SUMMARY
Marfan’s Syndrome is a multisistemic pathology of connective tissues, a dominant autosomal transmission, first discovered by a French pediatrician, Antoine Bernard-Jean Marfan, who first found in some of his patients a disproportionate alteration of inferior infertility. This alteration was caused by the mutation of the FBN1 gene, located on the long arm of the chromosome 15, which encodes for an extracellular matrix protein, fibrin-1. Later it was discovered that the disease could occasionally be due also to the mutation of the TGFBR2 gene, which encodes for a TGF-beta receptor 1. The estimated incidence of the disease is 2–3 subjects affected every 10,000, in the absence of predilection ratial, ethnic, geographic and gender. It is believed that some 15,000 people in Italy suffer from Marfan Syndrome. The disease is characterized by a wide range of clinical manifestations that affect different organs. The study evaluates through a literature review the manifestations in the oral cavity of the marfan syndrome and the correct management of the patient during dental maneuvers.
Keywords: Marfan Syndrome, dentistry, oral health, oral management
Introduction
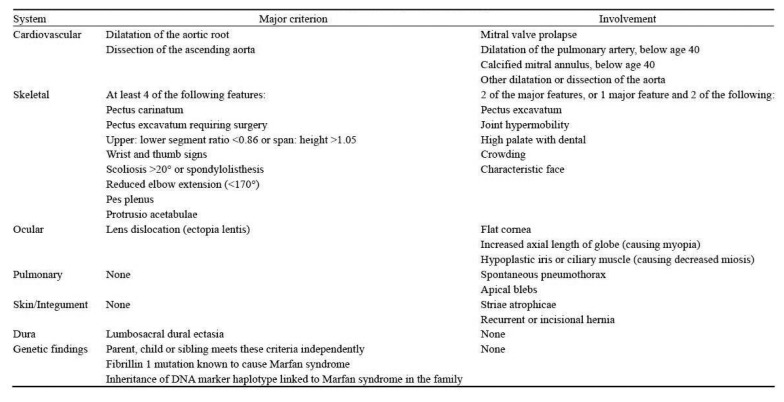
Marfan’s Syndrome is a multisistemic pathology of connective tissues, a dominant autosomal transmission, first discovered by a French pediatrician, Antoine Bernard-Jean Marfan, who first found in some of his patients a disproportionate alteration of inferior infertility. This alteration was caused by the mutation of the FBN1 gene, located on the long arm of the chromosome 15, which encodes for an extracellular matrix protein, fibrin-1. Later it was discovered that the disease could occasionally be due also to the mutation of the TGFBR2 gene, which encodes for a TGF-beta receptor (1). The estimated incidence of the disease is 2–3 subjects affected every 10,000, in the absence of predilection ratial, ethnic, geographic and gender. It is believed that some 15,000 people in Italy suffer from Marfan Syndrome. The disease is characterized by a wide range of clinical manifestations that affect different organs. Timely diagnosis and early treatment help significantly improve the quality and longevity of these patients. Therefore, knowledge of the various signs and symptoms and their radiological aspects allow for early diagnosis that can improve the life of patients with the disease (2). The diagnosis of Marfan’s Syndrome, not always simple, especially in the first months of life, has for many years been based on Ghent’s 1996 nosology, which is based on the combination of “major manifestations” and “minor manifestations”. The criteria considered to be higher are those with high specificity and therefore rare signs and symptoms in the general population. Minor criteria, on the contrary, are those signs and symptoms that are relatively common even in the general population. In sporadic cases, the diagnosis required the presence of at least two major criteria in different apparatus and the involvement of a third device; in family cases, the presence of a single major criterion, or the demonstration of a mutation of the FBN1 gene, was required. Currently, the diagnosis of Marfan’s Syndrome is based on revised Ghent nosology (Figure 1), simplified with respect to the former, which evaluates the two cardinal clinical aspects of syndrome (aortic aneurysm or aortic dissection, ectopia lentis), positivity to the genetic test and a systemic parameter, a term used to refer to all of the other cardiovascular and ocular manifestations typical of the syndrome and those involving all other organs and organs. The systemic parameter determines systemic involvement when the available 20 total points are equal to or greater than 7.
Figure 1.
Ghent nosology.
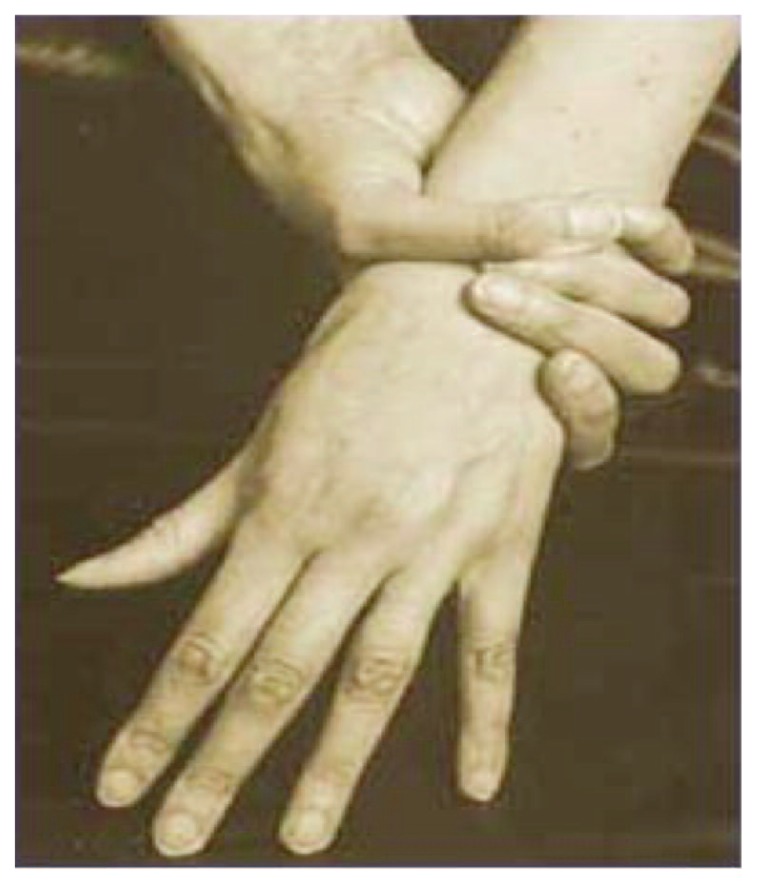
In the sporadic cases, the diagnosis of Marfan’s Syndrome is performed in cases of: aortic diameter at Valsalva breast level characterized by Z-Score > 2 or presence of aortic dissection and ectopia lentis, aortic diameter at the level of Valsalva breast characterized by Z-score > 2 or presence of aortic dissection and presence of genetic mutation of FBN1 gene, aortic diameter at Valsalva breast level characterized Z-scores > 2 or presence of aortic dissection and positive systemic parameter (> 7 points), presence of ectopia lentis and presence of genetic mutation of the FBN1 gene. In family cases, the diagnosis of Marfan Syndrome occurs in cases of: ectopia lentis, positive systemic parameter (> 7 points), aortic diameter of the Valsalva breast libellum with Z-score > 2 over the age of 20, and > 3 under 20 years of age. Although the revised Ghent nosology is simplified, a multidisciplinary approach is required, for a correct diagnosis of Marfan’s Syndrome, involving cardiologist, orthopedic, ophthalmologist, pediatrician, geneticist. Marfan’s Syndrome mainly involves musculoskeletal, ocular and cardiovascular systems (3). The most frequent musculoskeletal manifestations are aracnodactygia which is one of the most frequent clinical manifestations. Aracnodactyla is also recognized for the positivity of the thumb sign [the distal phalanx of the thumb protrudes beyond the closed ulnar ribs around the thumb itself (Figure 2)], and by the wrist mark (the distal fingers of thumb and small overlap if seated to surround the wrist). Another musculoskeletal manifestation is dolicostenomelia i.e. excessive growth in length of long, hollow bones (hands and feet undergo an abnormal elongation with metacarps and phalanxes out of measure). Frequently, scoliotic deformations can be very severe in this type of patient. It affects approximately 62% of the total patient when it is associated with chondrosis or chest wall deformity can contribute to cardiopulmonary impairment and restriction of luminous volume. Another important manifestation is the deformity of the chest wall and is present in 66% of patients, very pathognomonic is the presence of the so-called pectus carinatum or pectus excavatum both due to the longitudinal stretching of ribs. Another musculoskeletal clinical manifestation is the acetabular protrusion that consists of a deformity of the coxo-femoral joint. Radiographically they appear to be an invasion of the acetabulum and the femoral head inside the pelvic cavity. At clinical level, patients exhibit a marked hyperthermia but at the same time difficulties in abduction and rotation. The last cardinal symptom is articular hypermobility i.e. the ability to extend some or all joints beyond normal physiological limits due to ligament laxity. Another major group of symptoms are cardiovascular manifestations. Very important is the presence of ectasia of the ascending aorta, i.e. the elongation or enlargement of the thoracic artery; it mainly affects the root of the artery closest to the heart muscle but may also involve the fibrous ring located above the aorta, Valve opening: in this case there is anu-aortic ectasia. Histologically, the aorta walls show degeneration of the elastic tissue and necrosis of muscle cells. Such dilation is the primary cause of aortic valve failure and affects approximately 60–80% of adults with Marfan Syndrome. Another cardiovascular symptom is aortic dissection, that is, the slimming of the average tonaca of the aotical vessel, with the formation of a second lumen called “false light”. The classification is based on the aorta zone where this pathology occurs and is subdivided into the classifications of De Bakey and Stanford. About 70% of the dissection occurs in the ascending tract, which is also the most dangerous point for the survival of the patient. It is one of the most frequent factors leading to the death of patients with Marfan Syndrome. The last important symptom also from the dental point of view is the prolapse of the mitral valve; it is a disorder that prevents the proper functioning of the mitral valve. The valve flaps are less elastic than normal and can not close perfectly: the mitral valve, therefore, does not remain either tightly closed or open. Most prolapse does not cause any problems. In rare cases however, the blood can flow in the vein opposite to the normal one because the valve does not close well: therefore, palpitations, shortness of breath, chest pain, and other symptoms. Arthritis and endocarditis often occur among the complications of this disorder. The ectopia lentis, axial myopia and retinal detachment are particularly noteworthy. Ectopia lentis is a complication due to the alteration of the connective tissues present at the eyepiece level. It is a dislocation by the crystalline, which often results in a significant reduction in visual acuity. It can be progressive, and in some cases it is observed in concomitance with pupil dislocation, usually in the opposite direction to the dislocation of the crystalline (ectopia lentis et pupillae). It has a prevalence of 75% of patients with Marfan Syndrome, and it is a complication rarely present in other pathologies, and is one of the major criteria for diagnosing the disease. Another ocular symptom is axial myopia, that is, an ametropia, because the light rays coming from a distant object do not focus correctly on the retina, but in front of it.
Figure 2.
Wrist sign.
The consequence is that the observed objects tend to appear blurred and the vision improves by reducing the distance from which you look. It is defined as axial when these characteristics are attributed to an increase in the axial length of the eye (characteristic of many Marfan patients). Ultimate eye complication is the retinal detachment that represents one of the most serious emergencies affecting the eye and sight. It occurs when a retina layer rises by dragging with the blood vessels that feed on oxygen and nourish the eye. Only 48 hours after detachment begins cell death, and this causes progressive loss of vision. The lung manifestations of Marfan Syndrome are spontaneous pneumothorax, congenital bronchial malformation, pulmonary cysts. Spontaneous pneumothorax is a rare complication (4–15% of cases) for patients affected by this syndrome. It is a benign pleural pathology and consists in the presence of air in the pleural space resulting in pulmonary collapse. In Marfan patients it is probably due to the fact that the alteration at the level of collagen leads to an alteration at the level of the bronchial walls, with a decrease in their resistance, resulting in a trap of respiratory airflow. Congenital malformation of bronchi refers to any morphofunctional alteration that results in errors during development and differentiation. Last pulmonary manifestations are pulmonary cysts which are a rare occurrence in patients with such syndrome. It involves the presence of cystic pockets with a rather thin wall and containing air or liquid. Skin manifestations are limited to the presence of stretch marks that represent streaks on the skin. For patients with Marfan Syndrome there is a predisposition to strife development at an early age, without weight modification. Nervous system manifestations are hardcore Ectasia, which is one of the major criteria for disease diagnosis. It was observed in 63–90% of cases, and consists of a dilation of the dental bag or nerve roots, accompanied by erosion and arachnoid cysts. The hard mother, being a connective membrane, in the syndrome is altered in its elastin composition, and hence in its resistance. Under the effect of the pulsation of the liquor and the force of gravity, the dense bag can then be expanded to varying degrees by setting up a dural ectasia framework which, by analyzing the magnetic resonance imaging, is a more or less focal extension of the channel spinal. Dural ectasia can potentially develop throughout the spinal column. In fact, almost always it is seen in the inferior and sacral lumbar region, probably because of the greater influence of the severity factor. Sometimes it is limited to the focal dilation of the dural coating of the nerve root, near their spinal column exit: the so-called “radicular cysts” (4–6). The purpose of this study is to analyze the or-dental symptoms with their frequency in the patient affected by such syndrome and how to perform dental treatments safely by adopting appropriate arrangements. In addition, the position of the dentist is analyzed as part of the multidisciplinary team also for early diagnosis of the disease in the child.
Materials and methods
A bibliographic search on Medline was performed using as keywords “Marfan Syndrome, Dentistry, Oral Health”. The search engine selected 53 articles out of which 26 articles were excluded because they did not deal with the topic being studied. Twenty seven articles dealing with oral health and the skull-facial manifestations of the patient with such syndrome and their management during dental procedures have been considered. Time exclusion criteria have not been adopted.
Results
The different studies selected have indeed shown several changes in the oral-facial level of patients with Marfan’s syndrome compared to the general population. Among the various studies we can consider the case report and the study performed by De Coster et al., in which 23 patients were considered and examined at the University Hospital in Ghent, Belgium. In this study, the average age of patients was 26.17 +/− 13.6 years, with the largest group consisting of 8 patients aged 15 and 20 years. These patients were subjected to a complete dental visit and it turned out that patients did not have any major distinctions as to the overall level of oral hygiene in the different groups considered. Regarding caries, in patients with Marfan Syndrome, a fairly prevalent disorder has been described, in particular developed with respect to deciduous teeth. The study also found that structural defects and hypocalcified or hypomineralized enamel areas are frequently found in 35.78% of cases. Most of these deficits were found at the level of the upper central incisors, and the premolaris both maxillary and mandible, in the form of localized hypoplasmic patches. In the control group, however, only 13% of the cases achieved similar results, showing a statistically significant difference (P = 0.0446). On the contrary, no anomaly has been found with regard to dentin level alterations or opalescent discoloration. An explanation of the lack of significant alteration in comparison between Marfan patients and control group patients can be clarified by embryogenic theory, since enamel is an ectodermal source tissue, while the connective tissue is of mesodermal origin. Genetic alterations at the enamel level are caused by the mutation of some enamel-specific genes, including AMBN, AMELX, ENAM, TUFT1. All the studies conducted have therefore shown no apparent correlation between the genes coding for collagen and fibrin, and the enamel proteins. There were also non-traumatic deformities found in the Marfan group compared to the control group (but no gender preference). Further to conclude the study, De Coster analyzed the presence of pulp and showed a greater incidence in the affected patients than the control group (7). Concurrently in the study conducted by Bauss et al., 21 endoscopic radiographs were evaluated, of which 20.7% had pulpys and 7.9% had pulp obliteration (significantly different when compared with the control group). In the Marfan group some characteristic finds were found regarding the topographic distribution of the endodontal anomaly, as 48.82% of the cases showed one or more unilateral abnormalities, and in 30.43% there was a clear asymmetric defective trend. The control group did not present such anomalies of the endodont (8). The oral mucosa of patients with Marfan Syndrome has streaks and characteristic pigmentation. However, no patients had any difficulties in post-digestive healing, as well as difficulties in dental extraction or eruption problems (9). With regard to dental abnormalities, several studies including that of De Coster et al., (10) and other studies including that of Tsang state that dental elements are more frequently affected by dental anomalies, both of number and especially of form (in particularly radical deformations of the root character), and of structure (enamel hypoplasia on all, followed by imperfect dentinogenesis) (11, 12, 33). Mallineri’s study is always high in the general population of dental anomalies (34). A recent cephalometric study performed on a population of Marfan patients has also demonstrated a significant association between syndrome with retrograde and micrognathic cases (13). Many studies including those of Taddei, De Coster, Pirinen etc. deal with facial alterations in patients with Marfan Syndrome. It is for this reason that facial alterations are included among Ghent’s lower criteria in the skeletal system. All studies agree that Marfan patients have dolicocephaly, that is, the anatomic condition in which the skull box assumes a narrow and elongated shape in the antero-posterior direction. Specifically, this configuration is defined when the ratio between the width and the length of the head assumes a maximum value of 75–76%. The presence of a narrow skull with dolicocephaly is highly reported in many patients with Marfan Syndrome. Other features found in the facial mass are malaria hypoplasia and retrograde. The first condition is represented by a projection deficit of the zygomatic region, which alters the harmonic aspect of the face. With the term hypoplasia malare instead, it indicates a backward position of the jaws in the antero-posterior direction. It may be relative or false (when it is only apparent to the skeletal counterpart) or absolute and true (referring to the vertical drawn from the root of the nose). It is determined by the upper jaw, the jaw, or both. Another facial manifestation is the enophthalmic i.e. the displacement of the ocular bulb towards the inside of the orbit, more in depth than its normal condition. In Marfan patients, it can often evolve into diplopia. Numerous studies deal with the occlusal appearance and conformation of the oral cavity of patients with Marfan Syndrome (14–18). Khonsari et al. and De Coster studies have shown in Marfan Syndrome patients the tendency to the presence of the ogival palate which is one of the most commonly encountered in such studies. It is a malformation characterized by the reduction of the cross-section of the palatine vault, with accentuation of the height of the upper jawbone. Another characteristic conformation of the oral cavity of these patients is the presence of back crossbits which is often found in association with the ogival palate. It is a malocclusion characterized by an inverse buco-lingual relationship between one or more teeth of a mandibular jaw, and one or more teeth of the opposite jawbone. It is frequently accompanied by deviation of the lower median line for lateral sliding of the mandible. Frequent response is the tendency to the 2nd skeletal class of Angle, characterized by incorrect relationships between the upper jaw and jaw. Class 2 malocclusions can then be further distinguished in 2 subdivisions, depending on the presence or absence of overjet. The common condition for all class 2 malocclusions is the presence of a retrograde jaw, due in most cases to insufficient mandibular growth, although coexisting with a contemporary superior development of the upper jaw. Frequent response in the patients analyzed in these studies is the presence of open bite associated with other orthodontic problems mentioned above. For open bite is a vertically distorted normal occlusal relationship, characterized by anterior bend due to lack of contact between the antagonist teeth. Frequent feedback is also the presence of significant dental folls and numerous misalignments (16, 19–21). A single study deals with the presence of obstructive sleep apnea which is quite common in these patients as a result of skeletal alterations (14). De Coster and Shakya and Gorlin treat hypermobility of temporomandibular joint which, with the passage of time, may be responsible for dysfunction of temporomandibular joint including subluxation, frontal dislocation of the articular disk, osteoarthritis. They are due to joint malformation and hyperlassiness of ligaments: these alterations may cause a mouth opening block, concomitant with pain during chewing and clicking in the opening (22–24). It has been observed in the Rose study that these patients have a higher prevalence for periodontal disease when compared to control groups made up of healthy patients (25). This condition may be partly due to negligence on the part of such patients with regard to home oral hygiene maneuvers, and partly due to the etiopathogenesis of the disease itself, as well as the defect suffered by collagen and connective tissues. Marfan’s Syndrome has an effect on the oral cavity, particularly in periodontal disease, as periodontal ligament appears to be a soft, cellular and richly vascular connective tissue, located between the cement and the alveolar bone. It is a fabric that performs two fundamental functions, namely that of distribution and reabsorption of forces during chewing and dental contact, associated with ensuring a minimum degree of dental mobility. It is composed of numerous cells (including fibroblasts, cementoblasts, epithelial cells and nerve cells) and collagen fiber bundles that can be divided into four main groups in relation to their organization: alveolar ridge fibers, horizontal fibers, oblique fibers and apical fibers. Periodontal tissue cells also express the genes coding for fibrillin-1 and fibrillin-2 proteins, as a result any genetic mutation at these tissues may result in periodic oral changes in the periodontal tissue. De Coster analyzes the prevalence of gingival inflammation in a group of 23 patients by comparing them with a control group of 69 patients. He found a greater presence of gum bleeding in the survey (7). Staufenbien’s study analyzed the level of clinical attack in a group of 51 patients with Marfan Syndrome and compared it with a control group of 31 patients. The study found that in fact patients with Marfan syndrome have more gum bleeding but the prevalence of periodontitis is almost equal to the control group (26). To conclude, several articles deal with the dental treatment of the patient with such syndrome. This group of patients generally has a number of systemic and oro-facial alterations that lead to increased treatment difficulties in the dental field. Patients with Marfan Syndrome also have a high risk of developing cardiovascular disease following surgery, including dental surgery. Aortic dissection, heart failure, and other cardiac valve diseases are the most frequent cause of death in patients with such syndrome. It is therefore of fundamental importance to know these risks and to supplement the treatment of such complications in odontostomatological therapies. Numerous studies have found a high frequency of facial anomalies, including a high incidence of caries, periodontal disease, dentinal disorders, pulmonary disorders and other orthodontic alterations. In these patients it is very important to follow a proper prevention protocol and appropriate therapy to prevent the disease from becoming worse. Correct management of chronic pathology is crucial in preventing bacteremia in Marfan patients, especially in younger age. As with all cardiopathic patients, the use of local anesthetics with vasoconstrictors should be carried out with caution (27). Normally the most used vasoconstrictor in dentistry is adrenaline. In 1964, the conclusions drawn from a working conference of the American Dental Association were that vasoconstrictors at the concentrations normally used in dentistry do not have contraindications if they are administered correctly and after aspiration. Subsequently, some studies have confirmed the validity of this conclusion, while others have noted the occurrence of alterations in blood pressure or complications following the use of vasoconstrictor anesthetics in cardiac patients. The use of vasoconstrictor anesthetics aims to prolong the effect and depth of anesthesia. In fact, the appearance of pain can lead to an increase in adrenaline release from adrenal glands up to 20–40 times (Perusse, 1992). For this reason, the use of anesthetic with vasoconstrictor is recommended. Another important aspect in treating these patients is the fact that they, being subject to various cardiac pathologies, exhibit a high risk of bacterial endocarditis as a result of any dental surgery that causes bleeding. This complication is caused by the bacterial colonization of cardiac valve or endothelial wall. Mortality despite the therapy is high. Dental therapies that cause bleeding cause transient bacteremia and for this reason some bacteria (Streptococcus Viridans and Staphilococcus Aureus) can colonize the platelet vegetation present on pre-existing valve lesions. It is believed that 1 case of 5 of the bacterial endocarditis is associated with dental therapies and that in most cases the disease appears within 2 weeks of the intervention (28, 29). In patients at risk of developing bacterial endocarditis, prophylaxis is indicated for all dental interventions that cause mucous bleeding and provoke transient bacteremia. Reduction of inflammation and infectious process with local measures at the site of intervention (oral hygiene enhancement, flushing and sulphoxide irrigation with chlorhexidine) is indicated as it reduces the amount of bacteremia. An active antibiotic is used on the main beta-hemolytic streptococcus bacteria endocarditis agents. The standard oral administration is usually sufficient and accepted more favorably by dentist and patient; parenteral alternative regimen is indicated in particular situations. The intravenous parenteral regimen is reserved for patients with high risk cardiopathies or extensive interventions due to high bacteremia due to local infection or inflammation.
One shot therapy is usually used or two doses close to six hours (first dose and subsequent halved). The duration can be extended up to 5–7 days in particular situations that exhibit prolonged risk of bacteremia (sutures, second-order healing due to extensive interventions) using amoxicillin 500 mg for 4 days. To provide maximum protection at each session, treatment should be completed during the peak plasma concentration of the antibiotic (1–2 hours before surgery). Patients with this syndrome have a high risk of developing cardiovascular disease following dental interventions. Aortic dissection, heart failure, and other cardiac valve diseases are the most frequent cause of death. It is therefore crucial to know these risks and to integrate the treatment of such odontostomatological complications. In counteracting cardiological disorders, given the high susceptibility to aortic damage, Marfan patients often take antihypertensive drugs such as beta-blockers and ACE inhibitors. In dental surgery, stress and anxiety caused by the dental session itself and/or the excessive administration of vasoconstrictors may cause a hypertensive crisis and precipitate acute complications of associated pathologies (myocardial ischaemia, stroke). Antihypertensive or sedative therapies can cause postural hypotension, syncope and falls trauma. Antihypertensive therapies can cause pharmacological interactions with vasoconstrictors associated with anesthetic. Given the above considerations regarding cardiac problems it is apparent that stress can cause major complications in these patients. The activation of the sympathetic nervous system generates an alarm reaction characterized by cardiovascular reactions mediated by adrenergic stimulation. In fact, emotional or reduced cardiovascular functional reserve should be evaluated for the possibility of drug-induced sedation with nitrous oxide or benzodiazepine (in adult: diazepam 5–10 mg per hour or 30 minutes before surgery) and these interventions should be short and programmed preferably in the morning (29–32).
Discussion
The patient with Marfan Syndrome, given the wide symptom, needs to be treated by a multidisciplinary team. As far as the dental aspect is concerned, all the studies analyzed agree that they have a DMFT high compared to the rest of the general population. Regarding the health of periodontal tissues, there are contrasting studies. De Costen’s study states that the index of gingival inflammation is higher than that of the control group and for this reason also states arbitrarily that they are more susceptible to periodontal disease. In fact, in this respect, the Staufebien study clarifies that inflammatory indices are higher due to dental clotting but clinical attack level indexes are almost equal between the two groups. All studies agree that these patients need periodontal maintenance and frequent hygiene sessions about every 5/6 months. They also need an early and inter-orthodontic approach considering the many skeletal manifestations of the disease. From here emerges the figure of the orthodontist to allow an early diagnosis of the diseases seen in the multiple oral manifestations. All studies agree on proper management of pharmacological anxiety to avoid fearsome cardiovascular complications (33, 34).
Conclusion
Dental treatments can be carried out safely by implementing small cautions that could avoid important cardiovascular complications. In addition, Marfan’s patient needs an intercultural orthodontic program as a child to allow a harmonious development of the skeletal component and also frequent recalls of periodic dental hygiene and visits due to the high frequency of caries and the high inflammation of the peri-odontium.
References
- 1.Matyas G, De Paepe A, Halliday D, Boileau C, Pals G, Steinmann B. Evaluation and application of denaturing HPLC for mutation detection in Marfan syndrome: Identification of 20 novel mutations and two novel polymorphisms in the FBN1 gene. Hum Mutat. 2002 Apr;19(4):443–56. doi: 10.1002/humu.10054. [DOI] [PubMed] [Google Scholar]
- 2.De Coster PJ, Martens LC, De Paepe A. Orofacial manifestations of congenital fibrillin deficiency: pathogenesis and clinical diagnostics. Pediatr Dent. 2004 Nov-Dec;26(6):535–7. [PubMed] [Google Scholar]
- 3.Faivre L, Collod-Beroud G, Ades L, Arbustini E, Child A, Callewaert BL, Loeys B, Binquet C, Gautier E, Mayer K, Arslan-Kirchner M, Grasso M, Beroud C, Hamroun D, Bonithon-Kopp C, Plauchu H, Robinson PN, De Backer J, Coucke P, Francke U, Bouchot O, Wolf JE, Stheneur C, Hanna N, Detaint D, De Paepe A, Boileau C, Jondeau G. The new Ghent criteria for Marfan syndrome: what do they change? Clin Genet. 2012 May;81(5):433–42. doi: 10.1111/j.1399-0004.2011.-01703.x. Epub 2011 Jun 2. [DOI] [PubMed] [Google Scholar]
- 4.Frydman M. The Marfan syndrome. Isr Med Assoc J. 2008 Mar;10(3):175–8. [PubMed] [Google Scholar]
- 5.Ha HI, Seo JB, Lee SH, Kang JW, Goo HW, Lim TH, Shin MJ. Imaging of Marfan syndrome: multisystemic manifestations. Radiographics. 2007 Jul-Aug;27(4):989–1004. doi: 10.1148/rg.274065171. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HC. Marfan Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2001. Apr 18, 1993–2017. [updated 2017 Feb 2] [Google Scholar]
- 7.De Coster PJ, Martens LC, De Paepe A. Oral manifestations of patients with Marfan syndrome: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002 May;93(5):564–72. doi: 10.1067/moe.2002.121430. [DOI] [PubMed] [Google Scholar]
- 8.Bauss O, Neter D, Rahman A. Prevalence of pulp calcifications in patients with Marfan syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Dec;106(6):e56–61. doi: 10.1016/j.tripleo.2008.06.029. Epub 2008 Sep 20. [DOI] [PubMed] [Google Scholar]
- 9.Poole AE. Craniofacial aspects of the Marfan syndrome. Birth Defects Orig Artic Ser. 1989;25(4):73–81. [PubMed] [Google Scholar]
- 10.De Coster P, Martens L, De Paepe A. Novel dental anomalies associated with congenital contractural arachnodactyly: a case report. Pediatr Dent. 2004 Nov-Dec;26(6):478. author reply 478. [PubMed] [Google Scholar]
- 11.Tsang AK, Taverne A, Holcombe T. Marfan syndrome: a review of the literature and case report. Spec Care Dentist. 2013 Sep-Oct;33(5):248–54. doi: 10.1111/scd.12018. Epub 2013 Feb 28. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Garcia A, Castellanos-Cosano L, Machuca-Portillo G, Posada-de la Paz M. Impact of rare diseases in oral health. Med Oral Patol Oral Cir Bucal. 2016 Sep 1;21(5):e587–94. doi: 10.4317/medoral.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utreja A, Evans CA. Marfan syndrome-an orthodontic perspective. Angle Orthod. 2009 Mar;79(2):394–400. doi: 10.2319/112707-558.1. [DOI] [PubMed] [Google Scholar]
- 14.Taddei M, Alkhamis N, Tagariello T, D’Alessandro G, Mariucci EM, Piana G. Effects of rapid maxillary expansion and mandibular advancement on upper airways in Marfan’s syndrome children: a home sleep study and cephalometric evaluation. Sleep Breath. 2015 Dec;19(4):1213–20. doi: 10.1007/s11325-015-1141-y. Epub 2015 Feb 15. [DOI] [PubMed] [Google Scholar]
- 15.Docimo R, Maturo P, D’Auria F, Grego S, Costacurta M, Perugia C, Chiariello L. Association between Oro-Facial Defects and Systemic Alterations in Children Affected by Marfan Syndrome. J Clin Diagn Res. 2013 Apr;7(4):700–3. doi: 10.7860/JCDR/2013/5656.2885. Epub 2013 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Coster P, De Pauw G, Martens L, De Paepe A. Craniofacial structure in Marfan syndrome: a cephalometric study. Am J Med Genet A. 2004 Dec 15;131(3):240–8. doi: 10.1002/ajmg.a.30393. [DOI] [PubMed] [Google Scholar]
- 17.Pirinen S. Genetic craniofacial aberrations. Acta Odontol Scand. 1998 Dec;56(6):356–9. doi: 10.1080/000163598428310. [DOI] [PubMed] [Google Scholar]
- 18.Crean SJ, Firrozeai R, Hopper C. The role of three-dimensional computed tomographic reconstruction in orthognathic surgery planning. Br J Oral Maxillofac Surg. 1997 Oct;35(5):376–7. doi: 10.1016/s0266-4356(97)90423-x. [DOI] [PubMed] [Google Scholar]
- 19.Poole AE. Craniofacial aspects of the Marfan syndrome. Birth Defects Orig Artic Ser. 1989;25(4):73–81. [PubMed] [Google Scholar]
- 20.Gazit E, Lieberman M. Severe maxillary arch constriction in a patient with Marfan’s syndrome: report of case. ASDC J Dent Child. 1981 Jul-Aug;48(4):292–3. [PubMed] [Google Scholar]
- 21.Crosher R, Holmes A. Marfan syndrome: dental problems and management. Dent Update. 1988 Apr;15(3):120, 122. [PubMed] [Google Scholar]
- 22.Shakya S, Ongole R, Sumanth KN, Denny CE. Chronic bilateral dislocation of temporomandibular joint. Kathmandu Univ Med J (KUMJ) 2010 Apr-Jun;8(30):251–6. doi: 10.3126/kumj.v8i2.3570. [DOI] [PubMed] [Google Scholar]
- 23.De Coster PJ, Van den Berghe LI, Martens LC. Hypermobility and temporomandibular disorders: inherited connective tissue disease as a model with maximum expression. J Orofac Pain. 2005 Winter;19(1):47–57. [PubMed] [Google Scholar]
- 24.Barr M. Temporomandibular joint dysfunction and oro-facial pain. Aust Dent J. 1979 Jun;24(3):190–1. [PubMed] [Google Scholar]
- 25.Rose LF, Mealey B, Minsk L, Cohen DW. Oral care for patients with cardiovascular disease and stroke. J Am Dent Assoc. 2002 Jun;133(Suppl):37S–44S. doi: 10.14219/jada.archive.2002.0378. [DOI] [PubMed] [Google Scholar]
- 26.Staufenbiel I, Hauschild C, Kahl-Nieke B, Vahle-Hinz E, von Kodolitsch Y, Berner M, Bauss O, Geurtsen W, Rahman A. Periodontal conditions in patients with Marfan syndrome - a multicenter case control study. BMC Oral Health. 2013 Oct 28;13:59. doi: 10.1186/1472-6831-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perusse R, Goulet JP, Turcotte JY. Contraindications to vasoconstrictors in dentistry: Part I. Cardiovascular diseases. Oral Surg Oral Med Oral Pathol. 1992 Nov;74(5):679–86. doi: 10.1016/0030-4220(92)90365-w. [DOI] [PubMed] [Google Scholar]
- 28.Hirota Y, Sugiyama K, Niwa H, Matsuura H. Systemic management of Marfan’s syndrome during dental treatment: a case report. Anesth Pain Control Dent. 1993 Summer;2(3):162–70. [PubMed] [Google Scholar]
- 29.Sugiyama K, Hirota Y, Shibutani T, Niwa H, Idoji Y, Matsuura H. Systemic management of a patient with Marfan’s syndrome during dental treatment. Osaka Daigaku Shigaku Zasshi. 1988 Dec;33(2):496–504. [PubMed] [Google Scholar]
- 30.Crosher R, Holmes A. Marfan syndrome: dental problems and management. Dent Update. 1988 Apr;15(3):120, 122. [PubMed] [Google Scholar]
- 31.Scott DC. Frontier dentistry. Part 3: Marfans syndromean oral perspective. Ont Dent. 1982 Apr;59(4):54–9. 62. [PubMed] [Google Scholar]
- 32.Dowling JN, Lee WS, Sacco RJ, Ho M. Endocarditis caused by Neisseria mucosa in Marfan’s syndrome. Ann Intern Med. 1974 Nov;81(5):641–3. doi: 10.7326/0003-4819-81-5-641. [DOI] [PubMed] [Google Scholar]
- 33.Sachdev MS, Sood NN, Kumar H, Ghose S. Bilateral aniridia with Marfan’s syndrome and dental anomalies-a new association. Jpn J Ophthalmol. 1986;30(4):360–6. [PubMed] [Google Scholar]
- 34.Mallineni SK, Jayaraman J, Yiu CK, King NM. Concomitant occurrence of hypohyperdontia in a patient with Marfan syndrome: a review of the literature and report of a case. J Investig Clin Dent. 2012 Nov;3(4):253–7. doi: 10.1111/j.2041-1626.2012.00148.x. [DOI] [PubMed] [Google Scholar]