Abstract
Background:
There are limited data to guide clinicians in differentiating tumefactive multiple sclerosis (TMS) from CNS neoplasms. Identifying distinguishing features will inform diagnosis and management and avoid unnecessary diagnostic biopsy. Our study aimed to determine the clinical and radiologic features that differentiate TMS from glioma and CNS lymphoma (CNSL) in patients who present with tumefactive lesions.
Methods:
We retrospectively reviewed all patients with tumefactive lesions and histologically proven or clinically diagnosed TMS, glioma, or CNSL at our tertiary center from 1999 to 2012. Two independent blinded neuroradiologists rated MRI brain scans at presentation. We correlated patients' demographic, clinical, laboratory, and radiologic data to final diagnosis.
Results:
A total of 133 patients (10 TMS, 85 glioma, 38 CNSL) were analyzed. Patients with TMS were younger and a greater proportion were women. Presenting symptoms did not distinguish between diagnoses. TMS lesions were smaller compared to glioma and CNSL, had no or mild mass effect, and were always associated with contrast enhancement. Radiologic features that were more frequent in TMS lesions were incomplete rim (open-ring) enhancement, incomplete peripheral diffusion restriction, and mixed T2 signal and CT hypoattenuation of MRI-enhancing components (all p < 0.05).
Conclusions:
Radiologic features but not presenting symptoms are useful in distinguishing TMS from CNS neoplasms.
Tumefactive multiple sclerosis (TMS) is a rare subtype of multiple sclerosis (MS) characterized by large tumefactive demyelinating lesions that are often indistinguishable from CNS neoplasms, especially glioma and CNS lymphoma (CNSL), necessitating brain biopsy for definitive diagnosis.
Clinically, patients with TMS are reported to present polysymptomatically, with motor and cognitive symptoms predominating.1 Such presentations are nonspecific and may occur in patients with CNS lesions of other etiologies, including glioma and CNSL.
Radiologically, TMS is defined by lesion size more than 2 cm with associated mass effect, edema, or ring enhancement.2 An open-ring enhancement pattern is described to be highly specific for demyelinating disease3 while T2-hypointense patterns in ring-enhancing lesions may help distinguish glioma from MS.4 More recent data suggest that in ring-enhancing lesions, peripheral diffusion restriction is more common in demyelinating disease than in tumors.5 A systematic examination of radiologic features in patients with tumefactive demyelinating disease, gliomas, and lymphoma showed that unenhanced CT plus MRI improved diagnostic accuracy over contrast-enhancing MRI alone to distinguish the diagnoses.6
Nevertheless, there is still insufficient evidence to guide physicians in distinguishing TMS accurately from CNS neoplasms. Hence, in patients presenting with tumefactive lesions, exclusion of gliomas, CNSL, or other brain tumors by invasive brain biopsies is often required before specific therapy can be initiated. Our study aims to identify clinical and radiologic distinguishing features to guide clinicians' diagnosis and management and avoid unnecessary diagnostic biopsy in patients who present with tumefactive lesions.
METHODS
Study design and patients
We retrospectively reviewed patients with TMS, glioma, and CNSL at the National Neuroscience Institute from 1999 to 2012. Inclusion criteria were (1) histologic diagnosis of demyelinating disease, glioma, and lymphoma, or clinical diagnosis of TMS made by a neurologist; and (2) MRI brain scan with tumefactive lesions performed within a week of presentation available for review. In patients with histologic diagnosis of demyelinating disease, a clinical diagnosis of MS defined by revised McDonald criteria is required7; specifically excluded were patients with acute disseminated encephalomyelitis. In patients with TMS without histologic confirmation, a minimum of 2 years follow-up is required if patients were either treatment-naive or on active treatment; patients who were previously treated with immunotherapy but were no longer on active treatment must meet a minimum follow-up duration of 1 year. These follow-up periods allowed for exclusion of patients who were eventually found to have alternative diagnoses.
Cases that fulfill the above histologic or clinical diagnoses were further screened for radiologic features of tumefactive lesions: (1) white matter or periventricular location; (2) size more than 2 cm; and (3) presence of mass effect, perilesional edema, or ring enhancement.2 Cases of glioma with predominant gray matter involvement were excluded.
Clinical data
Data on patients' demographics, family and personal medical history, and presenting and constitutional symptoms were collected. Cognitive symptoms were broadly defined by the presence of memory impairment or cortical dysfunction such as aphasia, apraxia, neglect, and personality changes.2 Laboratory investigations including systemic inflammatory (C-reactive protein and erythrocyte sediment rate), immune-mediated (rheumatoid factor and antinuclear, anti-dsDNA, anticardiolipin, lupus anticoagulant, anti-Ro, anti-La, and antineutrophil cytoplasmic antibodies), and infective (syphilis immunoglobulin G, HIV) markers, and CSF results were recorded. As CNSL presents differently in immunocompetent and immunocompromised hosts,8 HIV-positive patients were excluded from study analysis.
Radiologic examination
The first MRI scan after symptom presentation was reviewed. At our institution, MRI brain scans were performed on a 1.5T MRI scanner (Signa HDxt, General Electric Medical Systems, Milwaukee, WI) or a 3T whole body MRI system (Achieva 3.0, Philips Medical Systems, Best, the Netherlands). Imaging protocols included axial T1-weighted (W) spin-echo (SE) (repetition time [TR]/echo time [TE]/field of view [FOV]; 500–640 ms/10–12 ms/220–240 mm; matrix 1.5T/3.0T; 320 × 224/256 × 200), axial T2W fast/turbo SE (TR/TE/FOV; 3,000 ms/83–100 ms/240 mm; matrix 1.5T/3.0T; 384 × 224/436 × 306), coronal fluid-attenuated inversion recovery (FLAIR) (TR/TE/inversion time/FOV; 10,000–11,000 ms/125–128/2,200–2,800/240 mm; matrix 1.5T/3.0T; 256 × 192/368 × 186), single shot echoplanar diffusion-weighted imaging in the axial plane (TR/TE/FOV; 3,378–8,000 ms/71–73 ms/240–250 mm; diffusion gradient encoding in 3 orthogonal directions; b = 1,000 s/mm2; matrix 1.5T/3.0T; 128 × 128/124 × 123), axial gradient echo (TR/TE/FOV; 678–800 ms/15–25 ms/240 mm; matrix 1.5T/3.0T; 256 × 192/256 × 256), and axial and coronal postgadolinium T1W SE (matrix 1.5T/3.0T; axial: 256 × 256/240 × 188; coronal: 320 × 224/264 × 193). MRI scans, which were performed at outside institutions, were imported into our picture archiving and communication system (PACS). Two senior neuroradiologists, each with more than 10 years of subspecialty experience, blinded to patients' diagnoses, evaluated MRI scans independently for (1) number of tumefactive lesions; (2) presence of other white matter lesions at periventricular, juxtacortical, and infratentorial regions; (3) location of the largest tumefactive lesion; (4) 3 size measurements of the largest tumefactive lesion ([a] diameter of the whole lesion on T2W image, [b] diameter of the discernible lesion that could be distinguished from the surrounding vasogenic edema, and [c] diameter of the enhancing component); (5) presence and severity of mass effect in the supratentorial region or the posterior fossa; (6) presence and pattern of rim and central enhancement and presence of vessels passing through the lesion; (7) T1W signal intensity of the lesion relative to white matter; (8) T2W signal intensity of the lesion relative to gray matter; (9) T2W signal intensity of the enhancing component; and (10) presence and characteristics of peripheral and central restricted diffusion. In the supratentorial region, mild mass effect was defined by presence of sulcal effacement, while moderate to severe mass effect displayed subfalcine or uncal herniation. At the brainstem, mild mass effect was defined by narrowing of basal cisterns, moderate mass effect by additional distortion of fourth ventricle, and severe mass effect by development of hydrocephalus. Where CT brain scan was available for review, the CT attenuation of the MRI-enhancing component in relation to cortical/basal ganglia gray matter was also rated.
The 2 neuroradiologists also independently recorded their radiologic diagnosis for each of these lesions based on their overall impression. They were allowed to discuss and make a joint final radiologic diagnosis if their initial diagnoses differed. Cases that could not be confidently assigned a final radiologic diagnosis were recorded separately.
Statistical analysis
Patients were sorted into TMS, glioma, and CNSL groups based on their histologic or clinical diagnoses according to the study inclusion criteria. Demographic, clinical, laboratory, and radiologic variables were compared across these groups for significant differences. Univariate analysis was performed using analysis of variance for continuous parametric or Kruskal-Wallis test for continuous nonparametric variables, and χ2 test for categorical parametric variables to test for overall significant differences. Pairwise comparisons of TMS against glioma and TMS against CNSL were done for significant variables using independent t test for parametric or Mann-Whitney U test for nonparametric variables. All statistical tests were performed using STATA version 10.1. Statistical tests were 2-tailed and statistical significance was set to p < 0.05 for overall tests, and Bonferroni-corrected significance was set to p < 0.025 for the multiple pairwise comparisons.
Standard protocol approvals, registrations, and patient consents
This study was approved by the local institutional review boards (IRBs), namely SingHealth Centralized Institutional Review Board and National Healthcare Group Domain-Specific Review Board. Patient informed consent had been waived by both IRBs.
RESULTS
A total of 138 patients met inclusion criteria. Five were HIV-positive, and excluded from the study; 133 patients (10 TMS, 85 glioma, 38 CNSL) were analyzed. Of the 10 patients with TMS, 5 had histologic confirmation while 5 were diagnosed clinically.
Clinical characteristics
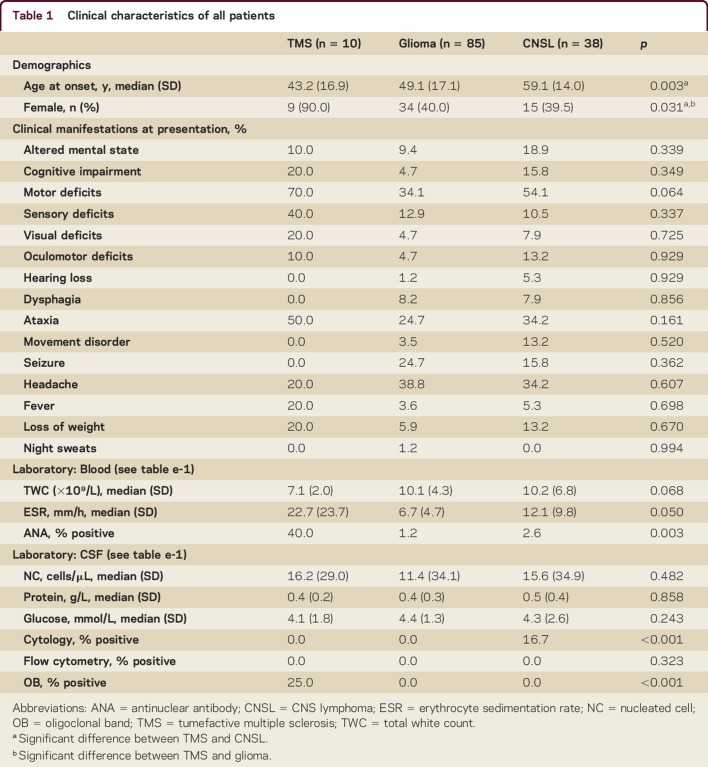
The median age at disease onset was 43 (TMS; SD 17), 49 (glioma; SD 17), and 59 (CNSL; SD 14) years. The majority of patients with TMS were women (90%) compared to patients with glioma (40%) and CNSL (39%). There were no differences in presenting symptoms, including cognitive (TMS 30% vs glioma 12% vs CNSL 26%, p = 0.349), motor (70% vs 34% vs 54%, p = 0.064), and sensory (40% vs 13% vs 11%, p = 0.337) deficits, between the groups (table 1).
Table 1.
Clinical characteristics of all patients
Laboratory characteristics
The majority of patients did not have inflammatory, immune, or infective markers. One of 10 glioma patients and 1 of 5 CNSL patients who were tested for antinuclear antibodies (ANA) had positive results; 1 of 4 glioma patients also tested positive for anti-Ro antibody.
CSF evaluation was performed for the majority of patients with TMS (90%) but only in a minority of patients with glioma (22%) and CNSL (45%). Median CSF white cell count, protein, and glucose levels were not significantly different between the groups. CSF cytology was positive in 2 of 12 patients with CNSL; no patients with glioma (n = 9) or TMS (n = 9) had positive CSF cytology. Two (25%) of 8 patients with TMS were CSF oligoclonal band (OCB) positive. Only 1 of 59 CNSL and 2 of 49 glioma patients had CSF OCB tested; all were negative (table 1 and table e-1 at Neurology.org/cp).
Radiologic characteristics
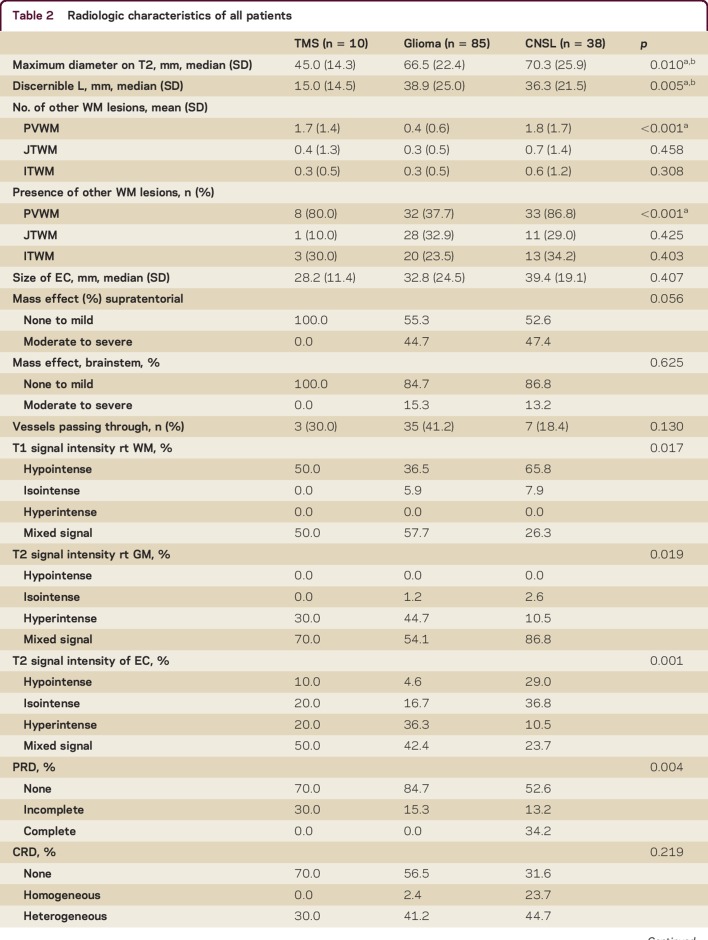
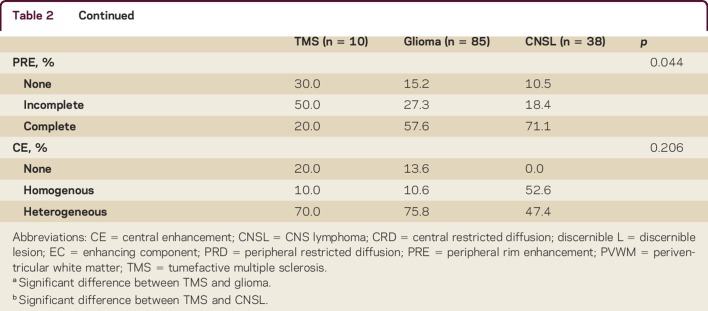
Tumefactive lesions in TMS were smaller than glioma and CNSL lesions. Median T2-weighted whole lesion diameter in TMS (4.5 cm) was smaller compared to glioma (6.6 cm) and CNSL (7.0 cm) (p = 0.010) (table 2). Median discernible lesion margin was also smaller in TMS (1.5 cm) compared to glioma (3.9 cm) and CNSL (3.6 cm) (p = 0.005). Moderate to severe mass effect was present in both supratentorial and brainstem lesions of glioma (supratentorial 45%; infratentorial 15%) and CNSL (47%; 13%) but not TMS (both 0%); results are not significant (figure 1).
Table 2.
Radiologic characteristics of all patients
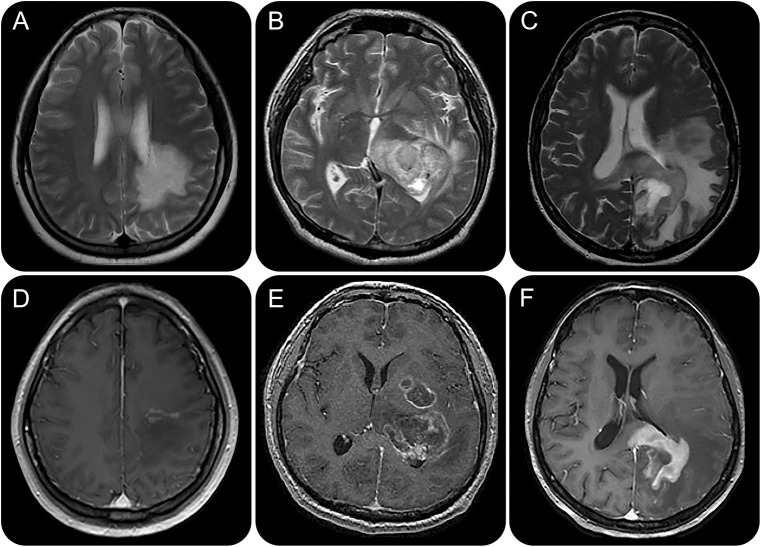
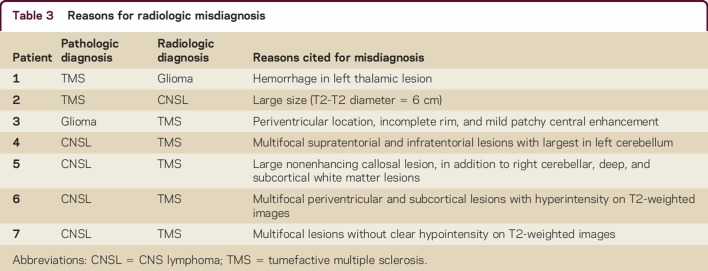
Figure 1. Axial T2-weighted MRI of tumefactive multiple sclerosis (TMS) lesions, glioma and CNS lymphoma.
Axial T2-weighted MRI of TMS lesions (A) are smaller with less mass effect compared to glioma (B) and lymphoma (C). Postgadolinium axial T1-weighted MRI of TMS lesions (D) shows more central heterogeneous and incomplete peripheral rim enhancement compared to glioma (E) and lymphoma (F).
All TMS and CNSL compared to 78% of glioma lesions were contrast-enhancing (p = 0.102). Higher proportions of TMS (50%) demonstrated incomplete peripheral rim (open-ring) enhancement compared to glioma (27%) and CNSL (18%) (p = 0.044). There were more TMS (70%) and glioma (76%) lesions with heterogeneous central enhancement compared to CNSL (47%) lesions (p = 0.206) (figure 1). There was no significant difference in presence of blood vessels passing through the lesions between groups.
Diffusion restriction was more common in TMS (60%) and CNSL (76%) than glioma (45%) lesions (p = 0.026). All TMS and glioma compared to a minority of CNSL (28%) lesions with peripheral restricted diffusion demonstrated an incomplete pattern (p = 0.016); all TMS and a majority of glioma (95%) lesions compared to a smaller proportion of CNSL (65%) lesions with central restricted diffusion had heterogeneous pattern (p = 0.128).
All TMS and a majority of glioma (94%) and CNSL (92%) lesions had hypointense or mixed T1 signals relative to white matter (p = 0.017); the rest of the glioma (6%) and CNSL (8%) lesions had isointense signals. Similarly, all TMS and the majority of glioma (99%) and CNSL (97%) lesions had hyperintense or mixed T2-weighted signals relative to gray matter (p = 0.019); the remaining glioma (1%) and CNSL (3%) lesions were isointense.
CT scans were available for review in 5 of 10 (50%) TMS, 48 of 85 (56%) glioma, and 24 of 38 (63%) CNSL patients. All TMS MRI-enhancing components demonstrated CT hypoattenuation compared to cortical/basal ganglia gray matter, while the majority of glioma (52%) and CNSL (83%) were either isodense or hyperdense.
Neuroradiologic diagnosis
Based on our neuroradiologists' read, 2 cases of TMS were misdiagnosed as malignant tumors, while 5 cases of tumor (1 glioma; 4 CNSL) were misdiagnosed as demyelinating disease. The reasons for the misdiagnosis are detailed in table 3 and figures 2 and 3. In addition, there were 8 cases where CNSL was misdiagnosed as glioma or vice versa.
Table 3.
Reasons for radiologic misdiagnosis
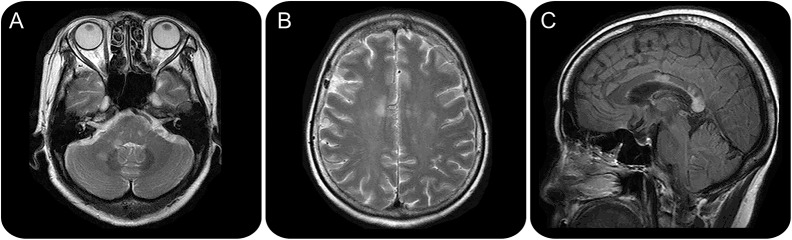
Figure 2. Patient 1 (table 3) with tumefactive multiple sclerosis (TMS) misdiagnosed as glioma.
Axial gradient echo MRI (A) of a left thalamic lesion demonstrates susceptibility, in keeping with intralesional hemorrhage. Postgadolinium axial T1-weighted MRI (B) shows complete rim as well as heterogeneous central enhancement. The location and features of the lesion are unusual for TMS.
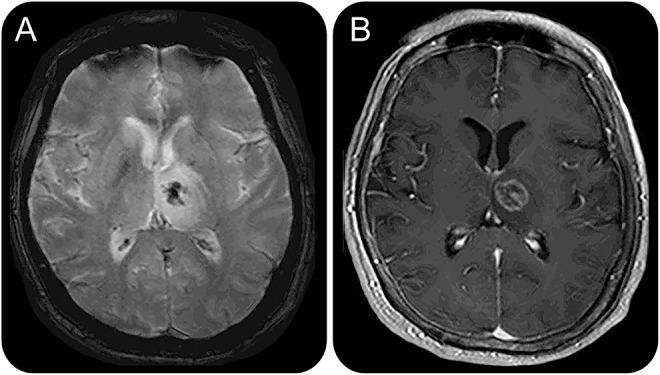
Figure 3. Patient 5 (table 3) with lymphoma misdiagnosed as tumefactive multiple sclerosis (TMS).
Axial T2-weighted (A, B) and sagittal fluid-attenuated inversion recovery (C) MRI show multiple lesions in the right brainstem and cerebellar white matter regions (A), juxtacortical and deep white matter (B), and multiple lesions in the corpus callosum (C). These imaging findings, in addition to the main nonenhancing lesion at the corpus callosum (not shown), are suggestive of an underlying demyelinating disease.
DISCUSSION
Our study demonstrated that TMS lesions were smaller compared to glioma and CNSL, had no or mild mass effect, and were always associated with contrast enhancement. Consistent with existing literature, peripheral rim enhancement in TMS was typically incomplete, and peripheral diffusion restriction incomplete3,5,6; MRI-enhancing components had mixed MRI T2-weighted signal intensity and CT hypoattenuation.6 Patients with TMS were younger and a greater proportion were women. Individual symptoms at presentation did not distinguish TMS from CNS neoplasms. However, our results demonstrated some differing radiographic characteristics from existing literature. In contrast to a study that showed moderate to marked mass effect in approximately 16% of biopsied TMS lesions, none of the TMS lesions in our study showed moderate or severe mass effect.2 Also, we did not evaluate the presence of spinal lesions, which was present in 38% of patients who had MRI spine in their study.2CSF analysis is a useful adjunct in distinguishing TMS from other pathology.
Neuroimaging is essential in the diagnostic process of a tumefactive lesion. Although there are no pathognomonic imaging signs for TMS, certain characteristics may be helpful in distinguishing it from other space-occupying lesions.9 Previous case series and reports of TMS have suggested the use of various magnetic resonance (MR) modalities, including conventional MRI,2,10,11 magnetization transfer imaging,12,13 diffusion tensor imaging,14 and magnetic resonance spectroscopy (MRS),12,15,16 to differentiate TMS from neoplasms. Case-control studies provided more robust data and demonstrated that unenhanced CT6 and MRS17 added value to conventional MRI in distinguishing TMS from neoplasms. A study of a cohort of 64 children also showed that the combination of clinical (abnormal neurologic examination and multifocal neurologic deficits) and radiologic (presence of multiple lesions, absence of cortical involvement, and decrease in lesion size or detection of new lesions on serial imaging) features helped distinguish TMS from tumor.18 However, these clinical and imaging findings do not provide an unambiguous diagnosis. It is important to note unusual features such as hemorrhage can occur in TMS, as in one of our TMS cases, which was misdiagnosed as a glioma (figure 2). The combination of multiple white matter lesions, absence of cortical involvement, and lack of hyopointensity on T2W image was not consistently helpful in distinguishing TMS from tumor, particularly lymphoma, in some of our cases (table 2 and figure 3).
Our study provides additional imaging features to distinguish between these conditions. In addition to routine MRI studies, we recommend CT imaging, particularly in cases with contrast-enhancing lesions. Comparing the CT attenuation of the MR-enhancing component of the lesion with cortical gray matter or basal ganglia may help distinguish TMS from CNS neoplasm; hypoattenuation of the lesion relative to gray matter would make TMS more likely than CNS neoplasm.6
CSF analysis is a useful adjunct in distinguishing TMS from other pathology. The presence of unmatched OCBs in the CSF favors a diagnosis of demyelination over neoplasm. CSF analysis is also important for detecting cytologic evidence of neoplasm or infection.9 Despite limited availability of CSF evaluation in our cohort, CSF cytology was diagnostic in 2 (17%) of 12 CNSL patients, and CSF OCB was positive in 2 (25%) of 8 patients with TMS. Hence we advocate CSF analysis in the initial evaluation of patients who present with tumefactive lesions.
The diagnosis of tumefactive lesions is challenging but distinguishing among demyelinating, neoplastic, or other etiologies is imperative for definitive treatment. In clinical practice, patients without a preexisting diagnosis of MS, with inconclusive or suspicious imaging, with negative CSF OCBs, and in whom a diagnosis of MS would be unusual will require biopsy for diagnosis.9 Tumefactive lesions highly suspicious for CNS neoplasms are also often biopsied for histologic confirmation before definitive treatment is given. However, brain biopsies are not without risks and furthermore, the results can sometimes be inconclusive or even misleading. Hence, in patients who are presumptively diagnosed with TMS, a trial of pulsed corticosteroids with surveillance imaging is sometimes given, which may potentially delay treatment of a neoplasm.
In the current assessment of patients with brain tumefactive lesions, the decision for biopsy vs a trial of immunotherapy is dependent on the clinical judgment of neurologists, neurosurgeons, and neuroradiologists. In our cohort, 4% of glioma and CNSL cases could have been misdiagnosed as TMS, and would not have undergone biopsy. Although relying on the clinical impression of the radiologists appears to be reasonably reliable, it is highly dependent on the radiologists' experience and is subject to error. The imaging characteristics identified in our study hence provide neuroradiologists with more specific imaging features that can potentially improve radiologic diagnostic accuracy and facilitate clinicians in their decisions to recommend brain biopsy or proceed with immunotherapy.
Clarifying the clinicoradiologic differences of TMS from brain tumors would aid multidisciplinary team discussions involving neurology, neurosurgery, neuroradiology, and neuropathology, which most academic medical centers currently use to make diagnostic and therapeutic decisions in such complex cases. Our small TMS sample size made it difficult to design a reliable clinicoradiologic algorithm using clinical and radiologic parameters from conventional MRI to accurately distinguish TMS from gliomas or CNSL in patients presenting with tumefactive lesions.
Our study has several limitations. First, pathologic confirmation was lacking in 5 (50%) of 10 patients with TMS. However, clinical and laboratory data as well as follow-up of at least 2 years supported an inflammatory rather than neoplastic etiology. Second, given the retrospective nature of our study, a considerable amount of laboratory data, especially in the glioma and CNSL groups, were unavailable. Nonetheless, a few glioma and CNSL patients had positive autoimmune markers, including ANA and anti-Ro antibody, demonstrating that the presence of autoantibodies does not exclude malignancies, and should not preclude biopsy. Third, our results cannot be generalized to brain tumors other than gliomas and CNSL. However, this comparison is the most clinically relevant as gliomas and CNSL are most commonly indistinguishable from TMS. In addition, gliomas are the most common primary malignant brain tumors and CNSL are highly treatable. Fourth, variability in scan parameters and resolution were unavoidable as a proportion of the MRI scans were performed at other institutions and imported into our PACS. However, it was important to assess the first MRI scan after presentation as it would most likely influence the initial management decisions. Finally, our study did not examine advanced imaging modalities such as diffusion tensor imaging, perfusion studies, angiography, and MRS, which have been reported to be useful in distinguishing TMS from other etiologies.5,14,16,19–24 However, we wanted to focus on studying the use of clinical and radiologic parameters from conventional MRI as the advanced imaging modalities are not readily available outside academic or tertiary hospitals, which also do not have the relevant expertise for interpretation of these scans.
In patients presenting with tumefactive lesions, radiologic features such as relatively smaller size lesions with no or mild mass effect, incomplete peripheral rim enhancement, incomplete peripheral diffusion restriction, mixed MRI T2W signal intensity, and CT hypoattenuation are useful in differentiating TMS from CNS neoplasms. However, brain biopsy may still be necessary in cases of diagnostic uncertainty. Future prospective collaborative studies with larger cohorts of patients with TMS would be useful to determine the radiologic differences of TMS lesions from brain tumors and may aid in the development of a clinicoradiologic diagnostic algorithm.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Wai-Hoe Ng and Dr. Tchoyoson Lim for providing their databases, Dr. Boon-Ping Toe and Dr. Shih Zhu Yiin for screening cases for radiologic inclusion criteria, and Dr. Hong-Choon Oh for designing the data collection form.
Footnotes
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
X. Lin: participated in design of the study, acquisition and analysis of the data, and drafting and revision of the manuscript. W-Y. Yu: participated in design of the study, acquisition and analysis of the data, and revision of the manuscript. L. Liauw: participated in design of the study, acquisition and analysis of the data, and revision of the manuscript. R.J. Chander: participated in design of the study, statistical analysis of the data, and revision of the manuscript. W.E. Soon: participated in design of the study, acquisition and analysis of the data, and revision of the manuscript. H.Y. Lee: participated in acquisition and analysis of the data and revision of the manuscript. K. Tan: participated in design and supervision of the study, acquisition and analysis of the data, and revision of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
X. Lin reports no disclosures. W.-Y. Yu received a speaker honorarium from Medlab Asia, Singapore. L. Liauw, R.J. Chander, W.E. Soon, and H.Y. Lee report no disclosures. K. Tan has served on scientific advisory boards for UCB, Novartis, and Biogen Idec. and has received funding for travel from UCB, Novartis, Merck Serono, and Biogen Idec. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Hayashi T, Kumabe T, Jokura H, et al. Inflammatory demyelinating disease mimicking malignant glioma. J Nucl Med 2003;44:565–569. [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008;131:1759–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masdeu JC, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology 2000;54:1427–1433. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz KM, Erickson BJ, Lucchinetti C. Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology 2006;48:143–149. [DOI] [PubMed] [Google Scholar]
- 5.Abou Zeid N, Pirko I, Erickson B, et al. Diffusion-weighted imaging characteristics of biopsy-proven demyelinating brain lesions. Neurology 2012;78:1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DS, Na DG, Kim KH, et al. Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology 2009;251:467–475. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol 2011;101:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy TA, Chataway J. Tumefactive demyelination: an approach to diagnosis and management. J Neurol Neurosurg Psychiatry 2013;84:1047–1053. [DOI] [PubMed] [Google Scholar]
- 10.Tsui EY, Leung WH, Chan JH, Cheung YK, Ng SH. Tumefactive demyelinating lesions by combined perfusion-weighted and diffusion weighted imaging. Comput Med Imaging Graph 2002;26:343–346. [DOI] [PubMed] [Google Scholar]
- 11.Altintas A, Petek B, Isik N, et al. Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler 2012;18:1448–1453. [DOI] [PubMed] [Google Scholar]
- 12.Enzinger C, Strasser-Fuchs S, Ropele S, Kapeller P, Kleinert R, Fazekas F. Tumefactive demyelinating lesions: conventional and advanced magnetic resonance imaging. Mult Scler 2005;11:135–139. [DOI] [PubMed] [Google Scholar]
- 13.Metafratzi Z, Argyropoulou MI, Tzoufi M, Papadopoulou Z, Efremidis SC. Conventional MRI and magnetisation transfer imaging of tumour-like multiple sclerosis in a child. Neuroradiology 2002;44:97–99. [DOI] [PubMed] [Google Scholar]
- 14.Miron S, Tal S, Achiron A. Diffusion tensor imaging analysis of tumefactive giant brain lesions in multiple sclerosis. J Neuroimaging 2013;23:453–459. [DOI] [PubMed] [Google Scholar]
- 15.Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol 2007;28:272–277. [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra HS, Jain KK, Agarwal A, et al. Characterization of tumefactive demyelinating lesions using MR imaging and in-vivo proton MR spectroscopy. Mult Scler 2009;15:193–203. [DOI] [PubMed] [Google Scholar]
- 17.Lu SS, Kim SJ, Kim HS, et al. Utility of proton MR spectroscopy for differentiating typical and atypical primary central nervous system lymphomas from tumefactive demyelinating lesions. AJNR Am J Neuroradiol 2014;35:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yiu EM, Laughlin S, Verhey LH, et al. Clinical and magnetic resonance imaging (MRI) distinctions between tumefactive demyelination and brain tumors in children. J Child Neurol 2014;29:654–665. [DOI] [PubMed] [Google Scholar]
- 19.Adamek D, Radwanska E, Rog T, Grzywna E, Herman-Sucharska I. Tumefactive demyelinating lesion: trying to find unity in diversity: comparison of two cases. Clin Neurol Neurosurg 2014;116:90–92. [DOI] [PubMed] [Google Scholar]
- 20.Sinha MK, Garg RK, Bhatt ML, Chandra A. Tumefactive demyelinating lesion: experience with two unusual patients. J Postgrad Med 2010;56:146–149. [DOI] [PubMed] [Google Scholar]
- 21.Hyland M, Bermel RA, Cohen JA. Restricted diffusion preceding gadolinium enhancement in large or tumefactive demyelinating lesions. Neurol Clin Pract 2013;3:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini J, Chatterjee S, Thomas B, Kesavadas C. Conventional and advanced magnetic resonance imaging in tumefactive demyelination. Acta Radiol 2011;52:1159–1168. [DOI] [PubMed] [Google Scholar]
- 23.Kiriyama T, Kataoka H, Taoka T, et al. Characteristic neuroimaging in patients with tumefactive demyelinating lesions exceeding 30 mm. J Neuroimaging 2011;21:e69–e77. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita S, Kimura E, Hirano T, Uchino M. Tumefactive multiple sclerosis. Intern Med 2009;48:1113–1114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.