Abstract
Objective
HIV-associated neurocognitive disorders (HAND) have historically been characterized as a subcortical condition that does not affect semantic memory; however, recent evidence suggests that the cortical regions that support semantic memory may be affected in HIV.
Method
The current study examined the effects of HAND on semantic memory in 85 HIV+ individuals with HAND, 193 HIV+ individuals without HAND, and 181 HIV– individuals who completed the Boston Naming Test (BNT) and the Famous Faces subtest of the Kauffman Adolescent and Adult Intelligence Test (KAIT-FF).
Results
Linear regressions revealed a significant adverse effect of HAND on total scores on the BNT and the KAIT-FF (all ps < .01). Analyses of BNT errors showed that individuals with HAND committed more semantically related errors as compared to the other two study groups (all ps < .05). However, there were no group differences in rates of visually based errors, which are more commonly observed in traditional subcortical diseases (all ps > .10).
Conclusions
Results indicate that HAND may impose adverse effects on individuals’ object naming and identification abilities suggestive of mild semantic deficits that parallel traditional cortical diseases such as Alzheimer’s disease.
Keywords: HIV, Semantic memory, Neuropsychological assessment, AIDS-related dementia
Although combination antiretroviral therapy (cART) has transformed HIV into a chronic manageable illness, individuals living with HIV are at increased risk of developing a number of complications, including HIV-associated neurocognitive disorders (HAND). Prevalent in as many as 50% of HIV+ adults (Heaton et al., 2010), HAND is marked by mild-to-moderate deficits in the domains of executive functions (e.g., cognitive flexibility, novel problem solving, and inhibition), episodic learning and memory, attention/working memory, and psychomotor speed and coordination (Heaton et al., 2010). In contrast, cognitive abilities such as basic visuoperception, receptive language, and constructional praxis, as well as the brain regions (i.e., posterior neocortex) that facilitate these functions, tend to be relatively spared in HIV+ individuals (Woods, Moore, Weber & Grant, 2009). As such, the neuropsychological profile of HAND is consistent with its effects on the fronto-striatal-thalamo-cortical circuits, bearing similarities to disorders such as Huntington’s disease (HD) (e.g., Tröster & Woods, 2010).
Nevertheless, controversy has arisen as to whether the expression of HAND in the cART era has undergone a shift from the classic “subcortical” neuropsychological profile to a more “cortical” presentation (Brew, 2004; Brew, Crowe, Landay, Cysique, & Guillemin, 2009; Valcour & Paul, 2006). This recent controversy proposes a subtle shift toward posterior neocortical (e.g., temporal and parietal lobe) neuropathology as a contributing—if not primary—mechanism of HAND in the cART era (e.g., Kieburtz et al., 1996). Although the exact mechanism of this proposed change is unknown, it is argued that as HIV+ individuals are living longer they are more vulnerable to neuropathological changes in mediotemporal and posterior cortical regions, which may increase their risk of Mild Cognitive Impairment (e.g., Sheppard et al., 2015) and Alzheimer’s disease (AD). For example, cART-era data suggests that HIV is associated with increased beta-amyloid deposition (Green et al., 2005) and alterations in the structure and function of the temporal lobes (e.g., Maki et al., 2009). Thus it is possible that HIV+ persons may be susceptible to deficits typically associated with temporal (e.g., rapid forgetting, dysnomia) and parietal (e.g., spatial cognition) lobe functioning (Brew, 2004).
Support for the shifted “cortical” hypothesis stems from studies showing HIV-associated deficits on tests of spatial cognitive (e.g., visuoperceptual) abilities thought to be reliant on the posterior parietal lobe (e.g., mental rotation; Olesen, Schendan, Amick, & Chronin-Golumb, 2007), as well as on tests of verbal and visual episodic memory (Sacktor et al., 2000). These findings have led some authors to argue that rather than the classic “subcortical” pattern (e.g., executive dysfunction, bradyphrenia) historically observed in younger and middle-aged HIV+ adults (e.g., Kieburtz et al., 1996; Ragin et al., 2005), the profile of neurocognitive impairment among HIV+ adults in the cART era may be more consistent with that seen in “cortical” dementias characterized by rapid forgetting, visuoperceptual deficits, and a degradation of semantic memory. Given that subcortical and cortical regions are highly interconnected, the concept of a “cortical” hypothesis is, of course, broad and flawed in many ways. Certainly, the neuropsychological presentation of damage to cortical areas can vary (e.g., FTD vs. AD; Léger & Banks, 2014) and neurocognitive profiles often fail to support such a simple distinction (Arango-Lasprilla et al., 2006). In the HIV literature however, this “cortical” label is meant to describe AD-like profiles and while this notion remains divisive there are few prospectisve, rigorous studies of this controversy, which serves as a useful heuristic to guide hypothesis-driven work.
Deconstruction of memory functioning is historically used to draw inferences about the cortical versus subcortical nature of various neuropsychological conditions (Butters et al., 1990). In general, the episodic memory deficits associated with frontal-striatal-thalamo-cortical circuit dysfunction reflect executive dyscontrol of encoding and retrieval within episodic memory (e.g., Delis et al., 1995), whereas deficits due to medial-temporal-lobe dysfunction are primarily due a disruption more fundamental to encoding and retention of new information (e.g., Chan et al., 1993). To this end, HIV-associated episodic memory deficits tend to reflect a mixed encoding and retrieval profile that is consistent with a frontostriatal neuropathogenesis (e.g., Doyle et al., in press). Specifically, HAND is most commonly marked by diminished free recall in the setting of near normal retention and recognition (e.g., Woods et al., 2006). Moreover, impairments in free recall are characterized by diminished use of strategic organizational strategies, such as semantic clustering (e.g., Peavy et al., 1994), which may increase in magnitude with the severity of HAND (Gongvatana et al., 2007). Two recent multi-disease sample studies also failed to support the cortical hypothesis, reporting that the pattern of HIV-associated episodic learning and memory deficits were more consistent with Parkinson’s and Huntington’s diseases than with Alzheimer’s disease or mesial temporal lobe epilepsy (Ciccarelli et al., 2016; Doyle et al., in press).
Degradation of semantic memory is a well-established feature of typical “cortical” dementias (Bayles & Tomoeda, 1983) and is not widely considered a feature of HAND. A seminal and widely cited pre-CART study by White and colleagues (1997) found that semantic memory was relatively spared in HIV-associated dementia (HAD), as demonstrated by normal performance on the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983). Moreover, both Scott and colleagues (2011) and Sheppard and colleagues (2017) observed null effects of HIV on confrontation naming. The few studies that have shown evidence of semantic memory deficits in HIV tend to reveal that the impairment is due to executive dyscontrol of those processes rather than a degradation of the semantic memory stores themselves. For example, Iudicello, Woods, Deutsch, Grant, and The HIV Neurobehavioral Research Program HNRP Group (2012) found that HIV-associated impairments in semantic fluency were driven by deficits in switching rather than semantic clustering. Similarly, Sadek and colleagues (2004) reported that HAD was associated with mild deficits in remote memory for famous faces and public events. However, the pattern of semantic memory deficits in HAD revealed no evidence for a temporal gradient, which was consistent with individuals with HD (i.e., a subcortical condition) and different from individuals with probable AD who showed the expected temporal gradient. Thus, consistent with the above-described profile of HIV-associated episodic memory deficits, the pattern of impairments observed in semantic memory is hypothesized to stem from deficient retrieval from semantic memory stores secondary to frontostriatal circuit neurotoxicity.
The vast majority of research in HIV disease has shown a relative sparing of semantic memory; however several limitations of this emerging literature limit conclusions that can be drawn regarding the effects of HAND on semantic memory. All of these cART-era studies on the cortical hypothesis have included relatively small sample sizes and none have focused specifically on HAND, which is the subgroup of HIV-infected persons with the greatest involvement of the central nervous system. Further, no error analyses have been conducted examining the nature of semantic memory task scores, use of which may increase specificity and diminish the risk of type II error. As such, the likelihood of detecting an effect in these studies was reduced and it is possible that the effect of HAND on semantic knowledge may be better observed through use of a more evaluative approach, such as error analysis.
Indeed, semantic processes may involve an intricate relationship between both executive and traditional storage components (see Ralph, Jefferies, Patterson, & Rogers, 2017 for a review). Acknowledging this complexity, Hodges, Salmon, and Butters (1991) noted that the use of total scores and broad, largely undefined, categories of errors may fail to distinguish between difficulties at various processing levels within semantic memory (e.g., confrontation naming errors that are based on visual misperceptions versus semantic-category failures). By applying a more comprehensive error coding system, based on the framework of Kohn and Goodglass (1985), Hodges and colleagues (1991) were able to distinguish the semantic memory deficits in HD (i.e., a prototypical subcortical disease with primarily perceptual deficits) from those observed in AD (i.e., a prototypical cortical disease with semantically based errors). Similarly, evaluating the qualitative nature of confrontation naming errors in HAND may provide understanding of potential semantic memory problems in this population.
With the above “subcortical” versus “cortical” controversy in mind, the proposed study has two primary objectives. First, the current study aims to explore the cortical hypothesis controversy by examining the semantic memory performance as measured by the BNT (total score and errors) and the Famous Faces subtest of the Kaufman Adolescent and Adult Intelligence Test in HIV+ individuals with and without HAND, and HIV– individuals. Second, the current study will investigate the HIV disease correlates of any observed semantic memory deficits in HAND. Viewed through the lens of the cortical hypothesis, we would expect participants to demonstrate a general stepwise decline in performance on semantic memory measures (i.e., HIV– > HIV > HAND) reflecting a progressive impairment of executive dysfunction and degradation of semantic processes. Alternatively, the traditional perspective of frontostriatal dysfunction would predict more specific declines indicative of subcortical pathology. Specifically HIV+ individuals and participants with HAND may demonstrate increased visual based errors compared to HIV– individuals on the BNT (as seen in HD), while there may be no significant differences between groups on the KAIT. In considering these competing hypotheses, the current study takes an exploratory approach to elucidate the neuropsychological profile of semantic memory in HIV.
Method
Participants
Study participants included 459 adults, aged 18–75 years, recruited from the University of California San Diego HIV Neurobehavioral Research Program. Participants were excluded if they received an estimated verbal IQ score < 70 on the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001), reported histories of neuromedical (e.g., seizure disorder, stroke, at least moderate traumatic brain injury), current substance use disorder, or severe psychiatric (e.g., psychosis) disorders that might affect cognition. Participant demographic and disease characteristics are displayed in Table 1.
Table 1.
Participant demographic and disease characteristics
| Total sample (N = 459) | HAND+ (n = 85) | HAND– (n = 193) | HIV– (n = 181) | p | Group differences | |
|---|---|---|---|---|---|---|
| Age (years) | 43.5 (12.7) | 44.2 (12.2) | 44.7 (10.8) | 41.9 (14.5) | — | — |
| Education (years) | 13.7 (2.6) | 13.1 (2.6) | 13.6 (2.6) | 14.2 (2.5) | .005 | H + < HIV– |
| Ethnicity | ||||||
| African-American | 23.3% | 28.2% | 20.2% | 24.3% | — | — |
| Asian | 2.2% | 0 | 3.1% | 2.2% | — | — |
| Hispanic | 16.1% | 15.3% | 16.1% | 16.6% | — | — |
| White | 57.5% | 55.3% | 60.1% | 56% | — | — |
| Gender (men) | 78.0% | 82.4% | 89% | 64.1% | <.0001 | H+ , H– > HIV– |
| Estimated verbal IQ (WTAR) | 102.8 (11.5) | 98 (12.2) | 104.3 (11.2) | 103.5 (10.8) | <.0001 | H–, HIV– > H + |
| POMS total (of 200) | 54.7 (36.1) | 72.4 (42.4) | 57.6 (36.2) | 43.1 (28) | <.0001 | H + > H– > HIV– |
| Major depression | 48.8% | 65.9% | 54.4% | 34.8% | <.0001 | H+, H– > HIV– |
| Generalized anxiety | 10% | 11.8% | 15% | 3.9% | <.001 | H + , H– > HIV– |
| Substance dependence | 49% | 57.6% | 52.9% | 40.9% | .01 | H+, H– > HIV– |
| Hepatitis C Virus | 15% | 21.69% | 17.46% | 9.5% | .02 | H+, H– > HIV– |
| Estimated duration of infection (months) | — | 148.8 (96.7) | 150.8 (101.1) | — | — | — |
| AIDS (n = 278) | — | 52.9% | 55.4% | — | — | — |
| CD4 count (cells/L) | — | 580.8 (307.3) | 569.4 (263.7) | — | — | — |
| Nadir CD4 (cells/L) | — | 234.6 (204.2) | 217.1 (173.9) | — | — | — |
| cART status | — | 80% | 86% | — | — | — |
| Plasma RNA detectable | — | 27.2% | 25.3% | — | — | — |
| Among subjects on cART | — | 12.3% | 15.2% | — | — | — |
Note. WTAR = Wechsler test of adult reading; POMS = profile of mood states; AIDS = acquired immune deficiency syndrome; CD4 = cluster of differentiation 4; cART = combination antiretroviral therapy; H+ = HAND; H– = HAND.
The human research ethics office of the University of California San Diego and the University of Houston approved this study. All participants provided written, informed consent.
HAND Diagnosis
HIV+ individuals were classified as having HAND if they met Frascati research (see Antinori et al., 2007) criteria for one of three conditions: asymptomatic neurocognitive impairment (ANI; n = 53), HIV-associated mild neurocognitive disorder (MND; n = 27), and HIV-associated Dementia (HAD; n = 5). Within the 278 HIV+ individuals, 85 (30.5%) were classified as having HAND (i.e., HAND+ group), and 193 were not cognitively impaired (i.e., HAND– group). The semantic memory measures (i.e., BNT and KAIT) were not used in the diagnosis of HAND. Descriptive data from the neuropsychological battery used to diagnose HAND is provided for each study group in Table 2.
Table 2.
Descriptive data from the individual neuropsychological measures used to derive diagnoses of HIV-associated neurocognitive disorders (HAND)
| Neuropsychological domains & measures | HAND+ (n = 85) | HAND– (n = 193) | HIV– (n = 181) | Group differences* | Demographics adjusted |
|---|---|---|---|---|---|
| Attention domain | |||||
| WMS-III Digit Span | 47.6 (8.2) | 50.5 (8.9) | 53.6 (9.4) | H+ < H– < HIV– | Age |
| CVLT-II Trial 1 | 37.8 (11.6) | 47.6 (10.3) | 49.4 (13.6) | H + < H– = HIV– | Sex, Age |
| Executive Domain | |||||
| Tower of London Total Moves | 42.5 (11.9) | 52.0 (9.9) | 48.1 (10.7) | H+ < HIV– < H– | Age |
| Action (verb) Fluency | 40.3 (8.4) | 48.0 (8.9) | 47.1 (9.3) | H+ < H– = HIV– | Ed. |
| Trail Making Test Part B | 41.4 (11.8) | 52.3 (9.7) | 51.4 (10.8) | H+ < H– = HIV– | Race, Sex, Age, Ed. |
| Learning Domain | |||||
| CVLT-II Trial 1–5 | 43.4 (9.9) | 53.1 (9.3) | 54.6 (11.4) | H+ < H– = HIV– | Sex, Age |
| WMS-III Logical Memory I | 43.4 (11.1) | 53.3 (8.5) | 55.0 (11.5) | H+ < H– = HIV– | Age |
| Memory Domain | |||||
| WMS-III Logical Memory II | 44.5 (11) | 54.9 (9.1) | 57.2 (11.2) | H+ < H– = HIV– | Age |
| CVLT-II Long Delay Free Recall | 40.7 (12.8) | 50.2 (10.2) | 51.5 (10.7) | H+ < H– = HIV– | Sex, Age |
| Speed of Processing Domain | |||||
| Tower of London Total Execution Time | 40.2 (10.4) | 49.9 (7.6) | 47.2 (9.5) | H+ < HIV– < H– | Age |
| Trail Making Test Part A | 43.9 (10) | 52.2 (9.5) | 52.2 (10) | H+ < H– = HIV– | Race, Sex, Age, Ed. |
| Motor Domain | |||||
| Grooved Peg Board (Dom) | 40.0 (9.9) | 50.0 (9.7) | 48.6 (10.3) | H+ < H– = HIV– | Race, Sex, Age, Ed. |
| Grooved Peg Board (Non Dom) | 40.1 (9.6) | 50.2 (9.7) | 48.9 (11.5) | H+ < H– = HIV– | Race, Sex, Age, Ed. |
Note: Data are presented as mean T-scores (standard deviation) for each study group. *All omnibus group differences accompanied by ps < .0001. WMS-III = Wechsler Memory Scale, 3rd edition; CVLT-II = California Verbal Learning Test, 2nd edition. Ed = education. H+ = HAND. H– = HAND–.
Measures of Semantic Memory
Boston Naming Test (BNT)
All participants completed the BNT (Kaplan, Goodglass, & Weintraub, 1983), a 60-item, widely used neuropsychological assessment of confrontation naming. The test includes 60 black-and-white drawings of objects and animals graded and ordered from easiest to most difficult. The images were presented in standard order and participants were instructed to respond with the name for the object represented in the image. For each image, participants were allowed 20 s to provide a response, unless the participant indicated that they did not know the word before time elapsed. Points were awarded based on whether or not the response was correct, and error scores were designed to characterize incorrect responses. The test was administered according to the following standard protocol: if a person was unable to name an object, a phonemic cue consisting of the initial sound of the target word, was provided. A predetermined semantic cue (“an ocean animal” for octopus; “used for air travel” for helicopter, etc.) was provided only when the participant’s response reflected a misperception of the pictures or a lack of recognition (i.e., “I don’t know what it is”). All responses were graded on a 0 or 1 scale to reflect an incorrect or correct answer respectively. BNT errors were categorized using criteria described by Hodges and colleagues (1991) and duplicated below. In the case of multiple errors on an individual item, only the first response to the target (i.e., before stimulus or phonemic cueing) was classified and used in analysis.
Error classifications
Nonresponse: includes “don’t know” and nonresponse.
Visual error: responses visually similar to the target and from a different semantic category (e.g., “spear” for asparagus). Also included were whole-part responses where participants named either a part of the target item (“blocks” for pyramids) or something incidentally present in the picture (e.g., “door” for knocker).
Ambiguous visual/semantic category errors: responses from the same semantic category as the target and visually similar such that the error could be either perceptually or semantically based (e.g., “hippopotamus” for rhinoceros).
Semantic: within-category errors: responses from the same semantic category as the target but clearly not visually similar (e.g., “lettuce” for asparagus).
Semantic: superordinate errors: responses denoting the general class or category to which objects belong (e.g., “musical instrument” for accordion).
Semantic: associative errors: responses showing an obvious association with the target item including statements of action or function (e.g., “painting” for easel), physical attributes (e.g., “ice” for igloo), contextual associates (e.g., “ocean” for octopus), and specific subordinate or proper noun examples of the target (e.g., “Vesuvius” for volcano).
- Semantic: circumlocutory errors: multiword responses showing accurate identification of the target by physical attribute, function or action (e.g., “doctors use them to listen to your heart” for stethoscope).
- If the distinction between error types 6 and 7 was unclear, we applied the following criterion: does the response describe a specific item? If it did, the error was categorized as a circumlocutory response. Also included in this category were acceptable slang terms, synonyms and creative neologisms (e.g., “squeezebox” for accordion).
Phonemic errors: mispronunciations or distortions of the target name sharing at least one syllable (e.g., “iglow” for igloo).
Perseverations: re-utterance of a response (correct or incorrect) that had previously been used to name one of the previous five pictures.
Unrelated errors: in which no clear connection between the target and response could be determined (e.g., “a mess” for snail).
The classification of error responses was performed independently by two of the authors (S.M.T., V.K.). Kappa analyses were conducted among a randomly selected 10% of the sample to assess inter-reliability of error classifications. Agreement between these two coders across 450 independently scored items was >85% (M = 0.93, Range = 0.88–0.96, 95% CI [0.90–0.94]; all ps < .0001). Discrepancies were discussed between raters and with the senior author and only one raters errors scores were used in analyses.
Kaufman Adolescent and Adult Intelligence Test-Famous Faces Test (KAIT)
Of the total sample (N = 459), 446 individuals completed the KAIT Famous-Faces Test (Kaufman & Kaufman, 1993). Participants were shown 21 pictures of famous persons and provided with a verbal cue regarding their identities. Examinees were instructed to then identify the face or faces. For example, the participant may be shown an image of Abraham Lincoln and asked to “name this president of the United States.” The subtest measures general factual knowledge and has been carefully constructed to minimize cultural and socioeconomic bias. Scores range from 0 to 21.
Data Analysis
The primary study hypotheses related to the effects of HAND on semantic memory were tested using multivariable regression. All variables listed in Table 1 were examined as possible covariates for each of the linear regressions on the semantic memory outcome variables. Variables were included as covariates if they differed between the study groups and were significantly associated with semantic memory outcomes. Years of education, sex, WTAR estimated verbal IQ, POMS total score, and the rates of lifetime diagnosis of MDD and GAD differed significantly between the HAND status groups (i.e., HAND+ , HAND–, and HIV–; all ps < .05). However, among these potential covariates, only education, lifetime MDD, and sex were also significantly related to the semantic memory outcome variables (all ps < .05). Thus, only these three variables were included as covariates for the regression analyses. In other analyses, normally distributed variables were evaluated using Analyses of Variance (ANOVA) and all variables that were non-normally distributed as indicated by a Shapiro–Wilk tests of normality using an alpha level of 0.05 were analyzed used Wilcoxon Rank Sum tests. Post-hoc analyses were conducted using Tukey’s Honest Significant Differences tests or Steel–Dwass tests for parametric and nonparametric distributions, respectively. To address the second aim of the study, separate bivariate association analyses were performed to examine the relationship of disease characteristics to semantic memory. For continuous disease variables, we used Pearson correlations (or Spearman’s rho for non-normally distributed variable). For categorical disease variables, we used independent samples t-test (or Wilcoxon rank sums). The critical alpha level for hypothesis testing was set at 0.05 for all analyses.
Results
BNT
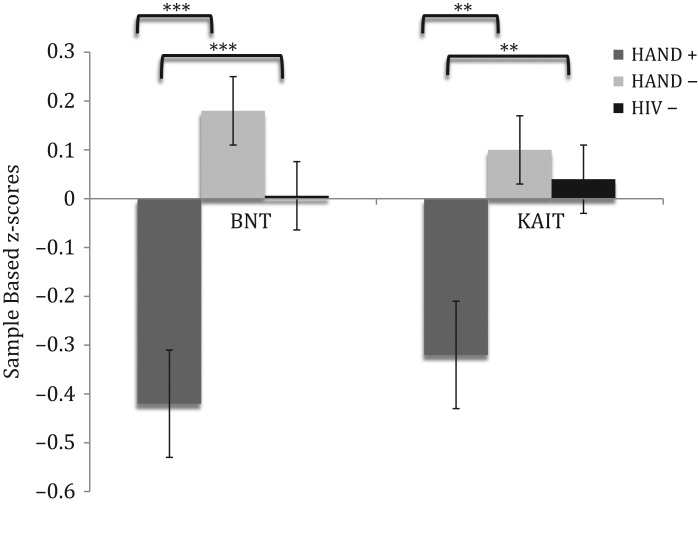
The overall regression model was significant for total score on the BNT (Adj R2 = 0.20, p < .0001; see Table 3). Significant contributors to this model included education, lifetime MDD and HAND status (all ps < .05). Individuals in the HAND– (β = 4.84, p = .0008, Cohen’s d = .601) and HIV– groups (β = 3.38, p < .0001, Cohen’s d = −.401) earned significantly higher scores (i.e. better performance) compared to HAND+ participants (see Fig. 1).
Table 3.
Results of linear regression analyses examining performance on Boston Naming Test (BNT) and Kaufman Adolescent and Adult Intelligence Test (KAIT) Famous Faces
| Variable | Adj R2 | F | β | p |
|---|---|---|---|---|
| BNT total | 0.195 | 23.33 | <.0001* | |
| HAND status | 11.74 | <.0001* | ||
| HAND+ vs. HIV– | 3.38 | .0008* | ||
| HAND+ vs. HAND– | 4.84 | <.0001* | ||
| Covariates | ||||
| Education* | 73.37 | 8.57 | <.0001* | |
| Sex | 3.46 | −1.86 | .063 | |
| Lifetime MDD* | 16.31 | −4.04 | <.0001* | |
| KAIT Famous Faces Total | 0.197 | 22.84 | <.0001* | |
| HAND Status | 5.56 | .004* | ||
| HAND+ vs. HIV– | 2.93 | .004* | ||
| HAND+ vs. HAND– | 3.15 | .002* | ||
| Covariates | ||||
| Education | 82.49 | 9.08 | <.0001* | |
| Sex | 10 | −3.16 | .002* | |
| Lifetime MDD | 11.39 | −3.37 | .0008* |
*p < .05.
Fig. 1.
Sample based z-score comparison of total score performance across study groups on the Boston Naming Test (BNT) and the Famous Faces subtest of the Kaufman Adolescent and Adult Intelligence Test (KAIT-FF). HAND = HIV-associated neurocognitive disorders. ***p < .001; **p < .01.
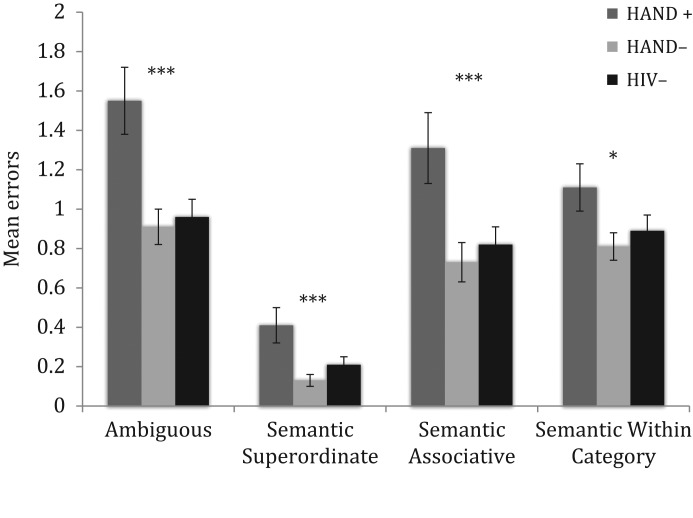
Results of error analyses are shown in Fig. 2. Overall models (with the above covariates included) examining the ten BNT error types showed significant group differences across ambiguous (Adj R2 = 0.08, p < .0001), semantic superordinate (Adj R2 = 0.05, p < .0001), semantic associative (Adj R2 = 0.06, p < .0001), and semantic within category (Adj R2 = 0.05, p < .0001) errors. HAND– participants and HIV– individuals committed significantly less ambiguous errors, semantic superordinate, and semantic associative based errors compared to participants with HAND (all ps < .05; Cohen’s d [0.304–0.462]). Results also show that the HAND+ group made significantly more semantic within category errors compared to the HAND– group (β = −2.15, p = .03). There were no significant differences between the HIV– and the HAND– groups in any of the above-mentioned BNT errors (all ps > .10).
Fig. 2.
Comparison of mean number of selected Boston Naming Test errors committed across HAND (HIV-associated neurocognitive disorders) status groups. **p < .01; *p < .05.
KAIT
The overall regression model was significant for total score on the KAIT Famous Faces subtest (Adj R2 = 0.20, p < .0001; see Table 3). Significant contributors to this model included sex, lifetime diagnosis of MDD, education, and HAND status (all ps < .05). The HAND– (β = 3.15, p = .002, Cohen’s d = .412) and HIV– participants (β = 2.93, p = .004, Cohen’s d = −.357) earned significantly higher scores (i.e., better performance) compared to the HAND+ group (see Fig. 1).
Correlates of Semantic Memory Performance
Demographic characteristics outlined in Table 1 (age, sex, education, and ethnicity) were examined as potential correlates of semantic memory within the HAND group (n = 85). Results showed that younger age (rs = .46, p < .0001) and fewer years of education (rs = .45, p < .0001) and non-Caucasian ethnicity (df = 79, t = −4.20, p < .0001) were significantly related to poorer KAIT performance. Similarly, results revealed significant relationships between lower BNT scores and younger age (rs = .42, p < .0001), fewer years of education (rs = .38, p < .0001), and non-Caucasian ethnicity (df = 83, t = −5.31, p < .0001). Neither the KAIT-FF nor the BNT was related to sex (ps > .10). In regards to disease characteristics, estimated duration of HIV infection was the only variable significantly associated with BNT (rs = .32, p = .003) and KAIT (rs = .23, p = .04) scores, with longer duration of infection corresponding to better semantic memory. Post-hoc analyses showed that when age and estimated duration of infection were included within a linear regression predicting BNT (β = 3.04, p = .003) and KAIT-FF (β = 6.18, p < .0001), age emerged as the only significant predictor. No other HIV disease or treatment variable shown in Table 1 was significantly related to the BNT and KAIT semantic tasks (all ps > .10).
Discussion
While HIV has historically been viewed as a subcortical disease, recent data suggest a subtle shift toward neocortical (e.g., temporal and parietal lobe) neuropathology as a contributing mechanism to HAND. To date, neuropsychological support for this so-called “cortical hypothesis” has been limited. In fact, many rigorous studies investigating HIV (not considering HAND) have failed to observe any significant deficits in traditionally cortical neurocognitive constructs such as semantic memory (e.g., Iudicello et al., 2012; Scott et al., 2011). Results of the current study, however, provide compelling neuropsychological evidence of cortical dysfunction in HIV+ persons with HAND, specifically moderate deficits in semantic memory (i.e., confrontation naming and knowledge). Individuals with HAND performed worse than HIV+ participants without HAND as well as their HIV– counterparts on both the BNT and the KAIT famous faces measures. These findings were accompanied by medium effect sizes and were independent of demographic (e.g., sex and education) and other clinical factors (e.g., major depression).
It has been proposed that errors on the BNT can be understood across specific stages of the naming process (e.g., Gainotti, Micelj, Caltagirone, Silveri & Masullo, 1981; Kohn & Goodglass, 1985; Riddoch, Humphrey, Coltheart & Funnell, 1988) and may provide conceptual insight into the nature of semantic difficulties observed in HAND. In general, the naming process can be viewed across four stages. The first stage, considered to be perceptual, involves the evaluation of the structural features of the target picture or object. Deficits at this first stage are evident in typical subcortical disorders such as HD (Hodges et al., 1991). However, we did not observe higher rates of visually based errors in HAND in the current study, perhaps suggesting normal function at the perceptual analysis stage. Following the perceptual stage, a semantic phase occurs in which the visual perception of the item is matched with broad superordinate category knowledge (e.g., vegetable, animal, and furniture) before more detailed and identifying semantic information is retrieved. Superordinate naming errors at this stage suggest a defect in the semantic process interfering with access of information past only broad category membership. Similarly, semantic-associative errors suggest that while an individual is able to access some appropriate semantic category knowledge, it is not sufficient to generate a correct descriptive response or a category exemplar of the target item. Next, the target word that corresponds to the identified semantic concept is retrieved in the lexical stage. At this phase, semantic within-category errors result from a defect at a late stage of the semantic process indicating that appropriate activation of the semantic knowledge network has occurred, but the correct target word cannot be accessed. Lastly, errors during the phonemic or word production stage imply impaired articulatory processing of phonological information.
Interestingly, participants with HAND in the current study demonstrated high rates of semantically related errors as compared to the other two groups. The heightened occurrence of semantic superordinate and semantic associative errors specifically (shown to occur rarely in subcortical disorders such as HD; Hodges et al., 1991) suggest a defect in the semantic phase of the naming process. It is important to note that the absence of visually based errors alone within the HAND group does not suggest an absence of subcortical dysfunction (indeed, a quick review of Table 2 shows otherwise). Rather the absence of visually based errors paired with increased semantic difficulties indicate neurocognitive dysfunction in HAND similar to that observed among cortical diseases such as AD marked by degradation of semantic knowledge including object naming and identification abilities.
Modern semantic theories emphasize that control of semantic cognition is effected by a distributed neural network which interacts with, however is largely independent from, the system responsible for semantic representation (Ralph et al., 2017). In this context, findings of the current study may suggest a disruption between these systems among individuals with HAND. For example, some errors observed among our HAND group indicate difficulty in deploying semantic knowledge (i.e., superordinate errors; naming), while others suggest that our HAND participants were able to access information regarding action or manipulation of the target item (i.e., semantic associative; “painting” for “pallet”). With the understanding that semantic performance necessitates coordinated interaction between the two components, it is possible that dysfunction may exist in both systems in HAND. Results of earlier studies have also indicated this complex presentation of semantic deficits. Sadek and colleagues (2004) noted mild semantic deficits in HAD while lacking the aspect of a temporal gradient commonly observed among disorders with traditional semantic difficulties. Likewise Iudicello and colleagues (2012) reported HIV-associated impairments in semantic fluency switching (i.e., an executive retrieval system), but not semantic clustering. It may be the case that semantic deficits in HAND result from degradation in some aspects of semantic memory, but not others, and may also demonstrate qualities of executive dysregulation of semantic memory. As the current study did not examine any executive control or manipulation hypotheses, the potential affect of executive dyscontrol on the observed semantic difficulties in HAND cannot be dismissed.
Evidence in support the cortical hypothesis draws heavily from research of aging adults and it is posited that older HIV-infected individuals may be more vulnerable to cortical dysfunction and thus more likely to show deficits associated with temporal and parietal functioning, such as rapid forgetting and semantic ability (Brew, 2004). However, the results of the current study showed that older age was associated with “better” semantic memory. Post- hoc analyses revealed no evidence that HAND imparts different effects on semantic memory in older HIV+ adults (i.e., >50 years of age) compared to their younger counterparts (ps > .10). These results are consistent with findings from the cognitive psychology literature that semantic memory is not typically impaired by the aging process (e.g., Nyberg, Backman, Erngrund, Olofsson, and Nilsson, 1996; Tulving, 1995). In fact, it has been suggested that semantic memory tends to somewhat improve across midlife (e.g., Nilsson et al., 1997). Of similar note, disease correlate analyses in the current study revealed that the significant positive relationship of EDI to performance on the KAIT and BNT was an artifact of age. It is possible that the BNT measure itself may have contributed to this result. For example, positive age effect may be a result of younger individuals being less familiar with items on the BNT (Schmitter-Edgecombe, Vesneski, & Jones, 2000). Together, findings suggest an adverse affect on semantic performance specific to HAND that is not moderated by age.
To the author’s knowledge, this is the first study to identify semantic memory deficits in HAND. Results reveal qualitative differences in the nature of semantic memory impairment among HIV+ individuals with and without HAND, seemingly not influenced by age or separate disease factors. Moreover, findings provide the first evidence of support for the cortical hypothesis in HAND. However, our study sample consisted largely of white men with an average of at least a high school education and may limit the generalizability of these findings as the HIV epidemic increasingly affects women and racial/ethnic minorities (CDC, 2015). The observational and inferential nature of our results also represents a limitation, as they do not allow for the determination of the precise mechanisms of semantic deficits in HAND. Relatedly, while we recognize that the use of simple “subcortical” and “cortical” distinctions allow for quick classification in research and clinical settings, these heuristics are also ambiguous and not directly tied to biomarkers. Future studies may therefore examine the relationship of qualitative aspects of semantic memory performance with imaging data and/or postmortem neuropathology in HAND. Further, double dissociations are ideal in demonstrating differences in performance patterns between two groups. As a double dissociation was not evaluated in the current study we cannot rule out the possibility that semantic errors are more sensitive to naming deficits than others and are thus affected first by HAND. Additionally, as previous studies have suggested some semantic tasks are generally unimpaired in persons with subsyndromic HIV infection (e.g., Damos, John, Parker, & Levine, 1997), future studies may wish to investigate differences in semantic memory performance as a multifactorial process across severity of HAND (e.g., ANI, MND, and HAD). Moreover, investigations examining the sensitivity of other neuropsychological measures to HAND are needed to assess the extent of cortical dysfunction in HIV disease. Despite its limitations, results of the current study provide support to the growing body of evidence suggesting the possibility of a shift towards more cortical pathology during the advanced stages of HIV and warrant further investigation of the cognitive mechanisms underlying HAND.
Acknowledgments
The authors are grateful to the UC San Diego HIV Neurobehavioral Research Program (HNRP) Group (I. Grant, PI) for their infrastructure support of the parent R01. In particular, we thank Donald Franklin, Dr. Erin Morgan, Clint Cushman, and Stephanie Corkran for their assistance with data processing, Drs. Scott Letendre and Ronald J. Ellis for their assistance with the neuromedical aspects of the parent project, and Dr. J. Hampton Atkinson and Jennifer Marquie Beck and their assistance with participant recruitment and retention. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank the study volunteers for their participation.
Funding
This work was supported by the National Institutes of Health under Grants R01-MH073419 and P30-MH62512.
Conflict of Interest
The authors have no financial conflicts of interest related to this work.
References
- Arango-Lasprilla J. C., Rogers H., Lengenfelder J., Deluca J., Moreno S., & Lopera F. (2006). Cortical and subcortical diseases: Do true neuropsychological differences exist? Achieves of Clinical Neuropsychology, 21, 29–40. [DOI] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayles K. A., & Tomoeda C. K. (1983). Confrontation naming impairment in dementia. Brain and Language, 19, 98–114. [DOI] [PubMed] [Google Scholar]
- Brew B. J. (2004). Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS (London, England), 18, S75–S78. [PubMed] [Google Scholar]
- Brew B. J., Crowe S. M., Landay A., Cysique L. A., & Guillemin G. (2009). Neurodegeneration and ageing in the HAART era. Journal of Neuroimmune Pharmacology, 4, 163–174. [DOI] [PubMed] [Google Scholar]
- Butters N., Grant I., Haxby J., Judd L. L., Martin A., McClelland J., et al. (1990). Assessment of AIDS-related cognitive changes: Recommendations of the NIMH workshop on neuropsychological assessment. Journal of Clinical and Experimental Neuropsychology, 12, 963–978. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015). HIV surveillance Report, 2013, 25.
- Chan A. S., Butters N., Paulsen J. S., Salmon D. P., Swenson M. R., & Maloney L. T. (1993). An assessment of the semantic network in patients with Alzheimer’s disease. Journal of Cognitive Neuroscience, 5, 254–261. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N., Limiti S., Fabbiani M., Baldonero E., Milanini B., Lamonica S., et al. (2017). Verbal list learning and memory profiles in HIV-infected adults, Alzheimer’s disease, and Parkinson’s disease: An evaluation of the “cortical hypothesis” of NeuroAIDS. Applied Neuropsychology Adult, 24(5), 410–419. doi:10.1080/23279095.2016.1189424. [DOI] [PubMed] [Google Scholar]
- Damos D. L., John R. S., Parker E. S., & Levine A. M. (1997). Cognitive function in asymptomatic HIV infection. Archives of neurology, 54, 179–185. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Peavy G., Heaton R., Butters N., Salmon D. P., & Taylor M. (1995). The HNRC Group. Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? Evidence using the California Verbal Learning Test. Assessment, 2, 151–165. [Google Scholar]
- Doyle K. L., Woods S. P., McDonald C., Leyden K., Holden H., Morgan E. E., et al. The HNRP Group (in press). Verbal memory profiles in HIV-associated Neurocognitive Disorders: A comparison with Huntington’s Disease and Temporal Lobe Epilepsy. Applied Neuropsychology: Adult. [DOI] [PMC free article] [PubMed]
- Gainotti G., Micelj G., Caltagirone C., Silveri M. C., & Masullo C. (1981). The relationship between type of naming error and semantic-lexical discrimination in aphasic patients. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 17, 401–409. [DOI] [PubMed] [Google Scholar]
- Gongvatana A., Woods S. P., Taylor M. J., Vigil O., & Grant I., The HNRC Group. (2007). Semantic clustering inefficiency in HIV-associated dementia. Journal of Neuropsychiatry and Clinical Neurosciences, 19, 36–42. [DOI] [PubMed] [Google Scholar]
- Green D. A., Masliah E., Vinters H. V., Beizai P., Moore D. J., & Achim C. L. (2005). Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS (London, England), 19, 407–411. [PubMed: 15750394]. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Clifford D. B., Franklin D. R. Jr., Woods S. P., Ake C., Vaida F., et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology, 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. R., Salmon D. P., & Butters N. (1991). The nature of the naming deficit in Alzheimer’s and Huntington’s disease. Brain, 114, 1547–1558. [PubMedPMID: 1832072]. [DOI] [PubMed] [Google Scholar]
- Iudicello J. E., Woods S. P., Deutsch R., & Grant I., The HIV Neurobehavioral Research Program (HNRP) Group. (2012). Combined effects of aging and HIV infection on semantic verbal fluency: A view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology, 34, 476–488. http://doi.org/10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., & Weintraub S. (1983). Boston Naming Test. Philadelphia: Lea & Febiger. [Google Scholar]
- Kaufman A. S., & Kaufman N. L. (1993). Kaufman adolescent and adult intelligence test In Manual. Circle Pines: American Guidance Service. [Google Scholar]
- Kieburtz K., Ketonen L., Cox C., Grossman H., Holloway R., Booth H., et al. (1996). Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology, 53, 155–158. [DOI] [PubMed] [Google Scholar]
- Kohn S. E., & Goodglass H. (1985). Picture-naming in aphasia. Brain and Language, 24, 266–283. [DOI] [PubMed] [Google Scholar]
- Léger G. C., & Banks S. J. (2014). Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dementia and Geriatric Cognitive Disorders, 37(0), 104–112. http://doi.org/10.1159/000354368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P. M., Cohen M. H., Weber K., Little D. M., Fornelli D., Rubin L. H., et al. (2009). Impairments in memory and hippocampal function in HIV-positive vs. HIV-negative women: A preliminary study. Neurology, 72, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L.-G., Bäckman L., Erngrund K., Nyberg L., Adolfsson R., Bucht G., et al. (1997). The Betula prospective cohort study: Memory, health, and aging. Aging, Neuropsychology, and Cognition, 4(1), 1–32. [Google Scholar]
- Nyberg L., Bäckman L., Erngrund K., Olofsson U., & Nilsson L. G. (1996). Age differences in episodic memory, semantic memory, and priming: Relationships to demographic, intellectual, and biological factors. Journals of Gerontology Series B: Psychological Sciences & Social Sciences, 51, 234–240. [DOI] [PubMed] [Google Scholar]
- Olesen P. J., Schendan H. E., Amick M. M., & Cronin-Golomb A. (2007). HIV infection affects parietal-dependent spatial cognition: Evidence from mental rotation and hierarchical pattern perception. Behavioral Neuroscience, 121, 1163–1173. [DOI] [PubMed] [Google Scholar]
- Peavy G., Jacobs D., Salmon D. P., Butters N., Delis D. C., Taylor M., et al. the HNRC Group. (1994). Verbal memory performance of patients with Human Immunodeficiency Virus infection: Evidence of subcortical dysfunction. Journal of Clinical and Experimental Neuropsychology, 16, 508–523. [DOI] [PubMed] [Google Scholar]
- Ralph M. A., Jefferies E., Patterson K., & Rogers T. T. (2017). The neural and computational bases of semantic cognition. Nature Reviews of Neuroscience, 18, 42–55. doi:10.1038/nrn.2016.150. PubMed PMID: 27881854. [DOI] [PubMed] [Google Scholar]
- Ragin A. B., Wu Y., Storey P., Cohen B. A., Edelman R. R., & Epstein L. G. (2005). Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. Journal of Neurovirology, 11, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch M. J., Humphrey G. W., Coltheart M., & Funnell E. (1988). Semantic systems or system? Neuropsychological evidence re-examined. Cognitive Neuropsychology, 5, 3–25. [Google Scholar]
- Sacktor N., Schifitto G., McDermott M. P., Marder K., McArthur J. C., & Kieburtz K. (2000). Transdermal selegiline in HIV-associated cognitive impairment: Pilot, placebo-controlled study. Neurology, 54, 233–235. [DOI] [PubMed] [Google Scholar]
- Sadek J. R., Johnson S. A., White D. A., Salmon D. P., Taylor K. I., Delapena J. H., et al. (2004). Retrograde amnesia in dementia: Comparison of HIV-associated dementia, Alzheimer’s disease, and Huntington’s disease. Neuropsychology, 18, 692–699. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M., Vesneski M., & Jones D. W. (2000). Aging and word-finding: a comparison of spontaneous and constrained naming tests. Achieves of Clinical Neuropsychology, 15(6), 479–493. PubMed PMID: 14590203. [PubMed] [Google Scholar]
- Scott J. C., Woods S. P., Weber E., Dawson M. S., Bondi M. W., & Grant I., The HNRC Group. (2011). Neurocognitive consequences of HIV infection in older adults: An evaluation of the “cortical” hypothesis. AIDS and Behavior, 15, 1187–1196. [PubMed: 20865313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. P., Iudicello J. E., Morgan E. E., Kamat R., Clark L. R., Avci G., et al. HIV Neurobehavioral Research Program (HNRP) Group. (2017). Accelerated and accentuated neurocognitive aging in HIV infection. Journal of Neurovirology, 23, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. P., Woods S. P., Bondi M. W., Gilbert P. E., Massman P. J., Doyle K. L. (2015). Does older age confer an increased risk of incident neurocognitive disorders among persons living with HIV disease? The Clinical Neuropsychologist, 29, 656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. (2001). Manual for the Wechsler Test of Adult Reading (WTAR). San Antonio: The Psychological Corporation. [Google Scholar]
- Tröster A., & Woods S. P. (2010). Neuropsychology of movement disorders and motor neuron disease In Armstrong C. (Ed.), Handbook of medical neuropsychology: Applications of Cognitive Neuroscience (pp. 315–334). New York: Springer. [Google Scholar]
- Tulving E. (1995). Organization of memory: Quo vadis? In Gazzaniga M. S. (Ed.), The cognitive neurosciences (pp. 839–847). Cambridge, MA: MIT Press. [Google Scholar]
- Valcour V., & Paul R. (2006). HIV infection and dementia in older adults. Clinical Infectious Disease, 42, 1449–1454. [DOI] [PubMed] [Google Scholar]
- White D. A., Taylor M. J., Butters N., Mack C., Salmon D. P., Peavy G., et al. (1997). Memory for verbal information in individuals with HIV-associated dementia complex. Journal of Clinical and Experimental Neuropsychology, 19, 357–366. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Moore D. J., Weber E., & Grant I. (2009). Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychological Review, 19(2), 152–168. doi:10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Morgan E. E., Dawson M., Scott J. C., & Grant I., HNRC Group. (2006). Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. Journal of Clinical and Experimental Neuropsychology, 28, 1030–1042. doi:10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]