Abstract
Background
Children with brain tumors present a complex set of factors when considering treatment decisions, including type and location of tumor and age of the child. Two-thirds of children will survive, but historically have had poorer neurocognitive and quality-of-life outcomes when compared with survivors of other childhood cancers. Delaying or forgoing cranial radiation completely is thought to lead to improved neurobiobehavioral outcomes, but there is still relatively little research in this area.
Objectives
The objectives of this study were to review and consolidate what is known about the effects of cranial radiation and chemotherapy on normal brain tissue and to synthesize that information relative to neurobiobehavioral findings in children with brain tumors.
Methods
A literature search using PubMed and PsycINFO from 2000 to 2011 was done using a variety of terms related to childhood brain tumor treatment and outcome. A total of 70 articles were reviewed, and 40 were chosen for inclusion in the review based on most relevance to this population.
Results
Both cranial radiation and certain chemotherapy agents cause damage to or loss of healthy neurons, as well as a decrease in the number of progenitor cells of the hippocampus. However, in general, children treated with chemotherapy alone appear to have less of a neurobiobehavioral impact than those treated with cranial radiation.
Conclusions
The trend toward delaying or postponing cranial radiation when possible may improve overall neurocognitive and quality-of-life outcomes.
Implications for Practice
Nurses require knowledge of these issues when discussing treatment with families and with caring for long-term survivors.
Keywords: Brain neoplasm, Cognitive aspects, Cranial irradiation, Drug therapy, Nervous system injury, Neurobehavioral manifestations, Quality of life
Brain tumors are the second most common type of cancer in children, affecting approximately 1 in every 30 000 to 40 000 youth in the United States.1 For survivors, neurocognitive deficits are common, affecting 40% to 100% of children, depending on age at treatment and history of cranial radiation or types of chemotherapy,2 and may not become fully realized until years after treatment has ended. Neurocognitive deficits have been linked to poor educational attainment, difficulty finding employment, and behavioral and social difficulties, all of which may contribute to poor quality of life (QOL).3 Central nervous system (CNS) injury after treatment of a brain tumor can be linked to the initial presence of the tumor and resulting edema or hydrocephalus or to any therapeutic modality such as surgical intervention, cranial radiation, or chemotherapy.
More recently, investigators have studied the physiological processes involved when giving chemotherapy and radiation to eradicate or provide prophylaxis against malignancies in the CNS. Cranial radiation, the cornerstone of pediatric brain tumor therapy for years, contributed to a 5-year survival rate of approximately 66%. However, as long-term survival was achieved, radiation to the brain was also identified as an important causative factor for neurocognitive deficits, ranging from global loss of intelligence quotient (IQ) points to attention problems.4–6 More severe neurocognitive deficits, specifically difficulty with abstract thinking, correlate with younger age at treatment and higher doses of radiation.7
Cranial radiation may be more damaging to the developing brain of a child than to the fully developed adult brain.8 This issue has been so strongly recognized that many young children are now treated on protocols with chemotherapy alone.9,10 Yet, even less is known about late effects of chemotherapy on the child’s developing brain. The rapid postnatal brain development that occurs during early childhood makes children particularly vulnerable to neurocognitive damage.11,12 Multiplication of glial cells and myelination of axons begin during gestation and continue during early childhood to the age of 5 to 7 years, even extending into the third decade in certain areas of the brain such as the prefrontal cortex. If normal brain development is interrupted by an insult, such as that of neurotoxic chemotherapy or cranial radiation, the branching and myelination processes of the developing brain can be delayed or thwarted, and ultimately, brain growth and functional outcome will be limited.11 In addition, during a time of rapid brain growth, it requires less of an insult to make a more dramatic lasting effect.11,13–15
Because radiation therapy results in deleterious consequences, high-dose chemotherapy followed by autologous hematopoietic stem cell rescue (AuHCR) has become more common as a frontline treatment for brain tumors in children younger than 6 years.9 This has resulted in a 5-year survival rate (57%–79%) that is within the range achieved by radiation and chemotherapy.10 Medulloblastoma has proven to be particularly sensitive to this treatment, resulting in improved survival and neurocognitive outcomes in very young children.16 The chemotherapy agents most commonly used in high-dose regimens for childhood brain tumors are cisplatin (CDDP), cyclophosphamide (CPM), etoposide, methotrexate (MTX), thiotepa, carboplatin, and topotecan.10,17,18 Few studies have examined the neurotoxic effects of high doses of this type of chemotherapy in children, focusing instead on more acute organ toxicities and survival statistics.
This article reviews the literature on the effects of cranial radiation and chemotherapy agents used to treat brain tumors in children on healthy brain tissue and discuss outcomes after treatment. There are limitations to the interpretation of the body of clinical research available on this population. Most of the research on CNS injury and neurocognitive outcomes due to pediatric cancer therapy involves subjects treated with a combination of cranial radiation, chemotherapy, and corticosteroids, making it difficult to distinguish between direct and interaction effects of each intervention. Thus, included in this review are relevant research studies in animal models and in general pediatric oncology.
A literature search was performed in PubMed and PsycINFO for the years 2000 to 2011, using a combination of key words: neuronal injury, neuronal loss, chemotherapy, radiation, neurocognitive, quality of life, brain tumors in children, childhood, and pediatric. Inclusion criteria for in vitro and rodent studies were clinically relevant treatment, and inclusion criteria for human studies were treatment for pediatric brain tumors. Exclusion criteria included duplicate or very similar findings to other articles. Some earlier important works were also included; one, a classic early article from 1963, establishing a link between cranial radiation, CNS injury, and behavior change, and a few brain tumor studies from the 1990s to demonstrate critical findings.
CNS Structure/Function
As a brief review, the CNS includes the brain and spinal cord. The brain itself is made up of neurons and glial cells that differentiate from neural progenitor cells (Figure 1). Neurons have a cell body, an axon, and many dendrites, and they send (via axons) and receive (via dendrites) signals by forming synapses with other cells. Astrocytes and oligodendrocytes are types of glial cells. Astrocytes provide a supportive structure and nutrients to neurons, whereas oligodendrocytes form the myelin sheaths that surround and insulate axons, facilitating transmission of synaptic signaling.19 White matter consists of myelinated axons, whereas gray matter is largely made up of cell bodies.
Figure 1.

Differentiation of neuronal cells. Reprinted by permission from Macmillan Publishers Ltd: ONCOGENE (Umberto Galderisi U, Jori, FP, Giordano A. Cell cycle regulation and neural differentiation. Oncogene. 2003;22:5208–5219), copyright (2003).
Although different parts of the brain have specific functions (Figure 2), there is some overlap. Cognition, specifically, requires the interaction of thinking, memory, perception, and language. Because many brain regions are involved in this process, dysfunction in 1 area can create a disability that may or may not be overcome.20
Figure 2.

Functional area of the brain. Source: http://www.ftdrg.org/caregivers/ftd/overview-of-brain-structure-and-function-in-ftd/. Accessed November 1, 2011. © Frontier, 2008. Permission granted by Professor Hodges (November 2, 2011).
The presence of a brain tumor alone, with or without resulting hydrocephalus, may injure normal brain tissue. Once the open sutures and fontanels of the infant’s skull close, the presence of a mass in the brain will cause increased intracranial pressure when it reaches a certain volume. Vasogenic edema, as a result, may cause damage to healthy brain tissue, if not treated in a timely manner.21 Delicate brain tissue may also be injured during surgical resection of the tumor, manifesting as either traumatic brain injury or ischemic injury.22
CNS Injury From Cranial Radiation and Chemotherapy
In Vitro and Rodent Studies of Radiation/Chemotherapy Effect on CNS
Basic science research has provided much of the evidence regarding neurotoxic effects of cancer therapies in children. It demonstrates that both cranial radiation and certain chemotherapy agents affect a variety of normal healthy brain cells, in addition to eradicating cancer cells. A summary of this literature is found in Table 1.
Table 1.
Neurotoxic Effects of Cranial Radiation (CRT) and Chemotherapy on In Vitro Cells and Rodents
| Study | Origin of Specimen(s) | Agent/Dose | Effect on CNS | Comments/Limitations |
|---|---|---|---|---|
| CRT: in vitro; Panagiotakos et al,23 2007 | Adult human radiated tissues compared with nonradiated glial tissue near lesion | 18- to 21-Gy focal CRT (radiosurgery) |
Loss of oligoprogenitors 2 mo post-CRT, still seen up to 7 y post-CRT. Progressive degradation of myelin sheaths over time, possibly related to axonal damage | Limitation: findings may be complicated by effects of aging. Demyelination occurred prior to vascular necrosis |
| CRT: rodents; Amano et al,24 2002 | 210 male rats, 6 wk of age; 30 age-matched controls | Random assignment to 1-, 2-, or 3-Gy single dose | Brain weight of irradiated rats 50%–62% of controls over 60 d. Apoptosis in SVZ ↑ 6 h post-CRT, disappeared within 2 d | Single dose of CRT may cause brain growth retardation. Limitation: 1-time low dose of CRT not clinically relevant |
| Monje et al,25 2002 | Adult rats | 10-Gy single dose CRT (approximates 2 Gy in humans) |
Surviving proliferating cells in DG, SGZ 38% at 1 mo post-CRT,a 52% at 2 mo post-CRTb compared with nonirradiated controls | Radiation detrimental to neural stem cells, to signaling process for neurogenesis |
| Ordy et al,26 1963 | 40 female mice, 10 controls | 80–720 Gy to various brain areas | 7 d After 720 Gy, early cell pyknosis, vascular congestion; after 7 mo, nerve cell bodies gone; 21 d after 80 Gy, pyknotic granular cells, proliferation of astrocytes, swelling of endothelial nuclei, granular cells disappeared | One of first to postulate that CRT injury may be influenced by dose of radiation, time since radiation |
| Panagiotakos et al,23 2007 | Adult rat brains | Single-dose 25-Gy WBRT | Progenitor cells in SVZ ↓ by 89% 1 d after WBRT. 15 mo later, average no. of progenitor cells in irradiated rats 15% that of controls. In olfactory bulb, delayed ↓ neuroblasts after 2 wk likely due to ↓ neurogenesis; restored after 6 mo. Endothelial cells ↓b 1 d after CRT, normal levels 2 mo later | Demonstrates effect of loss of neural progenitor cells in SVZ on nonirradiated areas of brain (olfactory bulb) outside hippocampus with no significant recovery over time |
| Rao et al,27 2011 | 1-mo-old male mice; nonirradiated controls | 4 Gy/d WBRT for 5 consecutive days | 83.4% ↓ in migrating neurons at 72 h and 1 mo post-CRTc 88.3% ↓ in proliferating cells of HC at 72 hb and 1 moa post-CRT | Simulated clinical dose of CRT in children |
| Rola et al,28 2004 | 21-d-old mice | 2- to 10-Gy WBRT | 48 h after initial dose, ↓c in proliferating cells in SGZ: 35% ↓ with 2 Gy, 93% ↓ with 10 Gy. Lossc of immature neurons: 12% after 2 Gy, 75% after 10 Gy. At 1, 3 mo post-WBRT, ↓ neuronal differentiation.a At 3 mo, ↑ activated microglia,b representing inflammatory response | May help to explain greater impact of radiation on younger brain |
| Rubin et al,29 1994 | Male rats, 225–250 g | 60-Gy CRT | Extensive leakage of contrast agent across BBB at 2 wk; some resolution at 6–8 wk. WM necrosis moderate-severe beginning at 24 wk post. At 24 wk post-CRT, increased leakage, cortical atrophy | Limitation: large single dose of radiation not representative of real-life therapy, but given to detect injury in shortest period possible |
| Chemo: in vitro, Dietrich et al,30 2006 | Nondividing brain tissue cells and cancer cell lines | Carmustine 0–200 μM CDDP 0–100 μM |
Carmustine, CDDP toxic to normal progenitor cells, 40%–90% ↓ in normal neuron viability; also toxic to oligodendrocytes | No tumor cell lines more sensitive to chemotherapy agents than normal progenitor cells were, even at low levels of exposure |
| James et al,31 2008 | Rat hippocampal, cortical neurons | Taxol CDDP MTX For 2, 5, or 9 d (dose not specified) | Taxol-treated cells: fewer secondary dendrites, shortened primary dendrites CDDP-treated cells: less dendritic branching, shortened dendrites MTX-treated cells: 40% likelihood of having only one major dendrite and shortened primary dendrites | All agents caused degeneration of neurites, changes to actin cytoskeleton of neurons. No microglial activation seen, suggesting lack of CNS inflammation |
| Rzeski et al,32 2004 | Neuronal cell cultures | 5- to 100-μM doses of MTX ifosfamide CDDP CPM thiotepa, and vinblastine: 0.1–1 μM | Cell shrinkage, death to neurons dose dependent with all agents; vinblastine > CDDP | Limitation: concentrations of chemo agents > clinical doses, but may be representative of combination therapy over several courses |
| Wick et al,33 2004 | Postmitotic primary cerebellar granule neurons, astrocytes from 8-d-old rat brains | Lomustine CDDP VCR topotecan | All agents ↓ cerebellar granule cell viability after 72 h of exposure due to apoptosis: VCR > topotecan > CDDP > lomustine. For reducing primary astrocytes: topotecan > CDDP > VCR > lomustine. For malignant glioma cell reduction: VCR > topotecan > CDDP > lomustine | Rare study on CNS effect of VCR. Difficult to explain high sensitivity of cerebellar granule cells to certain agents as compared with astrocytes |
| Chemo: rodents, Dietrich et al,30 2006 | 6- to 8-wk old mice | IP carmustine: 30 mg/kg, CDDP: 15 mg/kg, cytarabine: 0.1 μm concentration in CSF (equivalent to human conventional treatment) | Carmustine: 16.1-fold ↑ apoptotic cells in SVZ for at least 6 wk, 13.3-fold ↑ in CC, 3.8-fold ↑ in DG. CDDP: similar effect in DG, modest ↑ in CC, no significant ↑ in SVZ. Cytarabine: 2.4-fold ↑ in apoptotic oligodendrocytes, significant apoptosis in SVZ up to 56 d later, in DG and CC until 14 d later | Agents given systemically, not IT, not generally thought to cross BBB. Effect on cells of CNS is concerning as many forms of cancer treated with these agents |
| Mignone and Weber,34 2006 | Twelve 6-wk-old mice | Thiotepa: 1 mg/kg, 5 mg/kg, 10 mg/kg IP vs saline (3 mice per group) | Dose-related ↓ of up to 80% of neurons of DG: 52%c at 1 mg/kg, 71%–83%c at 5–10 mg/kg | Very large documented ↓ of proliferating cells in DG has potential to affect cognition |
| Rzeski et al,32 2004 | 7-d-old rats | IP CDDP: 5/10/15 mg/kg, CPM: 200/400/600 mg/kg, thiotepa: 15/30/45 mg/kg, ifosfamide: 50–500 mg/kg | Swelling of dendrites within 4 h of administration of each agent. Widespread degeneration of neurons with CDDP 5 mg/kg,b 10 mg/kg,c 15 mg/kgc;with thiotepa 15,a 30,a 45a; with CPM 400,b 600c; with ifosfamide 300,a 500c; Appeared due to apoptosis | Limitation: chemo dosage given exceeds usual clinical doses in pediatrics, but not in HD regimens given to adults |
Abbreviations: BBB, blood-brain barrier; CC, corpus callosum; CDDP, cisplatin; CNS, central nervous system; CPM, cyclophosphamide; CRT, cranial radiation therapy; CSF, cerebrospinal fluid; DG, dentate gyrus; HD, high-dose; IP, intraperitoneal; IT, intrathecal; MTX, methotrexate; SGZ, subgranular zone; SVZ, subventricular zone; VCR, vincristine; WBRT, whole-brain radiation therapy; WM, white matter.
P < .01.
P < .05.
P < .001.
A major cause of cellular injury results from the process of oxidative stress and inflammation triggered by radiation and/or chemotherapy. Within hours to days after exposure to cranial radiation, ongoing release of proinflammatory cytokines from damaged cells supports a chronic state of inflammation in the rodent brain.35 During the cycle of oxidative stress and inflammation, cell membrane breakdown and cell death, apoptosis (programmed cell death), demyelination, and loss of integrity of the blood-brain barrier (BBB) are common. Disruption of the BBB also contributes to edema and cell membrane breakdown and loss, as the regulatory mechanisms that control passage through this important barrier fail, creating a cycle of continuing injury.
Pathological changes in the mouse brain after cranial radiation were noted 5 decades ago26 with the findings that extreme doses of cranial radiation (720 Gy) caused widespread destruction of a variety of brain cells that were gradually replaced with fluid-filled cavities within 7 months of treatment. Even significantly less (80 Gy) but still very high doses of cranial radiation induced signs of active inflammation in the brain at 21 days after treatment.26 Although such high radiation doses are not used today, this 1963 study provided important early information about the chronic injurious nature of radiation.
There is evidence that several chemotherapy agents cause injury in a similar manner. Administration of intrathecal and systemic MTX has been linked to the presence of oxidative stress markers in the cerebrospinal fluid of children on treatment for acute lymphoblastic leukemia.36,37 Disruption of axonal and dendritic networks appeared in neuronal cultures exposed to ifosfamide, vinblastine, cyclophosphamide, CDDP, MTX, and thiotepa.31,32 Although this may not overtly cause cell death, it renders the affected neurons dysfunctional.
Cyclophosphamide appears to be more toxic to the young, developing brain than to the adult brain, causing brain tissue loss in many more areas in younger rodents than in older ones.32 Cerebellar granule cells, tiny neurons found in the cerebellum, are similarly affected by chemotherapy, and many die within 72 hours after exposure to lomustine, CDDP, vincristine (VCR), and topotecan.33 These cells were particularly sensitive to low concentrations of VCR. In addition, VCR was toxic to astrocytes. Vincristine is a known potent peripheral neurotoxin,38 but there has been little research on its effects on cells of the CNS.
Although cranial radiation results in generalized white and gray matter loss in the brain, its effect on the hippocampus is of particular importance, because this is a primary site of neurogenesis, the generation of new neurons from progenitor cells, after the early postnatal period. Specific areas of the hippocampus where proliferating (growing and dividing) cells may be found include the subventricular zone, subgranular zone, and dentate gyrus. A single low dose of radiation to the adult rat brain (1–3 Gy) led to significantly less brain growth over time, with an acute decrease in the number of cells in the subventricular zone.24 Proliferating cells decreased markedly up to 2 months after a single 10-Gy dose of cranial radiation.25 After exposure to radiation, remaining progenitor cells have a stronger tendency to differentiate into glial cells than into neurons, which may reflect an alteration of the signaling process.25 The exact mechanism for the failure of neurogenesis is unknown but may be related to alterations in the microenvironment of the hippocampus and the presence of proinflammatory cytokines or to interference with normal molecular and cellular interactions.25 Moreover, this reduction in hippocampal proliferating cells due to radiation given early in life persisted into adulthood.27 These processes may help to explain the more severe neurocognitive effects of radiation found in children, as compared with adults.27,28 Even in adults, loss of oligoprogenitor cells, precursors to the cells that form myelin, persisted for up to 7 years after radiation therapy.23
As with radiation, chemotherapy appears to preferentially affect cells of the hippocampus. Methotrexate injures neural progenitor cells in the hippocampus,39 which is believed to account for delayed neurotoxicities (progressive cognitive dysfunction, neuropathies, cerebral atrophy, cerebellar toxicity) associated with that treatment. Systemic administration of thiotepa, an antimitotic agent that effectively crosses the BBB, resulted in significant neuronal loss in the mouse hippocampus.30,34 Cisplatin exposure caused an increase in apoptosis of cells in the dentate gyrus,30 The effects of CDDP, carmustine and cytarabine, given separately and in combination, on both quiescent brain tissue cells and cancer cell lines suggest that these agents are even more toxic to neural progenitor cells than to the cancer cells, decreasing viability by up to 90% for at least 6 weeks after chemotherapy exposure.30
In summary, the literature suggests that cranial radiation results in both acute and chronic injury to a wide variety of cells, leading to demyelination and areas of necrosis of the brain. Both chemotherapy and radiation appear to target neural progenitor cells in the hippocampus, leading to notable decreases in the number of new neurons. As a site of memory consolidation, hippocampal cell loss is detrimental to cognition. The time progression of damage to normal cells after treatment with chemotherapy indicates that CNS cell death may occur within 24 hours of treatment and continue for up to 6 weeks and may cause more widespread neuronal degeneration in the young brain than in the adult brain. The multiple mechanisms by which cell injury occurs, including cell shrinkage, axonal and dendritic disruption, and apoptosis of cerebellar granule cells and neural progenitor cells, resulting in white and gray matter loss, have also been explicated.
Pediatric Brain Tumor Studies of Radiation/Chemotherapy Effect on the CNS
A summary of the literature on the effects of CNS cellular injury and loss after brain tumor therapy in childhood is found in Table 2. One of the most commonly reported effects of cancer treatment evident on pathological examination and with certain brain imaging techniques is a loss of white matter, or demyelination. After cranial radiation, demyelination appears within 5 months, with vascular structural changes and necrosis occurring about 9 months later.43 Five years after radiation, significant structural damage to the brain appeared and continued to progress.43 After treatment with 35- to 40-Gy cranial radiation, normal-appearing white matter (NAWM) continued to decrease at a rate of 0.3 mL/y over 5 years.44
Table 2.
Neurotoxic Effects of Cranial Radiation (CRT) and Chemotherapy in Children
| Study | Origin of Specimen(s) | Chemotherapy Agent(s) and Dose(s) | Effect on Neurons | Comments/Limitations |
|---|---|---|---|---|
| Kellie et al,40 2005 | 12 children with BT diagnosed >36 mo of age, median follow-up: 7.5 y. Mean age at diagnosis: 7.6 y | HD-MTX 8 g/m2 × courses etoposide 200 mg/m2 × courses CBDCA 700 mg/m2 × courses. Also conventional WBRT 35–50.4 Gy | 66%: Grade I leukoencephalopathy, 33%: grade II. Grade II treated with higher doses of WBRT. WM changes in grade II began within 1 y of WBRT with growing subarachnoid space, appearance of lacunae 5–6 y post-WBRT | Difficult to attribute toxicity to either treatment modality. Limitation: small sample size, multimodal treatment |
| Monje et al,41 2007 | Human hippocampi on autopsy of 3 children (2 with MBl (both 7 yo), 1 with AML (10 mo) | AML: 13.2 Gy TBI, with 10 chemo agents MBl: 23.4 Gy CSRT with boost to PF, 6 chemo agents |
100-fold ↓ in neurogenesis compared with age-, gender-matched controls 10-fold ↓ in neurogenesis compared with controls |
First study to demonstrate impact of CNS therapies on human hippocampal neurogenesis. Limitations: unable to assess for neuronal maturation |
| Nagel et al,42 2004 | 25 children with MBl, mean age at diagnosis 8.27 y | All received 55.8 Gy to tumor bed, 23.4- to 39.6-Gy WBRT, plus adjuvant chemo: HD CPM CDDP VCR | Time since diagnosis related to right, left HC volume,a declining until 2 y post-CRT, then resuming + growth. Girls had steeper ↓ in right,b leftc HC volume | First study to evaluate HC development in this population. Girls may be more affected than boys. Limitation: no control group |
| Oi et al,43 1990 | Postmortem study of 34 children, mean age 6.5 y, all with glioma | 65%: mean dosage 40.63-Gy WBRT or WB/CSRT; 35% did not receive any treatment | 32% of irradiated brains showed demyelination; 27%: necrosis or focal vacuoles; 18%: cortical atrophy; 18%: endothelial proliferation; 9%: vascular thrombosis; 5%: telangiectatic vascular proliferation, thickening. Findings of this sort rare in nonirradiated brains. Abnormalities most severe in WM | Limitation: did not relate damage seen to CRT dose or site |
| Reddick et al,44 2005 | 52 children with MBl, mean age at CRT 8.3 y, mean age at examination 13 y; 26 healthy controls | All received 35- to 40-Gy CRT; 73% received 1 or more of CBDCA, etoposide, VCR, CPM, CCNU, topotecan, CDDP | Quadratic random coefficient model: volume of NAWM as function of time since CRT,a time age at CRT,a presence of shunt.c Deficita in NAWM compared with controls at one timepoint | Limitations: single section of imaging only where CRT dose not uniform, wide range of chemo agents also given |
Abbreviations: AML, acute myelocytic leukemia; BT, brain tumor; CBDCA, carboplatin; CCNU, lomustine; CDDP, cisplatin; CNS, central nervous system; CPM, cyclophosphamide; CRT, cranial radiation therapy; CSRT, craniospinal radiation therapy; HC, hippocampus; HD, high-dose; MBl, medulloblastoma; MTX, methotrexate; NAWM, normal-appearing white matter; PF, posterior fossa; TBI, total body irradiation; VCR, vincristine; WB, whole brain; WBRT, whole-brain radiation therapy; WM, white matter.
P < .001.
P < .05.
The volume of the hippocampus was also affected by radiation, continuing to decrease bilaterally on magnetic resonance imaging for 2 to 3 years after diagnosis, but then returning to a normal growth pattern.42 On autopsy examination of the hippocampi of children, a 100-fold decrease in neurogenesis after total body irradiation (13.2 Gy) and a 10-fold decrease in neurogenesis after 23.4-Gy craniospinal radiation and focal cranial radiation were observed. As is typical in most clinical samples, these patients were also treated with chemotherapy and with corticosteroids, known inhibitors of neurogenesis, so these differences may not solely be attributable to radiation.41
There is also evidence of gray and white matter loss in children treated for brain tumors with standard dose chemotherapy combined with cranial radiation.7 With higher doses of MTX and cranial radiation, an increased incidence of leukoencephalopathy (white matter changes related to endothelial cell loss and demyelination) was seen at a median 7.5 years after therapy.40 Those children with more severe changes had all received higher doses of radiation. Because both cranial radiation and MTX have been associated with leukoencephalopathy, an interaction effect of both treatments may exist.
Model of Injury of Cancer Treatment to CNS
Figure 3 demonstrates the similarities in the injurious processes of chemotherapy and radiation to healthy brain tissue. An acute cranial radiation effect is an increased expression of intracellular adhesion molecule 1 (ICAM-1) within 24 hours of exposure, which contributes to disruption of the BBB.35 The integrity of the BBB is compromised by damage to vascular endothelial cells, leading to edema, further inflammation, and tissue hypoxia.4 In response to cell injury, acute and chronic oxidative stress processes damage cellular mitochondria and result in the formation of oxidative products and enzymes such as lipid peroxidase, which contribute to continuing cell membrane breakdown.36 Like radiation, chemotherapy induces oxidative stress in the CNS45–47 through the release of cytokines such as tumor necrosis factor and interleukin 1β, contributing to demyelination35 and white matter loss.
Figure 3.
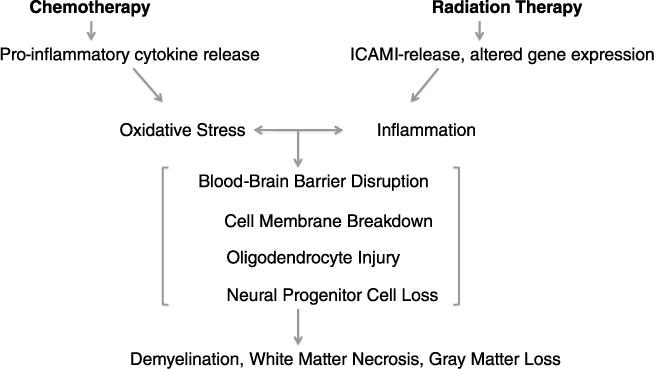
Mechanisms of radiation/chemotherapy injury to healthy brain tissue.
Chronic oxidative stress secondary to radiation or chemotherapy also inhibits hippocampal neurogenesis, which appears related to memory dysfunction.30,34,48 Therefore, the 2 common mechanisms of injury from chemotherapy and radiation resulting from oxidative stress are CNS cell injury and/or death leading to white and gray matter loss, and decreased hippocampal neurogenesis leading to a drop in the development of new neurons. These 2 separate mechanisms contribute to the most common neurocognitive deficits in brain tumor survivors. Damage to the hippocampus or to its progenitor cells results in memory problems, specifically with short-term memory, which can be very detrimental to learning.49 Loss of gray and white matter in certain areas of the brain may result in attention and executive functioning problems50–52 and decreased IQ.
CNS Cell Loss and Neurobiobehavioral Outcome
It is important to investigate the impact of CNS injury on developmental and neurocognitive outcomes. The loss of cells in cortical and subcortical regions in the brain induced by chemotherapy and radiation predicts neurocognitive deficits in children with brain tumors. Life-threatening illness and aggressive treatment at a young age can affect future physical, emotional, cognitive, and psychosocial health.
Neurocognitive deficits in children with brain tumors have been almost exclusively studied in those who received a combination of radiation and chemotherapy or with radiation alone. Children treated with cranial radiation before the age of 5 years have much poorer neurocognitive outcomes than those treated in older childhood and adolescence.53 Research on rodent models of the relationship of treatment-induced CNS injury and loss to neurocognitive deficits is summarized in Table 3.
Table 3.
Effect of Cancer Treatment on Neurobiobehavioral Status in Rodents
| Study | Origin of Specimen(s) | Treatment | Effect on Neurons/Cognition | Comments/Limitations |
|---|---|---|---|---|
| Ordy et al,26 1963 | 50 female mice, 10 controls | 80–720 Gy to various brain areas | Difference in mean overall scores of open-field latency between subjects and controls. Beginning in month 5, bilateral parietal CRT group scored highera on open-field latency. Right cerebellar CRT group significantly slower on locomotor wheela | Very early study relating CRT to behavior changes. Delayed behavioral effects on mice after radiation may be due to necrosis, vascular damage after high doses of radiation |
| Rao et al,27 2011 | 1-mo-old male mice and controls | 4-Gy WBRT for 5 consecutive days | Impairment of nonspatial learning at 1 mob and 5 moa post-CRT | First study to demonstrate continued ↓ in memory behavior long-term after early fractionated CRT to young rodents. |
| Rola et al,28 2004 | Twenty-four 21-d-old mice; 12 subjects, 12 controls | 5-Gy WBRT to subjects, controls sham-irradiated | Irradiated mice had deficits in memory retention 3 mo after WBRT,a greater numbers of immature neurons after behavioral training than nonirradiated micea | Increased immature neurons in behaviorally trained mice after WBRT likely due to learning-enhanced survival rather than physical activity of training, may lead to improved cognitive functioning. Limitation: single-dose radiation not representative of pediatric therapy |
| Seigers et al,54 2008 | Adult male rats | HD-MTX 250 mg/kg, controls received saline | Animals treated with MTX: longer latency timeb for spatial memory. Control rats had better novel recognitionb | Longer latency time indicates impaired spatial memory, failure to distinguish familiar object from novel reflects ↓ HC functioning |
| Seigers et al,55 2009 | Adult male rats | HD-MTX 250 mg/kg, controls received saline | Rats treated with MTX spent less time in platform quadrant,b had less freezing behavior, indicating fearc | Results from both tests indicate retrograde amnesia, impaired memory consolidation after learning specific behaviors, due to MTX |
| Winocur et al,56 2006 | 25 female adult mice | MTX 37.5 mg/kg IP and 5-fluorouracil 75 mg/kg IP each week for 3 wk. Control mice given IP saline injections | Chemo-treated mice had longer latencies, more errors on spatial memory testing on day 1, but similar to controls as time went on. On delayed NMTS, chemo group had treatment × delay interaction on latencyc and errorc scores | Spatial memory, conditional rule learning, longest delay of nonspatial memory test affected by chemo. Limitations: with administration of 2 drugs, cannot ascribe effects to either. Differences in spatial memory could be attributed to hyperarousal or hyperactivity |
Abbreviations: CRT, cranial radiation therapy; HC, hippocampus; HD, high-dose; IP, intraperitoneal; MTX, methotrexate; NMTS, nonmatching to sample learning; WBRT, whole-brain radiation therapy.
P < .01.
P < .05.
P < .001.
Rodent Models of Neurobiobehavioral Effects After Radiation and/or Chemotherapy
In an early study of adult rodent behavior after cranial radiation, mice treated with 80 Gy demonstrated small but significantly different behavior changes, depending on the area of the brain treated. For example, rodents who received cerebellar radiation had locomotor problems, whereas those radiated in the parietal areas had more difficulty with tasks involving memory.26 Young mice (21 days old) experienced memory retention deficits that correlated with a decrease in neurogenesis in the subgranular zone after a single 5-Gy dose of whole-brain radiation.28 Five consecutive days of 4-Gy whole-brain radiation resulted in long-term impairment of nonspatial learning in 1-month-old mice.27
Systemic high-dose MTX caused a loss of healthy neurons in the hippocampi of rats as well as concurrent changes in neurocognitive functioning, specifically, difficulties with spatial learning and object recognition tasks.54 High-dose MTX–treated adult mice had an inadequate response to fear conditioning, which represented cognitive and memory deficits.55 Mice treated with combined standard-dose MTX and 5-fluorouracil had difficulty with spatial memory tests on the day after treatment, but these deficits normalized with time.56
Pediatric Studies of Neurobiobehavioral Effects After Radiation and/or Chemotherapy
A summary of the studies of the relationship of treatment-induced CNS injury and loss to neurocognitive deficits in children is presented in Table 4.
Table 4.
Effect of Cancer Treatment on Neurobiobehavioral Status in Children
| Study | Origin of Specimen(s) | Treatment | Effect on Neurons/Cognition | Comments/Limitations |
|---|---|---|---|---|
| Barrera et al,57 2005 | 800 childhood cancer survivors (average age at diagnosis: 2 years, average age at study: 12 y). 36.6% leukemia, 15.2% CNS tumor, 48% other cancers. 923 age-, gender-matched controls | 21.3% IT MTX only 15.6% CRT and IT MTX 9% CRT only | Those with BT, leukemia: highest odds ratios of repeated/failed grade in schoola; attending learning disabled programa; academic problems.a Those who received CRT had higher odds ratiosb of above categories | Limitations:mailed questionnaires without objective measurement, survivors had wide range of diagnoses and treatment. An imperfect downstream indicator of neurocognitive deficits |
| Benesch et al,58 2009 | 23 children with MBl (78%) or ependymoma (22%) at median 56 mo after diagnosis; 8 children with glioma diagnosed with surgery only were controls | No treatment information, only that itwas multimodality | No significant difference in quality-of-life scores in relation to neurocognitive testing results, no significant difference between groups on KINDL | Limitations: small n; 74% were male; no treatment info |
| Fouladi et al,50 2004 | 127 children with MBl or supratentorial PNET | 36% HR: 36 Gy CSRT, 55.8 Gy boost; 59% AR: 23.4 Gy CSRT, 55.8 Gy boost; all received HD chemo: CDDP 75 mg/m2 × 4 CPM 4g/m2 × 4 VCR 3mg/m2 × 4; some also received topotecan | 17% had WML: 32% grade I, 68% grade II; most common locations pons, cerebellum. 14% of patients with WML had neurologic symptoms. Cumulative index of patients with WML at 1 y: 15%, at 2 y: 17.5%. No difference by risk group, diagnosis, cumulative dose of CPM. IQ ↓c in those with WMLs, but not in those without; decline in mathb for those with WMLs but not without | Those with WMLs had significant cognitive decline over time as compared with those without WMLs. Limitation: cannot determine whether WMLs due to CRT, HD chemo, or combination |
| Khong et al,59 2003 | 9 MBl survivors (mean age at diagnosis 7.8 y, mean age at study 10.8 y) 12 healthy age-matched controls | CRT (30.6- to 40-Gy WBRT with 50.4–54 Gy boost to PF) and chemotherapy (VCR, CDDP, and/or etoposide, CPM) | FA ↓ by 12.4%–19% in all areas in subjects as compared with controls. In children <5 y at treatment, 26.7% ↓ in supratentorial FA compared with controls, 23.2% ↓ in those >5 y at treatment. Severe deterioration in academic performance correlated with 46.2% ↓ in supratentorial FA | First study to show DTI sensitivity to neurotoxicity, may be correlated to academic performance. Limitations: small sample size, subjects treated with range of radiation doses and chemotherapy agents |
| Khong et al,60 2006 | 12 MBl, 18 leukemia survivors, 55 healthy age-matched controls; mean age at study 13.1 y | BT treated with CSRT (23.4–40 Gy), WBRT (23.4–40 Gy), PF CRT (50–55.8 Gy) and chemo (VCR, CPM, CDDP, etoposide or CCNU, CDDP, VCR). Leukemia treated with chemo (including IT and HD-MTX); 50% received CSRT (12–24 Gy) | Test scores among brain tumor survivors < ALL survivors who received CRT < ALL without CRT < controls but not statistically significant. Difference in WM FA between subjects and controls significantly correlated with FSIQ,a VIQ,b PIQa | Follow-up of previous study. Wide variability in range of radiation doses and chemo agents but demonstrated differences in neurocognitive outcome related to treatment modality |
| Mabbott et al,61 2005 | 53 survivors of PF tumors, mean age at diagnosis 6.6 y | 26%: reduced-dose CRT (23.4–30.2 Gy), 64%: standard-dose CRT (34–41.4 Gy), 8%: dose unknown. All received boost to PF of 45–55.8 Gy; 74% received chemotherapy | Older age at diagnosis correlated with better reading,b school performance scores by parents.b Longitudinal analysis: decline in math,c spelling,c reading.c Parents’/teachers’ school functioning ratings ↓ over time, with ↑ social,c attentionb problems | Longitudinal data demonstrate continued academic declines over time. Limitation: mixed therapies |
| Mabbott et al,62 2006 | 8 children with MBl (7 males, 1 female), mean age at diagnosis 7.5 y, mean time to study 2.5 y; 8 healthy controls | 50% received 36–36.6 Gy CSRT; 50% received 23.4 Gy CSRT; 100% received PF boost to 55.4 Gy. All received either etoposide/CDDP/CPM/VCR or CCNU/VCR/CDDP | Initial mean IQ 17.5 points < controls; mean decline over 2.5 y: 8 patients.a ↓ IQ related to ↑ ADC.b Correlation of low IQ, low FA.b FA in all ROIs lower in subjects than controls | Deficits in IQ outcome related to CRT may be result of tissue compromise. FA, ADC sensitive measures of tissue damage related to loss of progenitor cells, loss of myelin. Limitations: small sample size, only 1 female included, higher mean IQ at baseline of control group may have skewed results |
| Maddrey et al,63 2005 | 16 MBl survivors, mean age at diagnosis 7.2 y, mean age at study 22.2 y | All received CRT. 56% received unspecified chemotherapy | Mean IQ = 75, mean vocabulary score extremely low average, mean Block Design within mental retardation range. 67%: impairment in global intellectual functioning, 92%: impairment on 1 test of attention, 79%–86%: impaired on executive function tests | Limitations: small sample size, cross-sectional design, multimodal therapy without specific agents defined |
| Mulhern et al,7 2001 | 42 children with MBl, mean age at diagnosis 8.2 y, mean age at study 13.4 y | All received 23.4–36-Gy CRT with PF boost 49–54 Gy. 69% received chemo with 1 or more of: CDDP, etoposide, CPM, CBDCA, VCR, PCB, PDN | 70% of correlationc between IQ, age at CRT explained by NAWM volume; 90% for that between factual knowledge, age at CRT; 78% for verbal abstract thinking; 48% for nonverbal abstract thinking; model not significant for NAWM related to verbal memory, sustained attention | Limitations: small sample size, inconsistent number of studies done among subjects |
| Mulhern et al,51 2004 | 37 BT survivors; median age at diagnosis 6.5 y, median time since treatment 5.7 y | 18 received chemo: CDDP, CBDCA, CPM, VCR, nitrogen mustard, PCB, PDN. All received varying doses of focal CRT to tumor (49.2–70.2 Gy) and/or WBRT (23.4–44 Gy) | Subjects: lower norms on CCPT Overall index,a and 7/10 component indicesa relative to norms. Decreased NAWM volume associated with lower CCPT scores | First study to describe relationship between NAWM, attention; may be due to loss of NAWM over time after treatment or failure to develop NAWM age-appropriately. Limitations: cross-sectional, CCPT not comprehensive in assessment, ROIs did not include some areas involved in attention |
| Mulhern et al,64 2005 | 111 children with MBl, median age at diagnosis 7.4 y. 67% AR, 33% HR (with metastatic disease or residual tumor after surgery) | AR: 23.4 Gy CSRT, 36 Gy to PF, 55.8 Gy to tumor bed. HR: 36–39.6 Gy CSRT, 55.8 Gy to PF; all received HD CPM, CDDP, VCR | Tested postoperatively and at 1, 2, and 5 y after diagnosis. AR: mean loss of −0.4 patients/y compared with HR with mean loss of −8.2 patients/y.c Those ≥7 y at diagnosis declined in reading,b spelling.c Those <7 y declined in IQ,b reading,a spelling,a and mathc | Multi-institutional longitudinal study, models suggest AR subjects had better overall neurocognitive function. Limitations: some missing values, more young children had complication of PF syndrome |
| Penn et al,65 2008 | 37 children with BT (parents of 37 children, 27 children themselves completed tool). Median age at timepoint 1–9.4 y. Matched to healthy controls | No information about type of treatment | Differences in parent report of health-related QOL at 1, 6, 12 mo after diagnosis compared with controls.b For self-report at time 1, difference in all summary scores, school domain compared with controls.c At 6 mo, difference for total score, physical summary, school domainc | Longitudinal study showed discrepancy between child and parent report. Limitations: small sample size, no treatment-specific information |
| Reddick et al,52 2003 | 40 BT survivors, median age at study 12.8 y | CRT with or without chemotherapy | Correlations between WM volume and attentiona and IQ.c Memory not significantly correlated to WM volume | Developed model of therapy where ↓ NAWM correlates to attention deficits, which result in ↓ FSIQ, academic achievement. Limitations: mixed therapy, chemo agents not specified |
| Sands et al,66 1998 | 10 BT survivors, mean age 5 y 8 mo, mean time off-therapy 37.8 mo | 5 cycles of VCR CDDP VP-16 For bone marrow ablation: CBDCA, thiotepa, etoposide | Overall mean IQ 87.1 (19th percentile compared with peers). VIQ mean 88.6; PIQ mean 87.7, both low-average to borderline; 83%: high average to average on reading; 83%: impaired range on confrontational naming, expressive picture vocabulary; 77.8% within normal limits per behavioral checklist | Encouraging but overall mixed results in this early study of outcomes after HD chemo without CRT. Limitation: small sample size |
| Sands et al,67 2001 | 43 children with CNS germ cell tumors; average age at diagnosis 14.4 y, mean age at study 21 y | All received CBDCA, etoposide, bleomycin ± CPM; 67% received cranial CRT (G25–55.8 Gy) | Age at diagnosis correlated with FSIQ,b VIQ,c PIQ,c mathc | This study of therapy in older children demonstrated average results in most IQ scales. Limitations: two-thirds of subjects received mixed therapy, small sample size |
| Sands et al,18 2010 | 24 BT survivors, mean age at diagnosis 3 y | Received either VCR/CDDP/etoposide/CPM; or those agents plus HD-MTX; or VCR/CBDCA/temozolomide; and HD CBDCA, HD thiotepa, HD etoposide 33% received CRT | Time since diagnosis inversely related to FSIQ,c VIQ,c PIQ,c reading,c delayed verbal memory,b delayed verbal recognitionc | Limitation: those treated with CRT not separated from those who were not |
| Sands et al,68 2011 | 25 BT survivors; mean age at study timepoint 1: 8.1 y, at T2 (n = 19): 13.5 y | CDDP VCR VP-16 CPM, CBDCA, thiotepa, 28% received CRT | General Health mean scores “at risk” at T2, high percentage of children at-risk for withdrawal, social skills, leadership, somatization subscales. Younger age at diagnosis correlated with poor adaptability,c leadership skills.b Longer time at follow-up correlated with increased hyperactivity,b attention problemsc | Generally positive findings of quality of life in those treated on more current protocols. Limitations: small sample size, some received CRT, no baseline assessment done prior to therapy for comparison |
| Stargatt et al,69 2007 | 35 children with PF tumors, mean age at diagnosis 9.5 y; 43% MBl, 37% pilocytic astrocytoma, 14% ependymoma, 6% other; 23 completed entire study | 34% had surgery only; 6% had surgery, CRT; 57% had surgery, CRT, chemo; 54% received 50–59.6 Gy to tumor site; 3% received 39.6 Gy | CRT group had ↓ IQ points from diagnosis to 3 y later,b with no significant ↓ IQ in 1st y, ↑ in 2nd y,b ↓ in 3rd yb; Loss of attention spanb over time in CRT group | Longitudinal study providing data up to 3 y after treatment. Limitation: mixed therapy modalities |
| Ward et al,47 2009 | 31 children diagnosed with BT at < 3 y of age. Mean age at diagnosis 1.69 y, mean age at study 11 y | Surgery and/or chemotherapy, agents not specified | Those with > 1 surgical procedure had ↓ PIQ,c executive functioning.a Younger age at treatment correlated with ↓ FSIQ,c VIQ,c executive functioning.c Chemotherapy not related to cognitive outcome | Limitations: chemo agents not specified, no description of numbers of those treated with surgery vs chemo and surgery |
Abbreviations: ADC, apparent diffusion coefficient; ALL, acute lymphoblastic leukemia; AR, average risk; BT, brain tumor; CBDCA, carboplatin; CCNU, lomustine; CCPT, Connors Continuous Performance Test; CDDP, cisplatin; CNS, central nervous system; CPM, cyclophosphamide; CRT, cranial radiation therapy; CSRT, craniospinal radiation therapy; DTI, diffusion tensor imaging; FA, fractionated anisotropy; FSIQ, full-scale IQ; HD, high-dose; HR, high-risk; IQ, intelligence quotient; IT, intrathecal; KINDL, German QOL assessment tool; MBl, medulloblastoma; MTX, methotrexate; NAWM, normal-appearing white matter; PCB, procarbazine; PDN, prednisone; PF, posterior fossa; PIQ, performance IQ; PNET, primitive neuroectodermal tumor; ROI, region of interest; T2, timepoint 2; VCR, vincristine; VIQ, verbal IQ; WBRT, whole-brain radiation therapy; WM, white matter; WML, white matter lesions.
P < .001.
P < .01.
P < .05.
For children, school achievement is an indirect indicator of cognitive function. A report on more than 800 Canadian childhood cancer survivors demonstrated that those with a history of a CNS tumor treated with chemotherapy and cranial radiation were significantly more likely to have utilized special educational services than were survivors of other cancers.57 The academic subject accounting for the greatest difference between survivors and controls was mathematics. Children who were older at the time of diagnosis had better reading skills and were rated more highly by their teachers for academic performance than those who were younger.61 Those with ventriculoperitoneal shunts for tumor-related hydrocephalus had significantly lower math scores than those without shunts. Math, spelling, reading scores, and attention continued to decline over time, possibly related to continuing, chronic white matter changes.52 As additional evidence of progressive deficits over time, children with medulloblastoma treated with higher doses of CRT (36–39.6 Gy neuraxis and 55.8 Gy to tumor bed) lost more IQ points per year over 5 years than did those treated with a lower dose of neuraxis cranial radiation.64
Older children with germ cell tumors treated mainly with chemotherapy, although some also received cranial radiation, scored within the average range on full-scale IQ, verbal IQ, reading, math, and spelling.67 Younger age at diagnosis was related to lower scores in math and overall IQ.67
Significant correlations between white matter volume, attention, and IQ have been noted in brain tumor survivors treated with chemotherapy and cranial radiation.52 White matter loss is strongly correlated with adverse neurocognitive outcomes.60 A decrease in white matter of just 3.3% predicted an IQ score of 85 or less, approaching borderline deficiency. Indicators of white matter loss were seen in widespread areas of the brain, including the cerebellar hemispheres, pons, medulla, frontal and parietal periventricular structure, and corona radiata in children with medulloblastoma treated with chemotherapy and radiation 1 to 6 years earlier.60 Fractional anisotropy values, indicating loss of white matter integrity, correlated significantly with younger age at diagnosis, poorer school performance and longer time off-therapy,59 and with full-scale IQ, verbal IQ and performance IQ.60 These findings have been supported using alternate indicators of tissue damage with diffusion tensor imaging. Another indicator of microstructural CNS damage, the absolute diffusion coefficient, is significantly related to decreased IQ in brain tumor survivors as compared with healthy controls.62
Attention problems are fairly common in survivors of childhood brain tumors. Total volume of NAWM after chemotherapy and radiation was a strong predictor of attention problems,51 whereas 70% of the correlation between IQ and age at cranial radiation was explained by NAWM.7 White matter lesions became evident in a sample of brain tumor survivors at a median time of 7.8 months after radiation, and many (73%) resolved within another 6 months. A decline in IQ was also significantly related to the presence of these lesions.50
There are few studies to date on the long-term outcome of children with brain tumors who were treated with only chemotherapy. One study showed no significant loss of IQ points over time after chemotherapy, as compared with those who received radiation.69 Treatment for a brain tumor with chemotherapy before the age of 3 years resulted in mean IQ and memory scores within the average range, but in executive functioning significantly below the standard mean.47 Those who underwent more than 1 surgical resection had lower IQ, memory, and executive function scores. Lower socioeconomic status was related to lower scores on IQ and memory scores.47
Children with brain tumors who were an average of 3 years posttreatment showed low-average to average neurocognitive functioning after high-dose chemotherapy with AuHCR.66 Mean performance was in the average range for most academic skills, whereas fine motor skills and processing speed were in the low average range.66 Another group treated in the same manner displayed similar performance on intelligence testing and academic achievement and normal scores on behavioral and social-emotional functioning at a mean of 39.7 months after therapy.18
Quality of life in survivors of childhood brain tumors may be affected by many variables, including the treatment, frequent medical procedures, and hospitalizations at a young age. Findings are mixed regarding whether impairments in specific areas of neurocognitive functioning may impact QOL. More than 80% of medulloblastoma survivors studied had impaired executive functioning, and 92% were impaired on at least 1 subtest of attention, but despite these findings, neither self-reported nor caregiver-reported QOL was significantly diminished.63 A longitudinal study found that QOL scores of children with brain tumors improved such that, at 12 months after diagnosis, there was no significant difference between subjects and healthy controls in any domain.65 This particular study is relevant to the resolution of effects of acute treatment on QOL but does not measure QOL in the long-term survivor. No significant difference was seen in QOL scores between children with brain tumors treated with multimodal therapy (surgery, cranial radiation, and chemotherapy) and those treated with surgery only.58 Older children treated with a combination of cranial radiation and chemotherapy for germ cell tumors had low-average psychosocial functioning, borderline physical functioning, and impaired self-esteem on measures of QOL. Age at diagnosis was a factor related to lower scores on psychosocial and physical domains of QOL. Studies of children with brain tumors treated on the Head Start protocols, which utilize high-dose chemotherapy regimens and AuHCR, show that QOL scores were generally positive, but younger age at diagnosis and longer time off treatment correlated with risk for behavior and attention problems.68
As noted, there is good evidence to suggest that children treated with radiation alone or both chemotherapy and radiation have poor neurocognitive outcomes. Late effects of chemotherapy on cognitive centers in the brain in children include neurocognitive deficits in the areas of visual processing, visual motor skills, and memory and executive functioning. The addition of cranial radiation therapy leads to a more global loss of IQ points. In the few studies of children with brain tumors treated with chemotherapy only, memory and executive functioning deficits were observed, but overall neurocognitive function and QOL were generally within average range.
Implications for Practice
Nurses are often sought out by family members to explain treatment regimens and acute and chronic effects of therapy. As the numbers of childhood cancer survivors increase, our involvement in long-term follow-up care is crucial. Educating families to recognize the signs of neurocognitive problems in children is just as important as teaching about signs of other toxicities. Nurses can educate parents to be aware of subtle difficulties in school and to discuss concerns with teachers and healthcare providers. These difficulties may include academic, social, mood, and behavioral problems. Interaction with education specialists and teachers by the nurse to explain the possibility of neurocognitive late effects is recommended.
Standard guidelines for children who have received cranial radiation and/or MTX or high doses of cytarabine recommend regular neuropsychological testing and follow-up as needed.70 Nurses should advocate for any child with a brain tumor to have a neuropsychological testing battery after treatment and as recommended thereafter and encourage parents to be vigilant about this. Results of testing are used to develop Individualized Education Programs at school in order to ensure the best learning environment for each child. There is a wide range of possible interventions, ranging from simple techniques to more complex cognitive behavioral therapies, computerized interventions, and medications to improve attention and memory. Early intervention, in conjunction with newer therapies, may help to mitigate neurocognitive deficits and social problems.
Summary and Conclusions
Survival of a childhood brain tumor is often the result of administration of toxic therapies that not only eradicate cancer cells but also affect the healthy tissue of the child’s developing brain both acutely and in an ongoing manner. Radiation to the brain causes a progressive loss of healthy CNS cells because of a chronic state of inflammation, oxidative stress, and a loss of neural progenitor cells. Many chemotherapeutic agents cause injury to healthy brain cells by similar mechanisms. Neurocognitive deficits, often resulting in poor academic achievement and social isolation, may interfere with QOL in this population. Educational success is directly related to social proficiency and successful transition to adulthood and independence.71 Difficulty making friends and maintaining relationships may lead to withdrawal from social situations and subsequent isolation. Survivors of childhood brain tumors are less likely to marry or to be employed than are healthy peers and more likely to experience depression and anxiety.2,3,71 Although there is evidence to suggest that young children treated with chemotherapy alone may encounter fewer neurocognitive deficits, there may be long-term difficulties in the areas of memory, attention, and executive functioning. Little is known about long-term neurobiobehavioral outcomes in children who were treated with high-dose chemotherapy and AuHCR, which is becoming more common as a frontline treatment. As this treatment technique gains popularity, it will be important to define its long-term effects.
There are several limitations to the state of the science upon review of the literature. There are no studies to document loss of healthy brain tissue in children with brain tumors who were treated solely with chemotherapy. Most brain tumor studies have examined children treated with a combination of radiation and chemotherapy. However, available results indicate improved QOL in those treated without radiation.
Because the number of children diagnosed each year with brain tumors is relatively small, and so many variables affect outcomes, multi-institutional studies are critical to achieve sample sizes with adequate power to demonstrate significance. Future research must seek to minimize variability in tumor location, pathology, and treatment among subjects as much as possible in order to attribute certain toxicities to specific agents with more confidence. This approach will allow research to progress to develop more interventions to maximize long-term neurocognitive outcomes and QOL.
Acknowledgments
Dr Baron Nelson received funding support for this project from award no. F31NR011560 from the National Institute of Nursing Research and a grant from Alex’s Lemonade Stand Foundation.
The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Dr Mary Baron Nelson, Children’s Hospital Los Angeles, California; School of Nursing, University of California, Los Angeles.
Dr Peggy Compton, School of Nursing, University of California, Los Angeles.
Dr Sunita K. Patel, City of Hope, Duarte, California.
Dr Eufemia Jacob, School of Nursing, University of California, Los Angeles.
Dr Ronald Harper, Department of Neurobiology and David Geffen School of Medicine, University of California, Los Angeles.
References
- 1.Singer M, Byrne J. The epidemiology of brain tumors in children. http://childhoodbraintumor.org/THE%20EPIDEMIOLOGY%20OF%20BRAIN%20TUMORS_Part%201.pdf. Accessed July 1, 2008.
- 2.Anderson NE. Late complications in childhood central nervous system tumour survivors. Curr Opin Neurol. 2003;16(6):677–683. doi: 10.1097/01.wco.0000102623.38669.e5. [DOI] [PubMed] [Google Scholar]
- 3.Fuemmeler BF, Elkin TD, Mullins LL. Survivors of childhood brain tumors: behavioral, emotional and social adjustment. Clin Psych Rev. 2002;22(4):547–585. doi: 10.1016/s0272-7358(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 5.Moore BD, 3rd, Copeland DR, Ried H, Levy B. Neurophysiological basis of cognitive deficits in long-term survivors of childhood cancer. Arch Neurol. 1992;49(8):809–817. doi: 10.1001/archneur.1992.00530320033009. [DOI] [PubMed] [Google Scholar]
- 6.Waber DP, Tarbell NJ, Kahn CM, Gelber RD, Sallan SE. The relationship of sex and treatment modality to neuropsychological outcome in childhood acute lymphoblastic leukemia. J Clin Oncol. 1992;10:810–817. doi: 10.1200/JCO.1992.10.5.810. [DOI] [PubMed] [Google Scholar]
- 7.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19(2):472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- 8.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 9.Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplant. 2008;41:167–172. doi: 10.1038/sj.bmt.1705953. [DOI] [PubMed] [Google Scholar]
- 10.Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start.” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–1175. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 11.Kramer J, Moore IM. Late effects of cancer therapy on the central nervous system. Semin Oncol Nurs. 1989;5(1):22–28. doi: 10.1016/0749-2081(89)90019-3. [DOI] [PubMed] [Google Scholar]
- 12.Trask CL, Kosolfsky BE. Developmental considerations of neurotoxic exposures. Neurol Clin. 2000;18(3):541–562. doi: 10.1016/s0733-8619(05)70210-3. [DOI] [PubMed] [Google Scholar]
- 13.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than the adult brain? Dev Neurosci. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hossain MA. Molecular mediators of hypoxic-ischemic injury and implications for epilepsy in the developing brain. Epilepsy Behav. 2005;7(2):204–213. doi: 10.1016/j.yebeh.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Gajjar A, Mulhern RK, Heideman RL, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol. 1994;12:1212–1216. doi: 10.1200/JCO.1994.12.6.1212. [DOI] [PubMed] [Google Scholar]
- 16.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23(10):1149–1159. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniscer A, Nicolaides TP, Dunkel IJ, et al. High-dose chemotherapy with autologous stem cell rescue in the treatment of patients with recurrent non-cerebellar primitive neuroectodermal tumors. Pediatr Blood Cancer. 2004;42:261–267. doi: 10.1002/pbc.10369. [DOI] [PubMed] [Google Scholar]
- 18.Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–436. doi: 10.1002/pbc.22318. [DOI] [PubMed] [Google Scholar]
- 19.Kandel ER. Nerve cells and behavior. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 19–35. [Google Scholar]
- 20.Kandel ER. The brain and behavior. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th. New York: McGraw-Hill; 2000. pp. 5–18. [Google Scholar]
- 21.Laterra J, Goldstein GW. Ventricular organization of cerebrospinal fluid: blood-brain barrier, edema and hydrocephalus. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th. New York: McGraw-Hill; 2000. pp. 1288–1301. [Google Scholar]
- 22.Chamberlain MC. Neurotoxicity of cancer treatment. Curr Oncol Rep. 2010;12:60–67. doi: 10.1007/s11912-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 23.Panagiotakos G, Alshamy G, Chan B, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE. 2007;2(7) doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amano T, Inamura TCW, Kura S, et al. Effects of single low dose irradiation on subventricular zone cells in juvenile rat brain. Neurol Res. 2002;24:809–816. doi: 10.1179/016164102101200771. [DOI] [PubMed] [Google Scholar]
- 25.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 26.Ordy JM, Samarajski T, Zeman W, Collins RL, Curtis HJ. Long-term pathologic and behavioral changes in mice after focal deuteron irradiation of the brain. Radiat Res. 1963;20(1):30–42. [PubMed] [Google Scholar]
- 27.Rao AAN, Ye H, Decker PA, Howe CL, Wetmore C. Therapeutic doses of cranial irradiation induce hippocampus-dependent cognitive deficits in young mice. J Neurooncol. 2011;105(2):191–198. doi: 10.1007/s11060-011-0582-9. [DOI] [PubMed] [Google Scholar]
- 28.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Rubin P, Gash DM, Hansen JT, Nelson DF, Williams JP. Disruption of the blood-brain barrier as the primary effect of CNS irradiation. Radiother Oncol. 1994;31:51–60. doi: 10.1016/0167-8140(94)90413-8. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich J, Han R, Yang Y, Mayer-Proeschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(22):21–22, 23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James SE, Burden H, Burgess R, et al. Anti-cancer drug induced neurotoxicity and identification of Rho pathway signaling modulators as potential neuroprotectants. Neurotoxicology. 2008;29:605–612. doi: 10.1016/j.neuro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzeski W, Pruskil S, Macke A, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004;56(3):351–360. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- 33.Wick A, Wick W, Hirrlinger J, et al. Chemotherapy-induced cell death in primary cerebellar granule neurons but not in astrocytes: in vitro paradigm of differential neurotoxicity. J Neurochem. 2004;91:1067–1074. doi: 10.1111/j.1471-4159.2004.02774.x. [DOI] [PubMed] [Google Scholar]
- 34.Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thiotepa. Brain Res. 2006;1111:26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 35.Nordal RA, Wong S. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys. 2005;62(1):279–287. doi: 10.1016/j.ijrobp.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Miketova P, Kaemingk K, Hockenberry M, et al. Oxidative changes in cerebral spinal fluid phosphatidylcholine during treatment for acute lymphoblastic leukemia. Biol Res Nurs. 2005;6(3):187–195. doi: 10.1177/1099800404271916. [DOI] [PubMed] [Google Scholar]
- 37.Moore IM, Miketova P, Hockenberry M, Pasvogel A, Carey ME, Kaemingk K. Methotrexate-induced alterations in beta-oxidation correlate with cognitive abilities in children with acute lymphoblastic leukemia. Biol Res Nurs. 2008;9:311–319. doi: 10.1177/1099800407313268. [DOI] [PubMed] [Google Scholar]
- 38.Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy: clinical and electrophysiological observations. Brain. 1973;96:69–86. doi: 10.1093/brain/96.1.69. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. The Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 40.Kellie SJ, Chaku J, Lockwood LR, et al. Late magnetic resonance imaging features of leukoencephalopathy in children with central nervous system tumours following high-dose methotrexate and neuraxis radiation therapy. Eur J Cancer. 2005;41:1588–1596. doi: 10.1016/j.ejca.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 42.Nagel BJ, Palmer SL, Reddick WE, et al. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. Am J Neuroradiol. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- 43.Oi S, Kokunai T, Ijichi A, Matsumoto S, Raimondi AJ. Radiation-induced brain damage in children—histological analysis of sequential tissue changes in 34 autopsy cases. Neurol Med Chir. 1990;30:36–42. doi: 10.2176/nmc.30.36. [DOI] [PubMed] [Google Scholar]
- 44.Reddick WE, Glass JO, Palmer SL, et al. Atypical white matter volume development in children following craniospinal irradiation. Neurooncology. 2005;7(1):12–19. doi: 10.1215/S1152851704000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carey ME, Haut MW, Reminger SL, Hutter JJ, Theilmann R, Kaemingk KL. Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2008;29(4):792–797. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16(4):279–290. doi: 10.1053/sonu.2000.16582. discussion 279–291. [DOI] [PubMed] [Google Scholar]
- 47.Ward C, Phipps K, de Sousa C, Butler S, Gumley D. Treatment factors associated with outcomes in children less than 3 years of age with CNS tumours. Childs Nerv Syst. 2009;25(6):663–668. doi: 10.1007/s00381-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 49.Squire LR. Memory and brain systems: 1969–2009. J Neurosci. 2009;29(41):12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fouladi M, Chintagumpala M, Laningham FH, et al. White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol. 2004;22(22):4551–4560. doi: 10.1200/JCO.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 51.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 52.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 53.Duffner PK, Horowitz MA, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neurooncology. 1999;1(2):152–162. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Seigers R, Schagen SB, Coppens CM, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal and familial characteristics. Cancer. 2005;104(8):1751–1760. doi: 10.1002/cncr.21390. [DOI] [PubMed] [Google Scholar]
- 58.Benesch M, Spiegl K, Winter A, et al. A scoring system to quantify late effects in children after treatment for medulloblastoma/ependymoma and its correlation with quality of life and neurocognitive functioning. Childs Nerv Syst. 2009;25:173–181. doi: 10.1007/s00381-008-0742-1. [DOI] [PubMed] [Google Scholar]
- 59.Khong P, Kwong DLW, Chan GCF, Sham JST, Chan F, Ooi G. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. Am J Neuroradiol. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- 60.Khong P, Leung LHT, Fung ASM, Fong DYT, Qiu D, Kwong DLW. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 61.Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 62.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neurooncology. 2006:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 64.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 65.Penn A, Lowis SP, Hunt LP, et al. Health-related quality of life in the first year after diagnosis in children with brain tumours compared with matched healthy controls: a prospective longitudinal study. Eur J Cancer. 2008;44:1243–1252. doi: 10.1016/j.ejca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Sands SA, van Gorp WG, Finlay JL. Pilot neuropsychological findings from a treatment regimen consisting of intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. Childs Nerv Syst. 1998;14:587–589. doi: 10.1007/s003810050277. [DOI] [PubMed] [Google Scholar]
- 67.Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ cell tumors: from the First International CNS Germ Cell Tumor Study. Neurooncology. 2001;3:174–183. doi: 10.1093/neuonc/3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sands SA, Pasichow KP, Weiss R, et al. Quality of life and behavioral follow-up study of Head Start I pediatric brain tumor survivors. J Neurooncol. 2011;101:287–295. doi: 10.1007/s11060-010-0260-3. [DOI] [PubMed] [Google Scholar]
- 69.Stargatt R, Rosenfeld JV, Maixner W, Ashley D. Multiple factors contribute to neuropsychological outcome in children with posterior fossa tumors. Dev Neuropsychol. 2007;32(2):729–748. doi: 10.1080/87565640701376151. [DOI] [PubMed] [Google Scholar]
- 70.Landier W, Bhatia S, Eshelman DA, et al., editors. Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Arcadia, CA: Children’s Oncology Group; 2008. [Google Scholar]
- 71.Gurney JG, Krull K, Kadan-Lottick N, Nicholson S, Nathan PC, Zebrack B. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]


