Abstract
Background
Bone marrow (BM)-derived mesenchymal stromal cells (MSCs) have shown potential to differentiate into various cell types, including smooth muscle cells (SMCs). The extracellular matrix (ECM) represents an appealing and readily available source of SMCs for use in tissue engineering. In this study, we hypothesized that the ECM could be used to induce MSC differentiation to SMCs for engineered cell-sheet construction.
Methods
Primary MSCs were isolated from the BM of Wistar rats, transferred and cultured on dishes coated with 3 different types of ECM: collagen type IV (Col IV), fibronectin (FN), and laminin (LM). Primary MSCs were also included as a control. The proportions of SMC (a smooth muscle actin [aSMA] and SM22a) and MSC markers were examined with flow cytometry and Western blotting, and cell proliferation rates were also quantified.
Results
Both FN and LM groups were able to induce differentiation of MSCs toward smooth muscle–like cell types, as evidenced by an increase in the proportion of SMC markers (aSMA; Col IV 42.3 ± 6.9%, FN 65.1 ± 6.5%, LM 59.3 ± 7.0%, Control 39.9 ± 3.1%; P = 0.02, SM22; Col IV 56.0 ± 7.7%, FN 74.2 ± 6.7%, LM 60.4 ± 8.7%, Control 44.9 ± 3.6%) and a decrease in that of MSC markers (CD105: Col IV 64.0 ± 5.2%, FN 57.6 ± 4.0%, LM 60.3 ± 7.0%, Control 85.3 ± 4.2%; P = 0.03). The LM group showed a decrease in overall cell proliferation, whereas FN and Col IV groups remained similar to control MSCs (Col IV, 9.0 ± 2.3%; FN, 9.8 ± 2.5%; LM, 4.3 ± 1.3%; Control, 9.8 ± 2.8%).
Conclusions
Our findings indicate that ECM selection can guide differentiation of MSCs into the SMC lineage. Fibronectin preserved cellular proliferative capacity while yielding the highest proportion of differentiated SMCs, suggesting that FN-coated materials may be facilitate smooth muscle tissue engineering.
Keywords: cell culture, co-culture, flow cytometry, scanning electron microscopy
Introduction
Heart failure remains a frequent and life-threatening disorder despite recent medical and surgical advances. Interest in myocardial regenerative therapy as a means to improve left ventricular (LV) function in patients with end-stage heart disease is growing. We previously reported that a cell-sheet engineering approach using co-cultured smooth muscle cells (SMCs) and endothelial progenitor cells (EPCs) induced functional recovery of distressed myocardium in a small animal heart failure model. Recapitulating the natural interaction between EPCs and SMCs created structurally mature, functional microvasculature. This method, however, required that SMCs be obtained from the thoracic aorta, which is not possible in the clinical arena [1].
To resolve this problem, bone marrow (BM)-derived mesenchymal stem cells (MSC) have shown potential to differentiate into various cell types, including SMCs [2]. Additionally, it is known that the extracellular matrix (ECM) represents a powerful regulator of SMC phenotypic modulation for tissue engineering [3,4].
Tissue engineering has become an essential field of research for effective regenerative therapy [5]. In the past decade, tissue engineering therapeutic products created from cells and scaffolds have been widely investigated, and several products have become commercially available. Researchers have explored scaffold-based tissue engineering, such as the biodegradable scaffold [6,7], decellularized tissues [8], hydrogel and cell mixtures [9], bioprinting technology [10] and fiber-based tissue engineering [11]. Conversely, our group has used scaffold-free cell-sheet technology for clinical translation [12]. The cell-sheet is created on, and removed from specialized dishes that are covalently grafted with a temperature-responsive polymer, poly (N-isopropylacrylamide; PIPAAm), which changes from hydrophobic to hydrophilic by simply lowering the temperature without any enzymes [13]. The cell-sheet technology fabricates three-dimensional (3-D) tissues from densely adherent cells without an artificial scaffold and enzymatic digestion. The cell-sheet is easily manipulated and has a robust ability to integrate with native tissues by retaining cell-cell junctions, as well as the ECM on the basal surface of the cell-sheet.
In this study, we hypothesized that the ECM could stimulate transdifferentiation of MSCs into SMCs to facilitate construction of an SMC supported, angiogenic bi-level cell-sheet.
Materials and methods
Isolation of MSCs and EPCs
The carotid artery of male Wistar rats (8 weeks old, 250–300 g; Charles River) was dissected and transected under isoflurane anesthesia. BM mononuclear cells were isolated from the long bones (8 weeks old, 250– 300 g; Charles River), filtered through a 40 um cell strainer (Falcon), and centrifuged at 300g for 7 min. Red blood cells (RBCs) were excluded using 1x RBC lysis buffer (eBioscience, #00-4337-57) for 10 min at 4°C. Remaining cells were cultured in a medium with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, #11995-040) containing 10% fetal bovine serum (FBS; Sigma-Aldrich) and gentamicin on non-coated culture dishes for 24 h at 37°C. Following incubation, the adherent cells were washed and then cultured in a medium with DMEM containing 10% FBS and gentamicin. A purified population of MSCs was obtained 10 to 14 days after the initiation of culture. MSC was determined in accordance with the criteria of the International Society for CellularTherapy [2].
EPCs were isolated and cultured, as described previously [1]. Briefly, BM mononuclear cells were isolated from the long bones of Wistar rats by density gradient centrifugation with Histopaque 1083 (Sigma-Aldrich) and cultured in endothelial basal medium-2 supplemented with EGM-2 SingleQuot (Lonza) containing human epidermal growth factor, 5% FBS, vascular endothelial growth factor (VEGF), basic human fibroblast growth factor, recombinant human long R3 insulin-like growth factor-1, ascorbic acid and gentamicin on vitronectin (Sigma-Aldrich, V0132-50VG)–coated dishes. The combination of endothelium-specific media and the removal of non-adherent BM mononuclear cells were intended to select for the EPC phenotype.
ECM-driven trans-differentiation of MSCs into SMCs
The primary rodent MSCs were transferred and cultured in a medium with DMEM and 10% FBS on 60-mm culture dishes coated with 1 of 3 different types of ECM: fibronectin (FN group, BD Biosciences), Collagen IV (Col IV group, BD Biosciences) and laminin (LM group, BD Biosciences) at 37°C in a humidified atmosphere of 5% CO2 in air. Primary MSCs were also included in this study (Control group). MSCs were plated at a density of 4–6 × 103 cells/cm2. MSC growth medium was used as the nutrient medium and all media were exchanged every 48–72 h.
Phenotypes of trans-differentiated SMCs and cultured MSCs assessed with flow cytometry
To elucidate the phenotypes of cultured MSCs and trans-differentiated SMCs, flow cytometry was employed using markers specific for MSCs, EPCs, and SMCs. Cell assessment was performed after 10 to 14 days’ culture on each plate. Single-cell suspensions of 106/mL were fixed with Fixation/Permeabilization Diluent (eBioscience, 00-5223-56) for 30 min on ice. Following washing with 10% FBS in phosphate-buffered saline (PBS), cells were incubated with an optimal concentration of rabbit polyclonal anti-alpha smooth muscle actin antibody (Abcam, ab5694, 1:100), rabbit polyclonal anti-SM22 alpha antibody (Abcam, ab14106, 1:100), rabbit monoclonal anti-Caldesmon antibody (Abcam, ab32330, 1:100), mouse monoclonal anti-CD105 antibody (Abcam, ab156756, 1:100), rabbit polyclonal anti-CD73 antibody (Abcam, ab175396, 1:100) or rabbit polyclonal anti-CD45 antibody (Abcam, ab10558, 1:100) diluted in 10% FBS in PBS for 2 h on ice. After washing 2 times with 10% FBS in PBS, cells were incubated with donkey anti-rabbit immunoglobulin (Ig)G heavy and light chains (H&L) (Alexa Fluor 488) preadsorbed (Abcam, ab150065), and donkey anti-mouse IgG H&L (Alexa Fluor 488; Abcam, ab150105) for 2 h on ice. The percentage of cells expressing each cell surface antigen was analyzed with a Becton Dickinson FACSCalibur flow cytometer. Data analysis was performed using FlowJo vX (Tree Star Inc) [1]. Control samples consisted of cells with fluorescein isothiocyanate (FITC)-conjugated rat IgG2b κ isotype control (BD Pharmingen, #556923, 1:100) or Alexa Fluor 647 rat IgG IgG2b κ isotype control (BD Pharmingen, #557691, 1:100) diluted in 10% FBS in PBS.
Phenotypes of cultured EPCs assessed with flow cytometry
To elucidate the phenotypes of EPCs, flow cytometry was employed using markers specific for EPCs. Single-cell suspensions of 106/mL were fixed with Fixation/ Permeabilization Diluent (eBioscience 00-5223-56) for 30 min on ice. Following washing with 10% FBS in PBS, the cells were incubated with an optimal concentration of a mouse monoclonal anti-CD31 antibody (Abcam ab64543, 1:100) diluted in 10% FBS in PBS for 2 h on ice. After washing twice with 10% FBS in PBS, cells were incubated with donkey anti-mouse IgG H&L (Alexa Fluor 488; AB150105, 1:100 dilution; Abcam) for 2 h on ice. The percentage of cells expressing each cell surface antigen was analyzed with a Becton Dickinson FACSCalibur flow cytometer. Data analysis was performed using FlowJo vX [1]. Control samples consisted of cells with FITC-conjugated rat IgG2b k isotype control (BD Pharmingen, #556923, 1:100) diluted in 10% FBS in PBS.
Characteristics of trans-differentiated SMCs and cultured MSCs assessed with Western blotting
Western blotting was performed to identify SMC-specific cytoskeletal protein expression in MSCs, which were cultured on an ECM-coated or a non-coated dish. Cultured cells were homogenized using Halt Protease Inhibitor Single-Use Cocktail (ThermoScientific) diluted in T-PER Tissue Protein Extraction Reagent (ThermoScientific) after 10 to 14 days’ culture on each plate. The protein concentration for the lysate was estimated using Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Inc.), as described previously [14]. The proteins were denatured by boiling with sodium dodecyl sulfate (SDS) and 2-mercaptoethanol solution. An equal concentration of proteins in NuPAGE 4–12% Bis-Tris Gel (Life Technologies) was applied to samples to perform electrophoresis. The protein was transferred by iBlot Dry Blotting System (LifeTechnologies). The blotting membrane was blocked with 5% skim milk at room temperature for 60 min, then immunoblotted using rabbit polyclonal anti-alpha smooth muscle actin antibody (Abcam, ab5694, 1:400 dilution), rabbit polyclonal anti-SM22 alpha antibody (Abcam, ab14106, 1:1000 dilution), rabbit monoclonal anti-Caldesmon antibody (Abcam, ab32330, 1:1000 dilution), mouse monoclonal anti-CD105 antibody (Abcam, ab156756, 1:1000 dilution) or mouse monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) horseradish peroxidase (HRP)-conjugated antibody (Abcam, ab9484, 1:5000 dilution). Secondary donkey anti-rabbit IgG H&L (HRP; Abcam, ab16284, 1:1000 dilution) or anti-mouse IgG HRP-linked antibody (Cell SignalingTechnology, #7076P2, 1:1000 dilution) were used for detection using SuperSignal West Dura Extended Duration Substrate (ThermoScientific). The labeled membrane was stripped using Restore Western Blot Stripping Buffer (ThermoScientific), then re-probed. To assess the intensity of bands for these proteins semi-quantitatively, densitometric analysis was performed using ChemiDoc XRS + (Bio-Rad Laboratories, Inc.) with Imaging Lab Software Version 5.1 (Bio-Rad Laboratories, Inc.). The intensity level of detected protein bands was standardized by dividing by the intensity level of GAPDH.
Cell proliferation assay for trans-differentiated SMCs and cultured MSCs
To examine cell proliferation of cultured MSCs and trans-differentiated SMCs, flow cytometry was performed using Click-iT Plus Edu Flow Cytometry Assay Kit (LifeTechnologies, C10634) according to the manufacturer’s instructions. Cell proliferation for each plate was assessed after 120 h. Single-cell suspensions of 106 cells/mL were fixed with Click-iT fixative for 15 min and Click-iT saponin-based permeabilization for 15 min at room temperature. Following washing with 1% bovine serum albumin (BSA) in PBS, cells were incubated with Click-iT Plus reaction cocktail for 30 min at room temperature. Then, cells were suspended with Click-iT saponin-based permeabilization. For the detection of EdU with Alexa Fluor 647 picolyl azide, the percentage of cells was analyzed with a Becton Dickinson FACSCalibur flow cytometer. Data analysis was performed using FlowJo vX [1].
Creation of bi-level cell-sheets
SMCs were plated at 1.5 × 105/cm2 in a 35-mm Upcell dish, which was grafted with temperature-responsive polymers (CellSeed), and then cultured in EPC-specific medium. After 24 h of culture at 37°C and 5% CO2, EPCs were added at 1.5 × 105/cm2 onto the Upcell dish, which was already confluent with SMCs. After an additional 24 h in culture, the dishes were transferred to room temperature for 30 min to create a bi-level cell-sheet.
Visualization of EPC-SMC bi-level cell-sheet
To confirm densely adherent cells without an artificial scaffold in a cell-sheet and ECM deposited on the basal surface of a cell-sheet, cell-sheets were visualized with scanning electron microscopy (SEM) to confirm that: 1) cells were densely adherent despite lacking an artificial scaffold, and 2) ECM was deposited on the basal surface of the cell-sheet. For comparative purposes, “SMC only” cell-sheets were included in this experiment. Samples for SEM were fixed for 24 h at 4°C with 4% Paraformaldehyde and 2% Glutaraldehyde in 0.1 mol/L Sodium Cacodylate Buffer (pH 7.2) after lifting up a bi-level cell-sheet from the Upcell dish. Cell-sheets were rinsed in the same buffer and post-fixed for 1 h with 1% aqueous OsO4. After dehydration in an ascending ethanol series (50, 70, 90, 100% [2x]; 10 min each), samples were critical point dried with liquid CO2 in a Tousimis Autosamdri-815B apparatus (Tousimis), mounted with colloidal graphite on 15-mm aluminum stubs (Electron Microscopy Sciences) and sputter-coated with 50 Å of gold-palladium using a Denton DeskII Sputter Coater (Denton Vacuum). Visualization was performed with a Zeiss Sigma FESEM (Carl Zeiss Microscopy) operated at 2–3 kV, using inLens SE detection at a working distance of 5–6 mm. Images were captured in TIFF format using a store resolution of 2048 × 1536 and a line averaging, noise reduction algorithm.
Statistical analyses
Continuous variables are expressed as the mean ± standard error (SE). Comparisons between two groups were made using the Wilcoxon-Mann-Whitney U test because of small sample sizes. The Kruskal-Wallis test was used for comparisons between 3 groups, followed by the post hoc pairwise Wilcoxon-Mann-Whitney U tests. We corrected for multiple testing using the Bonferroni procedure. P < 0.05 was considered to be statistically significant. All statistical calculations were performed using JMP 9.0 (SAS Institute Inc.).
Animal care and biosafety
Wistar rats were obtained from Charles River Laboratories. Food and water were provided ad libitum. This investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committee of Stanford University (protocol 28921).
Results
Phenotypic modulations after ECM-driven trans-differentiation of MSCs into SMCs
The proportions of SMC and MSC markers were examined using flow cytometry. Initially, isolated BM cells were cultured for 10 to 14 days to induce MSCs. Both FN and LM groups were able to induce differentiation of MSCs toward smooth muscle–like cell types, as determined by the increase in the proportions of SMC markers (alpha smooth muscle actin (SMA); Col IV 42.3 ± 6.9%, FN 65.1 ± 6.5%, LM 59.3 ± 7.0%, Control 39.9 ± 3.1%; P = 0.02; SM22; Col IV 56.0 ± 7.7%, FN 74.2 ± 6.7%, LM 60.4 ± 8.7%, Control 44.9 ± 3.6%; P = 0.15) (Figure 1) and the decrease in that of MSC markers (CD105: Col IV 64.0 ± 5.2%, FN 57.6 ± 4.0%, LM 60.3 ± 7.0%, Control 85.3 ± 4.2%; P = 0.03).
Figure 1.
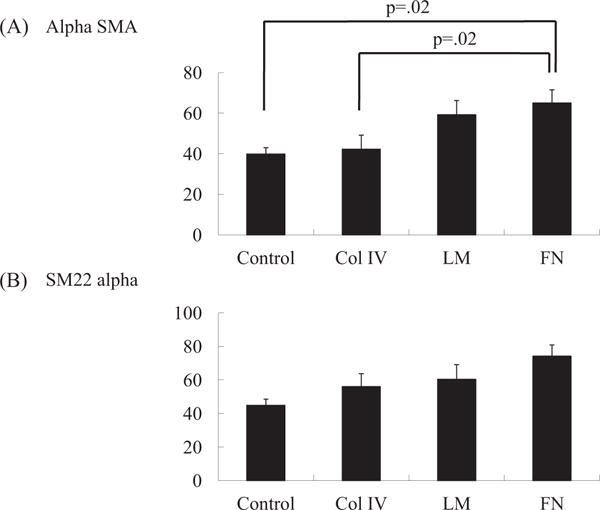
Flow cytometry was performed to elucidate the phenotypes of cultured MSCs and trans-differentiated SMCs. (A) The percentage of alpha SMA was greatest in the FN group (n = 6 in each; P = 0.02; Kruskal-Wallis test), which was significantly higher than the Col IV and Control groups (P = 0.02 and P = 0.02, respectively). (B) The percentage of SM22 alpha was highest in the FN group, although there was no significant difference between groups (n = 6 in each; P = 0.15; Kruskal-Wallis test).
Alterations of cellular characteristics after trans-differentiating MSCs into SMCs with ECM
SMC-specific cytoskeletal protein expression was analyzed using Western blotting in trans-differentiated MSCs, which were cultured on an ECM-coated or a non-coated dish (Figure 2). The expression of alpha SMA, SM22 alpha and Caldesmon was highest in the FN group (P = .01, P = .01 and P = .17, Kruskal-Wallis test), whereas the expression of CD105 was the highest in the control group (P = .02, Kruskal-Wallis test).
Figure 2.
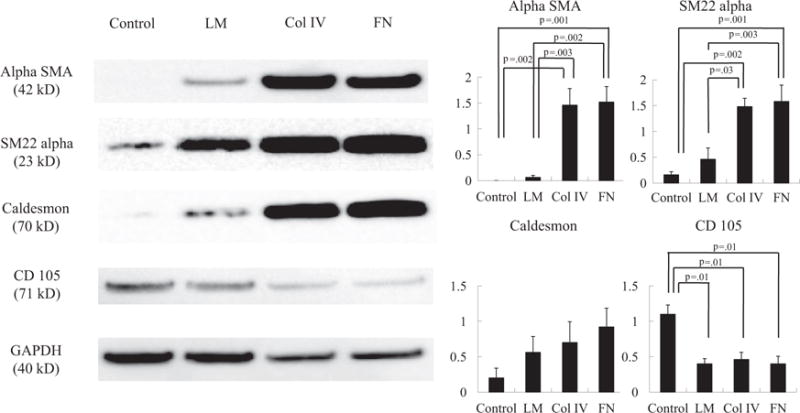
Representative immunoblots for alpha SMA, SM22 alpha, Caldesmon and CD105 between Col IV, FN, LM and control groups (n = 6 in each). The expression of alpha SMA (P = 0.01; Kruskal-Wallis test), SM22 alpha (P = 0.01; Kruskal-Wallis test) and Caldesmon (P = 0.17; Kruskal-Wallis test) was highest in the FN group, whereas the expression of CD105 was highest in the control group (P = 0.02; Kruskal-Wallis test). GAPDH staining was performed to demonstrate equivalent protein loading between the 4 groups.
Cell proliferation after ECM-driven trans-differentiation of MSCs into SMCs
The proliferation of MSCs and SMCs was estimated using flow cytometry. The LM group showed a decrease in overall cell proliferation, whereas both the FN and Col IV groups remained similar to that of the control (Col IV, 9.0 ± 2.3%; FN, 9.8 ± 2.5%; LM, 4.3 ± 1.3%; Control, 9.8 ± 2.8%; n = 6 in each; P = .17, Kruskal-Wallis test).
Characterization of cultured EPCs
Flow cytometric analysis demonstrated that 73.3% ± 2.7% of cultured EPCs were CD31+.
Creation of bi-level cell-sheet, composed of co-cultured EPCs and trans-differentiated SMCs
A confluent co-cultured bi-level cell-sheet, made of EPCs and trans-differentiated SMCs, was spontane ously detached as an intact cell-sheet from an UpCell dish (Figure 3A). SEM revealed the presence of densely adherent cells without an artificial scaffold in the cell-sheet. Furthermore, morphological differences were noted between individual cells, different cell types, and the upper versus lower surfaces of cell-sheets. Examination of EPC-SMC bi-level cell-sheets and SMC-only cell-sheets suggested that SMC morphology varies regionally from spherical to flattened, and SMCs differentiate into an elongated and spindle-shaped dense sheet-like construct with cellular extensions forming a densely adherent layer. In multilayer cell-sheets, outer layers contain more spherical cells than lower layers in which the cells become elongated and flattened. This suggests an influence of both developmental stage and cell-subtrate interaction on cell differentiation and morphology. EPCs form a smooth confluent layer with cell borders in close contact with each other, thereby forming a thin film-like layer on top of the SMCs (Figure 3B–E).
Figure 3.
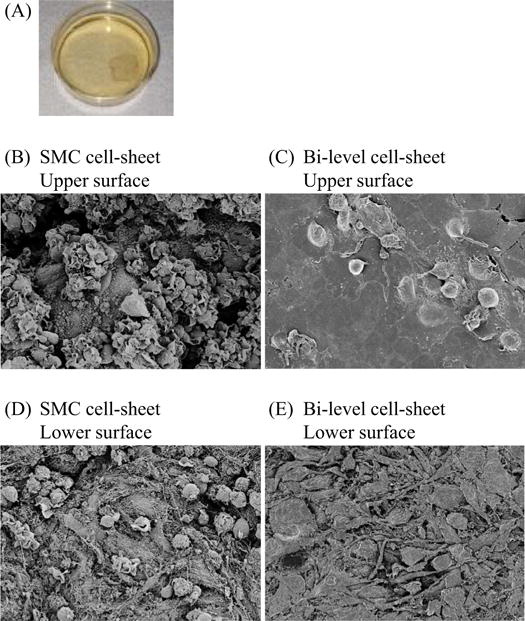
Characterization of a bi-level co-cultured cell-sheet made of EPCs and trans-differentiated SMCs in vitro. (A) Confluent SMCs topped with confluent EPCs were cultured in a 35-mm Upcell dish, which was grafted with temperature-responsive polymers (CellSeed) at 37°C and 5% CO2. After transfer to another incubator, set at 20°C, a confluent bi-level cell-sheet was spontaneously detached from an UpCell dish to release the cultured cells as an intact cell-sheet. (B)–(E) SEM images of the upper vs lower surfaces of the bi-level EPC-SMC cell-sheet and “SMC only” cell-sheet. SEM revealed the presence of densely adherent cells without an artificial scaffold in the cell-sheet, as well as morphological differences between individual cells, and different cell types. (B) Upper surface of “SMC only” cell-sheet: cells vary from spherical to flattened, and differentiate into elongated and spindle-shaped cells that establish a dense sheet-like construct with cellular extensions forming a densely adherent layer. (C) Upper surface of bi-level “EPC-SMC” cell-sheet: cells form a smooth confluent layer with cell borders in close contact with each other, thereby developing a thin film-like construct on top of the SMCs. (D) Lower surface of “SMC only” cell-sheet: cells are mostly elongated and flattened. In multilayer cell-sheets the lower cells are more spindle-shaped and flattened than the upper cells, which are predominantly spherical. This suggests an influence of both developmental stage and cell-subtrate interaction on cell differentiation and morphology. (E) Lower surface of bi-level “EPC-SMC” cell-sheet: mostly flattened and elongated cells are visible, similar to the lower surface of the “SMC only” cell-sheet. White bar = 10 μm.
Discussion
This study revealed that a significant up-regulation of SMC-specific protein expression, while simultaneously maintaining proliferative capacity, was obtained with in vitro simply by culturing BM- derived MSCs on FN-coated dishes. Futhermore, a confluent co-cultured bi-level cell-sheet, made of isolated EPCs and trans-differentiated SMCs, was effectively created using cell-sheet technology, and densely adherent cells without an artificial scaffold were observed in the cell-sheet construct. Furthermore, the EPC-SMC bi-level cell-sheet construct retained regional morphologic differences between individual cells, different cell types, and the upper versus lower surfaces of cell-sheets.
The bi-level cell-sheet engineering technology, made of co-cultured SMCs and EPCs, yields architecturally mature and functional microvasculature via maintenance of natural interactions between EPCs and SMCs, thereby improving cardiac function [1]. Considering this technology’s potential for clinical translation, the ability to acquire the requisite cells in a non-invasive, efficient fashion is crucial. To address this, the use of somatic adult stem cells (e.g., MSCs) has been suggested for use in regenerative medicine [15]. MSCs are an attractive autologous cell source for cell-based regenerative therapy due to a strong ability to proliferate actively in vitro and differentiate into various cells, including chondrocytes, osteocytes, adipocytes, skeletal myoblasts and cardiomyocytes [16,17]. Moreover, MSCs are isolated in a minimally invasive manner from various tissues, including BM, adipose tissue, umbilical cord, amniotic fluid and peripheral blood. Here, we demonstrate that isolated MSCs can be effectively trans-differentiated into the SMC lineage by simply culturing on commercially available FN-coated dishes, providing promising evidence that this method can be used in the clinical arena. A possible methodological limitation is that our flow cytometry and Western blotting results were somewhat discordant. This is probably because marked variations in cell surface fluorescence were noted, even when experimental conditions were carefully standardized.
Little is known about the mechanism by which FN and LM induce trans-differentiation of MSCs into SMCs more efficiently than Col IV; however, various possible explanations can be found in the literature. Previous studies indicate that LM and FN are more likely to enhance the intracellular signaling pathways of phosphatidylinositol 3-kinase (PI3K)/Akt activation through integrin, which is associated with trans-differentiation of MSCs into SMCs [18]. On the other hand, collagen IV enhances the FAK-ERK signaling pathway, which is associated with better MSC proliferation [19].
In the past decade, tissue engineering therapeutics created from cells and scaffolds have been widely investigated. Cell-sheet technology has been to regenerate various damaged tissues, and clinical trials have been performed in several areas, including the cornea, epithelium [20],myocardium [12], and esophagus [21]. Briefly, confluent cells on a temperature-responsive culture surface can be harvested as an intact and contiguous cell-sheet by simply reducing the temperature without protease treatment. The significant features of the cell-sheet are that cell-cell junctions and ECM components mediating cell adhesion are retained in the cell-sheet without scaffolds, whereas conventional cell harvesting using enzymatic digestion (e.g., trypsin) disrupts all cell-cell junctions and adhesive proteins between cells. In this study, we performed SEM to observe the surface of the cell-sheet in a detailed fashion, by which we confirmed the presence of densely adherent cells in the cell-sheet, as well as morphological differences between individual cells and different cell types. The above-mentioned results suggest that these retained cells and proteins behave as adhesive agents and accelerate the adhesion between the cell-sheet and transplanted site, thereby enabling transplantation of this cell-sheet construct onto the surface of the beating heart without suturing [22]. Another critical aspect is that the bi-level cell-sheet demonstrated a different cellular arrangement. The SMC layer establishes a dense sheet-like construct, whereas the EPC layer develops a thin film-like construct. These differences are likely affected by their different cellular properties, level of oxygenation, growth factors and cell-cell and cell-matrix interactions.
In conclusion, ECM is useful for the differentiation of MSCs into SMCs, and FN yielded the highest purification while preserving cellular proliferative capacity. This suggests that FN-coated materials may be quite useful for smooth muscle tissue engineering. Moreover, a bi-level cell-sheet construct, made of isolated EPCs and differentiated SMCs, was successfully created with the use of cell-sheet technology. These findings greatly enhance the translatability of bi-level cell-sheet technology.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) Grant 1R01HL089315-01 (Y.J.W.), the American Heart Association Great Rivers Affiliate Postdoctoral Fellowship co-sponsored by the Claude R. Joyner Fund for Young Medical Researchers (#12POST12060567;Y.S.) and the Uehara Memorial Foundation for Research Fellow, Japan (201340160; Y.S.).
Footnotes
Disclosure of interests: None.
References
- 1.Shudo Y, Cohen JE, MacArthur JW, Atluri P, Hsiao PF, Yang EC, et al. Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation. 2013;128(11 Suppl. 1):S59–68. doi: 10.1161/CIRCULATIONAHA.112.000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, et al. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs. 2010;191:269–80. doi: 10.1159/000260061. [DOI] [PubMed] [Google Scholar]
- 4.Thyberg J, Hedin U, Sjolund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–90. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Atluri P, Trubelja A, Fairman AS, Hsiao PF, MacArthur JW, Cohen JE, et al. Normalization of post-infarct biomechanics utilizing a novel tissue engineered angiogenic construct. Circulation. 2013;128(26 Suppl. 1):S95–104. doi: 10.1161/CIRCULATIONAHA.112.000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stock UA, Vacanti JP. Tissue engineering. Current state and prospects. Annu Rev Med. 2001;52:443–51. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 8.Neumann A, Sarikouch S, Breymann T, Cebotari S, Boethig D, Horke A, et al. Early systemic cellular immune response in children and young adults receiving decellularized fresh allografts for pulmonary valve replacement. Tissue Eng Part A. 2014;20:1003–11. doi: 10.1089/ten.tea.2013.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JE, Purcell BP, MacArthur JW, Jr, Mu A, Shudo Y, Patel JB, et al. Circ Heart Fail. 2014;7:619–26. doi: 10.1161/CIRCHEARTFAILURE.113.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 11.Onoe H, Okitsu T, Itoh A, Kato-Negishi M, Gojo R, Kiriya D, et al. Metre-long cell-laden microfibers exhibit tissue morphologies and functions. Nat Mater. 2013;12:584–90. doi: 10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 12.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, et al. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–4. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 13.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly (N-iso-propylacrylamide) J Biomed Mater Res. 1993;27:1243–51. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 14.Shudo Y, Miyagawa S, Ohkura H, Fukushima S, Saito A, Shiozaki M, et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A. 2013;20:728–39. doi: 10.1089/ten.tea.2012.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Gu J, Fujibayashi A, Yamada KM, Sekiguchi K. Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt-and MEK1/ERK-dependent pathways. J Biol Chem. 2002;277:19922–8. doi: 10.1074/jbc.M200383200. [DOI] [PubMed] [Google Scholar]
- 19.Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem. 2000;275:38040–7. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–96. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 21.Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engieered cell sheets. Gastroenterology. 2012;143:582–8. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Shudo Y, Miyagawa S, Nakatani S, Fukushima S, Sakaguchi T, Saito A, et al. Myocardial layer-specific effect of myoblast cell-sheet implantation evaluated by tissue strain imaging. Circ J. 2013;77:1063–72. doi: 10.1253/circj.cj-12-0615. [DOI] [PubMed] [Google Scholar]


