Abstract
Previous research has shown that the medial temporal lobes (MTL) are more strongly engaged when individuals think about the future than about the present, leading to the suggestion that future projection drives MTL engagement. However, future thinking tasks often involve scene processing, leaving open the alternative possibility that scene-construction demands, rather than future projection, are responsible for the MTL differences observed in prior work. This study explores this alternative account. Using functional magnetic resonance imaging, we directly contrasted MTL activity in 1) high scene-construction and low scene-construction imagination conditions matched in future thinking demands and 2) future-oriented and present-oriented imagination conditions matched in scene-construction demands. Consistent with the alternative account, the MTL was more active for the high versus low scene-construction condition. By contrast, MTL differences were not observed when comparing the future versus present conditions. Moreover, the magnitude of MTL activation was associated with the extent to which participants imagined a scene but was not associated with the extent to which participants thought about the future. These findings help disambiguate which component processes of imagination specifically involve the MTL.
Keywords: future thinking, hippocampus, imagination, medial temporal lobes, scene construction
Introduction
There is now considerable evidence suggesting that the medial temporal lobes (MTL) are involved not just in remembering past events but also in other aspects of cognition, including imagining hypothetical events or scenarios in the future. Functional imaging studies of healthy adults demonstrate striking overlap in engagement of the MTL for past and future thinking (Szpunar et al. 2007; Botzung et al. 2008; Addis et al. 2009; Weiler et al. 2010), and amnesic patients with MTL damage who cannot remember past events in rich detail are also unable to vividly imagine future events (Tulving 1985; Klein et al. 2002; Kwan et al. 2010; Race et al. 2011).
Together, these studies have led to the proposal that “mental time travel” (i.e., the act of mentally projecting oneself into the past and future; Tulving 1983) may be a critical feature responsible for MTL involvement in such studies (see Schacter et al. 2012 for review). Although some functional magnetic resonance imaging (fMRI) research involving the comparison of future versus present imagining provides some support for the importance of the MTL in future temporal projection, this research conflates episodic demands with future projection. For example, in a recent study that showed stronger activation in the MTL and other default mode regions in a future versus present contrast, the future condition involved imagining specific events, whereas the present condition involved imagining conceptual information (Xu et al. 2016). Thus, it is unknown whether future projection per se was responsible for the MTL findings. Indeed, in another study, Andrews-Hanna and colleagues found greater engagement of an MTL subsystem of the default mode network when individuals thought about the future than when they thought about the present, but as the authors state, this effect was driven by differences in scene construction (i.e., the need to imagine a scenario within a spatial context), which was more strongly elicited for the future condition (Andrews-Hanna et al. 2010). Accordingly, participants’ ratings of scene construction accounted for a large portion of the variance in MTL subsystem activity.
Other evidence also challenges the importance of mental time travel per se for preferential MTL involvement. For example, in a small sample (N = 5), Nyberg et al. (2010) observed no differences in MTL activity when comparing conditions that require imagining the past and future with a present condition though we speculate that the use of the same event probe across all conditions in this study (the same “walk through the park” probe was used for every experimental condition) may have made it difficult for participants to engage fully in the appropriate temporal projection required in each distinct condition. D'Argembeau and colleagues also did not observe differences in MTL recruitment when contrasting consideration of past or future personal traits relative to those pertaining to the present (D'Argembeau et al. 2010b; also see e.g., Mitchell et al. 2011) or when comparing specific future events with atemporal routine activities (D'Argembeau et al. 2010a), though the latter was not a future versus present comparison per se.
Relevant to this issue, Hassabis et al. (2007) found that amnesic patients with MTL lesions were just as impaired at imagining atemporal scenarios (i.e., scenarios that have no temporal designation) as they were at imagining future scenarios. Such a finding further challenges the notion that the MTL is necessary for mental time travel per se, although a caveat of this study is that one cannot rule out the possibility that the atemporal condition also elicited thinking about the future. Critically, irrespective of experimental condition, the patients’ narratives lacked spatial coherence—a finding that led the authors to propose that the critical feature that drives MTL activity is not mental time travel but instead scene construction.
The goal of this study was to disambiguate the involvement of the MTL in scene construction versus mental time travel, hereafter referred to as “future projection” given our focus on future thinking. Critically, we do so by experimentally manipulating demands on scene construction and future projection, an approach not previously taken. We examined MTL activity in 1) high scene-construction and low scene-construction imagination conditions matched in future projection demands and 2) future-oriented and present-oriented imagination conditions matched in scene-construction demands. If MTL activity in prior studies of future versus present thinking reflects differential demands on scene construction, then MTL activity should be greater when scene construction demands are high, but should not be modulated by future projection demands. Alternatively, if MTL activity reflects mental time travel into the future, then MTL activity should be greater in the future-oriented than in the present-oriented imagination condition.
Materials and Methods
Participants
Twenty-seven healthy, right-handed, native English speakers (10 male) with a mean age of 19.6 (±3.4) years and a mean education of 15.0 (±3.2) years participated in the study. Participants were recruited from Boston University and Northeastern University through campus flyers and online postings. Participants were given a detailed phone screen prior to participating in the study and were excluded from participation if they had any MRI contraindications, a major psychiatric condition, or a neurological condition. One subject was excluded due to questionable effort on the task (i.e., lack of variability in responses), resulting in a total of 26 participants. The session lasted approximately 2.5 h (approximately 1.5 h in the scanner), and participants were paid $60 for their participation in the study. The VA Boston Healthcare System Institutional Review Board approved all experimental procedures, and all participants provided informed consent.
Task Paradigm and Procedure
The task was composed of 4 experimental conditions and a low-level control task, similar to that of Andrews-Hanna et al. (2010). In each experimental condition, participants made forced choice self-referential decisions that required 1) high scene construction, 2) low scene construction, 3) future orientation, or 4) present orientation (see Appendix A for examples of trial probes). This design allowed for 2 critical contrasts, one involving high and low scene-construction imagination conditions with future thinking held constant and the other involving future- and present-oriented imagination conditions with scene construction held constant (as determined by piloting and participant ratings; see below). In the low-level control condition, participants made forced choice decisions about whether a number was odd or even (Stark and Squire 2001). The specific instructions administered to participants are provided in the Supplementary Materials. (Notably, we did not employ a 2×2 design, in which scene-construction and future projection demands are manipulated orthogonally. As elaborated on in the Discussion, behavioral piloting conducted before the present fMRI study motivated this decision.)
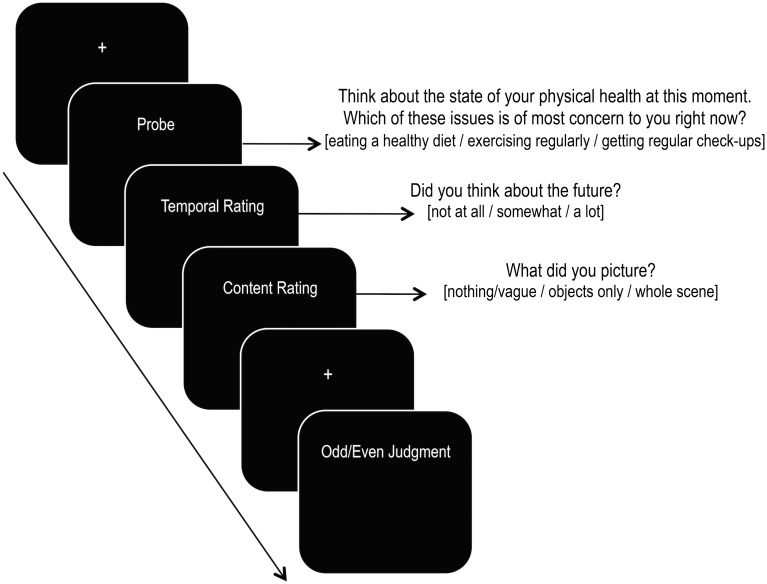
All stimuli were presented using a PC computer with E-prime (version 2.0) and an MRI-compatible projector and screen. Participants made their responses using an MRI-compatible button box placed in their right hand. For all 4 experimental conditions, a trial started with a crosshair fixation (1050 ms), followed by the probe along with the 3 corresponding response options for 9400 ms (Fig. 1). Appropriate timing for each trial was determined through behavioral pilot experiments and reference to other studies involving a similar design (Andrews-Hanna et al. 2010). Conditions were matched for the average number of words presented across the probe and corresponding response options (high scene construction: 33.8 words; low scene construction: 34.8 words; future: 31.9 words; present: 33.7 words) and did not significantly differ across contrasts of interest (high versus low scene construction: P = 0.57; future versus present: P = 0.20).
Figure 1.
Overview of the experimental paradigm used during scanning (also see Appendix A and Supplementary Materials). The example depicted is taken from the “present” condition.
Although the total trial duration was 9400 ms, participants were asked to make a button response indicating their choice as soon as they made their decision. Following a 50 ms interstimulus interval (ISI), a rating question appeared for 4150 ms (“Did you think about the future?”) with the corresponding response options (“not at all”; “somewhat”; “a lot”); hereafter referred to as “temporal” rating.
Following a 50 ms ISI, a second rating question appeared for 4150 ms (“What did you picture?”) with the corresponding response options (“nothing/vague”; “objects only”; “whole scene”; hereafter referred to as “content” rating). Prior to the scan, participants were given fuller explanations of these labels {“I didn't really picture anything or vague images at most”; “I pictured isolated objects or people, but not a scene”; “I pictured a whole scene [objects and surrounding background]”; also see Supplementary Materials for the complete instructions for the rating questions}. The content rating was followed by a 50 ms ISI. Both for the probe and for the ratings, the trial duration was fixed (i.e., the stimuli remained on the screen even after the participant made a response). Rating questions were used for subsequent brain-behavior analyses (see below) and also served as a manipulation check to confirm that the conditions differed/matched as expected. The latter is particularly relevant given that prior studies comparing future versus present thinking often did not include participant ratings to confirm that the future condition indeed evoked more future thinking.
Each experimental trial was followed by a low-level control trial, which consisted of a crosshair fixation for 1000 ms followed by a 50 ms ISI and then the sequential presentation of 5 numbers over a duration of 9450 ms (each digit was presented for 1790 ms with a 100 ms ISI). Participants made button responses to indicate whether a given number was odd or even.
A total of 20 trials per experimental condition were presented across 5 runs, with 4 trials per condition randomly assigned to each run (16 trials per run). Within a given run, trials from the 4 conditions were intermixed and presented in a different random order for each participant. The presentation order of the 5 runs was also randomized for each participant. (Due to technical reasons related to data acquisition, 1 run from 3 subjects and 2 runs from another subject could not be used.)
Immediately prior to the scan, participants were provided with the task instructions and completed 5 practice trials in a private testing room to familiarize themselves with the materials and procedure. Participants completed an additional 5 practice trials in the scanner to help them acclimate to the scanning environment and the button box used to make responses. After the scan, participants were debriefed about the task, which ensured that no participants had difficulty reading the screen, using the button box, or felt rushed during the task.
Image Acquisition
Images were collected on a 3.0 Tesla Siemens Trio scanner equipped with a 32-channel head coil and located at the Jamaica Plain campus of the VA Boston Healthcare System. A high-resolution T1-weighted magnetization-prepared rapid gradient-echo sequence was acquired in the sagittal plane (TR = 2530 ms, TE = 3.32 ms, TI = 1100 ms, flip angle = 7 degrees, sections = 176, slice thickness = 1 mm, matrix = 2562, FOV = 256 mm, voxel size = 1 mm3). Five whole brain functional scans were acquired parallel to the anterior–posterior commissural plane using a multiband echo-planar imaging sequence sensitive to the blood oxygenation level–dependent (BOLD) signal (Moeller et al. 2010; Xu et al. 2013; multiband = 6; TR = 1050 ms, TE = 34.80 ms, flip angle = 65°, slices = 72, slice thickness = 2 mm, FOV = 208, matrix = 1042, voxel size = 2 mm3, volumes = 452, phase encoding = anterior–posterior). To correct for image distortion, a brief scan using the same parameters was also acquired although the phase encoding direction was inverted (posterior–anterior).
Data processing and Analyses
Behavioral
Reaction time data were analyzed using paired t-tests for the critical contrasts of interest (high versus low scene construction; future versus present). To account for multiple comparisons, we used Bonferroni correction (corrected P = 0.025 for the 2 comparisons).
For analysis of the 2 rating question types (content and temporal), data were converted to proportions. Given the categorical and ordinal nature of the data for the content and temporal questions, respectively, and given that the 3 levels of responses were not statistically independent, we performed 3 separate paired t-tests (one at each level of response option) for both questions for each contrast. Bonferroni correction was employed for these analyses (corrected P = 0.004 for 12 comparisons).
FMRI Univariate Analyses
Functional imaging data were preprocessed and analyzed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) software library (FSL version 4.1; www.fmrib.ox.ac.uk/fsl). FSL's topup tool was used to estimate susceptibility fields. Images were motion corrected using MCFLIRT (Jenkinson et al. 2002), and then the estimated susceptibility field correction was applied to the functional time series using applytopup. The BOLD time series was skull stripped using FSL's Brain Extraction Tool (BET) and bias-field corrected using FMRIB's Automated Segmentation Tool (FAST). Subsequent fMRI data processing was carried out using FSL's FMRI Expert Analysis Tool (FEAT) version 6.00, with the following pre-statistics processing applied: spatial smoothing using a Gaussian kernel of FWHM 4.0 mm; grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor; high-pass temporal filtering (Gaussian-weighted least squares straight line fitting, with sigma = 30.0 s). Next, in a 2-step registration process, each functional image was co-registered to the participant's same-session T1-weighted structural image using FMRIB Linear Image Registration Tool (FLIRT). Between-subject registration was accomplished by alignment of functional images to the MNI152 standard space template and further refined using the FMRIB Nonlinear Image Registration Tool (FNIRT). Images were visually inspected to confirm proper registration to MNI space. Time series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al. 2001). Trial onset times were convolved with a single gamma hemodynamic response function, with the trial duration modeled as the participant's response time for each trial. Thus, only trials with responses were included in the imaging analyses (see Results). Subject level analysis was carried out using a fixed effects model, by forcing the random effects variance to zero in FLAME (FMRIB's Local Analysis of Mixed Effects; Beckmann et al. 2003; Woolrich et al. 2004). The general linear model consisted of 4 task regressors (one for each experimental condition) and additional regressors of no-interest, which included the low-level control condition, content and future ratings, and trials in which no response was made.
Group-level analysis was carried out using FLAME stage 1 (Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008). The resulting statistical images for all reported univariate analyses (e.g., high > low scene construction) were compared using paired t-tests and thresholded using clusters determined by Z > 1.96 and a (corrected) cluster significance threshold of P = 0.05 (Worsley 2001). Given that our primary hypotheses pertained to both potential differences and lack of differences in the MTL, we selected this threshold to strike a balance between the ability to reject the null hypothesis for high versus low scene construction and to accept the null hypothesis for future versus present.
FMRI Multivariate Analyses
If the MTL is sensitive to scene-construction demands but not to future projection, activation in the MTL should correlate with the extent to which participants endorsed thinking about a scene but should be unrelated to the extent to which participants endorsed thinking about the future. Alternatively, if the MTL is sensitive to future projection, activation should be related to the extent to which participants endorsed thinking about the future. To explore the brain regions that covaried with scene construction and future projection, we used partial least squares (PLS) analysis to examine correlations between voxel signal and subjective ratings across all experimental conditions simultaneously. PLS is a data-driven, flexible multivariate technique in which relationships between patterns of whole brain activity and multiple variables of interest (e.g., experimental conditions) are expressed as latent variables (LVs), which represent similarities and differences in patterns of activation in relation to these selected variables of interest (see Krishnan et al. 2011 for review). Such an approach allowed us to examine 1) whether subjective ratings are related to voxel signal within each experimental condition and 2) whether the magnitude of a given association between subjective ratings and voxel signal differed across conditions.
A “behavioral” PLS analysis (brain-behavior analysis) was run separately for the content rating and for the temporal rating, such that each PLS was computed based on a correlation matrix involving the covariance of voxel signal and the given rating question for each condition across participants. For the first PLS, for each participant, we entered the proportion of “scene” responses from the content rating within each condition, whereas for the second PLS, we entered the proportion of temporal ratings associated with the highest endorsement of future thinking (i.e., “a lot”) within each condition. Thus for both PLS analyses, we entered participants’ responses from only 1 level of response, which is the most suitable approach given the categorical and ordinal nature, respectively, of the content and temporal ratings. For this analysis, we only included rating responses for trials on which a probe response was made.
The LVs were computed via singular value decomposition (analogous to eigenvectors in principal components analysis). The reliability of an LV was assessed using 500 permutations (i.e., resampling without replacement to reassign the order of conditions within each subject); PLS was recalculated for each newly ordered sample to determine the probability that an LV occurred by chance. An LV was considered statistically reliable if the probability of the single value for the LV for a given permutation was less than 0.05 (also see e.g., McIntosh et al. 1996). The stability of each voxel's contribution to a given LV was assessed using a bootstrap estimation of the salience (voxel weight) standard errors with 100 resamplings. This involved resampling of participants with replacement for each voxel, while maintaining the assignment of conditions for each participant and rerunning the PLS following each resampling. The resulting bootstrap ratio for each voxel is analogous to a Z-score. As in other studies utilizing PLS (Seminowicz and Davis 2007; Poppenk et al. 2010; Spreng et al. 2010; Lombardo et al. 2015), the bootstrap ratio was considered reliable when it was above 1.96, which corresponds approximately to a 95% confidence interval (P = 0.05), although individual peak voxels typically had bootstrap ratios that were much higher. A voxel extent threshold > 30 contiguous voxels (4 mm3 space) was employed. This bootstrap ratio can be positive or negative, depending on the nature of its relationship to the pattern described by that LV. Because the voxel weights are calculated in a single analytic step involving the whole brain, it is not necessary to correct for multiple comparisons in PLS (see Krishnan et al. 2011 for a detailed review of the PLS procedure).
Results
Behavioral
Reaction Time
Analyses of reaction time revealed that the 9400 ms epoch for the probe provided participants with sufficient time to make a response. Participants were significantly slower for high (M = 6617 ms, SD = 712) versus low (M = 6495 ms, SD = 690) scene-construction probes, t (25) = 2.73, P = 0.01. There was no significant difference in reaction time for future (M = 5909 ms, SD = 727) versus present (M = 5964 ms, SD = 797) probes (P = 0.48). The mean number of responses made, across conditions, out of 20 trials, was as follows: for high scene construction, 17.5 (SD = 2.9; Total = 456), for low scene construction, 18.0 (SD = 2.5; Total = 468), for future, 18.5 (SD = 2.2; Total = 480), and for present, 18.4 (SD = 2.1; Total = 479).
Content Ratings
Participants’ ratings across the 4 conditions and 2 content questions are displayed in Table 1. As expected, participants endorsed thinking about a scene significantly more often in the high scene-construction condition than in the low scene-construction condition, t (25) = 14.02, P < 0.0001. By contrast, participants endorsed thinking about objects/people to a greater extent in the low than in the high scene-construction condition, t (25) = 8.41, P < 0.0001. Participants also reported thinking about nothing/vague images to a greater extent in the low than in the high scene-construction condition t(25) = 5.50, P < 0.0001.
Table 1.
The proportion of participant responses for the content (“What did you picture”) and temporal (“Did you think about the future?”) rating questions
| Content rating | Temporal rating | |||||
|---|---|---|---|---|---|---|
| Nothing/vague | Objects | Scenes | Not at all | Somewhat | A lot | |
| High scene construction | 0.09 (0.13) | 0.10 (0.11) | 0.81 (0.17) | 0.34 (0.24) | 0.42 (0.23) | 0.24 (0.22) |
| Low scene construction | 0.25 (0.20) | 0.39 (0.16) | 0.36 (0.20) | 0.26 (0.18) | 0.50 (0.21) | 0.24 (0.23) |
| Future | 0.24 (0.20) | 0.46 (0.17) | 0.30 (0.20) | 0.08 (0.12) | 0.41 (0.26) | 0.51 (0.30) |
| Present | 0.24 (0.20) | 0.47 (0.17) | 0.28 (0.17) | 0.51 (0.23) | 0.38 (0.23) | 0.11 (0.17) |
For the comparison of the future and present conditions, there were no significant differences in any of the content response options (all Ps > 0.60; Table 1).
The mean number of responses made, across conditions, out of 20 trials for the content rating, was as follows: for high scene construction, 17.1 (SD = 3.0; Total = 444), for low scene construction, 17.5 (SD = 2.8; Total = 455), for future, 18.0 (SD = 2.4; Total = 469), and for present, 17.8 (SD = 2.3; Total = 464). Only trials with responses were included in the subsequent imaging analyses involving participant ratings.
Temporal Ratings
As expected, participants endorsed thinking about the future “a lot” to a greater extent in the future than in the present condition, t (25) = 8.59, P < 0.0001, and participants endorsed thinking about the future “not at all” to a greater extent in the present than in the future condition, t (25) = 8.62, P < 0.0001. The intermediate condition “a little” did not differ across conditions (P = 0.58).
For the comparison of the high and low scene-construction conditions, participants endorsed thinking about the future “a lot” and “a little” to a similar extent (all Ps > 0.13); yet, participants unexpectedly endorsed thinking about the future “not at all” to a greater extent in the high than in the low scene-construction condition, t (25) = 2.46, P = 0.02, although this did not survive Bonferroni correction. Nonetheless, to ensure that these differences did not impact our fMRI results, we entered future ratings (“not at all”) as a covariate in contrasts comparing these conditions, which did not change the pattern of results (see below). The mean number of responses made, across conditions, out of 20 trials for the temporal rating, was as follows: for high scene construction, 16.9 (SD = 3.3; Total = 439), for low scene construction, 17.4 (SD = 2.9; Total = 453), for future, 18.0 (SD = 2.4; Total = 467), and for present, 18.0 (SD = 2.4; Total = 467).
FMRI Univariate Results
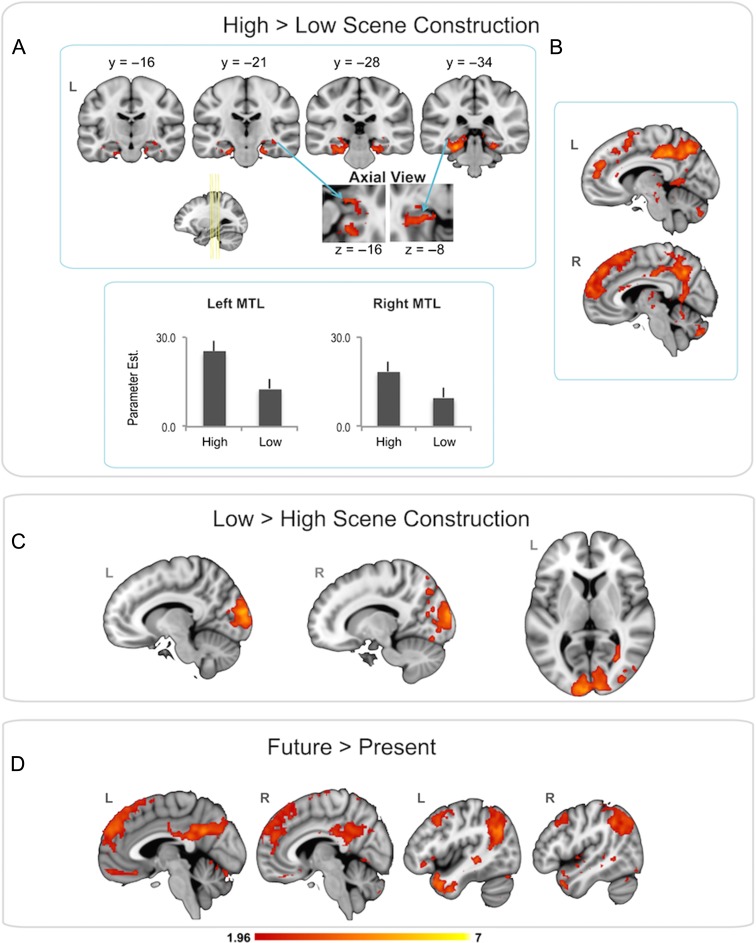
The contrast of high > low scene construction revealed differences in activation in the MTL bilaterally (anterior and posterior hippocampus and MTL cortices; see Fig. 2A), which extended from clusters with local maxima in the lateral temporal cortices (Supplementary Table 1). This pattern did not change when we entered future ratings as a covariate in the analysis (Supplementary Figure 1).
Figure 2.
(A) Greater MTL activity during high > low scene construction (for this figure, an MTL mask was applied to highlight activation in this region). (B) Greater activation in midline default mode regions during high > low scene construction. (C) The low > high scene construction contrast revealed activation in ventral visual regions. (D) The future > present contrast revealed no differences in MTL activity, but activation differences were observed in lateral parietal regions and ventromedial PFC, as well as other default mode regions. The color bar denotes Z-scores.
If the MTL is indeed involved in scene construction, differences in this region should no longer be significant when scene ratings are used as a covariate in the analysis. Indeed, when we extracted the signal from the MTL (left and right) for the high and low scene-construction conditions versus baseline and entered these data into a 2×2 within-subjects ANCOVA (condition, laterality), including scene ratings in the model as a covariate, the expected condition difference [i.e. significantly greater MTL activity for high versus low scene construction, F (1, 25) = 12.1, P = 0.002] was no longer observed, F (1, 24) = 0.24, P = 0.63.
Beyond the MTL, this contrast revealed differences in large clusters that mainly comprised default mode regions, including bilateral dorsomedial prefrontal cortex (PFC), lateral temporal cortex (including temporal pole), posterior cingulate, retrosplenial cortex, and precuneus (Fig. 2B). This activation also extended into bilateral, dorsolateral, and ventrolateral PFC. The peak maxima are shown in Supplementary Table 1. Given the large size of the clusters, a more complete picture of the spatial extent of the activation for this contrast is provided by a whole brain montage (Supplementary Figure 2).
The opposite contrast (low > high scene construction) revealed activation differences mainly within the ventral visual stream, encompassing lateral (e.g., middle occipital gyrus) and medial (e.g., cuneus) occipital regions, bilaterally (see Figure 2C; Supplementary Table 1; Supplementary Figure 3).
For the contrast of future > present, no differences were observed anywhere in the MTL, even at a more liberal statistical threshold (p = 0.10). For this contrast, differences were observed in bilateral ventromedial PFC and bilateral superior and inferior parietal lobule, as well as several of the aforementioned default mode regions denoted for the high versus low scene-construction contrast (Fig. 2D; Supplementary Table 2; Supplementary Figure 4). The opposite contrast (present > future) revealed no significant differences in activation in any regions.
As a note of caution, our primary hypotheses in this article pertain to the MTL. We consider our whole brain effects exploratory in nature and they should be interpreted as preliminary given the recent controversy pertaining to appropriate cluster correction thresholds as they relate to univariate approaches (Eklund et al. 2016).
FMRI Multivariate Results
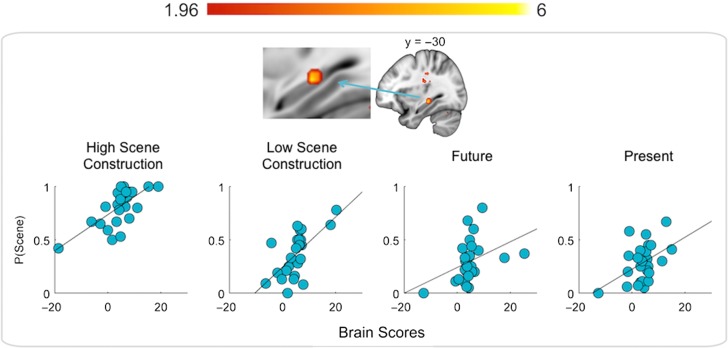
When scene ratings were entered into a behavioral PLS analysis, one significant LV emerged that explained 44% of the cross-block covariance (P = 0.036; Fig. 3). Critically, the extent to which participants endorsed thinking about a scene was positively correlated with signal in the MTL, across conditions, with peak localization in the left posterior hippocampus (Poppenk et al. 2013). Additionally, positive correlations were observed with activity in lateral (bilateral) and medial (left) occipital regions and right middle frontal gyrus. A negative association was observed with right medial PFC, approximating anteromedial PFC (Supplementary Table 3). The correlations were significant for all conditions, and the magnitude of the associations did not significantly differ across conditions, as can be gleaned from the overlapping confidence intervals across conditions (Supplementary Table 4).
Figure 3.
The figure depicts the results from the behavioral (“scene” ratings) PLS analyses. The brain image depicts a cluster in the left posterior hippocampus that positively correlated with scene ratings. The scatter plots illustrate the distribution of brain scores (x-axis) versus scene ratings (y-axis) for each experimental condition. Brain scores, which are conceptually similar to factor scores in principle components analysis, indicate how strongly a participant expresses the brain activity pattern within each condition. The correlations were significant for all conditions (high scene construction: r = 0.69, low scene construction: r = 0.64, future: r = 0.39, present: r = 0.46). The magnitude of the associations did not significantly differ across conditions (Supplementary Table 4). The color bar denotes bootstrap ratios.
When future ratings were entered into a behavioral PLS analysis, a single significant LV also emerged that explained 42% of the cross-block covariance (P = 0.026). This LV revealed no evidence that the extent to which participants endorsed thinking about the future related to MTL activity. Instead, future ratings were positively associated with activity in right inferior parietal lobule as well as lateral and medial frontal regions (left dorsolateral and medial PFC; right precentral gyrus, right dorsal anterior cingulate). A negative association was also observed in the left rostral anterior cingulate (Supplementary Figure 5; Supplementary Table 5). A significant correlation was observed across 3 of the 4 conditions (i.e., the high scene-construction condition did not contribute to the pattern, as denoted by the confidence interval encompassing zero for this condition; see Supplementary Table 4); the magnitude of the association did not significantly differ for the latter 3 conditions (also see Supplementary Table 4).
Discussion
Using fMRI, we directly contrasted MTL activity for 1) high and low scene-construction imagination conditions matched in future projection demands and 2) future- and present-oriented imagination conditions matched in scene-construction demands. In doing so, we sought to clarify whether scene construction, rather than future projection, could account for the difference in MTL activation observed in prior work. We found that the MTL was more active when individuals engaged in high versus low scene construction, whereas MTL differences were not observed for future versus present imagination, even at a reduced statistical threshold. Moreover, multivariate (i.e., PLS) analysis revealed that MTL activity covaried with the extent to which participants pictured a scene but was not associated with the extent to which participants thought about the future. Together, these findings suggest that the MTL is sensitive to scene-construction demands but is not modulated by the future projection aspect of mental time travel per se.
The observed preferential MTL engagement associated with high scene-construction demands is in line with other neuroimaging findings demonstrating robust MTL involvement during the imagination of scenes in comparison with the imagination of acontextual objects (Hassabis et al. 2007). Such evidence has been taken as support for the argument that scene construction is the primary function of the MTL, and more specifically, of the hippocampus (Maguire and Mullally 2013). We note, however, that our data are also compatible with other views that postulate a fundamental role for the hippocampus in relational (Cohen and Eichenbaum 1993), contextual (Ranganath 2010), and spatial (O'Keefe and Nadel 1978) processes that are likely necessary for scene construction.
Activation differences for the high versus low scene-construction contrast were distributed across the long axis of the hippocampus and MTL, although only the posterior hippocampus (Poppenk et al. 2013) was specifically modulated by participants’ subjective scene ratings in the multivariate analysis. That is, greater activation in this region was associated with higher ratings of scene construction across participants. Although this finding is consistent with a larger literature demonstrating a well-established role of the posterior MTL in spatially rich mental construction and spatial processing more broadly (Hayes et al. 2007; Poppenk et al. 2013; Sheldon and Levine 2016; but see Zeidman and Maguire (2016) for a somewhat different viewpoint on anterior versus posterior MTL contributions to mental construction), the fact that we did not observe an anterior versus posterior dissociation in our activation contrast limits support for a long axis specialization for scene construction.
Nonetheless, it is intriguing that the area of the posterior hippocampus that correlated with scene ratings in this study demonstrates striking overlap with an area of the hippocampus shown to be strongly engaged for constructed episodic events imagined in the context of a recombination task (Gaesser et al. 2013). Given the proposed importance of scene construction to episodic imagining (Maguire and Mullally 2013), it is interesting to speculate that the hippocampal activation associated with construction demands reflects associated demands on scene processing. Critically, although other fMRI studies also show a more anterior localization within the hippocampus for constructive processing (van Mulukom et al. 2013), the study by Gaesser et al. is unique in that it controls for possible effects of novelty and encoding, which may account for anterior hippocampal engagement in other studies.
In considering reasons for the lack of consistency between the activation contrast and PLS findings with regard to the longitudinal axis of the hippocampus, it is important to note that our high and low scene-construction conditions may differ in other aspects such as the extent to which they elicit processing of affective, action-based, and other sensory information. Accordingly, it is possible that these differences may also be responsible for the greater activation in the hippocampus for high versus low scene construction. For example, our high scene-construction condition may have elicited greater affective content, which is known to recruit the anterior hippocampus (Sheldon and Levine 2016). However, in the absence of corroborating behavioral data (e.g., ratings of emotional intensity), this interpretation is speculative. An alternative possibility is that because the anterior MTL tends to be more vulnerable to susceptibility artifacts than the posterior MTL (Olman et al. 2009), greater error variance associated with this portion of the MTL may have obscured an association with scene ratings. Our study was not designed to tease apart anterior versus posterior hippocampal contributions to imagination, but this is an interesting topic for future research.
Notably, methods used to elicit construction of imagined events in fMRI studies range widely in their cueing approach, from single word cues (Addis et al. 2007) to multiple word cues (e.g., person, location, object; Gaesser et al. 2013) to very specific events (e.g., “Imagine you are lying on a sandy beach in a tropical bay”; Hassabis et al. 2007). Similar to Hassabis et al. (2007), our study uses very specific events but with the additional provision of specific response options. These various cueing methods differ in their demands on scene construction, and in particular, in the extent to which scene information is provided within the cue (which could conceivably affect the localization of activation along the long axis). Yet, there is no clear cue-related pattern in hippocampal location across studies. However, it should be noted that studies of imagination also differ in other respects, such as in their chosen baseline condition or the extent to which novelty and encoding demands are matched across conditions within a study. Such methodological differences may make it more difficult to detect cue-related differences.
Although we have classified 2 of our conditions in the context of demands on scene construction, another way to conceptualize the difference between these conditions is in terms of “event specificity,” with the high scene-construction condition involving imagining a (hypothetical) specific autobiographical event (i.e., an episode) and the low scene-construction condition involving imagining (hypothetical) non-event-specific autobiographical information. Indeed, we used event specificity as a way to manipulate the demands on scene construction because imagination of scene-based information is thought to be a dominant process that is critical for the imagination of specific episodes. Relatedly, these conditions can also be conceptualized as “episodic” and “semantic,” although according to a recent taxonomy, semantic imagining that pertains to autobiographical information is considered as an intermediate state, falling between episodic and non-autobiographical semantic processing (Szpunar et al. 2014).
In contrast to the observed differences in MTL activity for high versus low scene construction, no MTL differences were observed for future versus present imagination—conditions that were deliberately matched in scene-construction demands. This finding aligns with and extends prior studies suggesting that the MTL may not be involved in future-oriented mental time travel (Hassabis et al. 2007; D'Argembeau et al. 2008; Nyberg et al. 2010). It also dovetails with the notion that previously reported greater MTL recruitment for future versus present imagination is a result of greater scene construction and/or episodic demands in the future condition (Andrews-Hanna et al. 2010). Such task demands seem to be a byproduct of differences inherent to the types of stimuli used to evoke future versus present thinking in some studies (Andrews-Hanna et al. 2010; Xu et al. 2016) and is not likely to be an inherent feature of future thinking per se. Notably, additional studies have observed MTL involvement in future thinking, but because these studies do not aim to isolate the involvement of the MTL in temporal orientation per se, they conflate episodic imagining and future projection and cannot address the issue at hand (Schacter et al. 2012).
Although the comparison of future versus present imagination yielded no differences in MTL activity, differences were observed in other default mode regions (as was also the case for high versus low scene construction). Intriguingly, the future versus present contrast uniquely revealed differences, bilaterally, in superior and inferior parietal lobule, and activation in the right inferior parietal lobule was positively associated with higher endorsement of future thinking in participants’ subjective ratings in the PLS analysis. These findings accord well with prior literature implicating lateral parietal regions in future thinking. For instance, D'Argembeau et al. (2010b) observed activation differences for future relative to present thinking in the inferior parietal cortex, while Nyberg et al. (2010) observed differences in intraparietal sulcus for a contrast involving mental time travel (past and future) versus present thinking (but see Ersner-Hershfield et al. 2009). Given prior work showing that some parietal regions are involved in perspective taking and empathy (Ruby and Decety 2001; Rabin et al. 2010), it is interesting to speculate whether activation differences in parietal regions for future versus present reflect a difference in perspective associated with shifting to a “future self.”
The future versus present contrast also revealed differences in the ventromedial PFC bilaterally {encompassing Brodmann areas (BA) 10, 11, 32}. This finding is consistent with 2 other studies, one that observed greater activity in this region for both personal and nonpersonal future events relative to (atemporal) routine activities (D'Argembeau et al. 2010a) and another that found greater activation in this region for far versus near future events (D'Argembeau et al. 2008). Theoretical ideas about the function of the ventromedial PFC have emphasized its role in leveraging distributed knowledge in the service of meaning generation (see Roy et al. 2012; Benoit et al. 2014 for discussion)—a process that may be particularly important for future thinking given that future eventualities are otherwise unknown or abstract (also see Trope and Liberman 2010). The hypothetical nature of future preferences compared with currently held preferences in this study would likewise place heavier demands on combining conceptual knowledge.
Yet, “reduced” activation in this region for future relative to present thinking has been observed in other studies involving imagining future versus present events (Mitchell et al. 2011) and in the evaluation of future versus present character traits (Ersner-Hershfield et al. 2009; D'Argembeau et al. 2010b). These findings have been interpreted in the context of self-referential processing and the putative involvement of the ventromedial PFC in this process (Northoff et al. 2006). That is, given that the future is more abstract, it might engender less extensive self-referential thinking than the present, thereby leading to “less” involvement of this region in future relative to present thinking (D'Argembeau et al. 2010b). Another related interpretation of these data comes from the view that the ventromedial PFC is involved in representing value information, which arguably is more concrete for the present relative to the future, thereby recruiting the ventromedial PFC to a greater extent (Mitchell et al. 2011). Notably, in the present study, the multivariate analysis revealed a negative association between ratings of future thinking and activation in left rostral anterior cingulate cortex in an area that was slightly more superior (BA24) than the ventromedial PFC cluster revealed in the future versus present (univariate) contrast. In other words, the “more” participants thought about the future, the “less” they activated the rostral anterior cingulate cortex. Although it is tempting to speculate that dissociations of function within the ventromedial PFC versus rostral anterior cingulate can account for our univariate (i.e., activation) versus multivariate findings, as well as the discrepancies in the literature at large (Mitchell et al. 2011), some of the aforementioned studies show activation that spans both these regions, thereby providing no supporting evidence for dissociation of function. Reconciling these discrepant findings is an important issue for future research, which will require the appropriate functional resolution to permit such fine-grained anatomical dissociations.
It is important to note that scene construction and future projection were not orthogonally examined in the context of a 2×2 design in this study because we found it impossible to achieve the necessary control to implement such a design. Specifically, in our pilot work, we found that in the future condition, low scene-construction probes consistently elicited higher ratings of future thinking than did high scene-construction probes. Thus, the interpretation of any difference between these conditions would be ambiguous. For this reason, we chose the alternative of creating 2 separate contrasts for which we could avoid such confounds. Namely, we opted to compare future and present imagination in conditions that were relatively low in scene construction demands. One consequence of this design choice is that it did not enable us to examine whether the MTL might contribute to future projection under conditions that are high in scene-construction demands. Of relevance, D'Argembeau et al. (2008) observed no MTL differences for a comparison of far versus near future episodic events (akin to our high scene-construction condition). A caveat of this study is that although participant ratings indicated that the far future condition indeed felt more distant in time, temporal extension is not synonymous with the need for future projection. Nonetheless, this study argues against a role of the MTL in future projection in the context of high scene construction.
Conclusion
The present findings not only elucidate the specific component processes of imagination that drive MTL engagement but also shed light on the neural substrates of future-oriented mental time travel. Although both processes elicit widespread brain activation, particularly within default mode regions, the MTL uniquely contributes to scene construction, whereas other regions, such as lateral parietal cortex and ventromedial PFC uniquely drive future projection. These findings help to elucidate the neurocognitive mechanisms associated with these aspects of higher order cognition.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
National Institutes of Mental Health (grant number H093431) and the Department of Veterans Affairs (Clinical Science Research and Development Service to M.V. and Rehabilitation Research & Development Service [grant number E7822W] to S.M.H.). D.J.P. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR).
Supplementary Material
Notes
D.J.P. and S.M.H equally contributed to this work. This work was further supported with resources and the use of facilities at the Neuroimaging Research for Veterans Center, VA Boston Healthcare System. The contents of this manuscript do not represent the views of the US Department of Veterans Affairs, the US Government, or the National Institutes of Health. The authors thank Rose Hopkins for research assistance. Conflict of Interest: None declared.
Appendix A. Examples of stimuli
| Condition | Probe | Response choices | ||
|---|---|---|---|---|
| High scene construction | Imagine taking your friend to a wine expo for their birthday. Which do you envision? | I'm helping my friend select wines | I'm eating cheese samples | I'm sneaking off to buy a birthday gift |
| Imagine you're at a party and you spill ink on your shirt. Which do you envision? | I'm scrubbing at the stain in the washroom | I'm calling someone for advice | I'm asking the host for club soda | |
| Imagine first arriving at your new home. Which do you envision doing during your first hour after your arrival? | I'm unpacking boxes | I'm introducing myself to neighbors | I'm arranging furniture | |
| Low scene construction | Imagine getting a month off from your regular work or school routine. How would you most likely spend this extra free time? | I would travel | I would do volunteer work | I would pursue hobbies |
| Imagine that you're a soccer coach for young children. Which would be your primary goal for the team? | That they learn good sportsmanship | That they improve their soccer skills | That they win a lot of games | |
| Imagine that a large new tax is imposed on fresh fruits and fresh vegetables. How will this tax affect your shopping behavior? | Buy more frozen food items | Buy more canned food items | No change | |
| Future | Think about whom you're most likely to be living within 5 years. Which best describes what your situation will be? | I will live with family members | I will live with people who are not related to me | I will live alone |
| Think about the future health of your family members. Who is most likely to have major health issues in 2 years? | Myself | A sibling | A parent | |
| Think about how much you will weigh in 5 years. Which of these is most likely to be true? | I will weigh more than now | I will weigh less than now | I will weigh the same | |
| Present | Think about the relationship you have with the people in your neighborhood. Which best describes your situation? | I know most of my neighbors | I know just a few of my neighbors | I don't know any of my neighbors |
| Think about the state of your physical health at this moment. Which of these issues is of most concern to you right now? | Eating a healthy diet | Exercising regularly | Getting regular check-ups | |
| Think about how you compare in age with most of your current friends. Which best describes your situation? | I'm similar in age to most of them | I'm younger than most of them | I'm older than most of them | |
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. 2009. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 47:2222–2238. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. 2007. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 45:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain's default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. 2014. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc Natl Acad Sci U S A. 111:16550–16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L. 2008. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn. 66:202–212. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. 1993. Memory, amnesia, and the hippocampal system. Cambridge: MIT. [Google Scholar]
- D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, Maquet P, Salmon E. 2010. a. The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci. 22:1701–1713. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Salmon E. 2010. b. Modulation of medial prefrontal and inferior parietal cortices when thinking about past, present, and future selves. Soc Neurosci. 5:187–200. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. 2008. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 40:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersner-Hershfield H, Wimmer GE, Knutson B. 2009. Saving for the future self: neural measures of future self-continuity predict temporal discounting. Soc Cogn Affect Neurosci. 4:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. 2013. Imagining the future: evidence for a hippocampal contribution to constructive processing. Hippocampus. 23:1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. 2007. Using imagination to understand the neural basis of episodic memory. J Neurosci. 27:14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. 2007. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A. 104:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. 2007. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 17:873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. 2002. Memory and temporal experience: the effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Social Cognition. 20:353–379. [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. 2011. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 56:455–475. [DOI] [PubMed] [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. 2010. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 48:3179–3186. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, Campbell K, Courchesne E. 2015. Different functional neural substrates for good and poor language outcome in autism. Neuron. 86:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. 2013. The hippocampus: a manifesto for change. J Exp Psychol Gen. 142:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. 1996. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 3:143–157. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Schirmer J, Ames DL, Gilbert DT. 2011. Medial prefrontal cortex predicts intertemporal choice. J Cogn Neurosci. 23:857–866. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 63:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 31:440–457. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Kim AS, Habib R, Levine B, Tulving E. 2010. Consciousness of subjective time in the brain. Proc Natl Acad Sci U S A. 107:22356–22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford: Clarendon Press. [Google Scholar]
- Olman CA, Davachi L, Inati S. 2009. Distortion and signal loss in medial temporal lobe. PLoS One. 4:e8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 17:230–240. [DOI] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FI, Moscovitch M. 2010. Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci. 30:4707–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. 2010. Common and unique neural correlates of autobiographical memory and theory of mind. J Cogn Neurosci. 22:1095–1111. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. 2011. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 31:10262–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. 2010. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 20:1263–1290. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. 2001. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 4:546–550. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. 2012. The future of memory: remembering, imagining, and the brain. Neuron. 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. 2007. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 97:3651–3659. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Levine B. 2016. The role of the hippocampus in memory and mental construction. Ann N Y Acad Sci. 1369:76–92. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. 2001. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Spreng RN, Schacter DL. 2014. A taxonomy of prospection: introducing an organizational framework for future-oriented cognition. Proc Natl Acad Sci U S A. 111:18414–18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. 2007. Neural substrates of envisioning the future. Proc Natl Acad Sci U S A. 104:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y, Liberman N. 2010. Construal-level theory of psychological distance. Psychol Rev. 117:440–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. 1983. Elements of episodic memory. New York: Oxford University Press. [Google Scholar]
- Tulving E. 1985. Memory and consciousness. Can Psychol. 26:1–12. [Google Scholar]
- van Mulukom V, Schacter DL, Corballis MC, Addis DR. 2013. Re-imagining the future: repetition decreases hippocampal involvement in future simulation. PLoS One. 8:e69596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. 2010. When the future becomes the past: differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res. 212:196–203. [DOI] [PubMed] [Google Scholar]
- Woolrich M. 2008. Robust group analysis using outlier inference. Neuroimage. 41:286–301. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. 2001. Statistical analysis of activation images In: Jezzard P, Matthews PM, Smith SM, editors.. Functional MRI: an introduction to methods. New York: Oxford University Press; p. 251–270. [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Ugurbil K. 2013. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 83:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yuan H, Lei X. 2016. Activation and connectivity within the default mode network contribute independently to future-oriented thought. Sci Rep. 6:21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. 2016. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 17:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.