Abstract
Properly assessing mitochondrial health is crucial to understand their role in disease. MitoTracker green (MTG) and nonylacridine orange (NAO) are fluorescent probes which have been commonly used to assess mitochondrial mass. This is based on the assumption that both MTG and NAO accumulate in mitochondria regardless of the mitochondrial transmembrane potential (ΔΨm). Here, we utilized flow cytometry to evaluate the performance of these probes for assessment of mitochondrial mass relative to forward (FSC) and side scatter (SSC) in human peripheral blood lymphocytes (PBL). In isolated mitochondria, two subpopulations were identified by FSC and SSC measurements which were matched to subpopulations stained by MTG and NAO. The performance of these dyes was examined under oxidative and nitrosative stress induced by rotenone and NOC-18 while N-acetylcysteine (NAC) was employed as an antioxidant. Production of reactive oxygen species (ROS) and ΔΨm were monitored in parallel. With respect to representation of mitochondrial mass, neither MTG nor NAO was affected by ΔΨm. However, MTG showed significant correlation with cytosolic and mitochondrial ROS production and nitrosative stress. Our data suggest that NAO may be more suitable than MTG for assessment of mitochondrial mass by flow cytometry during oxidative stress.
Keywords: Flow cytometry, Mitochondrial mass, MitoTracker green, Nonylacridine orange, Oxidative stress
1. Introduction
Mitochondria play an integral role in health and disease. Many diseases, disorders, and cancers have been shown to involve some form of mitochondrial dysfunction. Properly assessing mitochondrial function is crucial to understand the organelle's complete role and the impact of mitochondrial dysfunction on both cellular health and a person's health. The use of flow cytometry provides scientist with a powerful tool for the measurement of mitochondrial function and health. The earliest mitochondrial specific dye, rhodamine 123, provided a means to specifically identify mitochondria. However, it was mitochondrial potential (ΔΨm) dependent [1]. While specifically staining mitochondria, rhodamine 123 is a poor marker for measuring biogenesis and function. Over the years, better mitochondrial stains have allowed researchers to measure more components of mitochondrial health including ΔΨm, mitochondrial mass, and mitochondrial ROS production, though the ability to confidently measure mitochondrial size independent of ΔΨm by flow cytometry has proven difficult as many candidate dyes have been shown to in part be ΔΨm dependent. Two of the current dyes used to measure mitochondrial mass are MitoTracker probes-green (MTG) and nonylacridine orange (NAO).
First developed in the mid-1990s, the MitoTracker family of dyes is classified as cell permeant mitochondrion selective dyes. MitoTracker Red and Orange are both positively charged suggesting that ΔΨm will affect their uptake by mitochondria, while MTG does not share this trait. Containing a mildly thiol-reactive chloromethyl moiety, MTG accumulates in the mitochondrial matrix regardless of potential [2], where it binds to free thiols on mitochondrial proteins. There is also data suggesting that MitoTracker Orange may directly inhibit complex I respiration [3]. This makes MitoTracker Red and Orange poor candidates to use when studying the mitochondrial mass in live cells, and this is why we focus here on MTG.
NAO was first described in the early 1980s [4], considered to be membrane potential independent, NAO localizes to the mitochondria where it binds to cardiolipin. However, reports have shown that at low concentration (∼0.1 μM) NAO reacts to changes in potential brought on by ΔΨm altering compounds, though this is not observed at higher concentrations of NAO [5]. NAO rapidly enters mitochondria with 50% of fluorescent signal from a 30 minute incubation being present after just 1 minute [6]. NAO does leak out of the mitochondria with 20% of the signal being lost after 1 hour in NAO-free media [6]. While not sensitive to ΔΨm NAO does appear to be affected by mitochondrial homeostasis [7].
Both MTG and NAO are considered to be excellent candidates for measuring mitochondrial mass independent of ΔΨm in live cells by flow cytometry. However, we have noticed some discrepancies in our data when measuring mitochondrial mass with these two markers suggesting that they may not be accurately measuring mitochondrial mass. Here, we look at NAO and MTG under different treatment conditions which lead to mitochondrial stress, through the use of an NO donor (NOC-18), a complex I inhibitor (rotenone), and an antioxidant/glutathione precursor, namely, N-acetylcysteine (NAC). We provide evidence that MTG and NAO florescence signal are not ΔΨm independent. However, MTG is oxidative stress dependent.
2. Materials and Methods
2.1. PBL Isolation and Culture
Healthy subjects were recruited from within SUNY Upstate medical university. Peripheral blood lymphocytes (PBL) were isolated as previously described [9]. Isolated PBL were incubated overnight at a concentration of 1 × 106 cells/ml in RPMI media (Cellgro, Manassas, VA, USA; catalog No. 15-040-CV) containing 10% fetal bovine serum (Invitrogen/GIBCO, Eugene, OR, USA; catalog No 26140), 2 mM L-glutamime (Cellgro; catalog No 25-005-cl), and 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml amphotericin B (Cellgro; catalog No. 30-004-CI). PBL were treated with the nitric oxide (NO) donor NOC-18 (Milipore, Billerica, MA, USA; catalog No. 487957) for 20 h. NAC (Sigma-Aldrich St Louis, MO, USA; catalog No. A7250) was titrated to pH 7.4 and used at a final concentration of 3 mM. Rotenone (Sigma-Aldrich; catalog No. 45656) was used at a final concentration of 3 μM. Both NAC and rotenone were incubated on PBL for either 2 h or 15 min. All treatment groups were set up to provide a single endpoint.
2.2. Mitochondrial Isolation
Treated PBL were washed twice with phosphate-buffered saline (PBS) before beginning the mitochondrial isolation. Mitochondria were isolated using a kit purchased from Pierce (Thermo Scientific Pierce, Rockford, IL, USA; catalog No. 89874)
2.3. Flow Cytometry Analysis
All flow cytometry experiments were performed on a BD LSR II (BD Biosciences, San Jose, CA, USA). The fluorescent probes were purchased from Invitrogen/Molecular Probes (Eugene, OR, USA) unless otherwise stated. ΔΨm was measured using 10 nM tetramethylrhodamine, methyl ester (TMRM) (catalog No. T668; ex543, em567) and 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (catalog No. D273; ex488, em525). Mitochondrial mass was evaluated with 150 nM MitoTracker Green (MTG) (catalog No. M7514; ex490, em516) and 2.5 μM nonylacridine orange (NAO) (catalog No. A1372; ex490, em540). The concentration of NO, a reactive nitrogen species (RNS), was measured by 1 μM 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM) (catalog No. D23844; ex495, em518). H2O2levels were evaluated using 10 μM 2′,7′-dichlorofluorescin diacetate (DCF-DA) (catalog No. C400; ex495, em529). Dihydrorhodamine 123 (DHR) (catalog No. D23806; ex507 em527) and dihydroethidium (HE) (catalog No. D11347; ex635 em610) were also used. Data were analyzed with FlowJo version 7.5.5 software (Tree Star Inc., Ashland, OR, USA).
2.4. Western Blotting
As many as 1 × 106 whole PBL were lysed in 25 μl of 1× cell lysis buffer (Cell Signaling, Danvers, MA, USA; catalog No. 9803S) containing protease inhibitors, then mixed 1:1 with 4× sample buffer. Samples were run on 12% SDS-PAGE gels before being transferred to 0.45 μm nitrocellulose membranes. Blots were probed with antibodies against voltage-dependent anion channel (VDAC), adenine nucleotide translocator (ANT), NADH:ubiquinone oxidoreductase core subunit S3 (NDUFS3) (Abcam, Cambridge, UK; catalog No. ab14711), and actin (EMD Millipore, catalog No. MAB1501R).
2.5. Statistics
Statistical analyses were carried out with GraphPad Prism version 5.04 (La Jolla, CA, USA). Paired t-tests were performed for each experiment and p values < 0.05 were considered significant.
3. Results
3.1. MTG Positively Correlates with ROS and RNS Concentrations
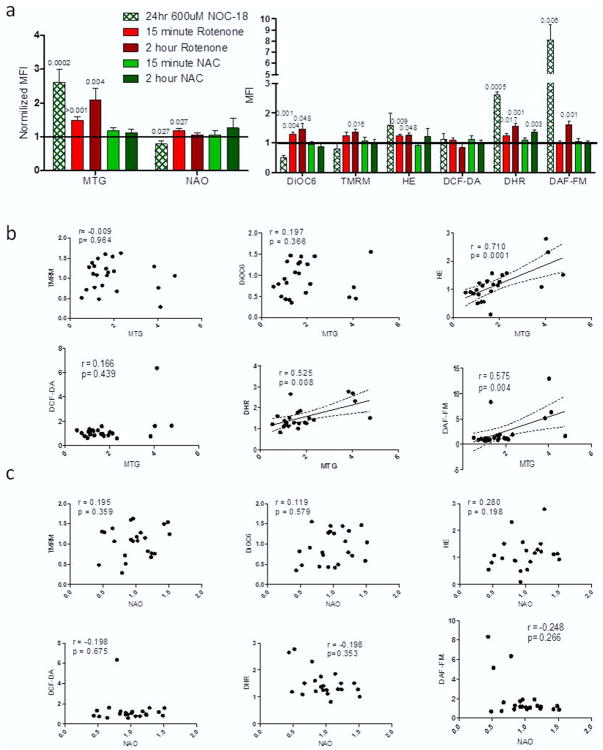
We first assessed the ability of both MTG and NAO to measure mitochondrial mass under different conditions of mitochondrial stress, PBL from healthy controls were treated for 24 h with either 600 μM of the NO donor NOC-18, 2 h or 15 min with 3 μM of the complex I inhibitor rotenone or 3 mM of the antioxidant NAC. Cells were washed and then stained for 1 h with either MTG or NAO along with TMRM, DiOC6, HE, DCF-DA, DHR, or DAF-FM to simultaneously measure mitochondrial mass, potential, and oxidative stress. Notably, NOC-18 induced a greater than 2.5-fold increase in mitochondrial mass measured by MTG compared to untreated controls (Figure 1a) (MTG normalized mean florescence intensity [MFI] = 2.596 ± 0.404; p = 0.041), while NAO stained mitochondria showed a significant decrease in mitochondrial size relative to untreated controls (NAO normalized MFI = 0.785 ± 0.104; p = 0.027). Rotenone treatment for 2 h had a greater than 2-fold increase in mass by MTG relative to untreated controls (MTG normalized MFI = 2.092 ± 0.351; p = 0.027), with no observable change with NAO staining. Rotenone treatment for 15 min caused a significant 1.5-fold increase in fluorescence by MTG (MTG normalized MFI = 1.486 ± 0.104; p < 0.001) and a 1.1-fold increase when measured by NAO (NAO normalized MFI = 1.177 ± 0.075; p = 0.027) compared to untreated controls. NAC treatment caused no change in MFI for either mass marker at the 2 h and 15 min time points.
Figure 1. Mitochondrial mass dyes correlate with ROS and RNS markers.
(a) Normalized levels of mitochondrial flow dyes for each of our treatment groups from 12 experiments. P values shown are from paired t-tests with p < 0.05. (b) Correlation results comparing MTG to ΔΨm (TMRM and DiOC6), ROS markers (HE and DHR), and RNS markers (DCF-DA and DAF-FM). (c) Correlation results comparing NAO to ΔΨm (TMRM and DiOC6), ROS markers (HE and DHR), and RNS markers (DCF-DA and DAF-FM). Correlations r and p values from Spearman statistical test are shown for all graphs with statistical significance being p < 0.05.
When the effects of each treatment on ΔΨm and cellular redox were measured, we noted that NOC-18 showed a significant decrease in potential by DiOC6 (MFI = 0.508 ± 0.061; p = 0.001), while increasing both superoxide/peroxynitrite and NO as measured by DHR and DAF-FM (DHR MFI = 2.608 ± 0.1, p = 0.0005; DAF-FM MFI = 8.104 ± 1.343, p = 0.006). PBL incubated 15 min with rotenone had increased ΔΨm measured by DiOC6 (MFI = 1.287 ± 0.062; p = 0.004), increased ROS levels as measured by HE and DHR (HE MFI= 1.229 ± 0.061, p = 0.009; DHR MFI = 1.243 ± 0.069, p = 0.017). After 2 h incubation with rotenone both markers for ΔΨm were elevated (DiOC6 MFI = 1.469 ± 0.190, p=0.048; TMRM MFI = 1.246 ± 0.115, p = 0.016), and we also observed that ROS and NO levels were elevated after 2 h (HE MFI = 1.247 ± 0.095, p = 0.048; DHR MFI = 1.557 ± 0.088, p = 0.001; DAF-FM MFI = 1.613 ± 0.104, p = 0.001). NAC treatments had minimal effects on the redox state of the PBL with only 2 h NAC showing a significant increase in DHR measurements (MFI = 1.359 ± 0.07; p = 0.003)
Correlation analysis was preformed between the two mass markers and the stains for ΔΨm and oxidative stress to determine if either MTG or NAO was a potential dependent mitochondrial stain. Confirming published data, neither MTG nor NAO significantly correlated to either TMRM or DiOC6 (Figure 1b and 1c). However, MTG has a strong positive correlation with markers for ROS and reactive nitrogen species (RNS). MTG showed positive correlation to ROS and RNS levels within the cell (HE r = 0.710, p = 0.0001; DHR r = 0.525, p = 0.008; DAF-FM r = 0.575, p = 0.004). Our results provided no evidence of correlations between NAO and our stains for mitochondrial stress. These data suggest that MTG MFI is affected by oxidative and NO stress, while NAO measurements appear to be unaffected by these flow cytometry markers.
3.2. Isolated Mitochondria Have Distinct Subpopulations
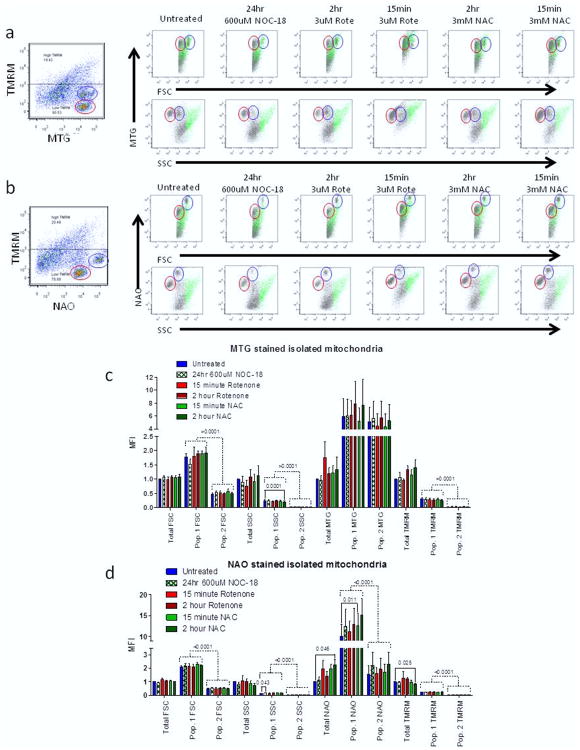
To get a more direct measurement of mitochondrial size by flow cytometry, we isolated mitochondria to run directly on the flow cytometer. After treatment with the different mitochondrial stress inducing compounds, we isolated mitochondria from our treatment groups with the use of a mitochondrial isolation kit. Isolated mitochondria were stained for 15 min on ice with MTG or NAO and TMRM. After staining we were able to measure mitochondrial size by forward scatter (FSC) and MTG or NAO (Figure 2a and 2b). No significant change in sizes was measured by FSC under either staining condition (Figure 2c and 2d). MTG showed no change in mass under any treatment condition in isolated mitochondria. However, NAO did show a greater than 2.25-fold increase in mitochondrial mass after 2 h NAC treatment (NAO MFI = 2.264 ± 0.385; p= 0.046) (Figure 2d). There was also a significant 16% decrease in ΔΨm in 2 h NAC treated isolated mitochondria (TMRM MFI = 0.845 ± 0.025; p = 0.025). While we found no evidence that ΔΨm affected our mass measurements by either NAO or MTG, our data does suggest that NAO measurement of isolated mitochondrial mass might be less accurate in the presence of a reducing agent such as NAC.
Figure 2. Isolated mitochondria have distinct subpopulations.
(a) Representative dot plots from isolated mitochondria stained with MTG and TMRM under each treatment group. (b) Representative dot plots from isolated mitochondria stained with NAO and TMRM under each treatment group. Mitochondria with high potential are shown in green, while low potential mitochondria are shown in grey. Two distinct sub populations of mitochondria are circled in blue (sub population 1) and red (sub population 2). (c) MTG stained mitochondria for total mitochondria as well as each sub population. (d) NAO stained mitochondria for total mitochondria as well as each sub populations. The t-test (solid lines) and 2-way ANOVA (dashed lines) with p < 0.05 are shown.
Analysis of dot plots from our isolated mitochondrial samples identified two small populations of mitochondria with very strong MTG and NAO signals but low TMRM signal (Figure 2a and 2b). Tracking these two populations shown in red and blue circles we noted that the size of both populations changed based on treatment conditions. Statistical analysis of these two populations by ANOVA showed that NAO but not MTG more closely tracked the differences seen in mass from both FSC and side scatter (SSC) measurements (Figure 2c and 2d). These data suggest that MTG may not accurately report mitochondrial mass, while NAO appears to be a more accurate measurement of changes in mitochondrial mass.
3.3. Mitochondrial Proteins Show Minimal Change after Treatment Conditions
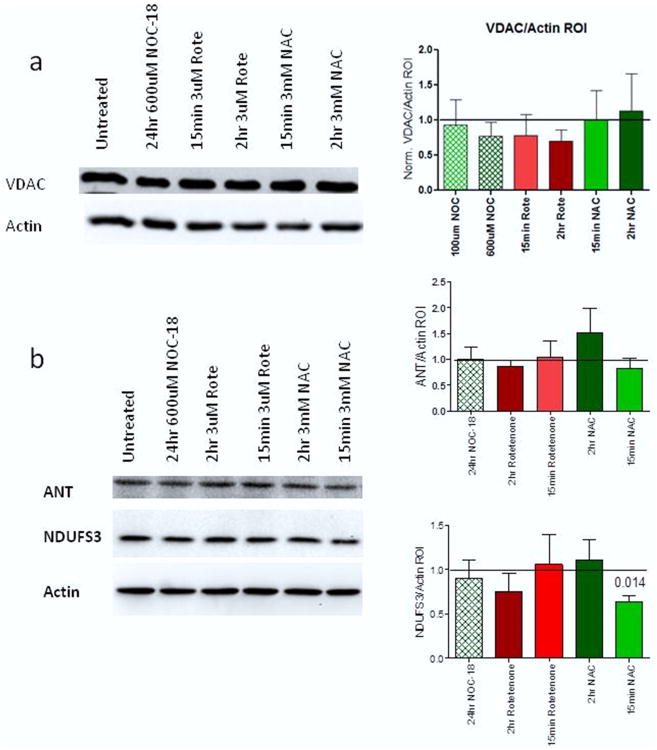
Next, we compared our flow cytometry staining data to mitochondrial protein levels as measured by western blot. Whole PBL were treated and protein levels of three different mitochondrial proteins were measured: (1) the outer mitochondrial membrane protein VDAC (Figure 3a); (2) the inner mitochondrial membrane protein ANT (Figure 3b); and (3) the electron transport chain complex I subunit NDUFS3 (Figure 3b). No significant changes in any protein expression were observed in either VDAC or ANT from any of the treatment groups. The only significantly changed protein was NDUFS3 with a 37% decrease after 15 min of NAC treatment (p = 0.014). These data suggest that while MTG and NAO under certain conditions show significant changes in mitochondrial size, the protein level remains relatively unchanged, leading us to the conclusion that redox state might be more significant for the accurate measurement of mitochondrial mass with MTG and NAO than ΔΨm.
Figure 3. Mitochondrial mass proteins show decreased NDUFS3 after NAC treatment.
(a) Representative western blot and actin normalized bar chart for VDAC protein levels. (b) Representative western blot and actin normalized bar charts for ANT and NDUFS3 protein levels respectively. P values shown are from paired t-tests with p < 0.05.
4. Discussion
Proper selection of stains for use in flow cytometry panels measuring mitochondrial function is crucial in everyone's efforts to provide meaningful and insightful data. Our data confirms that MTG and NAO localize to mitochondria independent of ΔΨm. However, MTG does not provide accurate measurements of mitochondrial mass under all conditions. In our whole cell studies we observed a correlation with MTG MFI and our markers for cytosolic and mitochondrial oxidative stress. Potentially MTG might be acting as a substrate for the reduction of oxidized mitochondrial proteins, like peroxiredoxin, thioredoxin, or glutaredoxin, and the increase in MTG fluorescence we are detecting could be due to one of two mechanisms: (1) oxidation by cysteines in mitochondrial proteins or (2) ROS generated by the electron transport chain (ETC). These mechanisms could be possible explanations for why others have reported increased MTG signal after altering mitochondrial function, independent of whether the compound enhanced or dissipated the ΔΨm [5]. Many of these treatments also alter the redox environment and may impact MTG fluorescence. Our data showed that the only treatment that did not result in a significant change in mitochondrial mass by MTG was the reducing agent NAC. NAC treatment caused minimal changes to ROS and RNS levels in mitochondria after 2 h (Figure 1a). Staining mitochondria in whole cells with NAO, which binds to cardiolipin, showed a decrease in fluorescence in samples treated with NO. While no significance was noted by our correlation analysis both DAF-FM and DHR (markers for NO and RNS) did show a negative trend. This is possibly due to the oxidation of cardiolipin by peroxynitrite [8] resulting in decreased binding sites for NAO.
When we isolated mitochondria after treatment we saw no change to mitochondrial mass by FSC, despite the large changes in mass from MTG staining of whole cells. The lack of change in mass by FSC but increased MTG staining could be a result of enhanced biogenesis of mitochondria coupled with fission of the mitochondria. However, the lack of measurable change in mitochondrial protein levels by western blotting suggests that this is not the case. When we further compare our mitochondrial mass measurements in whole cells to our western blot results we observe NAO mass measurements more closely resemble the changes seen in protein levels by western blotting, providing evidence that NAO is a more accurate mass marker than MTG.
While analysis of whole cells showed dramatic changes in MTG fluorescence, isolated mitochondria had greater changes in NAO signal. This suggests that NAO, while cell permeable, does not react the same way in whole cells as it does in isolated mitochondria, or that cardiolipin may become more exposed as a result of our isolation protocol. These discrepancies should be taken into account when selecting a mitochondrial mass dye for studies.
Our flow cytometric analysis of isolated mitochondria identified two subpopulations that strongly stained positive with both MTG and NAO but had minimal TMRM signal. These two populations were consistent in their location within the forward versus side scatter plots between each treatment group (Figure 2). These two populations of mitochondria might be from different subsets of cells within the isolated PBL. With different cell types having different work load requirements the mitochondrial structure in each subset would be different and might explain why we see these distinct groups. The use of isolated cell populations from PBL might help to elucidate where these populations derive from.
A significant decrease in NDUFS3 protein levels was measured by western blot after 15 min treatment with 3 mM NAC. This decrease was not present in our 2 h treated samples. With no difference seen in the level of either outer mitochondrial membrane protein VDAC, or inner mitochondrial membrane protein ANT after treatment with NAC, we propose that there may be a specific interaction between NAC and complex I. Given that we have previously described complex I respiration is directly inhibited by NAC [9], this decrease could be a result of NAC's inhibition of complex I. One possible mechanism this might occur through is NAC's ability to disrupt disulfide bonds [10], leading to loss of some complex I protein. Further work is needed to explore this hypothesis.
In this study, we have provided evidence that MTG does not accurately measure mitochondrial mass under all conditions and that this is not due to changes in ΔΨm, but rather is the result of altered oxidative and nitrosative stress. While both MTG and NAO may be good markers for mitochondrial mass under physiological conditions, we believe that it is critical to understand the ROS and RNS state of the mitochondria before using either of these dyes for determining mitochondrial mass by flow cytometry.
Acknowledgments
This work was supported in part by grant R01 AI072648 from the United States National Institutes of Health and the Central New York Community Foundation.
Abbreviations
- ANT
adenine nucleotide translocator
- DAF-FM
4-amino-5-methylamino-2′,7′-difluorescein
- DCF-DA
2′,7′-dichlorofluorescin diacetate
- DHR
dihydrorhodamine 123
- DiOC6
3,3′-dihexyloxacarbocyanine iodide
- HE
dihydroethidium
- MFI
mean florescence intensity
- MTG
MitoTracker green
- NAC
N-acetylcysteine
- NAO
nonylacridine orange
- NDUFS3, NADH
ubiquinone oxidoreductase core subunit S3
- PBL
peripheral blood lymphocytes
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TMRM
tetramethylrhodamine methyl ester
- VDAC
voltage-dependent anion channel
References
- 1.Ronot X, Benel L, Adolphe M, Mounolou JC. Mitochondrial analysis in living cells: the use of rhodamine 123 and flow cytometry. Biol Cell. 1986;57(1):1–7. doi: 10.1111/j.1768-322x.1986.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 2.Poot M, Zhang YZ, Kramer JA, Wells KS, Jones LJ, Hanzel DK, et al. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J HistochemCytochem. 1996;44(12):1363–72. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- 3.Poot M, Pierce RH. Detection of changes in mitochondrial function during apoptosis by simultaneous staining with multiple fluorescent dyes and correlated multiparameter flow cytometry. Cytometry. 1999;35(4):311–7. doi: 10.1002/(sici)1097-0320(19990401)35:4<311::aid-cyto3>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Septinus M, Berthold T, Naujok A, Zimmermann HW. Hydrophobic acridine dyes for fluorescent staining of mitochondria in living cells. 3. Specific accumulation of the fluorescent dye NAO on the mitochondrial membranes in HeLa cells by hydrophobic interaction. Depression of respiratory activity, changes in the ultrastructure of mitochondria due to NAO. Increase of fluorescence in vital stained mitochondria in situ by irradiation. Histochemistry. 1985;82(1):51–66. doi: 10.1007/BF00502091. [DOI] [PubMed] [Google Scholar]
- 5.Keij JF, Bell-Prince C, Steinkamp JA. Staining of mitochondrial membranes with 10-nonyl acridine orange, MitoFluor Green, and MitoTracker Green is affected by mitochondrial membrane potential altering drugs. Cytometry. 2000;39(3):203–10. doi: 10.1002/(sici)1097-0320(20000301)39:3<203::aid-cyto5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Benel L, Ronot X, Mounolou JC, Gaudemer F, Adolphe M. Compared flow cytometric analysis of mitochondria using 10-n-nonyl acridine orange and rhodamine 123. Basic ApplHistochem. 1989;33(2):71–80. [PubMed] [Google Scholar]
- 7.Cottet-Rousselle C, Ronot X, Leverve X, Mayol JF. Cytometric assessment of mitochondria using fluorescent probes. Cytometry A. 2011;79(6):405–25. doi: 10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]
- 8.Pope S, Land JM, Heales SJ. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? BiochimBiophysActa. 2008;1777(7–8):794–9. doi: 10.1016/j.bbabio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Doherty E, Oaks Z, Perl A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid Redox Signal. 2014;21(1):56–65. doi: 10.1089/ars.2013.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkham-Kamphorst E, Meurer SK, Gressner AM, Weiskirchen R. Disruption of intermolecular disulfide bonds in PDGF-BB dimers by N-acetyl-L-cysteine does not prevent PDGF signaling in cultured hepatic stellate cells. BiochemBiophys Res Commun. 2005;338(4):1711–8. doi: 10.1016/j.bbrc.2005.10.139. [DOI] [PubMed] [Google Scholar]