRS-III) and Hoehn & Yahr (H&Y) scale], daily intake of levodopa equivalent units, body-mass-index, and depression (BDI-II and HRSD) and fatigue levels (NMSS) (Table 1). None of the subjects had dyskinesias or were receiving medication with known action on the serotonergic system, or medication that affects the sleep-wake cycle including trazodone, melatonin, or stimulants.
All participants screened successfully to undertake PET and magnetic resonance imaging (MRI) scanning under standard criteria (http://www.mrisafety.com; https://www.gov.uk/government/publications/arsac-notes-for-guidance). Written informed consent was obtained from all study participants in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards and the research ethics committee. Permission to administer [11C]DASB was obtained from the Administration of Radioactive Substances Advisory Committee, UK.
2.2. Scanning procedures
Participants were scanned using a GE Discovery RX PET/CT scanner. The mean injected dose for [11C]DASB was 400 MBq and scanning began 30 s before tracer infusion as an intravenous bolus, generating 28 time frames of tissue data over 90 min. Subjects were positioned supine with their transaxial planes parallel to the line intersecting the anterior-posterior commissure line. Head position was maintained with the help of individualized foam holders and monitored by video. Subjects were scanned at rest in a quiet room with low light. Smoking and consumption of alcohol, coffee and other caffeinated beverages were not allowed for 12 h before scanning. Eating and drinking were not allowed for 8 h before PET scanning. All PD patients were scanned OFF medication and following an overnight withdraw of their normal medications.
MRI was performed using a clinical 1.5-Tesla MRI system (Siemens MAGNETOM Avanto) for the purposes of PET image co-registration and to facilitate localising the regions of interest (ROIs). The MRI protocol included volumetric T1 sequences (coronal and axial T1-spin echo: TR = 635 ms, TE = 17 ms, 5 mm slice thickness); T1 volumetric MPRAGE (TR = 1900 ms, TE = 3.53 ms, TI = 1100 ms, flip angle 15, 1 mm isotropic voxels), and T2-weighted sequences (axial T2-spin echo: TR = 4540 ms, TE = 97 ms, 5 mm slice thickness; axial FLAIR: TR = 9000 ms, TE = 114 ms, TI = 2500 ms, 5 mm slice thickness).
2.3. [11C]DASB PET analysis
Individual PET frames were corrected for head motion using frame-by-frame realignment using a frame with high signal-to-noise ratio as reference. Summed dynamic [11C]DASB images were created from the entire series of uptake image volumes. Parametric images of [11C]DASB distribution volume ratios (DVR) were generated using the Logan reference model with reference region for non-specific tracer-binding signal in the posterior cerebellar grey matter cortex avoiding inclusion of the vermis (Kish et al., 2005). Regional concentrations of radioactivity (kBq/ml) were obtained from the full dynamic scan and decay-corrected time-activity curves (TACs) were computed. PET images were anatomically co-registered and resliced to the corresponding T1-weighted MRI using the mutual information registration algorithm in the statistical parametric mapping (SPM) software package (Version 12).
Manual delineation of ROIs was performed on co-registered MRI and PET using ANALYZE medical imaging software (version 12, Mayo Foundation). ROIs were manually defined on both hemispheres for the caudate, putamen, ventral striatum, amygdala, thalamus, hypothalamus, globus pallidus, raphe nucleus, anterior cingulate cortex, posterior cingulate cortex, prefrontal cortex and insula. The binding potential of the specially bound radioligand relative to the non-displaceable radioligand (BPND) in the tissue was calculated as DVR-1 (Ginovart et al., 2001). Since changes in BPND could be associated with degeneration or volumetric loss, we confirmed there were no differences in grey matter volumes on individual subjects MRIs between groups of PD patients with and without sleep disturbances and healthy controls; therefore, partial volume correction was not applied.
2.4. Statistical analysis
Statistical analysis and graph illustration were performed with SPSS (version 20 Chicago, Illinois, USA) and GraphPad Prism (version 6.0) respectively. For all variables, variance homogeneity and Gaussianity were tested with Barrlett and Kolmogorov-Smirnov tests, and we proceeded with parametric tests as our PET and clinical data were normally distributed. Regional [11C]DASB BPND values among healthy controls, patients with PD with and without sleep dysfunction were compared using one-way ANOVA followed by Brown-Forsythe and Barrlett's test. P values for each variable were calculated following Bonferroni multiple-comparisons test. When ANOVA P values were significant, univariate comparisons and P values were calculated with Bonferroni post-hoc tests. Effect size was measured with Cohen's d. We interrogated correlations between PET and clinical data using Pearson's r correlation coefficient and we applied the Benjamini-Hochberg multiple-comparisons test to reduce false discovery rate; we set the false discovery rate cut-off at 0.05. All data are presented as mean ± standard deviation (SD). The α level was set at P < 0.05, corrected for multiple comparisons.
3. Results
Clinical measures between the two groups of patients with PD revealed no significant differences in age (P > 0.10; r = 0.12), MMSE scores (P > 0.10; r = 0.48), UPDRS-III scores (P > 0.10; r = −0.20), daily intake of levodopa equivalent units (P > 0.10; r = 0.005), body-mass-index scores (P > 0.10; r = 0.069), HRSD scores (P > 0.10; r = −0.12) and BDI-II scores (P > 0.10; r = −0.18) (Table 1).
3.1. Regional [11C]DASB binding
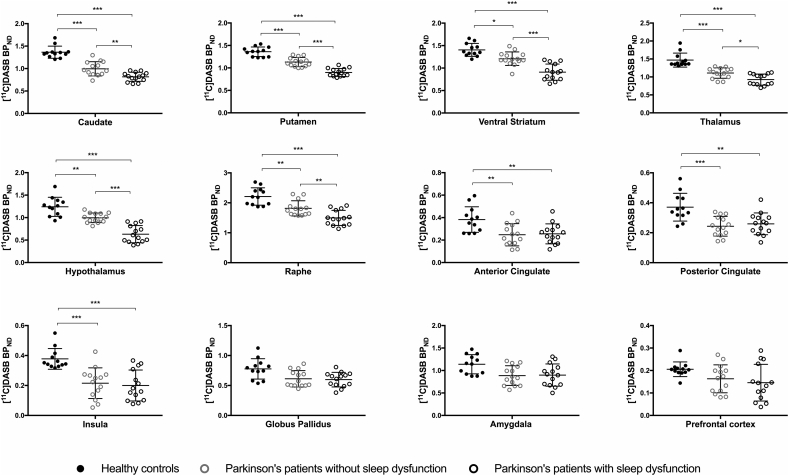
Mean [11C]DASB BPND values in ROIs were decreased by 14–25% in PD patients without sleep dysfunction and by 32–49% in Parkinson's patients with sleep dysfunction, compared to healthy controls (Table 2). Parkinson's patients with sleep dysfunction had decreased [11C]DASB BPND values, after correction for multiple comparisons, in the caudate (P = 0.004; effect size d = 1.33), putamen (P < 0.001; effect size d = 2.32), ventral striatum (P < 0.001; effect size d = 1.99), thalamus (P = 0.017; effect size d = 1.22), hypothalamus (P < 0.001; effect size d = 2.34) and raphe nuclei (P = 0.009; effect size d = 1.27) compared to Parkinson's patients without sleep dysfunction (Table 2, Fig. 2, Fig. 3). While decreased [11C]DASB BPND was observed in the anterior and posterior cingulate and the insula between patients with PD and healthy controls, there were no differences in [11C]DASB BPND between Parkinson's patients with and without sleep dysfunction in these areas. The prefrontal cortex, globus pallidus and amygdala showed no difference between group means as determined by ANOVA analysis (Table 2; Fig. 2). There were no volumetric differences in any regions of interest between the groups of patients with PD and healthy controls (P > 0.10) (Supplemental data: Table e-1).
Table 2.
[11C]DASB binding potential values in groups of Parkinson's disease patients with and without sleep dysfunction and healthy controls.
| ROI | Healthy controls (mean ± SD) | PD control (mean ± SD) | PD sleep dysfunction (mean ± SD) | HC vs PD control (% change; P value) | HC vs PD sleep dysfunction (% change; P value) | PD control vs PD sleep dysfunction (% change; P value) |
|---|---|---|---|---|---|---|
| Caudate | 1.364 (±0.13) | 0.994 (±0.16) | 0.816 (±0.1) | −27.1% P < 0.001 |
−40.1% P < 0.001 |
−17.8% P = 0.004 |
| Putamen | 1.360 (±0.11) | 1.131 (±0.10) | 0.899 (±0.1) | −16.9% P < 0.001 |
−33.9% P < 0.001 |
−20.5% P < 0.001 |
| Ventral striatum | 1.404 (±0.14) | 1.208 (±0.15) | 0.909 (±0.18) | −14.0% P = 0.011 |
−35.5% P < 0.001 |
−24.8% P < 0.001 |
| Thalamus | 1.469 (±0.19) | 1.109 (±0.15) | 0.926 (±0.15) | −24.6% P < 0.001 |
−37.0% P < 0.001 |
−16.5% P = 0.017 |
| Hypothalamus | 1.240 (±0.21) | 0.996 (±0.11) | 0.632 (±0.19) | −19.7% P = 0.003 |
−49.1% P < 0.001 |
−36.6% P < 0.001 |
| Raphe | 2.212 (±0.29) | 1.815 (±0.25) | 1.497 (±0.25) | −17.9% P = 0.002 |
−32.3% P < 0.001 |
−17.5% P = 0.009 |
| Insula | 0.378 (±0.07) | 0.215 (±0.10) | 0.200 (±0.10) | −43.0% P < 0.001 |
−47.0% P < 0.001 |
−7.1% P > 1.0 |
| Anterior cingulate | 0.383 (±0.11) | 0.247 (±0.10) | 0.256 (±0.09) | −35.3% P = 0.005 |
−33.2% P = 0.008 |
3.3% P > 1.0 |
| Posterior cingulate | 0.371 (±0.09) | 0.244 (±0.07) | 0.259 (±0.07) | −34.3% P < 0.001 |
−30.0% P = 0.002 |
6.5% P > 1.0 |
| Globus pallidus | 0.776 (±0.17) | 0.611 (±0.14) | 0.598 (±0.12) | NS* | NS* | NS* |
| Amygdala | 1.139 (±0.21) | 0.776 (±0.22) | 0.898 (±0.25) | NS* | NS* | NS* |
| Prefrontal cortex | 0.205 (±0.03) | 0.163 (±0.06) | 0.146 (±0.08) | NS* | NS* | NS* |
P values are Bonferroni corrected for multiple-comparisons. NS* = not significant ANOVA. PD = Parkinson's disease. PD control = Parkinson's disease patients without sleep dysfunction; PD sleep = Parkinson's disease patients with sleep dysfunction.
Fig. 2.
Regional [11C]DASB BPND decreases in groups of Parkinson's patients with and without sleep dysfunction. Column bar graphs showing mean [11C]DASB non-displaceable binding potential (BPND) in brain regions between Parkinson's patients with and without sleep disturbances and healthy controls. Error bars represent mean ± SD. *P < 0.05; **P < 0.001; ***P < 0.001. All P values are Bonferroni corrected for multiple comparisons.
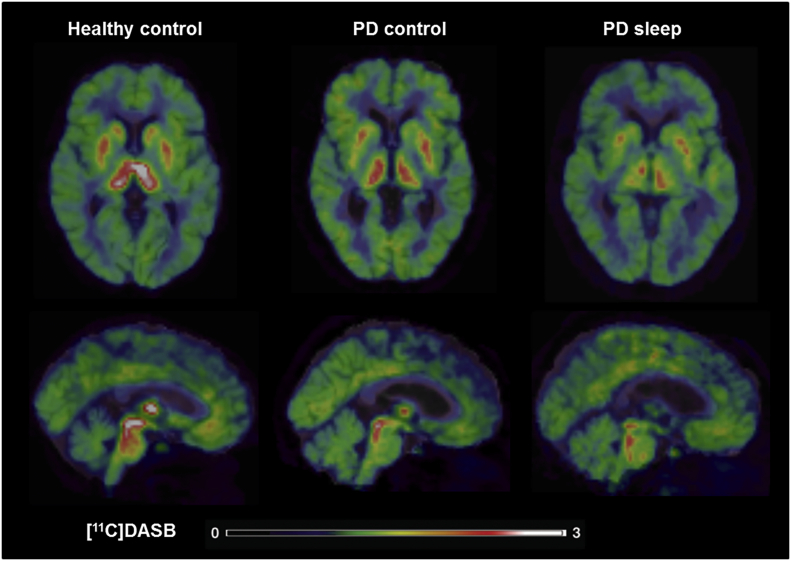
Fig. 3.
Loss of [11C]DASB BPND in Parkinson's patients with sleep dysfunction compared to patients without sleep dysfunction and healthy controls. [11C]DASB PET images co-registered and fused with 1.5-Tesla MRI images showing basal ganglia and brain stem regions in healthy controls (64 year old female; PDSS = 143; putamen BPND = 1.532; caudate BPND = 1.320; thalamus BPND = 1.755; raphe BPND = 2.693), Parkinson's patients without sleep dysfunction (62 year old male with disease duration of 4 years; PDSS = 95; UPDRS-III score OFF medication of 17; putamen BPND = 0.998; caudate BPND = 0.732; thalamus BPND = 1.282; raphe BPND = 1.595) and Parkinson's patients with sleep dysfunction (79 year old male with disease duration of 9 years; PDSS = 51; UPDRS-III score OFF medication of 56; putamen BPND = 0.828; caudate BPND = 0.664; thalamus BPND = 0.802; raphe BPND = 1.457). Colour bar reflects range of [11C]DASB BPND intensity.
3.2. Correlations
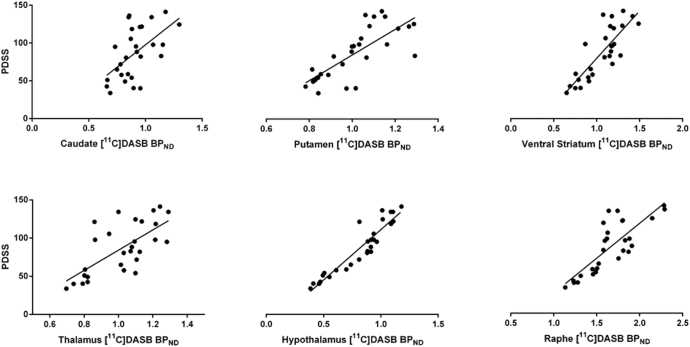
Lower individual PDSS scores, implying higher burden of sleep symptoms, correlated with lower [11C]DASB BPND values in the caudate (P < 0.001; r = 0.77), putamen (P < 0.001; r = 0.84), ventral striatum (P < 0.001; r = 0.86), thalamus (P < 0.001; r = 0.79), hypothalamus (P < 0.001; r = 0.90) and raphe nuclei (P < 0.001; r = 0.83) in all 28 patients with PD (Fig. 4).
Fig. 4.
Correlations between loss of [11C]DASB BPND and impaired sleep scores in patients with Parkinson's disease. Lower scores on the PDSS correlated with lower [11C]DASB BPND in the caudate (P < 0.001; r = 0.77), putamen (P < 0.001; r = 0.84), ventral striatum (P < 0.001; r = 0.86), thalamus (P < 0.001; r = 0.79), hypothalamus (P < 0.001; r = 0.90) and raphe nuclei (P < 0.001; r = 0.83). BPND = non-displaceable binding potential; PDSS = Parkinson's disease Sleep Scale.
4. Discussion
Our findings demonstrate that sleep dysfunction in PD is associated with reduced serotonergic function in the midbrain raphe, basal ganglia and hypothalamus. Using a [11C]DASB PET region of interest analytical approach, we found that SERT binding was decreased in the dorsal (caudate and putamen) and ventral striatum, thalamus, hypothalamus and raphe nuclei of Parkinson's patients with sleep dysfunction compared to patients without sleep dysfunction. Furthermore, higher burden of sleep symptoms correlated with reduced SERT binding in the caudate, putamen, ventral striatum, thalamus, hypothalamus and raphe nuclei. These are key brain regions involved in the mechanisms regulating sleep, supporting the role of serotonergic dysfunction in the pathophysiology of sleep disorders in PD.
Our results translate, into humans, preclinical findings highlighting the role of serotonergic dysfunction in the development of sleep disturbances. Neuronal activity in the basal ganglia is modulated by serotonin and dopamine during wakefulness and REM sleep (Monti, 2011). Preclinical studies showed that lesions within the basal ganglia led to alterations in the regulation of the sleep-wake cycle (Lazarus et al., 2013; Qiu et al., 2010). The dorsal striatum (caudate and putamen) enhances wakefulness, while the nucleus accumbens promotes sleep, and selective lesions in the external globus pallidus increase total wakefulness (Qiu et al., 2010). Furthermore, 5-HT receptors in the caudate, putamen and nucleus accumbens are thought to play a role in circadian rhythms (Monti, 2011). In the thalamus, 5-HT2 receptors are postulated to modulate both the excitation of the reticular nucleus leading to single-spike activity and waking, and the oscillatory activity of the reticular nucleus; implicating the thalamus in mechanisms for the arousing effects of serotonin (Ursin, 2002). In animal studies, lesions in the raphe nucleus resulted in decreased sleep which was associated with lower levels of serotonin (Jouvet, 1972). Thus, findings of decreased SERT binding in the raphe, striatum and thalamus in PD patients with sleep dysfunction supports their role in mechanisms modulating the sleep-wake cycle. Due to the selectively, and differential expression, of 5-HT receptor subtypes throughout the brain; serotonergic stimulation yields complex effects on the sleep-switch, exerting both sleep incompatible and sleep promoting effects (Portas et al., 2000; Leonard and Llinas, 1994; Strecker et al., 1999). Therefore, alterations in the serotoninergic system can lead to changes in sleep architecture and quality of sleep. Use of novel PET tracers to image and quantify the different 5-HT receptors subtypes in Parkinson's patients with sleep dysfunction would help to further elucidate underlying mechanisms.
Here, we report highest reductions in SERT binding in the hypothalamus in Parkinson's patients with sleep dysfunction. The hypothalamus plays a central role in the regulation of sleep and circadian rhythms, and receives serotonergic projections from the raphe nucleus (Saper et al., 2005). The posterior tuberomammillary nuclei and lateral hypothalamus also modulate the sleep-switch, acting predominantly to promote wakefulness (Espana and Scammell, 2011). Involvement of the hypothalamus and raphe nucleus in PD sleep dysfunction is consistent with involvement of Lewy body and neurite depositions which occurs early in the disease (Braak et al., 2003). Lewy body pathology has been reported in hypothalamic orexin neurons (hypocretin area) (Fronczek et al., 2007; Lessig et al., 2010). Therefore, Lewy body pathology in the hypothalamus could result in loss of orexin neurons, which also interacts with dopaminergic networks of the hypothalamus and is thought to function as an external regulator of the flip-flop switch promoting wakefulness (Nishino et al., 2000). Post-mortem studies have revealed loss of hypocretin neurons in PD brains and reduced levels of orexin in the cerebrospinal fluid (CSF), associated with sleep disorders, in patients with PD; implicating orexin neuronal loss and impaired orexin neurotransmission in PD pathophysiology (Fronczek et al., 2007; Asai et al., 2009). Further studies are required to fully understand regional loss of serotonin, as well as interaction with other regulators of sleep, in mechanisms underlying specific sleep disturbances in PD.
Recently, non-motor endophenotypes of PD including the Park sleep phenotype have been described (Sauerbier et al., 2016). Sleep disorders in PD are heterogeneous and complex, likely involving multiple neurotransmitter systems; thus, differentiating underlying pathophysiology of specific sleep phenotypes is challenging. A PET study with [11C]DASB found no association between severity of sleep disordered breathing and SERT binding (Lelieveld et al., 2012). Loss of striatal [123I]FP-CIT has been associated with severity of EDS, suggesting daytime sleepiness is associated with dopaminergic nigrostriatal degeneration (Happe et al., 2007). A [11C]DASB PET study showed no differences in raphe nucleus and striatal SERT binding in patients with and without RBD symptoms (Kotagal et al., 2012). In RBD, degeneration of cholinergic neurons in the basal forebrain and brainstem, and loss of noradrenergic neurons in the locus coeruleus played a more prominent role than serotonergic dysfunction (Kotagal et al., 2012). Furthermore, a [123I]FP-CIT single-photon emission computed tomography (SPECT), study in idiopathic RBD patients showed no difference in brainstem and thalamic binding, but significant reductions in the basal ganglia; leading the authors to conclude that the serotonergic system is not directly involved in RBD pathogenesis (Arnaldi et al., 2015). We provide evidence to suggest that serotonergic dysfunction is associated with sleep fragmentation, including sleep maintenance, nocturnal motor symptoms, nocturnal hallucinations and nocturia, as measured with the PDSS (Chaudhuri et al., 2002). Difficulty in maintaining sleep is often accompanied by a decrease in total sleep time and an increase in the number of awakenings during sleep and wakefulness after sleep onset. Serotoninergic dysfunction has previously been implicated to play a role in motor symptoms in PD (Politis et al., 2014; Loane et al., 2013), therefore could contribute to development of nocturnal motor symptoms. Taken our results together with previous studies, it's plausible that the development of nocturnal sleep disturbances is influenced by serotonergic dysfunction, whereas cholinergic, noradrenergic and dopaminergic pathology may underlie the pathophysiology of RBD and EDS. However, future larger studies assessing correlations between serotonergic function and subsets of the PDSS, in combination with other neurotransmitter systems, are required to highlight which specific aspect of sleep-wake disturbances are associated with serotonergic dysfunction.
To reduce the effects of possible confounding factors known to affect SERT binding, the two groups of patients with PD were carefully matched for age, disease duration, motor and non-motor symptom burden, and levodopa treatment intake. Furthermore, patients with fatigue, dementia, depression and abnormal weight gain or loss were excluded to reduce effects of known confounding factors of [11C]DASB binding and sleep. However, co-morbid non-motor and cognitive factors could predispose, exacerbate or follow sleep dysfunction in PD; these factors should be considered when interrupting the results. In order to investigate the role of serotonin dysregulating contributing to the pathophysiology of sleep disturbances in PD, and to limit the effects of possible confounding factors, the PD cohort studied was relatively homogenous. Therefore, larger studies in a more heterogeneous population of patients with PD are required to further understand the wider clinical application of these findings. Here, we defined the presence of sleep disturbances using the validated Parkinson's disease sleep scale. Further studies using specific sleep scales, and sleep electroencephalogram (EEG), are required to further investigate the role of serotonergic dysfunction in the development of specific types of sleep disturbances in patients with PD.
Sleep disturbances are highly prevalent among other neurodegenerative diseases including Alzheimer's disease and Lewy body dementia and Huntington's disease. Recent studies have highlighted the role of the glymphatic system in the removal of potentially neurotoxic protein waste, which accumulates during wakefulness, from the brain at a highest rate during sleep (Xie et al., 2013). Dysregulation of the glymphatic system, potentially due to sleep dysfunction, could result in abnormal accumulation of protein aggregates such as alpha-synuclein and beta-amyloid (Nedergaard and Goldman, 2016). Therefore, sleep dysfunction may not only be a side effect, but could contribute to a protein deposition and neurodegeneration. Alterations in the neurotransmitter systems could play a role in driving sleep dysfunction, subsequently contributing to dysregulation of the glymphatic system and the abnormal accumulation of protein aggregates underlying disease pathology. Studies investigating the involvement of the serotonergic system across neurodegenerative disorders could highlight if similar mechanisms underlie pathophysiology of sleep disturbances. Therapeutic strategies targeting serotonergic function may provide novel interventions to alleviate sleep disturbances with possible implications for disease pathology.
The following is the supplementary data related to this article.
Region of interest volumetric analysis between Parkinson's disease patients and healthy controls.
Author contributions
M. P. conceptualized the experimental design and organized the study. M.P., F.T. acquisition of the data. H.W., B.G., have analyzed the data. H.W. conducted the statistical analysis, interpretation of data and wrote the first draft of the manuscript. F.T. and K.C. interpretation of data and critical revision of the manuscript for important intellectual content. M.P. acquired funding for the study. All authors gave input and revised the manuscript.
Financial disclosures
This research was supported by the Lily and Edmond J. Safra Foundation (PNNWPGD). H. Wilson., B. Giordano., F.E. Turkheimer., K. Ray Chaudhuri., and M. Politis report no disclosures.
Acknowledgements
We thank all the participants who kindly agreed to take part in this study.
References
- Albin R.L., Koeppe R.A., Bohnen N.I., Wernette K., Kilbourn M.A., Frey K.A. Spared caudal brainstem SERT binding in early Parkinson's disease. J. Cereb. Blood Flow Metab. 2008;28:441–444. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- Arnaldi D., Fama F., De Carli F. The role of the serotonergic system in REM sleep behavior disorder. Sleep. 2015;38:1505–1509. doi: 10.5665/sleep.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H., Hirano M., Furiya Y. Cerebrospinal fluid-orexin levels and sleep attacks in four patients with Parkinson's disease. Clin. Neurol. Neurosurg. 2009;111:341–344. doi: 10.1016/j.clineuro.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Ballanger B., Strafella A.P., van Eimeren T. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch. Neurol. 2010;67:416–421. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chaudhuri K.R., Pal S., DiMarco A. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2002;73:629–635. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R.A., Scammell T.E. Sleep neurobiology from a clinical perspective. Sleep. 2011;34:845–858. doi: 10.5665/SLEEP.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R., Overeem S., Lee S.Y. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- Ginovart N., Wilson A.A., Meyer J.H., Hussey D., Houle S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: modeling strategies. J. Cereb. Blood Flow Metab. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Guttman M., Boileau I., Warsh J. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur. J. Neurol. 2007;14:523–528. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Halliday G.M., Blumbergs P.C., Cotton R.G., Blessing W.W., Geffen L.B. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson's disease. Brain Res. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- Happe S., Baier P.C., Helmschmied K., Meller J., Tatsch K., Paulus W. Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson's disease. J. Neurol. 2007;254:1037–1043. doi: 10.1007/s00415-006-0483-6. [DOI] [PubMed] [Google Scholar]
- Hipolide D.C., Moreira K.M., Barlow K.B., Wilson A.A., Nobrega J.N., Tufik S. Distinct effects of sleep deprivation on binding to norepinephrine and serotonin transporters in rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:297–303. doi: 10.1016/j.pnpbp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hogl B., Arnulf I., Comella C. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov. Disord. 2010;25:2704–2716. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
- Jahan I., Hauser R.A., Sullivan K.L., Miller A., Zesiewicz T.A. Sleep disorders in Parkinson's disease. Neuropsychiatr. Dis. Treat. 2009;5:535–540. doi: 10.2147/ndt.s4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb. Physiol. Biol. Chem. Exp. Pharmakol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- Kish S.J. Biochemistry of Parkinson's disease: is a brain serotonergic deficiency a characteristic of idiopathic Parkinson's disease? Adv. Neurol. 2003;91:39–49. [PubMed] [Google Scholar]
- Kish S.J., Furukawa Y., Chang L.J. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl. Med. Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kish S.J., Tong J., Hornykiewicz O. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kotagal V., Albin R.L., Muller M.L. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann. Neurol. 2012;71:560–568. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M., Chen J.-F., Urade Y., Huang Z.-L. Role of the basal ganglia in the control of sleep and wakefulness. Curr. Opin. Neurobiol. 2013;23:780–785. doi: 10.1016/j.conb.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld I.M., Muller M.L., Bohnen N.I. The role of serotonin in sleep disordered breathing associated with Parkinson disease: a correlative [11C]DASB PET imaging study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C.S., Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Lessig S., Ubhi K., Galasko D. Reduced hypocretin (orexin) levels in dementia with Lewy bodies. Neuroreport. 2010;21:756–760. doi: 10.1097/WNR.0b013e32833bfb7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane C., Wu K., Bain P., Brooks D.J., Piccini P., Politis M. Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology. 2013;80:1850–1855. doi: 10.1212/WNL.0b013e318292a31d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet A., Krack P., Lhommee E. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain. 2016;139:2486–2502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P., Visser M., Rodriguez-Blazquez C., Marinus J., Chaudhuri K.R., van Hilten J.J. SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson's disease. Mov. Disord. 2008;23:1681–1688. doi: 10.1002/mds.22110. [DOI] [PubMed] [Google Scholar]
- Menza M., Dobkin R.D., Marin H., Bienfait K. Sleep disturbances in Parkinson's disease. Mov. Disord. 2010;25(Suppl. 1):S117–122. doi: 10.1002/mds.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Nedergaard M., Goldman S.A. Brain drain. Sci. Am. 2016;314:44–49. doi: 10.1038/scientificamerican0316-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S., Ripley B., Overeem S., Lammers G.J., Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet (London, England) 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Pavese N., Metta V., Bose S.K., Chaudhuri K.R., Brooks D.J. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434–3443. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol. Dis. 2010;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology. 2010;75:1920–1927. doi: 10.1212/WNL.0b013e3181feb2ab. [DOI] [PubMed] [Google Scholar]
- Politis M., Loane C., Wu K., Brooks D.J., Piccini P. Serotonergic mediated body mass index changes in Parkinson's disease. Neurobiol. Dis. 2011;43:609–615. doi: 10.1016/j.nbd.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C. Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson's disease patients. J. Clin. Invest. 2014;124:1340–1349. doi: 10.1172/JCI71640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas C.M., Bjorvatn B., Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog. Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Qiu M.-H., Vetrivelan R., Fuller P.M., Lu J. Basal ganglia control of sleep–wake behavior and cortical activation. Eur. J. Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sauerbier A., Jenner P., Todorova A., Chaudhuri K.R. Non motor subtypes and Parkinson's disease. Parkinsonism Relat. Disord. 2016;22(Suppl. 1):S41–46. doi: 10.1016/j.parkreldis.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Selvaraj V.K., Keshavamurthy B. Sleep dysfunction in Parkinson's disease. J. Clin. Diagn. Res. 2016;10:OC09–12. doi: 10.7860/JCDR/2016/16446.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker R.E., Thakkar M.M., Porkka-Heiskanen T., Dauphin L.J., Bjorkum A.A., McCarley R.W. Behavioral state-related changes of extracellular serotonin concentration in the pedunculopontine tegmental nucleus: a microdialysis study in freely moving animals. Sleep Res. Online. 1999;2:21–27. [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Med. Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- Wallman M.J., Gagnon D., Parent M. Serotonin innervation of human basal ganglia. Eur. J. Neurosci. 2011;33:1519–1532. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q. Sleep drives metabolite clearance from the adult brain. Science (New York, N.Y.) 2013;342 doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Region of interest volumetric analysis between Parkinson's disease patients and healthy controls.