Abstract
Human parainfluenza virus type 3 (HPIV3) causes bronchiolitis, pneumonia, and croup in newborns and infants. Several studies have implicated intercellular adhesion molecule-1 (ICAM-1) in inflammation during infection by viruses. In this study, we investigated the potential for HPIV3 to induce ICAM-1 in HT1080 cells. FACS analysis showed that HPIV3 strongly induced ICAM-1 expression in these cells. The ICAM-1 induction was significantly reduced when the virions were UV inactivated prior to infection, indicating that ICAM-1 induction was mostly viral replication dependent. Culture supernatant of HPIV3-infected cells induced ICAM-1 at an extremely low level, indicating that virus-induced cytokines played only a minor role in the induction process. Consistent with this, potent inducers of ICAM-1 such as IFN-γ, TGF-β, and TNF-α were absent in the culture supernatant, but a significant amount of IFN type I was present. By using U2A cells, which are defective in IFN type I signaling, we confirmed that ICAM-1 induction by HPIV3 occurred in a JAK/STAT signaling-independent manner. These data strongly indicate that HPIV3 induces ICAM-1 directly by viral antigens in a cytokine-independent manner; this induction may play a role in the inflammation during HPIV3 infection.
Keywords: Human parainfluenza virus type 3 (HPIV3), Intercellular adhesion molecule-1 (ICAM-1), Inflammation
HUMAN parainfluenza virus type 3 (HPIV3), a paramyxovirus, can cause serious respiratory infections in newborns and young children including bronchiolitis, pneumonia, and croup. HPIV3 infection is associated with severe damage to the lung epithelium associated with the inflammatory and immune responses (8,16,17). Many acute viral infections, including airway infections with respiratory viruses, are characterized by early infiltration of infected tissues with inflammatory response cells such as polymorpho-nucleocytes and lymphocytes. These inflammation processes can cause cytotoxic tissue injury by releasing products stored in secretory granules as well as toxic free radicals (10,26,27).
ICAM-1, a member of the immunoglobulin supergene family, is an adhesive ligand for neutrophil and lymphocyte-associated integrins. The counterreceptors for ICAM-1 are the integrin lymphocyte function-associated antigen-1 (LFA-1), which is expressed on lymphocytes and monocytes, and the integrin Mac-1, which is expressed on polymorphonuclear cells. The interaction between ICAM-1 and LFA-1 and/or Mac-1 regulates in part the tight adhesion and transendothelial migration that leads to leukocyte infiltration into an area of inflammation. Thus, changes in the expression of adhesion molecules on circulating leukocytes are clearly important in controlling the formation of cellular exudate at sites of inflammation. Increased levels of ICAM-1 have been reported in a number of diseases, including infections, autoimmune diseases, cancer, and inflammatory reactions (6,10,22).
ICAM-1 is constitutively expressed on a wide variety of cell types and is upregulated in response to a variety of inflammatory mediators, including proinflammatory cytokines, hormones, cellular stresses, and virus infection (19). Moreover, ICAM-I has been shown to play an important role in viral infection. The ICAM family has been reported as a mediator for HIV-mediated syncytia formation (4,5). The mAb directed against ICAM-1, −2, and −3 to block syncytia formation both in PHA-activated lymphocytes infected in vitro with primary or laboratory strains of HIV and in a T-cell line that expresses HIV envelope and that is cocultured with PHA-activated lymphocytes. In HIV-infected individuals, the emergence of viral isolates with a greater capacity to induce syncytia formation in vitro is associated with rapid disease progression (4,5,21). ICAM-1 also has been reported to be a receptor for the major group of human rhinoviruses (2). Upregulation of ICAM-I on HeLa cells is associated with increased binding and susceptibility of the cells to major group rhinoviruses (2). Receptor blockade, in which anti-CAM-1 antibody is used to block endogenous ICAM-1, can significantly reduce the level of both reference stereotypes and clinical isolates of rhinovirus (11). In addition to the rhinoviruses, paramyxovirus family and other respiratory viruses also induce ICAM-1 expression (15). Respiratory syncytial virus (RSV) increases expression of ICAM-1 on a human alveolar epithelial cell line, A 549, by inducing the production of IL-1α, in an autocrine effect (18). Measles virus and parainfluenza virus 2 directly induce ICAM-1 through viral gene products(s) in human umbilical vein endothelial cells and tracheal epithelial cells, respectively (9,26). However, the mechanism whereby these viruses induce ICAM-1 expression remains unknown.
In a previous study, we reported that HPIV3 upregulates the expression of both MHC class I and class II on respiratory epithelial cells and human epithelium-like cells using a pathway independent of both STAT1 and CIITA (8). In this study, we investigated whether HPIV3 induces ICAM-1 expression on human epithelium-like HT1080 cells. Our data indicate that HPIV3 induces ICAM-1 directly in a cytokine-independent manner. This HPIV3-induced and cytokine-independent ICAM-1 expression may play a role in the virus-related inflammatory process.
MATERIALS AND METHODS
Biological Reagents
Human recombinant interferon-β (rIFN-β) was purchased from Biosource International (Camarillo, CA). Human recombinant interferon-γ (IFN-β) and human tumor necrosis factor-α (TNF-α) were purchased from Boehringer Mannheim (Indianapolis, IN).
Cell Lines and Culture Conditions
CV-1 (African green monkey kidney) cells were used for growing the virus and for plaque assays. HT1080 (ATCC CCL 121) is a fibrosarcoma cell line. U2A, a P48-defective cell line derived from parental HT1080 cell lines, was a gift from George Stark (Department of Molecular Biology, Lerner Research Institute, The Cleveland Clinic Foundation). All the cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 1% l-glutamine, 1% penicillin/streptomycin, and 10% fetal bovine serum.
Virus Stock and Infection
The HPIV3 viral stock HA-1 (NIH catalog No. 47784) was grown in the CV-1 cell line. The virions released in the culture medium were purified by centrifugation at 10,000 × g to remove cell debris followed by ultracentrifugation at 100,0000 × g for 2 h at 4°C using an SW 50.1 rotor, as described previously (1). The purified virus pellet was suspended in DMEM medium. IFNs were assayed in the purified virus pool by antivirus bioassay for IFN-I and enzyme-linked immunosorbent assay for IFN-γ. Virus titer was determined by plaque assay, and virus was aliquoted at a concentration of 108 PFU/ml. For some experiments, virus particles were inactivated with ultraviolet light as previously reported (8). HT1080 cells were infected with HPIV3 at 1.0 MOI in the same medium as used for growing the cells. The culture supernatants and cells were harvested at 48 h after infection for further experiments, as described below.
Flow Cytometry
The HT1080 cells were plated at 5 × 105 cells/well in 12-well plates. After 12 h, the cells were either infected with HPIV3 or treated with supernatant from UV-irradiated cultures of infected cells. After 48 h, the cells were harvested for ICAM-1 assay. The antibody used for staining ICAM-1 (CD54) antigens was murine mAb LB-2 conjugated directly to phycoerythrin (PE) (Becton Dickinson). Nonspecific background staining was determined using a control PE-conjugated isotype-matched Ig (Becton Dickinson). Cells were inoculated with the antibodies in a reaction mixture containing 1× PBS, 1% BSA, and 0.01% sodium azide for 30 min at room temperature. Flow cytometry was performed on a Becton-Dickinson FAC Scan (San Jose, CA) using Cyclops software (Cytomation, Fort Collins, CO). About 105 cells were analyzed for each sample.
Cytokine Assays
IFN-γ and TNF-α were assayed by ELISA (R&D Systems, Minneapolis, MN). IFN-I-mediated antiviral activity was determined for their ability to inhibit vesicular stomatitis virus-induced cytopathic effect on WISH cells, as described previously (8). Briefly, HT1080 cells were infected with HPIV3 at 1.0 MOI at 37°C and the culture supernatant was collected 48 h afterwards. The culture supernatant was UV irradiated to inactivate virions and was used to measure antiviral activity. The culture supernatant of uninfected cells served as the control. The assay was standardized with reference IFN of known activity. Cell viability was determined by staining with neutral red in PBS, eluting in 50% ethanol in 0.1 M NaH2PO4, and measuring the absorbance at OD 540 nm. The results are presented as percent protection calculated as: (A540 of the sample) − (A540 of the virus control)/(A540 of the cell control) − (A540 of the virus control) × 100.
RNase Protection Assays
Total RNA was isolated from cells using the RNA STAT-60 according to the manufacturer’s specifications (Tel-Test, Inc., TX). RNase protection assay was performed by using probes for TGF-β and GADPH, provided in the RiboQuanti™ Multi-Probe RNase Protection Assay (RPA) system (Pharmingen, San Diego, CA).
RESULTS
Induction of ICAM-1 Expression by HPIV3 in HT1080 Cells
We previously reported that HPIV-3 induces both MHC class I and MHC class II in human lung epithelial cells A549 and human fibrosarcoma cells HT1080 (8). In the case of MHC class II, we found that the induction through HPIV3 occurred directly by viral gene products in a STAT1- and CIITA-independent manner. The MHC class I induction, on the other hand, was mediated by viral antigens as well as endogenous IFN type I. Because ICAM-1, like MHC class I and class II, is involved in T-cell activation and antiviral immune response (6,10,12,27,28), we investigated whether ICAM-1 expression is induced in HPIV3-infected cells. HT1080 cells were infected with HPIV3 at 1.0 MOI; after 48 h the cells were harvested and ICAM-1 levels in these cells and in mock-infected cells were measured by FACS analysis using anti-ICAM-1 monoclonal antibody. As shown in Figure 1, a basal level of ICAM-1 expression measured as mean fluorescence intensity (MFI) was detected in mock-infected cells. In HPIV3-infected cells, MFI was about 3.8-fold higher. To determine whether ICAM-1 induction was dependent on viral replication, we then examined the production of infectious virions in these cells. The cells were infected with HPIV3 at an MOI of 1.0, and at 48 h postinfection progeny virions released into the medium were measured by plaque assay. About 1 × 106 to 1 × 107 PFU/ml of infectious virus particles were produced, indicating efficient replication of HPIV3 in these cells (Table 1). Next, we UV inactivated the virions and, after confirming the absence of infectious particles, used them to infect HT1080 cells. At 48 h postinfection, the cells were harvested and ICAM-1 expression was measured. As shown in Figure 1, UV-inactivated virions also induced ICAM-1 expression, but at a low level (1.7-fold) compared to mock-infected cells (Fig. 1). These data indicate that the induction of ICAM-1 by HPIV3 occurs in large part by viral antigens in a replication-dependent manner. Virus-induced cytokines may play an additional role in the induction process.
Figure 1.
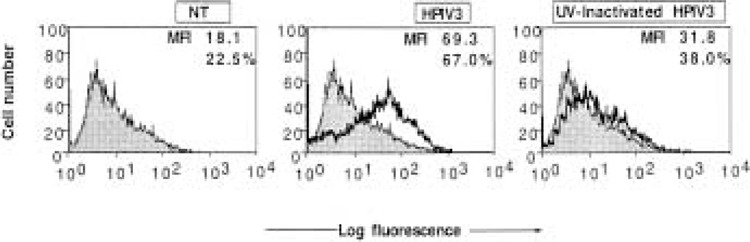
Flow cytometric analysis of ICAM-1 expression on HT1080 cells infected with either HPIV3 or UV-inactivated HPIV3. HT1080 cells (5 × 105 cells/ml) were infected with HPIV3 or UV-inactivate HPIV3 (1.0 MOI) and harvested at 48 h postinfection for ICAM-1 FACS assay. In each panel, the mean fluorescence intensity (MFI) and the percentage of cells staining for ICAM-1 are indicated. Results are representative of three independent assays.
TABLE 1.
PRODUCTION OF INFECTIOUS HPIV3 VIRIONS
| Time (h) | Viral Yield (PFU/ml) |
|---|---|
| 48 | 1 × 106–107 |
HT1080 cells (5 × 105/ml) were infected with HPIV3 at 1.0 MOI and after 48 h postinfection the progeny virions released into the medium were tested for their ability to produce plaque on a monolayer of CV-1 cells.
Role of Endogenous IFN-I in the HPIV3-Induced Expression of ICAM-1
Potential mechanisms underlying the inflammatory response during virus infection include synthesis of cytokines and other inflammatory mediators. Cell adhesion molecules including ICAM-1 are required for migration of inflammatory cells. In many cell types ICAM-1 expression is upregulated by proinflammatory cytokines such as TNF-α, IFN-γ, and IL-1, which are expressed in a variety of cells after exposure to viruses. Previous studies have showed that RVS and rhinovirus induced the expression of ICAM-1 in an autocrine fashion by inducing TNF-α, IFN-γ, and IL-1 (1,20,23,25,29). However, the induction of cytokines and consequently ICAM-1 expression varies with cell type and stimulus.
To investigate the role of virus-induced cytokines in the induction of ICAM-1 expression, we transferred the infected cell culture supernatant to a fresh monolayer of cells. After 48 h, the cells were harvested and ICAM-1 was measured by FACS assay. As shown in Figure 4, low levels of ICAM-1 were induced on fresh monolayer of cells by the infected cell culture supernatant. To investigate this further, we studied the induction of ICAM-1 by exogenous IFN-γ and other potent inducers. As shown in Figure 1, exogenous cytokines such as IFN-γ and TNF-α can induce significant ICAM-1 expression , whereas IFN-β is a poor inducer. Other researchers have also reported that TGF-β can induce significant ICAM-1 expression in these cells (24). Therefore, we investigated whether these cytokines were potential mediators of ICAM-1 expression induced by HPIV3 in HT1080 cells. First, we investigated the induction of these cytokines by HPIV3 in the culture supernatant of HT1080 cells. The culture supernatant was harvested at 48 h postinfection and tested by ELISA for the presence of IFN-γ and TNF-α. As shown in Table 2, IFN-γ and TNF-α were not detectable in control and HPIV3-infected HT1080 cell culture supernatant, but TNF-α was detected in infected human tracheal epithelial cells (about 100 U/ml), indicating that expression is cell type specific (data not shown). RNase protection assay detected no significant induction of TGF-β mRNA after HPIV3 infection (Fig. 3). Even though, we did not detect any level of IFN-γ, TNF-α, and TGF-β, which are important proinflammatory factors that induce ICAM-1, we did discover endogenous IFN-I in the culture supernatant of HPIV3-infected HT1080 cells. Using antivirus bioassay, we found that cells infected with HPIV3 (1.0 MOI) contained about 200–300 U/ml of IFN type I in the culture supernatant at 48 h postinfection (Table 2). These data demonstrate that HPIV3 infection induces HT1080 cells to secret IFN type I but not other proinflammatory cytokines. This IFN type I apparently contributes to the ICAM-1 induction by the infected cell culture supernatant.
Figure 4.
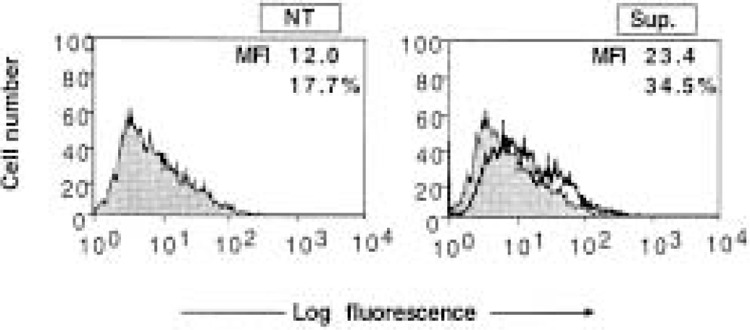
Flow cytometric analysis for ICAM-1 expression on HT1080 cells treated with the culture supernatant from HPIV3-infected cells. The culture supernatants from HPIV3-infected HT1080 cells and uninfected cells harvested at 48 h postinfection were inactivated by UV and transferred (20% volume) to fresh monolayers of HT1080 cells. The cells (5 × 105 cells/ml) were harvested after 48-h treatment for ICAM-1 FACS assay. In each panel, the mean fluorescence intensity (MFI) and the percentage of cells staining for ICAM-1 are indicated. The results are expressed as MFI expression levels and are representative of three independent assays.
TABLE 2.
CYTOKINE PRODUCTION IN THE CULTURE SUPERNATANT FROM HPIV3 INFECTIVE CELLS
| Cytokines | Unit/ml | |
|---|---|---|
| −HPIV3 | +HPIV3 | |
| IFN-γ | 0 | 0 |
| TNF-α | 0 | 0 |
| IFN-I | 0 | 200–300 |
HT1080 cells (5 × 105/ml) were infected with/without HPIV3 at 1.0 MOI and after 48-h postinfection the culture supernatants were collected for cytokine assay. IFN-γ and TNF-α were assayed by ELISA. IFN-I was assayed by antiviral bioassay.
Figure 3.

HPIV3 did not induce TGF-α mRNA expression on HT1080 cells. HT1080 cells were either untreated (left) or infected with HPIV3 (1.0 MOI) and assayed at 48 h. RNA was analyzed by RNase protection assays for TGF-β and GADPH.
ICAM-1 Induction by HPIV3 in Cells Defective in IFN Type I Signaling
The above results indicate that HPIV3 induces ICAM-1 expression, in large part, through viral antigens. The virus induced about 200-300 U/ml of IFN type I, raising the question of whether this endogenous IFN type I played a role in the induction process. To investigate this further, we used U2A (7,13,14) cells, which are defective in P48 and thereby defective in autocrine and paracrine function of endogenous IFN-I. As shown in Figure 5, HPIV3 induced ICAM-1 expression efficiently in U2A cells compared with parental cells. HPIV3 induces ICAM-1 on U2A cells at 3.8-fold, which is about the same level of induction observed in HPIV3-infected HT1080 cells (Fig. 1). Together, these data are consistent with our hypothesis that ICAM-1 expression by HPIV3 is viral antigen specific and occurs in a cytokine-independent manner.
Figure 5.
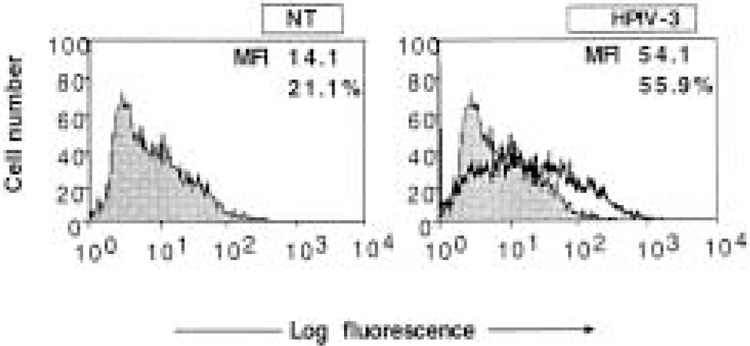
Flow cytometric analysis of ICAM-1 expression on U2A cells infected with HPIV3. U2A cells (5 × 105 cells/ml) were left uninfected or infected with HPIV3 (1.0 MOI), and at 48 h postinfection were assayed with FACS assay. Untreated cells served as the control. In each panel, the mean fluorescence intensity (MFI) and the percentage of cells staining for ICAM-1 are indicated. Results are representative of two independent assays.
DISCUSSION
In this study we investigated the induction of ICAM-1 expression in HT1080 cells. These are epithelial-like cells derived from human fibrosarcoma and originally subcultivated under conditions that eliminate the growth of fibroblasts and favor that of epithelial cells (1). We chose this cell line not only because it supports HPIV3 replication, but also be cause it has extensively been used to characterize the IFN-signaling pathway (7,12–14). In a previous study, we reported that MHC class I and class II molecules were induced by HPIV3 on HT1080 cells in a STAT1- and CIITA-independent manner (8). Virus-induced IFN type I played an additional role in the induction of MHC class I but not MHC class II (8). Because ICAM-1 is an important cell surface molecule and is induced by viruses (27,28), we investigated its induction by HPIV3. Infectious HPIV3 virions induced ICAM-1 by about 3.8-fold (Fig.1), where UV-inactivated virions induced ICAM-1 expression by only 1.7-fold (Fig.1). Culture supernatant from infected cells similarly increased ICAM-1 level by about 1.9-fold similar to the level induced by 100 U/ml of IFN-β (Fig. 2). The culture supernatant from HPIV3 infected cells contained about 100-300 U/ml of IFN-I, as assessed by antivirus bioassay, which may contribute to the ICAM-1 induction. Potent inducers of ICAM-1, such as IFN-γ, TNF-α, and TGF-β were not detected in HT1080 cells. Our data indicate that IFN-β can also induce ICAM-1, albeit at a low level (Fig. 2). By using cells lacking P48 and thereby defective in the autocrine and paracrine function of endogenous IFN-I, we found that HPIV3 can induce ICAM-1 in an IFN signaling-independent manner.
Figure 2.
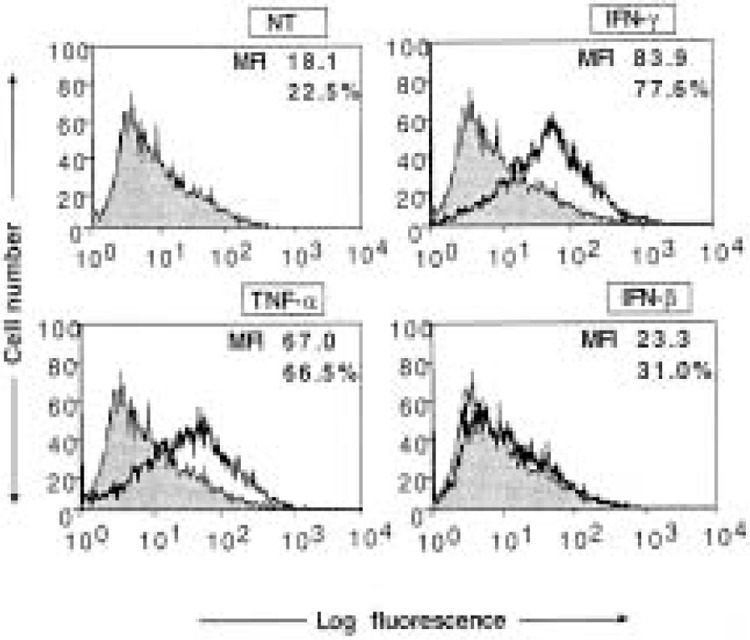
Flow cytometric analysis for ICAM-1 expression on HT1080 cells treated with IFN-γ, TNF-α, or IFN-β. HT1080 cells (5 × 105 cells/ml) were either untreated or treated with IFN-γ (100 U/ml), TNF-α (100 U/ml), or IFN-β (100 U/ml) and were harvested at 48-h culture for ICAM-1 assay. In each panel, the mean fluorescence intensity (MFI) and the percentage of cells staining for ICAM-1 are indicated. Results are representative of three independent assays.
Like HPIV3, several viruses have been shown to induce ICAM-1. Examples include cytomegalovirus-mediated induction of ICAM-1 on endothelial cells (3), measles virus-mediated induction on human umbilical vein endothelial cells (9), respiratory syncytial virus-mediated induction on human nasal epithelial cells (15), and parainfluenza virus type 2-mediated induction on human epithelial cells (26). In all these cases, induction of ICAM-1 expression was shown to be mediated by viral antigens directly in a cytokine-independent manner. In the case of respiratory syncytial virus, the viral envelope glycoproteins appear to be involved in the induction process. Moreover, induced expression of ICAM-1 is associated with increased adhesive activity of respiratory syncytial virus-infected nasal epithelial cells (15,18). In the case of HIV, the gp41 glycoprotein was shown to be involved in the ICAM-1 induction, and an elevated level of circulating ICAM-1 is observed in infected cells (4,6,21,22). Thus, it remains to be determined which protein or proteins of HPIV3 are involved in the ICAM-1 induction, and what role they play in the virus infection process.
ACKNOWLEDGMENTS
We thank George R. Stark for providing IFN signaling mutant cell lines, Amy Raber for FAC Scan analysis, and Yoshihiro Ohmor for discussion and valuable comments during this work. We thank Jessica Ancker for critical reading of the manuscript. This work was supported in part by United States Public Health Services grant AI3207 (A.K.B.).
REFERENCES
- 1. Becker S.; Koren H. S.; Henke D. C. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1, and interleukin-6. Am. J. Respir. Cell Mol. Biol. 8:20–27; 1993. [DOI] [PubMed] [Google Scholar]
- 2. Bella J.; Rossmann M. G. Review: Rhinoviruses and their ICAM receptors. J. Struct. Biol. 128:69–74; 1999. [DOI] [PubMed] [Google Scholar]
- 3. Burns L. J.; Pooley J. C.; Walsh D. J.; Vercellotti G. M.; Weber M. L.; Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation 67:137–144; 1999. [DOI] [PubMed] [Google Scholar]
- 4. Butini L.; De Fougerolles A. R.; Vaccarezza M.; Graziosi C.; Cohen D. I.; Montroni M.; Springer T. A.; Pantaleo G.; Fauci A. S. Intercellular adhesionated syncytia formation. Eur. J. Immunol. 24:2191–2195; 1994. [DOI] [PubMed] [Google Scholar]
- 5. Chen Y. H.; Bock G.; Vornhagen R.; Steindl F.; Katinger H.; Dierich M. P. HIV-1 gp41 enhances major histocompatibility complex class I and ICAM-1 expression on H9 and U937 cells. Int. Arch. Allergy Immunol. 104:227–231; 1994. [DOI] [PubMed] [Google Scholar]
- 6. Christensen J. P.; Johansen J.; Marker O.; Thomsen A. R. Circulating intercellular adhesion molecule-1 (ICAM-1) as an early and sensitive marker for virus-induced T cell activation. Clin. Exp. Immunol. 102: 268–273; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darnell J. E. Jr.; Kerr I. M.; Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421; 1994. [DOI] [PubMed] [Google Scholar]
- 8. Gao J.; De B. P.; Banerjee A. K. Human parainfluenza virus type 3 up-regulates major histocompatibility complex class I and II expression on respiratory epithelial cells: Involvement of a STAT1- and CIITA-independent pathway. J. Virol. 73:1411–1418; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harcourt B. H.; Rota P. A.; Hummel K. B.; Bellini W. J.; Offermann M. K. Induction of intercellular adhesion molecule 1 gene expression by measles virus in human umbilical vein endothelial cells. J. Med. Virol. 57:9–16; 1999. [DOI] [PubMed] [Google Scholar]
- 10. Holtzman M. J.; Look D. C.; Sampath D.; Castro M.; Koga T.; Walter M. J. Control of epithelial immune-response genes and implications for airway immunity and inflammation. Proc. Assoc. Am. Phys. 110: 1–11; 1998. [PubMed] [Google Scholar]
- 11. Huguenel E. D.; Cohn D.; Dockum D. P.; Greve J. M.; Fournel M. A.; Hammond L.; Irwin R.; Mahoney J.; McClelland A.; Muchmore E.; Ohlin A. C.; Scuderi P. Prevention of rhinovirus infection in chimpanzees by soluble intercellular adhesion molecule-1. Am. J. Respir. Crit. Care Med. 155:1206–1210; 1997. [DOI] [PubMed] [Google Scholar]
- 12. Kumar A.; Commane M.; Flickinger T. W.; Horvath C. M.; Stark G. R. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278:1630–1632; 1997. [DOI] [PubMed] [Google Scholar]
- 13. Leonard W. J.; O’Shea J. J. Jaks and STATs: Biological implications. Annu. Rev. Immunol. 16:293–322; 1998. [DOI] [PubMed] [Google Scholar]
- 14. Majumder S.; Zhou L. Z.; Chaturvedi P.; Babcock G.; Aras S.; Ransohoff R. M. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J. Immunol. 161:4736–4744; 1998. [PubMed] [Google Scholar]
- 15. Matsuzaki Z.; Okamoto Y.; Sarashina N.; Ito E.; Togawa K.; Saito I. Induction of intercellular adhesion molecule-1 in human nasal epithelial cells during respiratory syncytial virus infection. Immunology 88: 565–568; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moscona A.; Galinski M. S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J. Virol. 64:3212–3218; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakabayashi T.; Letterio J. J.; Geiser A. G.; Kong L.; Ogawa N.; Zhao W.; Koike T.; Fernandes G.; Dang H.; Talal N. Up-regulation of cytokine mRNA, adhesion molecule proteins, and MHC class II proteins in salivary glands of TGF-beta1 knockout mice: MHC class II is a factor in the pathogenesis of TGF-beta1 knockout mice. J. Immunol. 158:5527–5535; 1997. [PubMed] [Google Scholar]
- 18. Patel J. A.; Kunimoto M.; Sim T. C.; Garofalo R.; Eliott T.; Baron S.; Ruuskanen O.; Chonmaitree T.; Ogra P. L.; Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13:602–609; 1995. [DOI] [PubMed] [Google Scholar]
- 19. Roebuck K. A.; Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 66:876–888; 1999. [DOI] [PubMed] [Google Scholar]
- 20. Rothlein R.; Czajkowski M.; O’Neill M. M.; Marlin S. D.; Mainolfi E.; Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J. Immunol. 141:1665–1669; 1988. [PubMed] [Google Scholar]
- 21. Scheglovitova O.; Scanio V.; Fais S.; Papadia, S.; Abbate I.; Castilletti C.; Dianzani F.; Capobianchi M. R. Antibody to ICAM-1 mediates enhancement of HIV-1 infection of human endothelial cells. Arch. Virol. 140:951–958; 1995. [DOI] [PubMed] [Google Scholar]
- 22. Springer T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827–872; 1995. [DOI] [PubMed] [Google Scholar]
- 23. Subauste M. C.; Jacoby D. B.; Richards S. M.; Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J. Clin. Invest. 96:549–557; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki Y.; Tanigaki T.; Heimer D.; Wang W.; Ross W. G.; Murphy G. A.; Sakai A.; Sussman H. H.; Vu T. H.; Raffin T. A. TGF-beta 1 causes increased endothelial ICAM-1 expression and lung injury. J. Appl. Physiol. 77:1281–1287; 1994. [DOI] [PubMed] [Google Scholar]
- 25. Terajima M.; Yamaya M.; Sekizawa K.; Okinaga S.; Suzuki T.; Yamada N.; Nakayama K.; Ohrui T.; Oshima T.; Numazaki Y.; Sasaki H. Rhinovirus infection of primary cultures of human tracheal epithelium: Role of ICAM-1 and IL-1beta. Am. J. Physiol. 273:L749–759; 1997. [DOI] [PubMed] [Google Scholar]
- 26. Tosi M. F.; Stark J. M.; Hamedani A.; Smith C. W.; Gruenert D. C.; Huang Y. T. Intercellular adhesion molecule-1 (ICAM-1)-dependent and ICAM-1-independent adhesive interactions between polymorphonuclear leukocytes and human airway epithelial cells infected with parainfluenza virus type 2. J. Immunol. 149:3345–3349; 1992. [PubMed] [Google Scholar]
- 27. Tosi M. F.; Stark J. M.; Smith C. W.; Hamedani A. D.; Gruenert C.; Infeld M. D. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: Effects on neutrophil-epithelial cell adhesion. Am. J. Respir. Cell Mol. Biol. 7:214–221; 1992. [DOI] [PubMed] [Google Scholar]
- 28. Wilson I. A.; Stanfield R. L.; Jewell D. A.; Ghiara J. B.; Fremont D. H.; Stura E. A. Immune recognition of viral antigens. Infect. Agents Dis. 3:155–162; 1994. [PubMed] [Google Scholar]
- 29. Yamaya M.; Sekizawa K.; Suzuki T.; Yamada N.; Furukawa M.; Ishizuka S.; Nakayama K.; Terajima M.; Numazaki Y.; Sasaki H. Infection of human respiratory submucosal glands with rhinovirus: Effects on cytokine and ICAM-1 production. Am. J. Physiol. 277: L362–371; 1999. [DOI] [PubMed] [Google Scholar]


