Abstract
Apraxia of speech is a motor speech disorder thought to result from impaired planning or programming of articulatory movements. It can be the initial or only manifestation of a degenerative disease, termed primary progressive apraxia of speech (PPAOS). The aim of this study was to use task-free functional magnetic resonance imaging (fMRI) to assess large-scale brain network pathophysiology in PPAOS. Twenty-two PPAOS participants were identified from a prospective cohort of degenerative speech and language disorders patients. All participants had a comprehensive, standardized evaluation including an evaluation by a speech-language pathologist, examination by a behavioral neurologist and a multimodal imaging protocol which included a task-free fMRI sequence. PPAOS participants were age and sex matched to amyloid-negative, cognitively normal participants with a 1:2 ratio. We chose a set of hypothesis driven, predefined intrinsic connectivity networks (ICNs) from a large, out of sample independent component analysis and then used them to initialize a spatiotemporal dual regression to estimate participant level connectivity within these ICNs. Specifically, we evaluated connectivity within the speech and language, face and hand sensorimotor, left working memory, salience, superior parietal, supramarginal, insular and deep gray ICNs in a multivariate manner. The spatial maps for each ICN were then compared between PPAOS and control participants. We used clinical measures of apraxia of speech severity to assess for clinical-connectivity correlations for regions found to differ between PPAOS and control participants. Compared to controls, PPAOS participants had reduced connectivity of the right supplementary motor area and left posterior temporal gyrus to the rest of the speech and language ICN. The connectivity of the right supplementary motor area correlated negatively with an articulatory error score. PPAOS participants also had reduced connectivity of the left supplementary motor area to the face sensorimotor ICN, between the left lateral prefrontal cortex and the salience ICN and between the left temporal-occipital junction and the left working memory ICN. The latter connectivity correlated with the apraxia of speech severity rating scale, although the finding did not survive correction for multiple comparisons. Increased connectivity was noted in PPAOS participants between the dorsal posterior cingulate and the left working memory ICN. Our results support the importance of the supplementary motor area in the pathophysiology of PPAOS, which appears to be disconnected from speech and language regions. Supplementary motor area connectivity may serve as a biomarker of degenerative apraxia of speech severity.
Abbreviations: AES, Articulatory Error Score; AOS, Apraxia Of Speech; agPPA, Agrammatic/Nonfluent PPA; AQ, Aphasia Quotient; ASRS, Apraxia of Speech Severity Rating Scale; BNT, Boston Naming Test; FAB, Frontal Assessment Battery; FBI, Frontal Behavioral Inventory; ICN, Intrinsic Connectivity Network; MMSE, Mini-Mental State Examination; NPI-S, Neuropsychiatric Inventory – Severity; NVOA, Nonverbal Oral Apraxia; PCC, Posterior Cingulate Cortex; PFC, Prefrontal Cortex; PPA, Primary Progressive Aphasia; SMA, Supplementary Motor Area; TOJ, Temporal-Occipital Junction; TT, Token Test; UPDRS, Unified Parkinson Disease Rating Scale; WAB, Western Aphasia Battery
Keywords: Apraxia of speech, Functional connectivity, Intrinsic connectivity networks
Highlights
-
•
This is the first study of the network level connectivity changes underlying PPAOS.
-
•
We found changes in speech and language, face, working memory and salience networks.
-
•
Right SMA connectivity to speech and language areas correlated with AOS severity.
1. Introduction
Apraxia of speech (AOS) is a motor speech disorder resulting from impaired planning or programming of articulatory movements (Darley et al., 1969; McNeil et al., 2009; Wambaugh et al., 2006). In adults, AOS is usually seen as a result of focal injury, with stroke accounting for the majority of AOS cases (Duffy, 2013b; Schiff et al., 1983), or as a manifestation of a degenerative disease (Duffy, 2006; Duffy and Josephs, 2012). In neurodegenerative cases AOS is often embedded within a broader dysfunction of cognition, language or motor systems (Duffy, 2006). For example, it is one of the core criteria for the nonfluent/agrammatic variant of primary progressive aphasia (PPA), along with agrammatism (Gorno-Tempini et al., 2011), and is considered part of the new criteria for progressive supranuclear palsy (PSP) (Hoglinger et al., 2017) and corticobasal syndrome (CBS) (Armstrong et al., 2013), with ~50% of CBS participants experiencing speech changes over the course of their illness (Alexander et al., 2014).
However, AOS can also be the initial or only manifestation of a degenerative disease. Over the last decade this phenomenon, termed primary progressive apraxia of speech (PPAOS), has been characterized in great detail. These patients have a distinct clinical presentation and temporal evolution (Duffy and Josephs, 2012; Duffy et al., 2015; Josephs et al., 2014), with approximately half developing a CBS-PSP hybrid syndrome ~5 years into the illness (Josephs et al., 2014). They have also been found to differ from PPA patients using temporal acoustic measures (Duffy et al., 2017). While aphasia can develop, this usually happens after several years and AOS typically continues to dominate the clinical presentation (Josephs et al., 2014; Josephs et al., 2013). It is associated with bilateral supplementary motor area (SMA), dorsolateral premotor and primary motor cortex abnormalities on imaging, including atrophy (Josephs et al., 2012), hypometabolism (Josephs et al., 2012; Josephs et al., 2006) and flortaucipir (tau-PET) uptake (Utianski et al., 2018). Bilateral involvement of frontal white matter tracts has also been documented (Botha et al., 2015). PPAOS patients appear to almost always harbor an underlying 4-repeat tauopathy (Deramecourt et al., 2010; Josephs and Duffy, 2008; Josephs et al., 2006).
There is a growing body of evidence supporting the idea that degenerative diseases target large scale systems or networks in the brain (Seeley et al., 2009). Task-free functional MRI (TF-fMRI) has been used to assess functional connectivity in stroke-related AOS, where reduced connectivity between bilateral premotor cortex regions was found to correlate with AOS severity (New et al., 2015). It has also been applied to the nonfluent/agrammatic variant of PPA (agPPA), a disorder that is often associated with AOS, where functional connectivity predicted gray matter atrophy within a “speech production network” (Mandelli et al., 2016). Despite the aforementioned advances in our understanding of PPAOS, network or functional connectivity changes have not been explored in the disorder, a knowledge gap we aimed to address in the current study.
There are numerous methodological frameworks within which functional connectivity can be assessed, including seed-based analyses, which are typically model based, and data driven methods such as independent component analysis (ICA), which doesn't require the a priori selection of regions or seeds(Friston, 2009). When the goal is to assess connectivity between a limited set of predefined regions of interest (ROIs), seed-based analyses may be preferable. However, ICA has a distinct advantage when the objects to be studied are the intrinsic connectivity networks (ICNs) of the brain. This is due to the fact that the connectivity within an entire ICN can be quantified, as opposed to connectivity to a node thought to represent the ICN, as is the case in a seed based analysis (Leech et al., 2011). Prior work has shown the ICA may be more sensitive to group differences than seed based methods (Smith et al., 2014). In the current study we used a hybrid approach: we chose a set of hypothesis driven, predefined ICNs from a large, out-of-sample ICA and then used them to initialize a spatiotemporal dual regression (STR) to estimate participant level connectivity within these ICNs. Given that PPAOS is a relatively rare disorder that has never been subjected to functional connectivity analyses we felt this was the best compromise between hypothesis driven and data driven methods.
2. Materials and methods
2.1. Participants
Participants were members of a prospective cohort of degenerative speech and language patients evaluated at the Mayo Clinic Department of Neurology between 2010 and 2016. Details of the evaluation and diagnostic procedures are described elsewhere (Botha et al., 2015; Josephs et al., 2012). Briefly, participants with suspected degenerative speech or language disorders were prospectively recruited into the study. Each participant was interviewed and examined by a behavioral neurologist (KAJ), underwent detailed speech and language examination by a speech-language pathologist (EAS, JRD or HMC) and had MR imaging performed as part of a standardized protocol described below. As part of the neurologic evaluation the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), Frontal Assessment Battery (FAB) (Dubois et al., 2000), Frontal Behavioral Inventory(Kertesz et al., 1997), Ideomotor Apraxia (IMA) part of the Western Aphasia Battery (WAB) (Kertesz, 2007), Movement Disorder Sponsored Revision of the Unified Parkinson's Disease Rating Scale (UPDRS) Part 3 (Goetz et al., 2008) and the brief questionnaire form of the Neuropsychiatric Inventory (NPI-Q) (Cummings et al., 1994) were administered. The speech and language evaluation was recorded and reviewed by two speech-language pathologists who reached consensus on the presence and severity of dysarthria, the presence and severity of AOS, and the presence of nonverbal oral apraxia (NVOA) (Duffy, 2013b). The evaluation also included the WAB, with the aphasia quotient (WAB-AQ) serving as a measure of aphasia severity (Kertesz, 2007), the 22-item Token Test (TT)(De Renzi and Vignolo, 1962), the Boston Naming Test Short Form (BNT)(Lansing et al., 1999), the Apraxia of Speech Rating Scale (ASRS)(Strand et al., 2014), and a NVOA scale (Botha et al., 2014; Duffy, 2013a). Supplementary speech tasks, described previously (Duffy et al., 2015), were also administered, which included the repetition of mostly multisyllabic words (thirteen words, three repetitions each). This supplemental task was used to derive a quantitative measure of articulatory errors by taking the percentage of words with articulatory errors (articulatory error score or AES).
Based on the clinical examination alone, blinded to the results of imaging, a diagnosis of PPAOS was given if AOS was the predominant speech disturbance; mild dysarthria could be present but aphasia was absent (Botha et al., 2015; Josephs et al., 2012). In other words, the root criteria for PPA were not met (Gorno-Tempini et al., 2011). In addition, participants could not meet criteria for an alternative neurodegenerative disease, such as CBS (Armstrong et al., 2013), behavioral variant frontotemporal dementia (bvFTD) (Rascovsky et al., 2011), or possible/probable PSP (Hoglinger et al., 2017), to name but a few. Participants were also excluded if their imaging studies did not pass quality control (detailed below), if they were amyloid positive (SUVR ≥1.5) on Pittsburgh Compound B PET imaging, or if there was a structural MR abnormality that could confound connectivity analyses. A total of 30 participants with PPAOS were eligible for inclusion in the current study. Three were excluded because no TF-fMRI sequence was available or because the available sequence failed quality control. Four were excluded on account of being amyloid positive. One participant had a prior meningioma resected with left frontal gliosis and was excluded because this might potentially confound connectivity analyses.
For the imaging analysis, PPAOS participants were age and sex matched 1:2 to cognitively and neurologically normal amyloid PET negative participants from the Mayo Clinic Study of Aging (Roberts et al., 2008), who completed the same imaging protocol.
2.2. Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic Institutional Review Board, and consent enrollment into the study was given by all participants.
2.3. Image acquisition
All participants underwent a standardized 3.0 T MRI protocol which included a 3-dimensional magnetization-prepared rapid acquisition gradient-echo sequence (repetition time/echo time/T1, 2300/3/900 ms; flip angle, 8°; field of view, 26 cm; 256 × 256 in-plane matrix with a phase field of view of 0.94, slice thickness, 1.2 mm; and in-plane resolution, 1 mm) and task-free gradient echo-planar imaging (repetition time/echo time, 3000/30 ms; flip angle, 90°; slice thickness, 3.3 mm; in-plane resolution, 3.3 mm; and volumes, 103). Participants were instructed to keep their eyes open and not think of anything in particular during task-free fMRI scanning.
2.4. Image preprocessing
Participants whose task-free fMRI sequences did not meet the following criteria were excluded: fewer than 3 mm of translational movement, fewer than 3 degrees of rotational movement, and no evidence of obvious artifacts on visual inspection. In addition, motion was quantified for participants and controls using frame wise displacement (Power et al., 2012). We used this metric to compare motion between participants and controls, and to test whether or not any of our connectivity metrics correlated with motion.
Preprocessing and data analysis were done using elements of the Statistical Parametric Mapping (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), the Analysis of Functional NeuroImages (AFNI) software, and in-house developed software packages implemented in MATLAB v9.1 (Mathworks Inc., Natick, MA, USA). After the first 3 volumes were removed, to allow for steady state magnetization, each voxel's time-series was despiked using 3dDespike (AFNI). Slice time correction was performed next, followed by two-pass realignment to the mean EPI image. The gradwarp and biased corrected, non-accelerated structural image was then co-registered to the mean EPI image. Unified segmentation and normalization to the Mayo Clinic Adult Lifespan Template (MCALT, https://www.nitrc.org/projects/mcalt/) was performed, allowing for Mayo Clinic Study of Aging (MCSA) Functional Connectivity Atlas templates to be brought into subject space using the inverse warps (Jones et al., 2012). The white matter and cerebrospinal fluid (CSF) segmentations for each subject were used to create an anatomically-based “noise ROI” for use in component-based noise correction (Behzadi et al., 2007) by binarizing the segmentations at 0.9 and eroding this by two voxels in each direction. The voxel-wise time-series for the “noise ROI” was then extracted and entered into a principal component analysis. The first six principle components along with the six motion parameters and their temporal derivatives were used to create a nuisance regressor matrix (24 total regressors). Finally, we used 3dBandpass (AFNI) to simultaneously perform nuisance regression with the nuisance regressor matrix and bandpass filtering (0.009–0.08 Hz), which further reduces the motion confound by avoiding spectral misspecification (Hallquist et al., 2013). The same program was used for masking, time series variance normalization and within mask smoothing with a 6 mm full-width half-maximum Gaussian kernel.
2.5. Subject space spatial-temporal regression of networks of interest
Spatial independent component analysis is a popular data driven approach to TF-fMRI analysis. Typically, a group ICA is used to identify spatially-independent patterns in BOLD time-courses. Some of these components resemble well described ICNs, and these spatial maps can then be used to back-reconstruct subject-level networks. We previously performed a high dimensional group ICA in a large population-based cohort of cognitively unimpaired older adults (Jones et al., 2012). Briefly, 892 cognitively normal participants in the MCSA were included in the ICA, with an estimation of 54 components, which was run with 100 iterations to ensure stability. Each component was visually assessed and assigned a label based on a functional meta-analysis or discarded as artifactual. The result was a population based atlas of 31 ICNs (MCSA Functional Connectivity Atlas). All networks analyzed in the current study were taken from this atlas, thresholded and then used in a spatial temporal regression to back reconstruct participant level networks of interest. This out-of-sample group ICA allowed for an unbiased assessment of participant connectivity profiles.
We elected to use a multivariate framework for STR in order to quantify the connectivity within an ICN while controlling for the effect of the other ICNs included in our study. This has been shown to be important when characterizing the connectivity within and between ICNs (Leech et al., 2011). However, the multivariate approach at the single subject level lacks the degrees of freedom to include all 31 networks from the atlas. We thus chose nine networks based on prior studies of AOS and PPA. These are shown in Fig. 1, with names taken from the atlas unless otherwise specified. The speech and language ICN was chosen since it appears to be an early target in PPAOS participants, all of whom have motor speech involvement and many of whom develop aphasia (Josephs et al., 2014; Josephs et al., 2012). Similarly, the face and hand sensorimotor ICNs were chosen because of motor speech impairment, prior studies associating AOS with left primary motor cortex damage and the large proportion of participants who develop upper motor neuron signs during follow up (Graff-Radford et al., 2014; Josephs et al., 2014; Josephs et al., 2012; Schiff et al., 1983). The insular ICN was included based on prior work linking apraxia of speech to insular dysfunction (Dronkers, 1996; New et al., 2015).The superior parietal and supramarginal ICNs were included based on their roles in skilled motor movements and involvement in motor speech planning and execution, and the large proportion of participants who develop ideomotor apraxia during follow up (Josephs et al., 2014; Keller and Kell, 2016; Kroliczak and Frey, 2009; Simonyan and Fuertinger, 2015). The left working memory ICN was included based on prior work showing that left working memory dysfunction correlated with some measures of aphasia (Whitwell et al., 2015) and that executive control networks may be involved in some aspects of verbal and nonverbal orofacial movements (Basilakos et al., 2017). Given the selective vulnerability reported in frontotemporal dementia, and the fact that PPAOS is almost exclusively associated with frontotemporal lobar degeneration tau (FTLD-tau) pathology, we also included the salience network in our analysis (Josephs et al., 2005; Josephs and Duffy, 2008; Josephs et al., 2006; Seeley et al., 2009; Whitwell et al., 2011b; Zhou et al., 2010). Based on prior work showing subcortical network dysfunction in progressive supranuclear palsy, and the fact that many PPAOS participants develop PSP features during follow up, we included a network of deep gray structures (Josephs et al., 2014; Whitwell et al., 2011a). No explicit cerebellum-based network was included since each ICN map incorporates appropriate cerebellar regions, if applicable. Many of the areas included within these networks also feature prominently in existing models of speech production, such as the primary motor cortex, ventral premotor cortex, supplementary motor area, supramarginal gyrus, deep gray nuclei, and cerebellum in the DIVA model (Tourville and Guenther, 2011).
Fig. 1.
Three-dimensional brain renderings and representative slices showing the nine ICNs
The speech and language ICN (named “language” in the MCSA Atlas) included most of the canonical regions associated with language and speech, including the inferior frontal gyrus (Broca's area), posterior temporal region (Wernicke's area), left anterior temporal and dorsolateral premotor regions and left greater than right supplementary motor areas. Similarly, the left working memory, salience, hand and face sensorimotor, superior parietal, supramarginal (named “parietal operculum” in the MCSA Atlas) deep gray and insular ICNs included the expected regions based on prior studies. Renders created using Brain Net Viewer (Xia et al., 2013) (https://www.nitrc.org/projects/bnv/)
Abbreviations: ICN = Intrinsic Connectivity Network; MCSA = Mayo Clinic Study of Aging.
These 9 networks were identified at the single participant level, in subject space, by using STR as implemented in GIFT (Calhoun et al., 2001). During the STR, the average time course within each ICN of interest is extracted using the ICN maps that were moved to participant space during preprocessing. The time courses are then used in a multivariate voxel-level regression. In order to avoid biases introduced by differences in gray matter volume (e.g. due to atrophy in the PPAOS group), only voxels with >0.5 probability of containing gray matter were included in the STR, based on the subject-level gray matter segmentation. We also only included voxels where the mean EPI signal intensity was >100 so as to exclude areas of signal loss. In limiting the STR to these voxels we limited the potential influence of brain regions with poor signal. The STR produces participant level spatial maps for each ICN, where the value in each voxel represents the correlation between the time course of that voxel and the ICN time course, controlling for the effects of all other ICNs. The correlation coefficients are used as a measure of functional connectivity, and as such a given ICN spatial map quantifies the connectivity of each voxel to the network of interest while controlling for the effect of the other ICNs (Hlinka et al., 2011). In other words, voxels not included in the spatial map used as the initiator may be incorporated into the ICN during the STR. Each resulting spatial map for each participant was then transformed into a z-score map by standardizing across all voxels included in the mask described above. These z-score maps for each participant represent the spatial extent and magnitude of functional connectivity of a given ICN, and allows for group comparisons (Jones et al., 2016; Whitwell et al., 2015; Wiepert et al., 2017).
2.6. Network connectivity analysis
The spatial map for each subject was moved into MCALT space and smoothed with a 4 mm full-width half-maximum Gaussian kernel given that some smoothing was already applied during the final preprocessing step (smoothness estimates are discussed further below). We used SPM12 to perform voxel-level statistical analyses. The voxel-wise connectivity map of each network was obtained in controls by using a one-sample t-test. We used the uncorrected (p = 0.001) result for each network in controls to mask the analysis in PPAOS, as well as the comparison between groups, so as to only assess connectivity within a given and presumably normal ICN. Controls and PPAOS participants were compared to each other using a two sample t-test with age and sex included as covariates. For the direct comparisons between PPAOS participants and controls we applied correction for multiple comparisons at the cluster level. For each contrast, the median smoothness was estimated based on the residual files generated during the SPM analysis (median values across all 9 ICNs: 9.1874 mmx, 9.8558 mmy and 9.2559 mmz). These parameters and the mask file were entered into AlphaSim from the Resting-State fMRI Data Analysis Toolkit (RESTplus) v1.1 (http://restfmri.net/forum/RESTplusV1.2) which was used to run 5000 Monte-Carlo simulations to determine the appropriate cluster threshold for each contrast (Song et al., 2011).
2.7. Participant level connectivity parameters
The clusters identified in the two sample t-test comparing PPAOS and control participants were binarized and used to extract participant level connectivity metrics based on the z-score maps from the STR. In other words, for each participant we obtained the median z-score of functional connectivity between a given area within a network, identified by the voxel wise comparison between PPAOS and control participants, and the rest of the network. The effect of age on connectivity was assessed using the connectivity parameters extracted from control participants. The effect of motion was assessed by comparing maximal frame-wise displacement with the connectivity parameters in PPAOS and control participants.
2.8. Gray matter volume analyses
Although the STR and subsequent connectivity analyses were done within a gray matter mask, differences in gray matter volume due to age or disease could still influence the functional connectivity estimates. We explored this possibility in two ways. First, the gray matter volume within each cluster was calculated at the participant level using the gray matter segmentation map for each participant. These values were then compared to connectivity values for each cluster and included in models exploring the clinical correlates of changes in functional connectivity. Second, we used voxel-based morphometry (VBM) (Ashburner and Friston, 2000; Senjem et al., 2005) in SPM12 to directly contrast control and PPAOS participants and assessed whether any of our clusters overlapped with regions of atrophy in PPAOS participants.
2.9. Exploratory seed-based analysis
Although our choice of networks was based on prior research, and hypothesis driven, the possibility that the networks involved in PPAOS were not fully characterized by our analysis remained. As such, we conducted a whole brain exploratory analysis from the perspective of spherical seed (radius 6 mm) placed at the peak coordinates for any group differences in a seed-to-brain analysis in control participants. This was strictly exploratory, with the goal of identifying regions or networks connected to the areas where connectivity differed between PPAOS and control participants. If, for example, reduced connectivity within a network not included in our analysis gave rise to apparent differences within the networks we assessed, it might show up in the seed based analysis. We did not perform any group comparisons using these seed based analysis, given the obvious circularity.
2.10. Statistical analyses
All statistical analyses were done using MATLAB (v 9.1, Mathworks Inc., Natick, MA, USA) and R (v 3.4.1, http://www.R-project.org). These connectivity parameters were assessed against three clinical measures in PPAOS participants: the ASRS, the AES and the NVOA scale. Gray matter volume within each cluster was compared between control and PPAOS participants as well as to the connectivity parameters extracted from each cluster. Gray matter volumes were also added to the models assessing the association between clinical and connectivity parameters. Alpha was set at 0.05 and Bonferroni correction was used to correct for multiple comparisons.
3. Results
3.1. Participant details
Demographic, speech and language and neurologic evaluation results for the 22 included participants are shown in Table 1. Participants had no evidence for cognitive, behavioral or language impairment, while all participants had apraxia of speech, which was mild in most cases. Five (23%) had co-existing dysarthria and a majority of participants (14/22) did not have co-existing nonverbal oral apraxia (NVOA ≤29).
Table 1.
Demographic, neurologic and speech and language results for PPAOS participants.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | P21 | P22 | All | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||||||||||||||
| Sex | F | M | M | M | F | F | M | M | M | M | F | F | F | F | F | F | M | M | M | M | M | F | 10F, 12 M |
| Age, yrs | 84 | 77 | 75 | 81 | 79 | 71 | 75 | 53 | 62 | 65 | 79 | 69 | 81 | 75 | 73 | 59 | 55 | 61 | 56 | 69 | 70 | 70 | 70.50 [62.75,76.50] |
| Duration, yrs | 2 | 4 | 4 | 2 | 4 | 4 | 2 | 4 | 2 | 3 | 1.5 | 2 | 4 | 1 | 3 | 1 | 0.5 | 5 | 2 | 8 | 2 | 2 | 2.0 [2.0, 4.0] |
| Neurologic | |||||||||||||||||||||||
| MMSE, /30 | 30 | 30 | 30 | – | 29 | 30 | 30 | 27 | 30 | 30 | 30 | 29 | 30 | 29 | 29 | 27 | 30 | 30 | 30 | 30 | 30 | 29 | 30.00 [29.00,30.00] |
| FAB, /18 | 15 | 17 | 17 | 17 | 18 | 16 | 16 | 16 | 18 | 18 | 18 | 15 | 16 | 17 | 16 | 15 | 18 | 16 | 17 | 16 | 17 | 17 | 17.00 [16.00,17.00] |
| FBI, /72 | 2 | 3 | 4 | – | 20 | 2 | 12 | 25 | 8 | 2 | 4 | 6 | 6 | 3 | 22 | 7 | 2 | 5 | 3 | 25 | 9 | – | 5.50 [3.00,9.75] |
| NPI-S, /36 | 1 | 0 | 2 | 0 | 1 | 0 | 4 | 9 | 3 | 0 | 0 | 6 | 0 | 1 | 1 | 5 | 0 | 3 | 0 | 1 | 2 | 1 | 1.00 [0.00,2.75] |
| UPDRS III | 9 | 15 | 12 | 18 | 13 | 8 | 4 | 5 | 5 | 8 | 5 | 4 | 22 | 6 | 22 | 11 | 8 | 1 | 5 | 7 | 14 | 3 | 8.00 [5.00,12.75] |
| WAB Apraxia, /60 | 59 | 58 | 58 | 58 | 59 | 58 | 59 | 59 | 60 | 60 | 49 | 58 | 58 | 57 | 40 | 58 | 60 | 60 | 58 | 60 | 57 | 59 | 58.00 [58.00.59.00] |
| Speech and language | |||||||||||||||||||||||
| WAB AQ, /100 | 96 | 100 | 96.3 | 97.8 | 96.4 | 96 | 99.8 | 96.6 | 99.8 | 97.4 | 97 | 95.6 | 100 | 98.7 | 93.3 | 96.2 | 97.6 | 100 | 99.4 | 97.6 | 98.8 | 99 | 97.60 [96.33,99.30] |
| TT, /22 | 21 | 19 | 21 | 18 | 20 | 19 | 22 | 22 | 21 | 20 | 19 | 16 | 22 | 22 | 20 | 17 | 20 | 22 | 20 | 21 | 22 | 20 | 20.00 [19.25,21.75] |
| BNT, /15 | 15 | 13 | 13 | 15 | 15 | 15 | 15 | 12 | 15 | 15 | 14 | 13 | 13 | 14 | 15 | 14 | 15 | 15 | 14 | 15 | 15 | 15 | 15.00 [14.00,15.00] |
| AOS severity, /4 | 2 | 1 | 1 | 1 | 1.5 | 1 | 1 | 2 | 0.5 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1.5 | 1 | 1 | 3 | 1 | 1 | 1.00 [1.00,1.50] |
| ASRS, /64 | 21 | 11 | 20 | 9 | 16 | 9 | 12 | 25 | 7 | 10 | 15 | 14 | 15 | 15 | 12 | 5 | 13 | 16 | 12 | 28 | 17 | 12 | 13.50 [11.25,16.00] |
| AES, % | 12.8 | 5.1 | 12.8 | 0 | 7.7 | 15.4 | 7.7 | 41 | 7.7 | 12.8 | 30.8 | 30.8 | 2.6 | 7.7 | 7.7 | 23.1 | 26.5 | 12.8 | 23.1 | 40.5 | 12.8 | 15.3 | 12.80 [7.70,23.10] |
| Dysarthria severity, /4 | 1 | 0 | 0.5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 1.00 [0.5,1.00]a |
| NVOA, /32 | 27 | 31 | 29 | 32 | 30 | 32 | 31 | 24 | 32 | 32 | 32 | 32 | 32 | 32 | 14 | 21 | 28 | 32 | 31 | 24 | 25 | 31 | 31 [27.25,32.00] |
Participant level results (P1–P22) and group level results (median[Q1,Q3]) are shown. By design, PPAOS participants did not have behavioral, cognitive or language impairment. AOS severity was mild and approximately a quarter of participants had co-existing dysarthria. Most participants scored within normal limits on test of ideomotor and nonverbal oral apraxia.
Abbreviations: AES = Articulatory error score; AOS = Apraxia of speech; AQ = Aphasia quotient; ASRS = Apraxia of Speech Severity Rating Scale; BNT = Boston Naming Test; F = Female; FAB = Frontal Assessment Battery; FBI = Frontal Behavioral Inventory; M = Male; MMSE = Mini-Mental State Examination; NPI-S = Neuropsychiatric Inventory - Severity; NVOA = Nonverbal Oral Apraxia; TT = Token Test; UPDRS = Unified Parkinson Disease Rating Scale; WAB = Western Aphasia Battery; yrs. = Years.
Severity pooled only for participants that had dysarthria.
3.2. Task-free fMRI
Spatial maps for the 4 ICNs that differed between control and PPAOS participants are shown in Fig. 2. The speech and language ICN in both control and PPAOS participants included all of the expected areas: Broca's and Wernicke's area, the left anterior middle temporal lobe, supplementary and premotor regions. However, there appeared to be reduced connectivity involving the left SMA, and no right SMA inclusion within the ICN in PPAOS. On direct comparison, PPAOS participants indeed had reduced connectivity of the right SMA to the rest of the speech and language network compared to controls. There was an additional posterior temporal region of reduced connectivity in PPAOS compared to controls. Similarly, for the face ICN there was reduced SMA involvement in PPAOS, and on direct comparison an area in the left SMA was less connected to the ICN in PPAOS. Spatial maps for the left working memory ICN included areas of the posterior default mode network, such as the posterior cingulate and precuneus, in both control and PPAOS participants. On direct comparison a region at the temporal-occipital junction (TOJ) showed reduced connectivity in PPAOS, whereas an area in the posterior cingulate showed increased connectivity. For the salience ICN, PPAOS participants had reduced connectivity of the left lateral prefrontal cortex (left PFC) compared to controls. There were no significant differences in the remaining ICNs, shown in Fig. 3, or for connectivity involving the cerebellum, shown in Supplementary Fig. 1.
Fig. 2.
Three-dimensional brain renderings showing the ICNs that were different in PPAOS
Results of the single sample t-test for control and PPAOS participants for each ICN are shown separately on the left, uncorrected for multiple comparisons, with the color bar representing the voxel-level t-statistic and the horizontal white bar indicating the t-value cut off for FWEc p < 0.05. Results of the two sample t-test directly comparing PPAOS and control participant ICN maps are shown on the right, corrected for multiple comparisons at the cluster level (FWEc p < 0.05), with t-values again represented by the color scales. PPAOS participants had reduced connectivity of a region in the right SMA and the left posterior temporal gyrus to the rest of the speech and language ICN. PPAOS participants appeared to have reduced connectivity of bilateral SMAs to the face sensorimotor ICN, although only an area in the left SMA was statistically significant. Posterior DMN regions were incorporated into the left working memory ICN maps in both control and PPAOS participants. Controls had greater connectivity of a region at the left TOJ and PPAOS participants had increased connectivity in the dorsal PCC. Compared to controls, PPAOS participants had reduced connectivity of the left PFC and the salience ICN. Renders created using Brain Net Viewer (Xia et al., 2013) (https://www.nitrc.org/projects/bnv/)
Abbreviations: FWEc = Family-Wise Error Correction; ICN = Intrinsic Connectivity Network; PCC = Posterior Cingulate Cortex; PFC = Prefrontal Cortex; SMA = Supplementary Motor Area; TOJ = Temporal-Occipital Junction.
Fig. 3.
Three-dimensional brain renderings and representative slices showing the ICNs that did not differ between PPAOS and control participants
Results of the single sample t-test for control and PPAOS participants for each ICN are shown separately on the left, uncorrected for multiple comparisons, with the color bar representing the voxel-level t-statistic and the horizontal white bar indicating the t-value cut off for FWEc p < 0.05. The spatial maps for the hand sensorimotor (A), superior parietal (B), supramarginal (C), insula (D), and deep gray (F) ICNs included the expected regions. No differences were noted between PPAOS and control participants. Renders created using Brain Net Viewer (Xia et al., 2013) (https://www.nitrc.org/projects/bnv/)
FWEc = Family-Wise Error Correction; ICN = Intrinsic Connectivity Network.
3.3. Clinical-connectivity correlation
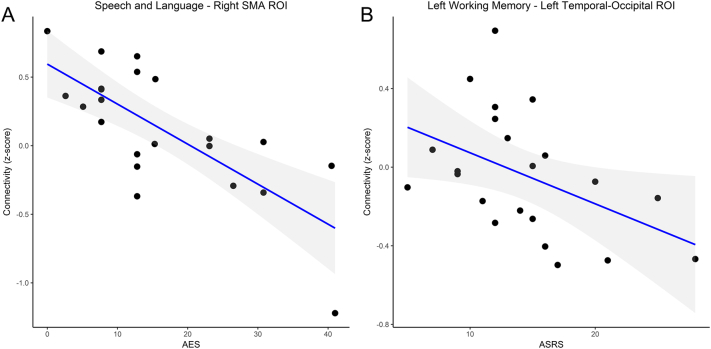
Next, we used the 6 areas that were found to be different in PPAOS compared to control participants (two areas of decreased connectivity to the speech and language ICN, one area of decreased connectivity to the face ICN, one area of decreased connectivity to the left working memory ICN, one area of decreased connectivity to the salience ICN and one area of increased connectivity to the left working memory ICN) and extracted the connectivity z-score for each participant. In other words, the connectivity between each of these areas and the relevant ICN was quantified. We then used these connectivity scores to assess for an age effect in control participants. There were no differences in motion parameters between control and PPAOS participants, and no correlation between motion and connectivity parameters extracted from the 6 clusters (Supplementary Table 1). Only the connectivity between the posterior cingulate and left working memory ICN was associated with age (Supplementary Fig. 2). We used a linear regression to assess the relationship between functional connectivity and the three clinical measures mentioned previously: the AES, ASRS and NVOA score. This amounted to 18 comparisons, and as such the Bonferroni corrected p-value for significance was set at 0.002778. Results for this analysis are shown in Table 2 and Fig. 4. Reduced connectivity between the right SMA and the speech and language ICN was associated with higher AES, denoting more severe AOS. Reduced connectivity between the left temporal-occipital junction and the left working memory ICN correlated with higher ASRS scores, also denoting more severe AOS, although this finding did not survive correction for multiple comparisons. The connectivity z-score for the other 4 areas was not associated with the ASRS or AES, and none of the cluster connectivity z-scores were associated with NVOA.
Table 2.
Relationship between connectivity z-score and ASRS, AES and NVOA.
| Region – ICN connectivity | ASRS |
AES |
NVOA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | R2 | p | Beta | R2 | p | Beta | R2 | p | |
| Right SMA – speech and language | −0.317 | 0.056 | 0.150 | −0.742 | 0.528 | <0.001 | 0.203 | −0.007 | 0.364 |
| Left temporal – speech and language | 0.092 | −0.041 | 0.683 | −0.039 | −0.048 | 0.864 | −0.291 | 0.039 | 0.189 |
| Left SMA – face | 0.171 | −0.019 | 0.446 | 0.307 | 0.049 | 0.165 | 0.034 | −0.049 | 0.881 |
| Left TOJ – left working memory | −0.456 | 0.168 | 0.033 | −0.212 | −0.003 | 0.343 | 0.126 | −0.033 | 0.577 |
| Left PCC – left working memory | 0.235 | 0.008 | 0.292 | 0.178 | −0.017 | 0.429 | 0.046 | −0.048 | 0.838 |
| Left lateral PFC – salience | −0.093 | −0.041 | 0.681 | 0.350 | 0.079 | 0.110 | −0.275 | 0.029 | 0.216 |
Standardized beta, adjusted R2 and p-values for models using the relevant clinical measure as the outcome, and the connectivity z-score as predictor. Significant correlations were found between the right supplementary motor area (SMA)-speech and language ICN connectivity and the AES, and between the left temporal-occipital (TOJ)-left working memory ICN connectivity and ASRS scores (see Fig. 4), although the latter did not survive correction for multiple comparisons. Models with gray matter volumes for each region included are show in Supplementary Tables 5–7.
Abbreviations: AES = Articulatory error score; ASRS = Apraxia of Speech Severity Rating Scale; NVOA = Nonverbal Oral Apraxia; PCC = Posterior Cingulate Cortex; PFC = Prefrontal Cortex; SMA = Supplementary Motor Area; TOJ = Temporal-Occipital Junction.
Fig. 4.
Scatterplot showing the relationship between the connectivity and clinical measures of AOS severity at the single participant level
A. Decreased connectivity between the right SMA cluster and the speech and language ICN was associated with higher AES, explaining approximately half of the observer variation. B. Decreased connectivity between the left temporal-occipital junction (TOJ) and the left working memory ICN we associated with higher ASRS scores, although this did not survive correction for multiple comparisons.
Abbreviations: AES = Articulatory Error Score; ASRS = Apraxia of Speech Severity Rating Scale; ICN = Intrinsic Connectivity Network; SMA = Supplementary Motor Area; TOJ = Temporal-Occipital Junction.
3.4. Gray matter volume and functional connectivity results
We calculated the gray matter volume within each of the clusters that were used to extract the connectivity parameters in Section 3.3 and used it to compare control and PPAOS participants and to explore the effect of gray matter volume on the connectivity results. While the groups were well separated using the connectivity parameters from each cluster (Supplementary Table 2), as would be expected since the clusters were based on the voxel-wise group comparison, gray matter volumes only differed between groups for the left SMA cluster (Supplementary Table 3). The only cluster where connectivity and gray matter volume were correlated was the right SMA cluster, although only in control participants (Supplementary Table 4). Gray matter volumes were added to the models described in Table 2. In terms of predicting ASRS values, results for the model involving the Left TOJ cluster, which were significant in Table 2, were no longer significant (Supplementary Table 5). However, the standardized beta for the connectivity term was unchanged and the gray matter term had minimal explanatory power. Results for models predicting AES values were comparable to those in Table 2, in that only the model involving the Right SMA cluster was significant (Supplementary Table 6). For this model, only the connectivity term was significant, and the beta for the gray matter volume suggested an effect in the opposite direction. As was the case in Table 2, none of the models were significantly predictive of NVOA (Supplementary Table 7).
Our VBM analysis revealed atrophy in PPAOS participants predominantly involving the left SMA, left dorsal premotor and left motor regions, with the right SMA and bilateral inferior frontal gyri involved to a lesser extent (Supplementary Fig. 3). We calculated the percentage of voxels within each of the 6 clusters described in Section 3.2 that overlapped with this group-level spatial map. Only the Left SMA cluster showed any overlap, although it did involve most of the voxels in the cluster (84.78%).
3.5. Exploratory seed-based analysis
We placed a spherical seed (radius 6 mm) at the peak coordinates for each of the 6 clusters and performed a seed-to-brain analysis. The t-maps for this analysis, thresholded at p(unc) = 0.001, are shown in Supplementary Fig. 4. In summary, each of the seed-based connectivity analyses resulted in spatial maps that included the regions of the relevant ICN for which a difference was noted in PPAOS.
4. Discussion
We explored changes in functional connectivity in PPAOS by evaluating nine ICNs using TF-fMRI. Our main findings were: (1) PPAOS was associated with reduced connectivity between an area in the right SMA and the rest of the speech and language network, and the degree of decreased connectivity correlated with a measure of AOS severity; (2) PPAOS was also associated with other connectivity changes involving the speech and language ICN, the face ICN, the left working memory ICN and the salience ICN; (3) PPAOS was not associated with connectivity changes in the other ICNs we assessed (insula, hand, supramarginal, superior parietal, deep gray). To the best of our knowledge this is the first study to assess network-level changes in PPAOS.
Prior FDG-PET and structural MRI studies found that PPAOS was associated with bilateral SMA, dorsolateral premotor and motor cortex hypometabolism and atrophy, as well as disruption of bilateral frontal white matter tracts (Botha et al., 2015; Josephs et al., 2014; Josephs et al., 2012; Josephs et al., 2006). As such, our report of network level connectivity changes in PPAOS involving the SMA is well in line with the existing literature. The fact that the connectivity between the right SMA and the rest of the speech and language ICN strongly correlated with the frequency of articulatory errors (AES) suggests an important functional role for this connection in articulation. A previous study found that decreased integrity of the Aslant tract, a white matter bundle that connects the inferior frontal gyrus (Broca's area) with the pre-SMA, SMA and premotor regions, was associated with a reduction in fluency in agPPA patients, measured as the mean length of the utterance and words per minute (Catani et al., 2013). AOS most likely contributed to this finding given the high proportion of agPPA participants with AOS and its effect on speech rate and words per breath group. Functional and structural connectivity between the inferior frontal gyrus and SMAs bilaterally was also found to correlate with longitudinal gray matter changes in agPPA (Mandelli et al., 2016). We also found that PPAOS was associated with reduced connectivity of a part of the left SMA and the bilateral face sensorimotor ICN, although this result may have been driven by left SMA atrophy and did not correlate with any of the clinical measures we evaluated. Nevertheless, in light of prior findings and the results of our study it is reasonable to posit that disconnection of the SMAs from other regions involved in verbal output is one of the network level changes underlying PPAOS.
Our results are in keeping with current models of articulation, which prominently feature the SMA and its connections to deep gray nuclei, lateral motor and premotor cortices, among others. For instance, in the DIVA model the SMA contains an “initiation map”, which gates articulator position commands (Tourville and Guenther, 2011). It is also consistent with activation studies, which consistently show activity in bilateral medial frontal lobes in addition to other language and sensorimotor regions (see (Price, 2012)for a review). Of note, a recent study found that activation in bilateral precentral and SMA regions was not selective for speech tasks and did not scale with articulation complexity, in contrast to the inferior frontal gyrus, suggesting that these areas may not support speech-specific computations (Basilakos et al., 2017). Indeed, it is likely that SMA and pre-SMA play important roles in many motor and non-motor systems, with different regions being involved in time coding (Mita et al., 2009) and temporal processing (Kotz and Schwartze, 2011), sequencing(Sohn and Lee, 2007), gating(Bohland et al., 2010), and so on, culminating in successful “motor planning” (Li et al., 2016). While the degree of activation in the SMA as a whole may not differ based on task or complexity (Basilakos et al., 2017), the specific areas activated and functional connectivity may well differ. Impairment of these functions, either by direct damage to the SMA or by reduced connectivity between the SMA and the rest of the speech and language network, may underlie the distorted substitutions, increased segmentation, groping, altered prosody, et cetera which characterizes AOS. The regional heterogeneity of the SMA (Chung et al., 2005) may also explain why the connectivity between the left SMA and the face sensorimotor ICN did not correlate with our clinical measures, which were focused on verbal and nonverbal oral movements. In our exploratory seed-based analysis, the right SMA seed was correlated with activity in many areas associated with speech and language, whereas the left SMA seed was correlated with bilateral sensorimotor regions including the arm and leg regions of the pre- and post-central gyri. This seed-to-brain map resembled the pattern on atrophy revealed by our VBM analysis, and did not involve the right SMA region discussed previously. Based on this and the results of our regional gray matter analyses, we suspect that the apparent reduction in left SMA connectivity is at least partially a result of disease related atrophy involving the sensorimotor system(s).
We must emphasize, however, that we are not suggesting all of the variable clinical features in AOS result from disruption of the SMA or its connections. There have been multiple prior studies linking AOS severity to atrophy, hypometabolism or infarction of the ventral and dorsolateral premotor cortex, primary motor cortex and insula (Ballard et al., 2014a; New et al., 2015; Whitwell et al., 2013), for instance, and the role of the inferior frontal gyrus (Hillis et al., 2004), deep gray nuclei and cerebellum should not be overlooked (Marien et al., 2014). Future functional connectivity studies using both task-free and task-related design, perhaps incorporating graph theory, will be crucial to determine the network connectivity changes underlying AOS and PPAOS (Ballard et al., 2014b; Simonyan and Fuertinger, 2015).
Our finding that a region of the posterior temporal lobe was relatively disconnected from the rest of the speech and language network was somewhat surprising, and did not appear to be due to atrophy. However, while PPAOS is typically associated with more anterior imaging abnormalities, some patients do develop more widespread hypometabolism and atrophy (Josephs et al., 2014). Assuming connectivity changes precede clinical changes, it may be that our sample contained a large enough subset of these participants to drive more posterior connectivity changes. Similarly, the finding of a region at the TOJ being disconnected from the left working memory ICN may seem incongruent with prior work on PPAOS. Although the negative correlation with ASRS did not survive correction for multiple comparisons, it is plausible that the working memory ICN plays some role in the ASRS. We have previously linked left working memory dysfunction to language measures in PPA (Whitwell et al., 2015), and the TOJ area reported here has been linked to target or goal directed eye and limb movements (Gallivan et al., 2013; Macaluso et al., 2007). Alternatively, especially given the indirect measure of connectivity involved in TF-fMRI, these results may simply reflect nonspecific disease related changes. The final area of reduced connectivity in PPAOS was found in the left LPFC in relation to the salience ICN. Although this did not correlate with measures of AOS or NVOA severity, this ICN has been implicated in bvFTD and PSP in prior studies (Bharti et al., 2017; Whitwell et al., 2011a; Whitwell et al., 2011b; Zhou et al., 2010). Given the association between PPAOS and 4-R tau, and the longitudinal development of bvFTD and PSP features in PPAOS participants, this connectivity change is not surprising. Although the area involved did not overlap with regions of atrophy from our VBM analysis, gray matter volumes within this cluster were lower in PPAOS compared to controls, suggesting that subtle atrophy may have contributed to this result.
The only area of increased connectivity we found in PPAOS involved the dorsal PCC and the left working memory ICN. This finding did not appear to be due to atrophy, and since the results for the comparison were masked with the connectivity maps from controls it is unlikely to represent the loss of anti-correlations (i.e. when a region that is usually anti-correlated with the network of interest loses the anti-correlation during disease, and results in spurious “increased connectivity”). As mentioned previously, the left working memory ICN map incorporated parts of the posterior DMN in control and PPAOS participants. As such this finding may represent altered PCC connectivity in the context of the left working memory ICN, the DMN or both. The posterior cingulate is an important hub of high connectivity, and much like the SMA is heterogeneous in its function and hence connectivity. In fact, the PCC cluster in the present study involves an area previously associated with the left frontoparietal control network at rest that was deactivated more significantly during a task than the rest of the PCC (Leech et al., 2012). It has been suggested that the PCC plays an important and active role in cognitive control (Leech et al., 2012), and together with other posterior DMN regions may play a central role in age and disease related connectivity changes (Jones et al., 2011; Jones et al., 2012). If network reconfiguration is occurring in PPAOS, whether adaptive and compensatory changes or maladaptive load shifting, the PCC is likely to be involved.
We did not find any connectivity changes in the other ICNs we evaluated, although this should be interpreted with caution. Given our interest in studying the network changes related to PPAOS specifically, we excluded patients that may have originally presented with PPAOS but subsequently developed sufficient aphasia, behavioral or motor impairment to meet criteria for an additional disorder. As such, our participants had little to no evidence for language involvement, ideomotor apraxia, behavioral disturbance, and so on. Our participants were, for the most part, early in the disease course, with mild AOS. It is entirely possible that changes in some of the ICNs we examined only occur later in the course of the disease and correlate with the development of the aforementioned clinical features. For example, ideomotor apraxia in the context of agPPA has been associated with involvement of the hand sensorimotor regions (Adeli et al., 2013). One might also expect more parietal involvement in those who develop CBS, involvement of the deep gray ICN in those who develop PSP-like features, or insula and salience network involvement in those who develop a bvFTD-like picture (Whitwell et al., 2011a; Zhou et al., 2010). Furthermore, the inherent heterogeneity of PPAOS might complicate clinical-connectivity correlation. Deficits in prosody, for example, likely have different correlates than articulatory errors. We did not have sufficient participants to explore subgroup analyses such as stratifying participants by age or by the detailed characteristics of their AOS. Lastly, not rejecting the null by not finding a statistically significant effect is not the same as proving the null. There may well be changes in the ICNs we examined that would only become “significant” with a larger sample size.
We have already hinted at some limitations of our study. The most obvious, and most frequently encountered in the case of PPAOS, is the small sample size. Given the relative rarity of the disorder, and the strict phenotyping required for studies such as ours, this is a hard limitation to overcome. Having said that, our sample size compares favorably with those of other functional connectivity studies in rare degenerative disorders such as PSP (Brown et al., 2017), CBS (Bharti et al., 2017), or posterior cortical atrophy (Migliaccio et al., 2016), as well as with prior studies of stroke-related AOS (New et al., 2015). Another potential limitation involves the a priori selection of nine ICNs. We based our choices on prior work on PPAOS, and explored the possibility that the differences we found may reflect changes in networks we did not evaluate through a seed-based analysis. We feel it is unlikely that large network changes were missed, and that the benefits of using a multivariate method of back-reconstructing participant level spatial maps outweigh the limitations (Leech et al., 2012; Leech et al., 2011). However, future studies exploring different ICNs, or using different methodologies, would be important in validating or challenging the results reported here. Finally, as is the case with TF-fMRI in degenerative disease in general, it is important to bear in mind that we are relied on an indirect measure of neural activity in the present study. Similarly, even under the best of circumstances autocorrelations in fMRI signal are but an imperfect and undirected measure of “connectivity”. It is entirely plausible that non-neuronal disease processes may result in alteration in the BOLD signal and hence TF-fMRI results, and that these may have contributed to some of our findings.
5. Conclusions
In summary, we used TF-fMRI to explore, for the first time, the network level connectivity changes underlying PPAOS. We found changes in the speech and language face sensorimotor, left working memory networks and salience networks. The connectivity between the right SMA and the rest of the speech and language network correlated with a measure of AOS severity, suggesting it may serve as a biomarker of disease severity.
Funding sources
This research was supported by the National Institutes of Health [grant numbers R01-DC010367, R01-DC12519, R21NS094684, U01 AG006786, R01 AG11378, and R01 AG041851], the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's disease Research Program, the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic and the Mayo Foundation for Medical Education and Research.
Acknowledgements
We would like to thank Sarah Boland for performing the neuropsychological testing and organizing all participants test schedules. Most importantly we would like to acknowledge the patients who participated in this study and their family members who made their participation possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.036.
Contributor Information
Hugo Botha, Email: Botha.Hugo@mayo.edu.
Rene L. Utianski, Email: Utianski.Rene@mayo.edu.
Jennifer L. Whitwell, Email: Whitwell.Jennifer@mayo.edu.
Joseph R. Duffy, Email: jduffy@mayo.edu.
Heather M. Clark, Email: Clark.Heather1@mayo.edu.
Edythe A. Strand, Email: edythestrand@gmail.com.
Mary M. Machulda, Email: Machulda.Mary@mayo.edu.
Nirubol Tosakulwong, Email: Tosakulwong.Nirubol@mayo.edu.
David S. Knopman, Email: knopman@mayo.edu.
Ronald C. Petersen, Email: peter8@mayo.edu.
Clifford R. Jack, Jr, Email: Jack.Clifford@mayo.edu.
Keith A. Josephs, Email: Josephs.Keith@mayo.edu.
David T. Jones, Email: Jones.david@mayo.edu.
Appendix A. Supplementary data
Supplementary material.
References
- Adeli A., Whitwell J.L., Duffy J.R., Strand E.A., Josephs K.A. Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. J. Neurol. 2013;260:1594–1600. doi: 10.1007/s00415-013-6839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S.K., Rittman T., Xuereb J.H., Bak T.H., Hodges J.R., Rowe J.B. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J. Neurol. Neurosurg. Psychiatry. 2014;85:925–929. doi: 10.1136/jnnp-2013-307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B., Boxer A.L., Dickson D.W., Grossman M., Hallett M., Josephs K.A., Kertesz A., Lee S.E., Miller B.L., Reich S.G., Riley D.E., Tolosa E., Troster A.I., Vidailhet M., Weiner W.J. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ballard K.J., Savage S., Leyton C.E., Vogel A.P., Hornberger M., Hodges J.R. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K.J., Tourville J.A., Robin D.A. Behavioral, computational, and neuroimaging studies of acquired apraxia of speech. Front. Hum. Neurosci. 2014;8:892. doi: 10.3389/fnhum.2014.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos A., Smith K.G., Fillmore P., Fridriksson J., Fedorenko E. Functional characterization of the human speech articulation network. Cereb. Cortex. 2017:1–15. doi: 10.1093/cercor/bhx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K., Bologna M., Upadhyay N., Piattella M.C., Suppa A., Petsas N., Gianni C., Tona F., Berardelli A., Pantano P. Abnormal resting-state functional connectivity in progressive supranuclear palsy and corticobasal syndrome. Front. Neurol. 2017;8:248. doi: 10.3389/fneur.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland J.W., Bullock D., Guenther F.H. Neural representations and mechanisms for the performance of simple speech sequences. J. Cogn. Neurosci. 2010;22:1504–1529. doi: 10.1162/jocn.2009.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H., Duffy J.R., Strand E.A., Machulda M.M., Whitwell J.L., Josephs K.A. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014;82:1729–1735. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H., Duffy J.R., Whitwell J.L., Strand E.A., Machulda M.M., Schwarz C.G., Reid R.I., Spychalla A.J., Senjem M.L., Jones D.T., Lowe V., Jack C.R., Josephs K.A. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex. 2015;69:220–236. doi: 10.1016/j.cortex.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Hua A.Y., Trujllo A., Attygalle S., Binney R.J., Spina S., Lee S.E., Kramer J.H., Miller B.L., Rosen H.J., Boxer A.L., Seeley W.W. Advancing functional dysconnectivity and atrophy in progressive supranuclear palsy. Neurol. Clin. 2017;16:564–574. doi: 10.1016/j.nicl.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C., Thompson C.K., Thiebaut de Schotten M., Dell'Acqua F., Weintraub S., Rogalski E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung G.H., Han Y.M., Jeong S.H., Jack C.R., Jr. Functional heterogeneity of the supplementary motor area. AJNR Am. J. Neuroradiol. 2005;26:1819–1823. [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Darley F.L., Aronson A.E., Brown J.R. Differential diagnostic patterns of dysarthria. J. Speech Hear. Res. 1969;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- De Renzi E., Vignolo L.A. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Deramecourt V., Lebert F., Debachy B., Mackowiak-Cordoliani M.A., Bombois S., Kerdraon O., Buee L., Maurage C.A., Pasquier F. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Duffy J.R. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- Duffy J.R. Third edition. ed. Elsevier; St. Louis, Missouri: 2013. Motor Speech Disorders : Substrates, Differential Diagnosis, and Management. [Google Scholar]
- Duffy J.R. Elsevier Health Sciences; 2013. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. [Google Scholar]
- Duffy J.R., Josephs K.A. The diagnosis and understanding of apraxia of speech: why including neurodegenerative etiologies may be important. J. Speech Lang. Hear. Res. 2012;55:S1518–1522. doi: 10.1044/1092-4388(2012/11-0309). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.R., Strand E.A., Clark H., Machulda M., Whitwell J.L., Josephs K.A. Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. Am. J. Speech Lang. Pathol. 2015;24:88–100. doi: 10.1044/2015_AJSLP-14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.R., Hanley H., Utianski R., Clark H., Strand E., Josephs K.A., Whitwell J.L. Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain Lang. 2017;168:84–94. doi: 10.1016/j.bandl.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Modalities, modes, and models in functional neuroimaging. Science. 2009;326:399–403. doi: 10.1126/science.1174521. [DOI] [PubMed] [Google Scholar]
- Gallivan J.P., Chapman C.S., McLean D.A., Flanagan J.R., Culham J.C. Activity patterns in the category-selective occipitotemporal cortex predict upcoming motor actions. Eur. J. Neurosci. 2013;38:2408–2424. doi: 10.1111/ejn.12215. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Jones D.T., Strand E.A., Rabinstein A.A., Duffy J.R., Josephs K.A. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. doi: 10.1016/j.bandl.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hlinka J., Palus M., Vejmelka M., Mantini D., Corbetta M. Functional connectivity in resting-state fMRI: is linear correlation sufficient? NeuroImage. 2011;54:2218–2225. doi: 10.1016/j.neuroimage.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., Mollenhauer B., Muller U., Nilsson C., Whitwell J.L., Arzberger T., Englund E., Gelpi E., Giese A., Irwin D.J., Meissner W.G., Pantelyat A., Rajput A., van Swieten J.C., Troakes C., Antonini A., Bhatia K.P., Bordelon Y., Compta Y., Corvol J.C., Colosimo C., Dickson D.W., Dodel R., Ferguson L., Grossman M., Kassubek J., Krismer F., Levin J., Lorenzl S., Morris H.R., Nestor P., Oertel W.H., Poewe W., Rabinovici G., Rowe J.B., Schellenberg G.D., Seppi K., van Eimeren T., Wenning G.K., Boxer A.L., Golbe L.I., Litvan I. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Machulda M.M., Vemuri P., McDade E.M., Zeng G., Senjem M.L., Gunter J.L., Przybelski S.A., Avula R.T., Knopman D.S., Boeve B.F., Petersen R.C., Jack C.R., Jr. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Vemuri P., Murphy M.C., Gunter J.L., Senjem M.L., Machulda M.M., Przybelski S.A., Gregg B.E., Kantarci K., Knopman D.S., Boeve B.F., Petersen R.C., Jack C.R., Jr. Non-stationarity in the "resting brain's" modular architecture. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Knopman D.S., Gunter J.L., Graff-Radford J., Vemuri P., Boeve B.F., Petersen R.C., Weiner M.W., Jack C.R., Jr., Alzheimer's Disease Neuroimaging I. Cascading network failure across the Alzheimer's disease spectrum. Brain. 2016;139:547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr. Opin. Neurol. 2008;21:688–692. doi: 10.1097/WCO.0b013e3283168ddd. [DOI] [PubMed] [Google Scholar]
- Josephs K.A., Boeve B.F., Duffy J.R., Smith G.E., Knopman D.S., Parisi J.E., Petersen R.C., Dickson D.W. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A., Whitwell J.L., Layton K.F., Parisi J.E., Hauser M.F., Witte R.J., Boeve B.F., Knopman D.S., Dickson D.W., Jack C.R., Jr., Petersen R.C. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A., Machulda M.M., Senjem M.L., Master A.V., Lowe V.J., Jack C.R., Jr., Whitwell J.L. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A., Machulda M.M., Senjem M.L., Lowe V.J., Jack C.R., Jr., Whitwell J.L. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A., Machulda M.M., Senjem M.L., Gunter J.L., Schwarz C.G., Reid R.I., Spychalla A.J., Lowe V.J., Jack C.R., Jr., Whitwell J.L. The evolution of primary progressive apraxia of speech. Brain. 2014;137:2783–2795. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C., Kell C.A. Asymmetric intra- and interhemispheric interactions during covert and overt sentence reading. Neuropsychologia. 2016;93:448–465. doi: 10.1016/j.neuropsychologia.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Kertesz A. PsychCorp.; San Antonio: 2007. Western Aphasia Battery (Revised) [Google Scholar]
- Kertesz A., Davidson W., Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Kotz S.A., Schwartze M. Differential input of the supplementary motor area to a dedicated temporal processing network: functional and clinical implications. Front. Integr. Neurosci. 2011;5:86. doi: 10.3389/fnint.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G., Frey S.H. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb. Cortex. 2009;19:2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing A.E., Ivnik R.J., Cullum C.M., Randolph C. An empirically derived short form of the Boston naming test. Arch. Clin. Neuropsychol. 1999;14:481–487. [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Braga R., Sharp D.J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Daie K., Svoboda K., Druckmann S. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532:459–464. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E., Frith C.D., Driver J. Delay activity and sensory-motor translation during planned eye or hand movements to visual or tactile targets. J. Neurophysiol. 2007;98:3081–3094. doi: 10.1152/jn.00192.2007. [DOI] [PubMed] [Google Scholar]
- Mandelli M.L., Vilaplana E., Brown J.A., Hubbard H.I., Binney R.J., Attygalle S., Santos-Santos M.A., Miller Z.A., Pakvasa M., Henry M.L., Rosen H.J., Henry R.G., Rabinovici G.D., Miller B.L., Seeley W.W., Gorno-Tempini M.L. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain. 2016;139:2778–2791. doi: 10.1093/brain/aww195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P., Ackermann H., Adamaszek M., Barwood C.H., Beaton A., Desmond J., De Witte E., Fawcett A.J., Hertrich I., Kuper M., Leggio M., Marvel C., Molinari M., Murdoch B.E., Nicolson R.I., Schmahmann J.D., Stoodley C.J., Thurling M., Timmann D., Wouters E., Ziegler W. Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M.R., Robin D.A., Schmidt R.A. Apraxia of speech: definition and differential diagnosis. In: McNeil M.R., editor. Clinical Management of Sensorimotor Speech Disorders. Thieme, New York. 2009. [Google Scholar]
- Migliaccio R., Gallea C., Kas A., Perlbarg V., Samri D., Trotta L., Michon A., Lacomblez L., Dubois B., Lehericy S., Bartolomeo P. Functional connectivity of ventral and dorsal visual streams in posterior cortical atrophy. J. Alzheimers Dis. 2016;51:1119–1130. doi: 10.3233/JAD-150934. [DOI] [PubMed] [Google Scholar]
- Mita A., Mushiake H., Shima K., Matsuzaka Y., Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat. Neurosci. 2009;12:502–507. doi: 10.1038/nn.2272. [DOI] [PubMed] [Google Scholar]
- New A.B., Robin D.A., Parkinson A.L., Duffy J.R., McNeil M.R., Piguet O., Hornberger M., Price C.J., Eickhoff S.B., Ballard K.J. Altered resting-state network connectivity in stroke patients with and without apraxia of speech. Neuroimage Clin. 2015;8:429–439. doi: 10.1016/j.nicl.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.O., Geda Y.E., Knopman D.S., Cha R.H., Pankratz V.S., Boeve B.F., Ivnik R.J., Tangalos E.G., Petersen R.C., Rocca W.A. The Mayo clinic study of aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff H.B., Alexander M.P., Naeser M.A., Galaburda A.M. Aphemia. Clinical-anatomic correlations. Arch. Neurol. 1983;40:720–727. doi: 10.1001/archneur.1983.04050110038005. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senjem M.L., Gunter J.L., Shiung M.M., Petersen R.C., Jack C.R. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. NeuroImage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Fuertinger S. Speech networks at rest and in action: interactions between functional brain networks controlling speech production. J. Neurophysiol. 2015;113:2967–2978. doi: 10.1152/jn.00964.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.V., Utevsky A.V., Bland A.R., Clement N., Clithero J.A., Harsch A.E., McKell Carter R., Huettel S.A. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. NeuroImage. 2014;95:1–12. doi: 10.1016/j.neuroimage.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J.W., Lee D. Order-dependent modulation of directional signals in the supplementary and presupplementary motor areas. J. Neurosci. 2007;27:13655–13666. doi: 10.1523/JNEUROSCI.2982-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand E.A., Duffy J.R., Clark H.M., Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J. Commun. Disord. 2014;51:43–50. doi: 10.1016/j.jcomdis.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville J.A., Guenther F.H. The DIVA model: a neural theory of speech acquisition and production. Lang. Cogn. Process. 2011;26:952–981. doi: 10.1080/01690960903498424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski R.L., Whitwell J.L., Schwarz C.G., Senjem M.L., Tosakulwong N., Duffy J.R., Clark H.M., Machulda M.M., Petersen R.C., Jack C.R., Jr., Lowe V.J., Josephs K.A. Tau-PET imaging with [18F]AV-1451 in primary progressive apraxia of speech. Cortex. 2018;99:358–374. doi: 10.1016/j.cortex.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J.L., Duffy J.R., McNeil M.R., Robin D.A., Rogers M.A. Treatment guidelines for acquired apraxia of speech: treatment descriptions and recommendations. J. Med. Speech-Lang. Pathol. 2006;14:xxxv–lxvii. [Google Scholar]
- Whitwell J.L., Avula R., Master A., Vemuri P., Senjem M.L., Jones D.T., Jack C.R., Jr., Josephs K.A. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat. Disord. 2011;17:599–605. doi: 10.1016/j.parkreldis.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Josephs K.A., Avula R., Tosakulwong N., Weigand S.D., Senjem M.L., Vemuri P., Jones D.T., Gunter J.L., Baker M., Wszolek Z.K., Knopman D.S., Rademakers R., Petersen R.C., Boeve B.F., Jack C.R., Jr. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology. 2011;77:866–874. doi: 10.1212/WNL.0b013e31822c61f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Duffy J.R., Strand E.A., Xia R., Mandrekar J., Machulda M.M., Senjem M.L., Lowe V.J., Jack C.R., Jr., Josephs K.A. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125:245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Jones D.T., Duffy J.R., Strand E.A., Machulda M.M., Przybelski S.A., Vemuri P., Gregg B.E., Gunter J.L., Senjem M.L., Petersen R.C., Jack C.R., Jr., Josephs K.A. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer's dementia. Neurobiol. Aging. 2015;36:1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiepert D.A., Lowe V.J., Knopman D.S., Boeve B.F., Graff-Radford J., Petersen R.C., Jack C.R., Jr., Jones D.T. A robust biomarker of large-scale network failure in Alzheimer's disease. Alzheimers Dement. (Amst) 2017;6:152–161. doi: 10.1016/j.dadm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D., Kramer J.H., Weiner M., Miller B.L., Seeley W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.