Abstract
Mitochondrial cytochrome c release in response to pro-apoptotic signals leads to the formation of a cytochrome c/Apaf-1/procaspase-9 complex (the apoptosome) and resultant activation of caspase-9 and caspase-3. Here we demonstrate that the molecular chaperone, Hsp27, inhibits this cytochrome c-mediated activation of caspase-3. Immunodepeletion of Hsp27 from cytochrome c-activated cytosols resulted in decreased caspase activity. Furthermore, immunoprecipitation of Hsp27 resulted in the coprecipitation of both cytochrome c and procaspase-3. In reciprocal experiments, immunoprecipitation of both procaspase-3 and cytochrome c resulted in coprecipitation of Hsp27, indicating two independent interactions. These results point to Hsp27 mediating its inhibition of procaspase-3 activation through its ability to sequester both cytochrome c and procaspase-3, and thus prevent the correct formation/function of the apoptosome complex.
Keywords: Apoptosis, Caspase, Cytochrome c, Hsp27, Stress
APOPTOSIS is a highly regulated form of cell death involving condensation of nuclear chromatin, cytoplasmic shrinkage, membrane blebbing, nuclear fragmentation, and, finally, formation of apoptotic bodies [for review see (18)]. Apoptosis is associated with activation of the caspase family of proteases, which consists of at least 14 caspases in mammalian cells. Caspases are synthesized as inactive zymogens and are converted to their active form through specific proteolytic cleavage [for review see (24)]. Upon activation they effect the demise of a cell through cleavage of specific target substrates leading to the highly regulated biochemical and morphological characteristics of apoptotic cell death [for review see (23)]. One of the mechanisms by which caspases are activated requires the release of cytochrome c from the mitochondria (8,26). Upon receiving an appropriate apoptotic signal cytochrome c escapes from the mitochondrial intermembrane space to the cytosol where it binds to Apaf-1 and promotes its oligomerization (9,11). Pro-caspase-9 is then recruited to the complex, resulting in its autoprocessing, followed by caspase-9-mediated cleavage and activation of pro-caspase-3 and the eventual demise of the cell (27,28). The caspase activating complex consisting of Apaf-1/cytochrome c/dATP/procasapse-9 has been termed the apoptosome (28).
The molecular pathways leading to caspase activation during apoptosis are tightly regulated. Emerging evidence has pointed to a role in this regulation for a family of proteins, termed heat shock proteins (Hsps), whose expression is associated with induced thermotolerance [for review see (5,19,25)] and inhibition of apoptosis (6,13,20). One of the major mammalian Hsps is a 27-kDa protein (Hsp27), a member of the family of small heat shock proteins displaying an in vitro ATP-independent molecular chaperone activity (7). Hsp27 is transiently induced in response to heat shock and other sources of stress and has been shown to play a role in the development of thermotolerance (21) and to protect cells from the deleterious effects induced by stress stimuli (1). Further studies have revealed that overexpression of Hsp27 can render cells resistant to apoptosis induced by a variety of stimuli including anti-Fas mAb, staurosporine, etoposide, actinomycin-D, and camptothecin (3,12,20). The mechanism by which Hsp27 inhibits stress-induced apoptosis is not fully understood. It has been reported that Hsp27 counteracts the stress-induced disruption of the actin microfilament network (10). Preville et al. (17) have demonstrated an ability to modulate the redox parameters within cells by increasing and upholding glutathione in a reduced form. Although these may account partially for the ability of Hsp27 to increase cellular resistance, they do not account for its ability to prevent the activation of caspases during apoptosis. Our group and others have observed an effect by Hsp27 on caspase activation downstream of cytochrome c release from mitochondria (3,22). However, recent evidence from our groups suggests that Hsp27 exerts its antiapoptotic effect primarily through prevention of mitochondrial cytochrome c release, at least in thermotolerant cells (22). In the present study we examined the mechanism by which Hsp27 affects caspase activation downstream of cytochrome c release from mitochondria in an in vitro system.
MATERIALS AND METHODS
Cell Culture, Heat Shock Conditions, and Cell Transfection
Jurkat cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere of 5% CO2 in air at 37°C. For heat shock, cell numbers were determined using a Neubauer hemocytometer and the density was adjusted to 106 cells/ml. The required numbers of cells were placed in culture flasks, which were sealed by wrapping parafilm around their lids. The flask was immersed in a water bath at the indicated temperatures (±0.5°C) for 1 h. After the incubation period, cells were resuspended in fresh medium and incubated at 37°C for various times. Cell viability was assessed by the ability of cells to exclude trypan blue dye. Cell morphology was evaluated by staining cytospin preparations and apoptotic/ necrotic cells were scored as described previously (20). Cell transfection was performed according to the protocol for DOTAP liposomal transfection reagent (Boehringer Mannheim). Cells were transfected with pSFFV expression plasmid (Clontech) containing the entire coding sequence of hsp27, or a corresponding control vector.
Western Blotting
Protein samples were subjected to SDS polyacrylamide gel electrophoresis and the proteins were transferred onto a nitrocellulose membrane. Western blotting was performed using mouse monoclonal antibodies to Hsp27 (StressGen Biotechnologies Corp.), PARP (BIOMOL), cytochrome c (a gift from Dr. R. Jemmerson), and rabbit polyclonal antibodies to caspase-3 (p17 antibody, a gift from Dr. D. W. Nicholson).
Immunoprecipitation
Samples containing 50 μg of protein in 100 μl of immunoprecipitation buffer (100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4, a cocktail of protease inhibitors, 0.5% Triton X-100, and 0.5% NP-40) were centrifuged at 20,000 × g for 15 min to remove particulate material. The supernatant was then incubated with anti-Hsp27 (1:200, StressGen), anti-caspase-3 (1:500, CPP32, Transduction Laboratories), anti-caspase-9 (1:500, StressGen), or cytochrome c (1:500, gift from Dr. R. Jemmerson) with constant agitation at 4°C for 1 h. This was followed by the addition of 25 μl of 50% protein A-Sepharose and incubation with constant agitation at 4°C for a further 1 h. The pellet was collected after five washes in immunoprecipitation buffer and prepared for Western blotting.
Preparation of the Cytosolic Fraction and In Vitro Caspase Activation With Cytochrome c and dATP
Cells were washed in PBS and resuspended in S-100 buffer (100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4, 10 μM aprotinin, 10 μM pepstatin A, 10 μM leupeptin, calpain inhibitor I, and 1 mM PMSF). After 10-min incubation on ice, cells were centrifuged at 900 × g for 5 min, followed by a further centrifugation of the supernatant at 100,000 × g for 45 min to obtain the cytosolic fraction. For in vitro caspase activation, 30 μl of the cytosolic fraction (2 mg/ml) was incubated with cytochrome c and dATP for 60 min at 30°C.
Caspase Assay
The activity of group II caspases, DEVDases, was determined fluorometrically using a modified version of the method developed by Nicholson et al. (14). Briefly, lysate from 1 × 106 cells and substrate (DEVD-AMC, 50 μM) were combined in reaction buffer [100 mM HEPES, 10% sucrose, 5 mM dithiothreitol (DTT), 0.0001% NP-40, and 0.1% 3-[(3-cholamidopropyl) dimethylammonio] propane-1-sulphonic acid (CHAPS), pH 7.25] and added in triplicate to a microtiter plate. Cleavage of the fluorogenic peptide substrate DEVD-AMC to liberate AMC in a fluorimeter using 355 nm excitation and 460 nm emission wavelengths. Fluorescence was measured at 70-s intervals over a 30-min period and fluorescence units were converted to pmol of AMC using a standard curve generated with free AMC. Data from triplicate samples were then analyzed by linear regression.
RESULTS
Induction of Hsp27 and Thermotolerance Is Associated With Inhibition of Caspase-3
In order to investigate the effect of Hsp27 induction on apoptosis, Jurkat cells were either heat shocked at 42°C for 1 h or maintained in culture at 37°C for the same period. After sufficient recovery time (12 h) apoptosis was induced by a subsequent heat shock at 44°C for 1 h. As demonstrated in Figure 1A, heat shocking at 42°C was associated with the induction of Hsp27. Subsequent Western blot analysis after induction of apoptosis demonstrated the cleavage of the caspase substrate PARP in cells maintained at 37°C, but not in those rendered thermotolerant by heat shock at 42°C, indicating that the thermotolerant cells were resistant to apoptosis and that caspases were inhibited. In order to further characterize this resistance, S-100 cytosols were prepared from thermotolerant and control cells and subsequently activated by adding cytochrome c/dATP. The addition of cytochrome c/dATP to cytosols from thermotolerant cells was associated with decreased caspase-3 activity in contrast to control cells (Fig. 1B). This result suggests that induction of Hsp27 is associated with inhibition of cytochrome c-dependent activation of caspase-3.
Figure 1.
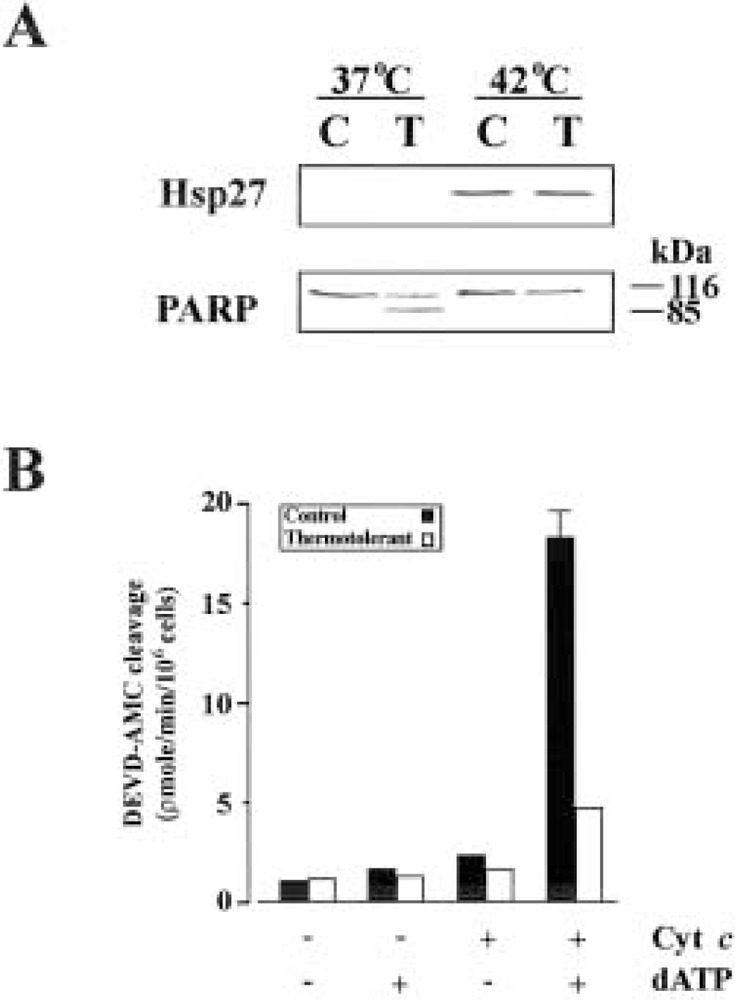
Induction of Hsp27 is associated with inhibition of cytochrome c-mediated activation of caspase-3. (A) Cells were maintained at 37°C or heat shocked at 42°C to induce Hsp27. After recovery cells were either further heat shocked at 44°C to induce apoptosis (T) or retained as controls (C). Equal amounts of protein were subjected to SDS-PAGE followed by Western blot analysis with specific antibodies against Hsp27 and PARP. (B) Cytosols prepared from control and thermotolerant cells were measured for DEVDase activity after the addition of cytochrome c/dATP.
Hsp27 Prevents Caspase-3 Activity by Interfering With Apoptosome Formation
To further investigate the role of Hsp27 in inhibiting apoptosis, Jurkat cells were transiently transfected with either the Hsp27 gene (Jurkat-Hsp27) or an empty vector as control (Jurkat-neo). Western blot analysis revealed that the levels of Hsp27 expressed in the transfected cells was comparable with those levels expressed after heat shock, but transfection with empty vector had no effect on Hsp27 expression levels (Fig. 2A). To assess if the transfected cells were as efficient in inhibiting caspase-3 activity, we measured cleavage of the peptide substrate DEVD-AMC after addition of cytochrome c/dATP to cytosols prepared from thermotolerant and transfected cells. As demonstrated by Figure 2B, the transfected cells were equipotent to thermotolerant cells in their ability to inhibit caspase-3 activity. To assess the mechanism of this inhibition, Hsp27 was immunodepeleted from S-100 cytosol of Jurkat-Hsp27 cells (data not shown) before and after the addition of cytochrome c/dATP. When Hsp27 was immunodepleted prior to the addition of cytochrome c/dATP a decrease in DEVD-AMC cleavage was observed in the cytosolic fraction from Jurkat-Hsp27 cells (Fig. 2C, left panel). However, the decrease in caspase activity was much more significant when Hsp27 was immunodepleted after the addition of cytochrome c/dATP (Fig. 2C, right panel). Taken together, these findings support a model that Hsp27 mediates its caspase inhibition by interacting with either cytochrome c/dATP, but also through an interaction with a component of the apoptosome other than cytochrome c/dATP.
Figure 2.
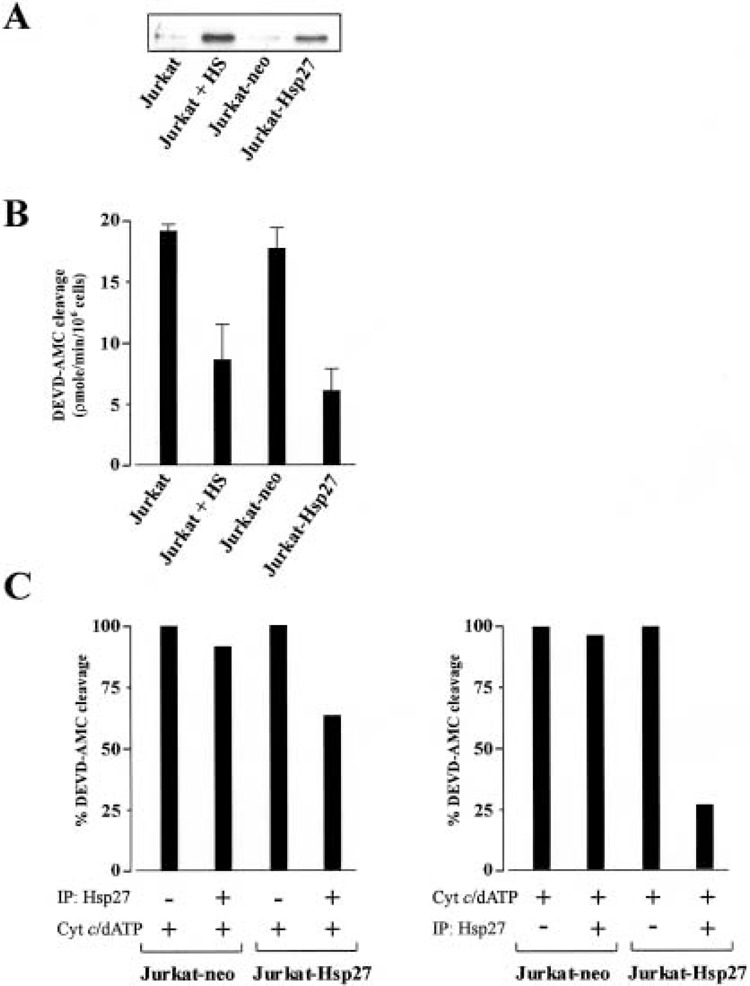
Hsp27 interferes with the formation of the apoptosome complex. (A) Western blot analysis of Hsp27 in thermotolerant and cells transiently transfected with Hsp27 gene. (B) Cytosols from thermotolerant and transfected cells were assayed for cleavage of DEVD-AMC after the addition of cytochrome c/dATP. (C) Jurkat-neo or Jurkat-Hsp27 cell cytosols were immunodepleted of Hsp27 prior to the addition of cytochrome c/dATP (left panel) or after the addition of cytochrome c/dATP (right panel). The immunodepleted extracts were assayed for cleavage of the fluorogenic substrate DEVD-AMC.
Coimmunoprecipitation of Hsp27 With Cytochrome c and Procaspase-3
A recent study demonstrated an interaction of Hsp27 with procaspase-3, preventing its processing into active caspase-3 (15). This interaction could account partially for our observation of the decrease in caspase-3 activity when Hsp27 is immunodepleted. However, this interaction would not account for the more significant decrease in caspase-3 activity when Hsp27 is immunodepleted after the addition of cytochrome c/dATP. To further characterize this inhibitory effect, immunoprecipitation studies were carried out with the different components of the apoptosome. Western blot analysis clearly demonstrates that Hsp27 coprecipitated with both cytochrome c and caspase-3 (Fig. 3A). In the reciprocal experiment, immunoprecipitation of Hsp27 resulted in the coprecipitation of cytochrome c and procaspase-3 (Fig. 3B). We did not detect an interaction between Hsp27 and Apaf-1 (data not shown), which is in agreement with a previous finding (15). These results are indicative of Hsp27 having two independent interactions with components of the apoptosome. This is substantiated by the finding that immunoprecipitation of cytochrome c does not result in the coprecipitation of procaspase-3 and vice versa (Fig. 3B).
Figure 3.
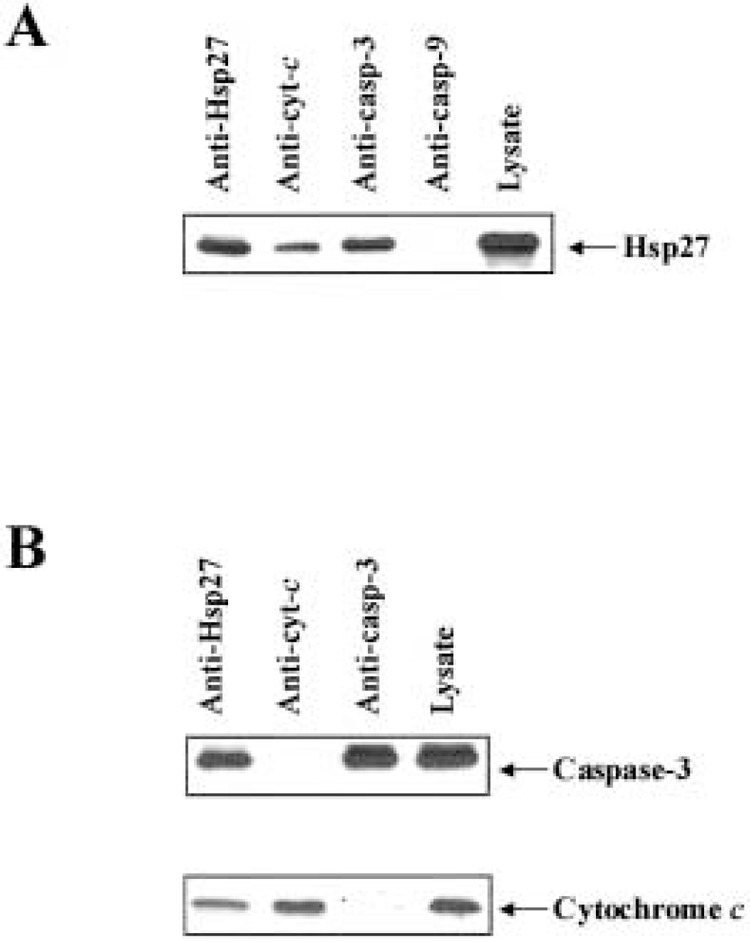
Hsp27 associates with both procaspase-3 and cytochrome c. (A) Jurkat-Hsp27 cell lysates were immunoprecipitated with anti-Hsp27, anti-cytochrome c, anti-caspase-3 (CPP32), or anti caspase-9, followed by Western blotting using antibody to Hsp27. (B) Western blot analysis using antibodies against caspase-3 (pl7) and cytochrome c, of proteins immunoprecipitated from cell lysate with anti-Hsp27, anti-cytochrome c, or anti-caspase-3.
DISCUSSION
The release of cytochrome c from the intermembrane space of mitochondria results in the formation of the apoptosome complex and the subsequent activation of caspase-3. Hsp27, a molecular chaperone, has been demonstrated to function both in vitro and in vivo as an endogenous inhibitor of apoptosis and caspases (3,15,22). However, the molecular mechanism of this inhibition is currently poorly understood. It has been reported that the protective effect of Hsp27 occurs after the release of cytochrome c (4) and prior to the activation of procaspase-9 (3). A more recent study has demonstrated an interaction between Hsp27 and procaspase-3 downstream of cytochrome c (15). The same study, and findings from our own group, also concluded that Hsp27 may act both upstream and/or downstream of cytochrome c release in a stimulus-dependent manner (15,22).
In this article we have presented data demonstrating the ability of Hsp27 to prevent the activation of procaspase-3 in an in vitro system. We have shown that immunoprecipitation of Hsp27 from the cytosolic fraction of cells transiently transfected with Hsp27 results in decreased caspase-3 activity following stimulation with cytochrome c/dATP. Our results are indicative of an interaction between Hsp27 and components of the apoptosome. Accordingly, in search for Hsp27 interactions with components of the apoptosome, immunoprecipitation studies revealed that Hsp27 can interact independently with both cytochrome c and procaspase-3. Interestingly, although the location of Hsp27 was previously thought to be mainly cytosolic, recent evidence has demonstrated a significant pool of Hsp27 located in the mitochondrial fraction of thermotolerant Jurkat cells where it is seen to block the loss of ΔΨm and the subsequent release of cytochrome c. This was confirmed by transfection of cells with hsp27 antisense, which prevented Hsp27 induction and failed to block the release of cytochrome c (22). It could be suggested that Hsp27 localized to mitochondria binds to cytochrome c, preventing its release into the cytosol and the subsequent activation of the apoptosome. This suggestion may be further substantiated by evidence that mitochondria are targets for the protective effects of thermotolerance against subsequent oxidative injury (16). Moreover, a recent study identified a novel set of sHsps that can associate with mitochondria of murine PC 12 cells during heat stress and protect against subsequent injury (2). Although the idea that Hsp27 acts at the level of the mitochondrion is contrary to other reports (3,4), it could be argued that this effect may only be seen when Hsp27 is expressed under physiological conditions (i.e., during thermotolerance), but not when overexpressed.
The interaction of Hsp27 with procaspase-3 was demonstrated by immunoprecipitation studies. This interaction is therefore in agreement with another report, demonstrating that this interaction is with the procaspase-3 molecule, and not the active form, to prevent its processing into active caspase-3 (15). In conclusion, our data demonstrate that Hsp27 inhibits cytochrome c-mediated caspase activation, by formation of two independent complexes, which sequester away two critical components of the apoptosome, cytochrome c and procaspase-3, thus preventing its correct formation.
ACKNOWLEDGMENTS
The authors are grateful to Elisabeth Peterson for excellent technical assistance and Dr. Adrienne Gorman for constructive discussions. We are grateful to Drs. Donald Nicholson (Merck Frosst Center for Therapeutic Research, Pointe-Claire-Dorval, Quebec) and Ronald Jemmerson (University of Minnesota Medical School, Minneapolis, MN) for providing antibodies to pl7 and cytochrome c, respectively.
REFERENCES
- 1. Arrigo A. P.; Landry J. Expression and function of the low molecular weight heat shock proteins. In: The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994:335–373. [Google Scholar]
- 2. Downs C. A.; Jones L. R.; Heckathorn S. A. Evidence for a novel set of small heat-shock proteins that associates with the mitochondria of murine PC 12 cells and protects NADH:Ubiquinone oxidoreductase from heat and oxidative stress. Arch. Biochem. Biophys. 365:344–350; 1999. [DOI] [PubMed] [Google Scholar]
- 3. Garrido C.; Bruey J. M.; Fromentin A.; Hammann A.; Arrigo A. P.; Solary E. Hsp27 inhibits cytochrome c dependent activation of procaspase-9. FASEB J. 13:2061–2070; 1999. [DOI] [PubMed] [Google Scholar]
- 4. Guénal I.; Sidoti-de Fraisse C; Gaumer S.; Mignotte B. Bcl-2 and Hsp27 act at different levels to suppress programmed cell death. Oncogene 15:347–360; 1997. [DOI] [PubMed] [Google Scholar]
- 5. Jäättelä M. Heat shock proteins as cellular lifeguards. Ann. Med. 31:261–271; 1999. [DOI] [PubMed] [Google Scholar]
- 6. Jäättelä M.; Wissing D.; Kokholm K.; Kalluunki T.; Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3 like proteases. EMBO J. 17: 6124–6134; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jakob U.; Gaestel M.; Engel K.; Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268:1517–1520; 1993. [PubMed] [Google Scholar]
- 8. Kluck R. M.; Bossy-Wetzel E.; Green D. R.; Newmeyer D. D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275:1132–11365; 1997. [DOI] [PubMed] [Google Scholar]
- 9. Kluck R. M.; Martin S. J.; Hoffman B. M.; Zhou J. S.; Green D. R.; Newmeyer D. D. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16:4639–4649; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lav oie J. N.; Gingras-Breton G.; Tanguay R. M.; Laundry J. Induction of Chinese hamster Hsp27 gene expression in mouse cells confers resistance to heat shock: Hsp27 stabilization of the microfilament organization. J. Biol. Chem. 268:3420–3429; 1993. [PubMed] [Google Scholar]
- 11. Li F.; Srinivasan A.; Wang Y.; Armstrong R. C.; Tomaselli K. J.; Fritz L. C. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J. Biol. Chem. 272:30299–30305; 1997. [DOI] [PubMed] [Google Scholar]
- 12. Mehlen P.; Schulze-Osthoff K.; Arrigo A. P. Small stress proteins as novel regulators of apoptosis. J. Biol. Chem. 271:16510–16514; 1996. [DOI] [PubMed] [Google Scholar]
- 13. Mosser D. D.; Caron A. W.; Bourget L.; Denis-Larose C.; Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17:5317–5327; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicholson D. W. Ali A.; Thornberry N. A.; Vaillancourt J. P.; Ding C. K.; Gallant M.; Gareau Y.; Griffin P. R.; Labelle M.; Lazebnik Y. A.; Munday N. A.; Saju S. M.; Smulson M. E.; Yamin T. T.; Yu V. L.; Miller D. K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37–43; 1995. [DOI] [PubMed] [Google Scholar]
- 15. Pandey P.; Farber R.; Nakazawa A.; Kumar S.; Bharti A.; Nalin C.; Weichselbaum R.; Kufe D.; Kharbanda S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19:1975–1981; 2000. [DOI] [PubMed] [Google Scholar]
- 16. Polla B. S.; Kantengwa S.; Francois D.; Salvioli S.; Franceschi C.; Marsac C.; Cossarizza A. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc. Natl. Acad. Sci. USA 93:6458–6463; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pre ville X.; Salvemini F.; Giraud S.; Chaufour S.; Paul C.; Stepien G.; Ursini M. V.; Arrigo A. P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 247:61–78; 1999. [DOI] [PubMed] [Google Scholar]
- 18. Samali A.; Gorman A. M.; Cotter T. G. Apoptosis: The story so far. Experientia 52:933–941; 1996. [DOI] [PubMed] [Google Scholar]
- 19. Samali A.; Orrenius S. Heat shock proteins: Regulators of stress response and apoptosis. Cell Stress Chap. 3:228–236; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samali A.; Cotter T. G. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 223:163–170; 1996. [DOI] [PubMed] [Google Scholar]
- 21. Samali A.; Holmberg C. I.; Sistonen L.; Orrenius S. Thermotolerance and cell death are distinct cellular responses to stress: Dependence on heat shock proteins. FEBS Lett. 461:306–310; 1999. [DOI] [PubMed] [Google Scholar]
- 22. Samali A.; Robertson J. D.; Peterson E.; Manero F.; van Zeijl L.; Paul C.; Cotgreave I. A.; Arrigo A. P.; Orrenius S. Hsp27 protects mitochondria of thermo-tolerant cells against apoptotic stimuli. Cell Stress Chap. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stroh C.; Schulze-Osthoff K. Death by a thousand cuts: An ever increasing list of caspase substrates. Cell Death Differ. 5:997–1000; 1998. [DOI] [PubMed] [Google Scholar]
- 24. Thornberry N. A.; Lazebnik Y. Caspases: Enemies within. Science 281:1312–1316; 1998. [DOI] [PubMed] [Google Scholar]
- 25. Vayssier M.; Polla B. S. Heat shock proteins chaperoning life and death. Cell Stress Chap. 3:221–227; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J.; Liu X.; Bhalla K.; Kim C. M.; Ibrado A. M.; Cai J.; Peng T. I.; Jones D. P.; Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 275:1129–1132; 1997. [DOI] [PubMed] [Google Scholar]
- 27. Zhou H.; Henzel W. J.; Liu X.; Lutschg A.; Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413; 1997. [DOI] [PubMed] [Google Scholar]
- 28. Zou H.; Li Y.; Liu X.; Wang X. An Apaf-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274:11549–11556; 1999. [DOI] [PubMed] [Google Scholar]


