TO THE EDITOR:
We read with great interest the article by Yoshioka et al published recently in Blood Advances and suggesting a critical role for S100A9 and neutrophils in granuloma formation.1 The authors used tasquinimod to block S100A9 activity and referred to it as an S100A9 inhibitor. Although this quinoline-3-carboxamide is a potent anti-inflammatory molecule, it is likely not a specific inhibitor of S100A9. In the first and only article to date describing the putative mechanism of S100A9 inhibition by tasquinimod-like quinoline-3-carboxamides, it was shown that laquinimod alleviates experimental autoimmune encephalitis in S100A9-deficient mice (Figure S8 in Bjork et al2). Tasquinimod itself has been reported to inhibit angiogenesis and upregulate expression of thrombospondin by tumor cells,3,4 two activities not associated with S100A9. Although it remains possible that tasquinimod activity does include interaction with S100A9, its specificity for this target is doubtful.
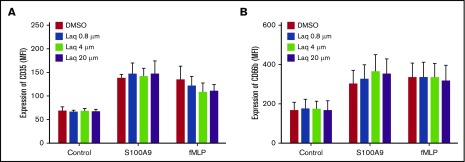
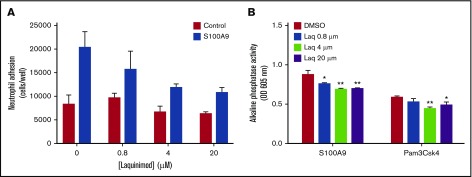
The mode of action of quinoline-3-carboxamides remains unclear.5 To confirm that they do not act solely by binding to S100A9, we analyzed the effect of laquinimod on various myeloid cell functions. Based on the measured increase in the expression of CD35 and CD66b specific and secretory granules, this quinoline-3-carboxamide failed to inhibit neutrophil degranulation induced by N-formylmethionine leucyl-phenylalanine or S100A9 (Figure 1). Meanwhile, laquinimod decreased S100A9-induced neutrophil adhesion to fibrinogen by 50% (Figure 2A). Laquinimod slightly inhibited S100A9-induced NF-κB activation in THP-1 cells (Figure 2B), indicating that it is not a good blocker of all S100A9 activities. More importantly, laquinimod also decreased NF-κB activation induced by the Toll-like receptor 1 (TLR1)/TLR2 agonist Pam3CSK4 in THP-1 cells, clearly demonstrating its lack of specificity for S100A9/TLR4 interactions. Laquinimod and tasquinimod differ only by the presence of a CF3 group, and both are presumed to act via inhibition of S100A9.
Figure 1.
The quinoline-3-carboxamide laquinimod does not inhibit neutrophil degranulation induced by S100A9 or fMLP. Human neutrophils were preincubated for 30 minutes with increasing concentrations of laquinimod (Laq) or with its diluent (dimethyl sulfoxide [DMSO]) and then stimulated for 30 minutes with 10 µg/mL of S100A9 or 10−7 M fMLP. The cells were then stained with anti CD35 (A) or anti CD66b (B) and analyzed by flow cytometry. Data shown are the mean fluorescence intensity (± standard error of the mean [SEM]) measured in 3 experiments performed on cells from different donors. MFI, mean fluorescence intensity.
Figure 2.
The quinoline-3-carboxamide laquinimod partially inhibits neutrophil adhesion and nuclear factor κB (NF-κB) activation in THP-blue cells stimulated with S100A9. (A) Neutrophils were labeled with calcein acetoxymethyl ester and preincubated with increasing concentrations of laquinimod or its diluent (DMSO) for 30 minutes. Cells were then added to fibrinogen-coated 96-well plates, and adhesion was allowed for 30 minutes in the presence or absence of S100A9 (10 µg/mL). After washings, adherent cells were lysed by adding deionized distilled water, and fluorescence was measured at λex = 485 nm and λem = 530 nm using a 96-well plate fluorescence reader. The number of neutrophils per well was determined using a standard curve of serially diluted calcein-AM-labeled neutrophils. Data are from 1 experiment representative of 4, with error bars (± SEM) for the 3 replicates. (B) Cells of the monocyte line THP-blue, which stably expresses an NF-κB/activator protein 1–inducible secreted embryonic alkaline phosphatase gene construct, were preincubated for 30 minutes with increasing concentrations of laquinimod (Laq) or with its diluent (DMSO). The cells were then stimulated for 20 hours with 10 µg/mL of S100A9 or 1 µg/mL of Pam3Csk4. Alkaline phosphatase activity secreted into the culture supernatant was then measured using Quanti-Blue according to the manufacturer’s instructions (Fisher Scientific, Ottawa, ON, Canada). Data shown are the optical density at 605 nm measured in 1 experiment representative of 3, with error bars (± SEM) for the 3 replicates (*P ≤ .05; **P ≤ .01; based on Dunnett’s multiple comparison test). OD, optical density.
We acknowledge that S100A9 is present in human and guinea pig granulomas. However, in light of the lack of specificity of quinoline-3-carboxamides for S100A9, the reported inhibition of lung granuloma formation in guinea pigs following treatment with tasquinimod cannot be attributed specifically to a direct effect on S100A9 activity. Proof of a role for S100A9 would require using a specific inhibitor of S100A9. Such an experiment should therefore be performed in order to support the conclusion that S100A9 has a critical role in granuloma formation.
Contribution: M.P. designed research and wrote the paper; J.-C.S. performed research and analyzed data; D.G. designed research and analyzed data; and P.A.T. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: P.A.T. holds a patent and is a shareholder of InflammatoRx Inc, a company developing an anti-S100A9 for the treatment of chronic inflammation.
Correspondence: Philippe A. Tessier, Université Laval, R-0709, CRCHUL, 2705 Laurier Blvd, Québec, QC G1V 4G2, Canada; e-mail: philippe.tessier@crchudequebec.ulaval.ca.
References
- 1.Yoshioka Y, Mizutani T, Mizuta S, et al. Neutrophils and the S100A9 protein critically regulate granuloma formation. Blood Adv. 2016;1(3):184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björk P, Björk A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7(4):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs JT, Pili R, Qian DZ, et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate. 2006;66(16):1768-1778. [DOI] [PubMed] [Google Scholar]
- 4.Olsson A, Björk A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, Al Ustwani O, Shen L, Pili R. Mechanism of action and clinical activity of tasquinimod in castrate-resistant prostate cancer. OncoTargets Ther. 2014;7:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]