Key Points
AL amyloidosis prevalence increased while incidence rates remained stable over a 9-year period (2007-2015).
Abstract
Amyloid light-chain (AL) amyloidosis is a rare disease caused by extracellular deposition of misfolded immunoglobulin light chains. This study aimed to provide an up-to-date estimate of prevalence and incidence of AL amyloidosis in the United States. Using claims databases from years 2007 to 2015, adults ≥18 years old with AL amyloidosis were included if they had (1) at least 1 inpatient or 2 outpatient claims consistent with AL amyloidosis and (2) received 1 AL-specific treatment. Prevalence was calculated as the number of AL patients divided by the number of enrollees on June 30th of each calendar year. Incidence was calculated as the number of patients with AL who were disease-free and enrolled with a health plan for 1 year prior, divided by the number of enrollees with enrollment from July 1st of the previous year to June 30th of each calendar year. The prevalence of AL amyloidosis increased significantly between 2007 and 2015, from 15.5 cases per million in 2007 to 40.5 in 2015, an annual percentage change (APC) of 12% (P < .001). The incidence ranged from 9.7 to 14.0 cases per million person-years (APC, 3%; P = .114) with no statistically significant increase. There was an increase in AL amyloidosis prevalence over a 9-year period coupled with stable incidence rates. Although there is no diagnosis code specific to AL amyloidosis and no validated method for identifying this condition using claims data, extrapolating from our data, there are at least 12 000 adults in the United States living with AL amyloidosis, and the number seems likely to rise.
Visual Abstract
Introduction
The amyloidoses refer to a group of rare disorders of protein folding characterized by extracellular tissue deposition of misfolded and aggregated autologous proteins as β-pleated sheet fibrils.1 The most common and severe type of systemic amyloidosis is amyloid light-chain (AL) amyloidosis, also known as primary amyloidosis. In AL amyloidosis, amyloid fibrils are derived from κ or λ monoclonal light chains, which are synthesized by a clonal population of plasma cells in the bone marrow.2 With the exception of the central nervous system, the toxic monoclonal light-chain proteins in AL can damage virtually all organs, most frequently the heart and kidneys. Cardiac dysfunction commonly manifests as heart failure.3 Renal involvement usually presents as nephrotic syndrome with progressive worsening of renal functions.
Patients with AL amyloidosis have a poor prognosis with an estimated median survival ranging from 6 months to 3 years depending on the patient population and data used.1,4-6 When symptomatic cardiac dysfunction is present, studies have found a median overall survival without treatment of ∼6 months.7 Cardiac biomarkers, including N-terminal pro brain natriuretic peptide [NT-proBNP] and cardiac troponins, have been used for assessing cardiac dysfunction severity and prognosis as well as staging.8,9 The extent of cardiac dysfunction is the main determinant of the prognosis of patients with AL amyloidosis, although symptomatic hepatic involvement and autonomic involvement also influence survival.2,10
The epidemiology of AL amyloidosis in the United States has not been well characterized. A study by Kyle et al with data from 1950 to 1989 on the general population residing in Olmsted County, MN is the most comprehensive epidemiological study to date.1 The authors of this study reported an AL amyloidosis incidence rate of 9 cases per million person-years, and inferred that ∼2200 new cases of AL amyloidosis occur annually in the United States.1 Other studies, although not population-based, have been conducted in European regions. For example, in 2 separate studies, Pinney et al11 and Hemminki et al12 estimated AL amyloidosis incidence rates to be ∼3 and 8.3 cases per million person-years in 2008 in England and Sweden, respectively. Therefore, the literature suggests an estimated incidence between 3 and 12 cases per million persons per year, and an estimated prevalence of 30 000 to 45 000 AL amyloidosis patients in the United States and the European Union.1,7,11-14 Additionally, studies have shown that men have a slightly increased rate of AL amyloidosis compared with women, and the majority of patients are over the age of 65 years.1,7,15
Given the lack of contemporary data on AL amyloidosis in the United States, this study sought to examine the prevalence (proportion of the population having the condition at a given time) and incidence (annual rate of newly identified cases) of AL amyloidosis over a 9-year period (2007-2015) using health insurance claims data. This epidemiological information on AL amyloidosis in the United States will be vital to (1) understanding the current burden of this life-threatening disease, and allow us to monitor future trends in prevalence and incidence rates and (2) bolstering awareness of AL amyloidosis, which may lead to more rapid identification and treatment and, in turn, enhance patient survival.
Methods
Data sources
Data from the Truven MarketScan Commercial and Medicare Supplement Databases (Truven Health Analytics, Ann Arbor, MI) from 1 January 2007 to 31 December 2015 were used to examine the prevalence and incidence of AL amyloidosis. These data sources were used in a previous publication.16
The MarketScan Commercial and Medicare Supplemental Databases are Health Insurance Portability and Accountability Act (HIPAA)-compliant administrative claims databases. The commercial data included medical encounters from ∼65 million individuals and their dependents insured by employer-sponsored plans (ie, non-Medicare eligible). Coverage was provided under a variety of fee-for-service, fully capitated, and partially capitated health plans, including preferred provider organizations, point-of-service plans, indemnity plans, and health maintenance organizations. The Medicare supplemental data included ∼5.3 million Medicare-eligible retired employees and their spouses with employer-sponsored Medicare supplemental plans. Given the deidentified nature of the data used in the present study, informed consent was not required by HIPAA rules.
Study population and measures
As there is no diagnosis code specific to AL amyloidosis, the following algorithm, which was used in a previously published study,16 was used to identify patients in each calendar year during the study period (1 January 2007 to 31 December 2015). Adults ≥18 years old with AL amyloidosis were identified if they had (1) at least 1 inpatient claim or 2 outpatient claims consistent with AL amyloidosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 277.30 or 277.39; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] codes E85.4x, E85.8x, or E85.9x [supplemental Table 1]) in any diagnosis field within each calendar year and (2) received a treatment recommended in expert guidelines17-21 for AL amyloidosis (bendamustine, bortezomib, carfilzomib, cyclophosphamide, dexamethasone, prednisone, doxycycline, lenalidomide, melphalan, pomalidomide, thalidomide, or hematopoietic stem cell transplant [HSCT]) on or after the first amyloidosis diagnosis in the study period. A similar population-identification algorithm was used in a recent publication and confirmed with clinical input.22
Prevalence was calculated as the number of AL patients divided by the number of adult enrollees on June 30th of each calendar year and reported as per million persons per year. Incidence was calculated as the number of patients with AL who were disease-free for 1 year and enrolled with a health plan for 1 year prior to the first AL amyloidosis diagnosis in the identification period (1 January 2008 to 31 December 2014), divided by the number of adult enrollees with enrollment from July 1st of the previous year to June 30th of each calendar year and reported as per million person-years. Considering the poor prognosis of AL amyloidosis, we did not require continuous enrollment in each study calendar year for the main analysis. A sensitivity analysis was performed requiring patients to have continuous enrollment in each calendar year (and in the year prior for incident cases).
Statistical analysis
Descriptive analyses were performed for this study. Yearly prevalence proportions were reported for each calendar year from 2007 through 2015. Yearly incidence rates were reported from 2008 through 2015. Rates stratified by age group and sex were also reported. In addition, we reported age-sex–adjusted prevalence and incidence based on 2010 US census data for the adult population (supplemental Table 2). Furthermore, for each sex, we reported age-adjusted rates based on the age distribution in the 2010 US census. To characterize trends in prevalent and incident rates of AL amyloidosis over time, we calculated an annual percentage change (APC) by fitting a linear regression line to the natural logarithm of the rates, using the calendar year as a regressor variable. APC is used to describe rates over time; with this approach, rates are assumed to change at a constant percentage of the previous year’s rate.
Results
Between 2007 and 2015, 7591 prevalent patients with AL amyloidosis were identified, ranging from 368 to 1080 cases per million per year (Table 1). Prevalent patients were a mean (standard deviation [SD]) age of 63 (12) years old; 55% were male; 34% were from the south; and 59% had commercial health insurance (Table 2). The mean (SD) age of prevalent patients was stable from 2007 to 2015 (Table 2).
Table 1.
Attrition table: patient identification
| Step | Description | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | All* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Patients with 1+ Dx† in each ID year | 1297 | 1966 | 2375 | 2501 | 3036 | 3181 | 3090 | 3265 | 2827 | 23 538 |
| B | Of A, patients with 1+ inpatient or 2+ outpatient Dx† in each ID year | 681 | 1113 | 1369 | 1456 | 1828 | 1915 | 1894 | 1932 | 1612 | 13 800 |
| C | Of B, patients with 1+ treatment‡ since the first Dx† | 374 | 601 | 753 | 847 | 1027 | 1086 | 1072 | 1068 | 830 | 7 658 |
| D | Of C, patients with age ≥18 y (prevalent cases) | 368 | 595 | 741 | 837 | 1021 | 1080 | 1063 | 1061 | 825 | 7 591‡ |
| E | Of D, patients with at least 1 y of continuous enrollment prior to the first Dx† date in each ID year (1 January 2008 to 31 December 2014) | n/a | 407 | 566 | 616 | 757 | 925 | 911 | 905 | 733 | 5 820 |
| F | Of E, patients with no Dx† in 1 y prior to the 1st Dx† date in ID year (incident cases) | n/a | 213 | 252 | 245 | 295 | 377 | 339 | 297 | 189 | 2 207 |
Dx, diagnosis; ID, identification; n/a, not applicable.
Patients might be included in multiple years
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM): 277.30, 277.39 or Tenth Revision (ICD-10-CM): E85.4x, E85.8x, E85.9x.
A total of 4006 unique patients.
Table 2.
Patient demographic characteristics among prevalent cases
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | All* | |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 368 | 595 | 741 | 837 | 1021 | 1080 | 1063 | 1061 | 825 | 7591 |
| Age, mean (SD), y | 63 (12) | 63 (12) | 62 (12) | 62 (12) | 64 (12) | 64 (12) | 65 (12) | 63 (12) | 64 (13) | 63 (12) |
| 18-34, n (%) | 5 (1.4) | 5 (0.8) | 6 (0.8) | 18 (2.2) | 17 (1.7) | 15 (1.4) | 8 (0.8) | 13 (1.2) | 12 (1.5) | 99 (1.3) |
| 35-54, n (%) | 73 (19.8) | 132 (22.2) | 176 (23.8) | 198 (23.7) | 195 (19.1) | 195 (18.1) | 194 (18.3) | 210 (19.8) | 161 (19.5) | 1534 (20.2) |
| 55-64, n (%) | 128 (34.8) | 230 (38.7) | 282 (38.1) | 300 (35.8) | 366 (35.8) | 387 (35.8) | 346 (32.5) | 392 (36.9) | 296 (35.9) | 2727 (35.9) |
| 65+, n (%) | 162 (44.0) | 228 (38.3) | 277 (37.4) | 321 (38.4) | 443 (43.4) | 483 (44.7) | 515 (48.4) | 446 (42.0) | 356 (43.2) | 3231 (42.6) |
| Female, n (%) | 168 (45.7) | 276 (46.4) | 346 (46.7) | 380 (45.4) | 467 (45.7) | 467 (43.2) | 466 (43.8) | 468 (44.1) | 389 (47.2) | 3427 (45.1) |
| Region, n (%) | ||||||||||
| Midwest | 129 (35.1) | 182 (30.6) | 244 (32.9) | 236 (28.2) | 282 (27.6) | 258 (23.9) | 248 (23.3) | 250 (23.6) | 193 (23.4) | 2022 (26.6) |
| Northeast | 45 (12.2) | 93 (15.6) | 135 (18.2) | 171 (20.4) | 226 (22.1) | 269 (24.9) | 242 (22.8) | 282 (26.6) | 205 (24.8) | 1668 (22.0) |
| South | 138 (37.5) | 217 (36.5) | 225 (30.4) | 276 (33.0) | 316 (31.0) | 351 (32.5) | 352 (33.1) | 382 (36.0) | 304 (36.8) | 2561 (33.7) |
| West | 56 (15.2) | 103 (17.3) | 137 (18.5) | 154 (18.4) | 197 (19.3) | 202 (18.7) | 221 (20.8) | 147 (13.9) | 123 (14.9) | 1340 (17.7) |
| Insurance type, n (%) | ||||||||||
| Commercial | 208 (56.5) | 377 (63.4) | 479 (64.6) | 527 (63.0) | 589 (57.7) | 608 (56.3) | 558 (52.5) | 626 (59.0) | 483 (58.5) | 4455 (58.7) |
| Medicare supplemental (Medigap)† | 160 (43.5) | 218 (36.6) | 262 (35.4) | 310 (37.0) | 432 (42.3) | 472 (43.7) | 505 (47.5) | 435 (41.0) | 342 (41.5) | 3136 (41.3) |
Patients might be included in multiple years
A Medicare supplemental insurance (Medigap) policy, sold by private companies, can help pay some of the health care costs that original Medicare does not cover (eg, copayments, coinsurance, and deductibles).
Similarly, incident patients were a mean (SD) age of 64 (13) years; 54% were male; 35% were from the south; and 56% had commercial health insurance (Table 3). The mean (SD) age of incident patients was stable from 2007 to 2015 (Table 3).
Table 3.
Patient demographic characteristics among incident cases
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | All | |
|---|---|---|---|---|---|---|---|---|---|
| N | 213 | 252 | 245 | 295 | 377 | 339 | 297 | 189 | 2207 |
| Age, mean (SD), y | 63 (12) | 64 (13) | 63 (13) | 65 (13) | 64 (13) | 65 (14) | 63 (12) | 63 (13) | 64 (13) |
| 18-34, n (%) | 3 (1.4) | 1 (0.4) | 6 (2.4) | 3 (1.0) | 7 (1.9) | 4 (1.2) | 4 (1.3) | 3 (1.6) | 31 (1.4) |
| 35-54, n (%) | 44 (20.7) | 66 (26.2) | 58 (23.7) | 44 (14.9) | 73 (19.4) | 72 (21.2) | 62 (20.9) | 39 (20.6) | 458 (20.8) |
| 55-64, n (%) | 88 (41.3) | 67 (26.6) | 78 (31.8) | 107 (36.3) | 121 (32.1) | 101 (29.8) | 98 (33.0) | 71 (37.6) | 731 (33.1) |
| 65+, n (%) | 78 (36.6) | 118 (46.8) | 103 (42.0) | 141 (47.8) | 176 (46.7) | 162 (47.8) | 133 (44.8) | 76 (40.2) | 987 (44.7) |
| Female, n (%) | 95 (44.6) | 122 (48.4) | 110 (44.9) | 150 (50.8) | 153 (40.6) | 153 (45.1) | 143 (48.1) | 94 (49.7) | 1020 (46.2) |
| Region, n (%) | |||||||||
| Midwest | 69 (32.4) | 85 (33.7) | 74 (30.2) | 89 (30.2) | 89 (23.6) | 77 (22.7) | 71 (23.9) | 43 (22.8) | 597 (27.1) |
| Northeast | 23 (10.8) | 40 (15.9) | 53 (21.6) | 56 (19.0) | 104 (27.6) | 71 (20.9) | 69 (23.2) | 41 (21.7) | 457 (20.7) |
| South | 79 (37.1) | 74 (29.4) | 78 (31.8) | 97 (32.9) | 127 (33.7) | 126 (37.2) | 122 (41.1) | 73 (38.6) | 776 (35.2) |
| West | 42 (19.7) | 53 (21.0) | 40 (16.3) | 53 (18.0) | 57 (15.1) | 65 (19.2) | 35 (11.8) | 32 (16.9) | 377 (17.1) |
| Insurance type, n (%) | |||||||||
| Commercial | 136 (63.8) | 136 (54.0) | 142 (58.0) | 157 (53.2) | 200 (53.1) | 177 (52.2) | 164 (55.2) | 119 (63.0) | 1231 (55.8) |
| Medicare supplemental* | 77 (36.2) | 116 (46.0) | 103 (42.0) | 138 (46.8) | 177 (46.9) | 162 (47.8) | 133 (44.8) | 70 (37.0) | 976 (44.2) |
A Medicare supplemental insurance (Medigap) policy, sold by private companies, can help pay some of the health care costs that original Medicare does not cover (eg, copayments, coinsurance, and deductibles).
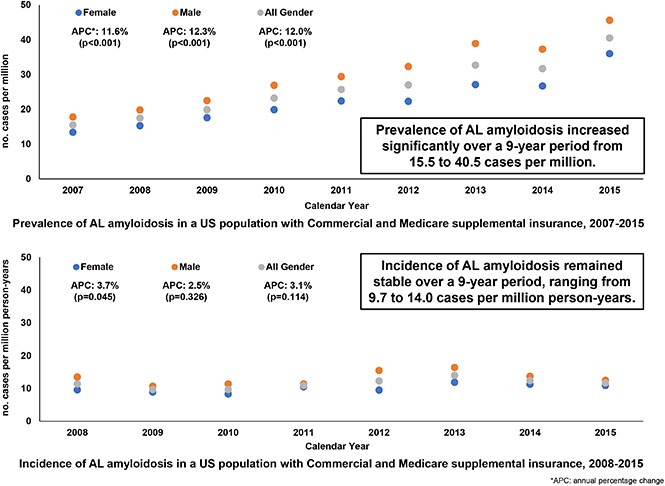
The unadjusted prevalence of AL amyloidosis increased significantly during the study period: from 15.5 in 2007 to 40.5 cases per million in 2015, an APC of 12% (P < .001) (Tables 4 and 5). When age- and sex-adjusted based on 2010 US census data for the adult population, the prevalence of AL amyloidosis increased significantly during the study period: from 20.1 in 2007 to 50.1 cases per million in 2015, an APC of 11.9% (P < .001) (Tables 4 and 5). Similarly, for each sex, when age-adjusted based on 2010 US census data for the adult population, the prevalence of AL amyloidosis increased significantly during the study period: from 17.3 in 2007 to 44.2 in 2015 (an APC of 11.5%; P < .001) for females and from 23.0 in 2007 to 56.5 in 2015 (an APC of 12.3%; P < .001) for males. Similar trends were found in the sensitivity analyses, which required patients to have continuous enrollment in each calendar year (and in the year prior for incident cases). (Tables available upon request.)
Table 4.
Prevalence of AL amyloidosis in years 2007 to 2011
| No. of cases per million (numerator/denominator*) | ||||||
|---|---|---|---|---|---|---|
| Sex | Age, y | 2007 | 2008 | 2009 | 2010 | 2011 |
| Female | 18-34 | 1.5 (5/3 414 356) | 0.6 (3/4 924 396) | 0.6 (3/5 358 001) | 2.3 (12/5 139 252) | 2.3 (13/5 768 546) |
| 35-54 | 6.6 (37/5 617 369) | 8.3 (65/7 859 203) | 11.3 (97/8 565 752) | 13.2 (108/8 210 092) | 11.6 (100/8 586 461) | |
| 55-64 | 26.5 (61/2 299 588) | 35.1 (114/3 244 578) | 35.0 (128/3 658 984) | 38.6 (140/3 627 100) | 44.5 (177/3 981 344) | |
| 65+ | 54.4 (65/1 195 298) | 47.9 (94/1 963 731) | 57.5 (118/2 052 079) | 55.5 (120/2 162 661) | 69.3 (177/2 555 455) | |
| All female | 13.4 (168/12 526 611) | 15.3 (276/17 991 908) | 17.6 (346/19 634 816) | 19.9 (380/19 139 105) | 22.4 (467/20 891 806) | |
| Male | 18-34 | 0.0 (0/3 152 398) | 0.4 (2/4 508 032) | 0.6 (3/4 876 950) | 1.3 (6/4 668 942) | 0.7 (4/5 442 930) |
| 35-54 | 7.2 (36/5 029 837) | 9.5 (67/7 060 318) | 10.3 (79/7 690 284) | 12.2 (90/7 360 450) | 12.2 (95/7 782 789) | |
| 55-64 | 31.9 (67/2 099 846) | 39.2 (116/2 957 222) | 46.6 (154/3 308 173) | 49.3 (160/3 244 501) | 53.1 (189/3 556 632) | |
| 65+ | 100.4 (97/965 927) | 85.4 (134/1 569 003) | 95.3 (159/1 667 958) | 116.3 (201/1 728 898) | 130.7 (266/2 035 214) | |
| All male | 17.8 (200/11 248 008) | 19.8 (319/16 094 575) | 22.5 (395/17 543 365) | 26.9 (457/17 002 791) | 29.4 (554/18 817 565) | |
| All sex | 18-34 | 0.8 (5/6 566 754) | 0.5 (5/9 432 428) | 0.6 (6/10 234 951) | 1.8 (18/9 808 194) | 1.5 (17/11 211 476) |
| 35-54 | 6.9 (73/10 647 206) | 8.8 (132/14 919 521) | 10.8 (176/16 256 036) | 12.7 (198/15 570 542) | 11.9 (195/16 369 250) | |
| 55-64 | 29.1 (128/4 399 434) | 37.1 (230/6 201 800) | 40.5 (282/6 967 157) | 43.7 (300/6 871 601) | 48.6 (366/7 537 976) | |
| 65+ | 75.0 (162/2 161 225) | 64.5 (228/3 532 734) | 74.5 (277/3 720 037) | 82.5 (321/3 891 559) | 96.5 (443/4 590 669) | |
| All patients | 15.5 (368/23 774 619) | 17.5 (595/34 086 483) | 9.9 (741/37 178 181) | 23.2 (837/36 141 896) | 25.7 (1021/39 709 371) | |
| US adult population | Age-sex adjusted† rate, no. of cases per million | |||||
| Female | 17.3 | 17.8 | 29.7 | 22.0 | 25.0 | |
| Male | 23.0 | 22.9 | 25.9 | 30.4 | 33.1 | |
| All | 20.1 | 20.2 | 23.2 | 26.1 | 28.9 | |
The denominator is the number of members enrolled with a health plan on June 30th of each calendar year.
Based on 2010 US census data. Overall rates were adjusted by age and sex distribution, and rates by sex were adjusted by age distribution within specific sex.
Table 5.
Prevalence of AL amyloidosis in years 2012 to 2015
| No. of cases per million (numerator/denominator*) | APC† | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Age, y | 2012 | 2013 | 2014 | 2015 | APC %† (95% CI) | P‡ |
| Female | 18-34 | 1.3 (8/6 027 440) | 1.2 (6/4 905 115) | 1.9 (10/5 174 857) | 2.5 (8/3 203 983) | 11.6 (−4.2 to 30.1) | .133 |
| 35-54 | 10.6 (91/8 598 579) | 13.0 (90/6 901 069) | 14.7 (104/7 087 336) | 21.1 (91/4 306 274) | 11.3 (5.8-17.2) | .002 | |
| 55-64 | 45.4 (183/4 034 960) | 50.7 (169/3 334 349) | 53.6 (187/3 487 785) | 65.4 (144/2 200 422) | 10.1 (8.1-12.2) | <.001 | |
| 65+ | 79.9 (185/2 313 999) | 98.0 (201/2 050 617) | 92.6 (167/1 803 890) | 134.3 (146/1 086 773) | 12.4 (8.4-16.6) | <.001 | |
| All female | 22.3 (467/20 974 978) | 27.1 (466/17 191 150) | 26.7 (468/17 553 868) | 36.0 (389/10 797 452) | 11.6 (9.5-13.8) | <.001 | |
| Male | 18-34 | 1.2 (7/5 750 069) | 0.4 (2/4 609 448) | 0.6 (3/4 942 947) | 1.3 (4/3 008 324) | 6.0 (−11.5 to 26.9) | .462 |
| 35-54 | 13.4 (104/7 785 622) | 17.0 (104/6 132 334) | 16.6 (106/6 375 666) | 18.5 (70/3 785 395) | 11.6 (9.1-14.1) | <.001 | |
| 55-64 | 57.0 (204/3 580 220) | 61.0 (177/2 903 982) | 66.7 (205/3 073 703) | 80.3 (152/1 893 874) | 10.5 (8.5-12.5) | <.001 | |
| 65+ | 159.4 (298/1 869 245) | 185.1 (314/1 696 636) | 184.8 (279/1 509 767) | 239.6 (210/876 397) | 13.2 (9.7-16.8) | <.001 | |
| All male | 32.3 (613/18 985 156) | 38.9 (597/15 342 400) | 37.3 (593/15 902 083) | 45.6 (436/9 563 990) | 12.3 (10.6-13.9) | <.001 | |
| All sex | 18-34 | 1.3 (15/11 777 509) | 0.8 (8/9 514 563) | 1.3 (13/10 117 804) | 1.9 (12/6 212 307) | 11.9 (−0.9 to 26.3) | .064 |
| 35-54 | 11.9 (195/16 384 201) | 14.9 (194/13 033 403) | 15.6 (210/13 463 002) | 19.9 (161/8 091 669) | 11.5 (7.9-15.2) | <.001 | |
| 55-64 | 50.8 (387/7 615 180) | 55.5 (346/6 238 331) | 59.7 (392/6 561 488) | 72.3 (296/4 094 296) | 10.2 (8.4-12.1) | <.001 | |
| 65+ | 115.5 (483/4 183 244) | 137.4 (515/3 747 253) | 134.6 (446/3 313 657) | 181.3 (356/1 963 170) | 12.9 (9.4-16.6) | <.001 | |
| All patients | 27.0 (1080/39 960 134) | 32.7 (1063/32 533 550) | 31.7 (1061/33 455 951) | 40.5 (825/20 361 442) | 12.0 (10.3-13.7) | <.001 | |
| US adult population | Age-sex adjusted§ rate, no. of cases per million | ||||||
| Female | 26.5 | 31.7 | 31.9 | 44.2 | 11.5 (9.2-13.9) | <.001 | |
| Male | 38.6 | 44.3 | 45.0 | 56.5 | 12.3 (10.6-14.0) | <.001 | |
| All | 32.4 | 37.8 | 38.3 | 50.1 | 11.9 (10.1-13.8) | <.001 | |
Number of members enrolled with a health plan on June 30th of each calendar year.
APC (for Tables 4 and 5).
P values are for the trend of APC, reflecting whether the APC is different from zero (for Tables 4 and 5).
Based on US 2010 census data. Overall rates were adjusted by age and sex distribution, and rates by sex were adjusted by age distribution within specific sex.
Between 2008 and 2015, 2207 incident patients with AL amyloidosis were identified, ranging from 189 to 377 cases per million person-years (Table 1). The unadjusted incidence of AL amyloidosis ranged from 9.7 to 14.0 cases per million person-years, with no statistically significant increase (APC, 3.1%; P = .114) (Tables 6 and 7). When age- and sex-adjusted based on 2010 US census data for the adult population, the incidence of AL amyloidosis ranged from 10.8 to 15.2 cases per million person-years, with no statistically significant increase (APC, 3.1%; P = .098) (Tables 6 and 7). Similarly, for each sex, when age-adjusted based on 2010 US census data for the adult population, the incidence of AL amyloidosis did not significantly increase during the study period (APC, 4.1%, P = .045 for females; APC, 2.3%, P = .331 for males). Similar trends were found in the sensitivity analyses. (Tables available upon request).
Table 6.
Incidence of AL amyloidosis in years 2008 to 2011
| No. of cases per million person-years (numerator/denominator*) | |||||
|---|---|---|---|---|---|
| Sex | Age, y | 2008 | 2009 | 2010 | 2011 |
| Female | 18-34 | 0.4 (1/2 328 356) | 0.0 (0/3 334 994) | 1.6 (5/3 179 448) | 0.9 (3/3 452 830) |
| 35-54 | 5.4 (24/4 458 641) | 6.6 (40/6 073 904) | 6.3 (37/5 867 133) | 4.6 (28/6 095 899) | |
| 55-64 | 20.8 (42/2 022 079) | 11.2 (31/2 756 439) | 13.5 (37/2 745 827) | 19.3 (57/2 958 448) | |
| 65+ | 26.7 (28/1 050 168) | 33.0 (51/1 543 818) | 20.5 (31/1 514 234) | 34.6 (62/1 792 636) | |
| All female | 9.6 (95/9 859 244) | 8.9 (122/13 709 155) | 8.3 (110/13 306 642) | 10.5 (150/14 299 813) | |
| Male | 18-34 | 0.9 (2/2 140 796) | 0.3 (1/3 048 029) | 0.3 (1/2 895 210) | 0.0 (0/3 172 642) |
| 35-54 | 5.1 (20/3 953 483) | 4.8 (26/5 404 324) | 4.0 (21/5 244 983) | 2.9 (16/5 449 578) | |
| 55-64 | 25.5 (46/1 801 702) | 14.5 (36/2 480 625) | 16.6 (41/2 473 344) | 19.0 (50/2 625 058) | |
| 65+ | 58.8 (50/849 850) | 54.5 (67/1 230 196) | 57.1 (72/1 261 151) | 55.3 (79/1 429 149) | |
| All male | 13.5 (118/8 745 831) | 10.7 (130/12 163 174) | 11.4 (135/11 874 688) | 11.4 (145/12 676 427) | |
| All sex | 18-34 | 0.7 (3/4 469 152) | 0.2 (1/6 383 023) | 1.0 (6/6 074 658) | 0.5 (3/6 625 472) |
| 35-54 | 5.2 (44/8 412 124) | 5.8 (66/11 478 228) | 5.2 (58/11 112 116) | 3.8 (44/11 545 477) | |
| 55-64 | 23.0 (88/3 823 781) | 12.8 (67/5 237 064) | 14.9 (78/5 219 171) | 19.2 (107/5 583 506) | |
| 65+ | 41.1 (78/1 900 018) | 42.5 (118/2 774 014) | 37.1 (103/2 775 385) | 43.8 (141/3 221 785) | |
| All patients | 11.4 (213/18 605 075) | 9.7 (252/25 872 329) | 9.7 (245/25 181 330) | 10.9 (295/26 976 240) | |
| US adult population | Age-sex adjusted† rate, no. of cases per million person-years | ||||
| Female | 10.4 | 10.4 | 8.7 | 11.5 | |
| Male | 15.2 | 12.5 | 12.9 | 12.5 | |
| All | 12.7 | 11.4 | 10.8 | 12.0 | |
Number of members enrolled with a health plan from July 1st of previous year to June 30th of the calendar year.
Based on US 2010 census data. Overall rates were adjusted by age and sex distribution, and rates by sex were adjusted by age distribution within specific sex.
Table 7.
Incidence of AL amyloidosis in years 2012 to 2015
| No. of cases per million person-years (numerator/denominator*) | APC† | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Age, y | 2012 | 2013 | 2014 | 2015 | APC %† (95% CI) | P‡ |
| Female | 18-34 | 0.7 (3/4 166 829) | 0.9 (3/3 310 183) | 1.2 (4/3 359 080) | 1.3 (3/2 304 754) | 10.8 (−6.4 to 31.2) | .178 |
| 35-54 | 5.5 (37/6 715 540) | 7.3 (38/5 216 573) | 6.9 (36/5 179 870) | 6.9 (24/3 472 683) | 3.2 (−2.5 to 9.2) | .226 | |
| 55-64 | 15.0 (50/3 328 882) | 17.6 (47/2 667 972) | 17.0 (45/2 651 934) | 19.7 (37/1 882 131) | 2.7 (−5.4 to 11.4) | .457 | |
| 65+ | 33.4 (63/1 886 895) | 39.2 (65/1 658 812) | 40.8 (58/1 421 356) | 31.9 (30/941 145) | 5.2 (−2.4 to 13.3) | .152 | |
| All female | 9.5 (153/16 098 146) | 11.9 (153/12 853 540) | 11.3 (143/12 612 240) | 10.9 (94/8 600 713) | 3.7 (0.1-7.5) | .045 | |
| Male | 18-34 | 1.0 (4/3 967 934) | 0.3 (1/3 118 956) | 0.0 (0/3 192 462) | 0.0 (0/2 189 512) | −4.5 (−43.0 to 59.9) | .794 |
| 35-54 | 6.0 (36/6 031 186) | 7.4 (34/4 591 304) | 5.7 (26/4 590 403) | 4.9 (15/3 036 880) | 3.9 (−6.6 to 15.5) | .413 | |
| 55-64 | 24.1 (71/2 949 686) | 23.4 (54/2 305 454) | 23.0 (53/2 309 138) | 21.1 (34/1 610 218) | 2.7 (−4.9 to 10.8) | .427 | |
| 65+ | 74.9 (113/1 508 927) | 71.6 (97/1 355 492) | 63.2 (75/1 186 149) | 60.3 (46/762 658) | 2.3 (−1.9 to 6.7) | .237 | |
| All male | 15.5 (224/14 457 733) | 16.4 (186/11 371 206) | 13.7 (154/11 278 152) | 12.5 (95/7 599 268) | 2.5 (−3.1 to 8.5) | .326 | |
| All sex | 18-34 | 0.9 (7/8 134 763) | 0.6 (4/6 429 139) | 0.6 (4/6 551 542) | 0.7 (3/4 494 266) | 7.4 (−13.8 to 33.9) | .456 |
| 35-54 | 5.7 (73/12 746 726) | 7.3 (72/9 807 877) | 6.3 (62/9 770 273) | 6.0 (39/6 509 563) | 3.5 (−3.5 to 10.9) | .274 | |
| 55-64 | 19.3 (121/6 278 568) | 20.3 (101/4 973 426) | 19.8 (98/4 961 072) | 20.3 (71/3 492 349) | 2.7 (−4.5 to 10.5) | .408 | |
| 65+ | 51.8 (176/3 395 822) | 53.7 (162/3 014 304) | 51.0 (133/2 607 505) | 44.6 (76/1 703 803) | 3.4 (−0.8 to 7.7) | .096 | |
| All patients | 12.3 (377/30 555 879) | 14.0 (339/24 224 746) | 12.4 (297/23 890 392) | 11.7 (189/16 199 981) | 3.1 (−1.0 to 7.3) | .114 | |
| US adult population | Age-sex adjusted§ rate, no. of cases per million person-years | ||||||
| Female | 10.9 | 13.1 | 13.3 | 12.0 | 4.1 (0.1-8.3) | .045 | |
| Male | 17.8 | 17.5 | 15.4 | 14.4 | 2.3 (−2.9 to 7.8) | .331 | |
| All | 14.2 | 15.2 | 14.3 | 13.2 | 3.1 (−0.8 to 7.1) | .098 | |
Number of members enrolled with a health plan from July 1st of previous year to June 30th of the calendar year.
APC (for Tables 6 and 7).
P value reflects whether the APC is significantly different from zero (for Tables 6 and 7).
Based on US 2010 census data. Overall rates were adjusted by age and sex distribution, and rates by sex were adjusted by age distribution within specific sex.
With regard to patient characteristics, male adults over the age of 65 years were found to have the highest prevalence and incidence rates of AL amyloidosis (Tables 4-7).
Discussion
The present study fills a gap in knowledge by adding contemporary data to the literature on the epidemiology of AL amyloidosis in the United States. To date, only 1 known population-based study of AL amyloidosis in the United States has been conducted, and data used are almost 30 years old.1 By increasing awareness of this fatal disease, clinicians may be more likely to recognize and treat it earlier, which may in turn extend patient survival and enhance quality of life.
Using health care claims databases, our analysis indicates that the adjusted (unadjusted) prevalence of AL amyloidosis increased significantly from 20.1 (15.5) cases per million in 2007 to 50.1 (40.5) cases per million in 2015, an annual percentage change of 12% (11.9%). Extrapolating from these data, there were at least 12 000 adults in the United States living with AL amyloidosis in 2015, and the number seems likely to continue rising based on the trajectory we found. Our finding that AL amyloidosis prevalence increased over the 9-year study period is supported by an earlier study in England by Pinney et al whose analyses also indicated a significant increase in prevalent cases from 2000 to 2008.11
In our analysis, the adjusted (unadjusted) incidence of AL amyloidosis did not increase significantly from 2007 to 2015; incidence rates ranged from 10.8 (9.7) to 15.2 (14.0) cases per million person-years, with an annual percentage change of 3.1%. This finding concurs with a previous study by Kyle et al that was conducted with 21 incident AL amyloidosis cases identified in Olmsted County, MN between 1950 and 1989, which found a slight but nonsignificant increase in age- and sex-adjusted incidence rates over the 4 decades.1 In the study on the general population living in Olmsted County from 1950 to 1989, the authors found an incidence rate of 9 cases per million person-years, and deduced that ∼2200 new cases of AL amyloidosis are diagnosed in the United States annually. Incidence rates found in our analyses are slightly higher than those found in England and Sweden,11,12 likely due to the fact that these 2 studies used older data and awareness of AL amyloidosis may have increased recently.
In our current study, prevalence and incidence of AL amyloidosis were higher in males than females; these findings are supported by several previous studies.1,4,5,7,15,19 For example, in a study by Weiss et al, 59% of the 1,430 AL amyloidosis patients identified in Sweden between 1995 and 2013 were men.4 In another Swedish study by Hemminiki et al with 813 AL amyloidosis patients identified between 2001 and 2008, men were found to have a 1.3 times higher incidence of the disease than women.12 As demonstrated by our analyses, prevalence and incidence did not differ across US geographic regions.
The mean age of AL amyloidosis patients in our study was 63 years. These findings are similar to those found in several prior analyses. For example, among the 1430 AL amyloidosis patients identified in Sweden between 1995 and 2013, the mean age at diagnosis was 66 years.4 Similarly, Real de Asúa et al reported a mean age of 66 years among 24 AL amyloidosis patients admitted to a hospital in Spain between 2000 and 2010.23 In another study with 232 AL amyloidosis patients with cardiac dysfunction, median age at presentation was 59 years.24
In this study, we observed an increase in AL amyloidosis prevalence over a 9-year period (2007-2015), coupled with stable incidence. Despite the fact that our study could not determine the mechanisms responsible for the observed change, a study by Muchtar et al with 1551 newly diagnosed AL amyloidosis patients seen at the Mayo Clinic between 2000 and 2014 found a significant improvement in overall survival over time, a change that could explain our findings.25 Continual enhanced survival is only possible if AL amyloidosis symptoms and cases continue to be identified earlier and characterized accurately. Awareness is essential for timely detection and treatment of amyloidosis.19 We further call for widespread education about the disease, as many institutions lack the training and resources to support these educational efforts.
This study has limitations. Most importantly, there is no diagnosis code in ICD-9-CM or ICD-10-CM specific to AL amyloidosis and no generally accepted or clinically validated method for identifying this condition using health insurance claims data. The codes for this study were selected with clinical expert input to eliminate as many non-AL amyloid patients as possible (eg, by excluding 277.31, familial Mediterranean fever, and E85.3, secondary systemic amyloidosis). We further required patients to have received treatment consistent with expert recommendations for AL amyloidosis. This requirement would be expected to decrease the sensitivity but increase the specificity of our identification algorithm. Nonetheless, patients with transthyretin-related hereditary amyloidosis would likely still have been included in our sample. To examine the number of transthyretin-related hereditary amyloidosis patients that may have been included in our sample, we conducted a sensitivity analysis excluding patients who received doxycycline without adjunctive chemotherapy and found a nonsignificant (5%) reduction in the sample size. We are planning to validate the algorithm we used in a future study using data collected from medical records. Until such a validation study is completed, or a more accurate algorithm is proposed, we believe our epidemiologic estimates represent an improvement on a decades-old study conducted in a limited geographic area. Second, our data set included commercially insured patients only who were either healthy enough to be employed or closely related to an employed person, so findings may not precisely reflect the US population due to this healthy-worker bias. Also, our data set included a small proportion of Medicare patients relative to commercially insured patients. Third, a larger proportion of patients aged ≥65 years were included in our study compared with the US adult population. This may be unrepresentative of the general population and overestimate prevalence and incidence given that these rates1 increase with advancing age. Fourth, the administrative claims used in this study were collected for reimbursement purposes and the completeness and accuracy of medical coding is subject to data coding restrictions and data entry error. Fifth, we were unable to examine mortality because these data were unavailable in claims, and we were unable to link the data due to privacy reasons. Lastly, because treatments for AL amyloidosis and multiple myeloma are similar, it is likely that a high number of prevalent AL amyloidosis patients have coexisting multiple myeloma, as observed in the existing literature.16,26,27
Despite these limitations, the current findings shed light on the epidemiology of this rare and life-threatening disease.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Jennifer Munday, an employee of Partnership for Health Analytic Research (PHAR), LLC, provided writing assistance. Sohum Gokhale, an employee of PHAR, LLC, provided technical assistance and proofreading of the manuscript.
Research funded by Prothena Biosciences Inc.
Authorship
Contribution: All authors were equally involved in the design of the study; E.C. conducted the statistical analyses; and all authors contributed equally in the interpretation of results and writing of the manuscript.
Conflict-of-interest disclosure: T.P.Q. is an employee of Prothena Biosciences Inc, which funded the research described in this manuscript. S.G. was employed by Prothena Biosciences Inc at the time the study was conducted and completed. M.S.B., E.C., and T.Y. are employees of the Partnership for Health Analytic Research, LLC, which received funding from Prothena to conduct the research described in this manuscript.
Correspondence: Tiffany P. Quock, Prothena Biosciences Inc, 331 Oyster Point Blvd, South San Francisco, CA 94080; e-mail: tiffany.quock@prothena.com.
References
- 1.Kyle RA, Linos A, Beard CM, et al. . Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79(7):1817-1822. [PubMed] [Google Scholar]
- 2.Rosenzweig M, Landau H. Light chain (AL) amyloidosis: update on diagnosis and management. J Hematol Oncol. 2011;4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1(6):1331-1341. [DOI] [PubMed] [Google Scholar]
- 4.Weiss BM, Lund SH, Bjorkholm M, et al. . Improved survival in AL amyloidosis: a population-based study on 1,430 patients diagnosed in Sweden 1995-2013. Blood. 2016;128(22):4448. [Google Scholar]
- 5.Janssen S, Van Rijswijk MH, Meijer S, Ruinen L, Van der Hem GK. Systemic amyloidosis: a clinical survey of 144 cases. Neth J Med. 1986;29(11):376-385. [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32(1):45-59. [PubMed] [Google Scholar]
- 7.Desport E, Bridoux F, Sirac C, et al. ; Centre National de Référence pour l’Amylose AL et les Autres Maladies par Dépôts d’Immunoglobulines Monoclonales. Al amyloidosis. Orphanet J Rare Dis. 2012;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohty D, Boulogne C, Magne J, et al. . Prognostic value of left atrial function in systemic light-chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2016;17(9):961-969. [DOI] [PubMed] [Google Scholar]
- 9.Palladini G, Dispenzieri A, Gertz MA, et al. . New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541-4549. [DOI] [PubMed] [Google Scholar]
- 10.Dingli D, Tan TS, Kumar SK, et al. . Stem cell transplantation in patients with autonomic neuropathy due to primary (AL) amyloidosis. Neurology. 2010;74(11):913-918. [DOI] [PubMed] [Google Scholar]
- 11.Pinney JH, Smith CJ, Taube JB, et al. . Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161(4):525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemminki K, Li X, Försti A, Sundquist J, Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12(1):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comenzo RL, Reece D, Palladini G, et al. . Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26(11):2317-2325. [DOI] [PubMed] [Google Scholar]
- 14.Lin HM, Gao X, Cooke CE, et al. . Disease burden of systemic light-chain amyloidosis: a systematic literature review. Curr Med Res Opin. 2017;33(6):1017-1031. [DOI] [PubMed] [Google Scholar]
- 15.Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126(12):e178-e182. [DOI] [PubMed] [Google Scholar]
- 16.Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Healthcare resource utilization and costs in amyloid light-chain amyloidosis: a real-world study using US claims data [published online ahead of print 2 Feb 2018]. J Comp Eff Res. doi:10.2217/cer-2017-0100. [DOI] [PubMed] [Google Scholar]
- 17.Weber N, Mollee P, Augustson B, et al. . Management of systemic AL amyloidosis: recommendations of the Myeloma Foundation of Australia Medical and Scientific Advisory Group. Intern Med J. 2015;45(4):371-382. [DOI] [PubMed] [Google Scholar]
- 18.Wechalekar AD, Gillmore JD, Bird J, et al. ; BCSH Committee. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015;168(2):186-206. [DOI] [PubMed] [Google Scholar]
- 19.Nienhuis HL, Bijzet J, Hazenberg BP. The prevalence and management of systemic amyloidosis in western countries. Kidney Dis (Basel). 2016;2(1):10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechalekar AD, Whelan C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017;7(3):e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN guidelines): systemic light chain amyloidosis. Fort Washington, PA: NCCN; 2014. [DOI] [PubMed] [Google Scholar]
- 22.Hari P, Lin HM, Asche CV, et al. . Treatment patterns and health care resource utilization among patients with relapsed/refractory systemic light chain amyloidosis. Amyloid. 2018;25(1):1-7. [DOI] [PubMed] [Google Scholar]
- 23.Real de Asúa D, Costa R, Galván JM, Filigheddu MT, Trujillo D, Cadiñanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol. 2014;6:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubrey SW, Cha K, Anderson J, et al. . The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141-157. [DOI] [PubMed] [Google Scholar]
- 25.Muchtar E, Gertz MA, Kumar SK, et al. . Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk RH. AL amyloidosis or multiple myeloma? An important distinction. Br J Haematol. 2014;164(5):748-749. [DOI] [PubMed] [Google Scholar]
- 27.Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


