Abstract
Objective:
In relapsing–remitting multiple sclerosis (RRMS), suboptimal adherence to injectable disease-modifying therapies (iDMTs; interferon β-1a/b, glatiramer acetate) is common, reducing their effectiveness. Patient retention on oral fingolimod and iDMTs was evaluated in PREFERMS, a randomized, parallel-group, active-controlled, open-label, 48-week study.
Methods:
Patients were included if they had RRMS, were aged 18–65 years and had Expanded Disability Status Scale score up to 6, enrolled at 117 US study sites, were treatment naïve or had received only one iDMT class. Patients were randomized 1:1 (fingolimod 0.5 mg/day; preselected iDMT) by interactive voice-and-web-response system without blinding, followed up quarterly, and allowed one study-approved treatment switch after 12 weeks, or earlier for efficacy or safety reasons. The primary outcome was patient retention on randomized treatment over 48 weeks. Secondary endpoints included patient-reported outcomes, brain volume loss (BVL), and cognitive function.
Results:
Analysis of 433/436 patients receiving fingolimod and 428/439 receiving iDMTs showed that patient retention rate was significantly higher with fingolimod than with iDMTs [352 (81.3%) versus 125 (29.2%); 95% confidence interval 46.4–57.8%; p < 0.0001]. The most common treatment switch was from iDMT to fingolimod for injection-related reasons. Patient satisfaction was greater and BVL less with fingolimod than with iDMTs, with no difference in cognitive function. Adverse events were consistent with established tolerability profiles for each treatment.
Conclusions:
In RRMS, fingolimod was associated with better treatment retention, patient satisfaction and BVL outcomes than iDMTs. Patients may persist with iDMTs, but many may switch treatment if permitted. Treatment satisfaction fosters adherence, a prerequisite for optimal outcomes.
Keywords: adherence, disease-modifying therapy, fingolimod, glatiramer acetate, interferon, randomized controlled trial, retention, multiple sclerosis
Introduction
In relapsing–remitting multiple sclerosis (RRMS), injectable disease-modifying therapies (iDMTs) interferon β-1a [Avonex (Biogen, Cambridge, MA, USA) or Rebif (EMD Serono, Rockland, MA, USA)], interferon β-1b [Betaseron (Bayer, Leverkusen, Germany) or Extavia (Novartis Pharma AG, Basel, Switzerland)], and glatiramer acetate [GA; Copaxone (Teva Pharmaceutical Industries, Petah Tikva, Israel)] are widely used first line,1–5 but suboptimal adherence is common (often for injection-related reasons) and reduces their effectiveness.1,2,4,6–8 Efficacy, safety, tolerability, and convenience influence adherence of patients to therapy,2,4,6,9,10 defined here as therapy retention rate. Retention is particularly important for effective therapy2,4,6,7,10,11 and is clinically meaningful when multiple treatment options are available.4,5
Switching between iDMTs is common,1,5 and newer high-efficacy oral therapies and infrequently administered intravenous therapies are alternatives to regular injections.4,5,12 Although often used second line, fingolimod [Gilenya (Novartis Pharma AG, Switzerland)] is approved first line in several countries (including the USA),12 is efficacious in early multiple sclerosis (MS),13 and shows a high rate of patient retention.9,14 Well-tolerated oral therapy could result in higher retention compared with iDMTs; however, no randomized controlled trials (RCTs) have demonstrated this. Prescription data from pharmacy registries and observational cohort studies suggest greater therapeutic retention with oral MS therapies than with iDMTs;14–16 however, interpretation of such data is limited by well-known biases associated with retrospective, nonrandomized data.
Here, we report findings from the 48-week Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis (PREFERMS), the first RCT comparing patient retention across injectable and oral therapies for RRMS.
Methods
Study participants
Patients aged 18–65 years, diagnosed with RRMS (2010 international panel criteria)17 and with an Expanded Disability Status Scale (EDSS) score up to 6, were enrolled from 117 centers in the USA between June 2012 and June 2014; last patient last visit was in July 2015. Eligible patients were treatment naïve or previously treated with no more than one class of study-approved iDMT. If appropriate, a negative pregnancy test and effective birth control methods were required; study sites and eligibility criteria are given in supplementary data 1.
Standard protocol approvals, registrations and patient consents
The study followed the Declaration of Helsinki and Good Clinical Practice guidelines, with applicable local regulations.18,19 Patients provided written informed consent before any assessments. Protocol amendments are in supplementary table 1. The study protocol and all amendments were centrally approved by Quorum Review IRB, and were reviewed at each center’s Independent Ethics Committee or Institutional Review Board.
Study design
PREFERMS was a randomized, open-label, active-controlled, parallel-group, multicenter study that followed up patients for 48 weeks. Visits occurred at screening, at baseline (day 0), and at weeks 4, 12, 24, 36, and 48 (supplementary figure 1 and supplementary table 2). Patient medical history, medication, and current disease status were obtained at screening. For patients previously treated with GA, an interferon iDMT was preselected during consultation with their physician before randomization. Similarly, GA was preselected for patients previously treated with an interferon (supplementary table 3); iDMTs of either class were preselected for treatment-naïve patients. Similar numbers of patients were preselected for each iDMT class [interferon, n = 420 (48%); GA, n = 455 (52%)].
Patients were randomized 1:1 to fingolimod 0.5 mg/day or to an iDMT using an interactive voice-and-web-response system (IVRS/IWRS) that automated random assignment of patient numbers to different treatment arms. After baseline assessment and patient eligibility confirmation, study medication was allocated via IVRS/IWRS and administered according to US prescribing information (supplementary table 3). To foster and track medication adherence, at each visit study drug accountability logs were maintained and updated by investigators, and patients were asked to return any unused drug and packaging; noncompliance was recorded as a protocol violation. The adherence rate in each group was also determined (duration of exposure excluding interruptions as a percentage of the study duration). At each visit, clinical, radiographic, cognitive, and patient-reported outcomes, and safety information were also collected (supplementary figure 1 and supplementary table 2).
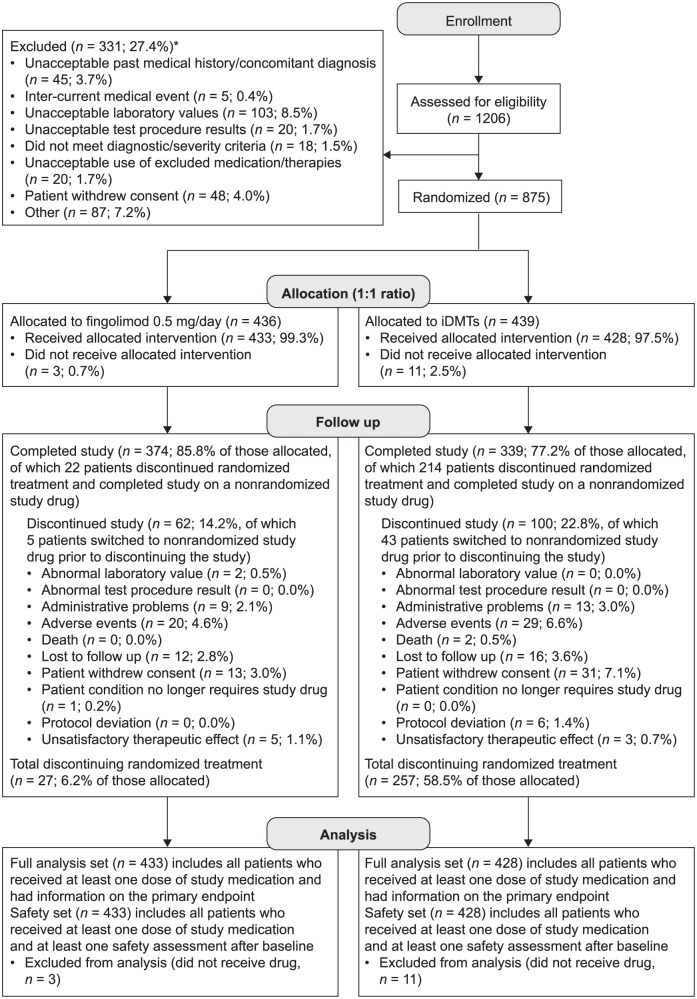
One on-study treatment switch was allowed. Before the week 12 visit, patients could switch from randomized treatment to another study drug only for efficacy or safety reasons. Thereafter, patients could switch treatment for any reason. The 12-week threshold was chosen to allow sufficient time for flu-like symptoms to abate and for patients to acclimatize to regular injections.4,20 Patients underwent ‘last assessment’ when they switched from randomized treatment and at the end of the study. New patients were not enrolled to replace those who withdrew. Patients were not excluded from analyses owing to protocol deviations according to the intent-to-treat principle (Figure 1).
Figure 1.
CONSORT patient flow diagram for PREFERMS.
*More than one reason for exclusion could be recorded for a patient.
CONSORT, Consolidated Standards of Reporting Trials; iDMT, injectable disease-modifying therapy; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying therapies in adults with Relapsing remitting Multiple Sclerosis.
The size, duration, and active comparator-controlled nature of PREFERMS, together with flexible treatment switching, were designed to determine whether starting fingolimod or an iDMT was associated with a higher therapeutic retention rate. A summary of the study design is presented in the PREFERMS video abstract (supplementary video 1).
Outcomes
The primary endpoint was patient retention on randomized treatment over 48 weeks. Secondary variables included reasons for discontinuation of randomized treatment; occurrence and persistence of drug-related adverse events (AEs); cognitive impairment [assessed by the Symbol Digit Modalities Test (SDMT)];21 changes in brain volume [measured as percentage change from baseline using magnetic resonance imaging (MRI)]; and patient-reported satisfaction [measured using the Medication Satisfaction Questionnaire (MSQ),22–24 a clinician-administered, single-item, seven-point Likert scale questionnaire, with a one-point change considered clinically meaningful]. All AEs and serious AEs (SAEs) were recorded by severity, duration, and relationship to study drug at each visit. Pre-existing medical conditions were classified as AEs only if they worsened after starting treatment. Exploratory endpoints included relapse (neurological abnormality present for ⩾24 h in the absence of fever or infection, occurring ⩾30 days after a preceding demyelinating event); cumulative number of newly active gadolinium-enhanced (Gd+) T1 lesions; number of new/enlarged T2 lesions; number of new active lesions; change in Gd+ lesion count; and changes in cortical gray-matter and thalamic volume measures.25–30 A central reader assessed all MRI data (NeuroRx, Montreal, Canada).
Statistics
Sample size and power calculations were based on retention rates from published studies;31–33 852 patients randomized 1:1 to fingolimod or iDMT had 84% power to detect an 8% difference in retention rates using a two-sided χ2 test with a significance level (α value) of 0.05. The study was powered only for the primary endpoint. For secondary and exploratory assessments, the last observation carried forward method was used to impute data missing at last assessment on randomized treatment. These analyses were used for hypothesis generation; no adjustments were made for multiple comparisons. Unless specified, all statistical tests assumed a two-sided alternative hypothesis, with a significance level (α value) of 0.05. All statistical analyses were performed under the direction of the corresponding author (BACC).
Retention rate over 48 weeks, and efficacy-related and patient-reported outcomes at last assessment on randomized treatment, were analyzed in the full analysis set (FAS; Figure 1). A Cochran–Mantel–Haenszel test assessed categorical variables, including the primary endpoint; a two-sample t test assessed continuous variables. Relapses and MRI lesion counts were analyzed using negative binomial regression,34 which accounted for time on study drug, and SDMT scores and volumetric MRI measures using analysis of covariance. Sensitivity analyses and adjustments for treatment exposure and covariates are in supplementary table 4 and in the figure legends.
AEs and SAEs were analyzed in the safety set (Figure 1), with all events coded using the Medical Dictionary for Regulatory Activities. Following a protocol amendment (supplementary table 1 and supplementary table 4), AE and SAE counts were adjusted for treatment exposure and calculated per patient year (number of days on study drug for all patients in the group divided by 365.25). Exposure was from randomization to first occurrence of an event or, if a specific event was not reported, the entire study duration.
Results
Patient disposition
Of 1206 patients screened, 331 (27.4%) were excluded (Figure 1 and supplementary table 5), most frequently because of unacceptable laboratory test results, potentially signifying coexisting systemic disease [n = 103 (8.5%)]. In the fingolimod and iDMT groups, 436 and 439 patients were randomized, respectively, with 433 (99.3%) and 428 (97.5%) in the FAS [total, 861 (98.4%)]. Patient attrition was acceptable, with 713 patients (81.5%) overall completing the study including the switch phase, 477 (54.5%) on randomized treatment and 236 (27.0%) having switched treatment (Figure 1). On-study drugs to which patients switched are summarized in supplementary table 6. Study-drug adherence rates were 95.5% in the fingolimod group (n = 423) and 90.6% in the iDMT group (n = 427).
Baseline demographics and characteristics
Patient demographic and baseline characteristics were similar between treatment groups (Table 1). Most randomized patients were women [n = 640 (73.1%)], and most were white [n = 710 (81.1%)]. Mean (SD) age was 41.7 (10.6) years; time since diagnosis was 4.3 (6.3) years; time since first symptoms was 7.25 (7.9) years; and EDSS score was 2.4 (1.5). Over half of patients had previously received iDMT treatment [fingolimod group, n = 223 (51.1%); iDMT group, n = 248 (56.5%), supplementary table 6].
Table 1.
Baseline patient demographics and characteristics in PREFERMS.
| Characteristic | Fingolimod 0.5 mg (n = 436) |
iDMT (n = 439) |
p value |
|---|---|---|---|
| Age, years | 41.5 (10.84) 41.0 (19–64) |
41.9 (10.39) 42.0 (18–65) |
0.6310 |
| Sex, n (%) | |||
| Male | 125 (28.7) | 110 (25.1) | 0.2282 |
| Female | 311 (71.3) | 329 (74.9) | |
| Race, n (%) | |||
| White | 355 (81.4) | 355 (80.9) | 0.6553 |
| Black | 69 (15.8) | 72 (16.4) | |
| Asian | 1 (0.2) | 1 (0.2) | |
| Native American | 1 (0.2) | 1 (0.2) | |
| Pacific Islander | 0 | 2 (0.5) | |
| Other | 10 (2.3) | 8 (1.8) | |
| Height, cm | 168.5 (8.99) 168 (128–193) |
167.5 (10.06) 167 (123–196) |
0.1388 |
| Weight, kg | 82.94 (20.1) 80.5 (44–163) |
83.56 (22.3) 80.7 (41–170) |
0.6651 |
| Body mass index, kg/m2 | 29.19 (6.70) 28.11 (18–60) |
29.76 (7.55) 28.34 (15–65) |
0.2335 |
| Duration of MS since diagnosis, years | n = 434 | n = 434 | |
| 4.42 (6.67) 1.4 (0–38) |
4.21 (5.94) 1.7 (0–34) |
0.6314 | |
| Duration of MS since first symptoms, years | n = 434 | n = 434 | |
| 7.29 (8.21) 4.2 (0–40) |
7.21 (7.66) 4.4 (0–42) |
0.8871 | |
| Number of relapses in the past year | n = 430 | n = 436 | |
| 0.6 (0.95) 0 (0–8) |
0.6 (0.94) 0 (0–10) |
0.6041 | |
| Number of relapses in the past 2 years | n = 430 | n = 436 | |
| 0.9 (1.51) 0 (0–14) |
0.9 (1.41) 0 (0–10) |
0.9502 | |
| Expanded Disability Status Scale score | n = 433 | n = 427 | |
| 2.36 (1.56) 2.0 (0–6.0) |
2.44 (1.51) 2.0 (0–7.0) |
ND | |
| T2 lesion volume, cm3 | n = 431 | n = 415 | |
| 7.65 (11.60) 3.20 (0–92) |
7.44 (10.17) 3.64 (0–77) |
ND | |
| Normalized brain volume, cm3 | n = 431 | n = 412 | |
| 1521.42 (83.9) 1519 (1241–1746) |
1511.19 (90.5) 1518 (1161–1732) |
ND | |
| Number of gadolinium-enhanced lesions | n = 429 | n = 414 | |
| 1.08 (3.75) 0 (0–42) |
0.85 (3.03) 0 (0–27) |
ND | |
Values are mean (SD) and median (range) unless stated otherwise. Treatment group comparisons were made using the Cochran–Mantel–Haenszel test for categorical variables and a two-sample t test for continuous variables.
iDMT, injectable disease-modifying therapy; MS, multiple sclerosis; ND, not determined; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis; SD, standard deviation.
Retention rates
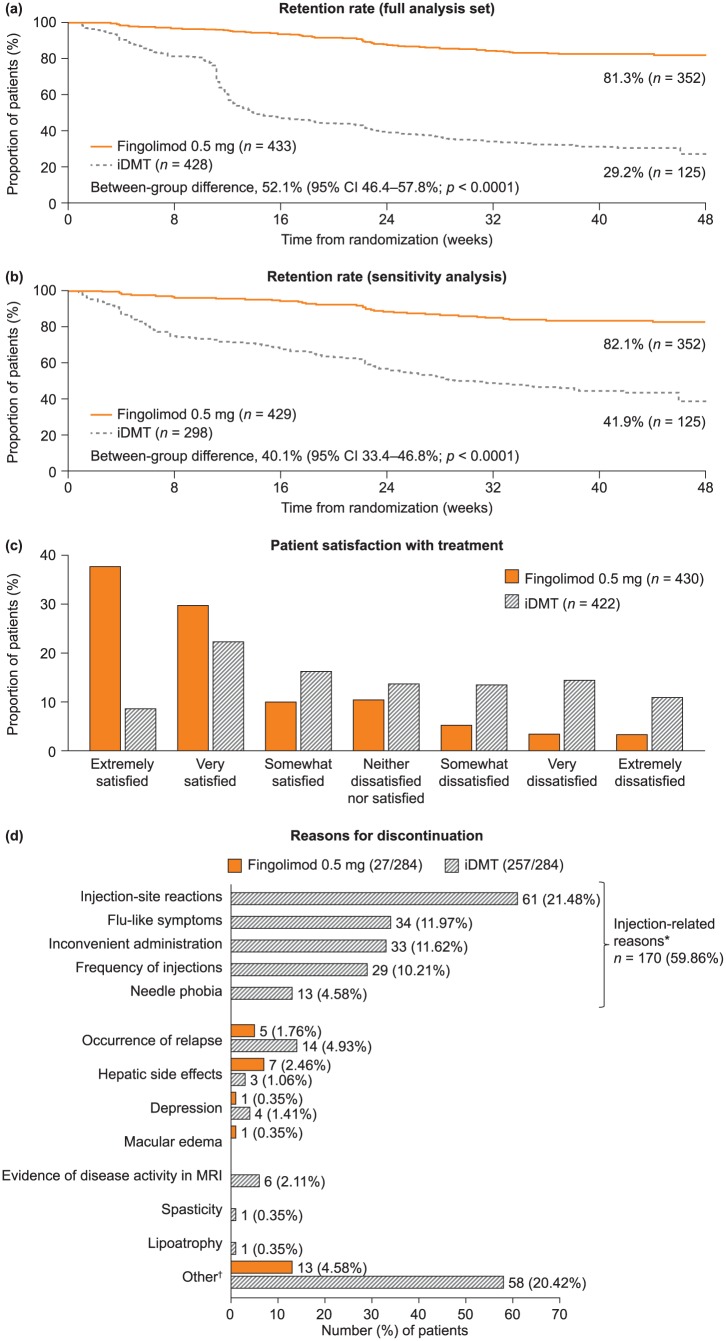
Retention rate on randomized treatment in the FAS was significantly higher with fingolimod than with iDMTs [352 patients (81.3%) versus 125 (29.2%); absolute difference (AD), 52.1%; 95% confidence interval (CI) 46.4–57.8%; number needed to treat (NNT), 1.92; p < 0.0001; Figure 2(a)]. During the randomized phase, mean duration of exposure to fingolimod was nearly twice that observed with iDMTs (301 versus 163 days). The significance of the primary endpoint was sustained: when a small number of protocol-deviation switches (in the first 12 weeks, not for safety or efficacy; n = 17) were excluded from the iDMT group [n/N (% retained): fingolimod, 352/433 (81.3%); iDMTs, 125/411 (30.4%); AD, 50.9%; 95% Cl 45.1–56.7%; NNT, 1.96; p < 0.0001]; when patients who switched treatment during days 77–110 (around the time when switching treatment for any reason became permissible) were excluded [n/N (% retained): fingolimod, 352/429 (82.1%); iDMT, 125/302 (41.4%); AD, 40.7%; 95% CI 34.0–47.3%; NNT, 2.46; p < 0.0001]; and when both of these groups of patients were excluded [Figure 2(b)]. We performed a second sensitivity analysis calculating retention rates in the two groups for weeks 16–48. Retention for fingolimod was 83.8% (n = 352) and for iDMT was 54.1% (n = 125). The between-group difference was 29.7% (95% CI 22.4–37.0%; p < 0.0001).
Figure 2.
Retention rates, patient satisfaction with treatment, and reasons for discontinuing randomized treatment in PREFERMS. (a) The primary outcome of retention rate in the two treatment groups as a Kaplan–Meier plot, statistically analyzed as a log-rank test adjusted for treatment. The data were also analyzed by a Cochran–Mantel–Haenszel test, a logistic regression and a Cox proportional hazard model, which were adjusted for treatment and treatment naïvety. The data were also measured by normal approximation performed using continuity correction. ****p < 0.0001 for all analyses. (b) Sensitivity analysis of the retention rate shown in panel (a) excluding patients who switched treatment for nonefficacy or safety reasons before week 12 in violation of the study protocol, and all patients switching treatment between days 77 and 110 in case any had enrolled with the intention of switching from iDMT to fingolimod as soon as it became permissible for any reason. (c) Patient-reported satisfaction as measured by the Medication Satisfaction Questionnaire at last assessment. The overall difference across categories between fingolimod 0.5 mg and iDMTs is significant (Cochran–Mantel–Haenszel test using modified ridit scores adjusted for treatment and treatment naïvety; p < 0.0001). (d) Primary reasons for discontinuing randomized treatment. Labels for each bar represent the number (percentage of the total number of patients discontinuing randomized treatment). *Injection-related reasons for discontinuation are listed on the left of the corresponding area of the graph. †Reasons for ‘Other’ stated in the fingolimod group include the following [n = 1 (0.35%), unless stated otherwise]: abnormal platelet counts; adverse drug reaction; AE of headache; AE of joint pain; AE of papilloma, hair loss, herpes simplex virus, and lymphopenia; anxiety; hypertension; lymphopenia [n = 2 (0.70%)]; mouth sores; possible diagnosis of macular edema; side effects; and tolerability. Reasons for ‘Other’ in the iDMT group include the following [n = 1 (0.35%), unless stated otherwise]: abdominal pain, chest pain, flushing; AE; AE of bilateral lower extremity edema; AE of headache; allergic reaction [n = 2 (0.70%)]; anxiety; anxiety and depression; anxiety and fatigue; arthralgia and myalgia; bruising; convenience; fatigue [n = 2 (0.70%)]; flu-like symptoms [n = 2 (0.70%)]; general body ache [n = 2 (0.70%)]; headache [n = 2 (0.70%)]; injection fatigue; injection pain [n = 8 (2.82%)]; injection reaction; intolerant; lack of efficacy; muscle aches; mood altering; needle phobia [n = 4 (1.41%)]; palpitations; panic and irritability; patient choice [n = 10 (3.52%)]; possible seizure exacerbation; rash; relapse [n = 3 (1.06%)]; stopped taking medication; and treatment dissatisfaction [n = 2 (0.70%)]. AE, adverse event; CI, confidence interval; iDMT, injectable disease-modifying treatment; MRI, magnetic resonance imaging; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis.
Patient satisfaction on randomized treatment
Patient satisfaction with treatment was greater with fingolimod than with iDMTs throughout the study: the distribution of responses in the fingolimod arm was more heavily weighted towards ‘very satisfied’ and ‘extremely satisfied’ than in the iDMT arm [p < 0.0001 at last assessment, Figure 2(c)].
Discontinuation of randomized treatment
Of 284 patients who discontinued randomized treatment, 27 (9.5%) switched from fingolimod (6.2% of patients randomized) and 257 (90.5%) switched from an iDMT (58.5% of patients randomized). Figure 2(d) summarizes the reasons for discontinuation. Side effects of iDMTs and their mode of administration were the primary reasons for discontinuation reported by 66.1% of patients who discontinued an iDMT, accounting for 59.9% of all discontinuations. Of 257 patients who discontinued an iDMT, 255 switched to fingolimod, one to GA and one to intramuscular interferon β-1a. Most of the 27 patients switching from fingolimod did so owing to the occurrence of relapse or AEs. Of those individuals who discontinued fingolimod, 16 switched to GA (59.3%) and 11 to interferon (40.7%). A breakdown of the reasons for patients reporting ‘other’ is included in the figure legend. The likelihood of discontinuation by discontinuation category (efficacy, safety, tolerability, or convenience) is shown in supplementary figure 2; the likelihood of discontinuing iDMTs versus fingolimod was significantly more likely in all of these categories except safety, for which the between-group difference was nonsignificant.
Annualized relapse rates
There was a nonsignificant trend towards lower annualized relapse rate (ARR) among patients on fingolimod, with a rate 30% lower than those on iDMTs [fingolimod ARR, 0.22; iDMT ARR, 0.31; ARR ratio (95% CI), 0.70 (0.47–1.05); p = 0.084; Figure 3(a)].
Figure 3.
ARR, key MRI measures, and volumetric brain changes at last assessment in PREFERMS.
Bars show mean (SD) except in (a), where they represent mean (95% Cl), with values listed below each graph together with n for each group. Statistics in (a)–(e): negative binomial regression adjusted for treatment, number of relapses in previous 2 years, screening Expanded Disability Status Scale score, and treatment naïvety, using duration (years) as an offset variable. Statistics in (f)–(h) performed on annualized rates with a rank analysis of covariance, adjusted for treatment, treatment naïvety, corresponding baseline values, and age. All data from last assessment. **p < 0.01; ***p < 0.001; ****p < 0.0001 versus iDMTs. ARR, annualized relapse rate; CI, confidence interval; Gd+, gadolinium enhanced; iDMT, injectable disease-modifying therapy; MRI, magnetic resonance imaging; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis; SD, standard deviation.
MRI lesion activity
On randomized treatment at last assessment, the new Gd+ lesion count was significantly lower with fingolimod than with iDMTs [mean (SD) fingolimod, 0.16 (0.82); iDMT, 0.39 (1.45); p < 0.0001; Figure 3(b)]. The new/enlarged T2 lesion count was significantly lower with fingolimod than with iDMTs [mean (SD) fingolimod, 1.76 (4.82); iDMT, 2.46 (6.24); p < 0.0001; Figure 3(c)], as was mean cumulative number of newly active lesions [mean (SD) fingolimod, 0.58 (2.06); iDMT, 1.55 (5.22); p < 0.0001; Figure 3(d)]. There was a significantly greater reduction from baseline in Gd+ lesion count with fingolimod than with iDMTs [mean (SD) fingolimod, −0.86 (3.56); iDMT, −0.41 (2.82); p < 0.0001; Figure 3(e)].
Changes in brain volume, cortical gray-matter volume and thalamic volume
Because of differences in exposure to fingolimod and iDMTs, analyses of brain volumetric changes were adjusted for treatment exposure. At last assessment, there was less brain volume loss (BVL) and cortical gray-matter volume loss from baseline with fingolimod than with iDMTs [Figure 3(f)–(g)]. Fingolimod did not significantly reduce thalamic volume loss [Figure 3(h)]. Volumetric MRI outcomes unadjusted for treatment exposure are presented in supplementary figure 3.
Changes in cognitive function
Small increases in SDMT score from baseline were seen on randomized treatment in both groups, with higher values in the oral test (up to three-point increases from baseline) than in the written version (all less than one-point increase from baseline). Increases were numerically greater with fingolimod than with iDMTs at all assessments, but the between-group differences were nonsignificant except at last assessment among patients taking the oral test [fingolimod (n = 73); iDMT (n = 65); least-squares mean difference (standard error), 3.1 (1.5); p = 0.033]. Sensitivity analyses accounting for upper limb impairment and visual acuity yielded similar results, with oral test results no longer significant (p = 0.051; supplementary figure 4).
Adverse events
In total, 91.0% of patients on fingolimod (394/433) and 82.9% of patients on iDMTs (355/428) experienced AEs during the randomized treatment phase. Most AEs were mild or moderate in severity, with no severe AEs reported in over 2.8% of patients. Safety outcomes for all treatments were consistent with the respective prescribing information. Macular edema (ME) was confirmed by optical coherence tomography in seven patients while on fingolimod (1.1%) and one patient on GA (0.2%). There were pre-existing risk factors in four of the seven fingolimod cases (uveitis in both eyes with inactive macular thickening; retinitis pigmentosa with macular degeneration and a legally blind left eye; astigmatism with intermittent blurred vision; controlled diabetes), and ME was bilateral in five cases. All seven patients discontinued fingolimod, and all recovered. Two patients on fingolimod (0.3%) experienced mild symptomatic bradycardia (dizziness; headache; confused state), one during first-dose monitoring, and one on study who had a history of cardiac murmur. Neither patient required treatment or discontinued fingolimod. To account for differences in exposure, rates of AEs were calculated per patient year. Thus, the proportions of patients who experienced AEs during the randomized-treatment phase on fingolimod (91.0%) and on iDMTs (82.9%) equated to rates of 4.008 and 7.011 AEs per patient year, respectively (Table 2). In total, 9.2% of patients on fingolimod and 23.4% on iDMTs experienced AEs leading to discontinuation of randomized treatment [0.112 and 0.540 per patient year, respectively (Table 2)].
Table 2.
Safety assessment summary in PREFERMS: rates of AEs, SAEs, and AEs causing discontinuation of randomized treatment.
| Preferred term | Rate (AE/patient year) |
|
|---|---|---|
| Fingolimod 0.5 mg (n = 433) | iDMT (n = 428) |
|
| Any AE | 4.008 | 7.011 |
| SAEs | 0.083 | 0.076 |
| Infections and infestations | 0.019 | 0.000 |
| Pneumonia | 0.006 | 0.000 |
| Metabolism and nutrition disorders | 0.006 | 0.000 |
| Dehydration | 0.006 | 0.000 |
| Nervous system disorders | 0.022 | 0.040 |
| MS relapse | 0.014 | 0.025 |
| Psychiatric disorders | 0.011 | 0.010 |
| Anxiety | 0.003 | 0.010 |
| Suicidal ideation | 0.006 | 0.000 |
| AEs leading to treatment discontinuation, total | 0.112 | 0.540 |
| General disorders and administration site conditions | 0.011 | 0.420 |
| Injection-site reaction | 0.000 | 0.131 |
| Influenza-like illness | 0.003 | 0.096 |
| Injection-site pain | 0.000 | 0.091 |
| Fatigue | 0.000 | 0.045 |
| Injection-site erythema | 0.000 | 0.035 |
| Injection-site pruritus | 0.000 | 0.035 |
| Musculoskeletal and connective tissue disorders | 0.006 | 0.040 |
| Myalgia | 0.003 | 0.030 |
| Nervous system disorders | 0.017 | 0.055 |
| Headache | 0.006 | 0.040 |
| Psychiatric disorders | 0.000 | 0.075 |
| Anxiety | 0.000 | 0.045 |
AEs causing study drug discontinuation are reported for events affecting at least 1% of patients in either treatment group. Rates of serious AEs are reported for events affecting at least two patients in either treatment group. Most of the between-group difference was attributable to injection-related reactions, fatigue, and influenzalike symptoms in the iDMT group. AE, adverse event; iDMT, injectable disease-modifying therapy; MS, multiple sclerosis; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis; SAE, serious adverse event.
The rates of SAEs per patient year were similar in the treatment groups (fingolimod, 0.083; iDMT, 0.076). SAEs were reported in 6.7% of patients on fingolimod and in 3.5% on iDMTs. Except for MS relapse (five patients in each group), no SAE was reported for more than two patients in either group. Pneumonia, dehydration, and suicidal ideation were each reported as SAEs for two patients in the fingolimod group. Anxiety was reported as an SAE for one patient in the fingolimod group and two patients in the iDMT group (Table 2). Serious opportunistic infections, such as progressive multifocal leukoencephalopathy, were not observed. Three patients died, one during screening (myocardial infarction), one from metastatic small-cell lung carcinoma (started on iDMT and switched to fingolimod), and one from cardiopulmonary arrest (iDMTs group). Deaths were unrelated to study medications.
A summary of the results is presented in the PREFERMS video abstract (supplementary video 1).
Discussion
A significantly higher retention rate over 48 weeks was observed in patients treated with fingolimod than among those on iDMTs. Between-group differences were anticipated,2,6,8,9,14–16,32,33 and retention rates of 86% for fingolimod and 78% for iDMTs were expected over 48 weeks, calculated from 6-month discontinuation rates reported in the EPOC study.32 Similar retention rates over 1–2 years have been reported elsewhere.15,16,33 Most patients switching therapy during randomized treatment switched to fingolimod rather than to an alternate iDMT. Moreover, treatment satisfaction was higher with fingolimod than with iDMTs. These data indicate a generally greater tolerance and acceptance of fingolimod than of injectable therapies. Other studies, including meta-analyses and registry publications, support these findings.2,15,16,27,32
The proportion of screening failure in PREFERMS was relatively high at 27%, although this was lower than was seen in the phase III TRANSFORMS trial of fingolimod that used similar eligibility criteria.27 This level of exclusion may hinder generalization of the findings to all clinical settings. Also, compared with routine clinical practice, an iDMT retention rate of 29.2% at 1 year is low. Owing to prescribing restrictions during the enrollment period (June 2012–June 2014), it is likely that some patients would not have been able to start or switch to fingolimod in a routine practice setting. Moreover, patients were aware at enrollment that they could switch to fingolimod after 12 weeks when switching for any reason became permissible. This may have biased recruitment in terms of preferred treatments or routes of administration. To address this, a sensitivity analysis excluded patients who switched treatment between weeks 11 and 16, and found that the retention rate with iDMT increased to 41.4%, but the between-group difference in retention relative to fingolimod remained highly significant. A second sensitivity analysis comparing retention rates during weeks 16–48 found similar highly significant results. Had we surveyed study participants regarding their preference for iDMT or fingolimod prior to randomization, such information could have been helpful in interpreting the decision to switch treatment. Nonetheless, we found that factors related to tolerability and convenience appeared to have the most influence on treatment retention. Injection-related issues dominated the reasons for discontinuing iDMTs in PREFERMS; thus, the preference for oral over injectable therapy might be expected. Nonetheless, the scale of this preference in PREFERMS was striking, and suggests that many patients receiving iDMTs would choose to switch to another therapy if given the opportunity.
There were trends towards better efficacy outcomes and treatment satisfaction when initiating and remaining on fingolimod than on iDMTs (numerically fewer brain lesions, reduced BVL and cortical gray-matter loss, and greater treatment satisfaction), and such treatment effects on cortical gray-matter volume and overall BVL are clinically relevant because they correlate with effects on disability progression and cognitive impairment.28,29,35,36 It is unexpected that cognition showed signs of improvement with fingolimod while thalamic volume on average decreased, but data were missing from both analyses so the two effects may not relate to the same subgroup of patients. Comparisons of therapeutic efficacy must acknowledge the greater treatment exposure to fingolimod than to iDMTs, and, although this did not affect the primary outcome or the adjusted safety assessments, the secondary and exploratory efficacy variables were affected. Owing to the large number of patients switching treatment, the iDMT group lost statistical power as the study proceeded, and had a shorter disease duration than the fingolimod group at last assessment. When adjusted for differences in treatment exposure, all volumetric MRI measures (total brain, gray matter, and thalamic volume loss) had decreased less with fingolimod than with iDMTs at the last assessment.
The study was open label owing to the different routes of administration in the treatment arms, and high rates of injection-related reactions complicated direct comparison of AE frequencies between treatments. Nevertheless, this design is informative in the setting wherein physicians may consider a switch to oral therapy for patients who poorly tolerate or dislike injectable medication and where payer policy allows.4,5,37 In patients at a relatively early stage of RRMS, PREFERMS showed that fingolimod is associated with a higher therapy retention rate than iDMTs, and revealed trends towards greater patient satisfaction and improved clinical and MRI outcomes with fingolimod.
Conclusion
Our findings indicate that, given the choice, many patients receiving iDMTs would consider switching to fingolimod. Whether this reflects a preference for oral therapy in general or for fingolimod in particular was not investigated. Patients with MS will benefit most in the long term if they adhere to therapy from an early stage of disease. There are nonmedical obstacles to switching MS therapy, but clinical practice might serve patients better if regular reviews to check treatment adherence, as well as safety and effectiveness, were routinely conducted.
Supplementary Material
Supplementary Material, Supplementary_files_final_version_PREFERMS for Phase IV study of retention on fingolimod versus injectable multiple sclerosis therapies: a randomized clinical trial by Bruce A.C. Cree, Douglas L. Arnold, Mark Cascione, Edward J. Fox, Ian M. Williams, Xiangyi Meng, Lesley Schofield and Nadia Tenenbaum in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors would like to thank the patients and study site staff who participated in PREFERMS and Dr Jeremy R. Bright at Oxford PharmaGenesis for editorial assistance in preparing the manuscript. The authors would also like to thank Dr Augusto Grinspan, formerly an employee of Novartis Pharmaceuticals Corporation, for his contribution to protocol development.
Footnotes
Author contributions: BACC (corresponding author): study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. DLA: study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. MC: study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. EJF: study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. IMW: data interpretation, drafted and revised the manuscript for intellectual content. XM (statistical analysis): study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. LS: study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. NT (principal investigator): study design and conceptualization, data analysis and interpretation, drafted and revised the manuscript for intellectual content. All authors approved the final version of the manuscript for publication. A list of co-investigators and contributors is given in Appendix 1 in the Supplement. Trial Registration: ClinicalTrials.gov identifier: NCT01623596.
Funding: PREFERMS was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Oxford PharmaGenesis, Oxford, UK provided editorial support, which was funded by Novartis Pharmaceuticals Corporation.
Conflict of interest statement: BACC has received personal compensation for consulting from AbbVie, Biogen, EMD Serono, GeNEuro, Novartis and Sanofi Genzyme. DLA has an equity interest in NeuroRx Research, and has received personal fees from Acorda Therapeutics, Biogen, EMD Serono, Genentech, Genzyme, Hoffmann-La Roche, MedImmune, Mitsubishi, Novartis, Receptos, and Sanofi-Aventis, and grants from Biogen and Novartis. MC has received research support or consulting fees from Acorda Therapeutics, Bayer HealthCare, Biogen Idec, EMD Serono, Genzyme, Genentech, Novartis, Roche, and Sanofi Aventis. EJF has received consultancy fees, honoraria, and travel or research support from Acorda Therapeutics, Bayer, Biogen, Chugai, Eli Lilly, EMD Serono, Genzyme, Novartis, Ono, Opexa Therapeutics, Roche, Sanofi, and Teva Neuroscience. IMW is an employee of Oxford PharmaGenesis. XM, LS, and NT are employees of Novartis Pharmaceuticals Corporation.
Supplemental Material: Supplementary material for this article is available online.
Contributor Information
Bruce A.C. Cree, UCSF Weill Institute for Neurosciences, Department of Neurology, 675 Nelson Rising Lane, San Francisco, CA 94158, USA.
Douglas L. Arnold, NeuroRx Research and Montreal Neurological Institute, Montreal, Quebec, Canada; Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
Mark Cascione, Tampa Neurology Associates, Tampa, FL, USA.
Edward J. Fox, Central Texas Neurology Consultants, Round Rock, TX, USA
Ian M. Williams, Oxford PharmaGenesis, Oxford, UK
Xiangyi Meng, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Lesley Schofield, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Nadia Tenenbaum, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
References
- 1. Coyle PK. Switching algorithms: from one immunomodulatory agent to another. J Neurol 2008; 255(Suppl. 1): 44–50. [DOI] [PubMed] [Google Scholar]
- 2. Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2011; 18: 69–77. [DOI] [PubMed] [Google Scholar]
- 3. Gajofatto A, Bacchetti P, Grimes B, et al. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler 2009; 15: 50–58. [DOI] [PubMed] [Google Scholar]
- 4. Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence 2010; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 2015; 15: 273–279. [DOI] [PubMed] [Google Scholar]
- 6. Cohen BA, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract 2007; 61: 1922–1930. [DOI] [PubMed] [Google Scholar]
- 7. Hansen K, Schussel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One 2015; 10: e0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci 2011; 38: 429–433. [DOI] [PubMed] [Google Scholar]
- 9. Lapierre Y, O’Connor P, Devonshire V, et al. Canadian experience with fingolimod: adherence to treatment and monitoring. Can J Neurol Sci 2016; 43: 278–283. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 11. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013; 3: pii: e001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novartis Phamaceuticals Corporation. Prescribing information – Gilenya®. 2016. Available at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gilenya.pdf,downloaded26-APR-2018
- 13. Agius M, Meng X, Chin P, et al. Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neurosci Ther 2014; 20: 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 2013; 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He A, Spelman T, Jokubaitis V, et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol 2015; 72: 405–413. [DOI] [PubMed] [Google Scholar]
- 16. Warrender-Sparkes M, Spelman T, Izquierdo G, et al. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler 2016; 22: 520–532. [DOI] [PubMed] [Google Scholar]
- 17. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Medical Association. Declaration of Helsinki – Ethical principles for medical research involving human subjects. Edinburgh: World Medical Association, 2016. [Google Scholar]
- 19. ICH. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1). 1996. Available at: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf,downloaded26-APR-2018 [PubMed]
- 20. Mohr DC, Likosky W, Boudewyn AC, et al. Side effect profile and adherence to in the treatment of multiple sclerosis with interferon beta-1a. Mult Scler 1998; 4: 487–489. [DOI] [PubMed] [Google Scholar]
- 21. Hughes AJ, Denney DR, Lynch SG. Reaction time and rapid serial processing measures of information processing speed in multiple sclerosis: complexity, compounding, and augmentation. J Int Neuropsychol Soc 2011; 17: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 22. Lee LK, Wu Y, Cutter GR, et al. Psychometric evaluation of the medication satisfaction questionnaire (MSQ) to assess satisfaction with glatiramer acetate among patients with multiple sclerosis. Ann Neurol 2016; 80: S137. [Google Scholar]
- 23. Vermersch P, Hobart J, Dive-Pouletty C, et al. Measuring treatment satisfaction in MS: is the Treatment Satisfaction Questionnaire for Medication fit for purpose? Mult Scler 2017; 23: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vernon MK, Revicki DA, Awad AG, et al. Psychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients. Schizophr Res 2010; 118: 271–278. [DOI] [PubMed] [Google Scholar]
- 25. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double- blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 26. Chard DT, Griffin CM, Rashid W, et al. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult Scler 2004; 10: 387–391. [DOI] [PubMed] [Google Scholar]
- 27. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 28. Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol 2008; 64: 247–254. [DOI] [PubMed] [Google Scholar]
- 29. Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 30. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 31. Evans C, Tam J, Kingwell E, et al. Long-term persistence with the immunomodulatory drugs for multiple sclerosis: a retrospective database study. Clin Ther 2012; 34: 341–350. [DOI] [PubMed] [Google Scholar]
- 32. Fox E, Edwards K, Burch G, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord 2014; 3: 607–619. [DOI] [PubMed] [Google Scholar]
- 33. Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011; 10: 520–529. [DOI] [PubMed] [Google Scholar]
- 34. Hilbe JM. Negative binomial regression. 2nd ed. New York: Cambridge University Press, 2011. [Google Scholar]
- 35. Hofstetter L, Naegelin Y, Filli L, et al. Progression in disability and regional grey matter atrophy in relapsing–remitting multiple sclerosis. Mult Scler 2014; 20: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014; 75: 43–49. [DOI] [PubMed] [Google Scholar]
- 37. Johnson KL, Kuehn CM, Yorkston KM, et al. Patient perspectives on disease-modifying therapy in multiple sclerosis. Int J MS Care 2006; 8: 11–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material, Supplementary_files_final_version_PREFERMS for Phase IV study of retention on fingolimod versus injectable multiple sclerosis therapies: a randomized clinical trial by Bruce A.C. Cree, Douglas L. Arnold, Mark Cascione, Edward J. Fox, Ian M. Williams, Xiangyi Meng, Lesley Schofield and Nadia Tenenbaum in Therapeutic Advances in Neurological Disorders