Abstract
Arachidonic acid metabolites have a myriad of biological actions including effects on the kidney to alter renal hemodynamics and tubular transport processes. Cyclooxygenase metabolites are products of an arachidonic acid enzymatic pathway that has been extensively studied in regards to renal function. Two lesser-known enzymatic pathways of arachidonic acid metabolism are the lipoxygenase (LO) and cytochrome P450 (CYP) pathways. The importance of LO and CYP metabolites to renal hemodynamics and tubular transport processes is now being recognized. LO and CYP metabolites have actions to alter renal blood flow and glomerular filtration rate. Proximal and distal tubular sodium transport and fluid and electrolyte homeostasis are also significantly influenced by renal CYP and LO levels. Metabolites of the LO and CYP pathways also have renal actions that influence renal inflammation, proliferation, and apoptotic processes at vascular and epithelial cells. These renal LO and CYP pathway actions occur through generation of specific metabolites and cell-signaling mechanisms. Even though the renal physiological importance and actions for LO and CYP metabolites are readily apparent, major gaps remain in our understanding of these lipid mediators to renal function. Future studies will be needed to fill these major gaps regarding LO and CYP metabolites on renal function.
Introduction
Fatty acids circulate in the plasma and are incorporated into cell membrane phospholipids. Arachidonic acid is the most abundant fatty acid present in cell membranes and incorporates into the sn-2 position of phospholipids. The release of arachidonic acid from cell membrane phospholipids by the action of phospholipases and subsequent enzymatic metabolism results in an array of metabolites. These 20 carbon polyunsaturated fatty acid metabolites are collectively known as eicosanoids named after the Greek word eicos means 20. Eicosanoids are generated from three enzymatic pathways: cyclooxygenase (COX), lipoxygenase (LO), and cytochrome P450 (CYP). These enzymatic pathways generate a wide range of eicosanoid metabolites that have numerous biological activities that greatly impact renal function (32, 43, 48, 113). It is well established that COX metabolites are important lipid mediators to renal function. Although not the focus of this article, eicosanoids prominently contribute to renal dysfunction in diseases such as hypertension, diabetes, acute kidney injury, and chronic kidney disease. For more information on the role of these metabolites to renal function and their impact on renal diseases, the reader is referred to several excellent review articles (43-45). This article will focus on the important contributions of LO- and CYP-derived eicosanoids to renal physiology.
Metabolic pathways: Genes, enzymes, and metabolites
The LO enzymatic pathway consists of a number of genes, enzymes, metabolites, and receptors. LO enzymes are a family of nonheme iron containing enzymes that insert molecular oxygen into polyunsaturated fatty acids including arachidonic acid (13, 32, 42). There are at least six human LO enzymes; 5-LO (gene: ALOX5), platelet-type 12-LO (gene: ALOX12), epidermal-type LO (gene: ALOXE3), 12(R)-LO (gene: ALOX12B), 12/15-LO (gene: ALOX15), and 15-LO type 2 (gene: ALOX15B) (13, 42). LO enzymes metabolize arachidonic acid to leukotrienes, hydroxyeicosatetraenoic acids, and lipoxins (Fig. 1). Leukocytes, mast cells, and macrophages are the primary cell types that generate LO metabolites (32, 42). Several reports have shown that renal mesangial and endothelial cells have capacity to generate leukotrienes (8, 22, 43, 71, 93). 12/15-LO products are also generated in glomerular mesangial cells, cortical tubules, and renal microvessels (32, 101).
Figure 1.
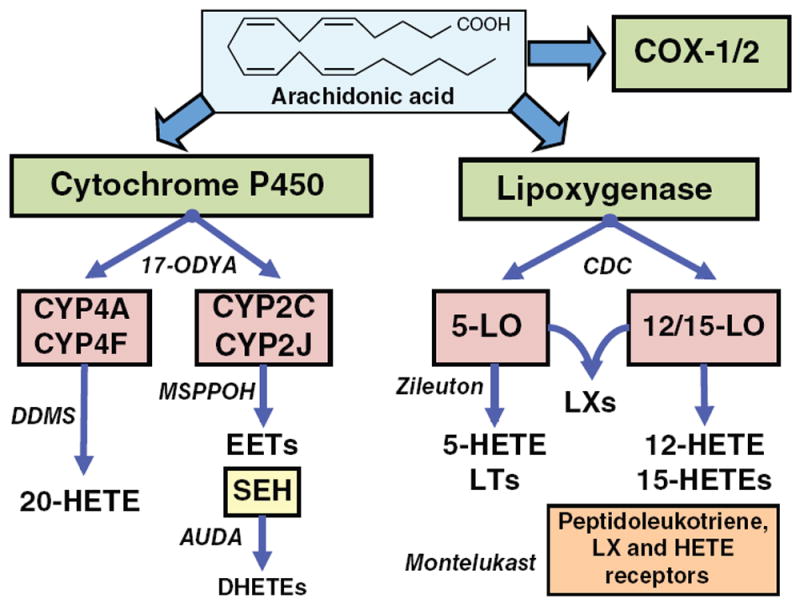
Diagram depicting pathways of arachidonic metabolism. CYP, LO, and COX, pathways can metabolize arachidonic acid. The contribution of COX-1 and COX-2 metabolites to renal function has been extensively reviewed (13, 78). CYP enzymes can generate 20-HETE and EETs. sEH can hydrate EETs to form dihydroxyeicosatrienoic acids (DHETEs). LO enzymes generate HETEs, leukotrienes (LTs) and lipoxins (LXs). Representative enzymatic inhibitors and receptor antagonists are named in italics.
Leukotriene biosynthesis is dependent on 5-LO generation of leukotriene A4 (LTA4) and requires the presence of 5-LO-activating protein (FLAP) a perinuclear membrane protein that transfers arachidonic acid to 5-LO (42). LTA4 is then converted to LTB4 by leukotriene 4A hydrolase or converted to cysteinyl leukotrienes LTC4, LTD4, and LTE4 via leukotriene C4 synthase and subsequent metabolism by γ-glutamyl leukotrienease, γ-glutamyl transpeptidase, and dipeptidase enzymes. 12-LO catalyzes the formation of 12-hydroperoxyeicosatetraenoic acid (12(S)-HpETE) that in turn is rapidly reduced to 12-hydroxyeicosatetraenoic acid (12(S)-HETE) (42). 12(S)-HpETE can also be converted to hepoxilin A3 or hepoxilin B3 that in some tissues can be metabolized to trioxilins. Like 12(S)-HpETE, 15-HpETE can convert a variety of hydroxyepoxyeicosatrienoic acids (HEETAs) and trioxiliins. 12/15-LO can convert arachidonic acid to both 12(S)-HETE and 15(S)-HETE. Dual lipoxygenation of arachidonic acid by either 5-LO and 12-LO or 15-LO and 5-LO results in the generation of trihydoxytetraene-containing eicosanoids named lipoxins (LXA4 and LXB4). Accumulating evidence indicates that LO-derived metabolites contribute importantly to renal function and pathologies associated with hypertension and diabetes (32, 99, 100).
The renal and biological actions of many of the LO metabolites are mediated through G-protein coupled receptors (42, 119). A number of receptors have been identified for many of the leukotrienes and lipoxins. Two receptors have been identified for LTB4 and termed BLT1 and BLT2. Cysteinyl leukotriene receptors, CYSLT1 and CYSLT2, have varying affinity for LTD4, LTC4, and LTE4. CYSLT1 receptor has a rank order potency of LTD4 > LTC4 > LTE4 whereas the CYSLT2 receptor has a rank order of LTD4 = LTC4 > LTE4 (42, 141). Lipoxins elicit their responses via the G-protein coupled ALX/FPR2 receptor (119). To date, there is limited knowledge on the contribution of BLT, CYSLT, and ALX/FPR2 receptors influence on renal function and their role in renal pathophysiological states remains to be determined.
CYP-derived eicosanoids can be separated into two primary enzymatic pathways; an epoxygenase pathway and hydroxylase pathway (Fig. 1). Epoxygenase enzymes of the CYP2J and CYP2C families generate four regioisomeric EETs in the kidney (18, 19, 123). The kidney epoxygenase enzymes in humans are CYP2C8 and CYP2J2 (18, 18). EETs are further metabolized to their corresponding diol, dihydroxyeicosatetraenoic acids (DHETs) by soluble epoxide hydrolase (sEH, gene: EPHX2) (56). As for the second CYP pathway, human kidneys express CYP4A and CYP4F hydroxylase enzymes (113, 124). The hydroxylase enzymatic pathway converts arachidonic acid to 20-HETE and other HETEs. CYP metabolites contribute to renal function through their actions on epithelial transport and vascular tone (5, 113). Although there is strong evidence that these eicosanoid metabolites are dependent on G proteins, up to now receptors for EETs, DHETS, or HETEs have not been identified.
Pharmacological and genetic manipulation of pathways
Pharmacological, genetic, and biochemical tools have been developed that allow for detailed studies to determine the contribution of eicosanoids to renal hemodynamic and epithelial function (19, 42, 50). An important aspect of these tools has been their use to evaluate the contribution of specific CYP and LO enzymes, metabolites, and receptors in regards to impact on renal function and interactions with hormonal and paracrine factors.
One major obstacle for determining the renal actions of CYP and LO metabolites was that these metabolites are relatively unstable and require storage at −80°C and under nitrogen or argon. Another impediment early on was the synthesis and availability of pure metabolites in sufficient amounts to conduct physiological studies. Although many experimental studies began to identify CYP and LO metabolites effects on renal hemodynamics and epithelial cell transport, the development of stable mimetics and selective antagonists allowed investigators to better determine the contribution of eicosanoid metabolites to kidney function (49, 50, 119). Findings from these studies have also determined structure-activity relationships and are assisting with identification of eicosanoid receptors. These CYP and LO agonists and antagonists are also being developed in a way that could eventually to lead to their use for combating renal or other diseases.
CYP and LO enzymatic inhibitors have been extremely beneficial in defining the contribution of specific pathways to renal blood flow and water and electrolyte regulation. Extensive experimental evaluation of the CYP and LO genes and proteins has led to excellent definition of the catalytic site for the enzymes resulting in the design and development of selective pharmacological inhibitors (4, 33, 50, 56, 119, 127). These pharmacological tools have been instrumental for evaluating the biological actions of CYP and LO enzymes in renal cell culture systems, isolated renal vascular, and tubular preparations, and in vivo. Like COX inhibitors, CYP and LO inhibitors have significant therapeutic potential. The 5-LO inhibitor, zileuton, is currently being used to treat asthma and sEH inhibitors are being evaluated for the treatment of hypertension, diabetes, and chronic obstructive pulmonary disease (34, 56).
Genetic manipulation of CYP and LO enzymes and receptors in mice has been another avenue to determine the importance of these eicosanoid metabolites to renal function. Various approaches have been utilized to eliminate or increase enzymatic or receptor protein expression in mice. Many of these genetic mice have human genes expressed either globally or in specific cell types in mice (2, 42, 80). These genetic mice have demonstrated gender differences in regards to CYP and LO pathways on renal function. For instance, two reports have shown that androgen-regulated Cyp4a enzyme contributes to renal function and blood pressure regulation (19, 138). This led to subsequent studies demonstrating gender differences in the CYP4A pathway and sodium excretion and blood pressure in humans (30, 37, 77, 78). In addition, these genetically manipulated mice have uncovered novel interactions between the various eicosanoid pathways, enzymes, and metabolites (19, 56).
Overview renal physiology: Hemodynamics, epithelial actions, inflammation, and apoptosis
Renal hemodynamics and epithelial transport are regulated by eicosanoid metabolites. Eicosanoids have very diverse biological actions in the kidney such that the overall effect on renal function depends on their regulation at the genetic, enzymatic, metabolite, and receptor level. The importance of eicosanoids is highlighted by the fact that nonsteroidal anti-inflammatory drugs that inhibit COX enzymes have adverse effects on kidney function (45). There have been numerous experimental studies that have focused on COX enzymes, metabolites, and receptors in regards to renal hemodynamic, water and electrolyte regulation, renal inflammation, and apoptosis (43-45, 98). CYP metabolites also influence renal function through actions on endothelial, vascular smooth muscle, glomerular, and epithelial cells to influence renal blood flow, glomerular filtration, and electrolyte transport (48, 113, 114, 138). The contribution of these CYP metabolites to renal function appears to be on a level similar to COX metabolites. LO metabolites on the other hand have been less well studied in regards to renal function; however, these metabolites have important glomerular and inflammatory actions (32, 101, 119). Consequently, manipulation of CYP and LO metabolites can be selectively manipulated to influence renal hemodynamics, water and electrolyte regulation, and inflammation.
In addition to the direct actions of CYP and LO metabolites on renal hemodynamics and epithelial transport, eicosanoid metabolites have important function to modify responses to hormonal, paracrine, and autocrine factors. Interactions between eicosanoids and the renin-angiotensin, endothelin, cytokine, and purinergic systems are just a few examples. CYP and LO pathways have also been implicated in alterations in renal function that occur with various disease states. There are excellent review articles on the contribution of eicosanoids in renal disease (11, 17, 51, 100, 113, 135); however, this review will focus on the renal hemodynamic and epithelial transport actions of CYP and LO metabolites.
Renal Hemodynamics
Renal blood flow and glomerular filtration rate (GFR) contribute importantly to the physiological role of the kidney to maintain water and electrolyte homeostasis. GFR determines the amount of plasma composed of water and electrolytes delivered to the renal tubule system for processing. The ability of the kidney tubular system to properly regulate fluids and electrolytes requires a constant renal blood flow and GFR in the face of changes in arterial pressure, termed autoregulation. This autoregulation of renal blood flow and GFR is accomplished through the interplay of two mechanisms, tubuloglomerular feedback and the myogenic response to adjust vascular resistance of the preglomerular afferent arteriole. Tubuloglomerular feedback is a mechanism that senses the delivery of fluid to the kidney tubule system and sends signals to the afferent arteriole to adjust resistance to increase or decrease GFR. Myogenic response is an inherent property of the afferent arteriole smooth muscle cells that sense pressure resulting in vasoconstriction or vasodilation to keep glomerular capillary pressure and GFR constant. Importantly, eicosanoid metabolites regulate renal blood flow and GFR. CYP metabolites play a significant role in autoregulation of renal blood flow and GFR (48, 50, 113). LO metabolites are generated in the glomerulus and influence GFR (101). We will highlight the contribution of CYP and LO metabolites to renal blood flow autoregulation and detail cell signaling mechanisms utilized to regulate renal microvascular function.
Renal blood flow and autoregulation
Eicosanoid metabolites have been implicated in renal blood flow autoregulation at the level of the macula densa and afferent arteriole (50, 102). Although COX-2 is expressed at the macula densa and contributes importantly to renin secretion, COX metabolites are not critical component in renal blood flow autoregulation (45,102). Likewise, glomerular LO metabolites have not been found to contribute to renal blood flow autoregulation (101). On the other hand, a critical role for CYP metabolites in renal blood flow autoregulation has been demonstrated and 20-HETE is a key component of the afferent arteriolar autoregulatory responses (50, 113) (Fig. 2). Initial experimental studies utilized the non-selective CYP inhibitor 17-ODYA infused into the renal artery and demonstrated that 17-ODYA eliminated renal blood flow and cortical blood flow autoregulation (151). Likewise, afferent arteriolar constrictor responses to increasing perfusion pressure were abolished by 17-ODYA resulting in increased glomerular capillary pressure (60). A drawback of these studies was that 17-ODYA could not distinguish between the contribution of epoxygenase EETs or hydroxylase 20-HETE pathways. Additional studies with relatively selective imidazole derivative of epoxygenase inhibitors failed to alter renal blood flow autoregulation (4). These findings support the notion and are consistent with the concept that the afferent arteriolar constrictor CYP metabolite 20-HETE rather than vasodilator EETs contributes to renal blood flow autoregulation.
Figure 2.
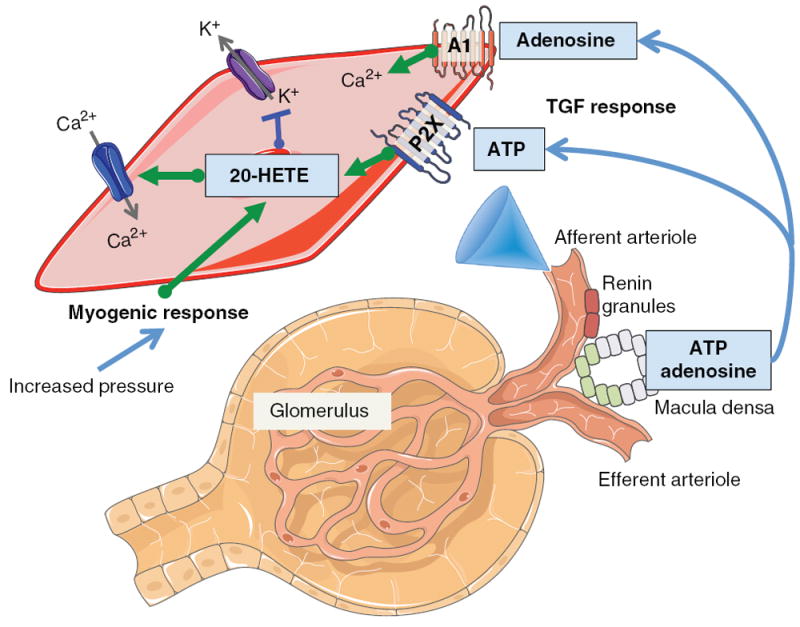
Diagram depicting renal blood flow autoregulatory tubuloglomerular feedback (TGF) and myogenic responses. Afferent arteriolar vascular smooth muscle cell depicts contribution of 20-HETE to myogenic and TGF responses. Increased afferent arteriolar transmural pressure triggers the myogenic response to increase 20-HETE levels. 20-HETE causes afferent arteriolar constriction via inhibition of large-conductance Ca2+-activated K+ channels and activation of L-type Ca2+ channels. TGF response is initiated by sensing cells at the macula densa resulting in release of adenosine and ATP. Adenosine activates adenosine type A1 receptors to increase intracellular Ca2+ and ATP activates purinergic P2X receptors to increase 20-HETE levels resulting in afferent arteriolar constriction.
A clear contribution for the CYP hydroxylase pathway and 20-HETE in the renal blood flow autoregulatory response was confirmed with the development of selective CYP hydroxylase inhibitors and 20-HETE antagonists (4, 54). Afferent arteriolar constrictor responses to increasing perfusion pressure was greatly attenuated by the selective hydroxylase inhibitor dibromo-dodecenyl-methylsulfimide (DDMS) (54). Whereas selective epoxygenase inhibition with 2-(2-propynyloxy)-benzenehexanoic acid (PPOH) or MS-PPOH resulted in an enhanced afferent arteriolar constrictor response when perfusion pressure was increased from 80 to 160 mmHg (54). More recently, the 20-HETE synthesis inhibitor, HET0016 or the 20-HETE antagonist, 6,15-20-HEDE completely blocked the myogenic response in rabbit and mouse afferent arterioles (38, 111). The addition of a 20-HETE agonist, 5,14-20-HEDE restored the myogenic response in afferent arterioles treated with HET0016 (38, 111). In regards to the tubuloglomerular feedback response, the 20-HETE agonist potentiated the afferent arteriolar constrictor response to increased sodium chloride delivery to the macula densa (38). Micropuncture studies demonstrated that 17-ODYA, but not inhibition of COX or LO, abolished the tubuloglomerular feedback response and that 20-HETE added to the tubular perfusate restored the feedback response (150). There is additional evidence for a contribution to tubuloglomerular feedback and fluid and electrolyte homeostasis. Infusion of 17-ODYA into the cortical interstitial space increases papillary blood flow, increase renal interstitial hydrostatic pressure, and increase sodium excretion (4,136). The increase in sodium excretion is likely secondary to increases in papillary blood flow and renal interstitial pressure since 20-HETE inhibits tubular sodium reabsorption (4, 114). Taken together, these findings indicate that 20-HETE produced locally contributes to modulating the afferent arteriolar myogenic and tubuloglomerular feedback responses.
Another paracrine factor that has been implicated in renal blood flow autoregulation is the purines adenosine and ATP. Like 20-HETE inhibition, ATP P2X receptor antagonists abolished afferent arteriolar autoregulatory responses (62). P2X receptors were found to be critical to both myogenic and tubuloglomerular feedback responses (62). Thus, experimental studies were conducted to determine if interactions between purinergic receptors and 20-HETE occurred at the level of the afferent arteriole. These studies found that CYP hydroxylase inhibition or 20-HETE antagonism significantly attenuated the afferent arteriolar constriction to ATP or the P2X receptor agonist α,β-methylene ATP (146). Increases in renal microvascular smooth muscle cell calcium in response to ATP or α,β-methylene ATP, but not the P2Y agonist, UTP, were markedly reduced by 20-HETE antagonism (145). These findings provide compelling evidence that 20-HETE is a critical smooth muscle cell-signaling component for the ATP P2X receptor-mediated afferent arteriolar autoregulatory response (Fig. 2).
Like 20-HETE and ATP P2X receptor interactions, other eicosanoid metabolites have significant interactions with paracrine and hormonal factors to influence renal blood flow. LO and CYP metabolites have been demonstrated to modulate renal blood flow and GFR responses to factors such as angiotensin II, endothelin-1, and bradykinin (48, 74). These interactions include LO and CYP metabolites as critical mediators of responses, as an opposing factor, or as a critical paracrine factor to transmit a signal from one cell type to another.
LO metabolite interaction with angiotensin II and renal blood flow has been extensively investigated (10, 12, 52, 70, 125, 128). Afferent arteriolar vasoconstriction to angiotensin II but not norepinephrine is attenuated by nonselective LO inhibition (52). Moreover, inhibition of the 12-LO pathway attenuates decreases in renal blood flow in response to angiotensin II (12). Unlike the afferent arteriole, norepinephrine constrictor responses in the renal arcuate artery were attenuated by LO inhibition (9). Experimental studies have implicated the LO metabolite 12(S)-HETE as a key mediator in renal vasoconstrictor responses to angiotensin II (9, 12, 52) (Fig. 3). 12(S)-HETE administration in the renal artery decreases renal blood flow and GFR (9). Angiotensin II but not norepinephrine increases renal microvascular 12(S)-HETE production (143). In addition, utilization of LO inhibitors demonstrated that 12(S)-HETE contributes to the afferent arteriolar response to angiotensin II (143). These interactions between the LO pathway and renal vasoconstrictors are also consistent with the ability of LXB4 and 7-cis-11-trans-LXA4 renal artery administration to decrease blood flow (10, 70). Renal vasoconstrictor actions of LXs are via activation of the LTD4 receptors (10). LTC4 and LTD4 also decrease renal blood flow and GFR when administered into the renal artery (10, 70). LXA4 via activation of the peptido-LT receptors can oppose the decrease in renal blood flow and GFR evoked by intrarenal infusion of LTD4 (109). Recent studies have demonstrated that the interaction between 12/15-LO and angiotensin II are dependent on activation of the angiotensin II type 1 (AT1) receptor (99,140). Likewise, 12/15-LO knockout mice have reduced vasoconstrictor responses to angiotensin II (6). This contribution of 12/15-LO metabolites to angiotensin II renal blood flow responses has important pathological implications because an elevated renin-angiotensin system has been linked to diseases such as hypertension, diabetic nephropathy, and renal inflammatory diseases.
Figure 3.
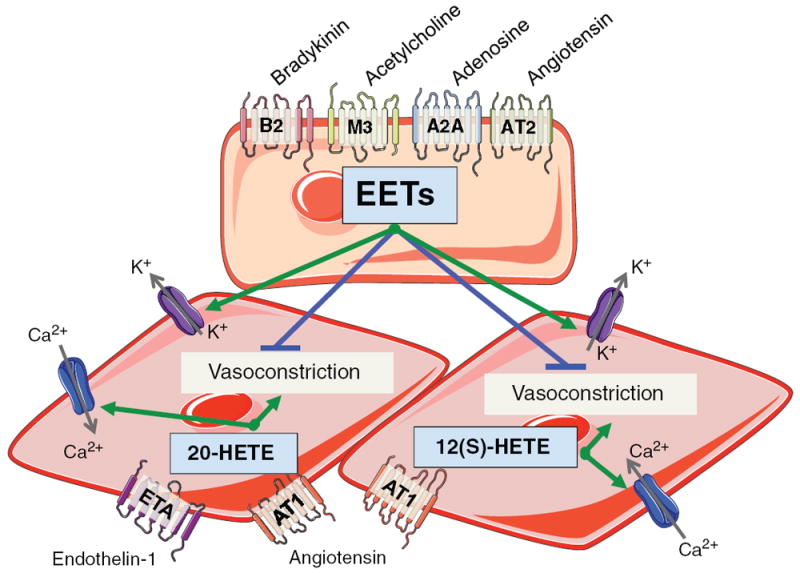
Diagram depicting renal vascular endothelial cell and vascular smooth muscle cell signaling for EETs, 20-HETE, and 12(S)-HETE. Bradykinin B2 receptor, acetylcholine muscarinic M3 receptor, adenosine A2A receptor, or AT2 receptor activation increases renal endothelial cell EET levels. EETs released from the endothelial cell act in a paracrine manner on vascular smooth muscle cells to activate large-conductance Ca2+-activated K+ channels and oppose vasoconstriction. ETA receptor activation increases renal vascular smooth muscle cell 20-HETE levels. AT1 receptor activation increases vascular smooth muscle cell 20-HETE and 12(S)-HETE activates. 20-HETE and 12(S)-HETE activate L-type Ca2+ channels and cause vasoconstriction.
CYP metabolite interactions with the renin-angiotensin system with regards to renal vascular function have also been demonstrated experimentally (52,90,107,147). Afferent arteriolar responses to angiotensin II are enhanced in the presence of nonselective CYP inhibition (52). The CYP metabolites that oppose angiotensin II renal vascular constriction appear to be endothelial-derived and angiotensin type 2 (AT2) receptor mediated (52). Afferent arterioles dilate to angiotensin II in the presence of AT1 receptor inhibition and this dilation is blocked by AT2 receptor or CYP inhibition (52). Likewise, the afferent arteriolar response to angiotensin II is enhanced by sEH inhibition to increase EET levels (147). Taken together, these studies support the concept that angiotensin II afferent arteriolar constriction and decrease in renal blood flow is opposed by AT2 receptor and endothelial release of EETs (Fig. 3).
A contribution of 20-HETE to the renal vasoconstrictor response to angiotensin II has been well established (66,107) (Fig. 3). Angiotensin II infusion into the isolated perfused rabbit kidney resulted in a significant release of 20-HETE, whereas vasopressin did not alter 20-HETE levels (107). In this regard, angiotensin II can increase vascular CYP4A expression and preglomerular microvessel 20-HETE levels (122). This response is AT1 receptor mediated since the AT1 receptor blocker reduces 20-HETE levels (25). Inhibition of 20-HETE also attenuates the renal interlobular artery response to angiotensin II by one-half (122). Interestingly, 20-HETE can also influence regulation of the vascular renin-angiotensin system (122). Cultured endothelial cells incubated with 20-HETE have a sixfold increase in angiotensin converting enzyme expression (122). Increased CYP4A2 expression in rat endothelial cells using a lentivirus expressing CYP4A2 cDNA with an endothelial cell specific promoter (VECAD-CYP4A2) resulted in increased plasma but not kidney angiotensin II levels (66, 122). These rats developed hypertension that was associated with decreased creatinine clearance (122). Thus increased renal vascular resistance in VECAD-CYP4A2 rats likely contribute to the hypertension. These findings support the notion of a complex feedback interaction between 20-HETE and angiotensin II where angiotensin II can increase 20-HETE and 20-HETE can increase endothelial cell angiotensin converting enzyme expression.
Endothelin-1 is another import factor that importantly influences renal hemodynamics that interacts with CYP metabolites (59, 108). These studies have determined that 20-HETE contributes to and EETs oppose endothelin-1 renal vasoconstriction (Fig. 3). Endothelin-1 results in increased 20-HETE efflux in the isolated perfused kidney and CYP hydroxylase inhibition attenuated endothelin-1-mediated increase in renal vascular resistance (108). Afferent arteriolar constrictor and renal microvascular increases in cytosolic calcium in response to endothelin-1 were greatly attenuated by the hydroxylase inhibitor DDMS (59). Experimental studies failed to demonstrate a contribution of LO metabolites to the renal arcuate artery response to endothelin-1 (32). On the other hand, selective epoxygenase inhibition with MS-PPOH resulted in enhanced endothelin-1 evoked afferent arteriolar constriction (59). In accordance with these findings, endothelial expression of human epoxygenase CYP2C8 and CYP2J2 enzymes in mice resulted in attenuation of the afferent arteriolar constriction to endothelin-1 (80). The ability of epoxygenase metabolites to oppose the renal vasoconstrictor actions does not occur at the vascular smooth muscle cell because MS-PPOH failed to alter the endothelin-1-mediated increase in calcium (59). Afferent arteriolar constrictor and renal microvascular smooth muscle cell responses to endothelin-1 are mediated through activation of the endothelin-1 type A (ETA) receptors (59). Taken as a whole, endothelin-1 renal vasoconstriction is in part mediated by 20-HETE contributing to increased vascular smooth muscle cell calcium and opposed by endothelial cell generated EETs.
CYP and LO metabolites also interact with the nitric oxide synthase and reactive oxygen species pathways. 12/15-LO can induce catalytic consumption of nitric oxide and prevent nitric oxide activation of cGMP (6, 101). Knockout of 12/15-LO in mice results in increased eNOS expression and nitric oxide bioavailability (6). LTD4 dilation of renal arteries has also been demonstrated to be endothelial- and nitric oxide-dependent (109). 20-HETE also interacts with nitric oxide and influences renal hemodynamics (3, 46, 54, 113). Nitric oxide and superoxide radicals can inhibit renal vascular production of 20-HETE and the decrease in 20-HETE levels partially mediates the cGMP-independent vasodilator effects of nitric oxide (3). 20-HETE in turn can increase vascular oxidative stress and endothelial dysfunction resulting in eNOS uncoupling (3). Although EETs appear to act independent of nitric oxide, increased endothelial cell EET generation occurs in eNOS gene deficient mice and minimizes the effect of decreased nitric oxide on renal and endothelial vascular function (54, 113). Renal microvascular interactions between CYP, LO, and nitric oxide exists and could have important implications in diseases that greatly effect the kidney like hypertension and diabetes.
Epoxygenase metabolites have been most studied for interactions with vasodilator mechanisms. 11,12-EET and 14,15-EET dilate afferent arterioles and have been identified as endothelial-derived hyperpolarizing factors (49, 50, 58). A number of experimental studies provide significant evidence that EETs contribute to acetylcholine, bradykinin, and adenosine afferent arteriolar vasodilator responses (24, 55, 112, 131, 134) (Fig. 3). Acetylcholine dilation of the afferent arteriole is greatly attenuated by epoxygenase inhibitors (131, 134). In agreement with these findings, afferent arteriolar dilation to acetylcholine is enhanced by overexpression of human CYP2C8 and CYP2J2 epoxygenase enzymes (80). Approximately one-third of the afferent arteriolar response to bradykinin is dependent on EETs with a larger nitric oxide contribution and smaller COX contribution (55). There is also an interaction with bradykinin and the renin-angiotensin system that appears to depend on EETs. Inhibition of angiotensin converting enzyme results in increased levels of bradykinin and renal microvascular dilation that is dependent on epoxygenase metabolites (49). In accordance with these findings, renal microvessels incubated with bradykinin have increased generation of EETs (55). Likewise, the epoxygenase inhibitor, MS-PPOH, also blunted bradykinin dilation of efferent arterioles (132). In contrast, 20-HETE inhibition with 20-HEDE enhances efferent arteriolar dilation to bradykinin (132). Additional experimental evidence demonstrated that 20-HETE was generated by the glomerulus to oppose efferent arteriolar dilation in response to bradykinin (132). EETs also contribute to the renal microvascular dilation in response to the purine adenosine (29). Adenosine increases renal microvessel EET production and the renal microvascular dilation in response to adenosine A2A receptor agonists is attenuated by epoxygenase inhibition (29). Taken together, these experimental outcomes demonstrate a contribution of CYP metabolites to renal microvascular responses to bradykinin and adenosine.
The effects of CYP and LO metabolites on renal blood flow and autoregulation have proved their importance to renal fluid and electrolyte homeostasis. These eicosanoids also interact by contributing to or opposing the renal hemodynamic actions of hormonal, paracrine, and autocrine factors. LO metabolites, 12(S)-HETE, 15-HETE, LTs, and LXs have effects to alter cell signaling at the renal glomerular, endothelial, and smooth muscle cell and have been implicated in inflammatory diseases and glomerulonephritis (9, 42). CYP hydroxylase generated 20-HETE also appears to have renal vascular glomerular, endothelial cell, and smooth muscle cell actions in hypertension (136). Lastly, EETs are important renal endothelial cell-derived metabolites that are important in maintaining proper vascular tone and glomerular function (49). Interestingly, 8,9-EET significantly blocked the increases in glomerular permeability in response to a circulating focal and segmental glomerulosclerosis permeability factor (FSPF) (120). This finding demonstrates the potential for manipulating eicosanoids to treat renal disease. The next section will provide detailed information on glomerular, endothelial, and vascular cell signaling mechanisms by which CYP and LO metabolites operate to control renal hemodynamics.
Renal microvascular cell signaling
Renal microvascular regulation is comprised of glomerular capillaries, afferent arterioles, efferent arterioles, and vasa recta. Eicosanoid metabolites have actions on endothelial, smooth muscle, and mesangial cells that regulate renal microvascular tone and glomerular filtration. Cell signaling mechanisms utilized by CYP and LO include effects on K+ and Ca2+ channels, cGMP and cAMP, and protein kinases. This section will detail LO and CYP metabolite renal microvascular regulation.
Glomerular mesangial cells and endothelial cells express LO enzymes that produce LTA4, 12(S)-HETE, and 15(S)-HETE (100, 101). At the level of the mesangial cell LO metabolites stimulate mesangial cell growth (32, 99, 101, 139). In this regard, mesangial cells derived from 12/15-LO−/− mice grow slower than wild-type mice. The decreased mesangial cell growth was associated with lower levels of superoxide, fibronectin, and transcriptional activities of activated protein-1 (AP-1) and cAMP response element-binding protein (CREB) (140). LTC4 is another LO metabolite that has been demonstrated to stimulate mesangial cell growth (9, 32). In addition, LTs can increase glomerular and peritubular capillary permeability (32). LTC4 and LTD4 receptors mediate the actions of LTs on glomerular mesangial cells, capillary permeability, and renal and glomerular vasoconstriction (32). LO metabolites 12(S)-HETE and 15(S)-HETE have been shown to constrict renal microvessels and glomerular mesangial cells (32, 87, 101, 143). However, 15(S)-HETE demonstrated a much weaker constrictor action on the afferent arteriole (143). There has been extensive investigation into the cell signaling mechanisms by which 12(S)-HETE causes renal microvascular constriction. 12(S)-HETE depolarizes renal arcuate artery vascular smooth muscle cells possibly through protein kinase C activation (87). Afferent arteriolar constriction and renal microvascular smooth muscle cell responses to 12(S)-HETE are greatly attenuated by diltiazem suggesting an important contribution for L-type Ca2+ channels (143) (Fig. 3). Additional studies with isolated renal microvascular smooth muscle cells determined that 12(S)-HETE elevates intracellular calcium through two mechanisms, a small intracellular calcium mobilization component and a large influx of extracellular calcium via L-type Ca2+ channels (143). The recent identification of GPR31 as the 12(S)-HETE receptor that couples to GTPγs will undoubtedly provide new knowledge on the role of LO metabolites in renal microvascular cell signaling mechanisms.
Renal arterioles, glomeruli, and vasa recta express CYP4A hydroxylase enzymes that generate 20-HETE (4, 61, 136). Other experimental studies determined that 20-HETE afferent arteriolar constriction required smooth muscle cell depolarization and an increase in intracellular calcium levels (7, 86, 148). 20-HETE inhibits renal microvascular smooth muscle cell Ca2+-activated K+ channels that results in cell membrane depolarization and activation of L-type Ca2+ channels (148) (Fig. 3). Other renal microvascular smooth muscle cell signaling mechanisms activated by 20-HETE include protein kinase C and extracellular signal regulated kinases (ERK) (4, 87). These findings are consistent with the findings that CYP hydroxylase and 20-HETE antagonists attenuate the renal microvascular intracellular calcium responses to ATP P2X receptor and endothelin-1 (59, 146). Although more recent studies have established the importance of 20-HETE renal vasoconstriction in renal and cardiovascular disease, future studies are necessary to fully comprehend the cell signaling mechanisms by which 20-HETE regulates renal microvascular function.
Despite the fact that epoxygenase inhibition demonstrates that the overall renal EET actions are vasodilation, there are differences in the direct renal vascular actions of the four EET regioisomers. Comparisons of the four regioisomeric EETs in most instances have demonstrated that 8,9-EET and 5,6-EET have renal vasoconstrictor or vasodilator actions and 11,12-EET and 14,15-EET have renal vasodilator actions (23, 36, 58, 69). Afferent arteriolar vasoconstriction induced by 5,6-EET is due to COX-dependent conversion to a thromboxane-like compound (58). Renal vascular vasodilation to 5,6-EET appears to be dependent on conversion by COX to a prostaglandin-like metabolite (23, 36). 8,9-EET also has actions on the glomerulus to reduce albumin permeability in response to administration of FSPF (120). On the other hand, 11,12-EET and 14,15-EET act directly on the afferent arteriolar smooth muscle to cause hyperpolarization and vasodilation (57, 58). Likewise, CYP2C enzymes produce kidney and renal vascular EETs with 11,12-EET and 14,15-EET as the predominant reigiosiomers. 11,12-EET and 14,15-EET comprise 65% of the total renal microvessel EET generation (55). Thus, 11,12-EET and 14,15-EET appear to be the dominant regioisomeric EETs acting on the afferent arteriole to evoke vasodilation and aligns with the findings with epoxygenase inhibitors.
11,12-EET and 14,15-EET are endothelial and CYP2C epoxygenase generated eicosanoids that have important actions to control afferent arteriolar function (50). Cell signaling mechanisms by which 11,12-EET causes renal vascular and afferent arteriolar dilation have been extensively studied. 11,12-EET-mediated renal microvascular dilation depends on activation of smooth muscle cell large-conductance Ca2+-activated K+ channels (KCa) channels (57, 149) (Fig. 3). Other studies determined that activation of KCa channels by 11,12-EET requires generation of cAMP and protein kinase A (57). More recently, 11,12-EET analogs were developed and allowed for determination of structure-activity relationships and more rigorous cell signaling studies (53). 11,12-EET analogs when evaluated in electrophysiological experiments were determined to activate renal microvascular smooth muscle cell KCa channels in the cell-attached mode (53). Afferent arteriolar dilations to a series of 11,12-EET analogs were inhibited by iberiotoxin but not chaybdotoxin, apamin, or TRAM-34. These findings supports the concept that 11,12-EET acts by activating renal microvascular large-conductance KCa channels and does not have actions on small- or intermediate-KCa channels. EET activation of vascular smooth muscle cell vallinoid type 4 (TRPV4) channels could contribute to large-conductance KCa channel activation and membrane hyperpolarization (49, 85). Besides these actions on renal microvascular smooth muscle cells, EETs can also have actions to increase endothelial cell K+ and TRP channel activity (49). TRPC3 and TRPC6 channel activation by EETs can lead to subsequent activation of endothelial cell small- or intermediate-KCa channels (49). These findings suggest that a coordinated action of EETs on endothelial and vascular smooth muscle cells is required for renal microvascular dilation.
EET analogs actions on afferent arterioles have provided very important information on structure-activity relationships and the development of EET antagonists. These EET analogs have been designed to have increase solubility and resist sEH and β-oxidative metabolism (16, 33, 53, 127). First generation EET analogs were methyl esters and sulfonamide substitutions of the carboxylic acid which obviated esterification and resisted β-oxidation (57). The next generation of EET analogs removed the 1,4-diene responsible for autoxidation and replaced the labile epoxide with bio-isosteres that resist metabolism (53). Studies of the second generation of EET analogs assessing vascular inflammation and dilation resulted in the following structural requirements: an acidic carboxyl group, Δ8olefin bond, 20-carbon chain length, and a cis epoxide (32, 53). On the other side, the nonselective EET antagonist, 14,15-EEZE, has been widely utilized and provided important findings on EETs and renal vascular function (16, 50). More recently, 14,15-DHE5ZE and 11,12,20-TH8ZE have been demonstrated to be 14,15-EET and 11,12-EET selective antagonists, respectively (16). The findings with EET analogs and selective EET antagonists along with other cell signaling experimental findings strongly suggests that EETs act through receptors to cause renal microvascular dilation.
In conclusion, there is a significant amount of evidence that CYP and LO metabolites contribute importantly to renal hemodynamics and mediate these actions through endothelial and vascular smooth muscle cell signaling mechanisms. Regrettably, there are still significant gaps in our knowledge of these eicosanoids in relation to renal hemodynamic function. Genetic animal models and novel pharmacological tools have been underutilized. Indeed, there is an overall lack of studies on different vascular segments such as glomerular mesangial cells and capillaries, efferent arterioles, and vasa recta. Although cell-signaling mechanisms for afferent arterioles have been defined, the identification and contribution of eicosanoid receptors is required to move the field forward. One example is the recent finding in mesenteric resistance arteries that CYSLT1R could be a novel mechanosensor that contributes to the myogenic response (126). The contribution of CYSLT1R to renal blood flow autoregulation and the afferent arteriolar myogenic response are not known. This issue is further complicated by the fact that novel biologically active CYP and LO metabolites are being found. Epoxygenase generated epoxy-derivatives can be formed from intermediates of the LO pathway. These LO intermediates can be metabolized to HEETAs, also referred to as hepoxylins (32). Another class of eicosanoids is the anti-inflammatory aspirin-triggered lipoxins (ATLs) with unknown renal vascular actions. Thus, there are numerous opportunities to evaluate the physiological role and basic mechanisms by which CYP and LO metabolites regulate renal blood flow and GFR.
Renal Tubular Transport
A primary function of the kidney is to regulate whole body fluid and electrolytes to maintain plasma volume and electrolyte concentrations within a narrow physiological range. Plasma is filtered across the glomerular capillaries into the proximal tubule for processing of water and electrolytes. Tubular epithelial cells transport electrolytes and water across apical and basolateral cell membranes in a complex and coordinated manner. Major electrolytes that are regulated include Na+, K+, H+, Ca2+, and Cl−. Regulation of these electrolytes and water are essential for proper physiological cell function. As part of the regulation of fluid and electrolyte regulation, the kidney also has endocrine functions. One major endocrine function is the regulation of renin secretion by the juxtaglomerular apparatus. Renin secretion will ultimately result in increased angiotensin II and aldosterone that act on various tubular segments to increased sodium and water conservation by the kidney. Eicosanoids are known to regulate tubular epithelial water and electrolyte transport as well as renin secretion (14, 43, 114). CYP metabolites and to a lesser extent LO metabolites make important contributions to renal tubular transport (32, 114).
CYP metabolites appear to contribute to pressure natriuresis, renin secretion, and regulate sodium excretion (113, 114, 136). Pressure natriuresis refers to the changes in sodium and water excretion that occur in response to a change in blood volume. For example, an increase in blood volume will result in an increase in cardiac output and arterial blood pressure. This increase in arterial blood pressure results in increased renal perfusion pressure that increases GFR leading to an increase in urinary water and sodium excretion in an attempt to decrease blood volume and arterial blood pressure. Previous studies have revealed that pressure natriuresis involves increases in renal medullary blood flow and interstitial hydrostatic pressure (113). A critical contribution for 20-HETE in pressure natriuresis has been hypothesized because 20-HETE is increased in response to an elevation in renal interstitial pressure and inhibits Na+ transport (113, 136). Interestingly, decreased 20-HETE levels have been linked to dysfunction in circadian renal sodium excretory rhythms and blood pressure control in clock−/− mice (103). Epoxygenase metabolites also are importantly involved in the regulation of urinary Na+ excretion (18). EETs are increased in response to elevations in dietary salt intake and have actions that would help to eliminate excessive Na+ from the body (18, 19).
Adenosine activation of A2A receptors appears to be a mechanism responsible for increasing EETs in response to increased salt intake (19, 50). EETs actions to dilate afferent arterioles and inhibit epithelial Na+ transport results in a natriuresis to maintain whole body Na+ levels constant. Another less well-studied biological action of EETs could also contribute to the increase in Na+ excretion. 14,15-EET inhibits renin secretion that would result in decreased actions of angiotensin II and aldosterone to conserve Na+ and water (50). The next sections of this review will detail the actions of CYP and LO metabolites on electrolyte transport at various renal tubular segments.
Proximal tubular transport
The first significant biological action attributed to CYP metabolites of arachidonic acid was inhibition of Na-K-ATPase activity (92, 117). In this landmark series of experimental studies the arachidonic acid metabolite was identified and isolated as an HPLC peak (117). Subsequent studies identified and verified 20-HETE as the CYP metabolite of arachidonic acid that inhibits Na-K-ATPase activity (117) (Fig. 4). 20-HETE inhibits Na-K-ATPase activity by enhancing protein kinase C-induced phosphorylation of serine23 on the Na-K-ATPase α-subunit (117). Dopamine, endothelin-1, and parathyroid hormone mediated Na-K-ATPase inhibition and decreased proximal tubule sodium reabsorption involve phospholipase A2 activation and 20-HETE generation (136, 138). Correspondingly, the natriuretic response to intrarenal dopamine infusion in Sprague-Dawley rats were significantly reduced by 20-HETE synthesis inhibitors ABT or HET0016 (35). Inhibition of proximal tubule sodium reabsorption by angiotensin II is also attenuated by CYP inhibitors which is consistent with the notion that 20-HETE participates in angiotensin II actions on sodium transport at the proximal tubule (114). 20-HETE also appears to contribute to the internalization of the proximal tubular sodium hydrogen exchanger 3 (NHE3) protein in response to renal perfusion pressure (114, 136). Thus, 20-HETE actions on proximal tubule transport would enhance sodium excretion.
Figure 4.
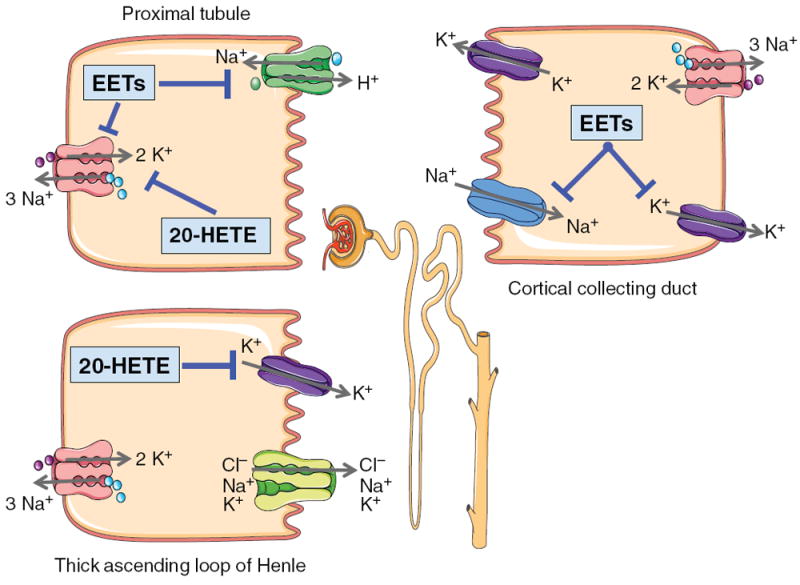
Diagram depicting renal proximal tubule, TALH, and CCD cell signaling for EETs and 20-HETE. Proximal tubule cell: 20-HETE inhibits basolateral Na-K ATPase activity to decrease sodium reabsorption. EETs inhibit basolateral Na-K ATPase activity and apical NHE3 to decrease sodium reabsorption. TALH: 20-HETE inhibits apical K+ channels to limit K+ availability for transport via the Na-K-2Cl− transporter resulting in decreased lumen positive transepithelial potential and passive reabsorption of cations. CCD: EETs inhibit apical ENaC and basolateral K+ channels to decrease sodium reabsorption.
Another proximal tubular cell physiological process that 20-HETE contributes to is mitogenesis (113, 114). The proximal tubular cell proliferative response to epidermal growth factor (EGF) is associated with increased 20-HETE synthesis (113). CYP hydroxylase inhibitors attenuate the rat proximal tubule cell proliferative response to EGF (1). Signal transduction pathways were evaluated in a well-characterized porcine proximal tubule cell line, LLC-PK1. 20-HETE and 20-HETE analogs activate the Raf/MEK/ERK and Akt pathways in LLC-PK1 cells (1). Activation of these pathways by 20-HETE was secondary to the activation of c-Src and EGF receptor (1). Interestingly, overexpression of Cyp4a12 in LLC-PK1 cells exacerbates cellular damage and caspase-3 activation following 4 h of ATP depletion and 2 h of recovery in serum free medium (1). The increased cell death was abolished by 20-HETE synthesis inhibition or free radical inhibition which supports the notion that the increased LLC-PK1 programmed cell death was partially dependent on Cyp4a12 generation of free radicals (1). On the other hand, while the 20-HETE synthesis inhibitor HET0016 exacerbated, 20-HETE protected human renal tubular cells (HK-2) from Adriamycin toxicity (129). These findings have led to an interest in manipulating 20-HETE for renal tubular pathologies such as acute kidney injury and polycystic kidney disease.
EETs also inhibit proximal tubule cell sodium absorption and induce mitogenesis (18, 19). In regards to proximal tubular cell transport, 11,12-EET and 5,6-EET are potent inhibitors of Na-K-ATPase activity (18) (Fig. 4). 5,6-EET inhibits sodium flux across cultured rabbit proximal tubule S1 segments (115). Additional findings in this report supports the concept that 5,6-EET acts as a second messenger that mediates angiotensin II induced natriuresis (115). Enhanced formation of 5,6-EET may affect the translocation of the NHE3 exchanger in the proximal tubule apical membrane (115). EETs inhibit amiloride-sensitive sodium transport and 86RB uptake in LLC-PK1 cells (18). EETs also contribute to dopamine effects to inhibit proximal tubule sodium absorption. Genetically modified mice to selectively decrease proximal tubule cell dopamine levels resulted in decreased renal and urinary EET levels (144). Dopamine also increased EET production and induced renal Cyp2c44 expression in mice (144). These findings are consistent with EETs contributing to dopamine-mediated natriuresis. A contribution of EETs to proximal tubule cell mitogenesis is based on the findings that EGF stimulates EET production and mitogenesis in proximal tubule cells (27). EETs stimulated thymidine incorporation in LLCPKc14 cells with 14,15-EET being the most potent (27). In addition, upregulation of G-protein-coupled receptor 40 (GPR40) enhances the mitogenic response to EETs in HEK293 cells (84). Sulfonamide (SI) EET analogs, 11,12-EET-SI and 14,15-EET-SI were also potent proximal tubule cell mitogens (27). Mitogenic actions of EETs are dependent on activation of Src kinase and initiation of a tyrosine kinase phosphorylation cascade (27). EETs also prevent cisplatin-induced apoptosis in LLC-PK1 cells by preventing mitochondrial trafficking of Bax and cytochrome C and caspase 3 activation (82). Taken together, EETs have actions at the proximal tubule that promote sodium excretion, prevent cell death, and enhance cellular regeneration.
LO metabolites can also influence proximal tubule transport and cell volume control. The 12-LO metabolite 12(S)-HETE also inhibits proximal convoluted tubule Na-K-ATPase activity (32, 81). 12(S)-HETE augments protein kinase C-induced phosphorylation of the Na-K-ATPase (81). A contribution for 5-LO and LTD4 on transport and cell volume control has been demonstrated using the stop-flow technique in renal proximal tubules isolated from goldfish (68). 5-LO inhibition or LTD4 receptor antagonism significantly reduced the rate of luminal fluid absorption (68). Renal proximal tubule transport in goldfish appears to be mediated by sodium glucose cotransport (68). Transepithelial isovolumetric substrate and water transport were attenuated by 5-LO inhibition but not by the administration of the LTD4 receptor antagonist L-660,771 (68). These data demonstrate that a 5-LO metabolite other than LTD4 is the regulator of transepithelial isovolumetric water transport in the proximal tubule. Endothelin-1 actions on lithium clearance as an index of proximal tubule sodium absorption also appear too dependent on 5-LO metabolites (110). A 5-LO inhibitor or the LTC4/D4 antagonists prevent the diuresis and natriuresis evoked by endothelin-1 (110). Likewise, infusion of LTD4 to rats causes a significant rise in urine flow and sodium excretion (110). Taken together, these data demonstrate that 5-LO and 12-LO metabolites inhibit proximal tubule sodium absorption.
LO metabolite actions at the proximal tubule cell extend beyond sodium and water transport processes. More recent studies in LLC-PK1 cells and 5-LO−/− mice, have demonstrated a contribution of 5-LO products to tubulointerstitial injury induced by albumin overload (79). 5-LO genetic deficient mice demonstrated decreased tubulointerstitial injury induce by albumin overload (79). In agreement with a contribution of 5-LO products to tubulointerstitial injury, LTB4 and LTD4 decreased albumin uptake in LLC-PK1 cells (79). Although these data provide evidence for physiological and pathological contributions for LO metabolites at the proximal tubule, experimental data is very limited and is an area that warrants additional research.
Loop of Henle, distal convoluted tubule, and collecting duct
Epithelial cell transport effects of 20-HETE distal to the proximal tubule further enhance natriuresis (114). 20-HETE is a primary metabolite and major regulator of chloride transport in the thick ascending loop of Henle (TALH) (41). Electrophysiological patch-clamp studies revealed that 20-HETE blocks 70pS apical membrane K+ channels to limit K+ availability for transport via the Na+-K+-2Cl− transporter in the TALH (41) (Fig. 4). These 20-HETE effects at the TALH segment reduce the transepithelial potential and reduce the driving force for passive reabsorption of cations. 20-HETE also inhibits the 10pS Cl− channel in the basolateral membrane of the medullary TALH (41). Consistent with these findings are that transepithelial potential and Cl− transport in the isolated perfused TALH are increased by CYP hydroxylase inhibition and decreased by 20-HETE (41). Likewise, a deficiency in TALH 20-HETE generation contributes to elevated loop Cl− reabsorption and this 20-HETE administration normalizes Cl− transport in the Dahl salt-sensitive rat (113,136). 20-HETE can also inhibit 50pS K+ channels in the TAHL (133). Adenosine A2a receptor activation can abolish the inhibitory effect 20-HETE on the 50pS K+ channels (133). Angiotensin II and bradykinin inhibitory actions on Na+ transport in the TALH are also dependent on 20-HETE (114). Interestingly, nitric oxide and carbon monoxides effects of Na+ transport in TALH depend on decreases in the formation of 20-HETE (114). Taken together these studies support the notion that 20-HETE actions on Na+ transport in TALH contribute to the pressure-natriuretic response.
A major cellular mechanism by which EETs regulate Na+ excretion appears to be through epithelial sodium channel (ENaC) and inward rectifying K+ channel inhibition (18, 20) (Fig. 4). 11,12-EET inhibits basolateral inwardly rectifying K+ channels and apical ENaC channels on the cortical collecting duct (CCD) epithelium (18, 20, 65). Likewise, Cyp2c44−/− and Cyp4a10−/− mice have decreased renal EET generation, a hyperactive ENaC, and a reduction in ERK1/2-mediated ENaC subunit phosphorylation (20, 97). In regards to EET regiosomeric actions on ENaC, 11,12-EET inhibits ENaC to a greater extent than 14,15-EET whereas 8,9-EET did not alter ENaC activity (18, 19). The EET analog, EET-A, has also been demonstrated to inhibit ENaC activity in cultured CCD cells and reduce kidney expression of ENaC subunits in rats with angiotensin II hypertension (20). 11,12-EET also decreases basolateral inwardly rectifying K+ channel activity resulting in cell membrane depolarization and a reduction in the driving force for Na+ entry across the apical membrane (18). Another renal epithelial cell action attributed to 11,12-EET is stimulation of apical large-conductance KCa epithelial channels that could contribute to renal K+ secretion (18). Inhibition of CYP epoxygenase with MS-PPOH abolishes the flow stimulated and large-conductance KCa channel dependent K+ secretion (18). These findings are consistent with the concept that 11,12-EET increases apical K+ conductance, thereby compensating the effect of decreased driving force for K+ secretion induced by 11,12-EET-mediated ENaC inhibition. EETs can also influence Cl− transport in collecting duct principal cells. In polarized Madin-Darbin canine kidney C7 cells, 5,6-EET but not other EET regioisomers increased short-circuit current when applied apically (106). Interestingly, the effect of 5,6-EET on Cl− transport was blocked by COX inhibition (106). Additional experiments demonstrated that 5,6-EET actions on Cl− transport were dependent on its conversion to 5,6-epoxy-PGE1 by COX (106). Taken together these findings clearly demonstrate a critical role for EETs in fluid and electrolyte at the level of the CCD.
There is limited experimental evidence on the tubular transport actions mediated by LO metabolites in the distal nephron segments. 12-LO protein is expressed in the distal convoluted tubules and generates 12(S)-HETE (39). Although angiotensin II and aldosterone did not alter 12-LO activity, vasopressin and cAMP significantly enhanced 12(S)-HETE generation in cultured mouse distal convoluted epithelial cells (39). Vasopressin is known to activate adenylate cyclase and stimulate sodium and water reabsorption in the distal nephrons through regulation of aquaporins and epithelial Na+ channels (39). In this scenario, 12(S)-HETE could act as a counter-regulatory by inhibiting Na+ transport in this nephron segment; however, this has yet to be verified. Overall, there is a paucity of data on LO metabolites and distal tubular transport.
Renal Inflammation and Apoptosis
Eicosanoid metabolites play crucial roles in renal inflammation and apoptosis (48, 49). The contributions for COX metabolites in this area have been extensively studied and there are excellent review articles (14, 15, 28). Although less is known about LO and CYP metabolites, it is becoming clear that these arachidonic acid metabolites also contribute significantly to renal inflammatory and apoptotic processes. LO metabolites are produced by inflammatory cells and contribute to renal inflammation and injury in diabetes (32, 100). CYP generated EETs have anti-inflammatory and antiapoptotic actions to prevent renal injury in pathological states (48, 49). In regards to inflammation, less is known about 20-HETE in the kidney and its actions are reported to be proinflammatory (64). This section will highlight CYP and LO metabolites and their cell signaling mechanisms that effect renal inflammation and apoptosis. Their actions in renal pathologies will be used as examples; however, we will focus on biological actions instead of the pathologies.
LO are generated by leukocytes, endothelial cells, and vascular smooth muscle cells and LO metabolites have proinflammatory actions (32, 100) (Fig. 5). 12-LO and 15-LO pathways have been implicated with renal injury associated with diabetes (100). LO metabolites contribute to glucose-induced monocyte adhesion to endothelial cells (100). Proinflammatory actions for LO metabolites have been demonstrated in acute and chronic renal disease (32, 32). Linoleic acid metabolites of 12/15-LO have proinflammatory actions on vascular smooth muscle cells (32). 13-HPODE increases transcription of the adhesion inflammatory molecule vascular cell adhesion protein-1 and monocyte chemoattractant protein-1 (MCP-1) via activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (67, 99). 12(S)-HETE increases vascular smooth muscle cell fibronectin levels through regulation of the transcription factor CREB in a p38 mitogen-activated protein kinase (MAPK)-dependent manner (67, 99). Reactive oxygen species generated by LO metabolites could mediate growth and inflammatory actions in vascular smooth muscle cells and endothelial cells (32, 99). In line with these findings, 12/15-LO−/− mice have decreased superoxide levels, reduced activation of MAPKs, and decreased endothelial monocyte binding compared to wild-type mice (6, 139). The 12-LO/15-LO pathway interacts with transforming growth factor-β (TGF-β) at the level of mesangial cells (75, 76). TGF-β can increase mesangial cell 12/15-LO mRNA and protein expression and 12(S)-HETE levels (76). 12(S)-HETE increased TGF-β and the TGF-β target transcription factor, p-Smad2/3 in a reciprocal manner (76) (Fig. 5). FLAP and cysLT generation are increased renal proximal tubular LLC-PK1 cells in response to aristolochic acid and appear to contribute to apoptosis (142). The FLAP inhibitor decreased aristolochic acid activation of ERK and significantly protected LLC-PK1 cells from apoptosis (142). On the whole, the actions of 12(S)-HETE contribute to renal inflammation and the recent findings of a 12(S)-HETE receptor suggest that receptor antagonists could be beneficial in combating renal injury associated with diabetes.
Figure 5.
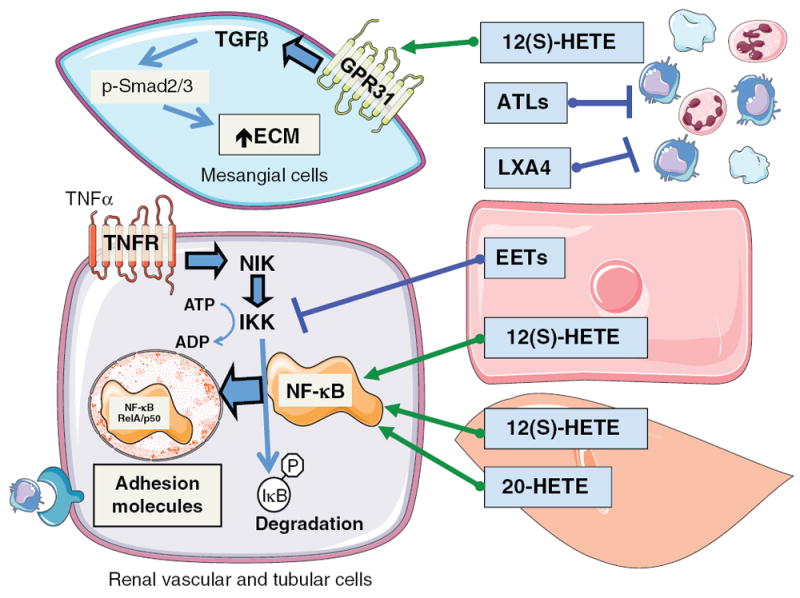
Diagram depicting mesangial cell hypertrophic and renal vascular and tubular cell inflammatory responses to CYP and LO metabolites. Circulating platelets, and leukocytes can generate LO metabolites, 12(S)-HETE, ATLs, and LXA. 12(S)-HETE acts on glomerular mesangial cells to activate GPR31 receptors resulting in increased TGF-β resulting in phosphorylation of Smad2/3 to increase extracellular matrix and hypertrophy. ATLs and LXA4 interfere with activation and migration of inflammatory cells. Renal endothelial cells generate EETs, endothelial and vascular smooth muscle cells generate 12(S)-HETE and vascular smooth muscle cells generate 20-HETE to act in a paracrine manner to influence the renal vascular and tubular cell inflammatory responses. TNF-α acting on the TNF receptor activates a sequence of cell signaling events resulting in NF-κB translocation to the nucleus to increase adhesion molecule levels to cause inflammatory cell infiltration. EETs act to prevent IKK phosphorylation and activation of NF-κB to decrease renal inflammation. 12(S)-HETE and 20-HETE increase NF-κB activation and translocation to the nucleus to increase renal inflammation.
On the other hand, 15-LO metabolites play a protective role in acute kidney injury. Lipoxins can also be produced locally at sites of renal inflammation (119). Interactions between neutrophils and platelets facilitate the generation of lipoxins and increased lipoxin generation has been associated with glomerulonephritis (119). Lipoxin generation can shift the glomerular response from inflammation to resolution and inhibition of monocyte recruitment. Polymorphonuclear neutrophil chemotaxis, adhesion, and migration across glomerular endothelial cells are reduced by lipoxins (119). Proliferations in response to platelet-derived growth factor (PDGF) and cytokine production in mesangial cells are inhibited by lipoxins (119). Likewise, structural analogs of LXA4 reduce neutrophil infiltration and preserve tubular epithelial cell integrity following renal ischemia reperfusion injury (74). This renal action for the LXA4 analog was associated with decreases in proinflammatory cytokine expression (74). In addition, to attenuating proinflammatory mediators, ATL and lipoxins cause potent activation and increased adhesion of monocytes without degranulation suggesting a protective action (74,119). ATL and lipoxins also enhance phagocytosis of apoptotic polymorphonuclear neutrophils by monocyte-derived macrophages promoting clearance of apoptotic leukocytes (74, 119). LXA4 also inhibits LTD4-induced mesangial cell proliferation by modulating transactivation of the PDGF receptor (32, 74). Antiproliferative effects of ATL and lipoxins can contribute to anti-inflammatory mechanisms through interference with the activation and migration of inflammatory cells. ATL treatment modifies the expression of renal cytokines, growth factors, adhesion molecules, and proteases (32). Likewise, overexpression of 15-LO in the rat kidney increases lipoxin production and protects the glomerular basement membrane form nephritic injury (32). Thus, lipoxins and ATLs can promote resolution by inhibiting renal polymorphonuclear neutrophil recruitment, enhance clearance of inflammatory cells, inhibit mesangial cell proliferation, and prevent glomerular extracellular matrix accumulation (Fig. 5).
CYP epoxygenase metabolites can be generated and metabolized in renal endothelial and epithelial cells and macrophages to exert proliferative, antiapoptotic, and anti-inflammatory actions (49, 104). Overexpression of CYP2C or EETs results in human and murine endothelial cell proliferation (49). Vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation is also associated with increased Cyp2c44 expression and EET generation (49). There appears to be a positive feedback loop because EETs can increase VEGF expression in endothelial cells (49). Endothelial cell proliferation results from EET activation of PI3K/Akt, MAPK, and cAMP/protein kinase A signaling pathways (49). EET activation of the EGF receptor appears to be upstream of these cell-signaling pathways in endothelial and proximal tubule epithelial cells (27, 49). 14,15-EET elicits the release of heparin-binding EGF-like growth factor in renal epithelial cells (27). Activation of the EGF receptor results in Akt activation and increased expression of cyclin D1 (27). MAPK phosphatase-1 inactivation of c-Jun N-terminal kinase has also been implicated in EET increases in cyclin D1 (27). These findings demonstrate that EETs increase endothelial and epithelial cell proliferations by EGF receptor transactivation and increased cyclin D1 signaling mechanisms.
EETs have been demonstrated to protect renal endothelial and epithelial cells from apoptosis. Tumor necrosis factor-α (TNF-α) induced endothelial cell apoptosis is attenuated by overexpression of CYP epoxygenases (49). EETs inhibition of ERK phosphorylation and activation of PI3K/Akt signaling contribute to antiapoptotic actions. EETs, EET analogs, Ephx2 deficiency, and sEH inhibition decrease renal apoptosis through p38 MAPK-regulated mitochondrial pathway (72, 73, 95). EET analogs attenuate cisplatin-induced nephrotoxicity through a reduction in Bcl-2 protein family mediated proapoptotic signaling, reduced renal caspase 12 expression, and reduced caspase 3 activity (72). Likewise, EETs in LLC-PK1 epithelial cells attenuates mitochondrial trafficking of Bax and cytochrome c resulting in decreased caspase 3 activity and oxidative stress (82). Interestingly, the renal endothelial and epithelial antiapoptotic actions of EETs appear to work in concert with anti-inflammatory actions (72, 73, 82).
EET anti-inflammatory actions have been well described and these actions appear to be very important in the kidney. Although endothelial and epithelial cells are the primary source for kidney EETs, macrophages also have the capacity to generate EETs (49). Human monocytes express CYP2J and this expression is increased by macrophage and granulocytecolony stimulating factors (49). EETs or sEH inhibition have been demonstrated to decrease renal inflammation in numerous pathologies (56, 95). The anti-inflammatory actions attributed to EETs or sEH inhibition is mediated through inhibition of phosphor-IKK-derived NFκB activation (Fig. 5). Peroxisome proliferator-activated receptor γ (PPARγ) activation also contributes to the endothelial cell anti-inflammatory effects of EETs and sEH inhibition (56, 95). Likewise, EET analogs and sEH inhibitors decrease renal macrophage infiltration and renal MCP-1 and TNF-α levels (56, 72, 73). More recently, the action of sEH inhibitors to decrease MCP-1, TNF-α, and intercellular adhesion molecule-1 (ICAM-1) in the kidneys appears to decrease renal interstitial fibrosis (95). Inhibition of sEH is anti-inflammatory, fibroprotective, and prevents tubular injury in obstructive nephropathy via down-regulation of NFκB, TGF-β, Smad3, inflammatory signaling pathways, and activation of PPARγ (95). These findings are in agreement with experimental studies demonstrating interactions between EETs and PPARs. Thus, increasing renal EET levels would have protective actions from inflammation in disease states.
20-HETE is a proinflammatory factor that has been less well studied in regards to renal inflammation and apoptosis. Human endothelial cell inflammation is stimulated by incubation with 20-HETE (64). Transduction of endothelial cells with CYP4A hydroxylases to increase 20-HETE levels results in increased ICAM-1 levels and oxidative stress (64). The potent neutrophil chemotactic factor, interleukin 8 (IL-8), is increased by 20-HETE through NF-κB and MAPK/ERK activation (64) (Fig. 5). 20-HETE also causes vascular remodeling of renal resistance arteries (31). On the other hand, 20-HETE prevents the effects of ethanol and TGF-β to increase glomerular permeability (91, 136). Protective actions for 20-HETE to combat Adriamycin toxicity of human renal tubular epithelial cells have also been demonstrated (129). These limited findings on 20-HETE and renal inflammation and apoptosis provide enough evidence that this area requires extensive evaluation in the future.
Genetic Rodent Models
There have been a number of genetic manipulations to rodents that have provided novel insight into the contributions of the LO and CYP enzymes to renal function. These genetic animals have also provided valuable insight concerning LO and CYP metabolites to cardiovascular and renal diseases. The involvement of these eicosanoid metabolites and genetically modified animals in pathological states can be found in excellent review articles (21, 42).
Various aspects of the LO pathway have been genetically modified in animals and consequences to biology, biochemistry, and pathology examined. The majority of experimental studies in these genetically modified mice have focused on inflammation and immune modulation. For instance, the 5-LO-deficient mice have been extensively studied in numerous vascular and inflammatory pathologies but information related to the kidney is very limited (42). Inflammatory responses induced by arachidonic acid were eliminated in 5-LO deficient mice but not wild-type mice by nonselective COX inhibition (40). These findings demonstrated links between prostaglandins and the 5-LO pathway in inflammation. Other LO enzymatic deficient mice include FLAP, LTA4 hydrolase, and LTC4 synthase-deficient mice. Genetic manipulation of leukotriene receptors has also been accomplished in mice. Again, these studies have focused on vascular inflammation, asthma, and arthritis (42). There have also been experimental studies related to the microvasculature with CysLT1 and CysLT2 receptor deficient mice and experimental findings support a role for these receptors to regulate microvascular leakage in inflammation (88, 94). Lastly, the 12/15-LO-deficient mice support a contribution for this pathway in the development of diabetes (32, 105, 118). A role for 12/15-LO to adipocyte inflammation and insulin resistance in response to a high fat diet has also been established (105). Experimental studies with the 12/15-LO-deficient mice and pharmacological inhibitors demonstrate a contribution to hypertension and renal disease (32, 42). Consequently, genetic manipulation of the LO enzymes and receptors have provided evidence for their contribution to inflammation and renal disease; however, these mice have been under utilized in determining contributions to renal vascular and fluid and electrolyte transport physiology.
CYP enzymatic pathway genetic manipulation has established an important contribution for hydroxylase, epoxygenase, and epoxide hydrolase enzymes to renal function and blood pressure control. Experimental studies in gene-deficient mice have also unveiled interactions between hydroxylase and epoxygenase enzymes (21). These findings have defined their physiological functions and provided significance to CYP enzymes on renal function and blood pressure control in humans.
Cyp4a genes in mice have been manipulated and a significant role to renal function has been found. Cyp4a14 was the first gene deficient mouse generated and was associated with increases in plasma androgens, upregulation of renal Cyp4a12(a) expression, and increases in urinary 20-HETE (47). Male and androgen specific hypertension was found in the Cyp4a14−/− mice due in part to 20-HETE actions to increase renal vascular resistance (47). Androgen driven overexpression Cyp4a12 has a similar 20-HETE driven renal vascular hypertensive phenotype (137). Increasing vascular CYP4A2 in rats also results increased 20-HETE levels and hypertension (63). Interestingly, the Cyp4a10 gene-deficient mice have no change in Cyp4a12 expression or urinary 20-HETE levels but have downregulated renal Cyp2c44 expression (97). Cyp4a10−/− mice develop salt-sensitive hypertension in response to high Na+ salt and have increased collecting duct ENaC activity (97). These findings provided support for the contribution of 20-HETE to increase renal vascular resistance and the first genetic evidence for interactions between the CYP hydroxylase and epoxygenase enzymatic pathways.
Additional support for vasodilator and natriuretic actions for EETs has been provided in mice with genetic manipulation of the epoxygenase and sEH enzymes. Gene deficiency in the sEH (Ephx2) or overexpressing human CYP2C8 or CYP2J2 in endothelial cells improves afferent arteriolar function and influences blood pressure control (80, 89, 121). Initial reports suggested a male-specific effect on blood pressure in Ephx2−/− mice; however, generation of Ephx2 on different genetic backgrounds failed to demonstrate sex-specific differences on blood pressure control (83, 121). In addition, overexpressing human CYP2C8 or CYP2J2 in endothelial cells had similar effects on afferent arteriolar responses to endothelin-1 and acetylcholine from male and female mice (80). More recently, Cyp2c44 gene deficient mice have been generated and demonstrate salt-sensitive hypertension associated with increased ENaC activity (20). Cyp2c44 is unique in that it generates primarily highly stereo-selective 11,12-EET and 14,15-EET and very small amounts of 8,9-EET (20). Thus, genetic manipulation of epoxygenase metabolites has provided strong support to the concept that 11,12-EET and 14,15-EET are the major contributors to renal vasodilator and natriuretic actions.
Even though genetic animal models have provided insight into CYP and LO metabolites to renal function and pathologies, these genetically manipulated animals have been largely underutilized. This underutilization is due to differences in genetic background, time required to receive mice that are cryopreserved, breeding and colony maintenance, and other expenses associated with genetic animals. Pharmacological tools therefore remain the predominant approach to determine the contribution of eicosanoids to renal function. Thus, there remains untapped and unexplored potential for experimental studies using these unique genetically modified CYP and LO mice that could provide valuable insight on the contribution of these metabolites to renal vascular and transport physiology.
Conclusion
Renal physiological function is clearly influenced by the vascular and tubular actions of LO and CYP metabolites of arachidonic acid. Their contribution to the ability of the kidney to maintain whole body fluid and electrolyte homeostasis is clear and evident. The generation of LO metabolites also are extremely important to renal inflammatory responses. These renal vascular and tubular actions depend on activation of LO metabolite specific receptors and cell signaling mechanisms. CYP metabolites are extremely important for proper sodium reabsorption and excretion. These CYP metabolite tubular transport actions depend on cell signaling mechanisms and effects on apical and basolateral ion channels. Unlike LO metabolites, CYP metabolite receptors have yet to be identified. Although there is a rapidly expanding tool-box of pharmacological agents and genetic animals, this has not been fully taken advantage of to better understand the contribution of CYP and LO metabolites to renal physiological function.
Expansion of the genetic, pharmacological and biochemical approaches to evaluate the CYP and LO pathways allows for experimental studies to determine and define their renal physiological relevance. Genetic tools and animal models are now readily available for LO and CYP enzymatic pathways. Mice deficient in the Cyp2c, Ephx2, and Cyp4a genes have already yielded unequivocal evidence of their role in hypertension and renal vascular and tubular transport (18, 19). Human CYP2C and CYP2J enzymes have been expressed in select tissues in mice including endothelial cells (80). Antisense nucleotide inhibition of CYP4A1/CYP4A2 expression lowers blood pressure in spontaneously hypertensive rats (136, 138).
Vascular CYP4A overexpression in rats causes renal endothelial dysfunction and increases blood pressure (138). Like CYP enzymatic pathways, LO genetic deficient mice have been generated. These include genetic knockout of genes for LO enzymes and receptors. Experimental studies in these LO mice have determined the contribution of this pathway to renal inflammation and diabetic nephropathy (32). Genetic mice with endothelial cell specific knockout or overexpression of the LO and CYP pathways already exist and renal function has been studied to some extent. Cell specific genetic manipulation of glomerular and tubular cells should soon be available and will yield interesting data on renal function.
Human and animal studies have demonstrate sex-specific differences with relation to CYP eicosanoids and renal function (21). There is strong evidence for androgen regulation of 20-HETE that contributes to renal vascular function and blood pressure control (47, 137). There has been conflicting data on sex-specific differences and the epoxygenase pathway. Recent findings have demonstrated that renal vascular EET levels are higher in female SHR compared to males (96). The functional consequences of this sex-specific difference in renal vascular EET levels remain unknown. Overall eicosanoid sex-specific differences in relation to renal hemodynamics and fluid and electrolyte transport remains an area that requires further evaluation.
Pharmacological tools have been developed and include enzymatic inhibitors, receptor antagonists, mimetics and agonists, and antagonists for LO and CYP pathways. These pharmacological tools have been instrumental in determining the renal physiological roles for LO and CYP enzymes, metabolites, and receptors. Development of many of these chemical compounds has rapidly progressed based on the identification and determination of the enzymatic and receptor protein structures. In the case of sEH inhibitors, there was such a rapid development that inhibitors reached the point of human clinical trials within a decade from determining their contribution to renal function (56). A major hurdle that has been overcome is the ability to utilize CYP and LO enzymatic inhibitors, receptor antagonists, mimetics and agonists, and antagonists in an in vivo experimental setting. These novel CYP and LO pharmacological tools can be now used to evaluate their contributions to renal function and to determine their therapeutic potential to combat renal diseases.
Another emerging area that requires further investigation is the interactions between the eicosanoid metabolic pathways. A number of studies have determined that COX enzymes metabolize EETs and 20-HETE and these metabolites have renal vascular and tubule transport activities (23, 36, 48, 58). It is also apparent that eicosanoid metabolites can regulate the enzymatic activity of other eicosanoid metabolic pathways. There is also the fact that eicosanoid metabolites of different pathways can act in synergy or opposition in regards to renal actions. For example, inhibitors of sEH can synergize and enhance the anti-inflammatory actions of COX-2 inhibitors (116). Although there have been advances in the ability to measure these various eicosanoid metabolites, most experimental studies focus on a single eicosanoid pathway. Thus, there is a need to pay greater attention to overall changes in eicosanoid metabolites and how these influence renal function. Future studies are undoubtedly warranted to determine the impact of novel CYP and LO metabolites and interactions between these enzymatic pathways and the COX pathway on renal function.
Although the renal physiological actions LO and CYP metabolites of arachidonic acid are the most abundant and well characterized, LO and CYP metabolites of other fatty acids are generated and could contribute significantly to renal vascular and tubular function. CYP epoxygenase metabolites of ω-3 polyunsaturated fatty acids can have renal anti-inflammatory actions (130). Fish oil or ω-3 polyunsaturated fatty acid diet rich in eicosapentaenoic acid and docosahexaenoic acid (DHA) coupled with sEH inhibitors lowers blood pressure and provides superior anti-inflammatory effects in hypertension (130). The DHA epoxide 19,20-epoxy docosapentaenoic acid (19,20-EDP) contributes to the antihypertensive action (130); however, the renal vascular and tubular actions of 19,20-EDP remain unknown. Another novel class of epoxygenase arachidonic acid metabolites is termed 2-epoxyeiocatrienoiylglycerols (2-EG) (26). The kidney generates 2-11,12-EG and 2-14,15-EG that activate cannabinoid CB1 and CB2 receptors with high affinity (26). Afferent arterioles dilate in response to 2-11,12-EG and this response is inhibited by CB1 receptor antagonism (26). Renal vascular and transport actions of these novel CYP and LO metabolites will unquestionably be a future focus.
CYP and LO metabolites have been extensively evaluated and these metabolites importantly impact renal hemodynamics and fluid and electrolyte balance. Improper regulation of CYP and LO metabolites also contributes to renal dysfunction and injury in many disease states. Despite the fact that there is significant evidence to support the notion that CYP and LO metabolites contribute to renal physiology and pathology there is still major gaps that persist. Finally, translational work will be required to apply these basic discoveries on CYP and LO metabolites into treatment for human diseases.
Acknowledgments
An American Heart Association Midwest Affiliate Grant-in-Aid and a postdoctoral fellowship from the Midwest Affiliate of the American Heart Association supported this work. Servier Medical Art was used to generate Figures 2-5 and is licensed by Servier under a Creative Commons Attribution 3.0 Unported License.
References
- 1.Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 4.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Roman RJ. Role of 20-Hydroxyeicosatetraeinoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regulatory, Integrative Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 6.Anning PB, Coles B, Bermudez-Fajardo A, Martin PE, Levison BS, Hazen SL, Funk CD, Kuhn H, O’Donnell VB. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol. 2005;166:653–662. doi: 10.1016/S0002-9440(10)62287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arima S, Omata K, Ito S, Tsunoda K, Abe K. 20-HETE requires increased vascular tone to constrict rabbit afferent arterioles. Hypertension. 1996;27:781–785. doi: 10.1161/01.hyp.27.3.781. [DOI] [PubMed] [Google Scholar]
- 8.Badr KF. Five-lipoxygenase products in glomerular immune injury. J Am Soc Nephrol. 1992;3:907–915. doi: 10.1681/ASN.V34907. [DOI] [PubMed] [Google Scholar]
- 9.Badr KF. Glomerulonephritis: Roles for lipoxygenase pathways in pathophysiology and therapy. Curr Opin Nephrol Hypertens. 1997;6:111–118. [PubMed] [Google Scholar]
- 10.Badr KF, DeBoer DK, Schwartzberg M, Serhan CN. (1989) Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: Evidence for competition at a common receptor. Proc Natl Acad Sci U S A. 1989;86:3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol. 2013;61:188–196. doi: 10.1097/FJC.0b013e318273b007. [DOI] [PubMed] [Google Scholar]
- 12.Bell-Quilley CP, Lin YS, Hilchey SD, Drugge ED, McGiff JC. Renovascular actions of angiotensin II in the isolated kidney of the rat: Relationship to lipoxygenases. J Pharmacol Exp Ther. 1993;267:676–682. [PubMed] [Google Scholar]
- 13.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 14.Breyer MD, Zhang Y, Guan YF, Hao CM, Hebert RL, Breyer RM. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl. 1998;67:S88–S94. doi: 10.1046/j.1523-1755.1998.06718.x. [DOI] [PubMed] [Google Scholar]
- 15.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: Subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 16.Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Konduru SR, Imig JD, Falck JR, Campbell WB. 11,12,20-Trihydroxy-eicosa-8(Z)-enoic acid: A selective inhibitor of 11,12-EET induced relaxations of bovine coronary and rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H1574–H1583. doi: 10.1152/ajpheart.01122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Câmara NO, Martins JO, Landgraf RG, Jancar S. Emerging roles for eicosanoids in renal diseases. Curr Opin Nephrol Hypertens. 2009;18:21–27. doi: 10.1097/MNH.0b013e32831a9df7. [DOI] [PubMed] [Google Scholar]
- 18.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 19.Capdevila JH, Pidkovka N, Mei S, Gong Y, Falck JR, Imig JD, Harris RC, Wang W. The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increased dietary salt. J Biol Chem. 2014;289:4377–4386. doi: 10.1074/jbc.M113.508416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capdevila J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. doi: 10.1097/MNH.0b013e32835d911e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: Genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediat. 2015 doi: 10.1016/j.prostaglandins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnini C, Accomazzo MR, Borroni E, Vitellaro-Zuccarello L, Durand T, Folco G, Rovati GE, Capra V, Sala A. Synthesis of cysteinyl leukotrienes in human endothelial cells: Subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J. 2011;25:3519–3528. doi: 10.1096/fj.10-177030. [DOI] [PubMed] [Google Scholar]
- 23.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–12266. [PubMed] [Google Scholar]
- 24.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther. 1992;260:104–109. [PubMed] [Google Scholar]
- 25.Carroll MA, Kang Y, Chander PN, Stier CT., Jr Azilsartan is associated with increased circulating angiotensin-(1-7) levels and reduced renovascular 20-HETE levels. Am J Hypertens. 2015;28:664–671. doi: 10.1093/ajh/hpu201. [DOI] [PubMed] [Google Scholar]
- 26.Chen JK, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, Falck JR, Capdevila JH, Harris RC. Identification of novel endogenous cytochrome P450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 28.Cheng HF, Harris RC. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 29.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y, Wu CC, Garcia V, Dimitrova I, Weidenhammer A, Joseph G, Zhang F, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. 20-HETE induces remodeling of renal resistance arteries independent of blood pressure elevation in hypertension. Am J Physiol Renal Physiol. 2013;305:F753–F763. doi: 10.1152/ajprenal.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falck JR, Koduru SR, Mohapatra S, Manne R, Atcha KR, Manthati VL, Capdevila JH, Christian S, Imig JD, Campbell WB. Robust surrogates of 14,15-epoxyeicosa-5,8,11-trienoic acid (14,15-EET): Carboxylate modifications. J Med Chem. 2014;57:6965–6972. doi: 10.1021/jm500262m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanning LB, Boyce JA. Lipid mediators and allergic diseases. Ann Allergy Asthma Immunol. 2013;111:155–162. doi: 10.1016/j.anai.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez MM, Gonzalez D, Williams JM, Roman RJ, Nowicki S. Inhibitors of 20-hydroxyeicosatetraenoic acid (20-HETE) formation attenuate the natriuretic effect of dopamine. Eur J Pharmacol. 2012;686:97–103. doi: 10.1016/j.ejphar.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulton D, Balazy M, McGiff JC, Quilley J. Possible contribution of platelet cyclooxygenase to the renal vascular action of 5,6-epoxyeicosatrienoic acid. J Pharmacol Exp Ther. 1996;277:1195–1199. [PubMed] [Google Scholar]
- 37.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O’Donnell CJ, Brown NJ, Waterman MR, Capdevila JH. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 38.Ge Y, Murphy SR, Lu Y, Falck J, Liu R, Roman RJ. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat. 2013;102-103:42–48. doi: 10.1016/j.prostaglandins.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Núñez D, Solé M, Natarajan R, Poch E. 12-Lipoxygenase metabolism in mouse distal convoluted tubule cells. Kidney Int. 2005;67:178–186. doi: 10.1111/j.1523-1755.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- 40.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci U S A. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu RM, Yang L, Zhang Y, Wang L, Kong S, Zhang C, Zhai Y, Wang M, Wu P, Liu L, Gu F, Zhang J, Wang WH. CYP-omega-hydroxylation-dependent metabolites of arachidonic acid inhibit the basolateral 10 pS chloride channel in the rat thick ascending limb. Kidney Int. 2009;76:849–856. doi: 10.1038/ki.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 43.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 44.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 45.Harris RC, Zhang MZ. Cyclooxygenase metabolites in the kidney. Compr Physiol. 2011;1:1729–1758. doi: 10.1002/cphy.c100077. [DOI] [PubMed] [Google Scholar]
- 46.Hercule HC, Wang MH, Oyekan AO. Contribution of cytochrome P450 4A isoforms to renal functional response to inhibition of nitric oxide production in the rat. J Physiol. 2003;551:971–979. doi: 10.1113/jphysiol.2003.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imig JD. Eicosanoids and renal vascular function in diseases. Clin Sci (Lond) 2006;111:21–34. doi: 10.1042/CS20050251. [DOI] [PubMed] [Google Scholar]
- 49.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imig JD. Epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, and renal microvascular function. Prostaglandins Other Lipid Mediat. 2013;104-105:2–7. doi: 10.1016/j.prostaglandins.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imig JD, Deichmann PC. Afferent arteriolar responses to ANG II involve activation of PLA2 and modulation by lipoxygenase and P-450 pathways. Am J Physiol. 1997;273:F274–F282. doi: 10.1152/ajprenal.1997.273.2.F274. [DOI] [PubMed] [Google Scholar]
- 53.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 56.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11, 12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 58.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 59.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 60.Imig JD, Zou A-P, Ortiz de Montellano PR, Sui Z, Roman RJ. Cytochrome P450 inhibitors alter the afferent arteriolar response to elevations in perfusion pressure. Am J Physiol Heart Circ Physiol. 1994;266:H1879–H1885. doi: 10.1152/ajpheart.1994.266.5.H1879. [DOI] [PubMed] [Google Scholar]
- 61.Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 62.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297:F875–F884. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 65.Jacobson HR, Corona S, Capdevila JH, Chacos N, Manna S, Womack A, Falck JR. Effects of epoxyeicosatrienoic acids on ion transport in the rabbit cortical collecting tubule. In: Braquet P, Garay RP, Frohlich JC, Nicosia S, editors. Prostaglandins, and Membrane Ion Transport. New York: Raven Press; 1985. pp. 311–318. [Google Scholar]
- 66.Kaide J, Wang MH, Wang JS, Zhang F, Gopal VR, Falck JR, Nasjletti A, Laniado-Schwartzman M. Transfection of CYP4A1 cDNA increases vascular reactivity in renal interlobar arteries. Am J Physiol Renal Physiol. 2003;284:F51–F56. doi: 10.1152/ajprenal.00249.2002. [DOI] [PubMed] [Google Scholar]
- 67.Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, Kashtan C, Adler SG. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen alpha5(IV) in experimental diabetic nephropathy and in glucose-stimulated podocytes. J Am Soc Nephrol. 2003;14:3178–3187. doi: 10.1097/01.asn.0000099702.16315.de. [DOI] [PubMed] [Google Scholar]
- 68.Kanli H, Terreros DA. Transepithelial transport and cell volume control in proximal renal tubules from the teleost Carassius auratus. Acta Physiol Scand. 1997;160:267–276. doi: 10.1046/j.1365-201X.1997.00155.x. [DOI] [PubMed] [Google Scholar]
- 69.Katoh T, Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF. Glomerular stereospecific synthesis and hemodynamic actions of 8,9-epoxyeicosatrienoic acid in rat kidney. Am J Physiol. 1991;261:F578–F586. doi: 10.1152/ajprenal.1991.261.4.F578. [DOI] [PubMed] [Google Scholar]
- 70.Katoh T, Takahashi K, DeBoer DK, Serhan CN, Badr KF. Renal hemodynamic actions of lipoxins in rats: A comparative physiological study. Am J Physiol. 1992;263:F436–F442. doi: 10.1152/ajprenal.1992.263.3.F436. [DOI] [PubMed] [Google Scholar]
- 71.Kelefiotis D, Bresnahan BA, Stratidakis I, Lianos EA. Eicosanoid-induced growth and signaling events in rat glomerular mesangial cells. Prostaglandins. 1995;49:269–83. doi: 10.1016/0090-6980(95)00049-g. [DOI] [PubMed] [Google Scholar]
- 72.Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27:2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan MA, Manthati V, Errabelli R, Pavlov TS, Staruschenko A, Falck JR, Imig JD. An orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt sensitive rat. Hypertension. 2013;62:905–913. doi: 10.1161/HYPERTENSIONAHA.113.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kieran NE, Maderna P, Godson C. Lipoxins: Potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim YS, Reddy MA, Lanting L, Adler SG, Natarajan R. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int. 2003;64:1702–1714. doi: 10.1046/j.1523-1755.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 76.Kim YS, Xu ZG, Reddy MA, Li SL, Lanting L, Sharma K, Adler SG, Natarajan R. Novel interactions between TGF-{beta}1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol. 2005;16:352–362. doi: 10.1681/ASN.2004070568. [DOI] [PubMed] [Google Scholar]
- 77.Laffer CL, Laniado-Schwartzman M, Wang MH, Nasjletti A, Elijovich F. 20-HETE and furosemide-induced natriuresis in salt-sensitive essential hypertension. Hypertension. 2003;41:703–708. doi: 10.1161/01.HYP.0000051888.91497.47. [DOI] [PubMed] [Google Scholar]
- 78.Laffer CL, Laniado-Schwartzman M, Wang MH, Nasjletti A, Elijovich F. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic Acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003;107:574–578. doi: 10.1161/01.cir.0000046269.52392.14. [DOI] [PubMed] [Google Scholar]
- 79.Landgraf SS, Silva LS, Peruchetti DB, Sirtoli GM, Moraes-Santos F, Portella VG, Silva-Filho JL, Pinheiro CS, Abreu TP, Takiya CM, Benjamin CF, Pinheiro AA, Canetti C, Caruso-Neves C. 5-Lypoxygenase products are involved in renal tubulointerstitial injury induced by albumin overload in proximal tubules in mice. PLoS One. 2014;9:e107549. doi: 10.1371/journal.pone.0107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow L, Lepp AN, Kannon A, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li D, Belusa R, Nowicki S, Aperia A. Arachidonic acid metabolic pathways regulating activity of renal Na(+)-K(+)-ATPase are age dependent. Am J Physiol Renal Physiol. 2000;278:F823–F829. doi: 10.1152/ajprenal.2000.278.5.F823. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Lu X, Nguyen S, Olson JL, Webb HK, Kroetz DL. Epoxyeicosatrienoic acids prevent cisplatin-induced renal apoptosis through a p38 mitogen-activated protein kinase-regulated mitochondrial pathway. Mol Pharmacol. 2013;84:925–934. doi: 10.1124/mol.113.088302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282:2891–2898. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma SK, Wang Y, Chen J, Zhang MZ, Harris RC, Chen JK. Overexpression of G-protein-coupled receptor 40 enhances the mitogenic response to epoxyeicosatrienoic acids. PLoS One. 2015;10:e0113130. doi: 10.1371/journal.pone.0113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma Y, Zhang P, Li J, Lu J, Ge J, Zhao Z, Ma X, Wan S, Yao X, Shen B. Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim Biophys Acta. 2015;1852:552–559. doi: 10.1016/j.bbadis.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 87.Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12-HETE on isolated dog renal arcuate arteries. Am J Physiol. 1991;261:H451–H456. doi: 10.1152/ajpheart.1991.261.2.H451. [DOI] [PubMed] [Google Scholar]
- 88.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 89.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuda H, Hayashi K, Wakino S, Kubota E, Honda M, Tokuyama H, Takamatsu I, Tatematsu S, Saruta T. Role of endothelium-derived hyperpolarizing factor in ACE inhibitor-induced renal vasodilation in vivo. Hypertension. 2004;43:603–609. doi: 10.1161/01.HYP.0000118053.42262.71. [DOI] [PubMed] [Google Scholar]
- 91.McCarthy ET, Zhou J, Eckert R, Genochio D, Sharma R, Oni O, De A, Srivastava T, Sharma R, Savin VJ, Sharma M. Ethanol at low concentrations protects glomerular podocytes through alcohol dehydrogenase and 20-HETE. Prostaglandins Other Lipid Mediat. 2015;116-117:88–98. doi: 10.1016/j.prostaglandins.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGiff JC, Quilley J. 20-HETE and the kidney: Resolution of old problems and new beginnings. Am J Physiol. 1999;277:R607–R623. doi: 10.1152/ajpregu.1999.277.3.R607. [DOI] [PubMed] [Google Scholar]
- 93.Molin DG, van den Akker NM, Post MJ. ‘Lox on neovascularization’: Leukotrienes as mediators in endothelial biology. Cardiovasc Res. 2010;86:6–8. doi: 10.1093/cvr/cvq054. [DOI] [PubMed] [Google Scholar]
- 94.Moos MP, Mewburn JD, Kan FW, Ishii S, Abe M, Sakimura K, Noguchi K, Shimizu T, Funk CD. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB J. 2008;22:4352–4362. doi: 10.1096/fj.08-113274. [DOI] [PubMed] [Google Scholar]
- 95.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moulana M, Hosick K, Stanford J, Zhang H, Roman RJ, Reckelhoff JF. Sex differences in blood pressure control in SHR: Lack of a role for EETs. Physiol Rep. 2014;2 doi: 10.14814/phy2.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakagawa K, Holla VJ, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nasrallah R, Clark J, Hébert RL. Prostaglandins in the kidney: Developments since Y2K. Clin Sci (Lond) 2007;113:297–311. doi: 10.1042/CS20070089. [DOI] [PubMed] [Google Scholar]
- 99.Natarajan R, Gonzales N, Lanting L, Nadler J. Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension. 1994;23:I142–I147. doi: 10.1161/01.hyp.23.1_suppl.i142. [DOI] [PubMed] [Google Scholar]
- 100.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 101.Natarajan R, Reddy MA. HETEs/EETs in renal glomerular and epithelial cell functions. Curr Opin Pharmacol. 2013;3:198–203. doi: 10.1016/s1471-4892(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 102.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 103.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23:1019–1026. doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295:E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nüsing RM, Schweer H, Fleming I, Zeldin DC, Wegmann M. Epoxyeicosatrienoic acids affect electrolyte transport in renal tubular epithelial cells: Dependence on cyclooxygenase and cell polarity. Am J Physiol Renal Physiol. 2007;293:F288–F298. doi: 10.1152/ajprenal.00171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oyekan A, Balazy M, McGiff JC. Renal oxygenases: Differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997;273:R293–R300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- 108.Oyekan AO, McGiff JC. Cytochrome P-450-derived eicosanoids participate in the renal functional effects of ET-1 in the anesthetized rat. Am J Physiol. 1998;274:R52–R61. doi: 10.1152/ajpregu.1998.274.1.R52. [DOI] [PubMed] [Google Scholar]
- 109.Pawloski JR, Chapnick BM. Antagonism of LTD4-evoked relaxation in canine renal artery and vein. Am J Physiol. 1993;265:H980–H985. doi: 10.1152/ajpheart.1993.265.3.H980. [DOI] [PubMed] [Google Scholar]
- 110.Perico N, Cornejo RP, Benigni A, Malanchini B, Ladny JR, Remuzzi G. Endothelin induces diuresis and natriuresis in the rat by acting on proximal tubular cells through a mechanism mediated by lipoxygenase products. J Am Soc Nephrol. 1991;2:57–69. doi: 10.1681/ASN.V2157. [DOI] [PubMed] [Google Scholar]
- 111.Ren Y, D’Ambrosio MA, Garvin JL, Peterson EL, Carretero OA. Mechanism of impaired afferent arteriole myogenic response in Dahl salt-sensitive rats: Role of 20-HETE. Am J Physiol Renal Physiol. 2014;307:F533–F538. doi: 10.1152/ajprenal.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren Y, Garvin J, Carretero OA. Mechanism involved in bradykinin-induced efferent arteriole dilation. Kidney Int. 2002;62:544–549. doi: 10.1046/j.1523-1755.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 113.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 114.Roman RJ, Alonso-Galicia M. P-450 Eicosanoids: A novel signaling pathway regulating renal function. News Physiol Sci. 1999;14:238–242. doi: 10.1152/physiologyonline.1999.14.6.238. [DOI] [PubMed] [Google Scholar]
- 115.Romero MF, Madhun ZT, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid 5,6 epoxy-eicosatrienoic acid mediates angiotensin-induced natriuresis in proximal tubular epithelium. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:205–208. [PubMed] [Google Scholar]
- 116.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na++K+)ATPase. Nature. 1985;314:620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- 118.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, Falck JR. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier. Prostaglandins Other Lipid Mediat. 2009;89:43–51. doi: 10.1016/j.prostaglandins.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 122.Sodhi K, Wu CC, Cheng J, Gotlinger KH, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-HETE and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 124.Stec DE, Flasch A, Roman RJ, White JA. Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am J Physiol Renal Physiol. 2003;284:F95–F102. doi: 10.1152/ajprenal.00132.2002. [DOI] [PubMed] [Google Scholar]
- 125.Stern N, Yanagawa N, Saito F, Hori M, Natarajan R, Nadler J, Tuck M. Potential role of 12 hydroxyeicosatetraenoic acid in angiotensin II-induced calcium signal in rat glomerulosa cells. Endocrinology. 1993;133:843–847. doi: 10.1210/endo.133.2.8344221. [DOI] [PubMed] [Google Scholar]
- 126.Storch U, Blodow S, Gudermann T, Mederos Y, Schnitzler M. Cysteinyl leukotriene1 receptors as novel mechanosensors mediating myogenic tone together withangiotensin II type 1 receptors-brief report. Arterioscler Thromb Vasc Biol. 2015;35:121–126. doi: 10.1161/ATVBAHA.114.304844. [DOI] [PubMed] [Google Scholar]
- 127.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17:1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takai S, Jin D, Kirimura K, Ikeda J, Sakaguchi M, Baba K, Fujita T, Miyazaki M. Effects of a lipoxygenase inhibitor, panaxynol, on vascular contraction induced by angiotensin II. Jpn J Pharmacol. 1999;80:89–92. doi: 10.1254/jjp.80.89. [DOI] [PubMed] [Google Scholar]
- 129.Tian T, Li J, Wang MY, Xie XF, Li QX. Protective effect of 20-hydroxyeicosatetraenoic acid (20-HETE) on adriamycin-induced toxicity of human renal tubular epithelial cell (HK-2) Eur J Pharmacol. 2012;683:246–251. doi: 10.1016/j.ejphar.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 130.Ulu A, StephenLee KS, Miyabe C, Yang J, Hammock BG, Dong H, Hammock BD. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2014;64:87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contributions of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney Int. 2003;63:2187–2193. doi: 10.1046/j.1523-1755.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Garvin JL, Falck JR, Ren Y, Sankey SS, Carretero OA. Glomerular cytochrome P-450 and cyclooxygenase metabolites regulate efferent arteriole resistance. Hypertension. 2005;46:1175–1179. doi: 10.1161/01.HYP.0000187531.93389.63. [DOI] [PubMed] [Google Scholar]
- 133.Wang M, Sui H, Li W, Wang J, Liu Y, Gu L, Wang WH, Gu R. Stimulation of A2a adenosine receptor abolishes the inhibitory effect of arachidonic acid on the basolateral 50-pS K channel in the thick ascending limb. Am J Physiol Renal Physiol. 2011;300:F906–F913. doi: 10.1152/ajprenal.00617.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Trottier G, Loutzenhiser R. Determinants of renal afferent arteriolar actions of bradykinin: Evidence that multiple pathways mediate responses attributed to EDHF. Am J Physiol Renal Physiol. 2003;285:F540–F549. doi: 10.1152/ajprenal.00127.2003. [DOI] [PubMed] [Google Scholar]
- 135.Ward NC, Rivera J, Hodgson J, Puddey IB, Beilin LJ, Falck JR, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 2004;110:438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 136.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu ZG, Li SL, Lanting L, Kim YS, Shanmugam N, Reddy MA, Natarajan R. Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: Potential role in diabetic nephropathy. Kidney Int. 2006;69:512–519. doi: 10.1038/sj.ki.5000137. [DOI] [PubMed] [Google Scholar]
- 140.Xu ZG, Yuan H, Lanting L, Li SL, Wang M, Shanmugam N, Kato M, Adler SG, Reddy MA, Natarajan R. Products of 12/15-lipoxygenase upregulate the angiotensin II receptor. J Am Soc Nephrol. 2008;19:559–569. doi: 10.1681/ASN.2007080939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamoto S. Mammalian lipoxygenases: Molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 142.Yang H, Dou Y, Zheng X, Tan Y, Cheng J, Li L, Du Y, Zhu D, Lou Y. Cysteinyl leukotrienes synthesis is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Toxicology. 2011;287:38–45. doi: 10.1016/j.tox.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Yiu SS, Zhao X, Inscho EW, Imig JD. 12-Hydroxyeicosatetraenoic acid participates in angiotensin II afferent arteriolar vasoconstriction by activating L-type calcium channels. J Lipid Res. 2003;44:2391–2399. doi: 10.1194/jlr.M300183-JLR200. [DOI] [PubMed] [Google Scholar]
- 144.Zhang MZ, Wang Y, Yao B, Gewin L, Wei S, Capdevila JH, Harris RC. Role of epoxyeicosatrienoic acids (EETs) in mediation of dopamine’s effects in the kidney. Am J Physiol Renal Physiol. 2013;305:F1680–F1686. doi: 10.1152/ajprenal.00409.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao X, Falck JR, Gopal VR, Inscho EW, Imig JD. P2X receptor-stimulated calcium responses in preglomerular vascular smooth muscle cells involves 20-hydroxyeicosatetraenoic acid. J Pharmacol Exp Ther. 2004;311:1211–1217. doi: 10.1124/jpet.104.070797. [DOI] [PubMed] [Google Scholar]
- 146.Zhao X, Inscho EW, Bondlela M, Falck JR, Imig JD. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H2089–H2096. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- 147.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 148.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K +channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 149.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 150.Zou A-P, Imig JD, Ortiz de Montellano PR, Sui Z, Falck JR, Roman RJ. Effect of P-450 ω-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Physiol. 1994;266:F934–F941. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 151.Zou A-P, Imig JD, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE impairs autoregulation of renal blood flow. Am J Physiol Renal Physiol. 1994;266:F275–F282. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]


