Abstract
Diverticulosis is a common anatomical condition, which appears to be age-dependent. Individuals who develop chronic gastrointestinal symptoms or complications are referred to as having diverticular disease. Although the diagnosis of this condition can be relatively straightforward, randomized controlled trials are scarce and management often follows tradition rather than principles of evidence-based medicine. This report deals with the topics discussed during a symposium held during the United European Gastroenterology Week (Barcelona, October 2017). During the meeting, the role of dysbiosis in the pathogenesis of diverticular disease and its treatment were thoroughly discussed, by examining the efficacy and mechanisms of action of the currently used drugs. Recent studies have shown the presence of dysbiosis in patients with diverticular disease and suggest an imbalance in favor of bacteria with pro-inflammatory and pathogenetic potential. These microbiota changes correlate with mucosal immune activation, mirrored by a marked increase of macrophages in colonic mucosa, both in the diverticular region and at distant sites. The low-grade inflammation, driven by bacteria-induced immune activation, could be involved in the pathophysiology of symptoms. As a consequence, pharmacological approaches targeting enteric bacteria (with poorly absorbed antibiotics, like rifaximin, or probiotics) or intestinal inflammation (with 5-ASA derivatives or rifaximin) have shown capability of controlling symptoms and also preventing complications, albeit more research is needed to establish the optimal regimen (daily dose and duration) of therapy. Well-designed randomized-controlled trials (RCTs), including homogeneous populations of patients, are therefore needed. The future of management of many GI diseases, including symptomatic uncomplicated diverticular disease, will rely on the so-called ‘microbiota-directed therapies’.
Keywords: diverticular disease, dysbiosis, mesalazine, microbiota, mucosal inflammation, rifaximin, probiotics
Introduction
In gastroenterology, as in other medical specialties, new potential therapies, both pharmaceutical and invasive, continually appear on the horizon, often with great initial enthusiasm. Over time, these will either prove to be failures or will find their appropriate level of acceptance in our therapeutic armamentarium. When faced with promising new therapies, we should always wonder whether they are effective and safe and whether they are really better than the current ones. One is, therefore, justified to attempt, from time to time, a critical review of the recent developments in the field in order to provide a glimpse of what may lie ahead. This symposium has been specifically designed to critically evaluate the management of diverticular disease in the third millennium.
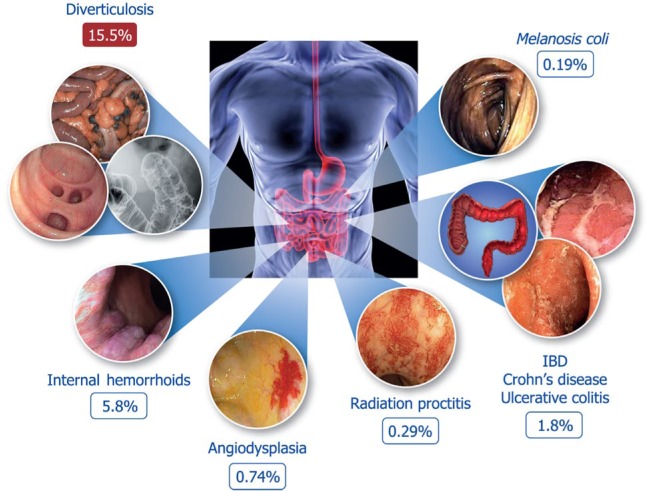
Diverticulosis is a common anatomical condition and, as our populations age, its prevalence is steadily increasing. Recent studies1 have shown that the presence of diverticula represents the most common non-neoplastic finding during screening colonoscopy (Figure 1). Among patients with diverticulosis, 15–25% are expected to develop diverticulitis in their lifetime, although a recent study suggests that this proportion may be much lower (i.e. <5%).2 Thus, diverticular disease is a challenge for both diagnosis and treatment in the daily clinical practice and represents a significant burden for healthcare systems.
Figure 1.
Most common non-neoplastic findings at screening colonoscopy (data from Bevan et al.1).
IBD, inflammatory bowel disease.
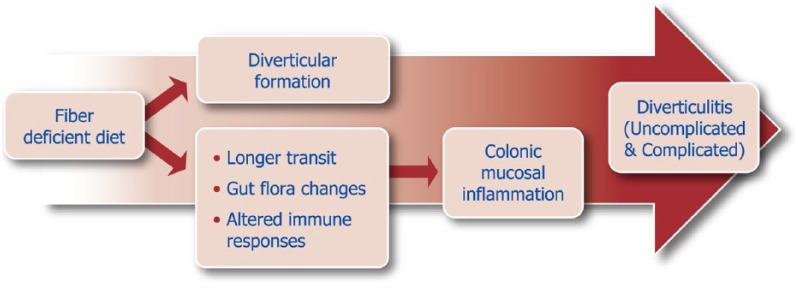
The spectrum of diverticular disease is wide, covering different clinical scenarios, each characterized by different symptomatology, severity and outcomes.3 The pathogenesis of chronic symptoms as well as the link between uncomplicated diverticulosis and symptoms are complex and still not fully understood. However, low-grade inflammation, driven by bacteria-induced immune activation,4,5 could be involved in the pathophysiology of symptoms (Figure 2). The recent clear demonstration of gut microbiota alterations in this condition6 has expanded the therapeutic armamentarium to include gut-selective antibiotics7 and anti-inflammatory drugs,8 albeit more research is needed to establish the optimal regimen (daily dose and duration) of therapy. The use of probiotics9 further extends the so-called ‘microbiota-directed therapies’, despite evidence of their efficacy still being low.
Figure 2.
Pathophysiology of diverticular disease: from fiber hypothesis to mucosal inflammation.
Although the diagnosis of diverticular disease can be relatively straightforward, randomized controlled trials of clinical management are scarce and management often follows tradition rather than principles of evidence-based medicine.10 Some practice guidelines do exist,11–22 but most of them are relatively old and rely mainly on expert opinion.
The aim of this symposium was to review the clinical presentation of diverticular disease, to discuss the role of dysbiosis in its pathogenesis and to define its treatment, by examining the efficacy and mechanisms of action of the currently used drugs.
To render the symposium more dynamic and deliver key messages in a more efficient way, an innovative formula of ‘questions and answers’, first developed by the Scientific Committee of the World Organization for Specialized Studies on Diseases of the Esophagus (OESO), and successfully used now for some decades, has been adopted.
What is the clinical picture of diverticular disease?
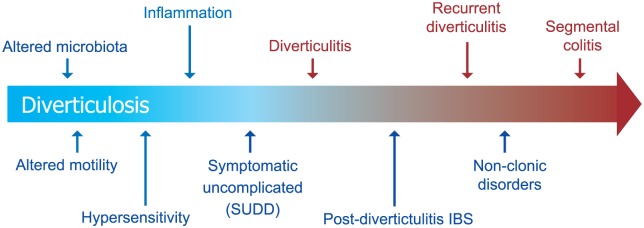
Diverticular disease is one of the most common gastrointestinal disorders in Western countries. The presence of diverticulosis, saccular protrusions in the colon, increases with age. Diverticulosis is uncommon before the age of 40 but is seen in approximately 60% of individuals aged over 70 years.23 Individuals with diverticulosis are at risk of developing diverticulitis (inflammation of one or a few diverticula and the surrounding colon) and diverticular bleeding (acute bleeding from a nutrient vessel in a diverticulum). They may also develop chronic gastrointestinal symptoms (abdominal pain, bloating or changes in bowel habits) termed symptomatic uncomplicated diverticular disease (SUDD).24 After an episode of diverticulitis, patients are at risk of developing a functional bowel syndrome.25 Rarely, patients with diverticulosis develop segmental colitis that closely resembles or even overlaps with inflammatory bowel disease26,27 (Figure 3).
Figure 3.
Clinical spectrum of diverticular disease.
IBS, irritable bowel syndrome; SUDD, symptomatic uncomplicated diverticular disease.
The vast majority of individuals with diverticulosis will remain asymptomatic. In a large US study of predominantly male subjects, over an 11-year follow-up period, only 4% of individuals with diverticulosis were diagnosed with diverticulitis.2 Diverticular bleeding is less common than diverticulitis. In one US nationwide study, the prevalence of diverticular bleeding was approximately one third that of diverticulitis.28 The incidence of SUDD and other problems in patients with diverticulosis is not well studied, but these may occur in 15% of patients with diverticulosis.24
The risk of diverticulitis in patients with diverticulosis is highest in young patients,2 although, because many more-older patients have diverticulosis, the majority of cases occur in older patients.28 Population-based studies indicate that about 20% of individuals with an incident episode of diverticulitis will experience a recurrence.29 The risk of recurrence increases with the number of episodes.29 Traditionally, recurrent attacks were felt to be more serious than incident events. However, recent data indicate that perforation and peritonitis are most likely to occur with the first or second episode.30 This finding has led to a less aggressive approach (with prophylactic segmental colectomy) for prevention of recurrence.
Diverticular bleeding is seen predominantly in older patients, with a peak prevalence in the ninth decade of life.28 Approximately 10% of patients will experience recurrence at 1 year,31 although the rate may be much higher in individuals in whom a definite source of bleeding is identified at the time of the initial bleed.32
As noted above, a subset of patients with diverticulosis develops chronic gastrointestinal symptoms. After an episode of diverticulitis, the risk of developing irritable bowel syndrome (IBS, whose symptoms overlap those of SUDD) or any functional bowel syndrome is increased.25 Patients with diverticulosis may also experience SUDD without a prior episode of diverticulitis.
How to differentiate between symptomatic uncomplicated diverticular disease and irritable bowel syndrome?
A meta-analysis33 of 81 studies found a global prevalence of IBS of 11.2% [95% confidence interval (CI), 9.8–12.8%] and a recent Italian survey34 reported that 59% of SUDD patients fulfilled the Rome III criteria for IBS.35 The question arises as to whether symptoms are attributable to the presence of diverticula or have to be attributed to the presence of a concomitant IBS. Although in most cases this question cannot be answered, some studies suggest that there may be some demographic and clinical features that help distinguish a subset of patients who may fit in one or the other clinical condition.
The wide overlap between IBS and SUDD is well depicted in older studies based on assessment of diverticulosis with barium enema.36 These studies showed that around one third of patients with colonic diverticula reported IBS-like symptoms, including recurrent abdominal pain and bloating, loose stools, hard stools, urgency, and straining, all frequently intermittent, with the exception of loose stools, which were present most of the time.36 These results have been confirmed in a recent multicenter survey in 598 SUDD patients in which 59% of patients fulfilled the Rome III criteria for IBS.34
The overlap between IBS and SUDD has also been highlighted in a study from the Mayo clinic,37 which points out that age is a critical factor. Indeed, diverticula develop with age and are predominant in the elderly population. Conversely, the incidence of IBS is higher in younger adults. Thus, the overlap between IBS and SUDD affects mostly elderly people. Indeed, in those of 65 years of age or older, the presence of IBS was associated with a ninefold higher odds for diverticulosis [odds ratio (OR) = 9.4, 95% CI 5.8–15.1]. Conversely, the odds value for diverticulosis in the younger groups (<65 years) was low (OR = 1.2, 95% CI 0.8–1.8).37 The role of sex as a discriminant factor in the identification of SUDD versus IBS patients has received little attention in ad hoc studies. Nonetheless, epidemiological studies have repeatedly shown that female sex predominates in IBS while older patients with SUDD could be predominantly male.
In order to identify clinical features that would help distinguish true SUDD from true IBS, and to avoid the relative importance of the above-mentioned demographic factors, a recent study38 matched patients for age and sex. Demographic and clinical characteristics (e.g. Rome III questionnaire, Bristol Stool Scale, characteristics of abdominal pain) were assessed. Interestingly, by matching patients by age and sex, only 10% of SUDD patients fulfilled the Rome III criteria for IBS. This was mainly due to the fact that those with SUDD, compared with IBS patients, had less frequent symptoms. In addition, abdominal pain did not improve with bowel movements in SUDD as well it did in IBS.38 One of the major findings of this study was the identification of a higher prevalence of long-lasting abdominal pain (more than 24 h) in those with SUDD compared with IBS patients. Conversely, IBS patients more frequently complained of short-lived abdominal pain. Other characteristics more frequently observed in SUDD were a more likely confinement to bed, higher requirement for urgent medical consultation and, as expected, higher rates of hospitalization, which was absent for IBS.38 These data have been confirmed in a more recent study39 showing that prolonged and moderate to severe (a score of more than 5 on a 0–10 scale) left lower-abdominal pain is the best symptom characterizing and differentiating SUDD from IBS. Another interesting observation relates to the distribution of abdominal pain on the abdominal wall. The study showed that SUDD patients have a well-localized pain in the left iliac fossa, while the pain of IBS is less localized and more diffuse in the abdomen.39
Psychological factors have been described to occur in both IBS and SUDD. There are limited comparative studies. One study from Nottingham24 found that when using the PHQ12 questionnaire, validated for the detection of somatization scores, patients with SUDD scored higher than asymptomatic diverticulosis and only slightly lower than IBS. These data suggest that psychosomatic symptoms are of limited value in discriminating the two entities.
Fecal calprotectin may also help to differentiate these two clinical conditions. This inflammatory marker was found to be increased in SUDD but not in the IBS-like group and its concentration correlated significantly with the severity of abdominal pain.39 Other pathophysiological features may include intestinal dysmotility, visceral hypersensitivity (present in both entities), the presence of low-grade intestinal inflammation (mostly mast cells in IBS, and macrophages in SUDD), neuro-immune interactions and changes in fecal and mucosal microbiota. However, comparative studies are lacking, and determination of the weight of these factors in SUDD versus IBS, as well as any assumptions, seem inappropriate at this time.
In conclusion, a proportion of patients with SUDD fulfill the criteria for IBS. Compared with IBS, SUDD patients are older, more frequently males, with severe, long-lasting (>24 h) and localized (mainly in the left lower quadrant) abdominal pain (Table 1). There are limited comparative data on tissue pathology, but while IBS has been associated with increased mast cells, SUDD is more frequently associated with increase in macrophages. After all, it remains unclear whether this distinction is of real clinical value, since management may not be different.
Table 1.
Differential features between irritable bowel syndrome and symptomatic uncomplicated diverticular disease.
| IBS | SUDD | |
|---|---|---|
| Demographics | Young: females > males |
Older: males ≥ females |
| Colon structural changes | No | Yes |
| Rome III criteria | 100% | 100%* |
| Pain pattern | Frequent recurrences, short lived |
Long remissions, prolonged (>24 h) |
| Pain location | Diffuse | Left lower quadrant |
| Bowel changes | Diarrhea and constipation | Diarrhea > constipation |
| Fecal calprotectin | Usually normal | Usually increased |
Only 15% of patients harboring diverticula.
IBS, irritable bowel syndrome; SUDD, symptomatic uncomplicated diverticular disease.
How is the gut microbiota altered in patients with diverticular disease?
The involvement of the gut microbiota in the development of symptoms and complications of diverticular disease has been frequently hypothesized but rarely demonstrated. In favor of a microbiota role in the pathogenesis of diverticular disease is the fact that most complications are bacterial in origin and are generally managed with therapies aimed at controlling infection.
There is only preliminary evidence of a shift in bacterial phyla abundance in SUDD, with a decrease in Bacteroidetes and an increase in Firmicutes.40 Interestingly, these changes are similar to those observed in patients with IBS.41 In addition, in line with the potential role of microbial pathogens in diverticular disease complications, a study showed a global increase in all fecal phyla, as well as an increase in Proteobacteria in patients with acute diverticulitis.42
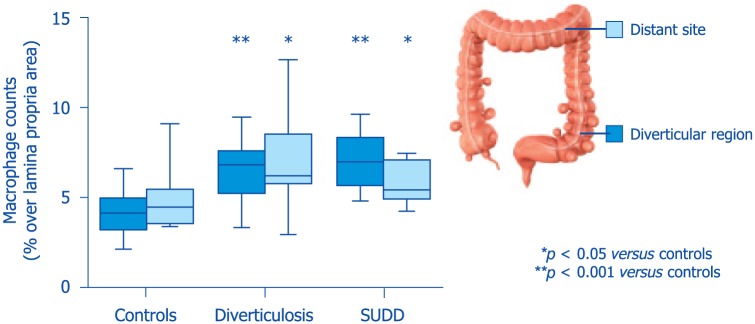
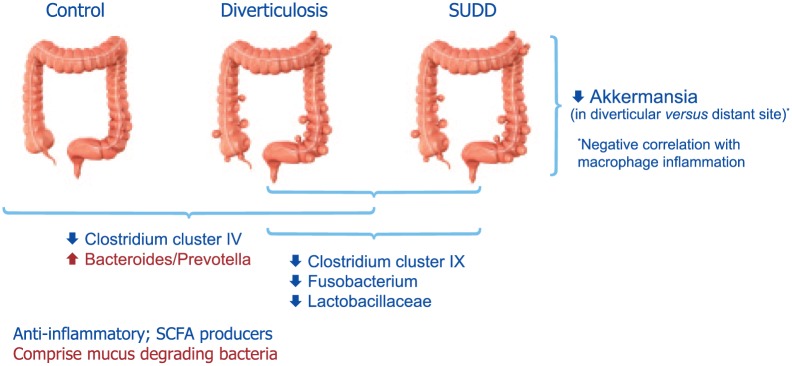
A recent descriptive, cross-sectional pilot study assessed gut low-grade inflammation, microbiota and the metabolome in patients with diverticular disease.6 The results showed that compared with controls, patients with diverticula (regardless of symptoms) had a >70% increase in colonic macrophages (Figure 4). Their fecal microbiota was depleted of Clostridium cluster IV, a class comprising several groups with potential anti-inflammatory properties. In addition, compared to asymptomatic patients, patients with SUDD showed a depletion in the groups Clostridium cluster IX, Fusobacterium and Lactobacillaceae, all bacterial groups with potential anti-inflammatory properties or producers of short chain fatty acids (Figure 5). Interestingly, depletion of microbiota members with anti-inflammatory activity were associated with mucosal macrophage infiltration. The results of this study also showed a decrease in the mucus-degrading bacteria Akkermansia in the colonic tract affected by the diverticula, compared with distant unaffected sites.6 A study by Tursi and colleagues43 assessed fecal microbiota from 15 patients with SUDD, 13 with asymptomatic diverticulosis and 16 healthy controls. Their results showed that the overall bacterial abundance of dominant bacterial groups, including Bacteroides/Prevotella, Clostridium coccoides, Bifidobacterium, Lactobacillus, and Escherichia coli, was not different among the three groups. Interestingly, the amount of Akkermansia muciniphila species was significantly higher in patients bearing diverticula compared with controls. Methodological and population differences exist between these two studies,6,43 which can explain differences in findings.
Figure 4.
Low-grade inflammation in diverticular disease (modified from Barbara et al.6).
SUDD, symptomatic uncomplicated diverticular disease.
Figure 5.
Microbiota composition in healthy subjects, subjects with diverticulosis or patients with symptomatic uncomplicated diverticular disease (data from Barbara et al.6).
SCFA, short-chain fatty acids; SUDD, symptomatic uncomplicated diverticular disease.
Six molecules have been identified in urinary metabolite analysis that are capable of distinguishing between patients with diverticular disease and healthy control subjects, with a success rate of greater than 95%. These metabolites may be considered biomarkers of the disease and be useful diagnostic tools in the near future.6 The potential clinical utility of metabolomics in the identification of diverticular disease biomarkers has been also suggested in another study44 in which analysis of nuclear magnetic resonance-based metabolomics data showed significant discrimination between healthy controls and diverticulosis, as well as between diverticulosis and SUDD. Nonetheless, the profile of metabolites identified in the two studies6,44 does differ, suggesting that more research in a larger sample size of patients is likely needed to define the specific metabolome and its diagnostic applicability in diverticular disease.
Taken together, these results indicate the presence of dysbiosis in patients with diverticular disease and suggest an imbalance in favor of bacteria with pro-inflammatory and pathogenetic potential, particularly in patients with symptomatic diverticular disease and at sites with most abundant presence of diverticula. These initial data pave the way for further large-scale studies, specifically aimed at identifying microbiota signatures with a potential diagnostic value in patients with diverticular disease.
What are the aims of treatment?
As mentioned above, the clinical spectrum of colonic diverticular disease is wide and ranges from asymptomatic diverticula to SUDD, uncomplicated diverticulitis and eventually, complicated diverticulitis. Therefore, the management, and the therapeutic approach are different, depending on the severity of the disease. Overall, prevention of progression, treatment of active disease and prevention of recurrence are the three key aims of treatment in any clinical manifestation of diverticular disease.
Asymptomatic diverticulosis does not need any pharmacologic treatment. However, healthy lifestyle (regular physical exercise, maintaining ideal body weight, abstention from smoking) and high-fiber diet are recommended to prevent its progression to SUDD and its complications.45 Most people with diverticulosis will not progress to symptomatic disease. The proportion of subjects with diverticulosis that eventually develop SUDD or acute diverticulitis is unknown. Consensus papers and reviews17,46,47 state that 80–85% of subjects with diverticulosis will remain asymptomatic throughout their life, whereas 15–20% of symptomatic patients will suffer from diverticular disease without inflammation, while the remainder will have diverticulitis. However, estimates of progression need to be based on more reliable studies. One study2 identified 2222 patients with baseline diverticulosis. Over an 11-year follow-up period, 95 patients developed diverticulitis (4.3%; 6 per 1000 patient-years). The median time to event was 7.1 years.
Pharmacological treatment of SUDD should reduce intensity and frequency of symptoms and prevent the progression to diverticulitis17,46,47 (Table 2). Most symptoms in SUDD are mild to moderate but they impair the patients’ quality of life. Typical symptoms of SUDD include pain (in the lower or left lower quadrant of the abdomen), bloating and changes in bowel habits, symptoms also observed in patients with IBS; a differential diagnosis between these two clinical entities should therefore be attempted.24,38,39
Table 2.
Aims of therapy in symptomatic uncomplicated diverticular disease.
| 1 | First differentiate SUDD from IBS |
| 2 | Get symptom relief and improve HRQL |
| 3 | Prevent progression to acute diverticulitis |
HRQL, health-related quality of life; IBS, irritable bowel syndrome; SUDD, symptomatic uncomplicated diverticular disease.
Progression to diverticulitis is uncommon and often benign. One prospective, long-term study,48 assessing the development of complications in patients with SUDD, pointed out that 97% of patients had mild or no symptoms after a median follow-up of 66 months. Acute diverticulitis appeared in only 2.5% of cases.
The objectives of therapy in patients who develop acute diverticulitis include the treatment of symptoms, the colonic infection, the prevention of complications and the recurrence of the condition47 (Table 3). Based on the severity of symptoms, ability to tolerate oral intake, presence of comorbidities and adequate outpatient support, the decision on whether patients should be hospitalized or receive ambulatory care should be carefully considered.49 Treatment of complications may require interventional radiology or surgery.
Table 3.
Aims of therapy in patients with acute diverticulitis.
| Prevention | • Appropriate management of risk factors • Appropriate management of SUDD and asymptomatic DD |
| Treatment | • Reduce unnecessary hospitalizations • Reduce inappropriate use of antimicrobials • Reduce duration of hospital stay • Reduce and likely prevent complications |
| Prevention of recurrence | • Appropriate management of risk factors |
DD, diverticular disease; SUDD, symptomatic uncomplicated diverticular disease.
Is there a role for diet and nutraceuticals?
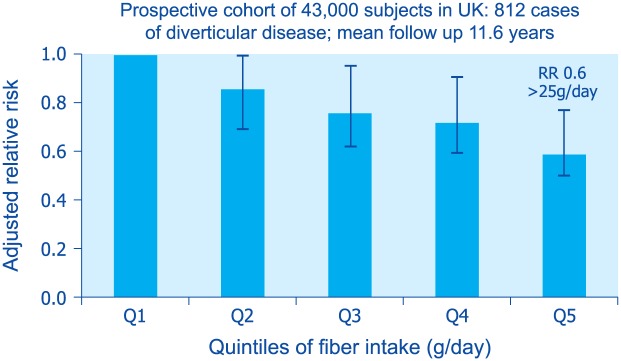
Diverticular disease has historically been considered a disease of diet and lifestyle. In the 1960s, Painter and Burkitt observed a striking difference in the prevalence of diverticular disease in the UK when compared with Africa and Asia.50 They attributed the high prevalence of diverticular disease in the West to insufficient fiber intake. Recent studies, however, indicate that after controlling for other risk factors, dietary fiber intake is not associated with the prevalence of diverticulosis detected at colonoscopy.23 On the other hand, a number of population-based studies have shown that fiber intake is inversely associated with the risk of diverticulitis51–53 (Figure 6). It is not clear whether a specific type or source of fiber is more beneficial in reducing the risk of diverticulitis.
Figure 6.
Relationship between fiber intake and risk of diverticulitis in the EPIC study (from Crowe et al.45).
RR, relative risk.
Red meat intake, particularly unprocessed red meat, is also associated with an increased risk of diverticulitis.54 The substitution of poultry or fish for one serving of red meat decreased risk by 20%. In one study, vegetarians were at a 30% decreased risk compared with omnivores.51
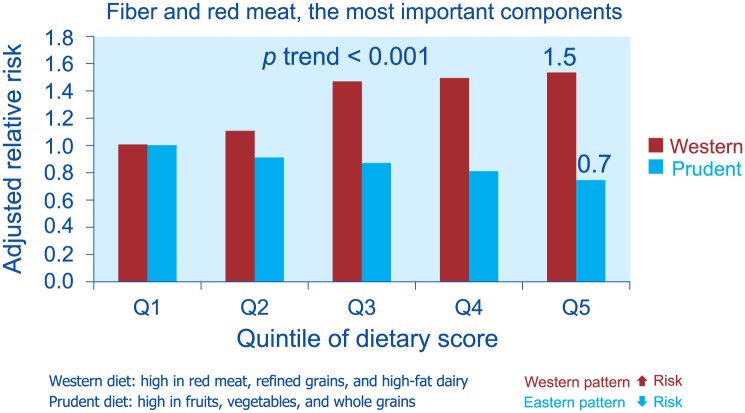
Dietary patterns as a whole, and not just specific components of the diet, seem to be related to the risk of diverticulitis. In a study,55 men in the highest quintile of Western dietary pattern (high in red meat, fat and refined grains) had a hazard ratio of 1.55 (95% CI 1.20–1.99) when compared with men in the lowest quintile, after adjustment for multiple potential confounders, including body mass index and physical activity level. Conversely, high scores of prudent dietary pattern (high in fruits, vegetables and whole grains) were at a decreased risk (HR 0.74, 95% CI 0.60–0.91), compared with low scores55 (Figure 7).
Figure 7.
Relationship between dietary patterns and risk of diverticulitis (from Strate et al.55).
After adjustment for smoking, which does increase the risk, there is no significant association between the consumption of alcohol and risk of diverticular disease.45
Vitamin D may also play a role in the development of diverticulitis. One retrospective case-control study56 found that the mean prediagnostic level of vitamin D was significantly lower for patients with acute diverticulitis (25 ng/ml) when compared with subjects with diverticulosis (29 ng/ml) and even lower in patients with diverticulitis requiring emergent surgery (23.5 ng/ml). In another study, linking hospital data to geographic areas, low UV light exposure was associated with a higher rate of diverticulitis, further supporting a role for vitamin D in this disease.57
A number of studies have investigated the use of probiotics in the treatment of SUDD and the prevention of recurrent diverticulitis. However, in general, the quality of the studies is poor or the outcomes and treatments heterogeneous, so that the results are difficult to compare and interpret.9 In one small randomized, unblinded trial, use of a polymicrobial lysate (given orally 2 weeks a month for 3 consecutive months) was associated with a decreased risk of recurrence.58
A 1-year treatment of patients harboring colonic diverticula with microencapsulated sodium butyrate (300 mg per day) was associated with fewer symptoms and fewer ultrasound diagnoses of diverticulitis when compared with placebo, with significant improvement in quality of life.59 In addition, the number of computed-tomography-scan-diagnosed episodes of diverticulitis and hospitalizations for diverticulitis were higher in the placebo group, although the differences were not significant. Further studies are needed to define the role of this nutraceutical in the prevention of diverticulitis.
Which drugs are effective?
In addition to fibers (both high-fiber diet and dietary supplements), the therapeutic armamentarium in diverticular disease relies on antibiotics, and more recently the poorly absorbed antibiotic rifaximin, mesalazine and probiotics, alone, or in combination.
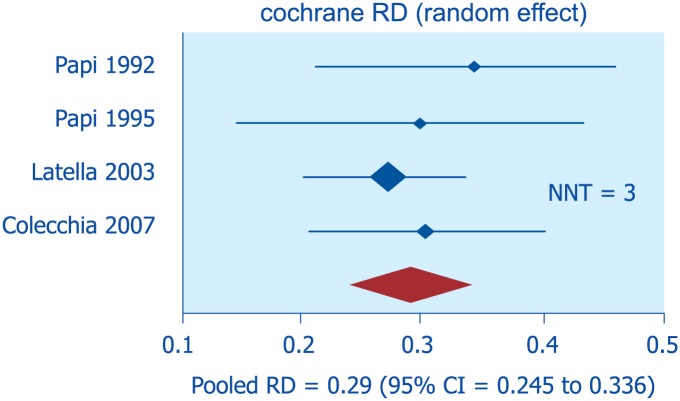
In asymptomatic diverticulosis, the prevention of progression to symptomatic disease or acute diverticulitis has been attempted with high-fiber intake and exercise, albeit with poor scientific evidence.60 No drug therapy is recommended at this stage. A meta-analysis of four RCTs (of which only one was placebo-controlled) showed that in patients with SUDD, rifaximin, in addition to soluble or insoluble fibers, is effective in reducing symptoms compared with fiber alone61 (Figure 8). The best results were obtained using a combination of soluble fibers, such as glucomannan (a soluble fiber extracted from the root of the konjac plant, able to absorb up to 200 times its weight in water), and rifaximin (given for 1 week every month).
Figure 8.
Efficacy of rifaximin for symptom relief in symptomatic uncomplicated diverticular disease: meta-analysis of randomized-controlled trials (from Bianchi et al.61).
CI, confidence interval; NNT, number needed to treat; RD, rate difference.
Mesalazine, an anti-inflammatory and antioxidant agent, has been used in patients with SUDD. In a recent systematic review,8 symptom relief with mesalazine was better than placebo, high-fiber diet, and low-dose rifaximin. The incidence of diverticulitis with mesalazine was lower only when compared with placebo8 (Table 4). Every-day mesalazine may be better than cyclic administration to prevent relapse.62 The combination of cyclic mesalazine and Lactobacillus casei DG seems to be better than placebo for maintaining remission of SUDD, but the small size of the study requires confirmation.63
Table 4.
Mesalazine for treatment of symptomatic uncomplicated diverticular disease and primary prevention of diverticulitis: results from a systematic review of randomized-controlled trials (data from Picchio et al.8).
| 6 RCTs including 1021 patients: (1) 526 patients were treated with mesalazine (2) 495 were treated with placebo or other therapies |
| Absolute risk reduction: significant only when mesalazine was compared with placebo, a high-fiber diet or low-dose (400 mg) rifaximin |
| The incidence of diverticulitis with mesalazine was significantly lower only when compared with placebo |
RCT, randomized-controlled trial.
The apparent colonic hypermotility in diverticular disease64,65 suggests that antispasmodic agents might improve abdominal pain, by decreasing muscular contraction. No randomized clinical trials are, however, available to confirm this benefit.
In acute uncomplicated diverticulitis (Hinchey, stage 0 or Ia), an outpatient management is now considered the optimal approach for most patients.66 Oral antimicrobials are often prescribed, but recent studies found no support to the routine use of antibiotics.67 A recent meta-analysis68 actually found that antibiotic use increases the length of hospital stay but is not associated with a reduction in overall or individual complication rates. The combination of ciprofloxacin and metronidazole is probably the most prescribed oral treatment.46,49 Admission to hospital and intravenous antibiotics are only recommended when the patient cannot tolerate oral feeding, is affected by severe comorbidities or does not improve with outpatient treatment.49 Most diverticulitis-associated abscesses can be treated with broad-spectrum antimicrobials or percutaneous drainage. Emergency surgery is considered standard treatment only in patients with acute peritonitis (Hinchey, stages III and IV).49
Prevention of recurrence of acute diverticulitis is a clear objective after recovery, but the best therapy has yet to be defined69 and several options (including, high-fiber diet, poorly absorbed antibiotics, anti-inflammatory agents, probiotics and surgery) have been proposed.10,70 An open-label, proof-of-concept study71 compared 3.5 g of high-fiber supplementation twice daily (b.i.d.), with or without 1 week per month of rifaximin (400 mg b.i.d.) for 1 year. Recurrences occurred in 10.4% of patients given rifaximin plus fibers versus 19.3% of patients receiving fiber alone. Mesalazine has also been tested in the prevention of recurrent acute diverticulitis. Most studies (including the two randomized, double-blind, placebo-controlled multicenter trials72,73) and a very recent meta-analysis74 failed to show a positive effect with mesalazine. In a small, retrospective, observational, long-term study,75 treatment with rifaximin (800 mg daily, 10 days a month) was more effective than mesalazine (2.4 g daily, 10 days a month) in preventing recurrence of acute diverticulitis.
It is worthwhile to emphasize that the treatment of diverticular disease relies mainly on data from uncontrolled studies, whose methodological quality is suboptimal. Indeed, only one long-term double-blind placebo-controlled study could be identified in the literature. Therefore, while the available studies show some evidence of symptom improvement with pharmacologic treatments, the best approach to SUDD (and especially to the primary and secondary prevention of acute diverticulitis) remains to be established.
How do effective drugs work?
There are several clinical scenarios that are relevant for the pharmacological and nonpharmacological treatment of diverticular disease. These include prevention of diverticulosis, treatment of SUDD, and primary as well as secondary prevention of diverticulitis. In these clinical settings, it is important to understand the rationale, the pharmacology and clinical benefits as well as the safety of commonly used drugs. The main classes of pharmacological therapies, currently investigated in diverticular disease, are anti-inflammatory drugs (mesalazine) and poorly absorbed antibiotics (namely rifaximin).
The justification for the use of aminosalicylates, such as mesalazine, is based on the assumption of low-grade inflammation in SUDD and symptom generation, whereas an overt inflammation may induce diverticulitis.76 Mesalazine is an anti-inflammatory agent, widely used as a pH- or time-dependent formulation in the treatment of ulcerative colitis.77 After oral or rectal administration, mesalazine is absorbed by colonic epithelial cells and its efficacy is related to its mucosal concentration. The main anti-inflammatory mechanisms of mesalazine, although not completely understood, are believed to be dependent on76:
(1) Reduction of the synthesis of endogenous prostaglandins and pro-inflammatory cytokines, including interleukin-1 and tumor necrosis factor (TNF);
(2) Inhibition of the activation of nuclear factor kappa-B (NF-κB) transcription factor family, involved in pro-inflammatory cytokine production;
(3) Activation of nuclear receptors (i.e. the gamma form of peroxisome proliferator-activated receptors), which downregulate inflammation and reduce inflammatory cytokine release;
(4) Antibacterial activity with inhibition of expression of bacterial genes involved in invasiveness, epithelial adherence, proliferation and antibiotic resistance.
The rationale for the use of antibiotics with high intraluminal availability is based on the evidence that diverticula are pouches of the colonic wall that, in predisposed individuals, favor fecal entrapment, bacterial overgrowth, and potential breakdown of the epithelial lining involved in bacterial translocation, mucosal inflammation and complications.78 This dogma is now supported by initial data showing the presence of dysbiosis in patients with diverticular disease.6 Rifaximin displays an extensive, evidence-based efficacy in the treatment of small intestine bacterial overgrowth (SIBO) and related (organic and functional) gastrointestinal disorders.79,80
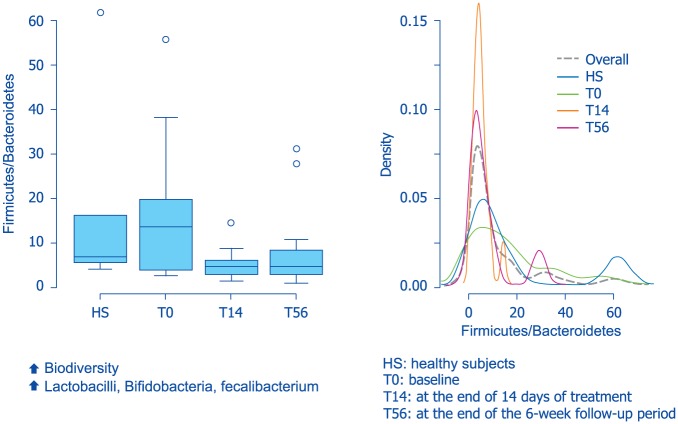
Rifaximin is a structural analogue of rifampin and exerts its antibiotic effects through inhibition of bacterial ribonucleic acid (RNA) synthesis by binding to the β-subunit of bacterial deoxyribonucleic acid-dependent RNA polymerase.81 Although rifaximin has antibiotic properties, it appears to have minimal negative impact on the overall gut microbiota. In addition, the drug has shown eubiotic effects since it stimulates the growth of beneficial bacterial species, including Lactobacilli and Bifidobacteria82,83 (Figure 9). Rifaximin has also shown good anti-inflammatory properties.84 In particular, it suppressed intestinal and systemic inflammation by preserving epithelial function (e.g. limiting bacterial translocation), but also through a direct anti-inflammatory activity. Subinhibitory concentrations of rifaximin have been shown to alter cytokine expression profiles (e.g. reduction in interleukin-8 and matrix metalloproteinase-9).80 Figure 10 summarizes the multiple mechanisms of action of rifaximin in diverticular disease. The safety profile of this drug is very good and adverse events have been rarely reported in the many trials conducted, with number needed to harm (NNH) of 9871.79
Figure 9.
Eubiotic effects of rifaximin on gut microbiota in patients with irritable-bowel-syndrome-associated constipation (from Soldi et al.82).
Figure 10.
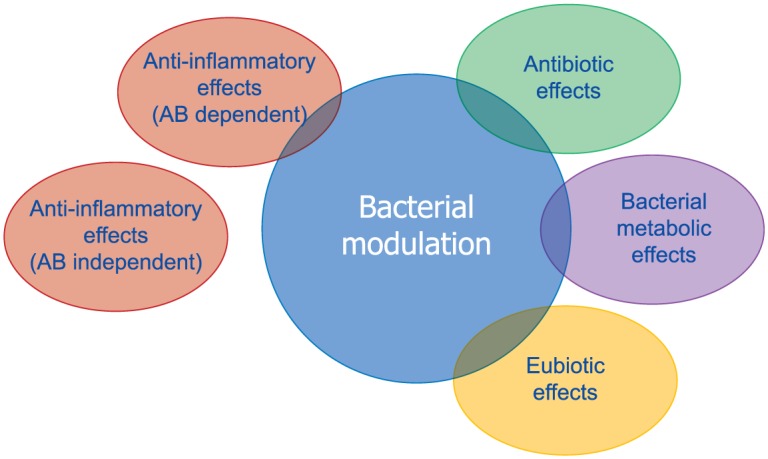
Multiple mechanisms of action of rifaximin in the treatment of diverticular disease (from Cuomo et al.80).
AB, antibiotic.
Which rifaximin?
All the studies in patients with SUDD have been performed by using the branded rifaximin formulation. The active ingredient contained in all rifaximin-based, brand name, medicinal products has been always characterized as a crystalline powder. Indeed, the European Pharmacopoeia, under the section ‘characteristics’, specifically states, ‘appearance: red-orange hygroscopic powder…’. The same monograph states that rifaximin is endowed with crystalline polymorphism. Currently, five polymorphic forms of rifaximin, designated as α, β, γ, δ, ε have been identified. They are all rifaximin hydrates, characterized by different water content.85 A noncrystalline form, designated as amorphous rifaximin, can also be generated through modifications of the synthetic and purification processes.
Amorphous or crystalline forms, despite containing the same active ingredient, may display very different chemical, physical and mechanical properties (for instance, solubility and bioavailability, hygroscopicity, chemical stability, hardness, etc.), with remarkable impact on their respective utilization, manipulation and absorption. In addition, possible interconversions among different polymorphs can impact seriously on the maintenance of the prespecified characteristics of a given product (for instance, therapeutic efficacy, in the case of drugs).86,87 In particular, by virtue of variations of some parameters (such as, for instance, pressure and relative humidity), a metastable form can be converted into a more thermodynamically stable form, or an anhydrous crystalline form can be converted into a hydrated crystalline form by adsorption of aqueous vapor from the environment. In some instances, the conversion of a crystalline form into another one can result in dramatic variations of the original properties.86,87
The different chemical-physical properties of polymorphs (stability, chemical reactivity, dissolution rate and solubility) can considerably modify the bioavailability of every molecule (thus affecting its pharmacokinetic and pharmacodynamic properties). Differences in solubility of the various crystalline and amorphous forms of rifaximin result in variations of their pharmacokinetics. Indeed, a study conducted in dogs showed that the systemic absorption of polymorph α and β is negligible, that of polymorph ε is sixfold higher, while that of polymorph γ is 400-fold higher.85 The amorphous form was evaluated in healthy volunteers, and its area under the curve (AUC) values documented a five- to sixfold higher systemic absorption than rifaximin-α.88
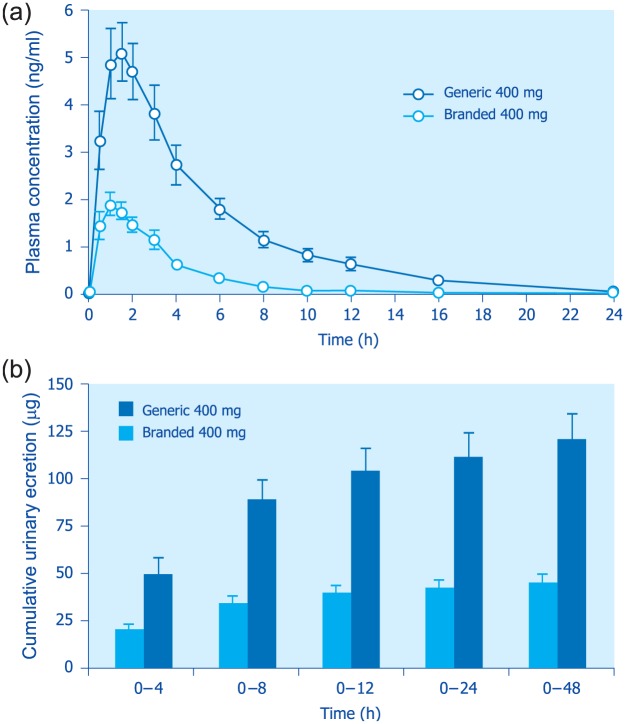
Taking all the above data into consideration, it is conceivable that the pharmacokinetic profiles of generic formulations that cannot contain rifaximin-α because of patent infringement, differ from those of the branded formulations. Indeed, patents covering the synthesis and pharmaceutical utilization of rifaximin polymorphs will expire in 2023. Generic formulations might thus contain the amorphous form, or a different crystalline form, or a mixture of different polymorphs. In the latter setting, systemic absorption would be fully unpredictable. In a study comparing a generic and the branded formulation,89 most pharmacokinetic parameters were significantly higher after administration of generic rifaximin than after rifaximin-α. In particular, the differences for Cmax (peak plasma concentration), AUC (area under the plasma drug concentration–time curve) and cumulative urinary excretion between the generic formulation and the branded product ranged from 165% to 345%89 (Figure 11). As a consequence, generic rifaximin does not possess the features of a poorly absorbed antibiotic.
Figure 11.
Mean rifaximin plasma concentration time (top panel) and cumulative urinary excretion (bottom panel) profiles following administration of 400 mg single-dose generic or branded (polymorph-α) rifaximin to healthy volunteers.
Each point or column represents the mean ± SEM (vertical lines) obtained from 24 subjects (from Blandizzi et al.89).
SEM, standard error of the mean.
Being the polymorph content related to the manufacturing process, the same considerations should be applied to those medicinal products containing rifaximin, whose origin of the active ingredient is different from that of the molecule contained in the branded formulations (Normix®, Spiraxin®, Xifaxan® and Flonorm®). In some South American countries (Argentina, Colombia, Venezuela and Perù), as well as in India and China, there are branded formulations of rifaximin whose summary of product characteristics (SPC) provides no clear information about the specific crystal structure of the active ingredient.
In any event, from a pharmacokinetic standpoint, none of the other rifaximin polymorphs (with the exception of the crystalline form β) might be regarded as a poorly absorbed antibiotic. As a consequence, their systemic absorption (which is, however, difficult to estimate) would not ensure the same safety in terms of both adverse effects and development of extragastrointestinal bacterial resistance.81,84,90 It is also important to emphasize that the branded formulation, employed in all clinical studies (both preregistration and postmarketing), contained a crystalline active ingredient with dissolution and pharmacokinetic profiles overlapping those of polymorph-α, known to be contained in the currently marketed formulation. For these reasons, the results obtained with the crystalline form α cannot be extended to the other polymorphs or the amorphous form. Therefore, clinical studies of therapeutic equivalence are required to document the actual interchangeability of different rifaximin formulations.91 Indeed, the FDA guidelines on this antibiotic,92 recently revised, recommend that the bioequivalence of generic formulations be evaluated by means of clinical studies based on a specific endpoint in patients affected by traveller’s diarrhea.
Summary and conclusions
The symposium was an interesting and exciting one, attracting a huge audience and stimulating a lively discussion. A large body of knowledge was delivered during the presentations. The key messages released during the meeting were:
(1) Diverticulosis is a common (>60% after age 70 years) condition and SUDD is a frequent and challenging disease, the symptoms of which often overlap those of IBS, sharing several common pathogenetic mechanisms;3
(2) Patients with diverticular disease show depletion of microbiota members with anti-inflammatory properties, including Clostridium cluster IV, Clostridium cluster IX, Fusobacterium and Lactobacillaceae;6
(3) Macrophages are markedly increased in patients with colonic diverticula both in the diverticular region and at distant sites;6
(4) Microbiota changes correlate with mucosal immune activation;34
(5) Pharmacologic treatment of SUDD relies on poorly absorbed antibiotics, anti-inflammatory drugs and, likely, probiotics.10,17,20
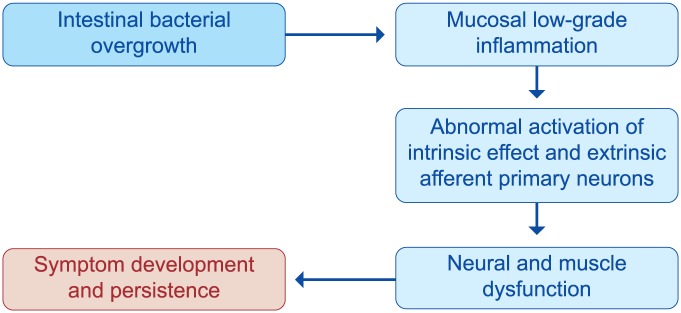
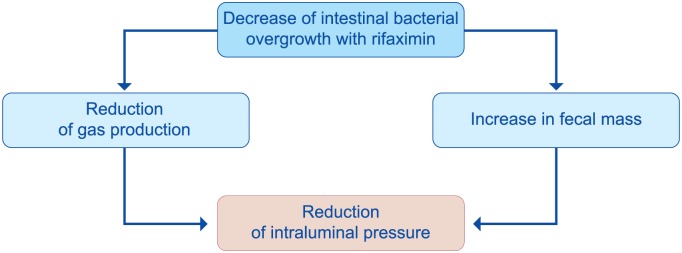
The rationale for the use of poorly absorbed antibiotics relies on the presence of SIBO (the most widely characterized form of dysbiosis) in patients with diverticular disease93,94 and its role in symptom development.95 Bacteria-induced immune activation will drive low-grade mucosal inflammation, which sensitizes both intrinsic primary efferent and extrinsic primary afferent neurons, generating neural and smooth muscle dysfunction. These disturbances will lead symptom development and persistence95 (Figure 12). Decreasing bacterial overgrowth with rifaximin79 has been shown to reduce colonic H2 production and gas-related symptoms. In addition, antibiotic therapy causes a rise in mean stool weight in patients on a constant fiber intake, most likely because of reduced fiber degradation, consequent to the decline in bacterial populations. Both effects will contribute to the reduction of intraluminal pressure and will lead to pain relief49 (Figure 13).
Figure 12.
Potential role of intestinal bacterial overgrowth in symptom development in patients with diverticular disease (derived from Colecchia et al.95).
Figure 13.
Benefits of poorly absorbed antibiotics in patients with diverticular disease (derived from Frieri et al.49).
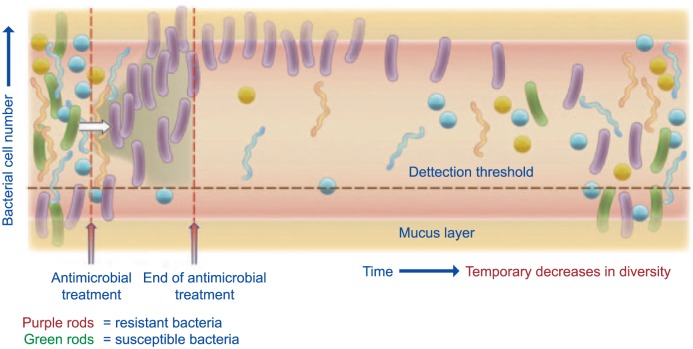
It is well known that systemic antimicrobials have many detrimental effects on gut microbiota.96 Both molecular- and cultivation-based approach-es have revealed ecological disturbances in the microbiota after antibiotic administration, in particular, for specific members of the bacterial community that are susceptible or alternatively resistant to the antibiotic in question. A disturbing consequence of antibiotic treatment has been a temporary decrease in microbial diversity and a long-term persistence of antibiotic-resistant genes97 (Figure 14). In this context, rifaximin appears to be a unique antibiotic, often referred to as ‘eubiotic’.83,98 Indeed:
Figure 14.
Representation of the impact of antimicrobial administration on bacterial community in the colon (from Jernberg et al.97).
Representation of the impact of antibiotic administration on the bacterial community of the colon. After the onset of treatment, an increase in resistant bacteria (purple rods) can be seen. This increase is due to either a susceptible bacterium (green rods) becoming resistant or resistant bacteria, already present in low levels, increasing in number due to their ability to survive the selective pressure provided by the antibiotic. The acquired resistance is often due to horizontal gene transfer or mutation events (white arrow). As a consequence of treatment, a temporary decrease in diversity can also be seen. Some bacteria may be protected from antibiotic exposure in the mucin layer (yellow shading) or in grooves in between the villi formed by host epithelial cells that line the intestinal channel (not shown). The figure is not drawn to scale and the timescale is relative.
(1) Long-term studies in IBS have shown that there were no clinically relevant changes in bacterial sensitivity to other antibiotic classes, no emergence of pathogenic bacteria, no occurrence of opportunistic infections, and no alteration of the overall microbiota;99
(2) In patients with Crohn’s disease, rifaximin, while not altering the overall structure of the human colonic microbiota, increased Bifi-dobacteria abundance and led to variation of metabolic profiles associated with potential beneficial effects on the host;100
(3) In selected patients with inflammatory bowel disease (IBD), diverticular disease and hepatic encephalopathy (HE), rifaximin did not alter the overall composition of microbiota, but increased the relative abundance of Lactobacilli;83
(4) Rifaximin is associated with improved cognitive function and endotoxemia in patients with minimal hepatic encephalopathy, which is accompanied by alteration of gut bacterial linkages with metabolites (metabolomic changes), without significant alterations in microbial abundance.101
Both the GRIMAD (Italian Group for the Study of Diverticular Disease) consensus17 and SICCR (Italian Society of Colon and Rectal Surgery)20 guidelines point out a benefit of rifaximin (in addition to soluble or insoluble fibers) in getting symptom relief for patients with SUDD, a recommendation shared by the Mexican14, Danish15 and Polish19 (but not German16) guidelines.
The role of low-grade inflammation (driven by bacteria-induced immune activation4,5) in symptom generation provides the rationale for use of mesalazine in patients with SUDD. However, despite a recent systematic review8 suggesting that symptom relief with this anti-inflammatory drug was better compared with placebo, a high-fiber diet, and low-dose rifaximin, the evidence was not considered enough by the consensus experts to recommend its use,17 although the SICCR guidelines suggest a benefit in some patients.20
Due to the large heterogeneity of the studies,9 the efficacy of probiotics in SUDD remains poor, since high-quality trials are few, with only two randomized, controlled investigations available.102 Large, well-designed, clinical studies are therefore needed to provide a strong evidence for probiotic use in diverticular disease.
In conclusion, recent pharmacological approaches targeting enteric bacteria (with poorly absorbed antibiotics, like rifaximin, or probiotics) or intestinal inflammation (with 5-ASA derivatives or rifaximin) have been shown capable of controlling symptoms and also preventing complications. The respective role of these drugs in the management of symptomatic diverticular disease needs to be better defined. In particular, it should be established for which patients rifaximin is most suitable and for which mesalazine is preferable. Also, those patients who can benefit most from the combined treatment should be identified. Well-designed RCTs, including homogeneous populations of patients, are therefore needed. The future of management of many GI diseases, including SUDD, will rely on the so-called ‘microbiota-directed therapies’.
Acknowledgments
We are indebted to Anna Bertelé, MD (Digestive Pathophysiology Unit, Maggiore University Hospital, Parma, Italy) for her invaluable help during the preparation of the manuscript.
Author contributions were made according to the International Committee of Medical Journal Editors.
Highlights concept: Carmelo Scarpignato.
Highlights design: Carmelo Scarpignato.
Drafting of the manuscript: Carmelo Scarpignato.
Critical revision of the manuscript for important intellectual content: Giovanni Barbara, Angel Lanas and Lisa L Strate.
Footnotes
Funding: The symposium was supported by Alfasigma SpA (Milan, Italy).
The company did not have any role in writing this report, whose responsibility is solely of the authors. The terms of the financial support included freedom for the authors to reach their own conclusions, and an absolute right to publish the results of their work, irrespective of any conclusions reached.
Conflict of interest statement: Carmelo Scarpignato is member of the Speakers’ Bureau and of the Scientific Advisory Board of Alfasigma SpA.
Giovanni Barbara is member of the Speakers’ Bureau of Alfasigma SpA.
Angel Lanas is member of the Speakers’ Bureau and of the Scientific Advisory Board of Alfasigma SpA.
Lisa L Strate has no conflicts of interest.
ORCID iD: Carmelo Scarpignato  https://orcid.org/0000-0001-5645-857X
https://orcid.org/0000-0001-5645-857X
Contributor Information
Carmelo Scarpignato, Clinical Pharmacology and Digestive Pathophysiology Unit, Department of Clinical and Experimental Medicine, University of Parma, Maggiore University Hospital, Cattani Pavillon, I-43125 Parma, Italy.
Giovanni Barbara, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Angel Lanas, Clinic Hospital Lozano Blesa, University of Zaragoza, Zaragoza, Spain.
Lisa L. Strate, Division of Gastroenterology, University of Washington, Seattle, WA, USA
References
- 1. Bevan R, Lee TJ, Nickerson C, et al. Non-neoplastic findings at colonoscopy after positive faecal occult blood testing: data from the English Bowel Cancer Screening Programme. J Med Screen 2014; 21: 89–94. [DOI] [PubMed] [Google Scholar]
- 2. Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 4. Cianci R, Iacopini F, Petruzziello L, et al. Involvement of central immunity in uncomplicated diverticular disease. Scand J Gastroenterol 2009; 44: 108–115. [DOI] [PubMed] [Google Scholar]
- 5. Spiller RC, Sloan TJ. Do diverticula provide a unique niche for microbiota which can lead to activation of the innate immune system? Gut 2017; 66: 1175–1176. [DOI] [PubMed] [Google Scholar]
- 6. Barbara G, Scaioli E, Barbaro MR, et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017; 66: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 7. Latella G, Scarpignato C. Rifaximin in the management of colonic diverticular disease. Expert Rev Gastroenterol Hepatol 2009; 3: 585–598. [DOI] [PubMed] [Google Scholar]
- 8. Picchio M, Elisei W, Brandimarte G, et al. Mesalazine for the treatment of symptomatic uncomplicated diverticular disease of the colon and for primary prevention of diverticulitis: a systematic review of randomized clinical trials. J Clin Gastroenterol 2016; 50(Suppl. 1): S64–S69. [DOI] [PubMed] [Google Scholar]
- 9. Scarpignato C, Bertelé A, Tursi A. Probiotics for the treatment of symptomatic uncomplicated diverticular disease: rationale and current evidence. J Clin Gastroenterol 2016; 50(Suppl. 1): S70–S73. [DOI] [PubMed] [Google Scholar]
- 10. Maconi G, Barbara G, Bosetti C, et al. Treatment of diverticular disease of the colon and prevention of acute diverticulitis: a systematic review. Dis Colon Rectum 2011; 54: 1326–1338. [DOI] [PubMed] [Google Scholar]
- 11. Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999; 94: 3110–3121. [DOI] [PubMed] [Google Scholar]
- 12. Kohler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc 1999; 13: 430–436. [DOI] [PubMed] [Google Scholar]
- 13. Murphy T, Hunt RH, Fried M, et al. Diverticular disease. WGO Practice Guidelines, http://www.worldgastroenterology.org/UserFiles/file/guidelines/diverticular-disease-english-2007.pdf (2007, accessed 24 April 2018)
- 14. Charua-Guindic L, Mazza-Olmos D, Orduna-Tellez D, et al. Gastroenterology. Diagnosis and treatment guidelines of diverticular disease of the colon. Treatment. Rev Gastroenterol Mex 2008; 73: 261–264. [PubMed] [Google Scholar]
- 15. Andersen JC, Bundgaard L, Elbrond H, et al. Danish national guidelines for treatment of diverticular disease. Dan Med J 2012; 59: C4453. [PubMed] [Google Scholar]
- 16. Kruis W, Germer CT, Leifeld L, et al. Diverticular disease: guidelines of the german society for gastroenterology, digestive and metabolic diseases and the german society for general and visceral surgery. Digestion 2014; 90: 190–207. [DOI] [PubMed] [Google Scholar]
- 17. Cuomo R, Barbara G, Pace F, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United European Gastroenterol J 2014; 2: 413–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vennix S, Morton DG, Hahnloser D, et al. Systematic review of evidence and consensus on diverticulitis: an analysis of national and international guidelines. Colorectal Dis 2014; 16: 866–878. [DOI] [PubMed] [Google Scholar]
- 19. Pietrzak A, Bartnik W, Szczepkowski M, et al. Polish interdisciplinary consensus on diagnostics and treatment of colonic diverticulosis (2015). Pol Przegl Chir 2015; 87: 203–220. [DOI] [PubMed] [Google Scholar]
- 20. Binda GA, Cuomo R, Laghi A, et al. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech Coloproctol 2015; 19: 615–626. [DOI] [PubMed] [Google Scholar]
- 21. Stollman N, Smalley W, Hirano I, et al. American Gastroenterological Association Institute Guideline on the Management of Acute Diverticulitis. Gastroenterology 2015; 149: 1944–1949. [DOI] [PubMed] [Google Scholar]
- 22. Sartelli M, Catena F, Ansaloni L, et al. WSES Guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg 2016; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 2012; 142: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther 2010; 32: 811–820. [DOI] [PubMed] [Google Scholar]
- 25. Cohen ER, Fuller G, Bolus R, et al. Tu1363 evidence for post-diverticulitis irritable bowel syndrome (Pdv-IBS): longitudinal analysis reveals higher incidence of IBS in DV cases vs. controls. Gastroenterology 2012; 142: S-811–S-812. [Google Scholar]
- 26. Lamps LW, Knapple WL. Diverticular disease-associated segmental colitis. Clin Gastroenterol Hepatol 2007; 5: 27–31. [DOI] [PubMed] [Google Scholar]
- 27. Tursi A. Segmental colitis associated with diverticulosis: complication of diverticular disease or autonomous entity? Dig Dis Sci 2011; 56: 27–34. [DOI] [PubMed] [Google Scholar]
- 28. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol 2016; 14: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol 2015; 110: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ritz JP, Lehmann KS, Frericks B, et al. Outcome of patients with acute sigmoid diverticulitis: multivariate analysis of risk factors for free perforation. Surgery 2011; 149: 606–613. [DOI] [PubMed] [Google Scholar]
- 31. Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1997; 92: 419–424. [PubMed] [Google Scholar]
- 32. Aytac E, Stocchi L, Gorgun E, et al. Risk of recurrence and long-term outcomes after colonic diverticular bleeding. Int J Colorectal Dis 2014; 29: 373–378. [DOI] [PubMed] [Google Scholar]
- 33. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721 e714. [DOI] [PubMed] [Google Scholar]
- 34. Annibale B, Lahner E, Maconi G, et al. Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis 2012; 27: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 35. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 36. Simpson J, Neal KR, Scholefield JH, et al. Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol 2003; 15: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 37. Jung HK, Choung RS, Locke GR, III, et al. Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol 2010; 105: 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuomo R, Barbara G, Andreozzi P, et al. Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest 2013; 43: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 39. Tursi A, Elisei W, Picchio M, et al. Moderate to severe and prolonged left lower-abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol 2015; 49: 218–221. [DOI] [PubMed] [Google Scholar]
- 40. Lopetuso LR, Petito V, Graziani C, et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders. Dig Dis 2018; 36: 56–65. [DOI] [PubMed] [Google Scholar]
- 41. Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 42. Daniels L, Budding AE, De Korte N, et al. Fecal microbiome analysis as a diagnostic test for diverticulitis. Eur J Clin Microbiol Infect Dis 2014; 33: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 43. Tursi A, Mastromarino P, Capobianco D, et al. Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol 2016; 50(Suppl. 1): S9–S12. [DOI] [PubMed] [Google Scholar]
- 44. Tursi A, Mastromarino P, Capobianco D, et al. Urinary metabolic profiling and symptomatic uncomplicated diverticular disease of the colon. Clin Res Hepatol Gastroenterol 2017; 41: 344–346. [DOI] [PubMed] [Google Scholar]
- 45. Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011; 343: d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tursi A, Papa A, Danese S. Review article: the pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment Pharmacol Ther 2015; 42: 664–684. [DOI] [PubMed] [Google Scholar]
- 47. Gargallo Puyuelo CJ, Sopena F, Lanas Arbeloa A. Colonic diverticular disease. Treatment and prevention. Gastroenterol Hepatol 2015; 38: 590–599. [DOI] [PubMed] [Google Scholar]
- 48. Salem TA, Molloy RG, O’Dwyer PJ. Prospective, five-year follow-up study of patients with symptomatic uncomplicated diverticular disease. Dis Colon Rectum 2007; 50: 1460–1464. [DOI] [PubMed] [Google Scholar]
- 49. Frieri G, Pimpo MT, Scarpignato C. Management of colonic diverticular disease. Digestion 2006; 73(Suppl. 1): 58–66. [DOI] [PubMed] [Google Scholar]
- 50. Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J 1971; 2: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol 2013; 11: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut 2014; 63: 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998; 128: 714–719. [DOI] [PubMed] [Google Scholar]
- 54. Cao Y, Strate LL, Keeley BR, et al. Meat intake and risk of diverticulitis among men. Gut 2018; 67: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology 2017; 152: 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maguire LH, Song M, Strate LE, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol 2013; 11: 1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg 2015; 150: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dughera L, Serra AM, Battaglia E, et al. Acute recurrent diverticulitis is prevented by oral administration of a polybacterial lysate suspension. Minerva Gastroenterol Dietol 2004; 50: 149–153. [PubMed] [Google Scholar]
- 59. Krokowicz L, Stojcev Z, Kaczmarek BF, et al. Microencapsulated sodium butyrate administered to patients with diverticulosis decreases incidence of diverticulitis–a prospective randomized study. Int J Colorectal Dis 2014; 29: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carabotti M, Annibale B, Severi C, et al. Role of fiber in symptomatic uncomplicated diverticular disease: a systematic review. Nutrients 2017; 9: pii: e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bianchi M, Festa V, Moretti A, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther 2011; 33: 902–910. [DOI] [PubMed] [Google Scholar]
- 62. Tursi A, Di Mario F, Brandimarte G, et al. Intermittent versus every-day mesalazine therapy in preventing complications of diverticular disease: a long-term follow-up study. Eur Rev Med Pharmacol Sci 2013; 17: 3244–3248. [DOI] [PubMed] [Google Scholar]
- 63. Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease–a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 2013; 38: 741–751. [DOI] [PubMed] [Google Scholar]
- 64. Bassotti G, Battaglia E, Spinozzi F, et al. Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis Colon Rectum 2001; 44: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 65. Bassotti G, Battaglia E, de Roberto G, et al. Alterations in colonic motility and relationship to pain in colonic diverticulosis. Clin Gastroenterol Hepatol 2005; 3: 248–253. [DOI] [PubMed] [Google Scholar]
- 66. Sanchez-Velazquez P, Grande L, Pera M. Outpatient treatment of uncomplicated diverticulitis: a systematic review. Eur J Gastroenterol Hepatol 2016; 28: 622–627. [DOI] [PubMed] [Google Scholar]
- 67. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. Epub ahead of print 11 January 2018. DOI: 10.1111/codi.14013. [DOI] [PubMed] [Google Scholar]
- 68. Mocanu V, Dang JT, Switzer N, et al. The role of antibiotics in acute uncomplicated diverticulitis: a systematic review and meta-analysis. Am J Surg 2018; pii: S0002-9610(17)31293-X. [DOI] [PubMed] [Google Scholar]
- 69. Unlu C, Daniels L, Vrouenraets BC, et al. Systematic review of medical therapy to prevent recurrent diverticulitis. Int J Colorectal Dis 2012; 27: 1131–1136. [DOI] [PubMed] [Google Scholar]
- 70. Elisei W, Tursi A. Recent advances in the treatment of colonic diverticular disease and prevention of acute diverticulitis. Ann Gastroenterol 2016; 29: 24–32. [PMC free article] [PubMed] [Google Scholar]
- 71. Lanas A, Ponce J, Bignamini A, et al. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: a proof-of-concept study. Dig Liver Dis 2013; 45: 104–109. [DOI] [PubMed] [Google Scholar]
- 72. Raskin JB, Kamm MA, Jamal MM, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology 2014; 147: 793–802. [DOI] [PubMed] [Google Scholar]
- 73. Kruis W, Kardalinos V, Eisenbach T, et al. Randomised clinical trial: mesalazine versus placebo in the prevention of diverticulitis recurrence. Aliment Pharmacol Ther 2017; 46: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Khan RMA, Ali B, Hajibandeh S, et al. Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis with trial sequential analysis of randomised controlled trials. Colorectal Dis. Epub ahead of print 9 March 2018. DOI: 10.1111/codi.14064. [DOI] [PubMed] [Google Scholar]
- 75. Festa V, Spila Alegiani S, Chiesara F, et al. Retrospective comparison of long-term ten-day/month rifaximin or mesalazine in prevention of relapse in acute diverticulitis. Eur Rev Med Pharmacol Sci 2017; 21: 1397–1404. [PubMed] [Google Scholar]
- 76. Barbara G, Cremon C, Barbaro MR, et al. Treatment of diverticular disease with aminosalicylates: the evidence. J Clin Gastroenterol 2016; 50(Suppl. 1): S60–S63. [DOI] [PubMed] [Google Scholar]
- 77. Criscuoli V, Modesto I, Orlando A, et al. Mesalazine for the treatment of inflammatory bowel disease. Expert Opin Pharmacother 2013; 14: 1669–1678. [DOI] [PubMed] [Google Scholar]
- 78. Humes DJ, Spiller RC. Review article: the pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther 2014; 39: 359–370. [DOI] [PubMed] [Google Scholar]
- 79. Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther 2017; 45: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cuomo R, Barbara G, Annibale B. Rifaximin and diverticular disease: position paper of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis 2017; 49: 595–603. [DOI] [PubMed] [Google Scholar]
- 81. Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy 2005; 51(Suppl. 1): 36–66. [DOI] [PubMed] [Google Scholar]
- 82. Soldi S, Vasileiadis S, Uggeri F, et al. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol 2015; 8: 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ponziani FR, Scaldaferri F, Petito V, et al. The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin. Dig Dis 2016; 34: 269–278. [DOI] [PubMed] [Google Scholar]
- 84. Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion 2006; 73(Suppl. 1): 13–27. [DOI] [PubMed] [Google Scholar]
- 85. Viscomi GC, Campana M, Barbanti M, et al. Crystal forms of rifaximin and their effect on pharmaceutical properties. Crystengcomm 2008; 10: 1074–1081. [Google Scholar]
- 86. Ilfiker R. Polymorphysm in pharmaceitical industry. Wiley-VCH 2006: 1–433. [Google Scholar]
- 87. Brittain HG. Polymorphism in pharmaceutical solids. 2nd ed. London: Informa Healthcare, 2009, pp.1–640. [Google Scholar]
- 88. Blandizzi C, Viscomi GC, Scarpignato C. Impact of crystal polymorphism on the systemic bioavailability of rifaximin, an antibiotic acting locally in the gastrointestinal tract, in healthy volunteers. Drug Des Devel Ther 2015; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blandizzi C, Viscomi GC, Marzo A, et al. Is generic rifaximin still a poorly absorbed antibiotic? A comparison of branded and generic formulations in healthy volunteers. Pharmacol Res 2014; 85: 39–44. [DOI] [PubMed] [Google Scholar]
- 90. DuPont HL. Rifaximin: an antibiotic with important biologic effects. Mini Rev Med Chem 2015; 16: 200–205. [DOI] [PubMed] [Google Scholar]
- 91. Snider DA, Addicks W, Owens W. Polymorphism in generic drug product development. Adv Drug Deliv Rev 2004; 56: 391–395. [DOI] [PubMed] [Google Scholar]
- 92. FDA. Draft guidance on rifaximin, https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm281455.pdf (Recommended November 2011, February 2012; revised March 2017, accessed 24 April 2018).
- 93. D’Inca R, Pomerri F, Vettorato MG, et al. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther 2007; 25: 771–779. [DOI] [PubMed] [Google Scholar]
- 94. Tursi A, Brandimarte G, Giorgetti GM, et al. Assessment of small intestinal bacterial overgrowth in uncomplicated acute diverticulitis of the colon. World J Gastroenterol 2005; 11: 2773–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Colecchia A, Sandri L, Capodicasa S, et al. Diverticular disease of the colon: new perspectives in symptom development and treatment. World J Gastroenterol 2003; 9: 1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001; 1: 101–114. [DOI] [PubMed] [Google Scholar]
- 97. Jernberg C, Lofmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010; 156: 3216–3223. [DOI] [PubMed] [Google Scholar]
- 98. Ponziani FR, Zocco MA, D’Aversa F, et al. Eubiotic properties of rifaximin: disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol 2017; 23: 4491–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pimentel M, Cash BD, Lembo A, et al. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci 2017; 62: 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010; 65: 2556–2565. [DOI] [PubMed] [Google Scholar]
- 101. Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013; 8: e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis 2016; 25: 79–86. [DOI] [PubMed] [Google Scholar]